Abstract
The Yersinia plasmid-encoded Yop virulon enables extracellular adhering bacteria to deliver toxic effector proteins inside their target cells. It includes a type III secretion system (Ysc), at least two translocator proteins (YopB, YopD), and a set of intracellular Yop effectors (YopE, YopH, YopO, YopM, and YopP). Infection of macrophages with a wild-type strain leads to low levels of tumor necrosis factor alpha (TNF-α) release compared to infection with plasmid-cured strains, suggesting that the virulence plasmid encodes a factor impairing the normal TNF-α response of infected macrophages. This effect is correlated with the inhibition of the macrophage mitogen-activated protein kinase (MAPK) activities. To identify the Yop protein responsible for the suppression of TNF-α release, we infected J774A.1 and PU5-1.8 macrophages with a battery of knockout Yersinia enterocolitica mutants and we quantified the TNF-α released. Mutants affected in secretion (yscN), in translocation (yopB and yopD), or in synthesis of all the known Yop effectors (yopH, yopO, yopP, yopE, and yopM polymutants) were unable to block the TNF-α response of the macrophages. In contrast, single yopE, yopH, yopO, and yopM mutants behaved like the wild-type strain. A yopP mutant elicited elevated TNF-α release, and complementation of the yopP mutant or the yop effector polymutant strain with yopP alone led to a drop in TNF-α release. In addition, YopP was also responsible for the inhibition of the extracellular signal-regulated kinase2 (ERK2) and p38 MAPK activities. These results show that YopP is the Yop effector responsible for the Yersinia-induced suppression of TNF-α release by infected macrophages.
Pathogenic yersiniae (Yersinia pestis, Y. pseudotuberculosis, and Y. enterocolitica) possess a complex plasmid-encoded anti-host system called the Yop virulon, which enables them to overcome the defense mechanisms of their host and to survive in the lymphoid tissues. This virulence apparatus allows extracellular adhering bacteria to deliver toxic effector proteins inside the target cells to damage them or disrupt their communications (for a review, see reference 14). The Yop virulon is composed of four elements that are (i) a type III secretion machinery called Ysc (for a review, see reference 13), allowing the bacteria to secrete the Yop proteins upon contact with the eukaryotic target cell; (ii) a set of proteins (YopB, YopD, and LcrV) required to translocate the effector proteins inside the eukaryotic cells (9, 24, 39, 45, 48, 53, 54); (iii) a control and recognition system consisting of YopN and LcrG (9, 10, 19, 39, 45, 49); and (iv) at least five effector proteins, namely, YopE, YopH, YpkA-YopO, YopM, and YopP (9, 23, 39, 45, 53–55). Recently, Holmström et al. (27) published data suggesting that the YopK protein of Y. pseudotuberculosis (YopQ in Y. enterocolitica) controls the size of the pore allowing translocation of Yop effectors into eukaryotic cells.
Until now, the intracellular action of each of the five translocated Yop effectors has not been completely elucidated. YopE, the first Yop effector to be shown to be targeted inside eukaryotic cells (45, 54), causes the destruction of the actin microfilament structures (44), but its molecular target as well as its enzymatic activity remain unknown. YopH, which is the effector that has been characterized best, is a protein tyrosine phosphatase (22) that acts on eukaryotic proteins such as the focal adhesion kinase and p130Cas (6, 38). YpkA-YopO is a serine-threonine kinase (20) whose target remains unknown. The enzymatic activities of YopM and YopP have not been identified yet. The Yop effectors act in concert to disarm the macrophages. For example, both the YopE cytotoxin and YopH contribute to impair phagocytosis (18, 42, 43). YopH is also involved in the inhibition of the respiratory burst of professional phagocytes (7, 26).
The release of cytokines is an important part of the immune response against an infection, and several studies have described the importance of tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and interleukin-12 (IL-12) in the immune response against Yersinia (1, 2, 8, 36). Indeed, in vivo neutralization of TNF-α or IFN-γ exacerbates the Yersinia infection (1–3). IL-12 also plays an essential role in the resistance against Y. enterocolitica infection by triggering the production of IFN-γ in natural killer and T cells (8). Wild-type yersiniae impair the normal TNF-α response of infected macrophages (4, 36, 37, 46), and this effect has been recently correlated to the inactivation of the macrophage mitogen-activated protein kinases (MAPKs) extracellular signal-regulated kinase (ERK1/2), p38, and c-Jun NH2-terminal kinase (JNK) (46). Yersiniae not only perturb the cytokine release of macrophages but also prevent T84 colon epithelial cells from releasing IL-8, which is a potent chemoattractant for polymorphonuclear neutrophils (51).
The Yersinia-induced suppression of TNF-α release has been attributed to various proteins. First, Nakajima and Brubaker (36) showed that mice infected with wild-type Y. pestis produce much less TNF-α than mice infected with Y. pestis cured of the pYV plasmid, indicating that a factor suppressing TNF-α synthesis is encoded by the pYV plasmid. Their further studies suggested that LcrV plays a critical role in this process (36, 37). Another study, conducted with cultured macrophages, confirmed that virulent Yersinia suppresses TNF-α release but identified the YopB protein as being responsible (4).
Since both YopB and LcrV are involved in the translocation machinery (9, 24, 48), one can speculate that their role is to translocate an intracellular effector that is responsible for suppression of TNF-α release. We thus decided to identify this effector with a series of knockout yop mutants. We show here that YopP is involved in this inhibition of TNF-α release by infected macrophages and that it is also involved in the inactivation of the ERK2 and p38 MAPK.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
This work was carried out with Y. enterocolitica E40(pYV40) (53), its isogenic ampicillin-sensitive derivative MRS40(pYV40) (49), and their various nonpolar mutants. The plasmids used here are listed in Table 1. Bacteria were grown in brain heart infusion; after overnight preculture, bacteria were diluted 1/20 in fresh brain heart infusion and allowed to grow for 30 min at room temperature, and synthesis of the Yop virulon was induced by incubation for 150 min at 37°C before infection.
TABLE 1.
