Abstract
Simple Summary
Current interventions for breast–cancer prevention are associated with adverse side effects that frequently deter women from selecting these evidence-based risk-reducing procedures. In addition, modifiable lifestyle changes can improve breast cancer risk but can be challenging in execution. Therefore, women who are considered high risk due to non-modifiable factors, such as BRCA1/2 mutations or a strong family history of breast cancer, are in need of alternative prevention procedures. Here, we review investigational preclinical and clinical approaches at a systemic and local level that focus on non-modifiable breast cancer risk-reducing interventions.
Abstract
One in eight women will develop breast cancer in the US. For women with moderate (15–20%) to average (12.5%) risk of breast cancer, there are few options available for risk reduction. For high-risk (>20%) women, such as BRCA mutation carriers, primary prevention strategies are limited to evidence-based surgical removal of breasts and/or ovaries and anti-estrogen treatment. Despite their effectiveness in risk reduction, not many high-risk individuals opt for surgical or hormonal interventions due to severe side effects and potentially life-changing outcomes as key deterrents. Thus, better communication about the benefits of existing strategies and the development of new strategies with minimal side effects are needed to offer women adequate risk-reducing interventions. We extensively review and discuss innovative investigational strategies for primary prevention. Most of these investigational strategies are at the pre-clinical stage, but some are already being evaluated in clinical trials and others are expected to lead to first-in-human clinical trials within 5 years. Likely, these strategies would be initially tested in high-risk individuals but may be applicable to lower-risk women, if shown to decrease risk at a similar rate to existing strategies, but with minimal side effects.
Keywords: breast cancer prevention, mammary gland, intraductal delivery, ductal tree, epithelial cell carcinogenesis, chemoprevention, endocrine therapy, transdermal gel
1. Introduction
For women in the US, breast cancer (BC) is the most prevalent cancer and the second-leading cause of cancer-related deaths. Projections for 2023 estimate that 55,720 women will be diagnosed with carcinoma in situ, 297,790 with invasive carcinoma, and 43,170 women will die from BC [1]. With the number of new diagnoses still on the rise, one in eight women will develop BC within their lifetime, but all women are at risk. Therefore, there is a need to develop new strategies for primary prevention with a focus on high-risk (>20%) individuals, but that can also be applied to moderate- (15–20%) and average (12.5%)-risk individuals.
There are well-established risk factors that contribute to the absolute risk of developing BC [2,3]. There are modifiable risk factors such as having a healthy diet, regular exercise, and limiting alcohol consumption [2,4]. Cumulative exposure of breast tissue to estrogen is an important risk factor for BC. This risk can be minimized by various actions such as having their first-born before age 30, limiting the use of hormonal birth control medications, and avoiding hormone replacement therapy. Non-modifiable risk factors that increase cumulative exposure to estrogen include younger age at menarche and older age at natural menopause [2]. There are non-modifiable genetic risk factors that include known mutations in a high-penetrant BC gene such as BRCA1 and BRCA2, cumulative interaction of risk-associated alleles of BC susceptible SNPs, and/or family history with multiple incidences of BC [5]. There are also non-modifiable risk factors related to the personal history of radiation therapy to the chest, and the number of breast biopsies [4].
For women in the US, a 1.67% increased risk over 5 years at any age or 20% increased risk over a 20-year period is considered a high-risk individual [3]. This review is focused on federal agency guidelines and regulatory approvals that pertain specifically to US women. It is worth noting that some of these guidelines are different in other countries. For example, for women in Europe, high risk is defined as a >30% lifetime risk [6]. This difference and other considerations may affect how risk-reducing interventions are applied, perceived, and complied with in different geographical regions and countries. Many non-modifiable factors contribute to this increased risk. BRCA mutation carriers are considered high-risk individuals (>50% chance of BC development) and are the most prevalent and counseled group for primary intervention. Women with other genetic predispositions including mutation in CDH1 (hereditary diffuse gastric cancer syndrome), PALB2, PTEN (Bannayan–Riley–Ruvalcaba, Cowden, or hamartoma tumor syndromes), TP53 (Li-Fraumeni syndrome), STK11 (Peutz–Jeghers syndrome) are also considered high-risk individuals and eligible for bilateral prophylactic mastectomy [7,8].
Guidelines for risk reduction of moderate (15–20%)-risk individuals are not as well-delineated in part due to the difficulty of identifying this subset of individuals and how modifiable risk factors such as diet, alcohol consumption, and BMI can affect and compound absolute risk [4,5]. However, women with genetic predispositions including mutations in ATM and CHK2, or who carry risk-associated alleles for multiple of the 92 susceptibility genes are generally considered moderate-risk individuals [9]. Genetic counseling based on multi-gene panels captures the most frequent mutations [7,8,10,11]. Recommendation for mammography and other monitoring modalities varies by risk [3,12].
There are several risk assessment models available that calculate an individual’s risk based on various risk factors [2,13]. The BCRAT tool (https://bcrisktool.cancer.gov/ (accessed on 30 December 2023)) uses the Gail model and is appropriate for women without a genetic predisposition or previous BC [14]. The IBIS tool (https://ibis.ikonopedia.com/ (accessed on 30 December 2023)) uses the Tyrer–Cuzick model and is appropriate for women with known or suspected genetic predisposition, including mutations in BRCA1 or BRCA2, using extensive personal and family history risk factors [15].
For high-risk women in the US, FDA-approved primary prevention strategies include surgical removal of the breasts and/or ovaries and the use of anti-estrogen therapies. Bilateral prophylactic mastectomy is currently the most effective procedure for preventing BC: it can reduce the incidence of BC by up to 90% in high-risk individuals [16]. Anti-estrogen treatments have been shown to reduce BC risk by up to 50% in high-risk women [16]. Though effective in risk reduction, less than 50% and less than 10% of high-risk individuals opt for surgical or hormonal interventions, respectively, with life-changing consequences and severe side effects as major contributing dissuading factors [16,17,18]. These prevention interventions are readily available but may be underused due to a lack of clinician or patient information regarding risk level, lack of clinical confidence to discuss appropriate prevention options, personal social dynamics, and fully informed choice, which can result in a low uptake of prevention methods [16]. Bilateral prophylactic mastectomy uptake is well documented in women who are BRCA mutation carriers. However, on average, only 20% of women at high risk without the BRCA mutations undergo this surgical procedure but have reported ranges from 11–50% [19,20]. Population studies on hormonal interventions have reported low uptake for eligible women (1–5%); however, this falls short when compared to high-risk proactive women interested in using these interventions, which can be as high as 40% [16,21]. A recent study conducted in Europe showed that women who were informed of their risk and provided information on preventive options within 8 weeks of risk identification had a large increase in uptake (77.5%) of hormonal interventions compared to much lower uptake in standard clinical settings (11–20)% [18,22,23,24]. Therefore, prevention interventions, either currently approved or investigational, should take into consideration education and informed decision-making in addition to clinically established risk reduction and management of adverse side effects.
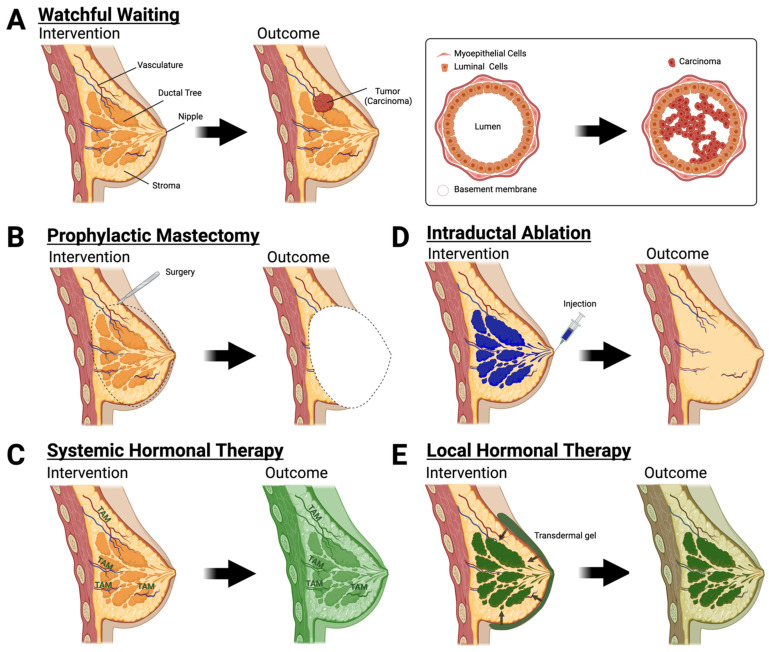
These primary prevention interventions treat the entire breast as a unit, but neoplasia originates in discrete regions within one or more ductal tree systems [25,26]. A woman’s breast contains the stroma and 8–12 ductal trees [27,28,29]. Surrounding the ductal tree is the stroma, which is composed of adipocytes, immune cells, endothelial cells, fibroblasts, and extracellular matrix (Figure 1A). The ductal trees are composed of luminal epithelial cells, myoepithelial cells, and mammary epithelial stem cells from which most BC arises and are not readily accessible without highly invasive surgical procedures. This review focuses on preclinical and clinical approaches that target the premalignant epithelial niche and minimize adverse side effects while maintaining the effectiveness of current surgical and systemic interventions for women with non-modifiable risk.
Figure 1.
Evidence-based and investigational approaches for primary prevention. (A) Watchful waiting with no preventative outcome. (B) Bilateral prophylactic mastectomy is an effective risk-reducing surgery. (C) Hormonal therapy such as tamoxifen (TAM) or raloxifene can reduce risk but with high systemic exposure (dark green shading). (D,E) Intraductal approaches for breast cancer prevention. (D) Intraductal injections ablate epithelial cells leaving the breast stroma intact. (E) Local hormonal therapy with moderate systemic exposure (light green shading).
2. Evidence-Based Interventions for Primary Prevention
We briefly review the limited number of FDA-approved drugs and interventions for primary prevention of BC. Due to adverse side effects, recommendation for these interventions is generally restricted to high-risk individuals.
2.1. Surgical Intervention
Surgical interventions target areas of the breast that are associated with a higher risk of BC development through the complete removal of the tissue. Due to the invasive nature of these procedures, only high-risk women consider these options. Dual mastectomy directly removes both breasts and in doing so removes the ductal tree, whereas salpingo-oophorectomy removes fallopian tubes and/or ovaries for reduced hormonal contribution in BC development. Here, we discuss the advantages and disadvantages of surgical intervention.
Bilateral prophylactic mastectomy. This procedure completely removes the breast tissue in both breasts, including the ductal tree and surrounding stroma. Bilateral prophylactic mastectomy is a highly invasive procedure that can be executed as a total mastectomy, skin-sparing mastectomy, or total skin-sparing mastectomy. In removing the entirety of the breast, this risk-reducing surgery removes the epithelial cells, the intended target cells, from which breast carcinomas arise [30,31]. With nipple reconstructive techniques available, total mastectomy is the most commonly selected choice [32]. However, despite risk reduction and available reconstructive surgery, less than 50% of women undergo this preventative procedure (Figure 1B) due to the pain, cosmetic, psychological, and social impact [17,33,34]. Pain, local, and systemic inflammation in response to mastectomy varies on the surgical procedure which can include musculoskeletal manipulation and tissue advancement for reconstruction in addition to the excision of the breast tissue. Bilateral prophylactic mastectomy studies focusing on patient-reported outcomes after breast reconstruction have noted higher body pain or breast discomfort and decreased sexual interest, but overall satisfaction with the procedure lowered cancer-related anxiety and increased satisfaction with breast cosmesis [35,36,37,38,39,40]. However, it is important to note that these reports vary due to factors such as number of patients, the timing of reconstructive surgery, and pre- vs. post-operation comparisons [35]. Nevertheless, the potential positive and negative impacts should be equally addressed with women for them to make fully informed decisions.
