Abstract
We conducted population-based surveillance for Candida bloodstream infections in Spain to determine its incidence, the extent of antifungal resistance, and risk factors for mortality. A case was defined as the first positive blood culture for any Candida spp. in a resident of Barcelona, from 1 January 2002 to 31 December 2003. We defined early mortality as occurring between days 3 to 7 after candidemia and late mortality as occurring between days 8 to 30. We detected 345 cases of candidemia, for an average annual incidence of 4.3 cases/100,000 population, 0.53 cases/1,000 hospital discharges, and 0.73 cases/10,000 patient-days. Outpatients comprised 11% of the cases, and 89% had a central venous catheter (CVC) at diagnosis. Overall mortality was 44%. Candida albicans was the most frequent species (51% of cases), followed by Candida parapsilosis (23%), Candida tropicalis (10%), Candida glabrata (8%), Candida krusei (4%), and other species (3%). Twenty-four isolates (7%) had decreased susceptibility to fluconazole (MIC ≥ 16 μg/ml). On multivariable analysis, early death was independently associated with hematological malignancy (odds ratio [OR], 3.5; 95% confidence interval [CI], 1.1 to 10.4). Treatment with antifungals (OR, 0.05; 95% CI, 0.01 to 0.2) and removal of CVCs (OR, 0.3; 95% CI, 0.1 to 0.9) were protective factors for early death. Receiving adequate treatment, defined as having CVCs removed and administration of an antifungal medication (OR, 0.2; 95% CI, 0.08 to 0.8), was associated with lower odds of late mortality; intubation (OR, 7.5; 95% CI, 2.6 to 21.1) was associated with higher odds. The incidence of candidemia and prevalence of fluconazole resistance are similar to other European countries, indicating that routine antifungal susceptibility testing is not warranted. Antifungal medication and catheter removal are critical in preventing mortality.
Candida species accounted for 8 to 10% of all nosocomial bloodstream infections (BSI) in the United States during the 1990s (8). The incidence of Candida BSI increased two- to fivefold in teaching hospitals and one- to fourfold in non-teaching hospitals in the United States during the 1980s (4, 5), and although there was a significant decrease in its annual incidence among intensive care unit (ICU) patients in the 1990s (41), the overall incidence did not decrease (7, 11, 13). In Europe, the incidence of Candida BSI in The Netherlands doubled between 1987 and 1995, but in Norway and Switzerland, it remained unchanged between 1991 and 1996 and 1991 and 2000, respectively (17, 36, 44).
Several population-based surveillance studies of Candida BSI have been reported in the United States (7, 11, 13). In contrast, most European studies have been conducted in selected hospitals (14, 17, 34, 40, 44) or have focused on specific groups of patients (16, 23, 42). Few population-based studies of Candida BSI have been conducted in Europe. Exceptions to this are a study from Iceland which reported a rise in annual incidence from 1.4 cases per 100,000 population between 1980 and 1984 to 4.9 cases per 100,000 population between 1995 and 1999 (3) and a study from Finland between 1995 and 1999 that detected an increase in annual incidence from 1.7 to 2.2 cases per 100,000 population (32).
Although Candida albicans continues to be the most common cause of Candida BSI, longitudinal studies have detected an increase in the incidence of BSI caused by other Candida species (28, 41). Compared with incidences from the 1980s, a larger proportion of Candida BSI are now caused by Candida glabrata in the United States (41) and by Candida parapsilosis and Candida tropicalis in European, Canadian, and Latin American hospitals (17, 27). The changing epidemiology of Candida BSI has generated concern about the emergence of azole drug resistance and its clinical relevance. The extent of fluconazole resistance in Europe has been analyzed in previous sentinel surveillance programs (12, 29, 31, 34, 39).
For the most part, the epidemiology of Candida BSI in Spain has only been studied in selected hospitals (2, 26, 38, 43). Population-based surveillance identifies all cases of disease regardless of the health care settings in which they occur. It minimizes bias resulting from the selection of only a subset of hospitals, and it permits newly affected patient groups to be identified. We conducted population-based active, prospective surveillance for Candida BSI in Barcelona, Spain, to describe the epidemiology of Candida BSI, to determine the distribution of the species involved and the prevalence of antifungal drug resistance, and to evaluate risk factors for mortality.
MATERIALS AND METHODS
Surveillance and data collection.
The study was conducted in the greater Barcelona area (population, 3.9 million) between 1 January 2002 and 31 December 2003. Fourteen major institutions participated, ranging in size from 214 to 1,295 beds. All blood cultures from which a Candida species was isolated were reported to the study coordinator (Dolors Rodríguez). The coordinator visited the hospital during the first week after the BSI was diagnosed to confirm the infection and again 3 weeks later to complete the case report form and record the outcome. A standardized case report form was used to abstract the medical records. Audits of clinical laboratories were periodically performed to ensure that all cases of candidemia were reported; cases found after the audits were added to the analysis. An audit of case patients' medical records was performed in July 2003 on 10% of the cases to verify data accuracy and completeness. To measure severity of illness, we used the Acute Physiology and Chronic Health Evaluation (APACHE) II score (6) for adult patients admitted to ICUs and the Karnofsky performance status scale for adults outside of an ICU (20). No standardized pediatric severity of illness score was measured during the study.
A case was defined as the incident isolation of any Candida species from the blood of a surveillance area resident. Candidemias that occurred >30 days after the initial case were considered new cases. Cases occurring either prior to or within 2 days of hospital admission were considered outpatient acquired. A case was defined as likely to be catheter related when (i) semiquantitative culture of the catheter tip yielded more than 15 CFU of a Candida species or (ii) simultaneous quantitative cultures of blood samples showed a ratio of ≥5:1 in CFU of blood samples obtained through the catheter and a peripheral vein (19).
To determine factors associated with BSI due to species other than C. albicans, we compared data for C. albicans with those from cases due to other Candida species by using univariate and multivariable analyses. Because of significant differences in demographics and crude mortality among C. parapsilosis cases, we excluded these cases from the multivariable analysis.