Plasmids
| Plasmid | Relevant characteristic(s) | References |
|---|---|---|
| pYV | ||
| pMSL41 | pYV40 yscNΔ169-177 (secretion mutant) | 53, 60 |
| pPW401 | pYV40 yopBΔ89-217 | 9 |
| pMSL44 | pYV40 yopDΔ121-165 | 34 |
| pAB4052 | pYV40 yopE21 | 34 |
| pSI4008 | pYV40 yopHΔ1-352 | 56 |
| pAB406 | pYV40 yopOΔ65-558 | 34 |
| pAB408 | pYV40 yopM23 | 34 |
| pMSK41 | pYV40 yopP23 | 34 |
| pABL402 | pYV40 yopQ17 | This work |
| pABL403 | pYV40 yopE21 yopHΔ1-352 yopOΔ65-558 yopP23 yopM23 | This work |
| pAB409 | pYV40 yopE21 yopHΔ1-352 yopOΔ65-558 yopP23 yopM23 yopBΔ89-217 | This work |
| Clones and vectors | ||
| pMSK13 | PyopE SD yopP+ (pTZ19R derivative) | 34 |
| pABL1 | pBluescript KS− yopQ+ (PCR fragment encoding YopQ cloned in BamHI-HindIII sites of the vector) | This work |
| pABL2 | pBluescript KS− yopQ17+ (directed mutagenesis of yopQ with oligo-Mipa 470) | This work |
| pABL3 | pMRS101 yopQ17+ (SalI-XbaI of pABL2 cloned in the same sites of the vector) | This work |
| Suicide vectors and mutators | ||
| pKNG101 | oriR6K sacBR+ oriTRK2 strAB+ | 30 |
| pMRS101 | oriR6K sacBR+ oriTRK2 strAB+ oriColE1 bla+ | 47 |
| pAB31 | pMRS101 yopHΔ1-352+ | 34 |
| pAB34 | pMRS101 yopOΔ65-558+ | 34 |
| pAB38 | pMRS101 yopM23+ | 34 |
| pMSK7 | pMRS101 yopP23+ | 34 |
| pPW75 | pKNG101 yopBΔ89-217+ | 51 |
| pPW52 | pKNG101 yopE21+ | 59 |
| pABL4 | pMRS101 yopQ17+ (NotI deletion of pABL3) | This work |
Construction of the yopQ mutant.
To mutagenize yopQ (33), we first amplified the gene by PCR with the amplimers Mipa 468 (5′-CGAAGATCTCACTCGTAGTGACGG-3′), introducing a BglII site, and Mipa 469 (5′-GGCAAGCTTTAATATAGCTTCATCCC-3′), introducing a HindIII site. The BglII-HindIII fragment was cloned into the BamHI-HindIII sites of pBluescript (KS−), yielding plasmid pABL1. By site-directed mutagenesis on plasmid pABL1 with the oligonucleotide Mipa 470 (5′-GCCGACTGTTCAAGAATTCGCGGTACATAAAGCAC-3′), we introduced an EcoRI site and a frameshift leading to a STOP codon after 17 amino acids of YopQ (yopQ17). A SalI-XbaI fragment of the resulting plasmid pABL2 was then cloned in the same sites of the pro-suicide vector pMRS101, yielding plasmid pABL3. Deletion of the NotI fragment of pABL3 gave pABL4, the yopQ mutator plasmid. The yopQ17 allele was crossed into the pYV plasmid of Y. enterocolitica MRS40(pYV40), yielding strain MRS40(pABL402).
Construction of the polymutant strains.
To construct the yopHOPEM polymutant strain, the yopE, yopH, yopO, yopM, and yopP genes were successively knocked out by allelic exchange in the MRS40 strain by using the suicide vectors pMRS101 and pKNG101 (30, 47). The various deletions are described in Table 1. The yopE gene was first mutated with the mutator pPW52 (59), yielding strain MRS40(pAB4052). Mutation of the yopH gene in this strain with the mutator pAB31 (34) yielded the double yopEH mutant MRS40(pAB404). The triple yopEHO mutant MRS40(pAB405) was then obtained by allelic exchange with the mutator pAB34 (34). We then mutated the yopP gene with mutator pMSK7 (34), yielding the yopEHOP mutant MRS40(pMSK46). The yopHOPEM strain MRS40(pABL403) was finally obtained by allelic exchange with the yopM mutator pAB38 (34). The yopHOPEMB polymutant was obtained by mutation of yopB (mutator pPW75 [51]) in strain MRS40(pMSK46), leading to strain MRS40(pMSK47), followed by mutation of yopM (mutator pAB38), leading to strain MRS40(pAB409).
Eukaryotic cell growth and infection conditions.
PU5-1.8 (ATCC TIB 61) and J774A.1 (ATCC TIB 67) mouse monocyte macrophage cell lines were routinely grown in RPMI 1640 medium (Seromed) supplemented with 10% fetal bovine serum (Gibco) and streptomycin (100 μg/ml) at 37°C under 8% CO2. At 20 h before infection, cells (5 × 105 cells/ml) were seeded either in 24-well tissue culture plates (1 ml/well) for the TNF-α assays or in 6-well tissue culture plates (4 ml/well) for the MAPK assays. At 1 h prior to infection, cells were washed once with RPMI and incubated further in RPMI without fetal bovine serum. Cells were then infected at a multiplicity of infection of 20 with bacteria grown for 30 min at room temperature and 150 min at 37°C. Following a 90-min infection period, gentamicin was added at a final concentration of 30 μg/ml for 2 h to kill extracellular bacteria.
TNF-α assay.
The amount of TNF-α released into RPMI culture supernatant was evaluated by a cytotoxic assay (5, 25) performed with the TNF-α-sensitive cell line WEHI 164 clone 13 (17). Briefly, 50 μl of cell culture supernatant was incubated with 50 μl of a mixture containing 30,000 WEHI cells, 2 μg of actinomycin D (Janssen Chemica) per ml, and 40 mM LiCl (UCB). After 20 to 24 h of incubation at 37°C, 50 μl of a 2.5-mg/ml solution of MTT [(3,(4,5-dimethylthiazol-2-yl)2,5-diphenyl-tetrazolium bromide)] (Merck & Co.) in phosphate-buffered saline was added to each well. After 2 h of incubation at 37°C, formazan crystals were dissolved by addition of 100 μl of lysis solution prewarmed to 37°C (2 volumes of 30% sodium dodecyl sulfate [SDS] and 1 volume of N,N-dimethyl formamide [DMF]); pH adjusted to 4.7 by adding 2.5% of 80% acetic acid and 0.25% 1 N HCl). After overnight incubation at 37°C, the optical density was read at 570 nm (reference wavelength, 650 nm). The standard curve was determined with rTNF-β (R and D Systems, Minneapolis, Minn.). For all of the experiments, the results were expressed as the percentage of the maximal response obtained. Since this cytotoxic assay measures a TNF-α-like activity, we also quantified the TNF-α release with a commercial enzyme-linked immunosorbent assay (ELISA) kit (Biotrak; Amersham).