Salpingo-oophorectomy. Surgical removal of the fallopian tubes and ovaries is a commonly recommended BC prevention for premenopausal BRCA mutation carriers that had a previously reported risk reduction of up to 50% [41]. These findings have been called into question after considering the salpingo-oophorectomy procedure as a time-varying covariate. Recent studies investigating BC risk reduction in BRCA mutation carriers following salpingo-oophorectomy have found minimal to no impact in women when taking into consideration the time-varying covariate [41,42,43]. Following salpingo-oophorectomy, women may experience symptoms typically associated with menopause due to the reduction of estrogen and progesterone production in the body. However, these symptoms are often reportedly tolerable and do not require further treatment [44]. Hormone replacement therapy for women experiencing severe symptoms is available. BRCA mutation carriers taking hormone replacement therapy after undergoing oophorectomy do not have an increased risk of BC if <45 years old but those >45 have an increased risk of triple-negative BC [45]. Other studies have shown that BRCA mutation carriers who receive only estrogen after an oophorectomy have no increased risk of BC. However, the risk of only progesterone use has yet to be determined [46,47].
2.2. Hormonal Modulation
Selective estrogen receptor modulators (SERMs), tamoxifen and raloxifene, are commonly used in the treatment of various diseases including BC and osteoporosis, and are the only two FDA-approved compounds for primary prevention of BC [5,48]. An estrogen-bound estrogen receptor (ER) forms a complex that hetero- or homo-dimerizes with a second estrogen-bound estrogen receptor. This complex is able to translocate into the nucleus and can act directly and indirectly on genes to promote cell growth, migration, and metastasis while simultaneously preventing functions such as apoptosis and necrosis [49,50,51]. These SERMs compete with and block the binding of estrogen to the ER within the breast epithelia and antagonize its functions (Figure 1C and Figure 2A); interaction of these SERMs with ERs in other cell types throughout the body can have partial antagonistic or even agonistic effects [52].
Figure 2.
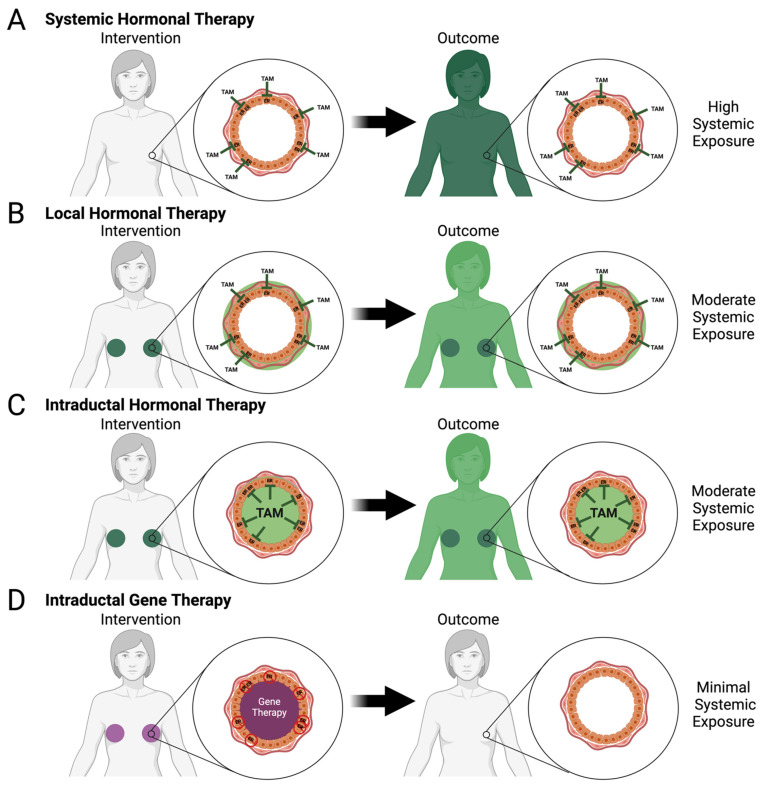
Systemic and local hormone therapy approaches for breast cancer prevention. (A) Systemic hormonal therapy with high systemic exposure (dark green shading). (B) Local hormonal therapy with concentrated exposure to the ductal tree (dark green shading) and moderate systemic exposure to the body (light green shading). (C,D) Intraductal delivery directly to the ductal tree by hormonal therapy with moderate systemic exposure (light green shading) or gene therapy with minimal systemic exposure (no shading).
Tamoxifen. Tamoxifen was originally developed in the 1960s as a contraceptive but was ultimately unsuccessful. In the 1990s, it was repurposed as a BC treatment due to its therapeutic benefits in reducing tumor recurrence at the origin site, lowering the incidence of cancer in the contralateral breast, and increasing overall survival. These benefits led to the selection of tamoxifen as a preventive agent for BC [53,54]. By 1992, the National Surgical Adjuvant Breast and Bowel Project had enrolled 13,175 high-risk women in a trial investigating the role of tamoxifen as an oral drug for the prevention of primary formation of BC. By the end of the five-year clinical trial, tamoxifen had reduced the incidence of invasive and non-invasive BC by 49% and 50%, respectively, compared to placebo groups [53]. Women who developed invasive BC had lower occurrence and tumor size in the tamoxifen-treated groups. Despite the reduction of BC incidence, increased hot flashes and vaginal discharge were reported among women in the tamoxifen-treated groups. More alarming was the associated risk of stroke and a 1.5-to-6.9-fold increased risk of developing endometrial cancer due to long-term exposure to tamoxifen [53,55,56]. Additional studies of tamoxifen as a preventive agent reported these adverse effects as major factors contributing to discontinuation of treatment, especially among women taking tamoxifen for primary prevention as opposed to adjuvant therapy for BC [57,58]. However, adverse effects ceased after treatment, and long-term follow-up studies showed extended tamoxifen protection for BC prevention [59,60,61].
Raloxifene. Similar to tamoxifen, raloxifene was originally developed for non-BC disease. Initially under investigation for treatment and prevention of osteoporosis in postmenopausal women, the Multiple Outcomes of Raloxifene Evaluation (MORE) study in 1998 was conducted to investigate bone fractures in 7700 post-menopausal women diagnosed with osteoporosis [62]. Interestingly, during this study, raloxifene was associated with a lower incidence of BC. Raloxifene treatment resulted in a 76% decrease in invasive BC after 3 years of treatment [62]. Like tamoxifen, reports of hot flashes along with leg cramps were higher among women taking raloxifene. Additionally, thromboembolic events were 3.1 times higher with raloxifene but did not increase the risk of endometrial cancer [62,63]. Direct comparison of tamoxifen and raloxifene revealed equal risk reduction of invasive BC, but endometrial cancer in postmenopausal women, thromboembolic events, and stroke occurred in both groups [64]. The raloxifene-treated group had a 30% lower rate of endometrial cancer, had fewer side effects on the uterus, and had a lower incidence of thromboembolic events which was consistent over the 81-month follow-up [64,65].
2.3. Watchful Waiting
Despite the evidence-based effectiveness of these surgical procedures and hormonal interventions, up to 50–70% of women presented with prevention and treatment options choose a watchful waiting strategy with enhanced surveillance [66,67]. These strategies may include annual or more frequent mammography and magnetic resonance imaging, monthly self-examination, and other monitoring protocols. Watchful waiting strategies do not reduce the risk of developing BC (Figure 1A) and up to 70% of high-risk individuals do not adhere to their enhanced surveillance protocols decreasing the benefit of early detection and intervention [68,69,70].
3. Investigational Approaches for Primary Prevention
There are several investigational approaches that aim to reduce or limit adverse side effects of current interventions and/or develop novel interventions with a high safety profile that may be offered more broadly to women seeking proactive options for primary prevention of BC. These approaches can be divided into systemic and local approaches. Systemic approaches consist of intravenous, intramuscular, or oral delivery of a drug, whereas local approaches consist of intraductal (ID) injection, transdermal application, or subcutaneous implant for drug delivery (Figure 1D,E, Figure 2B–D and Figure 3B–E). Some of these approaches have already reached the clinical trial stage (Table 1) as discussed below, whereas many are still at an early stage of development in preclinical animal models (Table 2 and Table 3).
Figure 3.
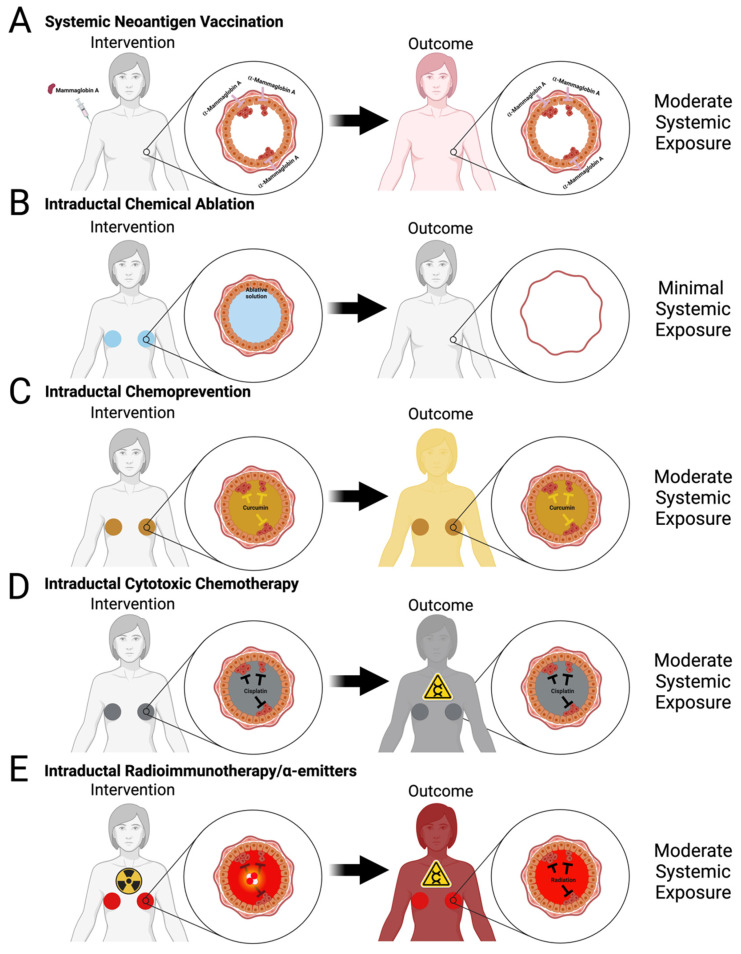
Systemic vaccination and intraductal approaches for breast cancer prevention. (A) Vaccines target cancer cell-expressed proteins and mount an immune response against (pre)malignant epithelial cells. (B–E) Intraductal injections provide direct delivery to epithelial cells that may become malignant. This form of delivery can be used with multiple solutions including ablative solutions (ethanol in (B)), chemopreventives (curcumin in (C)), cytotoxic chemotherapeutics (cisplatin in (D)), or radioimmunotherapy/α-emitters in (E). Intraductal chemical ablation and vaccines only require 1–2 injections and have minimal to moderate systemic exposure, unlike cytotoxic chemotherapeutics/radioimmunotherapeutics with potential iatrogenic carcinogenesis.