We also determined predictors of early and late mortality. We defined early mortality as death occurring 3 to 7 days after diagnosis and late mortality as death occurring between days 8 to 30. Patients that died on days 1 and 2 were excluded, as deaths occurring this early were unlikely to be related to treatment modality. Additionally, because of the significantly lower mortality observed with C. parapsilosis BSI, we excluded these cases from the analyses. For the late mortality study, we defined adequate treatment as the receiving ≥5 days of any antifungal medication in addition to catheter removal. Because a severity of illness score was not available for some cases, for mortality analyses we created an additional model that used other markers to adjust for severity of illness (location in the ICU, cardiac disease, renal failure, liver disease, and neurological disease).
Microbiological methods.
Detection of candidemia and species identification of isolates were performed at the participating laboratories according to their standard protocols (45). Isolates were sent to the Mycology Reference Laboratory (MRL), National Center for Microbiology, Madrid, Spain, for species confirmation and antifungal susceptibility testing. When MRL and submitting laboratory identifications differed, the MRL final identification was used for the purpose of this analysis. MICs of amphotericin B, fluconazole, and flucytosine were determined by the European Committee on Antimicrobial Susceptibility Testing broth microdilution method. These recommendations are based on National Committee for Clinical Laboratory Standards (NCCLS) reference procedure described in document M27-A2 but include some modifications to allow for automation of the method and to permit the incubation period to be shortened from 48 to 24 h (35). Isolates were classified as susceptible or as showing decreased susceptibility, by using interpretive breakpoints proposed by the NCCLS for flucytosine and fluconazole (21). The latter category included the susceptible dose-dependent (SDD), intermediate, and resistant categories of the NCCLS.
Statistical analysis.
Incidence and age-specific rates were calculated using denominator data obtained from the 2001 local census. Hospital-specific incidence was calculated using denominators from individual hospitals' data for the total number of patients discharged and patient-days for 2002 to 2003. Overall incidence was calculated using denominators of summed discharges and patient-days to calculate pooled mean rates.
Data were entered into Microsoft Access 2000. Statistical analysis was performed using SAS, version 8.2 (SAS Institute, Cary, N.C.). Chi-square or Fisher's exact test was used to compare categorical variables. Univariate and multivariable analyses were performed using the LOGISTIC procedure. For the multivariable analysis, candidate variables were identified as those (i) having a univariate significance at the P of 0.10 level, (ii) identified in an earlier published study, or (iii) believed to be clinically significant. By using this candidate list, multivariable analysis was conducted using forward, backward, and stepwise procedures, as well as by using various subsets of the data. Variables were assessed for correlation and for significant interactions. Final models were estimated using variables (risk or protective factors) whose coefficients were stable across the range of possible models.
RESULTS
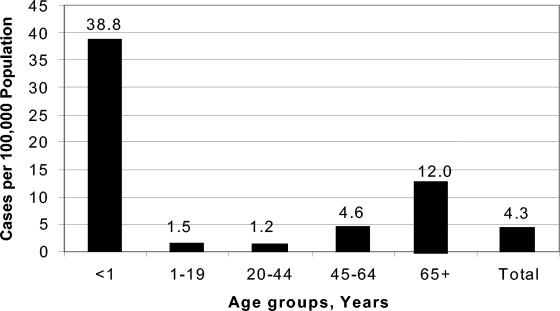
We detected 341 patients with Candida BSI. Four patients had a recurrent episode, resulting in 345 total cases. In six cases (2%), two different species of Candida were identified in the incident culture, and in 67 cases (20%), the patient had a concurrent bacteremia. The average annual incidence of candidemia was 4.3 cases per 100,000 population. Overall, the surveillance period included 4.7 million patient-days and 647,498 hospital discharges; the pooled mean rate of candidemia for the 14 hospitals was 0.53 episodes per 1,000 discharges and 0.73 episodes per 10,000 patient-days (Table 1). The age-specific incidence rate was highest in infants (38.8 cases per 100,000 population) and in those aged >65 years (12 cases per 100,000 population) (Fig. 1).
TABLE 1.
Incidence of candidemia and characteristics of hospitals included in surveillance for Candida bloodstream infections, Barcelona, Spain, 2002 to 2003
| Hospital | Number of:
|
Incidence per:
|
||||
|---|---|---|---|---|---|---|
| Beds | Discharges | Patient-days | Cases | 1,000 discharges | 10,000 patient-days | |
| A | 1,295 | 95,567 | 836,909 | 75 | 0.78 | 0.90 |
| B | 697 | 74,125 | 473,627 | 71 | 0.96 | 1.50 |
| C | 635 | 68,734 | 415,860 | 42 | 0.61 | 1.01 |
| D | 385 | 31,028 | 275,272 | 24 | 0.77 | 0.87 |
| Ea | 346 | 43,044 | 207,826 | 14 | 0.33 | 0.67 |
| F | 622 | 51,106 | 528,948 | 36 | 0.70 | 0.68 |
| G | 830 | 53,452 | 545,210 | 41 | 0.77 | 0.75 |
| H | 320 | 31,670 | 219,728 | 3 | 0.09 | 0.14 |
| I | 470 | 50,035 | 280,195 | 8 | 0.16 | 0.29 |
| J | 535 | 50,025 | 336,583 | 13 | 0.26 | 0.39 |
| K | 337 | 37,916 | 188,822 | 4 | 0.11 | 0.21 |
| L | 224 | 22,534 | 127,542 | 6 | 0.27 | 0.47 |
| M | 214 | 16,624 | 118,860 | 2 | 0.12 | 0.17 |
| N | 218 | 21,638 | 150,177 | 6 | 0.28 | 0.40 |
| Total | 647,498 | 4,705,558 | 345 | 0.53 | 0.73 | |
Pediatrics and obstetrics hospital.
FIG. 1.
Annual age-specific incidence of candidemia, Barcelona, Spain, 2002 to 2003.
