Abstract
Moraxella (Branhamella) catarrhalis is an important cause of otitis media and sinusitis in children and of lower respiratory tract infections in adults. Lipooligosaccharide (LOS) is a major surface antigen of the bacterium and elicits bactericidal antibodies. Treatment of the LOS from strain ATCC 25238 with anhydrous hydrazine reduced its toxicity 20,000-fold, as assayed in the Limulus amebocyte lysate (LAL) test. The detoxified LOS (dLOS) was coupled to tetanus toxoid (TT) or high-molecular-weight proteins (HMP) from nontypeable Haemophilus influenzae through a linker of adipic acid dihydrazide to form dLOS-TT or dLOS-HMP. The molar ratios of dLOS to TT and HMP conjugates were 19:1 and 31:1, respectively. The antigenicity of the two conjugates was similar to that of the LOS, as determined by double immunodiffusion. Subcutaneous or intramuscular injection of both conjugates elicited a 50- to 100-fold rise in the geometric mean of immunoglobulin G (IgG) to the homologous LOS in mice after three injections and a 350- to 700-fold rise of anti-LOS IgG in rabbits after two injections. The immunogenicity of the conjugate was enhanced by formulation with monophosphoryl lipid A plus trehalose dimycolate. In rabbits, conjugate-induced antisera had complement-mediated bactericidal activity against the homologous strain and heterologous strains of M. catarrhalis. These results indicate that a detoxified LOS-protein conjugate is a candidate for immunization against M. catarrhalis diseases.
Moraxella (Branhamella) catarrhalis (8, 14, 22) is recognized as the third-most-common pathogen causing otitis media and sinusitis in children, after Streptococcus pneumoniae and nontypeable Haemophilus influenzae (NTHi) (5, 23). This gram-negative diplococcus is also a cause of respiratory tract infections in adults (6, 43), especially those with chronic obstructive pulmonary diseases (39) or compromised immune systems (2, 22). The incidence of the diseases caused by M. catarrhalis appears to be increasing (37). Further, the percentage of clinical isolates producing beta-lactamase has increased over the last 20 years, with some geographical areas reporting an incidence rate as high as 90% (25). Currently, there is no vaccine for diseases caused by M. catarrhalis.
Vaccine development has been limited because there is little information about the protective antigens or an in vitro correlate of immunity against M. catarrhalis in humans. However, as for other bacterial pathogens (42), serum or bactericidal antibodies appear to be involved in immunity against M. catarrhalis diseases. For example, normal adults with natural immunity possibly resulting from colonization or infection have a lower carriage rate (1 to 6%) than children (50 to 78%) and elderly persons (>26%) and suffer fewer infections (20, 21, 23, 48). Children develop serum antibodies to M. catarrhalis gradually during the first 4 years of life (26). This seems to correlate with a decrease in the incidence of bacteremia and otitis media caused by M. catarrhalis (5, 13). Antibodies to whole cells, outer membrane proteins, and lipooligosaccharide (LOS) of M. catarrhalis have been detected in acute- and convalescent-phase sera of adult patients (12, 40, 41). The rise of antibodies to the above-mentioned antigens in paired sera during infection was variable, with about 50% of patients having an increase and only a few having a significant increase (≥4-fold). Most convalescent-phase sera (90%), however, demonstrated bactericidal activity against the corresponding M. catarrhalis isolate (9).
Efforts to date to study M. catarrhalis have focused primarily on surface antigens such as outer membrane proteins (4, 7, 31, 32, 38). Among them, a high-molecular-weight protein (UspA) and a major outer membrane protein (CD) have been studied since both are relatively conserved among different strains and are able to generate bactericidal antibodies (32, 38, 52). Passive immunization with monoclonal antibodies to UspA or immunization with UspA enhanced pulmonary clearance of M. catarrhalis strains in a murine challenge model (10, 32). Other surface antigens such as fimbriae have not been found in all clinical isolates (35), and it is still not clear whether a capsular polysaccharide (PS) exists (1).
LOS, a major surface component of M. catarrhalis, is a possible virulence factor in the pathogenesis of infections caused by this bacterium (15, 24). The LOS might be a potential vaccine candidate because serum antibodies to LOS developed in patients with M. catarrhalis infections (40), the convalescent-phase immunoglobulin G (IgG) anti-LOS from patients demonstrated bactericidal activity against M. catarrhalis strains (45), and the serological properties of LOS in humans suggest a less variable structure of LOS (40).
Three major antigenic types of M. catarrhalis LOSs can be distinguished (47), accounting for 95% of 302 strains (A, 61%; B, 29%; C, 5%). These LOSs contain an oligosaccharide (OS) linked to lipid A without an O-specific PS. Structural studies have shown that the OSs from all three serotypes are branched with a common inner core (17–19), and the lipid A portion is similar to that of other gram-negative bacteria (36a). In this study, we used strain 25238 LOS (type A) as a source of LOS (17, 36). LOS is toxic, and the detoxified LOS (hapten) is not immunogenic. Accordingly, LOS was detoxified and coupled to tetanus toxoid (TT) or high-molecular-weight proteins (HMP) to form conjugates designed to elicit serum LOS antibodies (41a). The detailed characterizations of the conjugates in vitro and in animal models are described.
MATERIALS AND METHODS
Bacterial strains.
Eleven wild-type strains of M. catarrhalis, ATCC 8176, 8193, 23246, 25238, 25239, 25240, 43617, 43618, 43627, 43628, and 49143, were purchased from the American Type Culture Collection (ATCC), Rockville, Md. Ten clinical isolates, M1 to M10, from patients with otitis media with effusions, were kindly provided by Goro Mogi, Oita Medical University, Oita, Japan.
LOS purification.
Type A strain ATCC 25238 was grown on chocolate agar at 37°C in 5% CO2 for 8 h and transferred to 250 ml of 3% tryptic soy broth (Difco Laboratories, Detroit, Mich.) in a 500-ml bottle. The bottle was incubated at 110 rpm in an incubator shaker (model G-25; New Brunswick Scientific Co., Edison, N.J.) at 37°C overnight. The culture was transferred to six 2.8-liter baffled Fernbach flasks, each of which contained 1.4 liters of tryptic soy broth. The flasks were shaken at 110 rpm and maintained at 37°C for 24 h. The culture was centrifuged at 15,000 × g and 4°C for 10 min to collect the cells.
The cell pellets were washed once with 95% ethanol, twice with acetone, and twice with petroleum ether (36) and dried to a powder. The LOS was extracted from cells (28), and the protein and nucleic acid contents of the LOS were less than 1% (44, 50).
Detoxification of LOS.
