Abstract
Exposure of bovine neutrophils to Pasteurella haemolytica leukotoxin (LKT) stimulates the production of leukotriene B4 (LTB4), which is believed to be an important chemotactic agent in the development of acute fibrinopurulent pneumonic infection in cattle. The involvement of phospholipase A2 (PLA2) in LKT-induced synthesis of LTB4 was studied by using bovine neutrophils labeled with 3H-arachidonate ([3H]AA). Incubation of isolated neutrophils with [3H]AA resulted in incorporation of radioactivity in the PLA2 substrates phosphatidylcholine, phosphatidylinositol, and phosphatidylethanolamine. Exposure of radiolabeled neutrophils to LKT caused concentration- and time-dependent release of radioactivity and redistribution of radioactivity in neutrophil membranes consistent with utilization of phosphoglyceride substrate and release of free fatty acid and eicosanoid products. These LKT-induced effects could be inhibited by pretreatment with arachidonyl trifluoromethyl ketone, an inhibitor of type IV cytoplasmic PLA2, and were dependent on extracellular calcium. These results support the conclusion that LKT-induced synthesis of LTB4 involves a calcium-mediated increase in PLA2 activity.
Pasteurella haemolytica is the primary etiologic agent of pneumonic pasteurellosis (11), a disease that causes substantial economic losses to the cattle feedlot and stocker industry (19). Pulmonary lesions caused by P. haemolytica infection are characterized by extensive infiltration of neutrophils and exudation of fibrin into airways and alveoli (36). Mobilization of neutrophils fails to effectively combat infection, and degranulation and lysis of these phagocytes releases damaging products that aggravate pulmonary damage (5, 30).
Chemotaxis of neutrophils and their inability to clear the infection may both be due to the action of P. haemolytica leukotoxin (LKT). This pore-forming RTX (repeats-in-toxin) cytotoxin is produced by log-phase bacteria and causes lysis of ruminant leukocytes and platelets (9, 10). Exposure of bovine neutrophils to low concentrations of LKT stimulates release of chemotactic eicosanoids, such as leukotriene B4 (LTB4) (18). Previous studies reported that LKT-induced synthesis of LTB4 by isolated bovine neutrophils was closely correlated with membrane damage and lysis (8), suggesting a common mechanism for these two important effects of LKT.
Eicosanoids are derived from the oxidation of arachidonic acid (AA), which is released from membrane phospholipids via the action of phospholipases. Hydrolysis of the ester linkage at the sn-2 position of plasma membrane phospholipids by phospholipase A2 (PLA2) is believed to be the rate-limiting step in eicosanoid synthesis (16). The action of phospholipases may also contribute to LKT-induced loss of plasma membrane integrity: hydrolysis of phospholipids by PLA2 leads to elaboration of lysophospholipids, which are known to cause detergent-like effects on membranes (35).
Mammalian leukocytes contain several types of PLA2 enzymes. The type most commonly involved in eicosanoid production is high-molecular-mass (85-kDa) cytosolic PLA2 (cPLA2) (2). If cPLA2 is involved in LKT-induced effects on bovine neutrophils, this enzyme would constitute a rational target for therapy to suppress the uncontrolled pulmonary exudation that contributes to lung damage. Therefore, the objectives of this study were to determine whether LKT caused increased activity of PLA2, by measuring the effect of LKT on the release of arachidonate from isolated bovine neutrophils, the distribution of phospholipid substrates in neutrophil membranes, and the effects of an inhibitor of cPLA2.
MATERIALS AND METHODS
Preparation of P. haemolytica LKT.
P. haemolytica biotype A, serotype 1 wild-type strain and an isogenic LKT-deficient mutant strain A, produced by allelic replacement of lktA with the β-lactamase (bla) gene (25), were grown in 150 ml of brain heart infusion broth to an optical density at 600 nm (OD600) of 0.8 to 1.0. Bacteria collected from the brain heart infusion cultures were inoculated into 250 ml of RPMI 1640 medium (pH 7.0, 2.2 g of NaHCO3 per liter) to an OD600 of 0.25. The RPMI cultures were grown at 37°C, and 70 oscillations/min to an OD600 of 0.8 to 1.0, and the culture supernatants were harvested following centrifugation at 8,000 × g for 30 min (Sorvall GS3 rotor; DuPont Co., Wilmington, Del.). This and all subsequent steps were conducted at 4°C. Culture supernatants were concentrated by addition of solid ammonium sulfate (361 g/liter) to yield 60% saturation, and the precipitated material was collected by centrifugation at 8,000 × g for 45 min (Sorvall GS3 rotor). Precipitates were resuspended in 3 ml of 50 mM sodium phosphate–0.1 M NaCl (pH 7.0) buffer and then dialyzed against 500 ml of the same buffer overnight. Dialyzed concentrated culture supernatants were stored frozen at −135°C.
LKT activity was quantified as toxic units (TU) in BL3 cells, as described previously (8). One TU was defined as the amount of LKT that caused 50% maximal leakage of lactate dehydrogenase (LDH) from 4 × 105 BL3 cells in 200 μl at 37°C after 2 h of incubation. The mean activity of undiluted LKT preparations used in this study was 6.6 × 105 ± 1.9 × 105 (standard deviation [SD]) TU/ml.
Preparation of bovine neutrophils.