Demographics, clinical characteristics, underlying patient comorbidities, and outcomes are summarized by species in Table 2. Thirty-seven cases (11%) were outpatient acquired, but only 115 patients (33%) were in an ICU when candidemia was diagnosed. Among inpatient candidemias and excluding those transferred from other institutions, the median time between admission to the current hospital and date of positive blood culture was 22 days (range, 3 to 234).
TABLE 2.
Demographics, clinical characteristics, and outcomes of candidemia cases by select species, Barcelona, Spain, 2002 to 2003
| Characteristic | All casesa | C. albicans | C. glabrata | C. tropicalis | C. krusei | C. parapsilosis |
|---|---|---|---|---|---|---|
| Median age (range) | 63 (0-90) | 66 (0-90) | 68 (0-90) | 66 (22-86) | 60 (29-79) | 36 (0-80) |
| Male | 203 (59) | 101 (58) | 21 (72) | 22 (67) | 7 (58) | 43 (55) |
| Age <1 yr | 32 (9) | 11 (6) | 1 (4) | 1 (3) | 0 | 19 (24) |
| Outpatientb | 37 (11) | 23 (13) | 4 (14) | 2 (7) | 0 | 6 (8) |
| Median days in hospital until candidemia (range) | 20 (0-288) | 20 (1-268) | 14 (0-64) | 16 (3-58) | 18.5 (4-34) | 28 (0-288) |
| Malignancy | 123 (36) | 61 (35) | 6 (20) | 18 (58) | 9 (75) | 21 (27) |
| Immunosuppressive therapy | 130 (39) | 64 (37) | 6 (21) | 16 (53) | 9 (75) | 29 (38) |
| Neutropeniac | 38 (11) | 10 (6) | 0 | 8 (26) | 8 (67) | 9 (12) |
| Renal failure | 117 (34) | 72 (41) | 11 (38) | 11 (36) | 3 (25) | 16 (21) |
| Transplant patient | 26 (8) | 3 (2) | 0 | 5 (15) | 2 (17) | 12 (15) |
| HIV infection | 15 (4) | 12 (7) | 1 (4) | 0 | 0 | 2 (3) |
| In ICU at diagnosis | 115 (33) | 59 (37) | 7 (28) | 10 (33) | 4 (33) | 31 (42) |
| Previous Candida colonization | 111 (33) | 75 (43) | 11 (38) | 11 (34) | 1 (10) | 9 (12) |
| Previous surgery | 157 (46) | 84 (49) | 14 (48) | 13 (42) | 4 (33) | 36 (46) |
| Vascular catheter at diagnosis | 302 (89) | 146 (86) | 24 (83) | 28 (88) | 12 (100) | 76 (97) |
| TPNd | 125 (40) | 57 (38) | 7 (27) | 7 (23) | 3 (25) | 43 (57) |
| Catheter related | 108 (31) | 53 (31) | 3 (10) | 7 (22) | 2 (17) | 42 (54) |
| Prior antibiotic therapy | 298 (88) | 156 (91) | 21 (72) | 26 (84) | 10 (91) | 70 (91) |
| Prior antifungal therapy | 57 (17) | 12 (7) | 7 (21) | 4 (13) | 9 (75) | 20 (26) |
| Death by day 3-7 | 74 (22) | 42 (24) | 9 (31) | 11 (33) | 2 (17) | 5 (6) |
| Overall mortality | 150 (44) | 83 (47) | 14 (50) | 19 (59) | 5 (46) | 22 (28) |
| Total cases | 345 (100) | 176 (51) | 29 (8) | 34 (10) | 12 (4) | 78 (23) |
All data are given as no. (%), except where a range is indicated.
Outpatient, cases with positive blood culture either prior to or at ≤2 days of hospitalization.
Absolute neutrophil count less than 0.5 × 109/liter.
TPN, total parenteral nutrition.
Among conditions known to be associated with candidemia, receiving immunosuppressive therapy was the most common (39%), followed by presence of a malignancy (36%), neutropenia (11%), and receiving a transplant (8%); central venous catheters (CVC) were in place in 302 cases (89%). Catheters were studied for the source of infection in 188 cases (54%); 108 of these cases (57.4%) were likely catheter associated.
Over 80% of patients received some antifungal treatment for candidemia, but 55 cases were never treated. Twenty-nine untreated patients (52.7%) died within the first 48 h, likely before the result of the first positive blood culture became available.
Species distribution.
C. albicans was the most common isolate (51%), followed by C. parapsilosis (23%), C. tropicalis (10%), C. glabrata (9%), and Candida krusei (4%). Other Candida species were isolated in 11 cases (3%) (Table 3). The local laboratories correctly identified the Candida species in 332 cases (96%).
TABLE 3.
In vitro susceptibilities of 351 Candida bloodstream isolates to amphotericin B, flucytosine, and fluconazolea
| Species | No. of isolates (%) | Amphotericin B
|
Flucytosine
|
Fluconazole
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | GM | MIC90 | Range | GM | MIC90 | Range | GM | MIC90 | ||
| C. albicans | 178 (51) | 0.03-0.25 | 0.06 | 0.12 | 0.125-4 | 0.17 | 0.25 | 0.125-8 | 0.16 | 0.25 |
| C. parapsilosis | 81 (23) | 0.03-0.25 | 0.19 | 0.25 | 0.125-1 | 0.15 | 0.25 | 0.125->64 | 0.41 | 1 |
| C. tropicalis | 36 (10) | 0.06-0.5 | 0.09 | 0.12 | 0.125-0.5 | 0.15 | 0.25 | 0.125->64 | 0.28 | 0.5 |
| C. glabrata | 31 (9) | 0.03-0.5 | 0.19 | 0.25 | 0.125-0.25 | 0.14 | 0.25 | 2->64 | 6.3 | 16 |
| C. krusei | 14 (4) | 0.25-0.5 | 0.3 | 0.5 | 2-4 | 2.43 | 4 | 32->64 | 52 | 64 |
| Others | 11 (3) | 0.03-0.25 | 0.08 | 0.25 | 0.125-4 | 0.41 | 2 | 0.125-16 | 1.06 | 16 |
| Total | 351 (100) | 0.03-0.5 | 0.08 | 0.25 | 0.125-4 | 0.18 | 0.5 | 0.125->64 | 0.39 | 8 |
Susceptibility data in micrograms per milliliter. Data are from 345 cases with 351 isolates (6 polyfungal candidemias). Results are reported as MIC ranges, geometric mean (GM) MIC values, and MIC90s.