Anhydrous hydrazine treatment of LOS removes esterified fatty acids from lipid A (29, 30). LOS (160 mg) was suspended in 16 ml of anhydrous hydrazine (Sigma Chemical Co., St. Louis, Mo.) and incubated at 37°C for 3 h with mixing. This suspension was cooled on ice and added dropwise with cold acetone until a precipitate formed. The mixture was centrifuged at 5,000 × g and 5°C for 30 min. The pellet was washed twice with cold acetone, dissolved in pyrogen-free water at a final concentration of 10 to 20 mg/ml, and then ultracentrifuged at 150,000 × g and 5°C for 3 h. The supernatant was passed through a column (1.6 by 90 cm) of Sephadex G-50 (Pharmacia LKB Biotechnology, Uppsala, Sweden) eluted with 25 mM ammonium acetate and monitored with a differential refractometer (R-400; Waters, Milford, Mass.). The eluate was assayed for carbohydrate by a phenol-sulfuric acid method (16). The carbohydrate-containing fractions were pooled, freeze-dried, and designated dLOS.
Derivatization of dLOS.
Adipic acid dihydrazide (ADH; Aldrich Chemical Co., Milwaukee, Wis.) was bound to dLOS to form adipic hydrazide (AH)-dLOS derivatives, using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide HCl (EDC) and N-hydroxysulfosuccinimide (sulfo-NHS) (Pierce) (27). dLOS (70 mg) was dissolved in 7 ml of 345 mM ADH (molar ratio of ADH to LOS is ∼100 to 1, based on an estimated Mr of 3,000 for dLOS) (17). Sulfo-NHS was added to a concentration of 8 mM, the pH was adjusted to 4.8, and EDC was added to a concentration of 0.1 M. The reaction mixture was stirred and maintained at pH 4.8 for 3 h. The reaction mixture was adjusted to pH 7.0 and passed through the G-50 column as described above. The eluate was assayed for carbohydrate and for AH (34). The peaks containing both carbohydrate and AH were pooled, freeze-dried, and designated AH-dLOS. AH-dLOS was measured for its composition, using dLOS and ADH as standards (16, 34).
Conjugation of AH-dLOS to proteins.
TT was obtained from Connaught Laboratories Inc., Swiftwater, Pa., and HMP was purified from NTHi strain 12 (3). AH-dLOS was coupled to TT or HMP to form conjugates (27). Briefly, AH-dLOS (30 mg) was dissolved with 3 ml of water and mixed with 15 mg of TT (5.9 mg/ml) or with 12 mg of HMP (4 mg/ml). The molar ratio of AH-dLOS to both TT (Mr, 150,000) and HMP (Mr, 120,000) was ∼100 to 1. The pH was adjusted to 5.4, and EDC was added to a concentration of 0.05 to 0.1 M. The reaction mixture was stirred, and the pH was maintained at 5.4 for 3 h. The reaction mixture was adjusted to pH 7.0, centrifuged, and passed through a column (1.6 by 90 cm) of Sephacryl S-300 in 0.9% NaCl. Peaks that contained both protein and carbohydrate were pooled and designated dLOS-TT or dLOS-HMP. Both conjugates were analyzed for their composition of carbohydrate and protein, using dLOS and bovine serum albumin (BSA) as standards (16, 44).
Preparation of hyperimmune sera.
Two New Zealand White rabbits (female, 2 to 3 kg) were injected subcutaneously and intramuscularly (two injecting sites) twice with a 4-week interval with 109 bacteria from strain 25238 plus incomplete Freund’s adjuvant (1:1). Blood samples were collected before and 2 weeks after each injection.
Ten female BALB/c mice were injected intraperitoneally three times at 2-week intervals with 108 bacteria from strain 25238. Blood samples were collected and pooled 1 week after the third injection.
Antigenicity.
Antigenicity of the dLOS and conjugates was tested by double immunodiffusion and/or enzyme-linked immunosorbent assay (ELISA), using rabbit hyperimmune serum to 25238 whole cells. Double immunodiffusion was performed in 0.8% agarose in phosphate-buffered saline (PBS; pH 7.4).
ELISA was performed as described previously (29), with modifications. Briefly, after LOS coating, the plate was blocked with 3% BSA in PBS for 1 h, and the rabbit serum (1/8,000) was added for 2 h of incubation before alkaline phosphatase-conjugated goat anti-rabbit IgG and IgM (Sigma) were added for 1 h of incubation. PBS containing 0.01% Tween 20 was used for washings between steps. Diluents for sera and phosphatase were 1% BSA in PBS with 0.01% Tween 20. After the enzyme substrate was added and the mixture was incubated for 30 min, the reactions were read by using a microplate autoreader at A405.
Immunogenicity.
Immunogenicity of the conjugates was examined in mice and rabbits. Five-week-old female mice (NIH Swiss), 10 to 20 per group, were injected subcutaneously with 5 μg of dLOS-TT or dLOS-HMP (carbohydrate), LOS, or dLOS plus TT or HMP (5 μg for protein) in 0.2 ml of 0.9% NaCl with or without Ribi-700 adjuvant (50 μg of monophosphoryl lipid A and 50 μg of synthetic trehalose dicorynomycolate; Ribi ImmunoChem Research, Inc., Hamilton, Mont.). The injections were given three times at 3-week intervals, and the mice were bled 14 days after the first injection and 7 days after the second and third injections.
Female New Zealand White rabbits, weighing 2 to 3 kg (two or three per group), were injected subcutaneously and intramuscularly twice with a 4-week interval with 50 μg of dLOS-TT or dLOS-HMP (carbohydrate), LOS, or dLOS plus TT or HMP (50 μg for protein) in 1 ml of 0.9% NaCl, with or without Ribi-700 adjuvant (250 μg each of monophosphoryl lipid A and synthetic trehalose dicorynomycolate). The rabbits were bled before and 2 weeks after each injection.
Serum anti-LOS levels were expressed as ELISA units, using strain 25238 LOS as a coating antigen. The mouse hyperimmune sera to strain 25238 whole cells as a reference were assigned values of 7,200 and 7,200 U/ml for IgG and IgM, respectively. The rabbit hyperimmune serum to strain 25238 whole cells as a reference was assigned values of 65,000 and 800 U/ml for IgG and IgM, respectively.
Serum TT or HMP antibodies were measured by ELISA with TT or HMP (5 μg/ml) as a coating antigen. The results are expressed as ELISA units on the basis of a reference mouse or rabbit serum produced by three injections of TT or HMP and assigned values of 2,000 and 10 ELISA U/ml for IgG and IgM, respectively.
Bactericidal assay.