Two healthy beef calves (200 ± 50 kg) served as blood donors for isolation of neutrophils. Neutrophils were isolated by hypotonic lysis as previously described (34). Briefly, the whole venous blood was collected in 60-ml syringes containing 5 ml of 10% sodium citrate and then centrifuged in 50-ml polypropylene conical tubes (Corning Incorporated, Corning, N.Y.) at 600 × g and 4°C for 30 min (Centra-GP8R; IEC, Boston, Mass.). The plasma, buffy coat, and top layer of erythrocytes were aspirated, leaving approximately 10 ml of the cell pellet in each tube. In the first cycle of hypotonic lysis, 20 ml of cold (4°C) sterile distilled water was added to each tube, the cell suspension was mixed for 50 to 60 s, 20 ml of double-strength phosphate-buffered saline (PBS) was added to restore the tonicity, and the suspension was then centrifuged at 200 × g and 4°C for 10 min. The cell pellet was resuspended in 5 ml of PBS after the supernatant was discarded. Thereafter, 10 ml of water was again added to each tube, suspensions were mixed for 50 to 60 s, tonicity was balanced with 10 ml of double-strength PBS, and then the material was centrifuged at 200 × g and 4°C for 10 min. The cell pellet was washed once with PBS and twice with modified Ca2+-free Hanks’ balanced salt solution (HBSS; Sigma Chemical Co., St. Louis, Mo.) containing 1 mM CaCl2, 0.5 mM MgCl2, and 50 μM EGTA. The cell pellet in each tube was resuspended in 3 ml of modified HBSS. The concentration of neutrophils was estimated by hemocytometer and adjusted to 2 × 107 cells/ml. The viability and purity of neutrophil suspensions were assessed by trypan blue exclusion. Proportions of viable neutrophils were greater than 95%.
Incorporation of [3H]AA into neutrophils.
Incorporation of [3H]AA into bovine neutrophils was accomplished by using a modification of the method described by Ramesha and Taylor (27). Briefly, [3H]AA (100 μCi/ml or 0.0010 mmol/ml of ethanol; Dupont NEN Research Products, Boston, Mass.) was added to neutrophils in suspension (2.0 × 107 cells/ml) at 0.5 μCi/ml, and the suspension was then incubated at 37°C for 30 min. Thereafter, the suspension was centrifuged (200 × g, 10 min), the cell pellet was washed twice with cold HBSS, and the neutrophils were resuspended in HBSS containing 0.5 mM MgCl2, 50 μM EGTA, and 1 mM CaCl2 at 1.0 × 107 to 1.5 × 107 cells/ml.
Effect of LKT on neutrophil phospholipase activity and membrane integrity.
Concentration- and time-dependent effects of LKT and controls were tested in 1.5-ml polypropylene microcentrifuge tubes. LKT-induced responses were distinguished by comparison with an LKT-deficient control preparation [LKT(−)]. Concentration-dependent effects of LKT on PLA2 activity and membrane integrity were studied by incubating [3H]AA-loaded neutrophils with dilutions (1:10, 1:100, 1:200, 1:500, 1:1,000, 1:2,000, 1:5,000, and 1:10,000) of LKT or LKT(−) for 60 min. The relationships between period of incubation and LKT-induced stimulation of phospholipases and loss of membrane integrity were measured by incubating neutrophils with LKT (1:100), LKT(−) (1:100), or the calcium ionophore A23187 (2.5 μM) for 0, 5, 15, 30, 60, or 90 min. Experiments included four replicates for each of the primary treatments.
Release of radioactivity served as a measure of phospholipase activity in intact neutrophils. At the completion of each incubation period, the experiment was terminated by centrifugation at 10,000 × g for 5 min, 100 μl of supernatant was suspended in 5 ml of liquid scintillation cocktail (Atomlight; Dupont NEN Research Products), and radioactivity was measured for 3 min by liquid scintillation counting (model LC5000TD apparatus; Beckman Instruments). Percent specific release of radioactivity was calculated by using the formula
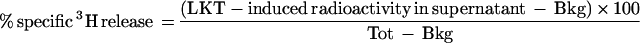 |
where Bkg is the radioactivity released by suspensions exposed to HBSS and Tot is the total radioactivity added to the sample.
The effect of P. haemolytica LKT on neutrophil plasma membrane integrity was assayed by measuring extracellular release of LDH. Extracellular LDH was assayed by transfer of 100 μl of incubation supernatant to wells of a flat-bottom 96-well microtiter plate. The plate was warmed to 37°C, 100 μl of LDH assay reagent (LD-L; 228 to 50 ml [Sigma]; rehydrated by addition of 25 ml of H2O) at 37°C was added, and the LDH activity was measured in a thermally controlled kinetic microtiter plate reader (Thermomax; Molecular Devices, Palo Alto, Calif.) at 340 nm for 2 min at 37°C. Data were reported as optical density × 10−3 per minute. Maximal LDH leakage was determined by replacing LKT with Triton X-100 (final concentration was 0.1% [vol/vol]), and background LDH leakage was determined by replacing LKT with the appropriate buffer control. Percent specific leakage of LDH was calculated by using the formula
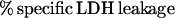 |
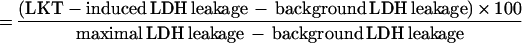 |
Effect of LKT on distribution of 3H-labeled membrane phospholipids.
The ability of LKT to activate phospholipases was further explored by comparing the effect of LKT on the distribution of 3H-labeled substrate and products in neutrophil membranes with those of positive (A23187) and negative [LKT(−)] controls. Bovine neutrophil suspensions were incubated at 37°C for 90 min with LKT (1:100), LKT(−) (1:100), A23187 (5 μM), and dimethyl sulfoxide (DMSO; 2%, final concentration), which served as a solvent control for A23187. Stimulation was terminated by addition of 3 ml of chloroform-methanol (1:2 [vol/vol]) and 0.1 ml of 9% formic acid, and lipids were extracted by using a modification of the method of Bligh and Dyer (4). Briefly, the mixture was vortexed for 2 min, 2 ml of chloroform was added, and the mixture was vortexed for 30 s, followed by further addition of 1 ml of water and mixing for 30 s. After centrifugation at 600 × g for 10 min, the chloroform phase was removed and evaporated under a stream of nitrogen. Lipid precipitates were resuspended in 50 μl of chloroform-methanol (9:1 [vol/vol]) and separated by thin-layer chromatography (TLC) on Silica Gel G plates (250-mm thickness, 20- by 20-cm plates; Alltech Associates Inc., Deerfield, Mass.) by development for 2 h in a solvent system consisting of chloroform-ethanol-water-triethylamine (30:34:8:35) (20). Silica gel bands (5 mm in width) were scraped into scintillation vials, and radioactivity was measured by liquid scintillation counting. Identification of lipids in radioactive bands was accomplished by comparison with parallel tracks containing standards (phosphatidylcholine [PC], phosphatidylinositol [PI], phosphatidylethanolamine [PE], free fatty acids [FFA], and neutral lipids [NL]; Sigma). Radioactivities in eluted bands corresponding to lipid standards were reported as counts per minute and as percentages of total radioactivity spotted onto the plates. Preliminary experiments confirmed that the distribution of 3H-labeled membrane constituents in untreated neutrophils did not change substantially between 30 and 120 min of incubation.