Compared to non-C. albicans species, cases with C. albicans were more frequently associated with prior colonization by the same Candida species (43 versus 22%, P < 0.01). Cases with C. parapsilosis were more likely to be infants (24 versus 5%, P < 0.01), more likely to have indwelling CVC (97 versus 88%, P = 0.02), and less likely to die within 30 days (28 versus 48%, P < 0.01) compared to other cases. C. krusei cases were more frequently neutropenic (67 versus 9%, P < 0.01) or more frequently had a malignancy (75 versus 35%, P < 0.01) when compared to non-C. krusei cases. C. tropicalis cases were also likely to be neutropenic (26 versus 10%, P = 0.01), have a malignancy (58 versus 4%, P < 0.01), or die within 30 days (59 versus 42%) compared to non-C. tropicalis cases, although this latter difference did not reach statistical significance (P = 0.06).
On multivariable analysis excluding C. parapsilosis cases, the only exposure significantly associated with non-C. albicans candidemia, compared to that with C. albicans, was previous treatment with fluconazole (odds ratio [OR], 3.3; 95% confidence interval [CI], 1.8 to 6.1; P < 0.01); previous antibiotic use was a protective factor for non-C. albicans candidemia compared to that with C. albicans (OR, 0.4; 95% CI, 0.2 to 0.8; P < 0.01).
Outcomes.
Overall, 150 patients (44%) died with 30 days; 74 (22%) died within 7 days of incident culture. Two hundred (58%) cases were evaluated for metastatic candidiasis; 21 (10%) had evidence of dissemination to the heart (29%), the kidney (19%), liver (19%), or eyes (14%). Autopsy was performed on 15 cases; disseminated candidiasis was documented in two (13%).
To investigate the correlation between severity of illness and mortality, we divided adult cases into three categories: ICU patients with an APACHE II score of >20, other hospitalized adults with APACHE II scores of <20 or any Karnofsky score, and adult outpatients (Table 4). A severity of illness category was available for 217 of 289 adults (75.1%).
TABLE 4.
Severity of illness categories and correlation to overall mortality among adult candidemic cases (n = 217)a
| Category (n) | Deaths (%) |
|---|---|
| Severe (51) | 41 (80) |
| Moderate (159) | 62 (39) |
| Mild (7) | 2 (29) |
Categories are divided in order of decreasing severity of illness.
On univariate analysis, numerous factors were found to be significantly associated with early mortality (Table 5). Upon multivariable analysis and controlling for the high and moderate severity of illness categories, treatment with an antifungal and having the catheter removed as a part of treatment were independently associated with lower odds of early death; having a hematological malignancy was associated with greater odds (Table 5).
TABLE 5.
Univariate and multivariable predictors of mortality at days 3 to 7 after candidemia, Barcelona, Spain, 2002 to 2003a
| Characteristic | Deaths (%) | Non-deaths (%) | Relative Risk (95% CI) | P value |
|---|---|---|---|---|
| Shock | 22 (61) | 29 (15) | 5.5 (3.0-10.0) | <0.01 |
| Renal failure | 17 (46) | 25 (13) | 3.9 (2.2-6.7) | <0.01 |
| Treatment with antifungal | 25 (68) | 188 (96) | 0.2 (0.1-0.3) | <0.01 |
| Catheter removal | 20 (61) | 142 (85) | 0.4 (0.2-0.7) | <0.01 |
| High severity of illness category | 12 (41) | 25 (19) | 2.3 (1.2-4.4) | 0.01 |
| Catheter-related candidemia | 5 (14) | 62 (32) | 0.4 (0.2-1.0) | 0.03 |
| Intubated | 15 (43) | 49 (25) | 2.0 (1.1-3.6) | 0.03 |
| Age >65 yr | 26 (68) | 100 (50) | 1.9 (1.0-3.7) | 0.03 |
| Neutropenia | 8 (22) | 19 (10) | 2.1 (1.1-4.2) | 0.04 |
| Bacteria in incident culture | 13 (34) | 38 (19) | 1.9 (1.0-3.4) | 0.04 |
| Hematologic malignancy | 9 (24) | 27 (13) | 1.8 (0.9-3.4) | 0.1 |
Excluding C. parapsilosis cases and deaths occurring at days 1 to 2. The multivariable analysis is as follows: for treatment with antifungal, the OR was 0.05, the 95% CI was 0.01 to 0.2, and P was >0.01; for hematologic malignancy, the OR was 3.5, the 95% CI was 1.1 to 10.4, and P was 0.03; and for catheter removal, the OR was 0.3, the 95% CI was 0.1 to 0.9, and P was 0.04. Multivariable analysis results are adjusted for severity of illness category.
Table 6 shows the univariate and multivariable predictors associated with late mortality. After controlling for severity of illness category, receiving adequate treatment was independently associated with lower odds of death at days 8 to 30; intubation was associated with higher odds. Similar associations were seen after creating multivariable models using other markers to adjust for severity of illness (data not shown).
TABLE 6.