Pre- and postimmune sera (mouse sera after three injections and rabbit sera after two injections) were inactivated at 56°C for 30 min and tested for bactericidal activity against strains of M. catarrhalis. A complement-mediated bactericidal assay was performed as described previously (29) except that a guinea pig serum (1:1 dilution, 10 or 20 μl per well) was used as a source of complement (Sigma) and the reaction plate was incubated at 37°C for 30 min before plating onto agar plates. The highest serum dilution causing >50% killing was expressed as the reciprocal bactericidal titer.
LAL assay.
The LOS and dLOS were tested by the Limulus amebocyte lysate (LAL) assay (33). All reagents were obtained from the Food and Drug Administration, Bethesda, Md. The sensitivity of the LAL assay is 0.2 endotoxin unit (EU)/ml.
SDS-PAGE and silver staining.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and silver staining were performed as described previously (46).
Statistical analysis.
Antibody levels are expressed as the geometric mean ELISA units or titers (reciprocal) of n independent observations ± standard deviation or range (n, <4). Significance was tested with the two-tailed t test, and P values smaller than 0.05 were considered significant.
RESULTS
Characterization of dLOS.
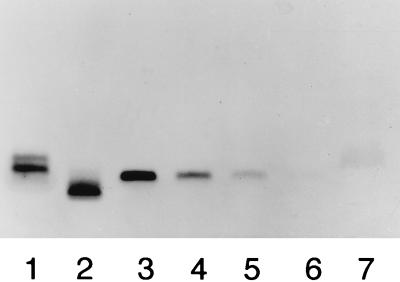
Silver staining of an SDS-polyacrylamide gel showed a single band of LOS (50 to 200 ng) (Fig. 1). In contract, as much as 20 μg of dLOS did not show a band at the location of LOS, which indicated that its residual LOS was less than 0.25%.
FIG. 1.
Silver-stained SDS-PAGE patterns of LOS and dLOS from M. catarrhalis 25238. Lanes 1 and 2 contain 200 ng each of Salmonella minnesota LPS Ra and Rc, respectively; lanes 3 through 6 contain 200, 100, 50, and 25 ng, respectively, of LOS; lane 7 contains 20 μg of dLOS from M. catarrhalis.
By the LAL assay, the LOS showed 20,000 EU/μg whereas the dLOS showed 1 EU/μg (20,000-fold reduction of toxicity). When a rabbit hyperimmune serum directed against strain 25238 whole cells was tested for reactivity with dLOS as shown in Fig. 2, dLOS yielded precipitation by double immunodiffusion, indicating that dLOS retained the antigenicity of the LOS (Fig. 2, wells 4 and 5).
FIG. 2.
Double immunodiffusion. The central well contains a rabbit hyperimmune serum against strain 25238 whole cells. Other wells: 1, LOS, 2 mg/ml; 2, dLOS-TT, 103 μg/ml; 3, dLOS-HMP, 220 μg/ml; 4 and 5, dLOS, 1 mg/ml and 200 μg/ml, respectively; 6, HMP, 500 μg/ml.
Characterization of AH-dLOS and the conjugates.
The molar ratio of AH to dLOS in AH-dLOS was 0.38, and the yield, on the basis of carbohydrate content, was 93%. The molar ratios of dLOS to TT and to HMP in two conjugate preparations were 19:1 and 31:1, and the yields were 8 and 19%, respectively, on the basis of carbohydrate content (Table 1). The two conjugates and the LOS formed identical precipitation lines by double immunodiffusion (Fig. 2, wells 1 to 3). The two conjugates showed similar levels of binding to a rabbit hyperimmune serum by ELISA (Table 1).
TABLE 1.
Composition, yield, and antigenicity of conjugates
| Conjugate | Amt (μg/ml) of:
|
Molar ratio,a dLOS/protein | Yieldb (%) | A405c (hyperimmune serum) | |
|---|---|---|---|---|---|
| dLOS | Protein | ||||
| dLOS-TT | 103 | 266 | 19 | 8 | 1.9 |
| dLOS-HMP | 220 | 280 | 31 | 19 | 1.3 |
Expressed as moles of dLOS per mole of protein, with molecular weights of 3,000 for dLOS, 150,000 for TT, and 120,000 for HMP.
Based on the starting amount of dLOS and the dLOS contained in the conjugates as measured by the phenol-sulfuric acid method.
The antigenicity of conjugates was expressed as ELISA reactivity at A405 when the conjugates were used as coating antigens (10 μg/ml) and a rabbit immune serum was used as a binding antibody (1/8,000). LOS (10 μg/ml) showed an A405 value of 1.1 under the same conditions.
LOS antibodies in mice.
A nonconjugated mixture of dLOS and TT or HMP did not elicit anti-LOS antibodies (Table 2). Both conjugates elicited low levels of anti-LOS IgG after the second but not the first injection, and there was a 50- to 100-fold rise of anti-LOS IgG after the third injection (P < 0.01). dLOS-TT and dLOS-HMP elicited similar levels of anti-LOS IgG after 3 injections. LOS alone and the conjugates elicited similar levels of anti-LOS IgG.
TABLE 2.
Murine antibody response to M. catarrhalis LOS elicited by conjugates
| Immunogena | Injection no. | Geometric mean (range) ELISA unitsb
|
|
|---|---|---|---|
| IgG | IgM | ||
| dLOS + TT | 1 | 1 | 1 |
| 2 | 1 | 1 (1–2) | |
| 3 | 3 | 5 (1–15) | |
| dLOS + HMP | 1 | 1 | 1 |
| 2 | 1 | 1 (1–2) | |
| 3 | 1 (1–2) | 1 | |
| dLOS-TT | 1 | 1 | 1 |
| 2 | 5 (1–19)* | 7 (2–23) | |
| 3 | 52 (6–447)** | 17 (3–91) | |
| dLOS-HMP | 1 | 1 | 1 (1–2) |
| 2 | 2 (1–8)* | 6 (2–19) | |
| 3 | 101 (15–691)** | 17 (5–63) | |
| dLOS-TT + adjuvant | 1 | 3 (1–11)* | 2 (1–4) |
| 2 | 210 (48–923)** | 16 (62–396) | |
| 3 | 470 (266–828) | 14 (8–25) | |
| dLOS-HMP + adjuvant | 1 | 3 (1–9)* | 9 (2–39) |
| 2 | 101 (26–389)** | 22 (6–84) | |
| 3 | 1,514 (299–7,658) | 32 (13–80) | |
| LOS | 1 | 1 | 3 (1–9) |
| 2 | 8 (2–40)* | 2 (1–4) | |
| 3 | 113 (20–630)** | 52 (12–230) | |
Ten to twenty mice for each group were given a total of three subcutaneous injections at 3-week intervals with 5 μg of conjugates, conjugates with Ribi adjuvant, LOS, or the mixture of dLOS and TT or HMP (5 μg of each). Blood samples were collected 2 weeks after the first injection and 1 week after the second and third injections.