Effect of cPLA2 inhibition on LKT-induced effects.
The cPLA2 inhibitor arachidonyl trifluoromethyl ketone (AACOCF3) was used to confirm the involvement of cPLA2 in LKT-induced eicosanoid synthesis (31). The effect of AACOCF3 on the release of radioactivity from [3H]AA-loaded neutrophils was tested by adding appropriate volumes of AACOCF3 (2 mM in 20% ethanol) to neutrophil suspensions to achieve concentrations of 0, 20, 40, 80, and 160 μM, preincubating the mixture for 15 min, and then exposing neutrophil suspensions (n = 4) to LKT (1:100) or A23187 (5 μM) for 30 min at 37°C. Thereafter, neutrophil suspensions were treated as described above for estimation of 3H release. Data were reported as percent decrease in supernatant activity expressed as a proportion of 3H release measured at 0 μM AACOCF3.
The effect of AACOCF3 on the distribution of radioactivity in [3H]AA-loaded neutrophils was investigated by preincubating neutrophil suspensions with 120 μM AACOCF3 or the DMSO solvent control (2%, final concentration) for 15 min and then exposing suspensions (n = 4) for 90 min at 37°C to LKT (1:100) or LKT(−) (1:100). Thereafter, lipids were extracted and separated by TLC as described above.
Finally, the effect of AACOCF3 on LKT-induced production of LTB4 was examined by preincubating unlabeled neutrophil suspensions with 120 μM AACOCF3 for 15 min and then exposing suspensions (n = 4) for 120 min at 37°C to LKT (1:100) or A23187 (5 μM). Experiments were terminated by centrifugation at 600 × g for 10 min at 4°C, and the amount of LTB4 in 50 μl of supernatant was assayed by radioimmunoassay (Dupont NEN Research Products) as described previously (8).
Involvement of calcium in LKT-induced activation of PLA2.
The extracellular Ca2+ dependence of LKT-induced release of 3H and LDH from radiolabeled neutrophils was tested by altering the concentration of calcium in the neutrophil suspension media. Neutrophils were suspended in calcium-free HBSS, HBSS with 1 mM CaCl2, HBSS with 1 mM EGTA, or HBSS with 3 mM CaCl2 and 1 mM EGTA. Additional CaCl2 and MgCl2 were not added as in previous experiments. The percent specific 3H release and percent specific LDH release were estimated after 30 min of incubation at 37°C as described above.
Statistical analyses.
Statistical analyses were conducted by using a commercially available microcomputer program (Systat, Evanston, Ill.). Concentration- and time-dependent effects of LKT and/or A23187 on 3H and LDH release and the effects of these stimulators on the distribution of radioactivity in neutrophil membranes and LTB4 synthesis were compared to corresponding negative controls by using t tests. The effect of AACOCF3 on release of 3H from intact neutrophils was investigated by comparing the response at each dosage level with that of the inhibitor-free control, using Dunnet’s test. The effects of AACOCF3 on the distribution of radioactivity in LKT-exposed neutrophils and extracellular Ca2+ dependency of LKT-induced responses were investigated by using the general linear model followed by comparison of means using Tukey’s test. Differences between means were declared significant at the P < 0.05 level.
RESULTS
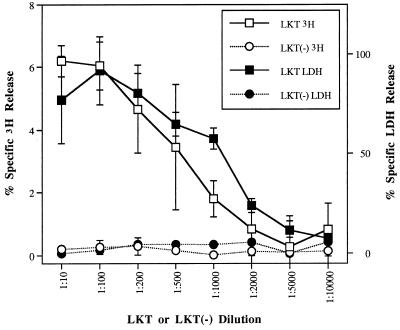
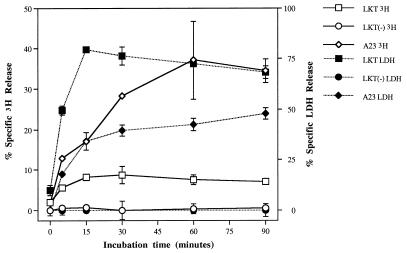
LKT caused 3H release (6.19% ± 0.50% at a dilution of 1:10) and LDH leakage (77.09% ± 21.92% at a dilution of 1:10) from bovine neutrophils in a dose-dependent manner, whereas LKT(−) failed to stimulate either 3H release or LDH leakage across the range of dilutions tested (Fig. 1). Compared with the response to A23187, exposure of [3H]AA-loaded neutrophils to LKT resulted in lower maximum percent specific 3H release (8.69 ± 2.14 at 30 min of incubation versus 37.11 ± 9.66 at 60 min for A23187) but higher maximum percent specific LDH release (79.38 ± 1.77 versus 47.92 ± 2.79 for A23187) (Fig. 2). Maximal responses for both 3H release and LDH release were achieved more rapidly by LKT-exposed neutrophils than by those exposed to A23187.
FIG. 1.