Immunoprecipitations, immunoblotting, and MAPK assays.
J774A.1 macrophages were infected with bacteria as described above. The culture supernatant was removed and centrifuged for 5 min to collect the cells that had detached during the infection period. Cells were lysed with 200 μl of cell extraction buffer (50 mM Tris [pH 7.5], 100 mM KCl, 1 mM Na3VO4, 10 mM NaF, 10 mM sodium pyrophosphate, 15 mM sodium β-glycerophosphate, 1 mM EGTA, 1 mM EDTA, 1% Triton X-100, 270 mM sucrose, 1 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride, leupeptin [2 μg/ml; Sigma], aprotinin [2 μg/ml; Sigma]). The pellet obtained after centrifugation of the culture supernatant was lysed with 50 μl of cell extraction buffer and added to the cell lysate. After centrifugation, the supernatant was collected and quickly frozen in liquid nitrogen. A Bradford assay was performed to evaluate the amount of protein present in each sample. The samples were stored at −80°C until immunoprecipitation was performed. To immunoprecipitate the MAPK, 300 μg of protein was diluted to a final volume of 500 μl in cell extraction buffer and then incubated with 1 μg of anti-ERK2 (Santa Cruz sc-154) or anti-p38 (Santa Cruz sc-535) antibodies for 1 h at 4°C. Then 40 μl of protein A-Sepharose beads was added for 2 h (4°C).
For the immunoblotting assays, beads were washed twice with cell extraction buffer and then mixed with SDS-polyacrylamide gel electrophoresis (PAGE) loading buffer. Proteins were separated on an SDS–12% PAGE gel and transferred to a nitrocellulose membrane. The membrane was then blocked with 5% nonfat dry milk in phosphate-buffered saline before probing with the anti-ERK2 or anti-p38 antibodies (dilution, 1/2,000). Immunoreactive bands were visualized by incubation for 1 h with goat anti-rabbit antibodies (1/1,000 dilution; Dako) conjugated to horseradish peroxidase followed by revelation with enhanced chemiluminescence reagents (Pierce).
For the MAPK assays, the beads were washed twice with cell extraction buffer and once with reaction buffer (10 mM Tris [pH 7.5], 1 mM MgCl2, 0.02 mM EDTA, 0.2 mM EGTA, 0.2 mM DTT). The pellet was then mixed with 50 μl of the reaction mixture (10 mM Tris [pH 7.5], 1 mM MgCl2, 0.02 mM EDTA, 0.2 mM EGTA, 0.2 mM DTT, 1 μM cAMP-dependent protein kinase inhibitor peptide [Santa Cruz Biotechnology], myelin basic protein [MBP; 0.5 mg/ml], [γ-32P]ATP [0.05 μM, 14 × 106 cpm/pmol]) and incubated for 20 min at 30°C. The reaction was stopped by addition of 25 μl of SDS-PAGE loading buffer and boiling for 5 min. Proteins were separated on an SDS–15% PAGE gel; the stained and dried gel was then subjected to autoradiography. For quantification, the gel was dried and analyzed with a phosphorimager.
RESULTS
Functional type III secretion and translocation mechanisms are required for the suppression of TNF-α release by infected macrophages.
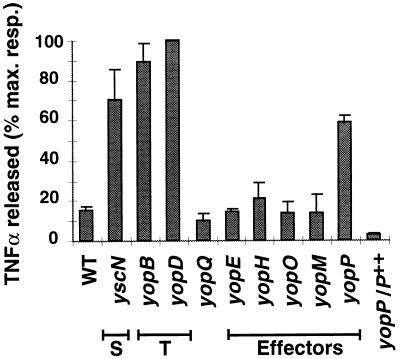
We first infected J774A.1 mouse monocytes/macrophages with the wild-type Y. enterocolitica E40(pYV40) strain and its isogenic yscN secretion mutant E40(pMSL41) (53, 60); after infection, the amount of TNF-α released in the cell culture supernatant was quantified by a biological assay with the TNF-α-sensitive WEHI 164 clone 13 cell line (17). Figure 1 shows that the TNF-α released upon infection with the wild-type strain was much lower than with the secretion mutant (yscN), thus confirming that a secreted Yop protein is involved in the inhibition of TNF-α release (46). We next tested yopB and yopD mutants to determine whether some Yop proteins need to be translocated into the cytosol of eukaryotic cells to trigger this phenomenon. The amount of TNF-α detected in the cell culture supernatants was comparable to that obtained with the secretion mutant (Fig. 1), indicating that a functional translocation apparatus is required for suppression of TNF-α release and thus suggesting that one or more Yop effectors are translocated to trigger the phenomenon. Similar results were obtained upon infection of PU5-1.8 macrophages (data not shown).
FIG. 1.
TNF-α-like activity released by Y. enterocolitica-infected J774A.1 macrophages. J774A.1 cells were infected with various Y. enterocolitica strains at a multiplicity of 20 bacteria per cell: WT, wild-type E40; yscN, secretion mutant (S); yopB and yopD, translocation mutants (T); yopQ, yopE, yopH, yopO, yopM, and yopP, single yop mutants; yopP/P++, yopP mutant complemented with the yopP overexpressing plasmid pMSK13. The infection procedure and the TNF-α assay were as described in Materials and Methods. Results are expressed as the percentage of the maximal response and are the mean values of three separate experiments. Error bars represent the standard deviation of the three experiments. Refer to Table 1 for the identification of the strains used.