The ELISA units were based on a reference serum against strain 25238; the LOS from strain 25238 was used as a coating antigen. Symbols: * versus **, P < 0.01.
Formulation of both conjugates with the Ribi adjuvant significantly enhanced their immunogenicity: two doses of the conjugates with adjuvant elicited IgG levels comparable to or higher than those elicited by three doses of the conjugates alone, and there was about a 9- to 15-fold rise of anti-LOS IgG after three injections (P < 0.01). dLOS-TT elicited a lower level of IgG than dLOS-HMP after three injections when formulated with the adjuvant (P < 0.05).
For IgM, conjugates elicited low to medium levels of anti-LOS after each injection, while LOS elicited high levels of anti-LOS IgM after the third injection. The Ribi adjuvant enhanced the levels of anti-LOS IgM in the conjugate groups.
Protein antibodies in mice.
For anti-TT antibodies, the mixture of dLOS and TT elicited a level of IgG higher than that elicited by dLOS-TT after each injection (Table 3). dLOS-TT elicited a low level of IgG after the first injection; that level rose significantly after the second and third injections (P < 0.01). The Ribi adjuvant enhanced the level of IgG in the dLOS-TT group. All immunogens elicited low levels of anti-TT IgM.
TABLE 3.
Murine antibody response to proteins (TT or HMP) elicited by conjugates
| Immunogena | Injection no. | Geometric mean (range) ELISA unitsb
|
|
|---|---|---|---|
| IgG | IgM | ||
| For assay of anti-TT titer | |||
| dLOS + TT | 1 | 11 (7–18)* | 1 |
| 2 | 303 (191–481)** | 1 (1–2) | |
| 3 | 729 | 2 (1–3) | |
| dLOS-TT | 1 | 1 (1–2)* | 1 |
| 2 | 34 (21–54)** | 1 | |
| 3 | 90 (35–237) | 2 (1–3) | |
| dLOS-TT + adjuvant | 1 | 14 (6–35)* | 3 |
| 2 | 303 (191–481)** | 11 (7–18) | |
| 3 | 2,430 | 27 (13–56) | |
| For assay of anti-HMP titer | |||
| dLOS + HMP | 1 | 2 (1–6)* | 2 (1–3) |
| 2 | 377 (149–952)** | 2 (1–3) | |
| 3 | 1,403 (645–3,051) | 10 (7–14) | |
| dLOS-HMP | 1 | 1 | 1 |
| 2 | 2 (1–8)* | 1 | |
| 3 | 11 (3–43)** | 1 | |
| dLOS-HMP + adjuvant | 1 | 4 (2–9)* | 2 (1–6) |
| 2 | 52 (30–92)** | 7 (3–17) | |
| 3 | 810 (389–1,685) | 11 (7–18) | |
See Table 2, footnote a.
The ELISA units were based on a reference serum against TT or HMP; TT or HMP was used as a coating antigen. Symbols: * versus **, P < 0.01.
For anti-HMP antibodies, the mixture of dLOS and HMP elicited a level of IgG higher than that of dLOS-HMP after each injection (Table 3). dLOS-HMP elicited a low level of IgG after the first injection; that level rose significantly after the second and third injections (P < 0.01). The Ribi adjuvant enhanced the levels of IgG in the dLOS-HMP group. All immunogens elicited low levels of anti-HMP IgM.
LOS antibodies in rabbits.
A mixture of dLOS, TT, and HMP or LOS alone elicited low levels of anti-LOS IgG or IgM antibodies after three injections (Table 4). dLOS-TT elicited a significant rise of anti-LOS IgG after the first and second injections (37- and 700-fold above the preimmune serum levels). dLOS-HMP showed lower levels of IgG than dLOS-TT (6- and 347-fold above the preimmune serum levels). The Ribi adjuvant enhanced the levels of anti-LOS IgG in both conjugate groups after each injection (40- to 2,000-fold above the preimmune serum levels), and there was no significant difference between the two conjugates with the adjuvant after two injections.
TABLE 4.
Rabbit antibody response to M. catarrhalis LOS elicited by conjugates
| Immunogena | Injection no. | Geometric mean (range) ELISA unitsb
|
|
|---|---|---|---|
| IgG | IgM | ||
| dLOS + TT + HMP | 0 | 10 (3–30) | 17 (10–30) |
| 1 | 10 (3–30) | 30 | |
| 2 | 52 (30–90) | 30 | |
| LOS | 0 | 6 (3–10) | 6 (3–10) |
| 1 | 10 (3–30) | 52 (30–90) | |
| 2 | 52 (30–90) | 90 | |
| dLOS-TT | 0 | 5 (3–10) | 14 (10–30) |
| 1 | 187 (90–270) | 90 | |
| 2 | 3,505 (2,430–7,290) | 187 (90–187) | |
| dLOS-HMP | 0 | 7 (3–10) | 10 |
| 1 | 43 (30–90) | 30 (10–90) | |
| 2 | 2,430 | 90 (30–270) | |
| dLOS-TT + adjuvant | 0 | 10 | 5 (3–10) |
| 1 | 810 (270–2,430) | 389 (270–810) | |
| 2 | 21,870 | 270 (90–810) | |
| dLOS-HMP + adjuvant | 0 | 10 (3–30) | 7 (3–10) |
| 1 | 389 (30–2,430) | 90 (30–270) | |
| 2 | 21,870 | 270 (90–810) | |
Two or three rabbits for each group were immunized subcutaneously and intramuscularly twice with a 1-month interval with 50 μg of conjugates, conjugates with Ribi adjuvant, LOS, or a mixture of dLOS, TT, and HMP (50 μg of each). Blood samples were collected before and 14 days after each injection.
See Table 2, footnote b.
For IgM, both conjugates elicited low levels of anti-LOS antibodies, and the conjugates with the Ribi adjuvant elicited low to medium levels of anti-LOS antibodies after each injection.
Protein antibodies in rabbits.
For anti-TT antibodies, the mixture of dLOS, TT, and HMP elicited a higher level of anti-TT IgG than that of dLOS-TT, especially after one injection (Table 5). dLOS-TT elicited a significant level of IgG after two injections (389-fold above the preimmune serum levels). The Ribi adjuvant enhanced the levels of IgG elicited by dLOS-TT fourfold after two injections. All immunogens elicited low levels of anti-TT IgM.
TABLE 5.
