Abstract
We report a long-lasting (8-month) reactivation of human herpesvirus 6 (HHV-6) infection in child who had undergone cord blood stem cell transplantation. The reactivation was characterized by high viral loads and by immediate-early mRNA positivity. HHV-6 infection was associated with a deep depletion of CD3, while the CD4/CD8 ratio remained substantially unchanged.
CASE REPORT
A 6-year-old male with relapsed acute lymphoblastic leukemia (ALL) and in second remission underwent transplantation of cord blood stem cells (CBSC) from his HLA-identical sister. The main clinical events observed during the patient's follow-up could be summarized as follows. On day +12 after transplantation, a first-grade graft-versus-host disease was easily controlled by cortisone. At day +15, the child suffered an acute febrile episode lasting 2 days, followed by a transient cytomegalovirus (CMV) infection from day +20 to day +23, documented by PP65 antigenemia and PCR positivity in blood samples and successfully treated with foscarnet. Finally, a hemorrhagic cystitis was retrospectively related to a simian virus 40 infection (3). The engraftment was complete with >500 polymorphonuclear leukocytes/μl on day +17. Currently, the child is alive and in good clinical condition.
The virological follow-up started on day +15 and lasted until day +289. A set of virological investigations (to detect the presence of, i.e., CMV, Epstein-Barr virus, human herpesvirus 6 [HHV-6] and HHV-7, adenovirus, and polyomaviruses) was performed weekly. For this study, peripheral blood mononuclear cell (PBMC) dry pellets stored at −80°C were analyzed. HHV-6 PCR was performed with primers encompassing three different HHV-6 genomic regions (U31, U42, and U46); amplicons were genotyped as strain B or A by a HindIII restriction endonuclease assay (6). The HHV-6 viral load was determined in duplicate with GeneAmp 5700 SDS software (Applied Biosystem, Foster City, Calif.). The annealing-extension temperature was 62°C, and the primers were HHV-6 U94(+) (5′-6-carboxyfluorescein-GAGCGCCCGATATTAAATGGAT-6-carboxytetramethylrhodamine-3′) and U94(−) (5′-6-carboxyfluorescein-GCTTGAGCGTACCACTTTGCA-6-carboxytetramethylrhodamine-3′). A plasmid containing the targeted sequences for HHV-6 and for GAPDH was used as a standard. The method had a 6-log dynamic range and a sensitivity of 20 copies/ml. The presence of viral transcripts corresponding to U42 and U16/17 genes was analyzed using 200 ng of cDNA obtained by reverse transcription from DNA-free RNA samples and amplified by nested PCR (7). Flow cytometric analysis was performed with monoclonal antibodies against CD8, CD4, CD3, and CD19 (Caltag Laboratories, Burlingame, Calif.).
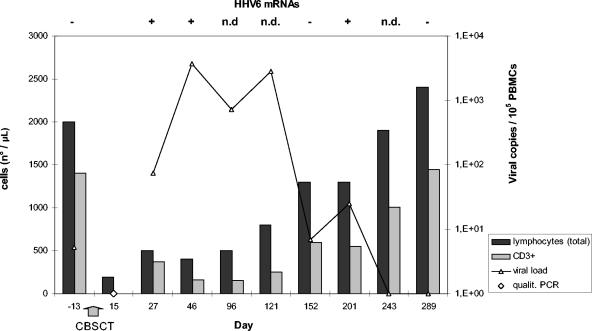
Figure 1 shows the kinetics of both HHV-6 infection and lymphocyte counts. On day +15, an undifferentiated febrile illness, unrelated to any bacterial or viral infection except for a HHV-6 infection discovered by a qualitative PCR on the whole buffy coat, was recorded. At 10 days later, the viral load was >103 copies/105 PBMCs coupled with the detection of HHV-6 immediate-early (IE) transcripts. The infection was a true secondary infection, since the child was HHV-6 positive before transplantation. HHV-6 pre- and posttransplant amplicons were genotyped as variant B. The cord blood cells of the donor were not infected. During the follow-up, the viral loads and the IE transcripts were compatible with a biphasic course of the infection. The active infection was considered resolved starting from day +243. A persistent lymphopenia lasting 4 months with comparatively low counts of CD3 lineage cells was recorded. The mean value of the CD4/CD8 ratio was 2.2 ± 0.49, with a range from 1.57 to 3.0, denoting a fairly low count of cytotoxic T lymphocytes.
FIG. 1.
HHV-6 virological and immunologic features of the child who had undergone CBSC transplantation.
In this case report, we describe a prolonged active HHV-6 infection in a child who had undergone CBSC transplantation, with a concomitant deficiency of the CD3 lymphocyte lineage.