YopP is involved in the inhibition of TNF-α release by infected macrophages.
To identify the Yop effector protein(s) responsible for the suppression of TNF-α release, both nonpolar mutants unable to synthesize the known effector proteins (YopE, YopH, YopO, YopM, and YopP) and a yopQ mutant were used to infect J774A.1 macrophages. The amounts of TNF-α detected upon infection with yopE, yopH, yopO, yopM, and yopQ mutants were comparable to those obtained with the wild-type strain. The only mutant that elicited a higher amount of TNF-α was the yopP mutant (Fig. 1). In order to complement the yopP mutation, plasmid pMSK13 (34) was introduced in trans in the yopP mutant. This complemented strain expresses yopP under the control of the strong yopE promoter and secretes higher amounts of YopP than the wild-type strain (34). Infection of J774A.1 macrophages with this complemented strain (yopP/P++) led to very low levels of TNF-α secretion, even lower than those observed after infection with the wild-type strain (Fig. 1). Again, similar results were obtained with the PU5-1.8 cell line (data not shown). Since the biological assay measures a TNF-α-like activity, the amount of TNF-α released was also quantified by an ELISA to confirm that our biological assay was specific; all the single yop effector mutants were tested, as well as secretion and translocation mutants, and the pattern of TNF-α release appeared to be the same as that observed with the biological assay (data not shown). Taken together, these results indicate that the YopP effector is involved in the inhibition of TNF-α release by infected macrophages.
Infection of macrophages with a Y. enterocolitica polymutant strain overexpressing yopP.
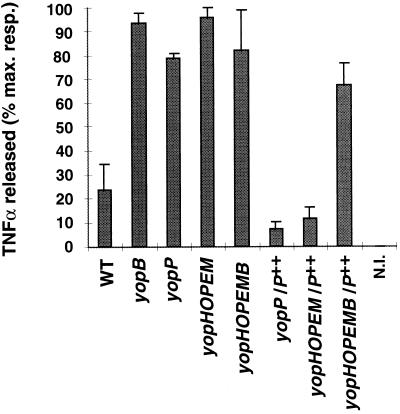
To confirm the role of YopP in the inhibition of TNF-α release, we constructed a Y. enterocolitica polymutant strain in which all the genes encoding known Yop effectors (YopE, YopH, YopO, YopM, and YopP) are mutated. Introduction of a plasmid encoding only one Yop protein in this yopHOPEM strain allows the overexpression of the protein of interest with a minimal background and avoids any interference due to the other known effectors. As a negative control, we constructed the yopHOPEMB mutant, which does not allow translocation of effector Yop proteins. We then introduced plasmid pMSK13, which overexpresses yopP, in both strains, and we infected J774A.1 macrophages. The wild-type strain, the yopB and yopP single mutants, and the yopHOPEM and yopHOPEMB polymutant strains were used as controls. As shown in Fig. 2, infection with the polymutant strains led to high levels of TNF-α release comparable to those obtained with the yopB mutant. Infection with the yopHOPEM strain overexpressing yopP (yopHOPEM/P++) induced levels of TNF-α as low as those seen with the complemented yopP mutant. Again, these values were lower than those obtained with the wild-type strain. On the other hand, infection with the yopHOPEMB strain overexpressing yopP (yopHOPEMB/P++) led to the release of levels of TNF-α as high as those recorded with the translocation mutant or the noncomplemented polymutant strains. These experiments indicated that YopP is the only Yop effector involved in the inhibition of TNF-α release and that it needs to be translocated into eukaryotic cells to play this role.
FIG. 2.
TNF-α-like activity released by J774A.1 cells upon infection with the Y. enterocolitica polymutant strains. Cells were infected with 20 bacteria per cell with the wild-type strain (WT), the yopB and yopP mutants as controls, the polymutant strains yopHOPEM and yopHOPEMB, and the complemented strains overexpressing yopP (yopP/P++, yopHOPEM/P++, and yopHOPEMB/P++). NI, noninfected cells. TNF-α was assayed by the biological method. Results are expressed as the percentage of the maximal response and are the mean values of three separate experiments. Error bars represent the standard deviation of the three experiments. Refer to Table 1 for the identification of the strains used.
YopP has an effect on the ERK2 and p38 MAPK activities.
While this study was in progress, Ruckdeschel et al. (46) showed that infection of J774A.1 macrophages with a virulent Y. enterocolitica strain led to the inactivation of the ERK1/2, JNK, and p38 MAPKs. They also showed that this MAPK inactivation parallels the inhibition of TNF-α release by the infected macrophages, suggesting that the suppression of the TNF-α release occurs through the inhibition of the MAPK activities. In order to see if YopP could also interfere with the MAPK cascades, we tested the ERK2 MAPK activity after infection with our various Y. enterocolitica strains.
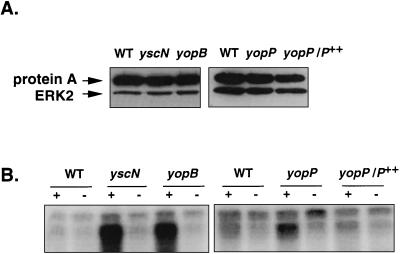
After infection of J774A.1 cells with the wild-type strain, the yscN secretion mutant, or the yopB translocation mutant, we prepared cell extracts, immunoprecipitated the ERK2 MAPK, and performed a kinase activity assay with [γ-32P]ATP and MBP as a substrate. To make sure that the phosphorylation of the MBP detected was really due to the immunoprecipitated ERK2 MAPK, we included control samples without anti-ERK2 antibodies. Figure 3B shows that the activity of the ERK2 MAPK isolated from macrophages infected with the wild-type strain was very weak. On the other hand, assays performed with lysates of macrophages infected with either the yscN or the yopB mutant indicated a high level of activity of ERK2. This first result confirmed that wild-type Yersinia has an inhibitory effect on the ERK2 MAPK activity and that functional secretion and translocation mechanisms are required for this effect. Figure 3A shows that the same amounts of ERK2 were immunoprecipitated in the different samples.
FIG. 3.