Rabbit antibody response to proteins (TT or HMP) elicited by conjugates
| Immunogena | Injection no. | Geometric mean (range) ELISA unitsb
|
|
|---|---|---|---|
| IgG | IgM | ||
| For assay of anti-TT titer | |||
| dLOS + TT + HMP | 0 | 10 | 10 |
| 1 | 270 | 90 | |
| 2 | 2,430 | 30 | |
| dLOS-TT | 0 | 3 | 3 |
| 1 | 7 (3–10) | 10 | |
| 2 | 1,168 (810–2,430) | 14 (10–30) | |
| dLOS-TT + adjuvant | 0 | 5 (3–10) | 3 |
| 1 | 14 (10–30) | 14 (10–30) | |
| 2 | 5,055 (2,430–21,870) | 10 (3–30) | |
| For assay of anti-HMP titer | |||
| dLOS + TT + HMP | 0 | 10 | 10 |
| 1 | 156 (90–270) | 30 | |
| 2 | 2,430 | 52 (30–90) | |
| dLOS-HMP | 0 | 10 (3–30) | 7 (3–10) |
| 1 | 14 (10–30) | 10 | |
| 2 | 810 | 10 | |
| dLOS-HMP + adjuvant | 0 | 7 (3–10) | 10 (3–30) |
| 1 | 130 (30–270) | 14 (10–30) | |
| 2 | 3,505 (2,430–7,290) | 30 | |
For anti-HMP antibodies, the mixture of dLOS, TT, and HMP elicited a higher level of IgG than that of dLOS-HMP, especially after one injection (Table 5). dLOS-HMP elicited a significant level of IgG after two injections (81-fold above preimmune serum levels). The Ribi adjuvant enhanced the levels of IgG elicited by dLOS-HMP fourfold after two injections. All immunogens elicited low levels of anti-HMP IgM.
Bactericidal activity of mouse and rabbit antisera.
In the mouse model, only 20% (4 of 20 mice) of conjugate-immunized sera or 45% (9 of 20 mice) of conjugate (with adjuvant)-immunized sera showed bactericidal activity against the homologous strain at a mean titer of 1:6 (1:2 to 1:32) after three injections of dLOS-TT or dLOS-HMP. There was a low correlation between LOS IgM ELISA levels and the bactericidal titers among 40 mice (r = 0.13, P = 0.02).
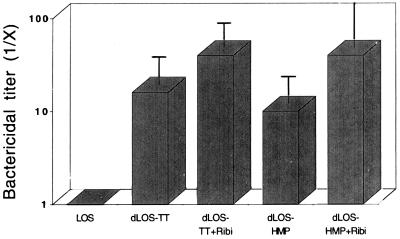
In the rabbit model, LOS (or dLOS)-immunized sera showed no bactericidal activity against the homologous strain 25238 (Fig. 3). In contrast, dLOS-TT-immunized sera showed bactericidal activity at mean titers of 1:16 (no adjuvant) and 1:40 (with adjuvant), while the dLOS-HMP-immunized sera showed bactericidal activity at mean titers of 1:10 and 1:40. There was a correlation between LOS IgG (but not IgM) ELISA levels and the bactericidal titers among 14 rabbits (r = 0.60, P = 0.02).
FIG. 3.
Bactericidal activity of conjugate-induced antisera against M. catarrhalis 25238. Rabbit antisera after two vaccinations of LOS, conjugates, and conjugates with Ribi adjuvant were tested, and each group contained two or three rabbits. The bactericidal titers were expressed as the fold increase above the value for preimmune sera based on the serum dilution causing >50% killing of the bacteria and expressed as the geometric mean and standard deviation for each group. The bactericidal titer for the hyperimmune sera elicited by whole cells was 1:1,600.
The bactericidal activities of the rabbit antisera elicited by dLOS-TT formulated with Ribi adjuvant were further assayed with 10 strains from ATCC and 10 clinical isolates from Japan. Ten of twenty strains were either complement sensitive (23246, 43617, and M9) or serum sensitive (43627, 43628, 49143, M4, M7, M8, and M10). With the remaining 10 strains, the rabbit antisera demonstrated bactericidal activities to four ATCC and five Japanese strains at a mean titer of 1:15 (1:2 to 1:32). No bactericidal activity was demonstrated by the antisera against one of the ATCC strains (25240).
DISCUSSION
Two approaches for detoxification of lipopolysaccharide (LPS) or LOS have been applied to obtain clinically acceptable PS or OS from LPS or LOS. Mild-acid treatment of LOS cleaves the lipid A portion from the LOS molecule at the 3-deoxy-d-manno-octulosonic acid–glucosamine linkage (27); mild-alkali treatment of LOS removes ester-linked fatty acids while preserving amide-linked fatty acids of lipid A (29, 30). We used the latter method for the detoxification of M. catarrhalis LOS because the resulting dLOS gave a better yield and immunogenicity after conjugation to protein carriers (data not shown). Treatment of M. catarrhalis LOS with hydrazine resulted in a 20,000-fold reduction in the level of endotoxin, which makes it clinically acceptable (51).
After detoxification, dLOS retained antigenic determinants but was not immunogenic in vivo. dLOS was rendered immunogenic when covalently bound to protein carriers. The resulting conjugates induced IgG antibody responses to LOS in mice and rabbits. In mice, the conjugates elicited levels of anti-LOS IgG similar to those elicited by LOS. However, in rabbits, levels of anti-LOS IgG following immunization with the conjugates were significantly higher than those after LOS. In addition, the two conjugates elicited similar levels of anti-LOS IgG except when dLOS-HMP was formulated with Ribi adjuvant. This latter formulation elicited higher levels of anti-LOS IgG than dLOS-TT after the third injection in mice, indicating that the HMP is a good protein carrier for conjugate vaccines. Both conjugates were more immunogenic in rabbits than in mice, and the immunogenicity of the conjugates in both animals was enhanced with the adjuvant, consistent with our previous studies (27, 29).
The complement-mediated bactericidal activities of the conjugate-induced antisera were examined with homologous strain 25238 and heterologous strains of M. catarrhalis from ATCC and Japan, using guinea pig serum as a source of complement. Most mouse antisera showed no bactericidal activities against the homologous strain. Addition of Ribi adjuvant to the conjugates increased the percentage of mice with bactericidal activity from 20 to 45%. Other sources of complement (53) such as human AB serum or infant rabbit serum were tested, but none of them were applicable because they were bactericidal to M. catarrhalis alone. All conjugate-induced rabbit sera showed bactericidal activity against the homologous strain 25238. Some representative rabbit sera also showed cross-bactericidal activity against 9 of 10 strains from ATCC and Japan. There was a correlation between LOS antibody levels and the bactericidal titers. Immunizing with meningococcal LOS-derived OS-TT conjugates (27) and NTHi dLOS-protein conjugates (29) induced serum IgG anti-LOS in mice and rabbits, but only the latter had bactericidal activity against both homologous and heterologous strains, using infant rabbit serum as a source of complement.