Effects of LKT and LKT(−) control preparations on release of 3H and LDH. Isolated neutrophils, loaded with [3H]AA, were exposed to dilutions of LKT or LKT(−) for 60 min (n = 4). Mean values describing release of 3H caused by LKT dilutions of ≤1:1,000 were significantly different from corresponding LKT(−) values. Except for that obtained with the 1:10,000 dilution, all percent specific LDH release values were significantly different from corresponding negative control values.
FIG. 2.
Time-dependent effects on release of 3H and LDH after exposure of isolated bovine neutrophils to LKT (1:10 dilution), LKT(−) (1:10 dilution), or A23187 (A23; 2.5 μM). All LKT and A23187 values derived from samples incubated for ≥5 min were significantly different from corresponding LKT(−) values.
TLC revealed that incubation of bovine neutrophils with [3H]AA resulted in labeling of the phospholipid, FFA, and NL components of lipid membranes (Table 1). In neutrophils exposed to LKT(−), the highest proportion of radioactivity was associated with PC (38.07% ± 0.44%) and lower proportions were associated with the FFA (7.02% ± 0.41%) and NL (14.63% ± 1.46%) constituents. Exposure to LKT caused a significant decrease in labeled PC and increases in labeled FFA and NL, consistent with metabolism of phospholipid substrate by phospholipases and production of free arachidonate and LTB4, which elute together with the FFA and NL standards (3), respectively. Exposure to A23187 caused an even greater transfer in radioactivity from PC to NL components, consistent with this ionophore’s ability to induce Ca2+-mediated production of eicosanoids.
TABLE 1.
Mean counts per minute and percentages of total radioactivity in each elution profile (n = 3) corresponding to PC, PI, PE, FFA, and NL standardsa
| Standard | Mean ± SD
|
|||||||
|---|---|---|---|---|---|---|---|---|
| LKT(−)
|
LKT
|
DMSO
|
A23187
|
|||||
| cpm | % | cpm | % | cpm | % | cpm | % | |
| PC | 103,052 ± 8,979 | 38.07 ± 0.44 | 82,032 ± 10,299 | 29.62b ± 0.26 | 109,896 ± 2,618 | 40.50 ± 0.60 | 47,168 ± 1,754 | 20.50b ± 0.63 |
| PI | 51,956 ± 3,421 | 19.22 ± 0.53 | 65,867 ± 9,046 | 23.76b ± 0.14 | 47,165 ± 1,629 | 17.39 ± 0.89 | 38,001 ± 1,762 | 16.53 ± 1.01 |
| PE | 51,011 ± 488 | 18.93 ± 1.57 | 46,701 ± 5,022 | 16.89 ± 0.58 | 33,471 ± 26,570 | 12.23 ± 9.69 | 24,943 ± 8,584 | 10.90 ± 3.96 |
| FFA | 19,056 ± 2,722 | 7.02 ± 0.41 | 27,139 ± 5,794 | 9.73b ± 0.80 | 18,755 ± 26,344 | 7.02 ± 9.91 | 30,373 ± 7,755 | 13.24 ± 3.50 |
| NL | 39,825 ± 7,241 | 14.63 ± 1.46 | 49,186 ± 5,791 | 17.77b ± 0.33 | 33,818 ± 3,854 | 12.45 ± 1.26 | 40,533 ± 7,391 | 17.54b ± 2.55 |
Bovine neutrophil suspensions were incubated at 37°C for 90 min with LKT (1:10), LKT(−) (1:10), A23187 (5 μM), and DMSO, and lipid extracts were subjected to TLC.
Significantly different from corresponding negative control value.
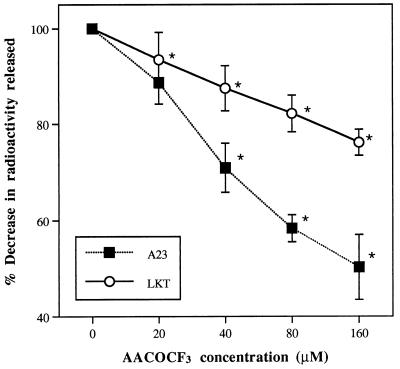
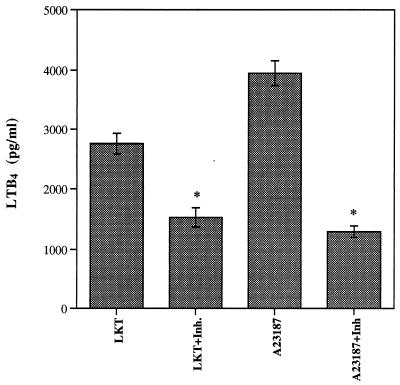
Pretreatment of neutrophils with AACOCF3 confirmed the involvement of cPLA2 in LKT-induced synthesis of eicosanoids. When [3H]AA-loaded neutrophils were pretreated with the inhibitor and subsequently exposed to LKT, the amount of radioactivity released decreased as the concentration of AACOCF3 was increased (Fig. 3). At a concentration of 160 μM, the amount of radioactivity released was 76.19% ± 2.66% of the value measured without the inhibitor. The calcium ionophore A23187 was even more susceptible to the inhibitory effects of AACOCF3; at 160 μM, release of radioactivity was decreased by approximately 50%. The effects of AACOCF3 on the distribution of radioactivity in neutrophil membranes further supported the involvement of cPLA2, by demonstrating inhibitory effects on the LKT-induced decrease in the PC substrate and the increase in the NL product (Table 2). However, AACOCF3 also caused significant increases in labeled FFA, suggesting that this inhibitor may affect enzymes and processes in addition to those involved in release of AA by cPLA2. Nevertheless, treatment of unlabeled neutrophils with AACOCF3 demonstrated that preservation of the PC substrate is correlated with LTB4 synthesis, as the LKT- and A23187-induced releases of LTB4 from intact neutrophils were both inhibited (Fig. 4).