The length of the active infection (about 8 months) is the longest reported so far (1, 5, 8). Moreover, the viral load was constantly over 103 copies/105 PBMCs, which is regarded as the threshold value for an active viral replication and is considered a good marker for the onset of or association with relevant clinical disorders (1). However, a quantitative PCR cannot truly distinguish between a low-level productive infection and an increased load of latently infected cells in immunocompromised patients. Thus, we confirmed the active HHV-6 replication by testing IE U42 U16/17 transcripts and we described the biphasic kinetics of infection. Applying this methodology to a series of 22 children who had undergone transplantation, we found seven cases of latent infection and three cases of reactivation (unpublished data). The HHV-6 infection was caused by the B variant and was established as a reactivation of a previous infection. The reactivation was definitively proved by the detection of HHV-6 DNA in an mRNA-negative pretransplantation sample, while the cord cells of the donor were free from HHV-6 footprints. This excludes the donor as a source of infection and confirms that the infused cord cells were truly naïve with respect to HHV-6.
During the 8-month posttransplantation period, HHV-6 was associated with a severe immunodeficiency, mainly with respect to T lymphocytes. The described depletion of CD3 lineage could be putatively ascribed to HHV-6 infection on the basis of the following findings. (i) There was no other factor, either iatrogenic or clinical, that can explain the selective T-lymphocyte reduction. Both the mild graft-versus-host disease, successfully controlled by a short course of cortisone treatment, and the CMV infection, promptly resolved by foscarnet treatment, seemed to be unrelated to the immunosuppression. (ii) The temporal relationship between the kinetics of HHV-6 infection and the counts of peripheral T lymphocytes was strictly coincidental. (iii) Several reported findings, both experimental and clinical, lead to the conclusive evidence that HHV-6 induces a defective proliferation and depletion of T lymphocytes, in particular, CD4 and CD8 mature cells (2, 4). Moreover, naïve cells may also sustain HHV-6 replication, suggesting that the effect of suppression on T lymphocytes could be wider and less selective (4). This model could be applied to the CBSC transplantation patients for whom true naïve cells were transfused (8). In our case, we observed an overall depletion of the CD3 lineage without specific depletion of CD8 or CD4, as expected. This effect could be consistent with an infection involving the progenitor cells of the donor T lineage, allowing an impaired T-cell maturation and possibly an impaired viral clearance.
In conclusion, this case report adds further evidence of the HHV-6 pathogenic role in CBSC transplantation recipients, including CD3 lymphopenia in the list of the disorders reported so far. In these patients, accurate molecular monitoring by viral load determination and, in selected cases, by mRNA detection is advisable. An appropriate therapy should be considered whenever relevant clinical events can be ascribed to active HHV-6 infection.
Acknowledgments
This work was supported by grant R.F. 2001/135 from the Italian Ministry of Health.
REFERENCES
- 1.Boutolleau, D., C. Fernandez, E. Andrè, B. M. Imbert-Marcille, N. Milpied, H. Agut, and A. Gautheret-Dejean. 2003. Human herpesvirus HHV-6 and HHV-7: two closely related viruses with different infection profiles in stem cell transplantation recipients. J. Infect. Dis. 187:179-186. [DOI] [PubMed] [Google Scholar]
- 2.Braun, D. K., G. Dominguez, and P. E. Pellett. 1997. Human herpesvirus 6. Clin. Microbiol. Rev. 10:521-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Comar, M., P. D'Agaro, M. Andolina, N. Maximova, F. Martini, M. Tognon, and C. Campello. 2004. Hemorrhagic cystitis in children undergoing bone marrow transplantation: a putative role for simian virus 40. Transplantation 78:544-548. [DOI] [PubMed] [Google Scholar]
- 4.Grivel, J. C., F. Santoro, S. Chen, G. Fagà, M. S. Malnati, Y. Ito, L. Margolis, and P. Lusso. 2003. Pathogenic effects of human herpesvirus 6 in human lymphoid tissue ex vivo. J. Virol. 77:8280-8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ihira, M., T. Yoshikawa, K. Suzuki, M. Ohashi, S. Suga, K. Horibe, N. Tanaka, H. Kimura, S. Kojima, K. Kato, T. Matsuyama, Y. Nishiyama, and Y. Asano. 2002. Monitoring of active HHV-6 infection in bone marrow transplant recipients by real time PCR; comparison to detection of viral DNA in plasma by qualitative PCR. Microbiol. Immunol. 46:701-705. [DOI] [PubMed] [Google Scholar]
- 6.Mirandola, P., P. Menegazzi, S. Merighi, T. Ravaioli, E. Cassai, and D. Di Luca. 1998. Temporal mapping of transcripts in herpesvirus 6 variants. J. Virol. 72:3837-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rotola, A., T. Ravaioli, A. Gonelli, S. Dewhurst, E. Cassai, and D. Di Luca. 1998. U94 of human herpesvirus 6 is expressed in latently infected peripheral blood mononuclear cells and blocks viral gene expression in transformed lymphocytes in culture. Proc. Natl. Acad. Sci. USA 95:13911-13916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sashihara, J., K. Tanaka-Taya, S. Tanaka, K. Amo, H. Miyagawa, G. Hosoi, T. Taniguchi, T. Fukui, N. Kasuga, T. Aono, M. Sako, J. Hara, K. Yamanishi, and S. Okada. 2002. High incidence of human herpesvirus 6 infection with a high viral load in cord blood stem cell transplant recipients. Blood 100:2005-2011. [PubMed] [Google Scholar]