Many studies have shown that PS (from bacterial capsule or LPS) or dLPS-protein conjugates are usually effective immunogens in mice and rabbits as well as in humans (30, 41a, 44a). Regarding the use of LOS as a component for conjugates, many factors may result in differences of antibody response generated in vivo. These include different bacterial strains and species, detoxification and conjugation methods, quality and composition of the conjugates, protein carriers, immunization routes, adjuvants, animal species, detecting methods, etc. In meningococcal LOS conjugate studies, Jennings et al. (33a) showed that dephosphorylated OS-TT conjugates, emulsified in Freund complete adjuvant, elicited bactericidal antibodies in rabbits to the homologous strain. Verheul et al. (48a) reported that OS-TT conjugates were immunogenic in rabbits, but none of the antisera was bactericidal for the homologous strain despite the use of different sources of complement. We synthesized an OS-TT conjugate which was immunogenic in both mice and rabbits, and the antiserum from the latter showed bactericidal activity against both homologous and heterologous strains (27).
Two available studies of NTHi LOS conjugates showed that an OS-CRM197 conjugate was a poor immunogen in mice (26a); however, dLOS-TT and dLOS-HMP prepared by us were highly immunogenic in both mice and rabbits without adjuvants (29). In addition, the rabbit antisera but not mouse antisera showed bactericidal activity against the homologous and heterologous strains. These preliminary published and unpublished data from meningococcal, NTHi, and M. catarrhalis LOS-derived conjugates indicate that dLOS conjugates are usually more immunogenic than OS conjugates, both conjugates are more immunogenic in rabbits than in mice, and rabbit antisera are more likely to be bactericidal than mouse antisera.
It has been documented that serum bactericidal LPS or PS antibodies confer immunity to many pathogens in humans, including H. influenzae type b, Neisseria meningitidis, Vibrio cholerae, and Shigella sonnei (12, 42). Recently, we have shown that systemic immunization with dLOS from NTHi conjugated to proteins confers protection from experimental otitis media on chinchillas (26b). In the case of M. catarrhalis infections, the role of anti-LOS antibodies in conferring protective immunity has not been elucidated (40). The convalescent-phase anti-LOS IgG from patients demonstrated bactericidal activity against M. catarrhalis strains (45). We suggest that dLOS-TT or dLOS-HMP induced serum anti-LOS antibodies (IgG) with bactericidal activity can transude to mucosal surfaces (49) of nasopharynges and lyse the inocula of strains causing otitis media (26b) and respiratory diseases caused by M. catarrhalis. For further evaluation of the dLOS-protein conjugate as a potential vaccine for human use, animal protection studies are planned, realizing that M. catarrhalis is an exclusively human pathogen (37a).
ACKNOWLEDGMENTS
We are grateful to Goro Mogi for providing clinical strains, Linda Winter for help in the purification of HMP, and Jianzhong Sun and Cassandra Phillips for help in the purification of LOS.
REFERENCES
- 1.Ahmed K, Rikitomi N, Ichinose A, Matsumoto K. Possible presence of a capsule in Branhamella catarrhalis. Microbiol Immunol. 1991;35:361–366. doi: 10.1111/j.1348-0421.1991.tb01566.x. [DOI] [PubMed] [Google Scholar]
- 2.Alaeus, A., and G. Stiernstedt.Branhamella catarrhalis septicemia in an immunocompetent adult. Scand. J. Infect. Dis. 23:115–116. [DOI] [PubMed]
- 3.Barenkamp S J. Immunization with high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae modifies experimental otitis media in chinchillas. Infect Immun. 1996;64:1246–1251. doi: 10.1128/iai.64.4.1246-1251.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhushan R, Craigie R, Murphy T F. Molecular cloning and characterization of outer membrane protein E of Moraxella (Branhamella) catarrhalis. J Bacteriol. 1994;176:6636–6643. doi: 10.1128/jb.176.21.6636-6643.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bluestone, C. D. 1986. Otitis media and sinusitis in children. Role of Branhamella catarrhalis. Drugs 31(Suppl. 3):132–141. [DOI] [PubMed]
- 6.Boyle F M, Georghiou P R, Tilse M H, McCormack J G. Branhamella (Moraxella) catarrhalis: pathogenic significance in respiratory infections. Med J Aust. 1991;154:592–596. doi: 10.5694/j.1326-5377.1991.tb121219.x. [DOI] [PubMed] [Google Scholar]
- 7.Campagnari A A, Shanks K, Dyer D W. Growth of Moraxella catarrhalis with human transferrin and lactoferrin: expression of iron-repressible proteins without siderophore production. Infect Immun. 1994;62:4909–4914. doi: 10.1128/iai.62.11.4909-4914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catlin B W. Branhamella catarrhalis: an organism gaining respect as a pathogen. Clin Microbiol Rev. 1990;3:293–320. doi: 10.1128/cmr.3.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman A J, Jr, Musher D M, Jonsson S, Clarridge J E, Wallace R J. Development of bactericidal antibody during Branhamella catarrhalis infection. J Infect Dis. 1985;151:878–882. doi: 10.1093/infdis/151.5.878. [DOI] [PubMed] [Google Scholar]
- 10.Chen D, McMichael J C, VanDerMeid K R, Hahn D, Mininni T, Cowell J, Eldridge J. Evaluation of purified UspA from Moraxella catarrhalis as a vaccine in a murine model after active immunization. Infect Immun. 1996;64:1900–1905. doi: 10.1128/iai.64.6.1900-1905.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen J J, Hansen N Q, Bruun B. Serum antibody response to outer membrane proteins of Moraxella (Branhamella) catarrhalis in patients with bronchopulmonary infection. Clin Diagn Lab Immunol. 1990;3:717–721. doi: 10.1128/cdli.3.6.717-721.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen D, Ashkenzai S, Green M S, Gdalevich M, Robin G, Slepon R, Yavzori M, Orr N, Block C, Ashkenazi I, Shemer J, Taylor D N, Hale T L, Sadoff J C, Pavliakova D, Schneerson R, Robbins J B. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet. 1997;349:155–159. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- 13.Communicable Disease Surveillance Centre. –1995. 1992. CDR Weekly Reports. Communicable Disease Surveillance Centre, London, England. [Google Scholar]