Table 1.
New approaches for primary prevention of breast cancer in clinical trials.
| Approach | Intervention | Active Agent | Number of Participants | Results | Reference(s) |
|---|---|---|---|---|---|
| Hormonal therapy | Local | Endoxifen gel | 90 | 1.9% Reduction in mammographic density |
NCT04616430, Completed [71] |
| 4-Hydroxytamoxifen transdermal gel | 194 | 52% Decrease in Ki-67 labeling index |
NCT03063619, Active [72] |
||
| Fulvestrant | 3 | N/A | NCT02540330, Terminated | ||
| Systemic | Aromatase inhibitors (Anastrozole) | 3864 | N/A | NCT00078832, Completed | |
| Aromatase inhibitors (Letrozole) | 55 | N/A |
NCT00579826 Completed |
||
| Chemoprevention | Retinoid (Fenretinide) | 20 | ≤50% Risk reduction | NCT01479192 [58,73] Terminated |
Preclinical models of primary prevention and local treatment of BC include genetically engineered mouse models, chemical carcinogen-induced rat models, and orthotopic cell line models. These models present different advantages and limitations. Genetically engineered mouse models (GEMMs) have, in principle, an inexhaustible potential of developing malignancy, whereas chemical carcinogen-induced and orthotopic models have a limited number of neoplastic cells. In some of these models, the line between primary prevention and local treatment is difficult to establish. Different GEMMs have been used to model human BC (Table 2 and Table 3). Several studies have used similar Brca1-deficient GEMMs as they are more relevant to high-risk BRCA mutation carriers. However, other GEMMs, including MMTV-PyMT, MMTV-Erbb2, and C3(1)-TAg, often considered more aggressive models, have also been used in this setting. Chemically induced rat models have been used for almost 40 years to study hormonal control [74] and more recently have been used to investigate local approaches for BC prevention (Table 3). Common limitations of these rodent models include the relative simplicity of linear architectures of their ductal trees, smaller ductal tree volumes with different surface area to volume ratios, different stromal density compared to human counterparts for translation of local intervention, duration, and frequency of chronic treatment in rodents vs. humans, and establishing effective dose for translation of systemic interventions.
Table 2.
Systemic approaches for breast cancer prevention in preclinical models.
| Approach | Active Agent | Experimental Model 1 | Level of Evidence 2 | Results | Reference(s) |
|---|---|---|---|---|---|
| Prophylactic Vaccine | α-Lactalbumin | MMTV-HER2 (n = 6) | A | Increased latency (p-value = 0.0004) |
[75] |
| MMTV-PyMT (n = 8) | D | Reduced tumor burden (p-value < 0.0006) |
|||
| 4T1 isograft (n = 8) | D | Reduced tumor burden until day 13 post injection (p value = 0.0006) | |||
| HER2 | MMTV-HER2 (n = 10) |
A | Increased latency (p-value < 0.01) |
[76] | |
| HER2 | MMTV-HER2 (n = 5–8) |
A | Increased latency (p-value < 0.02) |
[77] | |
| Chemoprevention | Erlotinib | Brca1fl/fl;Trp53+/−; MMTV-Cre (n = 13) | A | Increased latency (p-value = 0.0001) |
[78] |
| I-BET 762 | MMTV-PyMT (n = 13) | B | Increased latency (p-value < 0.05) |
[79] | |
| CCDO-Me | Brca1fl/fl;Tpr53+/; MMTV-Cre (n = 15) | A | Increased latency (p-value < 0.05) |
[80] | |
| RankL inhibitor | Brca1fl/fl;Trp53+/−; MMTV-Cre (n = 17) | A | Increased latency (p-value < 0.001) |
[81] | |
| RankL monoclonal antibody | Brca1fl/fl; MMTV-Cre (n = 9) | A | Increased latency (p-value < 0.001) |
[82] | |
| Cox-2 inhibitor | MMTV-Erbb2 (n = 24) |
A | Reduced tumor incidence (p-value = 0.003) |
[83] | |
| Curcumin | 4T1 isograft (n = 9) | C | Reduced tumor burden (p-value < 0.05) |
[84] | |
| Bisphosphonates (zolendronic acid and risdronate) | MDA-MB-231 xenograft (n = 12) | D | Reduced tumor burden (p-value < 0.05) |
[85] | |
| Rexinoids (Bexarotene) | MMTV-Erbb2 (n = 20) |
A | Increased latency (p-value < 0.0001) |
[86] | |
| MMTV-Erbb2 (n = 19) |
A | Increased latency (p-value < 0.001) |
[87] | ||
| JAK3 and EGFR inhibitor (WHI-P131 | DMBA-induced Balb/c mice (n = 20) | B | Increased latency (p-value = 0.0014) |
[88] | |
| Cytotoxic | Paclitaxel | DMBA-induced Balb/c mice (n = 20) | B | Increased latency (p-value = 0.0041) |
[88] |
Notes: 1 “n” denotes the number of animals in investigational treatment group instead of overall number of animals in all groups of the study. 2 A = animals were observed for 6 months to 2 years; B = for 8 weeks to 6 months; C = for 4 to 8 weeks; D = for <4 weeks. Abbreviations: Brca: breast cancer gene, CDDO-me: 2-Cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid methyl ester, Cox-2: Cyclooxygenase-2, Cre: cre-recombinase, DMBA: 4 7,12-Dimethylbenz[a]anthracene, EGFR: epidermal growth factor receptor, Erbb2: avian erythroblastic leukemia viral oncogene homolog 2, HER2: human epidermal growth factor receptor 2, I-Bet: inhibitor of Bromodomain and extra-terminal domain, Jak3: Janus Kinase 3, MMTV: Mouse Mammary Tumor Virus, Py-MT: polyoma middle tumor-antigen, RankL: receptor activator of nuclear factor kappa beta ligand, Trp53: Tumor Protein P53.
Table 3.
Intraductal and local approaches for breast cancer prevention in preclinical models.
| Intervention | Active Agent | Experimental Model 1 | Level of Evidence 2 | Results | Reference(s) |
|---|---|---|---|---|---|
| Chemoprevention | Oral-free curcumin | MNU-induced Sprague Dawley rats (n = 12) | A | Reduced tumor incidence (HR = 3.95, p-value 0.007) | [89] |
| Intraductal free curcumin | Reduced tumor incidence (HR = 2.85, p-value 0.020) | ||||
| Nanocurc encapsulated curcumin | A | Reduced tumor incidence (HR = 2.88, p-value 0.028) | |||
| Cytotoxic | Paclitaxel | MNU-induced Sprague-Dawley rats (n = 15) | A | Reduced tumor burden (p-value < 0.05) | [90] |
| Pegylated Liposomal Doxorubicin | MMTV-Erbb2 (n = 12) |
B | Reduced tumor incidence (HR = 6.40, p-value < 0.0001) | [91] | |
| MNU-induced Sprague Dawley rats (n = 15) | B | Reduced tumor incidence (p-value < 0.001) | [91] | ||
| MNU-induced Sprague Dawley rats (n = 5) | B | No change compared to control | [92] | ||
| 5-fluorouracil | MNU-induced Sprague Dawley rats (n = 5) | B | Reduced tumor incidence (HR = 3.30, p-value = 0.018) | ||
| Carboplatin | MNU-induced Sprague Dawley rats (n = 5) | B | Reduced tumor incidence (HR = 10.4, p-value < 0.0001) | ||
| Nanoparticle albumin-bound paclitaxel | MNU-induced Sprague Dawley rats (n = 5) | B | No change compared to control | ||
| Methotrexate | MNU-induced Sprague Dawley rats (n = 5) | B | No change compared to control | ||
| Nanoparticle albumin-bound paclitaxel | MNU-induced Sprague Dawley rats (n = 6) | B | Reduced tumor burden (p-value < 0.05) | [93] | |
| Cisplatin |
Brca1fl/flTrp53L/L; WAPcre (n = 20) |
A | Increased latency (p-value < 0.0001) | [94] | |
| Hormonal therapy | 4-hydroxytamoxifen (4-OHT) | MNU-induced Sprague Dawley rats (n = 20) | A | Reduce tumor incidence (p-value < 0.0001) | [91] |
| Fulvestrant | MIND MCF-7 xenograft (n = 3) |
B | Reduced tumor burden (p-value < 0.001) | [95] | |
| MNU-induced Sprague Dawley rats (n = 10) | B | Increased latency (p < 0.0001), reduced tumor incidence (HR = 2.08) | |||
| Fulvestrant and silastic tubing | MCF-7 xenograft (n = 8) |
C | Reduced tumor burden (p-value < 0.05) | [96] | |
| Suicidal gene vector | Adenovirus vector with thymidine kinase and gancyclovir | MNU-induced Wistar Furth rats (n = 30) |
A | (paradoxical) Decreased latency and increased tumor incidence | [97] |
| Gene silencing | Liposomal Hox1A siRNA silencing | C3(1)-TAg (n = 8) | B | Reduced tumor Incidence | [98] |
| Radioimmunotherapy | Radio-conjugated trastuzumab | MIND SUM225 xenograft (n = 3, 4) |
C | Dose-dependent reduced tumor burden | [99] |
| Targeted immunotoxin | Anti-transferrin receptor-antibody conjugated pseudomonas exotoxin | MIND MCF7 xenograft (n = 20) |
C | Increased latency and reduced tumor burden (p-value < 0.001) | [100] |
| Chemical ablation | Ethanol | C3(1)-TAg (n = 13) |
A | Increased latency (p-value < 0.0001), reduced incidence (HR = 4.76, p-value < 0.0001) | [101] |
Notes: 1 “n” denotes the number of animals in the investigational treatment group instead of the overall number of animals in all groups of the study. 2 A = animals were observed for 6 months to 2 years; B = for 8 weeks to 6 months; C = for 4 to 8 weeks; D = for <4 weeks. Abbreviations: Brca: breast cancer gene, Cre: cre-recombinase, Erbb2: avian erythroblastic leukemia viral oncogene homolog 2, Hox1A: homeobox protein 1A, MIND: Mouse Mammary Intraductal, MMTV: Mouse Mammary Tumor Virus, MNU: N-methyl-N-nitrosourea, siRNA: short interfering RNA, Trp53: Tumor Protein P53, WAPcre: Whey Acidic Protein Cre-Recombinase.
4. Systemic Approaches for Primary Prevention in Preclinical Models and Clinical Trials
Systemic interventions require ADME (absorption, distribution, metabolism, and excretion) processing of the drug leading to drug delivery throughout the body. There are many well-characterized drugs with known modes of action and systemic effects on the body. Scientists are now investigating the repurposing of some of these drugs for the primary prevention of BC.