Univariate and multivariable predictors of mortality at days 8 to 30 after candidemia, Barcelona, Spain, 2002 to 2003a
| Characteristic | Deaths (%) | Non-deaths (%) | Relative risk (95% CI) | P value |
|---|---|---|---|---|
| High severity of illness category | 14 (38) | 11 (12) | 2.6 (1.6-4.3) | <0.01 |
| Intubation | 22 (45) | 27 (18) | 2.4 (1.5-3.9) | <0.01 |
| Developed renal failure | 13 (27) | 12 (8) | 2.5 (1.6-4.1) | <0.01 |
| Septic shock | 13 (27) | 16 (11) | 2.1 (1.3-3.4) | <0.01 |
| Recurrent candidemia | 10 (20) | 12 (8) | 2.0 (1.2-3.4) | 0.02 |
| Neurologic disease | 13 (26) | 18 (12) | 1.8 (1.1-3.0) | 0.03 |
| Likely catheter related | 10 (20) | 52 (35) | 0.5 (0.3-1.0) | 0.04 |
| Adequate treatmentb | 30 (59) | 104 (69) | 0.7 (0.4-1.2) | 0.17 |
Excluding C. parapsilosis cases and deaths that occurred at days 0 to 7 and adjusting for severity of illness category. The multivariable analysis is as follows for intubation, the OR was 7.5, the 95% CI was 2.6 to 21.1, and P was <0.01; and for adequate treatment, the OR was 0.2, the 95% CI was 0.08 to 0.8, and P was 0.01.
Removal of catheter(s) in addition to having received ≥5 days of antifungal treatment.
Antifungal susceptibility testing.
All 351 isolates recovered were tested for susceptibility to amphotericin B, flucytosine, and fluconazole as summarized in Table 3. Amphotericin B MICs ranged from 0.03 to 0.5 μg/ml and flucytosine MICs ranged from 0.125 to 4 μg/ml. Isolates of C. krusei demonstrated the highest geometric mean MIC for amphotericin B and flucytosine with geometric mean values of 0.30 and 2.43 μg/ml, respectively. A total of 24 isolates (7%; 1 C. parapsilosis, 1 C. tropicalis, 6 C. glabrata, 14 C. krusei, 1 C. inconspicua, and 1 C. norvegensis) showed decreased susceptibility to fluconazole (MIC ≥ 16 μg/ml). Six isolates were classified as resistant to this compound (MIC, ≥64 μg/ml) and 18 as SDD (MIC, 16 to 32 μg/ml).
DISCUSSION
This is the first population-based description of Candida BSI in Spain. It provides more representative information on species distribution, clinical characteristics, antifungal resistance rates, and outcomes than previous reports that were based on selected hospitals (2, 26, 38, 43). The overall incidence of Candida BSI in Barcelona is lower than in the United States (4.3 cases per 100,000 population versus 6 to 10 cases per 100,000 population) (11, 13, 18) but is consistent with recent reports from Northern European countries (1.7 to 4.9 cases per 100,000 population) (3, 32).
In comparison with data from the United States, the lower population-based incidence rate reported here might be a result of demographic or medical practice differences between the two countries. For example, the proportion of outpatient candidemia was lower than in the United States (10.8 versus 28% within 24 h of admission), possibly due to a difference in frequency of outpatient CVC use (11). Although the rate of outpatient candidemia reported here was higher than in other European studies, our study, being population-based, is likely a better estimate of the true outpatient burden (14, 40).
Candida BSI rates among hospitalized patients in this study (0.73 episodes per 10,000 patient-days) were lower than reported incidence rates from the United States (1.5 episodes per 10,000 patient-days) (11) but similar to previously reported Candida BSI rates for other European countries. These have ranged from 0.27 episodes per 10,000 patient-days in Norwegian hospitals in 1996 to 0.35 episodes per 10,000 patient-days in France in 1995, 0.54 episodes per 10,000 patient-days in Switzerland in 2000, and 0.71 episodes per 10,000 patient-days in The Netherlands in 1995 (17, 34, 36, 44).
The high incidence of Candida BSI among infants observed here (38.8 cases per 100,000 population) is consistent with previous studies in the United States (11, 13). Mortality among this population was significantly lower than among adults, similar to other reports (15, 16). However, in contrast to those studies where C. albicans was the most common species isolated, the most frequent isolate that we recovered from neonates was C. parapsilosis (16 of 24 cases, 67%). This proportion is higher than that reported in the United States, where C. parapsilosis accounts for 27 to 45% of BSI in neonates and decreases sharply with age thereafter (11, 13).
Although candidemia is often associated with an ICU stay, only 115 patients (33%) were in an ICU when candidemia was diagnosed. This rate is similar to those reported recently in the United States (11) and supports the thinking that candidemia is not exclusively associated with critical care. A significant decrease in the incidence of C. albicans BSI and a significant increase in the incidence of C. glabrata BSI in ICU patients have also been reported in the United States (41).
In general, the underlying conditions and risk factors identified in this study were similar to those documented in other European studies (14, 26, 38, 40). The proportion of persons with human immunodeficiency virus (HIV) infection (4.4%) is similar to the rate of 6% reported in Northern Italy (40) but lower than proportions reported from the United States (8 to 10% of candidemic patients) (11, 13). This probably reflects the changing care of HIV infection, as fewer such patients now require admission to Spanish hospitals.
We found that 57% of catheters evaluated were likely the source of Candida BSI, highlighting the relevance of catheter-related sources in candidemia. Removal of an indwelling CVC is recommended whenever possible (16, 25), with some studies showing a clear benefit in terms of a reduction in duration of candidemia and a lower attributable mortality rate (9, 14). Other studies, however, do not support this consensus (24). In our study, removal of catheters in addition to receiving antifungal treatment was independently associated with a decreased risk for both early and late mortality. Our data, therefore, support current recommendations to remove CVC when Candida BSI is detected and to treat any candidemia with systemic antifungals (16, 25).
The distribution of Candida species was generally similar to reports from other European countries (29, 34, 40). An exception is the frequency of C. glabrata, which in our study was the fourth most-common species, compared to being the second most-common species in Switzerland (17), the United Kingdom (14), and the United States (7, 11, 13).