YopP is involved in the inhibition of the ERK2 MAPK. After infection, the ERK2 MAPK was immunoprecipitated from the lysate of cells infected with the wild-type strain (WT), the secretion mutant (yscN), the translocation mutant (yopB), the yopP mutant, and the complemented yopP mutant (yopP/P++). (A) Immunoblot showing immunoprecipitation of the ERK2 MAPK. The immunoprecipitated proteins were submitted to SDS-PAGE (12%), transferred to nitrocellulose membrane, and probed with the anti-ERK2 antibody. (B) MAPK activity of the immunoprecipitated ERK2 protein. The immunoprecipitated proteins were added to a kinase assay in the presence of [γ-32P]ATP and with MBP as a substrate. Proteins were then separated by SDS-PAGE (15%), and the gel was subjected to autoradiography. + and −, presence or absence of immunoprecipitating anti-ERK2 antibodies in the sample. A strong labeling of the MBP indicates a high kinase activity of the immunoprecipitated ERK2 MAPK.
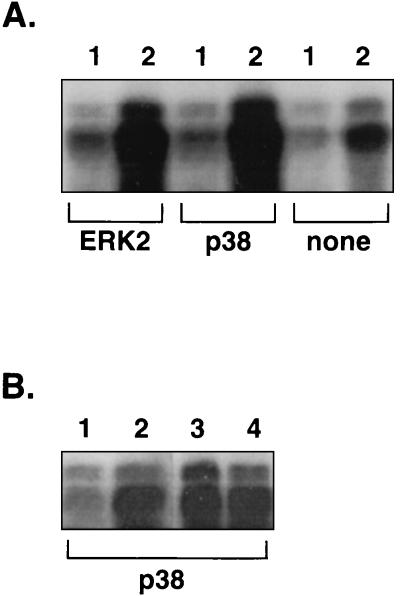
We then repeated the experiment with macrophages infected with either the yopP mutant or the complemented mutant (yopP/P++). Figure 3B shows that the ERK2 kinase activity was higher after infection with the yopP mutant than after infection with the wild-type strain and that the inhibitory effect could be restored by complementing the mutation in trans. Again, the same amounts of ERK2 were immunoprecipitated in the different samples, as assessed by immunoblotting (Fig. 3A). Since the level of activation of the ERK2 MAPK after infection with the yopP mutant was not as high as after infection with a yscN or a yopB mutant, we decided to repeat the experiment and to quantify this difference with a phosphorimager. The result of this experiment (expressed in phosphorimager units after subtraction of the background) shows that the amount of MBP radiolabeled by the lysate of cells infected with the yopP mutant (223,500 U) was indeed intermediate between the amounts obtained after infection with the wild-type strain (179,500 U) and after infection with the yopB mutant (305,800 U). The complementation of the yopP mutation with plasmid pMSK13 restored the same low level of activation as that observed with the wild-type strain (172,800 U), indicating that YopP affects the ERK2 MAPK activity. Results obtained with the yopHOPEM and yopHOPEMB polymutant strains overexpressing yopP lead also to the conclusion that YopP is involved: the ERK2 MAPK was inhibited upon infection with the yopHOPEM/P++ strain but not with the yopHOPEMB/P++ strain (Fig. 4A). Since in each case the amounts of immunoprecipitated ERK2 MAPK were equivalent (data not shown), these results really reflect the activation state of the ERK2 protein.
FIG. 4.
YopP is involved in the inhibition of the p38 MAPK. After infection, the ERK2 or p38 MAPK was immunoprecipitated from the lysate of cells infected with the yopHOPEM/P++ strain (lanes 1), the yopHOPEMB/P++ strain (lanes 2), the yopHOPEM strain (lane 3), or the yopHOPEMB strain (lane 4). The immunoprecipitated proteins were added to a kinase assay in the presence of [γ-32P]ATP and with MBP as a substrate. Proteins were then separated by SDS-PAGE (15%), and the gel was subjected to autoradiography. Panels A and B correspond to two separate experiments. ERK2 and p38, the antibodies used for immunoprecipitation; none, the control samples without immunoprecipitating antibodies. A strong labeling of the MBP indicates a high kinase activity of the immunoprecipitated ERK2 and p38 MAPKs.
We also monitored the influence of YopP on the activation of the p38 MAPK. We infected J774A.1 macrophages with the yopHOPEM/P++ and yopHOPEMB/P++ strains, and we performed MAPK assays of immunoprecipitated p38 instead of ERK2. As shown in Fig. 4A, bacteria producing YopP but no other Yop effector did not activate the p38 MAPK. In contrast, the isogenic strain deprived of the translocator YopB did activate p38. In each case, the amounts of immunoprecipitated p38 were equivalent, as assessed by immunoblotting with anti-p38 antibodies (data not shown). Moreover, infection of macrophages with either the yopHOPEM or the yopHOPEMB strains led to high levels of p38 MAPK activation (Fig. 4B), showing that the reduction in MAPK activity observed with the yopHOPEM/P++ strain is really due to expression of YopP. Taken together, these results show that the YopP protein is the only effector Yop involved in the inhibition of the ERK2 and p38 MAPK activities in Yersinia-infected macrophages.
DISCUSSION
TNF-α is a key cytokine in the development of the host’s immune and inflammatory response to infection. Secreted mainly by macrophages, TNF-α acts on various cell types involved in the host’s defense mechanisms. It stimulates the microbicidal activity of macrophages and polymorphonuclear neutrophils, and it acts on natural killer cells together with IL-12 to provoke the release of IFN-γ, which further increases the microbicidal activity of macrophages. In addition, it induces the expression of adhesion molecules on endothelial cells, and it is chemotactic for monocytes, contributing to the amplification of the inflammatory response (for a review, see reference 58). The ability of macrophages to produce TNF-α thus appears to be a critical step in the activation of the first line of defense against foreign organisms, and it is not surprising that several pathogens have evolved virulence mechanisms interfering with the host’s cytokine responses. Interference with the TNF-α production has been reported for various pathogens, among which are several bacteria, such as brucellae (11, 12), Listeria monocytogenes (15), Mycobacterium avium (50), and Bacillus anthracis (28). In the case of brucellae, it could be shown that after ingestion by the macrophages they prevent the induction of TNF-α synthesis by releasing a protein (12). Perturbations of the TNF-α response are also induced by parasites such as Leishmania donovani (16) and by viruses (21, 52).