4.1. Hormonal Therapy with Aromatase Inhibitors
Similar to tamoxifen and raloxifene, aromatase inhibitors are already approved for BC treatment. These drugs mimic androstenedione and bind to aromatase enzymes to prevent the conversion of testosterone to estrogens [102]. Type I inhibitors bind irreversibly to aromatase, whereas type II inhibitors have reversible, competitive inhibition [102]. Reduction in contralateral BC and overall survival improvement in the treatment of early-stage BC led researchers to investigate aromatase inhibitors for primary prevention. Currently, a type I inhibitor (exemestane) and two type II inhibitors (letrozole and anastrozole) are in clinical trials as preventive agents for BC. Exemestane clinical trials recruited 4500 high-risk, post-menopausal women for a median 3-year study [103]. Like tamoxifen, exemestane was taken orally and daily. Compared to the placebo, the exemestane group reported a 65% reduction in invasive BC incidence [103]. Hot flashes, fatigue, sweating, and insomnia were reported in both groups and mild bone density loss occurred in the exemestane group [104]. After a 5-year follow-up, no serious adverse effects, such as bone fractures, endometrial cancer, or thrombotic effects had occurred [103]. A phase 2 clinical trial (NCT00579826) with letrozole was recently completed and findings of this study have not yet been published.
The phase 3 clinical trial IBIS II (NCT00078832) with anastrozole involved 3864 post-menopausal women for a 10-year follow-up study [105]. Anastrozole was given orally and daily for 5 years. Compared to the placebo, the anastrozole group reported a 49% reduction in BC significantly within and after the first 5 years of treatment. Additionally, a significant decrease in non-BC was also observed. Arthralgia, joint stiffness, hot flashes, sweating, hypertension, and vulvovaginal dryness had higher reports among anastrozole groups compared to placebo treatment groups. Additionally, major adverse effects such as fractures, myocardial infarction, deep vein thrombosis, pulmonary embolism, stroke, and transient ischemic attacks were non-significantly different between anastrozole and placebo treatment groups [105]. Recently, anastrozole has received regulatory approval for BC prevention use in the United Kingdom; however, the FDA has yet to approve this or any other IA in a primary prevention setting [3].
4.2. Chemoprevention
Chemoprevention involves a variety of drugs used to prevent or delay the onset of cancer. These drugs are used as an alternative to invasive surgical procedures, such as bilateral prophylactic mastectomy, to lower a person’s risk of cancer development. Although chemoprevention drugs may have life-saving benefits, women can be hindered by the associated side effects. Therefore, most women who qualify for BC chemoprevention are high-risk individuals.
Retinoids and Rexinoids. Retinoids are vitamin A analogs that primarily bind to retinoid acid receptors for growth regulation, differentiation, and apoptosis. However, these drugs have limited use due to their toxicity. Fenretinide is a synthetic derivative of all-trans-retinoic acid that has been shown to accumulate in human breast tissue and prevent BC development in animal models [106,107,108] and has low toxicity compared to other retinoids. This led to a clinical trial for secondary prevention in pre- and post-menopausal women in 1987. A total of 1750 women participated in this 5-year study with up to a 15-year follow-up. Interestingly, pre-menopausal women had up to a 50% risk reduction of both ipsilateral and contralateral BC which was not seen in women over the age of 55 years. However, no significant difference was seen with distant metastases formation; new, non-breast, primary tumor formations; or overall mortality [58]. A follow-up study on the safety of fenretinide was conducted in 2800 women with stage I or ductal carcinoma in situ given the same dosage for 5 years. Under 20% of women reported diminished dark adaptations or dermatological effects, 13% reported gastrointestinal symptoms, which all decreased over the 5-year period, whereas 11% reported ocular surface disorders which had a slight increase in occurrence [73]. Liver function and lipid profile were also tested with no significant difference between the treatment and control groups. No significant differences were found between pre- and post-menopausal women for adverse effects [73]. These results led to a clinical trial for high-risk pre-menopausal women but it was terminated due to low patient accrual (NCT01479192).
Retinoid X receptor is another class of retinoid receptors that has different affinities for naturally occurring ligands and can dimerize with a plethora of receptors such as vitamin D, thyroid hormone, and liver X receptors. This vast access to regulator networks makes this an ideal target for cancer prevention and treatment [109]. Currently, bexarotene, otherwise known as LGD1069, is a rexinoid that is selective for the retinoid X receptor. It is the only FDA-approved rexinoid for the treatment of cutaneous T-cell lymphoma. However, preclinical studies of bexarotene for BC prevention have led to active, ongoing clinical trials (NCT03323658, NCT00055991) in patients at high risk for BC development [86,87].
Erlotinib. Women with BRCA mutations do not frequently express hormonal receptors and develop triple-negative BC. However, epidermal growth factor receptor (EGFR) is highly expressed in these triple-negative BCs and is suggested to be associated with BRCA1 mutations in this cancer subtype [110,111,112]. Erlotinib is a small molecule inhibitor that blocks the phosphorylation and activation of EGFR and has been tested as a chemopreventive agent in Brca1-deficient GEMM [78]. At three months of age, animals were given an oral daily dose of 100 mg/kg of erlotinib, equivalent to ~8 mg/kg in humans, to study tumor-free survival and tolerability of chronic erlotinib treatment. Alopecia was the main side effect observed and was only found in 30% of all animals [78]. Erlotinib treatment significantly delayed or prevented the formation of ER- tumors but had a minimal effect on the formation of ER+ tumors [78]. Implicit from these findings is that a combination treatment with hormone therapy and erlotinib could prevent the formation of both ER-dependent and ER-independent EGFR-dependent tumors. However, the combination treatment may also exacerbate the side effects of either drug.
Bromodomain inhibitors. Epigenetics acts on gene regulation that can impact cancer initiation and progression. Unlike genetic modifications, these events are considered reversible making them a desirable drug target. Several drugs have already been developed to modulate DNA methylation or histone modifications. One of the drug targets are chromatin readers such as the bromodomain and extra-terminal (BET) protein family whose conserved bromodomain binds to specific epigenetically modified sites [113]. BET inhibitors such as iBET 762 have recently shown promising efficacy in preclinical models for cancer treatments with clinical trials underway for breast and lung cancer treatments (NCT02964507, NCT01587703). Additional studies focused on iBET’s involvement in the tumor microenvironment. A 1-week short-term experiment showed an increase in helper T cells in the spleen of iBET-treated mice, but a 9-week study found a decrease in helper T cell population in the mammary glands of iBET-treated mice [79]. As a chemopreventive agent, oral treatment (60 mg/kg) of iBET significantly delayed mammary gland tumor formation by 3 weeks compared to control treatment in the MMTV-PyMT GEMM [79]. This 60 mg/kg dose was well tolerated with no signs of toxicity in mice [79].
Vitamins and Micronutrients. Vitamins and micronutrients have been found to impact various pathways in BC development. Among these, vitamin D3, folate, omega-3 polyunsaturated fatty acids, piperine, sulforaphane, indole-3-carbinol, epigallocatechine gallate, and quercetin along with curcumin have extensively been reviewed [114].
Curcumin. Curcumin is a hydroponic phenol derived from turmeric that has gained attention in the scientific community over the past decade for its effects as an anticancer agent. However, a large dose is required for effectiveness due to its low absorption. In vitro studies on curcumin have shown inhibition of migration, invasion, and angiogenesis, as well as induction of apoptosis and tumor suppressor genes [115]. Xenograft mouse models given oral doses of dendrosomal curcumin support these findings by showing reductions in tumor size, weight, and incidence compared to controls. Suppression of NF-kB, COX-2, and VEGF in these treated mice further supports curcumin as an anti-metastatic cancer agent [84]. Several clinical trials of curcumin in treatment of BC alone, or in combination with chemotherapy are currently active (NCT03980509, NCT03072992, NCT01740323, NCT01975363).
RankL. RankL is a protein secreted from osteoblasts which are most commonly known for their role in the formation of osteoclasts and bone remodeling. However, RankL is also expressed in the mammary gland during development and tumor formation [116,117,118]. Expression of RankL has been documented in BC cell lines and is associated with increased proliferation and poor survival in primary human BC [81]. Therefore, suppression of RankL is a promising new strategy in BC prevention. Xenograft mouse models given a RankL inhibitor, OPG-Fc, had increased tumor latency and reduced hyperplasia compared to the control mice. This was also supported in GEMMs treated with RankL-specific monoclonal antibodies [82].
4.3. Prophylactic Vaccines
In the late 2000s, there was a great interest in developing universal preventive vaccines against BC, in part spurred by the success of preventive vaccines against HPV-associated cervical cancer [119,120]. The Artemis project is the most well-known effort, supported by the National Breast Cancer Coalition and their initiative, to know how to eradicate BC by 2020 [121]. The scientific premise is to mount a specific immune response against mammary epithelial cells that would eliminate any neoplastic BC cells. As this initiative moved forward, the Artemis project Steering Committee in consultation with the FDA and other agencies raised concerns about clinical translation [122]. The main concern was the lack of a known antigen that would be expressed in 100% of the cells of all BC tumors. α-lactalbumin, mammaglobin-A, HER2, and survivin are among the best candidates, but studies suggest that none of them are represented in more than 80% of tumors [123]. More recently, the focus of the Artemis project has shifted from universal prophylaxis as primary prevention to targeted vaccination of specific subgroups of high-risk individuals for primary prevention or in specific patient subgroups to prevent metastatic recurrence [123]. Despite these caveats and challenges for a universal vaccine, progress has been made both in preclinical models and clinical trials that suggest the refinement and selection of the target population and combinatorial approach of vaccination, immunotherapy, and/or immune modulation could increase therapeutic efficacy in a preventive setting [119,120,124].
Preclinical models have shown a significant delay in tumor formation in mice vaccinated against α-lactalbumin [75,77] and HER2 [76,77]. α-lactalbumin is expressed at high levels exclusively during lactation in normal mammary epithelial cells, but α-lactalbumin expression is upregulated in about 50% of hormone receptor-positive and more than 70% of triple-negative BC tumors [119]. Subcutaneous injection of α-lactalbumin protein significantly delayed tumor formation in the MMTV-Erbb2 GEMM and elicited anti-α-lactalbumin-specific T cell response. However, α-lactalbumin vaccination can inhibit tumor growth but cannot delay tumor formation in more aggressive BC models [75,125]. In late 2020, a phase I clinical trial (NCT04674306) was started to investigate the safety of α-lactalbumin vaccine as adjuvant therapy in triple-negative BC patients at high risk of recurrence. HER2 is overexpressed in 20–30% of invasive BC tumors but expressed at very low levels in normal mammary epithelia. Intramuscular injections of virus-like particles studded with full-length HER2 or oncogenic variant Delta16HER2 extracellular domains in GEMMs significantly delayed or prevented tumor formation along with eliciting a robust anti-HER-2 production immune response [76]. A dendritic cell-mediated vaccine against HER2 also provided a tumor prevention effect in the full-length HER2-driven GEMM [77]. Mammaglobin A is a small glycoprotein that belongs to a family of epithelial secretory proteins. Mammaglobin A expression is upregulated in 40–80% of BCs [126]; unlike α-lactalbumin and HER2, mammaglobin A is more broadly expressed in normal mammary epithelial cells. Mammaglobin-A is exclusively expressed in BC cells, making it a suitable targeted protein for BC immunotherapy. Intramuscular injections of Mammaglobin A cDNA inhibit the growth of established tumors derived from multiple human BC cell lines in SCID-beige host animals and elicit a strong anti-Mammaglobin A T cell response [127,128]. Using this DNA vaccination approach (Figure 3A), anti-mammaglobin-specific T cells are readily detected in treated preclinical models, BC patients in phase 1 clinical trials, and metastatic BC patients in phase 1 clinical trials (NCT00807781) [89,129]. The results of this phase 1 clinical trial demonstrated the safety of this DNA vaccine and suggested a therapeutic effect based on extended progression-free survival of vaccinated participants [129]. These encouraging results have led to the ongoing phase 1b clinical trial (NCT02204098) investigating mammaglobin-A DNA in non-metastatic BC patients undergoing neoadjuvant endocrine therapy or chemotherapy [126]. However, this DNA vaccination approach has not been tested in a primary prevention setting in either preclinical models or in clinical trials.