FIG. 3.
Mean (±SD) percent decrease in radioactivity released from isolated bovine neutrophils exposed to LKT or A23187 (A23) in the presence of different concentrations of AACOCF3 (n = 4). ∗, radioactivity (disintegrations per minute) values are significantly different from corresponding inhibitor-free (0 μM AACOCF3) values.
TABLE 2.
Mean counts per minute and percentages of total radioactivity in each elution profile (n = 4) corresponding to PC, PI, PE, FFA, and NL standardsa
| Standard | Mean ± SDb
|
|||||
|---|---|---|---|---|---|---|
| LKT(−)
|
LKT
|
LKT + AACOCF3
|
||||
| cpm | % | cpm | % | cpm | % | |
| PC | 63,706 ± 18,981 | 37.53a ± 0.88 | 52,620 ± 6,935 | 31.82b ± 1.22 | 64,093 ± 3,762 | 34.85c ± 1.41 |
| PI | 35,564 ± 10,708 | 20.92a ± 0.35 | 32,501 ± 3,593 | 19.70a ± 0.97 | 35,283 ± 10,919 | 19.08a ± 5.37 |
| PE | 35,314 ± 10,382 | 20.83a ± 0.45 | 27,884 ± 4,742 | 16.81b ± 1.02 | 23,865 ± 2,639 | 12.94c ± 0.73 |
| FFA | 6,476 ± 1,785 | 3.83a ± 0.08 | 8,775 ± 494 | 5.34b ± 0.34 | 12,415 ± 941 | 6.74c ± 0.17 |
| NL | 17,877 ± 5,256 | 10.53a ± 0.57 | 24,042 ± 2,598 | 14.56b ± 0.09 | 24,557 ± 3,072 | 13.30c ± 0.54 |
Bovine neutrophils, loaded with [3H]AA, were preincubated with 120 μM AACOCF3 or DMSO solvent control (2%, final concentration) for 15 min and then exposed (n = 4) for 90 min at 37°C to LKT (1:10) or LKT(−) (1:10).
Values in the same row with different superscripts are significantly different.
FIG. 4.
Effect of AACOCF3 (Inh.) on synthesis of LTB4 induced by exposure of isolated bovine neutrophils to LKT (1:10) or A23187 (5 μM) for 120 min (n = 4).
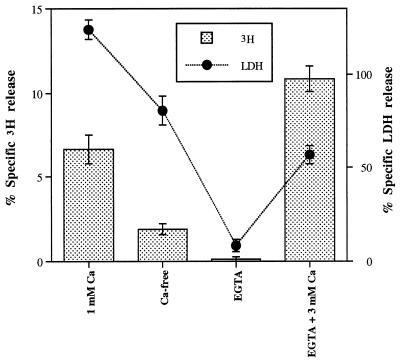
Release of radioactivity and LDH from LKT-exposed neutrophils was Ca2+ dependent (Fig. 5). Removal of Ca2+ from the incubation medium caused decreases in both 3H and LDH release. Further decreases in both responses were produced when EGTA was added to the Ca2+-free medium; LKT-induced effects were restored when a high concentration of Ca2+, exceeding the chelating capacity of EGTA, was added. These results were consistent with Ca2+-dependent catalysis of membrane phospholipids by cPLA2.
FIG. 5.
Extracellular calcium dependence of LKT-induced effects on percent specific 3H release and percent specific LDH release. Isolated neutrophils were exposed to a 1:10 dilution of LKT in buffer suspensions containing 1 mM CaCl2 (1 mM Ca), no Ca2+ (Ca-free), 1 mM EGTA and no Ca2+ (EGTA), or 1 mM EGTA and 3 mM CaCl2 (EGTA + 3 mM Ca). All treatments within each of the response variables were significantly different from one another.
DISCUSSION
P. haemolytica LKT is a member of the RTX group of exotoxins that are produced by a number of gram-negative bacteria. Previous studies have indicated that LKT causes cytolysis of ruminant leukocytes and platelets (9, 10) and, at sublytic concentrations, induces degranulation of neutrophils and generation of reactive oxygen derivatives (12, 22). Furthermore, exposure of bovine neutrophils to LKT stimulates the release of eicosanoids such as LTB4 (8, 18), which has been implicated as an important chemotactic agent for bovine neutrophils (17) and a mediator of inflammation in P. haemolytica infection (7).
Synthesis of LTB4 involves two important enzyme systems, PLA2 and 5-lipoxygenase. The former catalyzes the hydrolysis of cell membrane phospholipids to liberate AA, which is then further oxidized by the latter to LTB4 via the intermediate, 5(S)-hydroperoxy-6,8,11,14-(E,E,Z,Z)-eicosatetraenoic acid (15). Hydrolysis of membrane phospholipids by PLA2 is believed to be the rate-limiting step in eicosanoids synthesis (16) and, therefore, serves as a relevant focus for investigation of the mechanism of LKT-induced synthesis of LTB4 by bovine neutrophils.
Release of radioactivity from [3H]AA-loaded cells provides a convenient and sensitive method of studying phospholipase activity in a wide variety of cell types, including neutrophils. Incorporation of [3H]AA into the PC, PI, and PE fractions of the lipid membrane in the present study was consistent with the results of previous studies investigating remodeling of phosphoglycerides in neutrophils (6, 27). This incorporation profile is not related to pool size but rather reflects rates of phosphoglyceride turnover. In human neutrophils, relatively short incubation periods, such as that used in the present study, result in preferential incorporation of activity into PI and PC, whereas longer incubation periods result in more radioactivity being incorporated into PE. All three of these phosphoglycerides serve as important substrates for the PLA2 enzymes found in neutrophils (24). Thus, the results of the present study demonstrating LKT-induced release of incorporated [3H]AA and metabolites and redistribution of radioactivity from PC substrate to FFA and NL products provide strong evidence of activation of PLA2 by LKT. Although statistically significant, the extent to which LKT activated PLA2 was less than that predicted from previous studies (8), indicating that exposure of bovine neutrophils to LKT caused the production of large quantities of LTB4. However, this discrepancy can be explained by the limitations of measuring only the release and redistribution of labeled AA and metabolites. A comparison of gas chromatographic and radiometric assays concluded that the radiometric assay substantially underestimated PLA2 activity because it does not take into account the AA released from endogenous, unlabeled phosphoglyceride pools (27).