The importance of TNF-α in clearing a Yersinia infection (2) and the suppression of TNF-α release by virulent Yersinia (4, 36, 37, 46) are well established. We have confirmed here that type III secretion is required for the latter phenomenon to occur. In addition, we showed that functional translocation machinery (i.e., YopB and YopD) is required, implicating a translocated Yop effector in the suppression of TNF-α release. In good agreement with this hypothesis, we observed that a nonpolar yopP mutant induced the same level of TNF-α release as secretion or translocation mutants. The yopP mutation could be complemented in trans, and these observations could be repeated with a Y. enterocolitica polymutant strain overexpressing yopP but no other known Yop effector. These data show that YopP is the only Yop effector involved in the inhibition of TNF-α release by Y. enterocolitica-infected macrophages.
Previous studies (36, 37) suggested a role for LcrV in this phenomenon. An lcrV mutant (48) was included in our study (data not shown) and gave the same results as the translocation mutants (yopB and yopD); however, this result can simply be explained by the fact that an lcrV mutant is unable to secrete the YopB and YopD translocators (48), which are absolutely required for the phenomenon to occur. In the study by Ruckdeschel et al. (46), a strain either expressing the secretion machinery and the YopB, YopD, LcrV, and YopN proteins or expressing the secretion machinery and the YopB, YopD, LcrV, YopN, YopE, and YopH proteins, as well as the YadA adhesin, gave the same results as nonvirulent bacteria, indicating that LcrV is not the only protein required. The YopB protein has also been proposed to be responsible for the suppressive effect of Yersinia on TNF-α release (4). However, as mentioned above, strains expressing the secretion machinery and the YopB protein do not induce suppression of TNF-α release, indicating that YopB alone is not responsible for the phenomenon (46). Moreover, our results indicated that YopB plays an indirect role, acting as a translocator to allow intracellular targeting of YopP, the actual effector.
Several studies have reported a link between TNF-α production and MAPK activation (31, 32, 40, 41, 57, 61). Recently, Ruckdeschel et al. (46) suggested a correlation between the inhibition of TNF-α release and the inhibition of ERK1/2, p38, and JNK MAPK activities in macrophages infected by Y. enterocolitica. Our results also indicated that the activation state of the ERK2 MAPK was different upon infection with a wild-type strain or with translocation-defective mutants; moreover, we found that YopP is the effector involved in the inhibition of the ERK2 MAPK activity. Experiments performed with the polymutant strains showed that YopP is also involved in the lack of activation of p38. However, the observed difference in ERK2 activation is higher between the wild-type- and the yscN or yopB mutant-infected cells than between the wild-type- and the yopP mutant-infected cells. This could be explained in several ways. First, another translocated Yop could also reduce the MAPK activity. This hypothesis is weakened by the observation that YopP alone fully complements a yopHOPEM mutant for MAPK activity. Another explanation would be that the secretion and translocation mutants are more phagocytosed than the wild-type bacteria and than the yopP mutant (42, 43; data not shown). However, variations in the levels of internalization cannot account for the observed differences in the MAPK activities, since a yopHOPEM and the yopHOPEM/P++ strains are internalized to the same level (gentamicin protection assay [29]; data not shown) although they induce a dramatic difference in the MAPK activities (Fig. 4). The same argument can be extended to the yopP mutant and the yopP/P++ strain, which are also phagocytosed to the same extent (data not shown). These observations indicate that the increase in MAPK activity occurring upon infection with the yopP mutant is not due to an increased level of internalization of bacteria but to an intracellular action of the protein.
Activation of the MAPKs results from a sequential stimulation of several cytoplasmic protein kinases such as Raf-1 and MEK1/2 (mitogen activated, ERK-activating kinases) in the case of the ERK1/2 MAPKs. It is not yet known at what stage of the MAPK activation cascade Yersinia exerts its inhibitory effect. Ruckdeschel et al. (46) presented data suggesting that the inhibition of the MAPK activities occurs at least partially via a decrease in the activities of upstream kinases even to the level of Raf-1. The elucidation of the actual target of YopP and of its mechanism of action requires further investigation.
It was recently shown that yersiniae that infect macrophages are able to induce the death of their target cell by triggering apoptosis (34, 35). The induction of apoptosis requires functional secretion (34, 35) and translocation machineries (34). YopP in Y. enterocolitica and YopJ, its homolog in Y. pseudotuberculosis, were identified as being responsible for the triggering of apoptosis (34, 35). The fact that apoptosis and inhibition of TNF-α release involve the same Yop effector suggests that the reduction of TNF-α release could be a consequence of the cell death caused by the YopP protein. It remains to be determined if both phenomena are consequences of each other or are independent processes resulting from a single or distinct signaling event(s).
ACKNOWLEDGMENTS
We thank I. Lambermont and C. Kerbourch for excellent technical assistance and A. Boyd and C. Geuijen for their critical reading of the manuscript. We thank P. Van der Bruggen and C. Shaw-Jackson for the gift of the WEHI cell line and their advice concerning the TNF-α assay. We also thank C. Pierreux for his help and advice in the setting up of the MAPK assays.
This work was supported by the Belgian Fonds National de la Recherche Scientifique Médicale (Convention 3.4595.97); the Direction Générale de la Recherche Scientifique-Communauté Française de Belgique (Action de Recherche Concertée, 94/99-172); and by the Interuniversity Poles of Attraction Program—Belgian State, Prime Minister’s Office, Federal Office for Scientific, Technical, and Cultural Affairs (PAI 4/03). A.B. is supported as a research assistant from the Belgian Fonds National de la Recherche Scientifique.