5. Local Approaches for Primary Prevention in Preclinical Model and Clinical Trials
Each of the two mammary glands of a woman contains 8–12 ductal trees with the main duct of each tree opening at the nipple orifice [27,28]. The main function of these ductal tree systems is to produce, express, and deliver milk during lactation. Alveolar cells arranged in lobules at the ends of the ductal tree produce and secrete milk components into ductules that join into larger ducts and eventually the main duct to transport the milk outwardly. Breast carcinoma predominantly arises from the terminal ductal lobular units (TDLUs) [130,131] within a single ductal tree [26]. Remodeling of TDLUs during an individual’s childbearing years and aging influences cancer risk. Epidemiological and genetic susceptibility studies suggest that the number and differentiation state of mammary epithelial cells correlated with BC risk; for example, increased lobular involution (fewer TDLU) as a result of the aging process is protective against BC [132,133,134]. The opening at the nipple into the ductal tree offers a unique opportunity for BC prevention by directly targeting the epithelial cells of the ductal tree. In 2002, Sivaraman et al. introduced the concept of local ablation for primary prevention and local treatment of early BC by intraductal (ID) delivery of a suicidal gene system [97]. Although this study was unsuccessful in reducing tumor incidence, it unveiled a new approach and local strategy for BC prevention. Within the last twenty years, several groups have built on this local treatment concept that focuses on the ductal tree as a functional unit consisting of individual pre-malignant and/or malignant cells for primary prevention of BC (Table 3). A common theme of many of these local approaches is the delivery of the active compound at a lower dose that achieves the same efficacy on targeted cells and/or minimizes the undesired side effects of systemic exposure (Figure 1D,E, Figure 2B–D and Figure 3B–E).
5.1. Gene Therapy
Gene therapy utilizes DNA or RNA sequences to modify gene activity or expression. Some gene therapy approaches such as viral delivery of DNA or genetic editing of a patient’s genome may offer a more permanent treatment compared to continuous and multi-dose treatment of chemoprevention and endocrine therapies. Here, we discuss the ID delivery of components of different gene therapy platforms.
Suicide gene therapy. An adenoviral vector was used to intraductally deliver a thymidine kinase-based suicidal gene system in an N-methyl-N-nitrosourea (MNU)-induced rat model of BC [97]. The therapeutic intent was to prevent tumor formation by ablating the proliferating cells of the terminal end buds akin to human TDLUs. However, treated rats paradoxically developed tumors earlier than control rats despite the high transduction efficiency, high thymidine kinase expression, and 50–90% epithelial ablation by suicidal gene activation.
RNAi-based gene silencing. ID delivery of lipidoid nanoparticles containing small interfering RNAs (siRNAs) against Hox1A in the C3(1)-TAg GEMM resulted in a significant delay in tumor latency [98]. Hox1A is a homeodomain transcription factor involved in autocrine growth hormone dysregulation and was identified as an early driver of mammary cell carcinogenesis [98]. Knocking down Hox1A mRNA maintained hormone receptor status and reduced the proliferative rate of pre-malignant cells [85]. Remarkably, researchers were able to sustain this knockdown effect by repeatedly cannulating and delivering 20 μL of therapeutic siRNAs in nine biweekly procedures without causing any damage or perforation to ductal trees. This study also demonstrated the efficiency of in vivo transfection of the ductal tree using components of RNAi technology and opened the possibility of introducing components of other gene-silencing or -editing technologies.
CRISPR-based genetic editing. CRISPR/Cas9 is a versatile gene-editing tool to produce loss of function or knockout alleles in in vitro cell line systems and in vivo animal models [135]. CRISPR technology has already reached the clinical trial stage. Although the majority of these trials involved ex vivo editing of hematopoietic stem cells and T cells, in vivo editing of liver cells with a systemic delivery approach and eye cells with a local delivery approach have been successful [135]. ID delivery of the CRISPR system to target mammary epithelial cells has been applied to develop new models of BC and to study cooperative interactions of multiple driver genes [136,137,138,139,140]. ID delivery of CRISPR components (Cas9 mRNA and guide RNA) via lentiviral vector achieved up to 21.5% editing efficacy of the target gene [137]. An alternative approach to minimize the immunogenicity of de novo Cas9 expression is to intraductally deliver the guide RNA in a lentiviral vector in animals endogenously expressing Cas9 [137,140]. Up to 32.6% editing efficacy of the target gene may be achievable using this modified system [140]. Although further development of this technology will be required to identify a safe delivery system with an improved editing efficacy, ID delivery of CRISPR or similar system for editing and/or inactivation of early driver genes of mammary carcinogenesis such as estrogen receptor α (ESR1), HOXA1, and/or EGFR may offer new opportunities for BC prevention. It is tempting to speculate that such genetic editing approaches to dampen mitogenic activity of ER signaling may provide a local alternative to systemic hormonal control but with minimal adverse effects (Figure 2D).
5.2. Local Hormone Therapy
Different approaches are being investigated to minimize side effects of systemic hormone therapy. More readily available than the above-mentioned genetic editing approach is the local delivery of a selective estrogen receptor modulator (SERM) or degrader (SERD). Local delivery methods include topical transdermal gel application, ID injection, and slow-releasing drug implants (Figure 2B,C).
Transdermal Gel for hormone therapy. Topical application to the skin of the breast area of a gel containing active metabolite 4-hydroxy tamoxifen achieved a high local concentration of this SERM [141,142]. A phase 2 clinical trial (NCT00952731) divided 27 women diagnosed with DCIS into two groups to receive transdermal gel application or tamoxifen orally for six to ten weeks before surgery. Due to the early stage of the disease, the proliferation index Ki-67 was used as the endpoint rather than tumor incidence. Compared to pre-treatment tissue samples, the proliferative index was reduced by 52% in the transdermal gel group and by 61% in the tamoxifen group. At a local level, both groups had the same concentration of this SERM. Systemically, the transdermal gel group had reduced plasma concentrations of the SERM compared to the tamoxifen group. However, side effects such as hot flashes did not significantly differ between the two groups [59]. The timeframe of this study limited the assessment of long-term side effects, but this is currently being evaluated in a phase 2 clinical trial (NCT03063619) whose primary objective is to determine risk reduction in BC by transdermal gel application (Table 1, Figure 2B). An alternative study was conducted using z-form endoxifen, a tamoxifen metabolite with the highest affinity to the ER, to study BC prevention [71]. Groups of 30 women self-applied 10 mg, 20 mg, or a placebo topically to each breast daily for three months and were monitored for breast density changes, systemic side effects, and plasma concentration of endoxifen [71]. Before the end of the three-month period, there was a significant decrease in mammographic density of women applying 20 mg endoxifen which was not seen in the 10 mg treatment group. Dose-dependent plasma concentrations of endoxifen were observed in the treatment groups without systemic side effects [71]. However, severe skin reactions occurred in both treatment groups which caused almost all women (58 out of 60) to prematurely discontinue the gel application and no therapeutic window was identified [58]. Therefore, endoxifen has the potential to reduce BC incidence but requires further studies due to severe skin toxicity.
Intraductal hormone therapy. ID delivery of tamoxifen did not provide a protective effect on an MNU-induced rat model of BC most likely due to the lack of active metabolite production in the liver. However, ID delivery of 4-hydroxytamoxifen provided a protective effect comparable to subcutaneous injection of tamoxifen [91]. Fulvestrant acts as a selective estrogen receptor degrader (SERD). Fulvestrant binds with a higher affinity to the ER than tamoxifen and unlike tamoxifen is a pure antagonist by degrading ER upon binding [143]. ID delivery of fulvestrant in a mammary intraductal (MIND) ER+ MCF7 xenograft model provided superior protection than intramuscular injection, whereas ID delivery of fulvestrant provided a protective effect comparable to intramuscular injection in an MNU-induced rat model [95]. Locally delivered fulvestrant was more effective at inhibiting cell proliferation, angiogenesis, and decreasing ER expression. In both mouse and rat models, the total amount of fulvestrant received per animal was the same by ID delivery (fragmented dosing per ductal tree) and by intramuscular injection [95]. A phase 2 clinical trial (NCT02540330) was initiated to investigate the pharmacokinetics and local and systemic side effects of ID delivery compared to intramuscular injection (Table 1, Figure 2C). However, due to a business decision, the study was terminated after the recruitment of only three participants.
Slow-release implant of hormone therapy. To expand on the application of fulvestrant as a local long-term prevention treatment, bilateral fulvestrant-loaded silastic tubing was subcutaneously implanted next to the abdominal mammary glands of NSG mice [96]. One week after implantation, the mice were orthotopically injected with MCF7 cells in both fat pads of the abdominal glands [96]. A significant tumor growth reduction was observed in the fulvestrant-loaded silastic tubing group compared to vehicle control treatment [96]. Importantly, the treatment efficacy of fulvestrant-loaded implants was very similar to weekly treatment with subcutaneous injection of fulvestrant. This is an encouraging study to consider for local drug delivery to the mammary gland. However, more investigations are needed to have a more controlled and homogenous release of fulvestrant or other drugs throughout the entire mammary gland since a much more pronounced decrease in the Ki-67 proliferation marker was measured in tumor areas adjacent to the tubing implant [96].
5.3. Intraductal Chemotherapy and Targeted Treatments
Several research groups have used ID delivery of cytotoxic compounds, targeted agents, and/or targeted particles for both primary prevention of BC (Table 3, Figure 3C–E) and preclinical models for local disease control [144].
Infusion of cytotoxic agents. Cytotoxic compounds such as pegylated liposomal doxorubicin, carboplatin, or paclitaxel when ID delivered in the chemically induced (MNU) rat model significantly reduced tumor incidence [90,91,92]. Similarly, liposomal doxorubicin showed greater therapeutic efficacy at reducing tumor incidence when ID was delivered rather than systemic administration in the genetic MMTV-Erbb2 mouse model [91]. Several groups have already tested the feasibility of this approach in first-human clinical studies. Stearns et al. 2011 reported an 88% success rate by administering pegylated liposomal doxorubicin into one ductal tree per patient [92]. Love et al. 2013 reported a 96.6% success rate in administering pegylated liposomal doxorubicin into 5–8 ductal trees per patient) [145]. These studies provide strong support for the translational feasibility of ID delivery of cytotoxic or other solutions. Unfortunately, local cytotoxic treatment with pegylated liposomal doxorubicin, 5-fluorouracil, and/or cisplatin can induce tumors with long latency in non-transgenic animals [89,94,146]. This result has diminished enthusiasm for such local chemotherapy delivery unless it is needed to minimize systemic exposure [89,146].