Our findings confirm the reports of negligible fluconazole resistance among C. albicans bloodstream isolates (11, 27). Additionally, susceptibility results for non-C. albicans species were consistent with other studies (11, 27). A low level of fluconazole resistance was found among C. tropicalis and C. parapsilosis isolates, and a high level of resistance was detected among C. krusei isolates. Although only 3% of our C. glabrata isolates were resistant to fluconazole, the MICs for 16% fell within the SDD range (16 to 32 μg/ml), consistent with reports from sentinel surveillance (28) and results recently reported from 19 European centers where 5% of C. glabrata isolates were fluconazole resistant (31). These low rates of fluconazole resistance overall suggest that routine antifungal susceptibility testing for Candida species is not necessarily indicated in our population, particularly if C. albicans is isolated.
Amphotericin B MICs were consistent with reports from other European countries (3, 14). The decreased susceptibility of C. krusei to amphotericin B (MIC at which 90% of isolates were inhibited [MIC90], 0.5 μg/ml) is also consistent with previous reports (11, 13, 30).
The role of widespread azole use in the emergence of species other than C. albicans as causes of Candida BSI remains controversial. An increase in the proportion of C. glabrata BSI was first reported from the United States during the 1990s (1, 22, 33, 41). A similar trend was reported from The Netherlands (44), but two studies from Switzerland reported no shift to species other than C. albicans, despite a significant increase in fluconazole use (10, 17). In our multivariable analysis, we found that the risk of non-C. albicans, non-C. parapsilosis BSI was higher in cases previously treated with fluconazole, similar to several previous reports (1, 18, 23, 40). Taken as a whole, these results suggest that increases in the proportion of C. glabrata BSI in some countries might be related to increasing fluconazole usage, although other host and health care factors could also have an effect on this trend (37, 46, 47).
Our study was subject to limitations. First, severity of illness scores were not obtained on all cases. APACHE II and Karnofsky scores could not be calculated at times because the difficulty in obtaining these data or they were inappropriate. However, when we created models using other markers for severity of illness, our results were highly reproducible, thereby underlining the stability of our results. Second, the study relied on routine clinical care to document disseminated and metastatic candidiasis, making it difficult to determine the real number of cases of disseminated Candida infection.
This article is the first population-based surveillance for Candida BSI conducted in Spain and highlights the significant morbidity and mortality associated with this infection. Our results demonstrate that the incidence of candidemia in Spain is slightly higher than in other European countries but lower than in the United States and that the rate of fluconazole resistance is very low. Additionally, our findings indicate that adequate treatment of candidemia, with catheter removal and antifungal medication, is associated with improved outcomes, thereby supporting current treatment guidelines.
Acknowledgments
This study was supported by research grants from Pfizer, Inc., and Gilead Sciences and by the Sociedad Española de Enfermedades Infecciosas y Microbiologia Clinica (SEIMC).
Other members of the Barcelona Candidemia Project Study Group are Rana Hajjeh, Thomas Taylor (Centers for Disease Control and Prevention), Francesc Marco Reverter, C. Melcion Soler (Hospital Clinic-IDIBAPS, Barcelona, Spain), Margarita Salvado (Hospital del Mar, Barcelona, Spain), Amadeu Gene (Hospital Sant Joan de Deu, Esplugues, Barcelona, Spain), Dionisia Fontanals (Hospital Parc Tauli, Sabadell, Barcelona, Spain), Mariona Xercavins (Hospital Mutua de Terrassa, Terrassa, Barcelona, Spain), Lluis Falgueras (Hospital General de Catalunya, Sant Cugat del Valles, Barcelona, Spain), Marta de Ramon (Hospital General de Catalunya, Sant Cugat del Valles, Barcelona, Spain), Maria Teresa Torroella (Hospital General de Catalunya, Sant Cugat del Valles, Barcelona, Spain), Carles Alonso (Hospital Creu Roja, Hospitalet de Llobregat, Barcelona, Spain), Montserrat Sierra (Hospital de Barcelona, Barcelona, Spain), Joaquin Martinez-Montauti (Hospital de Barcelona, Barcelona, Spain), Maria Antonia Morera (Hospital de Terrassa, Terrassa, Barcelona, Spain), and Jordi de Otero (Hospital Creu Roja, Barcelona, Spain).
REFERENCES
- 1.Abi-Said, D., E. Anaissie, O. Uzun, I. Raad, H. Pinzcowski, and S. Vartivarian. 1997. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 24:1122-1128. [DOI] [PubMed] [Google Scholar]
- 2.Alonso-Valle, H., O. Acha, J. D. Garcia-Palomo, C. Farinas-Alvarez, C. Fernandez-Mazarrasa, and M. C. Farinas. 2003. Candidemia in a tertiary care hospital: epidemiology and factors influencing mortality. Eur. J. Clin. Microbiol. Infect. Dis. 22:254-257. [DOI] [PubMed] [Google Scholar]