- 14.Doern G V. Branhamella catarrhalis—an emerging human pathogen. Diagn Microbiol Infect Dis. 1986;4:191–201. doi: 10.1016/0732-8893(86)90098-2. [DOI] [PubMed] [Google Scholar]
- 15.Doyle, W. J. 1989. Animal models of otitis media: other pathogens. Pediatr. Infect. Dis. J. 81(Suppl.):S45–S47. [PubMed]
- 16.Dubois M, Gillis H, Hamilton J K, Rebers A A, Smith R. Colorimetric method for the determination of sugars and related substances. Anal Biochem. 1956;28:250–256. [Google Scholar]
- 17.Edebrink P, Jansson P E, Rahman M M, Widmalm G, Holme T, Rahman M, Weintraub A. Structural studies of the O-polysaccharide from the lipopolysaccharide of Moraxella (Branhamella) catarrhalis serotype A (strain ATCC 25238) Carbohydr Res. 1994;257:269–284. doi: 10.1016/0008-6215(94)80040-5. [DOI] [PubMed] [Google Scholar]
- 18.Edebrink P, Jansson P E, Rahman M M, Widmalm G, Holme T, Rahman M. Structural studies of the O-antigen oligosaccharides from two strains of Moraxella catarrhalis serotype C. Carbohydr Res. 1995;266:237–261. doi: 10.1016/0008-6215(94)00276-l. [DOI] [PubMed] [Google Scholar]
- 19.Edebrink P, Jansson P E, Widmalm G, Holme T, Rahman M. The structures of oligosaccharides isolated from the lipopolysaccharide of Moraxella catarrhalis serotype B, strain CCUG 3292. Carbohydr Res. 1996;295:127–146. doi: 10.1016/s0008-6215(96)90132-9. [DOI] [PubMed] [Google Scholar]
- 20.Ejlertsen T, Thisted E, Ebbesen F, Olesen B, Renneberg J. Branhamella catarrhalis in children and adults. A study of prevalence, time of colonization, and association with upper and lower respiratory tract infections. J Infect. 1994;29:23–31. doi: 10.1016/s0163-4453(94)94979-4. [DOI] [PubMed] [Google Scholar]
- 21.Eliasson, I. 1986. Serological identification of Branhamella catarrhalis. Serological evidence for infection. Drugs 31(Suppl. 3):7–10. [DOI] [PubMed]
- 22.Enright M C, McKenzie H. Moraxella (Branhamella) catarrhalis-clinical and molecular aspects of a rediscovered pathogen. J Med Microbiol. 1997;46:360–371. doi: 10.1099/00222615-46-5-360. [DOI] [PubMed] [Google Scholar]
- 23.Faden H, Harabuchi Y, Hong J J. Epidemiology of Moraxella catarrhalis in children during the first 2 years of life: relationship to otitis media. J Infect Dis. 1994;169:1312–1317. doi: 10.1093/infdis/169.6.1312. [DOI] [PubMed] [Google Scholar]
- 24.Fomsgaard J S, Fomsgaard A, Hoiby N, Bruun B, Galanos C. Comparative immunochemistry of lipopolysaccharides from Branhamella catarrhalis strains. Infect Immun. 1991;59:3346–3349. doi: 10.1128/iai.59.9.3346-3349.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fung C P, Powell M, Seymour A, Yuan M, Williams J D. The antimicrobial susceptibility of Moraxella catarrhalis isolated in England and Scotland in 1991. J Antimicrob Chemother. 1992;30:47–55. doi: 10.1093/jac/30.1.47. [DOI] [PubMed] [Google Scholar]
- 26.Goldblatt D, Turner M V V, Levinsky R J. Branhamella catarrhalis: antigenic determinants and the development of the IgG subclass response in childhood. J Infect Dis. 1990;162:1128–1135. doi: 10.1093/infdis/162.5.1128. [DOI] [PubMed] [Google Scholar]
- 26a.Green B A, Meredith E S, Edwards L, Jones K F. Nontypeable Haemophilus influenzae lipooligosaccharide conjugates as vaccine candidates against NTHi. In: Norrby E, Brown F, Chanock R M, Ginsberg H S, editors. Vaccines 94. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 125–129. [Google Scholar]
- 26b.Gu X-X, Sun J, Jin S, Barenkamp S J, Lim D J, Robbins J B, Battey J. Detoxified lipooligosaccharide from nontypeable Haemophilus influenzae conjugated to proteins confers protection against otitis media in chinchillas. Infect Immun. 1997;65:4488–4493. doi: 10.1128/iai.65.11.4488-4493.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu X-X, Tsai C-M. Preparation, characterization, and immunogenicity of meningococcal lipooligosaccharide-derived oligosaccharide-protein conjugates. Infect Immun. 1993;61:1873–1880. doi: 10.1128/iai.61.5.1873-1880.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu X-X, Tsai C-M, Apicella M A, Lim D J. Quantitation and biological properties of released and cell-bound lipooligosaccharides from nontypeable Haemophilus influenzae. Infect Immun. 1995;63:4115–4120. doi: 10.1128/iai.63.10.4115-4120.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu X-X, Tsai C-M, Ueyama T, Barenkamp S J, Robbins J B, Lim D J. Synthesis, characterization, and immunological properties of detoxified lipooligosaccharide from nontypeable Haemophilus influenzae conjugated to proteins. Infect Immun. 1996;64:4047–4053. doi: 10.1128/iai.64.10.4047-4053.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta R K, Szu S C, Finkelstein R A, Robbins J B. Synthesis, characterization, and some immunological properties of conjugates composed of the detoxified lipopolysaccharide of Vibrio cholerae O1 serotype Inaba bound to cholera toxin. Infect Immun. 1992;60:3201–3208. doi: 10.1128/iai.60.8.3201-3208.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helminen M E, Maciver I, Latimer J L, Cope L D, McCracken G H, Hansen E J. A major outer membrane protein of Moraxella catarrhalis is a target for antibodies that enhance pulmonary clearance of the pathogen in an animal model. Infect Immun. 1993;61:2003–2010. doi: 10.1128/iai.61.5.2003-2010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helminen M E, Maciver I, Latimer J L, Klesney-Tait J, Cope L D, Paris M, McCracken G H, Hansen E J. A large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J Infect Dis. 1994;170:867–872. doi: 10.1093/infdis/170.4.867. [DOI] [PubMed] [Google Scholar]
- 33.Hochstein H D, Elin R J, Cooper J F, Seligmann E B, Wolff S M. Further developments of Limulus amebocyte lysate test. Bull Parenter Drug Assoc. 1973;27:139–148. [PubMed] [Google Scholar]