Infusion of targeted agents. Pseudomonas exotoxin has been engineered to serve as an anti-cancer agent thanks to its effect on protein translation and cell death [147]. However, systemic delivery of this exotoxin has undesired effects that lead to inflammation and vascular leakage. Transferrin receptor is overexpressed in the majority of BC cells, in many cases starting at a preneoplastic stage. To harness the anti-tumoral effect of this exotoxin, but to keep it contained locally within the ductal tree, Wang et al. 2022 fused a monoclonal antibody targeting the human transferrin receptor to a 40 kDa fragment of this exotoxin [100]. ID delivery of this antibody–toxin conjugate shows therapeutic efficacy in HER2+ MIND models of MCF7 and SUM225 human BC cells that recreate the early stage of DCIS lesions [100]. A similar approach was utilized to deliver an a-emitter radionuclide, as the killing agent, conjugated to a HER2-targeting antibody (trastuzumab). a-emitters can cause irreparable double-strand DNA damage that leads to cell death [148]. The radionuclide used in this study was 225Ac with a half-life of 9.9 days [148]. ID delivery of this antibody–225Ac conjugate shows therapeutic efficacy in the HER2+ MIND model of SUM225 BC cells [99]. However, this treatment can induce tumor formation in the mammary gland or lung due to sustained radiation exposure [99]. As the field of radionuclides and conjugation chemistry continues to mature [148,149], the use of a-emitters with shorter half-life (<12 h) such as 211At and 212Pb could minimize the undesirable iatrogenic effects of trastuzumab-225Ac. Although these studies were proof-of-concept for clinical treatment of DCIS due to the requirement of transferrin receptor or HER2 expression, conjugating these cell-killing agents to other antibodies, peptides, or targeting moieties could expand their applications to primary prevention of BC and/or local control of uninvolved ductal trees in DCIS-affected breast. Similarly, antibody–drug conjugates [150] such as trastuzumab deruxtecan for HER2-low BC and sacituzumab govitecan for triple-negative BC could be considered for primary prevention.
Ductal tree ablation: We have been intrigued by the concept of a universal prophylactic intervention for several years. Our approach aims at combining the effectiveness of prophylactic mastectomy, with the universality of an ideal vaccine and delivery method of ID chemotherapy (Figure 1D and Figure 3B). We are investigating different chemical and thermal ablation approaches to locally kill mammary epithelial cells while causing minimal collateral tissue damage and side effects. We have extensively studied ethanol as a cell-killing solution for chemically ablating the ductal tree [101,151,152,153,154,155]. In clinical settings for ablation or sclerotherapy, tens of milliliters of 95–100% ethanol (EtOH) can be locally delivered to the target area [156,157,158,159,160,161,162,163,164,165,166,167,168,169,170]. In some procedures up to 50 mL of EtOH can be administered per session, indicating its low toxicity [156,166]. We have demonstrated in preclinical rodent models the feasibility of ablating the entire ductal tree system before epithelial cells become malignant [101,152,153]. Our study in the C3(1)-TAg GEMM showed that ID injection of 70% EtOH significantly delayed tumor formation and reduced tumor incidence [101]. ID injection of 70% EtOH provides similar or superior tumor risk reduction compared to other prevention interventions in this or similar GEMMs (Table 3; refs [91,98]). This chemical ablation approach has advantages over other ID approaches for clinical translation. It would be a one-time treatment in contrast to other ID approaches that require repeated administration of active agents which can be a challenge, especially for chemotherapy agents that may compromise ductal tree structure and leak out of intended targeted area in later cycles. Currently, there are no reports linking clinical uses of EtOH to iatrogenic cancer, but this is a concern for other local treatments that cause DNA damage such as radioimmunotherapy and chemotherapy (Figure 3D,E; refs [94,99,146,171]). The International Agency for Research on Cancer considers EtOH to be carcinogenic to humans [172]; however, this is based on chronic exposure to EtOH as an alcoholic beverage. EtOH is metabolized into acetaldehyde, a toxic chemical that causes DNA damage and DNA-protein cross-linking. This is considered a main contributing mechanism for EtOH-induced cancers but the exact molecular mechanism(s) of EtOH and increased cancer risk is not fully established [172]. No significant DNA damage was seen with acute EtOH exposure in mice [173,174]. Our studies showed no evidence of iatrogenic effects of EtOH injections in non-transgenic mice after 22 months of follow-up [101]. There are also some unique challenges with this chemical ablation approach. Image guidance will be required for precisely infusing each ductal tree with the appropriate volume. We and others have used different contrast agents for in vivo imaging of the ductal tree in rodents [101,151,152,153,154,175,176] and rabbits [177] after ID infusion. Controlling the diffusion of EtOH outside the ductal tree will be required to further minimize collateral tissue damage [101]. The use of ethyl cellulose as a gelling agent to limit ethanol diffusion has been reported for clinical treatment of venous malformation and in preclinical models of BC, cervical cancer, and liver cancer [152,159,160,161,162,163,164,165,178]. We have shown that ethyl cellulose is compatible with a 70% EtOH ablative solution and with imaging contrast agents such as tantalum oxide nanoparticles in both mouse and rat models [152,153].
Similar to chemical ablation, thermal ablation aims to target the ductal tree with minimal collateral damage and improved cosmesis. This technique uses physical energy to raise or lower the temperature to internally target local tumors instead of surgical removal [179]. Procedures such as microbubble solutions with high-intensity ultrasound or an iron rod nanoparticle solution coupled with a magnetic field have been previously used in cancer models such as pancreatic xenograft mouse models [180,181]. These thermal ablation techniques, coupled with their imaging abilities, may be applied toward targeting epithelial cells within the ductal tree for BC prevention.
6. Conclusions and Future Directions
BC is the leading cause of new cancer cases and is the second leading cause of cancer-related deaths in women. The limited, approved prevention interventions available for high-risk individuals can have severe and long-term side effects that deter many women from making proactive choices. Regrettably, BC prevention is an area of translational research that is currently underfunded by federal agencies [182]. Therefore, emerging and novel approaches for the primary prevention of BC that provide the same or superior protection while minimizing the side effects of current interventions should be pursued and prioritized. These approaches seek to eradicate BC and align well with NIH’s All of US Research Program—a nationwide initiative for precision health interventions that proactively prevent rather than treat disease in high-risk individuals. We provide a comprehensive review of emerging approaches for BC prevention based on non-modifiable risk factors that put women at a higher risk of developing BC. Virtually all of these studies were performed in rodent models of BC, which have some limitations and challenges for direct translation to at-risk individuals. Additional scalability and validation studies in larger animal models will be an important step in bridging the gap between discovery and clinical evaluation. Although currently underutilized, rabbit studies should be considered as an appropriate intermediate model. Evolutionarily, anatomically, and physiologically, rabbit mammary glands are more similar to humans than those of rodent models or other large animals such as cows and sheep [183,184]. Female rabbits have four pairs of mammary glands each containing four ductal trees [177], which can be cannulated for ID injection [177,185,186,187,188,189] using a procedure very similar to ID administration of contrast agents in clinical ductography [190,191] and chemotherapeutic agents in first-in-human clinical research [92,145]. Alternatively, or complementary to validation studies in larger animals, judicious determination based on the scientific rigor and clinical feasibility of these emerging approaches should be applied to prioritize those interventions more likely to have an impact on primary prevention of BC.
Acknowledgments
The authors would like to thank Mohamed Ashry and Elizabeth G. Phelps and other members of the Sempere Lab for their constructive feedback on this review. All figures were created with Biorender.com.
Author Contributions
Concept and design: E.K.Z. and L.F.S. Figures generated by E.K.Z. Drafting of the paper: E.K.Z. and L.F.S. Critical revision of the paper for important intellectual content: E.K.Z. and L.F.S. Final approval of the paper for submission: E.K.Z. and L.F.S. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported, in part, by the National Institute of Health R21 CA226579 and R01 CA258314 grants to LFS, and by the Doctoral Program in Environmental and Integrative Toxicological Sciences of the Institute for Integrative Toxicology 2021, 2022, 2023 Summer Research Stipend to EKZ.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J. Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Britt K.L., Cuzick J., Phillips K.A. Key steps for effective breast cancer prevention. Nat. Rev. Cancer. 2020;20:417–436. doi: 10.1038/s41568-020-0266-x. [DOI] [PubMed] [Google Scholar]
- 3.Michaels E., Worthington R.O., Rusiecki J. Breast Cancer: Risk Assessment, Screening, and Primary Prevention. Med. Clin. North Am. 2023;107:271–284. doi: 10.1016/j.mcna.2022.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Engmann N.J., Golmakani M.K., Miglioretti D.L., Sprague B.L., Kerlikowske K. Breast Cancer Surveillance C: Population-Attributable Risk Proportion of Clinical Risk Factors for Breast Cancer. JAMA Oncol. 2017;3:1228–1236. doi: 10.1001/jamaoncol.2016.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maas P., Barrdahl M., Joshi A.D., Auer P.L., Gaudet M.M., Milne R.L., Schumacher F.R., Anderson W.F., Check D., Chattopadhyay S., et al. Breast Cancer Risk From Modifiable and Nonmodifiable Risk Factors Among White Women in the United States. JAMA Oncol. 2016;2:1295–1302. doi: 10.1001/jamaoncol.2016.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Collaborating Centre for Cancer (UK) Familial Breast Cancer: Classification and Care of People at Risk of Familial Breast Cancer and Management of Breast Cancer and Related Risks in People with a Family History of Breast Cancer. National Collaborating Centre for Cancer; Cardiff, UK: 2013. [PubMed] [Google Scholar]