- 3.Asmundsdottir, L. R., H. Erlendsdottir, and M. Gottfredsson. 2002. Increasing incidence of candidemia: results from a 20-year nationwide study in Iceland. J. Clin. Microbiol. 40:3489-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee, S. N., T. G. Emori, D. H. Culver, R. P. Gaynes, W. R. Jarvis, T. Horan, J. R. Edwards, J. Tolson, T. Henderson, and W. J. Martone. 1991. Secular trends in nosocomial primary bloodstream infections in the United States. Am. J. Med. 91:86-89. [DOI] [PubMed] [Google Scholar]
- 5.Beck-Sague, C. M., and W. Jarvis. 1993. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980-1990. J. Infect. Dis. 167:1247-1251. [DOI] [PubMed] [Google Scholar]
- 6.Chang, R. W., S. Jacobs, and B. Lee. 1988. Predicting outcome among intensive care unit patients using computerized trend analysis of daily APACHE II scores corrected for organ system failure. Intensive Care Med. 108:435-449. [DOI] [PubMed] [Google Scholar]
- 7.Diekema, D. J., S. A. Messer, A. B. Brueggemann, S. L. Coffman, G. V. Doern, L. A. Herwalt, and M. A. Pfaller. 2002. Epidemiology of candidemia: 3-year results from the Emerging Infections and the Epidemiology of Iowa Organisms study. J. Clin. Microbiol. 40:1298-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edmond, M. B., S. E. Wallace, D. K. McClish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29:239-244. [DOI] [PubMed] [Google Scholar]
- 9.Eggiman, P., J. Garbino, and D. Pittet. 2003. Management of Candida species infections in critically ill patients. Lancet Infect. Dis. 3:772-785. [DOI] [PubMed] [Google Scholar]
- 10.Garbino, J., L. Kolarova, P. Rohner, D. Lew, P. Pichna, and D. Pittet. 2002. Secular trends of candidemia over 12 years in adult patients at a tertiary care hospital. Medicine (Baltimore) 81:425-433. [DOI] [PubMed] [Google Scholar]
- 11.Hajjeh, R. A., A. N. Sofair, L. H. Harrison, G. M. Lyon, B. A. Arthington-Skaggs, S. A. Mirza, M. Phelan, J. Morgan, W. Lee-Yang, M. A. Ciblak, L. E. Benjamin, L. T. Sanza, S. Huie, S. F. Yeo, M. E. Brandt, and D. W. Warnock. 2004. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol. 42:1519-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hazen, K. C., E. J. Baron, A. L. Colombo, C. Girmenia, A. Sanchez-Sousa, A. Palacio, C. Bedout, D. L. Gibbs, and The Global Antifungal Surveillance Group. 2003. Comparison of the susceptibilities of Candida spp. to fluconazole and voriconazole in a 4-year global evaluation using disk diffusion. J. Clin. Microbiol. 41:5623-5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kao, A. S., M. E. Brandt, W. R. Pruitt, L. A. Conn, B. A. Perkins, D. S. Stephens, W. S. Baughman, A. L. Reingold, G. A. Rothrock, M. A. Pfaller, R. W. Pinner, and R. A. Hajjech. 1999. The epidemiology of candidemia in two United States cities: results of a population-based active surveillance. Clin. Infect. Dis. 29:1164-1170. [DOI] [PubMed] [Google Scholar]
- 14.Kibbler, C. C., S. Seaton, R. A. Barnes, W. R. Gransden, R. E. Holliman, E. M. Johnson, J. D. Perry, D. J. Sullivan, and J. A. Wilson. 2003. Management and outcome of bloodstream infections due to Candida species in England and Wales. J. Hosp. Infect. 54:18-24. [DOI] [PubMed] [Google Scholar]
- 15.Kossoff, E. H., E. S. Buescher, and M. G. Karlowicz. 1998. Candidemia in a neonatal intensive care unit: trends during fifteen years and clinical features of 111 cases. Pediatr. Infect. Dis. J. 17:504-508. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Sastre, J. B., D. Gil, C. Coto, B. Fernandez, and Grupo de Hospitales Castrillo. 2003. Neonatal invasive candidiasis: a prospective multicenter study of 118 cases. Am. J. Perinatol. 20:153-164. [DOI] [PubMed] [Google Scholar]
- 17.Marchetti, O., J. Bille, U. Fluckiger, P. Eggiman, C. Ruef, J. Garbino, T. Calandra, M. P. Glausser, M. G. Tauber, and D. Pittet. 2004. Epidemiology of candidemia in Swiss tertiary care hospitals: secular trends, 1991-2000. Clin. Infect. Dis. 38:311-320. [DOI] [PubMed] [Google Scholar]
- 18.Marr, K. A., K. Seidel, T. C. White, and R. A. Bowden. 2000. Candidemia in allogeneic blood and marrow transplant recipients: evolution of risk factors after the administration of prophylactic fluconazole. J. Infect. Dis. 181:309-316. [DOI] [PubMed] [Google Scholar]
- 19.Mermel, L. A., B. M. B. Farr, R. J. Sherertz, I. I. Raad, N. O'Grady, J. S. Harris, and D. E. Craven. 2001. Guidelines for the management of intravascular catheter-related infections. Clin. Infect. Dis. 32:1249-1272. [DOI] [PubMed] [Google Scholar]
- 20.Motzer, R. J., M. Mazumdar, J. Bacik, W. Berk, A. Amsterdam, and J. Ferrara. 1999. Survival and prognostic stratification of 670 patients with advance renal cell carcinoma. J. Clin. Oncol. 17:2530-2540. [DOI] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard, 2nd ed. Document M27-A2. National Committee for Clinical Laboratory Standards,Wayne, Pa.