Release of AA from neutrophil membrane phosphoglycerides is believed to occur primarily via the actions of two types of PLA2, type II secretory PLA2 (sPLA2) and type IV cPLA2 (3, 24). Low-molecular-mass (∼14-kDa) sPLA2 requires millimolar concentrations of Ca2+ for optimal catalytic activity (13, 16), and has no fatty acid specificity in the sn-2 position of phosphoglyceride substrates, but prefers PE and, to a lesser degree, PI and PC (14). In contrast to sPLA2, cPLA2 has a higher molecular mass (∼85 kDa), requires nanomolar concentrations of Ca2+ for translocation from the cytosol to the nuclear envelope, and has no phospholipid substrate preference but prefers AA in the sn-2 position.
Inhibitory effects of AACOCF3 on LKT-induced release of 3H and LTB4 from intact neutrophils and catalysis of PC in neutrophil membranes provide further confirmation that exposure of bovine neutrophils to LKT activates PLA2. AACOCF3, an analog of AA in which the COOH group is replaced with COCF3, is a slow, tight-binding inhibitor of cPLA2. Although it has little effect on other phospholipases, including sPLA2 (1, 31), it also directly inhibits cyclo-oxygenase (28). This dual inhibitory effect on both the release of AA from membrane phosphoglycerides and further oxidation of AA to eicosanoids explains the effect of AACOCF3 on the distribution of radioactivity in LKT-exposed neutrophil membranes observed in the present experiment. Pretreatment with AACOCF3 preserved the PC but increased the FFA content of LKT-exposed neutrophil membranes. This effect is consistent with both partial inhibition of AA release and inhibition of eicosanoid synthesis, leading to accumulation of AA precursor. Although the inhibitory effects of AACOCF3 clearly implicate cPLA2 in the hydrolysis of radiolabeled phosphoglycerides, one must assume from the increase in radioactivity associated with the FFA fraction that other phospholipases, including sPLA2, may also be involved in the molecular pathogenesis of P. haemolytica LKT.
Experiments involving human neutrophils permeabilized with another pore-forming bacterial toxin, Staphylococcus aureus alpha-toxin, have provided strong evidence that cPLA2 is primarily responsible for providing AA precursor for LTB4 synthesis (2). However, the involvement of cPLA2 in providing substrate for leukotriene synthesis is less certain in other leukocyte types. In human monocytes, cPLA2 appears to play a more important role in the synthesis of prostaglandins than in that of leukotrienes; sPLA2 apparently is primarily responsible for providing the substrate for leukotriene formation (23). Nevertheless, it is clear from the inhibitory effect of AACOCF3 on LTB4 synthesis observed in the present study that, in bovine neutrophils, cPLA2 plays an important role in leukotriene biosynthesis.
A previous study (8) demonstrated that LKT-induced synthesis of LTB4 is dependent on extracellular Ca2+. The present study further extends our understanding of the involvement of Ca2+ in LKT-induced LTB4 synthesis by confirming that Ca2+ is necessary for activation of PLA2. Influx of Ca2+ into LKT-exposed neutrophils causes increased intracellular Ca2+ concentration, which serves as the stimulus for the oxidative burst (26). Calcium dependency of PLA2 function is consistent with the role of Ca2+ in stimulating translocation of cPLA2 or modulating the catalytic activity of sPLA2. The further reduction in responses observed in the present study when EGTA was added to the Ca2+-free suspension medium suggests that intracellular stores of Ca2+ may also contribute to the LKT-induced increase in intracellular Ca2+; extracellular EGTA causes rapid depletion of intracellular Ca2+ stores as Ca2+ rapidly diffuses down a concentration gradient from intracellular organelles to extracellular buffer (29).
The involvement of PLA2 in LDH release appears to be more complex than that of LTB4 synthesis. Extracellular leakage of high-molecular-mass LDH serves as a measure of plasma membrane integrity. The parallel LKT concentration dependencies of LDH release and 3H release suggested that a single mechanism, such as the elaboration of both AA and membrane-damaging lysophospholipids, may explain both responses. However, close examination of the relationship between incubation time and LDH release from LKT- or A23187-exposed neutrophils indicated that LKT caused more LDH release yet less 3H release than did A23187. The moderate degree of membrane damage caused by A23187 suggested that Ca2+-mediated activation of phospholipases and production of lysophospholipids probably contributed to membrane damage, but other mechanisms must be investigated to fully explain the lytic effect of LKT.
The central role of neutrophils in the development of fulminating pneumonic pasteurellosis is well supported. Experimental aerosol exposure to P. haemolytica induces rapid infiltration of neutrophils into the lung (33) and a marked increase in the neutrophil/macrophage ratio (21). These changes correlate well with reported histologic changes in which small airways become plugged with purulent exudate (21). There is reliable evidence indicating that mobilization of neutrophils does not effectively combat infection but contributes to development of lung lesions. Neutrophil depletion prior to inoculation with P. haemolytica protected calves from the development of gross fibrinopurulent pneumonic lesions (30), although less severe inflammatory changes still occurred (5). Thus, the neutrophil-mediated inflammatory response itself appears to be a major determinant of P. haemolytica pathogenicity, and identification of the mechanisms whereby LKT induces the synthesis of leukotrienes that cause chemoattraction of neutrophils into infected tissue is crucial to understanding the pathogenesis of pneumonic pasteurellosis. The results of the present study support the hypothesis that LKT-induced LTB4 synthesis involves Ca2+-dependent activation of cPLA2.