- 7.Rosenthal E.T., Evans B., Kidd J., Brown K., Gorringe H., van Orman M., Manley S. Increased Identification of Candidates for High-Risk Breast Cancer Screening Through Expanded Genetic Testing. J. Am. Coll. Radiol. 2017;14:561–568. doi: 10.1016/j.jacr.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher S., Hughes E., Wagner S., Tshiaba P., Rosenthal E., Roa B.B., Kurian A.W., Domchek S.M., Garber J., Lancaster J., et al. Association of a Polygenic Risk Score With Breast Cancer Among Women Carriers of High- and Moderate-Risk Breast Cancer Genes. JAMA Netw. Open. 2020;3:e208501. doi: 10.1001/jamanetworkopen.2020.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes E., Tshiaba P., Gallagher S., Wagner S., Judkins T., Roa B., Rosenthal E., Domchek S., Garber J., Lancaster J., et al. Development and Validation of a Clinical Polygenic Risk Score to Predict Breast Cancer Risk. JCO Precis. Oncol. 2020;4:585–592. doi: 10.1200/PO.19.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu C., Hart S.N., Gnanaolivu R., Huang H., Lee K.Y., Na J., Gao C., Lilyquist J., Yadav S., Boddicker N.J., et al. A Population-Based Study of Genes Previously Implicated in Breast Cancer. N. Engl. J. Med. 2021;384:440–451. doi: 10.1056/NEJMoa2005936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breast Cancer Association Consortium. Dorling L., Carvalho S., Allen J., Gonzalez-Neira A., Luccarini C., Wahlstrom C., Pooley K.A., Parsons M.T., Fortuno C., et al. Breast Cancer Risk Genes—Association Analysis in More than 113,000 Women. N. Engl. J. Med. 2021;384:428–439. doi: 10.1056/NEJMoa1913948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith R.A., Andrews K.S., Brooks D., Fedewa S.A., Manassaram-Baptiste D., Saslow D., Wender R.C. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2019;69:184–210. doi: 10.3322/caac.21557. [DOI] [PubMed] [Google Scholar]
- 13.Evans D.G., Howell A. Can the breast screening appointment be used to provide risk assessment and prevention advice? Breast Cancer Res. 2015;17:84. doi: 10.1186/s13058-015-0595-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gail M.H., Anderson W.F., Garcia-Closas M., Sherman M.E. Absolute risk models for subtypes of breast cancer. J. Natl. Cancer Inst. 2007;99:1657–1659. doi: 10.1093/jnci/djm228. [DOI] [PubMed] [Google Scholar]
- 15.Kurian A.W., Hughes E., Simmons T., Bernhisel R., Probst B., Meek S., Caswell-Jin J.L., John E.M., Lanchbury J.S., Slavin T.P., et al. Performance of the IBIS/Tyrer-Cuzick model of breast cancer risk by race and ethnicity in the Women’s Health Initiative. Cancer. 2021;127:3742–3750. doi: 10.1002/cncr.33767. [DOI] [PubMed] [Google Scholar]
- 16.Padamsee T.J., Wills C.E., Yee L.D., Paskett E.D. Decision making for breast cancer prevention among women at elevated risk. Breast Cancer Res. 2017;19:34. doi: 10.1186/s13058-017-0826-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans D.G., Gandhi A., Wisely J., Clancy T., Woodward E.R., Harvey J., Highton L., Murphy J., Barr L., Howell S.J., et al. Uptake of bilateral-risk-reducing-mastectomy: Prospective analysis of 7195 women at high-risk of breast cancer. Breast. 2021;60:45–52. doi: 10.1016/j.breast.2021.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jahan N., Jones C., Rahman R.L. Endocrine prevention of breast cancer. Mol. Cell. Endocrinol. 2021;530:111284. doi: 10.1016/j.mce.2021.111284. [DOI] [PubMed] [Google Scholar]
- 19.Skytte A.B., Gerdes A.M., Andersen M.K., Sunde L., Brondum-Nielsen K., Waldstrom M., Kolvraa S., Cruger D. Risk-reducing mastectomy and salpingo-oophorectomy in unaffected BRCA mutation carriers: Uptake and timing. Clin. Genet. 2010;77:342–349. doi: 10.1111/j.1399-0004.2009.01329.x. [DOI] [PubMed] [Google Scholar]
- 20.Metcalfe K.A., Mian N., Enmore M., Poll A., Llacuachaqui M., Nanda S., Sun P., Hughes K.S., Narod S.A. Long-term follow-up of Jewish women with a BRCA1 and BRCA2 mutation who underwent population genetic screening. Breast Cancer Res. Treat. 2012;133:735–740. doi: 10.1007/s10549-011-1941-0. [DOI] [PubMed] [Google Scholar]
- 21.Ralph A.F., Ager B., Bell M.L., Collins I.M., Andrews L., Tucker K., Phillips K.A., Butow P. Women’s preferences for selective estrogen reuptake modulators: An investigation using protection motivation theory. Patient Educ. Couns. 2014;96:106–112. doi: 10.1016/j.pec.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Gareth Evans D., McWilliams L., Astley S., Brentnall A.R., Cuzick J., Dobrashian R., Duffy S.W., Gorman L.S., Harkness E.F., Harrison F., et al. Quantifying the effects of risk-stratified breast cancer screening when delivered in real time as routine practice versus usual screening: The BC-Predict non-randomised controlled study ( NCT04359420) Br. J. Cancer. 2023;128:2063–2071. doi: 10.1038/s41416-023-02250-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donnelly L.S., Evans D.G., Wiseman J., Fox J., Greenhalgh R., Affen J., Juraskova I., Stavrinos P., Dawe S., Cuzick J., et al. Uptake of tamoxifen in consecutive premenopausal women under surveillance in a high-risk breast cancer clinic. Br. J. Cancer. 2014;110:1681–1687. doi: 10.1038/bjc.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howell A., Gandhi A., Howell S., Wilson M., Maxwell A., Astley S., Harvie M., Pegington M., Barr L., Baildam A., et al. Long-Term Evaluation of Women Referred to a Breast Cancer Family History Clinic (Manchester UK 1987–2020) Cancers. 2020;12:3697. doi: 10.3390/cancers12123697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tot T., Gere M., Hofmeyer S., Bauer A., Pellas U. Seminars in Cancer Biology. Volume 72. Academic Press; Cambridge, MA, USA: 2021. The clinical value of detecting microcalcifications on a mammogram; pp. 165–174. [DOI] [PubMed] [Google Scholar]
- 26.Tan M.P., Tot T. The sick lobe hypothesis, field cancerisation and the new era of precision breast surgery. Gland. Surg. 2018;7:611–618. doi: 10.21037/gs.2018.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Love S.M., Barsky S.H. Anatomy of the nipple and breast ducts revisited. Cancer. 2004;101:1947–1957. doi: 10.1002/cncr.20559. [DOI] [PubMed] [Google Scholar]
- 28.King B.L., Love S.M. The intraductal approach to the breast: Raison d’etre. Breast Cancer Res. 2006;8:206. doi: 10.1186/bcr1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper A.P. On the Anatomy of the Breast. Volume 1 Longman; London, UK: 1840. [Google Scholar]
- 30.Newman L.A., Kuerer H.M., Hung K.K., Vlastos G., Ames F.C., Ross M.I., Singletary S.E. Prophylactic mastectomy. J. Am. Coll. Surg. 2000;191:322–330. doi: 10.1016/S1072-7515(00)00361-6. [DOI] [PubMed] [Google Scholar]
- 31.Euhus D.M., Diaz J. Breast cancer prevention. Breast J. 2015;21:76–81. doi: 10.1111/tbj.12352. [DOI] [PubMed] [Google Scholar]
- 32.Alaofi R.K., Nassif M.O., Al-Hajeili M.R. Prophylactic mastectomy for the prevention of breast cancer: Review of the literature. Avicenna J. Med. 2018;8:67–77. doi: 10.4103/ajm.AJM_21_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuttle T.M., Habermann E.B., Grund E.H., Morris T.J., Virnig B.A. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: A trend toward more aggressive surgical treatment. J. Clin. Oncol. 2007;25:5203–5209. doi: 10.1200/JCO.2007.12.3141. [DOI] [PubMed] [Google Scholar]
- 34.Morrow M., Mehrara B. Prophylactic mastectomy and the timing of breast reconstruction. Br. J. Surg. 2009;96:1–2. doi: 10.1002/bjs.6463. [DOI] [PubMed] [Google Scholar]
- 35.Ticha P., Sukop A. Patient-reported outcomes in bilateral prophylactic mastectomy with breast reconstruction: A narrative review. Breast. 2023;73:103602. doi: 10.1016/j.breast.2023.103602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gandhi A., Duxbury P., Murphy J., Foden P., Lalloo F., Clancy T., Wisely J., Kirwan C.C., Howell A., Evans D.G. Patient reported outcome measures in a cohort of patients at high risk of breast cancer treated by bilateral risk reducing mastectomy and breast reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2022;75:69–76. doi: 10.1016/j.bjps.2021.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Gahm J., Wickman M., Brandberg Y. Bilateral prophylactic mastectomy in women with inherited risk of breast cancer--prevalence of pain and discomfort, impact on sexuality, quality of life and feelings of regret two years after surgery. Breast. 2010;19:462–469. doi: 10.1016/j.breast.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Gopie J.P., Mureau M.A., Seynaeve C., Ter Kuile M.M., Menke-Pluymers M.B., Timman R., Tibben A. Body image issues after bilateral prophylactic mastectomy with breast reconstruction in healthy women at risk for hereditary breast cancer. Fam. Cancer. 2013;12:479–487. doi: 10.1007/s10689-012-9588-5. [DOI] [PubMed] [Google Scholar]
- 39.Brandberg Y., Sandelin K., Erikson S., Jurell G., Liljegren A., Lindblom A., Linden A., von Wachenfeldt A., Wickman M., Arver B. Psychological reactions, quality of life, and body image after bilateral prophylactic mastectomy in women at high risk for breast cancer: A prospective 1-year follow-up study. J. Clin. Oncol. 2008;26:3943–3949. doi: 10.1200/JCO.2007.13.9568. [DOI] [PubMed] [Google Scholar]
- 40.McCarthy C.M., Hamill J.B., Kim H.M., Qi J., Wilkins E., Pusic A.L. Impact of Bilateral Prophylactic Mastectomy and Immediate Reconstruction on Health-Related Quality of Life in Women at High Risk for Breast Carcinoma: Results of the Mastectomy Reconstruction Outcomes Consortium Study. Ann. Surg. Oncol. 2017;24:2502–2508. doi: 10.1245/s10434-017-5915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi Y.H., Terry M.B., Daly M.B., MacInnis R.J., Hopper J.L., Colonna S., Buys S.S., Andrulis I.L., John E.M., Kurian A.W., et al. Association of Risk-Reducing Salpingo-Oophorectomy With Breast Cancer Risk in Women With BRCA1 and BRCA2 Pathogenic Variants. JAMA Oncol. 2021;7:585–592. doi: 10.1001/jamaoncol.2021.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mavaddat N., Antoniou A.C., Mooij T.M., Hooning M.J., Heemskerk-Gerritsen B.A., GENEPSO, Nogues C., Gauthier-Villars M., Caron O., Gesta P., et al. Correction to: Risk-reducing salpingo-oophorectomy, natural menopause, and breast cancer risk: An international prospective cohort of BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2020;22:25. doi: 10.1186/s13058-020-01259-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mai P.L., Miller A., Gail M.H., Skates S., Lu K., Sherman M.E., Ioffe O.B., Rodriguez G., Cohn D.E., Boggess J., et al. Risk-Reducing Salpingo-Oophorectomy and Breast Cancer Risk Reduction in the Gynecologic Oncology Group Protocol-0199 (GOG-0199) JNCI Cancer Spectr. 2020;4:pkz075. doi: 10.1093/jncics/pkz075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Modaffari P., Ponzone R., Ferrari A., Cipullo I., Liberale V., D’Alonzo M., Maggiorotto F., Biglia N. Concerns and Expectations of Risk-Reducing Surgery in Women with Hereditary Breast and Ovarian Cancer Syndrome. J. Clin. Med. 2019;8:313. doi: 10.3390/jcm8030313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michaelson-Cohen R., Gabizon-Peretz S., Armon S., Srebnik-Moshe N., Mor P., Tomer A., Levy-Lahad E., Paluch-Shimon S. Breast cancer risk and hormone replacement therapy among BRCA carriers after risk-reducing salpingo-oophorectomy. Eur. J. Cancer. 2021;148:95–102. doi: 10.1016/j.ejca.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 46.Kotsopoulos J., Gronwald J., Karlan B.Y., Huzarski T., Tung N., Moller P., Armel S., Lynch H.T., Senter L., Eisen A., et al. Hormone Replacement Therapy After Oophorectomy and Breast Cancer Risk Among BRCA1 Mutation Carriers. JAMA Oncol. 2018;4:1059–1065. doi: 10.1001/jamaoncol.2018.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gordhandas S., Norquist B.M., Pennington K.P., Yung R.L., Laya M.B., Swisher E.M. Hormone replacement therapy after risk reducing salpingo-oophorectomy in patients with BRCA1 or BRCA2 mutations; a systematic review of risks and benefits. Gynecol. Oncol. 2019;153:192–200. doi: 10.1016/j.ygyno.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 48.Sauter E.R. Breast Cancer Prevention: Current Approaches and Future Directions. Eur. J. Breast Health. 2018;14:64–71. doi: 10.5152/ejbh.2018.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roy S.S., Vadlamudi R.K. Role of estrogen receptor signaling in breast cancer metastasis. Int. J. Breast Cancer. 2012;2012:654698. doi: 10.1155/2012/654698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel H.K., Bihani T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol. Ther. 2018;186:1–24. doi: 10.1016/j.pharmthera.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 51.Chimento A., De Luca A., Avena P., De Amicis F., Casaburi I., Sirianni R., Pezzi V. Estrogen Receptors-Mediated Apoptosis in Hormone-Dependent Cancers. Int. J. Mol. Sci. 2022;23:1242. doi: 10.3390/ijms23031242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Das S.K.S., Singh Y., Kumar P., Thareja S. Elective Estrogen Receptor Modulators (SERMs) for the treatment of ER+ breast cancer: An overview. J. Mol. Struct. 2022;1270:133853. doi: 10.1016/j.molstruc.2022.133853. [DOI] [Google Scholar]