REFERENCES
- 1.Autenrieth I B, Beer M, Bohn E, Kaufmann S H E, Heesemann J. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect Immun. 1994;62:2590–2599. doi: 10.1128/iai.62.6.2590-2599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Autenrieth I B, Heesemann J. In vivo neutralization of tumor necrosis factor-alpha and interferon-gamma abrogates resistance to Yersinia enterocolitica infection in mice. Med Microbiol Immunol. 1992;181:333–338. doi: 10.1007/BF00191545. [DOI] [PubMed] [Google Scholar]
- 3.Autenrieth I B, Kempf V, Sprinz T, Preger S, Schnell A. Defense mechanisms in Peyer’s patches and mesenteric lymph nodes against Yersinia enterocolitica involve integrins and cytokines. Infect Immun. 1996;64:1357–1368. doi: 10.1128/iai.64.4.1357-1368.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beuscher H U, Rödel F, Forsberg Å, Röllinghoff M. Bacterial evasion of host immune defense: Yersinia enterocolitica encodes a suppressor for tumor necrosis factor alpha expression. Infect Immun. 1995;63:1270–1277. doi: 10.1128/iai.63.4.1270-1277.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyaert R, Vanhaesebroeck B, Suffys P, Van Roy F, Fiers W. Lithium chloride potentiates tumor necrosis factor-mediated cytotoxicity in vitro and in vivo. Proc Natl Acad Sci USA. 1989;86:9494–9498. doi: 10.1073/pnas.86.23.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black D S, Bliska J B. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 1997;16:2730–2744. doi: 10.1093/emboj/16.10.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bliska J B, Black D S. Inhibition of the Fc receptor-mediated oxidative burst in macrophages by the Yersinia pseudotuberculosis tyrosine phosphatase. Infect Immun. 1995;63:681–685. doi: 10.1128/iai.63.2.681-685.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohn E, Autenrieth I B. IL-12 is essential for resistance against Yersinia enterocolitica by triggering IFN-gamma production in NK cells and CD4+ T cells. J Immunol. 1996;156:1458–1468. [PubMed] [Google Scholar]
- 9.Boland A, Sory M-P, Iriarte M, Kerbourch C, Wattiau P, Cornelis G R. Status of YopM and YopN in the Yersinia Yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5-1.8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 1996;15:5191–5201. [PMC free article] [PubMed] [Google Scholar]
- 10.Boyd A, Sory M-P, Iriarte M, Cornelis G R. Heparan sulfate proteoglycans are cellular receptors for the Yersinia Yop virulon. Mol Microbiol. 1998;27:425–436. doi: 10.1046/j.1365-2958.1998.00691.x. [DOI] [PubMed] [Google Scholar]
- 11.Caron E, Gross A, Liautard J P, Dornand J. Brucella species release a specific, protease-sensitive, inhibitor of TNF-α expression, active on human macrophage-like cells. J Immunol. 1996;156:2885–2893. [PubMed] [Google Scholar]
- 12.Caron E, Peyrard T, Köhler S, Cabane S, Liautard J P, Dornand J. Live Brucella spp. fail to induce tumor necrosis factor alpha excretion upon infection of U937-derived phagocytes. Infect Immun. 1994;62:5267–5274. doi: 10.1128/iai.62.12.5267-5274.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornelis G R. Cross-talk between Yersinia and eukaryotic cells. In: McCrae M A, Saunders J R, Smyth C J, Stow N D, editors. Molecular aspects of host-pathogen interactions, SGM Symposium 55. Cambridge, England: Cambridge University Press; 1996. pp. 45–66. [Google Scholar]
- 14.Cornelis G R, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 15.Demuth A, Goebel W, Beuscher H U, Kuhn M. Differential regulation of cytokine and cytokine receptor mRNA expression upon infection of bone marrow-derived macrophages with Listeria monocytogenes. Infect Immun. 1996;64:3475–3483. doi: 10.1128/iai.64.9.3475-3483.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Descoteaux A, Matlashewski G. c-fos and tumor necrosis factor gene expression in Leishmania donovani-infected macrophages. Mol Cell Biol. 1989;9:5223–5227. doi: 10.1128/mcb.9.11.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espevik T, Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 18.Fällman M, Andersson K, Håkansson S, Magnusson K-E, Stendahl O, Wolf-Watz H. Yersinia pseudotuberculosis inhibits Fc receptor-mediated phagocytosis in J774 cells. Infect Immun. 1995;63:3117–3124. doi: 10.1128/iai.63.8.3117-3124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forsberg Å, Viitanen A M, Skurnik M, Wolf-Watz H. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol Microbiol. 1991;5:977–986. doi: 10.1111/j.1365-2958.1991.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 20.Galyov E E, Håkansson S, Forsberg Å, Wolf-Watz H. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature. 1993;361:730–732. doi: 10.1038/361730a0. [DOI] [PubMed] [Google Scholar]
- 21.Gooding L R. Virus proteins that counteract host immune defenses. Cell. 1992;71:5–7. doi: 10.1016/0092-8674(92)90259-f. [DOI] [PubMed] [Google Scholar]
- 22.Guan K L, Dixon J E. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 1990;249:553–556. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- 23.Håkansson S, Galyov E E, Rosqvist R, Wolf-Watz H. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol Microbiol. 1996;20:593–603. doi: 10.1046/j.1365-2958.1996.5251051.x. [DOI] [PubMed] [Google Scholar]
- 24.Håkansson S, Schesser K, Persson C, Galyov E E, Rosqvist R, Homble F, Wolf-Watz H. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact dependent membrane disrupting activity. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen M B, Nielsen S E, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 26.Hartland E L, Green S P, Phillips W A, Robins-Browne R M. Essential role of YopD in inhibition of the respiratory burst of macrophages by Yersinia enterocolitica. Infect Immun. 1994;62:4445–4453. doi: 10.1128/iai.62.10.4445-4453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmström A, Pettersson J, Rosqvist R, Håkansson S, Tafazoli F, Fällman M, Magnusson K-E, Wolf-Watz H, Forsberg Å. YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Mol Microbiol. 1997;24:73–91. doi: 10.1046/j.1365-2958.1997.3211681.x. [DOI] [PubMed] [Google Scholar]
- 28.Hoover D L, Friedlander A M, Rogers L C, Yoon I-K, Warren R L, Cross A S. Anthrax edema toxin differentially regulates lipopolysaccharide-induced monocyte production of tumor necrosis factor alpha and interleukin-6 by increasing intracellular cyclic AMP. Infect Immun. 1994;62:4432–4439. doi: 10.1128/iai.62.10.4432-4439.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isberg R, Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985;317:262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- 30.Kaniga K, Delor I, Cornelis G R. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene. 1991;109:137–141. doi: 10.1016/0378-1119(91)90599-7. [DOI] [PubMed] [Google Scholar]