This study, together with further elucidation of the role of other PLA2 enzymes and 5-lipoxygenase in LKT-induced synthesis of LTB4, is expected to identify pathways and mechanisms that can be targeted to develop strategies for the control of infections caused by P. haemolytica and similar bacteria. Antibacterial therapy of these infections may fail because inflammatory responses caused by bacteria change the composition of interstitial fluid and compromise host defenses (7), thus decreasing antibiotic activity (32). The use of agents to suppress uncontrolled pulmonary exudation mediated by inflammatory pathways such as activation of cPLA2 is likely to have the benefit of restoring effective neutrophil phagocytic function as well as enhancing the efficacy of antibacterial therapy.
ACKNOWLEDGMENTS
We thank P. Clinkenbeard for preparation of LKT and LKT(−) and G. Murphy for the P. haemolytica isogenic LKT-deficient mutant.
This work was supported by the U.S. Department of Agriculture NRICGP, agreement 95-37204-2134.
REFERENCES
- 1.Bartoli F, Lin H K, Ghomashchi F, Gelb M H, Jain M K, Apitz-Castro R. Tight binding inhibitors of 85-kDa phospholipase A2 but not 14-kDa phospholipase A2 inhibit release of free arachidonate in thrombin-stimulated human platelets. J Biol Chem. 1994;269:15625–15630. [PubMed] [Google Scholar]
- 2.Bauldry S A, Wooten R E. Leukotriene B4 and platelet activating factor production in permeabilized human neutrophils: role of cytosolic PLA2 in LTB4 and PAF generation. Biochim Biophys Acta. 1996;1303:63–73. doi: 10.1016/0005-2760(96)00077-x. [DOI] [PubMed] [Google Scholar]
- 3.Bauldry S A, Wykle R L, Bass D A. Phospholipase A2 activation in human neutrophils. J Biol Chem. 1988;263:16787–16795. [PubMed] [Google Scholar]
- 4.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem. 1959;37:911–923. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 5.Breider M A, Walker R D, Hopkins F M, Schultz T W, Bowerstock T L. Pulmonary lesions induced by Pasteurella haemolytica in neutrophil sufficient and neutrophil deficient calves. Can J Vet Res. 1988;52:205–209. [PMC free article] [PubMed] [Google Scholar]
- 6.Chilton F H, Murphy R C. Remodeling of arachidonate-containing phosphoglycerides within the human neutrophil. J Biol Chem. 1986;261:7771–7777. [PubMed] [Google Scholar]
- 7.Clarke C R, Lauer A K, Barron S J, Wyckoff J H., III The role of eicosanoids in the chemotactic response to Pasteurella haemolytica infection. J Vet Med B. 1994;41:483–491. doi: 10.1111/j.1439-0450.1994.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 8.Clinkenbeard K D, Clarke C R, Hague C M, Clinkenbeard P, Srikumaran S, Morton R J. Pasteurella haemolytica leukotoxin-induced synthesis of eicosanoids by bovine neutrophils in vitro. J Leukocyte Biol. 1994;56:644–649. doi: 10.1002/jlb.56.5.644. [DOI] [PubMed] [Google Scholar]
- 9.Clinkenbeard K D, Mosier D A, Confer A W. Effects of Pasteurella haemolytica leukotoxin on isolated bovine neutrophils. Toxicon. 1989;27:797–804. doi: 10.1016/0041-0101(89)90047-0. [DOI] [PubMed] [Google Scholar]
- 10.Clinkenbeard K D, Upton M L. Lysis of bovine platelets by Pasteurella haemolytica leukotoxin. Am J Vet Res. 1991;52:453–457. [PubMed] [Google Scholar]
- 11.Collier J R, Brown W W, Chow T L. Microbiologic investigation of natural epizootics of shipping fever of cattle. J Am Vet Med Assoc. 1962;140:807–810. [PubMed] [Google Scholar]
- 12.Czuprynski C J, Noel E J, Ortiz-Carranza O, Srikumaran S. Activation of bovine neutrophils by partially purified Pasteurella haemolytica leukotoxin. Infect Immun. 1991;59:3216–3133. doi: 10.1128/iai.59.9.3126-3133.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dennis E A. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- 14.Diez E, Chilton F H, Stroup G, Mayer R J, Winkler J D, Fonteh A N. Fatty acid and phospholipid selectivity of different phospholipase A2 enzymes studied by using a mammalian membrane as substrate. Biochem J. 1994;301:721–726. doi: 10.1042/bj3010721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford-Hutchinson A W, Gresser M, Young R N. 5-Lipoxygenase. Annu Rev Biochem. 1994;63:383–417. doi: 10.1146/annurev.bi.63.070194.002123. [DOI] [PubMed] [Google Scholar]
- 16.Glaser K B, Mobilio D, Chang J Y, Senko N. Phospholipase A2 enzymes: regulation and inhibition. Trends Pharmacol Sci. 1993;14:92–98. doi: 10.1016/0165-6147(93)90071-q. [DOI] [PubMed] [Google Scholar]
- 17.Heidel J R, Taylor S M, Laegreid W W, Silflow R M, Liggitt H D, Leid R W. In vivo chemotaxis of bovine neutrophils induced by 5-lipoxygenase metabolites of arachidonic and eicosapentaenoic acid. Am J Pathol. 1989;143:671–676. [PMC free article] [PubMed] [Google Scholar]