- 22.Nguyen, M. H., J. E. Peacock, A. J. Morris, D. C. Tanner, M. L. Nguyen, D. R. Snydman, M. M. Wagener, M. G. Rinaldi, and V. C. Yu. 1996. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am. J. Med. 100:617-623. [DOI] [PubMed] [Google Scholar]
- 23.Nolla-Salas, J., A. Sitges-Serra, C. Leon-Gil, J. Martinez-Gonzalez, M. A. Leon-Regidor, P. Ibanez-Lucia, and J. M. Torres-Rodriguez. 1997. Candidemia in non-neutropenic critically ill patients: analysis of prognostic factors and assessment of systemic antifungal therapy. Intensive Care Med. 23:23-30. [DOI] [PubMed] [Google Scholar]
- 24.Nucci, M., and E. Anaissie. 2001. Revising the source of candidemia: skin or gut? Clin. Infect. Dis. 33:1959-1967. [DOI] [PubMed] [Google Scholar]
- 25.Pappas, P. G., J. H. Rex, J. D. Sobel, S. G. Filler, W. E. Dismukes, T. J. Walsh, and J. E. Edwards. 2004. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 38:161-189. [DOI] [PubMed] [Google Scholar]
- 26.Peman, J., E. Canton, A. Orero, A. Viudes, J. Frasquet, and M. Gobernado. 2002. Epidemiology of candidemia in Spain: multicenter study. Rev. Iberoam. Micol. 19:30-35. (In Spanish.) [PubMed] [Google Scholar]
- 27.Pfaller, M. A., D. J. Diekema, R. N. Jones, H. S. Sader, A. C. Fluit, R. J. Hollis, S. A. Messer, and SENTRY Participant Group. 2001. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY Antimicrobial Surveillance Program. J. Clin. Microbiol. 39:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfaller, M. A., and D. J. Diekema. 2002. Role of sentinel surveillance of candidemia: trends in species distribution and antifungal susceptibility. J. Clin. Microbiol. 40:3551-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaller, M. A., and D. J. Diekema. 2004. Twelve-years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin. Microbiol. Infect. 10 (Suppl. 1):11-23. [DOI] [PubMed] [Google Scholar]
- 30.Pfaller, M. A., R. N. Jones, S. A. Messer, M. B. Edmond, and R. P. Wenzel. 1998. National surveillance of nosocomial bloodstream infection due to species of Candida other than Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE program. Diagn. Microbiol. Infect. Dis. 30:121-129. [DOI] [PubMed] [Google Scholar]
- 31.Pfaller, M. A., S. A. Messer, L. Boyken, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2004. Geographic variation in the susceptibilities of invasive isolates of Candida glabrata to seven systemically active antifungal agents: a global assessment from the ARTEMIS Antifungal Surveillance Program conducted in 2001 and 2002. J. Clin. Microbiol. 42:3142-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poikonen, E., O. Lyytokainen, V. J. Anttila, and P. Ruutu. 2003. Candidemia in Finland, 1995-1999. Emerg. Infect. Dis. 9:985-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price, M. F., M. T. La Rocco, and L. O. Gentry. 1994. Fluconazole susceptibilities of Candida species and distribution of species recovered from blood cultures over a 5-year period. Antimicrob. Agents Chemother. 38:1422-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richet, H., P. Roux, C. Des Champs, Y. Esnault, A. Andremont, and French Candidemia Study Group. 2002. Candidemia in French hospitals: incidence rates and characteristics. Clin. Microbiol. Infect. 8:405-412. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Tudela, J. L., F. Barchiesi, J. Bille, E. Chryssanthou, M. Cuenca-Estrella, D. Denning, J. P. Donnelly, B. Dupont, W. Fegeler, C. Moore, M. Richardson, and P. E. Verweij. 2003. Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Clin. Microbiol. Infect. 9:1-8.12691538 [Google Scholar]
- 36.Sandven, P., L. Bevanger, A. Digranes, P. Gaustad, H. H. Haukland, and M. Steinbakk. 1998. Constant low rate of fungemia in Norway, 1991 to 1996. J. Clin. Microbiol. 36:3455-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanglard, D., and F. C. Odds. 2002. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2:73-85. [DOI] [PubMed] [Google Scholar]
- 38.Sota, M., C. Ezpeleta, and R. Cisterna. 1999. Description of 165 episodes of fungemia: a multicenter study. Rev. Iberoam. Micol. 16:30-35. [PubMed] [Google Scholar]
- 39.Tortorano, A. M., A. L. Rigoni, E. Biraghi, A. Prigitano, M. A. Viviani, and FIMUA-ECMM Candidaemia Study Group. 2003. The European Confederation of Medical Mycology (ECMM) survey of candidemia in Italy: antifungal susceptibility patterns of 261 non-albicans Candida isolates from blood. J. Antimicrob. Chemother. 52:679-682. [DOI] [PubMed] [Google Scholar]
- 40.Tortorano, A. M., E. Biraghi, A. Astolfi, C. Ossi, M. Tejada, C. Farina, S. Perin, C. Bonaccorso, C. Cavanna, A. Ravallo, A. Grossi, and FIMUA Candidemia Study Group. 2002. European Confederation of Medical Mycology (ECMM) prospective survey of candidaemia: report from one Italian region. J. Hosp. Infect. 51:297-304. [DOI] [PubMed] [Google Scholar]
- 41.Trick, W. E., S. K. Fridkin, J. R. Edwards, R. A. Hajjeh, R. P. Gaynes, and National Nosocomial Infections Surveillance System Hospitals. 2002. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989-1999. Clin. Infect. Dis. 35:627-630. [DOI] [PubMed] [Google Scholar]
- 42.Viscoli, C., C. Girmenia, A. Marinus, L. Collette, P. Martino, B. Vandercam, C. Doyen, B. Lebeu, D. Spence, V. Kremery, B. De Pauw, and F. Menier. 1999. Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin. Infect. Dis. 28:1071-1079. [DOI] [PubMed] [Google Scholar]
- 43.Viudes, A., J. Peman, E. Canton, P. Ubeda, J. L. Lopez-Ribot, and M. Gobernado. 2002. Candidemia at a tertiary-care hospital: epidemiology, treatment, clinical outcome and risk factors for death. Eur. J. Clin. Microbiol. Infect. Dis. 21:767-774. [DOI] [PubMed] [Google Scholar]
- 44.Voss, A., J. A. Kluytmans, J. G. Koeleman, L. Spanjaard, C. M. Vandenbroucke-Grauls, H. A. Verbrugh, M. C. Vos, A. Y. Weersink, J. A. Hoogkamp-Korstanje, and J. F. Meis. 1996. Occurrence of yeast bloodstream infections between 1987 and 1995 in five Dutch university hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 15:909-912. [DOI] [PubMed] [Google Scholar]
- 45.Warren, N. G., and K. C. Hazen. 1999. Candida, Cryptococcus, and other yeasts of medical importance, p. 1184-1199. In R. P. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 46.White, M. H. 1997. Editorial response: the contribution of fluconazole to the changing epidemiology of invasive candidal infections. Clin. Infect. Dis. 24:1129-1130. [DOI] [PubMed] [Google Scholar]
- 47.Wingard, J. R. 1995. Importance of Candida species other than C. albicans as pathogens in oncology patients. Clin. Infect. Dis. 20:115-125. [DOI] [PubMed] [Google Scholar]