- 31.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 32.Lee J C, Young P R. Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J Leukocyte Biol. 1996;59:152–157. doi: 10.1002/jlb.59.2.152. [DOI] [PubMed] [Google Scholar]
- 33.Michiels T, Wattiau P, Brasseur R, Ruysschaert J M, Cornelis G. Secretion of Yop proteins by Yersinia. Infect Immun. 1990;58:2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mills S D, Boland A, Sory M-P, Van der Smissen P, Kerbourch C, Finlay B B, Cornelis G R. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monack D M, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakajima R, Brubaker R R. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect Immun. 1993;61:23–31. doi: 10.1128/iai.61.1.23-31.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakajima R, Motin V L, Brubaker R R. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect Immun. 1995;63:3021–3029. doi: 10.1128/iai.63.8.3021-3029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Persson C, Carballeira N, Wolf-Watz H, Fällman M. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 1997;16:2307–2318. doi: 10.1093/emboj/16.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Persson C, Nordfelth R, Holmström A, Håkansson S, Rosqvist R, Wolf-Watz H. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol Microbiol. 1995;18:135–150. doi: 10.1111/j.1365-2958.1995.mmi_18010135.x. [DOI] [PubMed] [Google Scholar]
- 40.Reimann T, Büscher D, Hipskind R A, Krautwald S, Lohmann Matthes M L, Baccarini M. Lipopolysaccharide induces activation of the Raf-1/MAP kinase pathway. A putative role for Raf-1 in the induction of the IL-1β and the TNFα genes. J Immunol. 1994;153:5740–5749. [PubMed] [Google Scholar]
- 41.Rose D M, Winston B W, Chan E D, Riches D W, Gerwins P, Johnson G L, Henson P M. Fcγ receptor cross-linking activates p42, p38, and JNK/SAPK mitogen-activated protein kinases in murine macrophages: role for p42MAPK in Fcγ receptor-stimulated TNFα synthesis. J Immunol. 1997;158:3433–3438. [PubMed] [Google Scholar]
- 42.Rosqvist R, Bölin I, Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988;56:2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosqvist R, Forsberg Å, Rimpilainen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990;4:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 44.Rosqvist R, Forsberg Å, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosqvist R, Magnusson K E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruckdeschel K, Machold J, Roggenkamp A, Schubert S, Pierre J, Zumbihl R, Liautard J-P, Heesemann J, Rouot B. Yersinia enterocolitica promotes deactivation of macrophage mitogen-activated protein kinases extracellular signal-regulated kinase-1/2, p38, and c-Jun NH2-terminal kinase. J Biol Chem. 1997;272:15920–15927. doi: 10.1074/jbc.272.25.15920. [DOI] [PubMed] [Google Scholar]
- 47.Sarker M R, Cornelis G R. An improved version of suicide vector pKNG101 for gene replacement in gram-negative bacteria. Mol Microbiol. 1997;23:409–411. doi: 10.1046/j.1365-2958.1997.t01-1-00190.x. [DOI] [PubMed] [Google Scholar]
- 48.Sarker M R, Neyt C, Stainier I, Cornelis G R. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J Bacteriol. 1998;180:1207–1214. doi: 10.1128/jb.180.5.1207-1214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarker, M. R., M.-P. Sory, A. Boyd, M. Iriarte, and G. R. Cornelis. LcrG is required for efficient internalization of Yersinia Yop effector proteins by eukaryotic cells. Infect. Immun., in press. [DOI] [PMC free article] [PubMed]
- 50.Sarmento A M, Appelberg R. Relationship between virulence of Mycobacterium avium strains and induction of tumor necrosis factor alpha production in infected mice and in in vitro-cultured mouse macrophages. Infect Immun. 1995;63:3759–3764. doi: 10.1128/iai.63.10.3759-3764.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schulte R, Wattiau P, Hartland E L, Robins-Browne R M, Cornelis G R. Differential secretion of interleukin-8 by human epithelial cell lines upon entry of virulent or nonvirulent Yersinia enterocolitica. Infect Immun. 1996;64:2106–2113. doi: 10.1128/iai.64.6.2106-2113.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith G L. Virus strategies for evasion of the host response to infection. Trends Microbiol. 1994;2:81–88. doi: 10.1016/0966-842x(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 53.Sory M-P, Boland A, Lambermont I, Cornelis G R. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc Natl Acad Sci USA. 1995;92:11998–12002. doi: 10.1073/pnas.92.26.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sory M-P, Cornelis G R. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994;14:583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 55.Sory, M.-P., C. Kerbourch, and G. R. Cornelis. Unpublished data.
- 56.Stainier I, Iriarte M, Cornelis G R. YscM1 and YscM2, two Yersinia enterocolitica proteins causing downregulation of yop transcription. Mol Microbiol. 1997;26:833–843. doi: 10.1046/j.1365-2958.1997.6281995.x. [DOI] [PubMed] [Google Scholar]
- 57.Trotta R, Kanakaraj P, Perussia B. Fcγ R-dependent mitogen-activated protein kinase activation in leukocytes: a common signal transduction event necessary for expression of TNFα and early activation genes. J Exp Med. 1996;184:1027–1035. doi: 10.1084/jem.184.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 59.Wattiau P, Cornelis G R. SycE, a chaperone-like protein of Yersinia enterocolitica involved in the secretion of YopE. Mol Microbiol. 1993;8:123–131. doi: 10.1111/j.1365-2958.1993.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 60.Woestyn S, Allaoui A, Wattiau P, Cornelis G R. YscN, the putative energizer of the Yersinia Yop secretion machinery. J Bacteriol. 1994;176:1561–1569. doi: 10.1128/jb.176.6.1561-1569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng Z M, Specter S. Dynamic production of tumour necrosis factor-alpha (TNFα) messenger RNA, intracellular and extracellular TNFα by murine macrophages and possible association with protein tyrosine phosphorylation of STAT1α and ERK2 as an early signal. Immunology. 1996;87:544–550. doi: 10.1046/j.1365-2567.1996.513591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]