- 33a.Jennings H J, Lugowski C, Ashton F E. Conjugation of meningococcal lipopolysaccharide R-type oligosaccharides to tetanus toxoid as route to a potential vaccine against group B Neisseria meningitidis. Infect Immun. 1984;43:407–412. doi: 10.1128/iai.43.1.407-412.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kemp A H, Morgan M R A. Studies on the detrimental effects of bivalent binding in a microtiter plate ELISA and possible remedies. J Immunol Methods. 1986;94:65–72. doi: 10.1016/0022-1759(86)90216-4. [DOI] [PubMed] [Google Scholar]
- 35.Marrs, C. F., and S. Weir. 1990. Pili (fimbriae) of Branhamella species. Am. J. Med. 88(Suppl. 5A):36S–40S. [DOI] [PubMed]
- 36.Masoud H, Perry M B, Brisson J R, Uhrin D, Richards J C. Structural elucidation of the backbone oligosaccharide for the lipopolysaccharide of Moraxella catarrhalis serotype A. Can J Chem. 1994;72:1466–1477. [Google Scholar]
- 36a.Masoud H, Perry M B, Richards J C. Characterization of the lipopolysaccharide of Moraxella catarrhalis. Structural analysis of the lipid A from M. catarrhalis serotype A lipopolysaccharide. Eur J Biochem. 1994;220:209–216. doi: 10.1111/j.1432-1033.1994.tb18616.x. [DOI] [PubMed] [Google Scholar]
- 37.McLeod D T, Ahmad F, Capewell S, Croughan M J, Calder M A, Seaton A. Increase in bronchopulmonary infection due to Branhamella catarrhalis. Br Med J. 1986;292:1103–1105. doi: 10.1136/bmj.292.6528.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a.Murphy T F. Branhamella catarrhalis: epidemiology, surface antigenic structure, and immune response. Microbiol Rev. 1996;60:267–279. doi: 10.1128/mr.60.2.267-279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy T F, Kirkham C, Lesse A J. The major heat-modifiable outer membrane protein CD is highly conserved among strains of Branhamella catarrhalis. Mol Microbiol. 1993;10:87–97. doi: 10.1111/j.1365-2958.1993.tb00906.x. [DOI] [PubMed] [Google Scholar]
- 39.Nicotra B, Rivera M, Luman J I, Wallace R J. Branhamella catarrhalis as a lower respiratory tract pathogen in patients with chronic lung disease. Arch Intern Med. 1986;146:890–893. [PubMed] [Google Scholar]
- 40.Rahman M, Holme T, Jonsson I, Krook A. Lack of serotype-specific antibody response to lipopolysaccharide antigens of Moraxella catarrhalis during lower respiratory tract infection. Eur J Clin Microbiol Infect Dis. 1995;14:297–304. doi: 10.1007/BF02116522. [DOI] [PubMed] [Google Scholar]
- 41.Rahman M, Holme T, Jonsson I, Krook A. Human immunoglobulin isotype and IgG subclass response to different antigens of Moraxella catarrhalis. APMIS. 1997;105:213–220. doi: 10.1111/j.1699-0463.1997.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 41a.Robbins J B, Schneerson R. Polysaccharide-protein conjugates: a new generation of vaccines. J Infect Dis. 1990;161:821–832. doi: 10.1093/infdis/161.5.821. [DOI] [PubMed] [Google Scholar]
- 42.Robbins J B, Schneerson R, Szu S C. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171:1387–1398. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 43.Sarubbi F A, Myers J V V, Williams J J, Shell C G. Respiratory infections caused by Branhamella catarrhalis. Selected epidemiologic features. Am J Med. 1990;88:9S–14S. doi: 10.1016/0002-9343(90)90254-b. [DOI] [PubMed] [Google Scholar]
- 44.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 44a.Svenson S B, Lindberg A A. Artificial Salmonella vaccines: Salmonella typhimurium O-antigen-specific oligosaccharide-protein conjugates elicit protective antibodies in rabbits and mice. Infect Immun. 1981;32:490–496. doi: 10.1128/iai.32.2.490-496.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka H, Oishi K, Sonoda F, Iwagaki A, Nagatake T, Matsumoto K. Biochemical analysis of lipopolysaccharides from respiratory pathogenic Branhamella catarrhalis strains and the role of anti-LPS antibodies in Branhamella respiratory infections. J Jpn Assoc Infect Dis. 1992;66:709–715. doi: 10.11150/kansenshogakuzasshi1970.66.709. [DOI] [PubMed] [Google Scholar]
- 46.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 47.Vaneechoutte M, Verschraegen G, Claeys G, Van Den Abeele A M. Serological typing of Branhamella catarrhalis strains on the basis of lipopolysaccharide antigens. J Clin Microbiol. 1990;28:182–187. doi: 10.1128/jcm.28.2.182-187.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaneechoutte M, Verschraegen G, Claeys G, Weise B, Van den Abeele A M. Respiratory tract carrier rates of Moraxella (Branhamella) catarrhalis in adults and children and interpretation of the isolation of M. catarrhalis from sputum. J Clin Microbiol. 1990;28:2674–2680. doi: 10.1128/jcm.28.12.2674-2680.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48a.Verheul A F M, Braat A K, Leenhouts J M, Hoogerhout P, Poolman J T, Snippe H, Verhoef J. Preparation, characterization, and immunogenicity of meningococcal immunotype L2 and L3,7,9, phosphoethanolamide group-containing oligosaccharide-protein conjugates. Infect Immun. 1991;59:843–851. doi: 10.1128/iai.59.3.843-851.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner D K, Clements M L, Reimer C B, Synder M, Nelson D L, Murphy B R. Analysis of immunoglobulin G antibody responses after administration of live and inactivated influenza A vaccine indicates that nasal wash immunoglobulin G is a transudate from serum. J Clin Microbiol. 1987;25:559–562. doi: 10.1128/jcm.25.3.559-562.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warburg O, Christian W. Isolation and crystallization of enolase. Biochem Z. 1942;310:384–421. [Google Scholar]
- 51.W.H.O. Expert Committee on Biological Standardization. Requirements for Haemophilus type b conjugate vaccines. WHO Tech Rep Ser. 1991;814:15–37. [Google Scholar]
- 52.Yang Y P, Myers L E, McGuinness U, Chong P, Kwok Y, Klein M H, Harkness R E. The major outer membrane protein, CD, extracted from Moraxella (Branhamella) catarrhalis is a potential vaccine antigen that induces bactericidal antibodies. FEMS Immunol Med Microbiol. 1997;17:187–199. doi: 10.1111/j.1574-695X.1997.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 53.Zollinger W D, Mandrell R E. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect Immun. 1983;40:257–264. doi: 10.1128/iai.40.1.257-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]