- 18.Henricks P A, Binkhorst G J, Drijver A A, Nijkamp F P. Pasteurella haemolytica leukotoxin enhances production of leukotriene B4 and 5-hydroxyeicosatetraenoic acid by bovine polymorphonuclear leukocytes. Infect Immun. 1992;60:3238–3243. doi: 10.1128/iai.60.8.3238-3243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hird D W, Weigler B J, Salman M D, Danaye-Elmi C, Palmer C W, Holmes J C, Utterback W W, Sischo W M. Expenditures for veterinary services and other costs of disease and disease prevention in 57 California beef herds in the National Animal Health Monitoring System (1988–1989) J Am Vet Med Assoc. 1991;198:554–558. [PubMed] [Google Scholar]
- 20.Korte C, Casey M L. Phospholipid and neutral lipid separation by one-dimensional thin-layer chromatography. J Chromatogr. 1982;232:47–53. doi: 10.1016/s0378-4347(00)86006-5. [DOI] [PubMed] [Google Scholar]
- 21.Lopez A, Maxie M G, Ruhnke L, Savan M, Thomson R G. Cellular inflammatory response in the lungs of calves exposed to bovine viral diarrhea virus, Mycoplasma bovis and Pasteurella haemolytica. Am J Vet Res. 1986;47:1283–1286. [PubMed] [Google Scholar]
- 22.Maheswaran S K, Weiss D J, Kannan M S, Townsend E L, Reddy K R, Whiteley L O, Srikumaran S. Effects of Pasteurella haemolytica A1 leukotoxin on bovine neutrophils: degranulation and generation of oxygen-derived free radicals. Vet Immunol Immunopathol. 1992;33:51–68. doi: 10.1016/0165-2427(92)90034-n. [DOI] [PubMed] [Google Scholar]
- 23.Marshall L A, Bolognese B, Winkler J D, Roshak A. Depletion of human monocyte 85-kDa phospholipase A2 does not alter leukotriene formation. J Biol Chem. 1996;272:759–765. doi: 10.1074/jbc.272.2.759. [DOI] [PubMed] [Google Scholar]
- 24.Mayer R J, Marshall L A. New insights on mammalian phospholipase A2(s); comparison of arachidonoyl-selective and -nonselective enzymes. FASEB J. 1993;7:339–348. doi: 10.1096/fasebj.7.2.8440410. [DOI] [PubMed] [Google Scholar]
- 25.Murphy G L, Whitworth L C, Clinkenbeard K D, Clinkenbeard P A. Hemolytic activity of the Pasteurella haemolytica leukotoxin. Infect Immun. 1995;63:3209–3212. doi: 10.1128/iai.63.8.3209-3212.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortiz-Carranza O, Czuprynski C J. Activation of bovine neutrophils by Pasteurella haemolytica leukotoxin is calcium dependent. J Leukocyte Biol. 1992;52:558–564. doi: 10.1002/jlb.52.5.558. [DOI] [PubMed] [Google Scholar]
- 27.Ramesha C S, Taylor L A. Measurement of arachidonic acid release from human polymorphonuclear neutrophils and platelets: comparison between gas chromatographic and radiometric assays. Anal Biochem. 1991;192:173–180. doi: 10.1016/0003-2697(91)90203-6. [DOI] [PubMed] [Google Scholar]
- 28.Riendeau D, Guay J, Weech P K, Lalibert J F, Yergey J, Li C, Desmarais S, Perrier H, Liu S, Nicoll-Griffith D, Street I P. Arachidonyl trifluoromethyl ketone, a potent inhibitor of 85-kDa phospholipase A2, blocks production of arachidonate and 12-hydroxyeicosatetraenoic acid by calcium ionophore-challenged platelets. J Biol Chem. 1994;269:15619–15624. [PubMed] [Google Scholar]
- 29.Rosales C, Brown E J. Calcium channel blockers nifedipine and diltiazem inhibit Ca2+ release from intracellular stores in neutrophils. J Biol Chem. 1992;267:1443–1448. [PubMed] [Google Scholar]
- 30.Slocombe R F, Malark J, Derksen F J, Robinson N E. Importance of neutrophils in the pathogenesis of acute pneumonic pasteurellosis in calves. Am J Vet Res. 1985;46:2253–2258. [PubMed] [Google Scholar]
- 31.Street I P, Lin H K, Lalibert J F, Ghomashchi F, Wang Z, Perrier H, Tremblay N M, Huang Z, Weech P K, Gelb M H. Slow- and tight-binding inhibitors of the 85-kDa human phospholipase A2. Biochemistry. 1993;32:5935–5940. doi: 10.1021/bi00074a003. [DOI] [PubMed] [Google Scholar]
- 32.Vaudaux P, Waldvogel F A. Gentamicin inactivation in purulent exudates: role of cell lysis. J Infect Dis. 1980;142:586–593. doi: 10.1093/infdis/142.4.586. [DOI] [PubMed] [Google Scholar]
- 33.Walker R D, Hopkins F M, Schultz T W, McCracken M D, Moore R N. Changes in leukocyte populations in pulmonary lavage fluids of calves after inhalation of Pasteurella haemolytica. Am J Vet Res. 1985;46:2429–2433. [PubMed] [Google Scholar]
- 34.Weiss J W, Kraemer R, Schmit K. Isolation of granulocytes and mononuclear cells from the blood of dogs, cats, and cattle. Vet Clin Pathol. 1989;18:33–36. doi: 10.1111/j.1939-165x.1989.tb00510.x. [DOI] [PubMed] [Google Scholar]
- 35.Weltzien H U. Cytolytic and membrane-perturbing properties of lysophosphatidylcholine. Biochim Biophys Acta. 1979;559:259–287. doi: 10.1016/0304-4157(79)90004-2. [DOI] [PubMed] [Google Scholar]
- 36.Yates W D G. A review of infectious bovine rhinotracheitis shipping fever pneumonia and viral-bacterial synergism in respiratory disease of cattle. Can J Comp Med. 1982;46:225–263. [PMC free article] [PubMed] [Google Scholar]