- 53.Fisher B., Costantino J.P., Wickerham D.L., Cecchini R.S., Cronin W.M., Robidoux A., Bevers T.B., Kavanah M.T., Atkins J.N., Margolese R.G., et al. Tamoxifen for the prevention of breast cancer: Current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J. Natl. Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 54.Fisher B., Costantino J.P., Wickerham D.L., Redmond C.K., Kavanah M., Cronin W.M., Vogel V., Robidoux A., Dimitrov N., Atkins J., et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl. Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 55.Cohen I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol. Oncol. 2004;94:256–266. doi: 10.1016/j.ygyno.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 56.Bergman L., Beelen M.L., Gallee M.P., Hollema H., Benraadt J., van Leeuwen F.E. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres’ ALERT Group. Assessment of Liver and Endometrial cancer Risk following Tamoxifen. Lancet. 2000;356:881–887. doi: 10.1016/S0140-6736(00)02677-5. [DOI] [PubMed] [Google Scholar]
- 57.Powles T., Eeles R., Ashley S., Easton D., Chang J., Dowsett M., Tidy A., Viggers J., Davey J. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet. 1998;352:98–101. doi: 10.1016/S0140-6736(98)85012-5. [DOI] [PubMed] [Google Scholar]
- 58.Veronesi U., Mariani L., Decensi A., Formelli F., Camerini T., Miceli R., Di Mauro M.G., Costa A., Marubini E., Sporn M.B., et al. Fifteen-year results of a randomized phase III trial of fenretinide to prevent second breast cancer. Ann. Oncol. 2006;17:1065–1071. doi: 10.1093/annonc/mdl047. [DOI] [PubMed] [Google Scholar]
- 59.Cuzick J., Forbes J.F., Sestak I., Cawthorn S., Hamed H., Holli K., Howell A. International Breast Cancer Intervention Study II: Long-term results of tamoxifen prophylaxis for breast cancer--96-month follow-up of the randomized IBIS-I trial. J. Natl. Cancer Inst. 2007;99:272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 60.Powles T.J., Ashley S., Tidy A., Smith I.E., Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J. Natl. Cancer Inst. 2007;99:283–290. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- 61.Cuzick J., Sestak I., Cawthorn S., Hamed H., Holli K., Howell A., Forbes J.F., Investigators I.-I. Tamoxifen for prevention of breast cancer: Extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16:67–75. doi: 10.1016/S1470-2045(14)71171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cummings S.R., Eckert S., Krueger K.A., Grady D., Powles T.J., Cauley J.A., Norton L., Nickelsen T., Bjarnason N.H., Morrow M., et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: Results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 63.Ettinger B., Black D.M., Mitlak B.H., Knickerbocker R.K., Nickelsen T., Genant H.K., Christiansen C., Delmas P.D., Zanchetta J.R., Stakkestad J., et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: Results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 64.Vogel V.G., Costantino J.P., Wickerham D.L., Cronin W.M., Cecchini R.S., Atkins J.N., Bevers T.B., Fehrenbacher L., Pajon E.R., Wade J.L., et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 65.Vogel V.G., Costantino J.P., Wickerham D.L., Cronin W.M., Cecchini R.S., Atkins J.N., Bevers T.B., Fehrenbacher L., Pajon E.R., Wade J.L., 3rd, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev. Res. 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quinlan A., O’Brien K.K., Galvin R., Hardy C., McDonnell R., Joyce D., McDowell R.D., Aherne E., Keogh C., O’Sullivan K., et al. Quantifying patient preferences for symptomatic breast clinic referral: A decision analysis study. BMJ Open. 2018;8:e017286. doi: 10.1136/bmjopen-2017-017286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCaffery K., Nickel B., Moynihan R., Hersch J., Teixeira-Pinto A., Irwig L., Barratt A. How different terminology for ductal carcinoma in situ impacts women’s concern and treatment preferences: A randomised comparison within a national community survey. BMJ Open. 2015;5:e008094. doi: 10.1136/bmjopen-2015-008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gomez S.L., Tan S., Keegan T.H., Clarke C.A. Disparities in mammographic screening for Asian women in California: A cross-sectional analysis to identify meaningful groups for targeted intervention. BMC Cancer. 2007;7:201. doi: 10.1186/1471-2407-7-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vadaparampil S.T., Miree C.A., Wilson C., Jacobsen P.B. Psychosocial and behavioral impact of genetic counseling and testing. Breast Dis. 2006;27:97–108. doi: 10.3233/BD-2007-27106. [DOI] [PubMed] [Google Scholar]
- 70.Van Bockstal M.R., Agahozo M.C., Koppert L.B., van Deurzen C.H.M. A retrospective alternative for active surveillance trials for ductal carcinoma in situ of the breast. Int. J. Cancer. 2020;146:1189–1197. doi: 10.1002/ijc.32362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bäcklund M., Eriksson M., Gabrielson M., Hammarström M., Quay S., Bergqvist J., Hellgren R., Czene K., Hall P. Topical Endoxifen for Mammographic Density Reduction-A Randomized Controlled Trial. Oncologist. 2022;27:e597–e600. doi: 10.1093/oncolo/oyac102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee O., Page K., Ivancic D., Helenowski I., Parini V., Sullivan M.E., Margenthaler J.A., Chatterton R.T., Jovanovic B., Dunn B.K., et al. A randomized phase II presurgical trial of transdermal 4-hydroxytamoxifen gel versus oral tamoxifen in women with ductal carcinoma in situ of the breast. Clin. Cancer Res. 2014;20:3672–3682. doi: 10.1158/1078-0432.CCR-13-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Camerini T., Mariani L., De Palo G., Marubini E., Di Mauro M.G., Decensi A., Costa A., Veronesi U. Safety of the synthetic retinoid fenretinide: Long-term results from a controlled clinical trial for the prevention of contralateral breast cancer. J. Clin. Oncol. 2001;19:1664–1670. doi: 10.1200/JCO.2001.19.6.1664. [DOI] [PubMed] [Google Scholar]
- 74.Russo J. Significance of rat mammary tumors for human risk assessment. Toxicol. Pathol. 2015;43:145–170. doi: 10.1177/0192623314532036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jaini R., Kesaraju P., Johnson J.M., Altuntas C.Z., Jane-Wit D., Tuohy V.K. An autoimmune-mediated strategy for prophylactic breast cancer vaccination. Nat. Med. 2010;16:799–803. doi: 10.1038/nm.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palladini A., Thrane S., Janitzek C.M., Pihl J., Clemmensen S.B., de Jongh W.A., Clausen T.M., Nicoletti G., Landuzzi L., Penichet M.L., et al. Virus-like particle display of HER2 induces potent anti-cancer responses. Oncoimmunology. 2018;7:e1408749. doi: 10.1080/2162402X.2017.1408749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bryson P.D., Han X., Truong N., Wang P. Breast cancer vaccines delivered by dendritic cell-targeted lentivectors induce potent antitumor immune responses and protect mice from mammary tumor growth. Vaccine. 2017;35:5842–5849. doi: 10.1016/j.vaccine.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burga L.N., Hu H., Juvekar A., Tung N.M., Troyan S.L., Hofstatter E.W., Wulf G.M. Loss of BRCA1 leads to an increase in epidermal growth factor receptor expression in mammary epithelial cells, and epidermal growth factor receptor inhibition prevents estrogen receptor-negative cancers in BRCA1-mutant mice. Breast Cancer Res. 2011;13:R30. doi: 10.1186/bcr2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang D., Leal A.S., Carapellucci S., Zydeck K., Sporn M.B., Liby K.T. Chemoprevention of Preclinical Breast and Lung Cancer with the Bromodomain Inhibitor I-BET 762. Cancer Prev. Res. 2018;11:143–156. doi: 10.1158/1940-6207.CAPR-17-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim E.H., Deng C., Sporn M.B., Royce D.B., Risingsong R., Williams C.R., Liby K.T. CDDO-methyl ester delays breast cancer development in BRCA1-mutated mice. Cancer Prev. Res. 2012;5:89–97. doi: 10.1158/1940-6207.CAPR-11-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Infante M., Fabi A., Cognetti F., Gorini S., Caprio M., Fabbri A. RANKL/RANK/OPG system beyond bone remodeling: Involvement in breast cancer and clinical perspectives. J. Exp. Clin. Cancer Res. 2019;38:12. doi: 10.1186/s13046-018-1001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nolan E., Vaillant F., Branstetter D., Pal B., Giner G., Whitehead L., Lok S.W., Mann G.B., Rohrbach K., Huang L.Y., et al. RANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriers. Nat. Med. 2016;22:933–939. doi: 10.1038/nm.4118. [DOI] [PubMed] [Google Scholar]
- 83.Howe L.R., Subbaramaiah K., Patel J., Masferrer J.L., Deora A., Hudis C., Thaler H.T., Muller W.J., Du B., Brown A.M., et al. Celecoxib, a selective cyclooxygenase 2 inhibitor, protects against human epidermal growth factor receptor 2 (HER-2)/neu-induced breast cancer. Cancer Res. 2002;62:5405–5407. [PubMed] [Google Scholar]
- 84.Farhangi B., Alizadeh A.M., Khodayari H., Khodayari S., Dehghan M.J., Khori V., Heidarzadeh A., Khaniki M., Sadeghiezadeh M., Najafi F. Protective effects of dendrosomal curcumin on an animal metastatic breast tumor. Eur. J. Pharmacol. 2015;758:188–196. doi: 10.1016/j.ejphar.2015.03.076. [DOI] [PubMed] [Google Scholar]
- 85.Stachnik A., Yuen T., Iqbal J., Sgobba M., Gupta Y., Lu P., Colaianni G., Ji Y., Zhu L.L., Kim S.M., et al. Repurposing of bisphosphonates for the prevention and therapy of nonsmall cell lung and breast cancer. Proc. Natl. Acad. Sc.i USA. 2014;111:17995–18000. doi: 10.1073/pnas.1421422111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu K., Zhang Y., Xu X.C., Hill J., Celestino J., Kim H.T., Mohsin S.K., Hilsenbeck S.G., Lamph W.W., Bissonette R., et al. The retinoid X receptor-selective retinoid, LGD1069, prevents the development of estrogen receptor-negative mammary tumors in transgenic mice. Cancer Res. 2002;62:6376–6380. [PubMed] [Google Scholar]
- 87.Li Y., Zhang Y., Hill J., Kim H.T., Shen Q., Bissonnette R.P., Lamph W.W., Brown P.H. The rexinoid, bexarotene, prevents the development of premalignant lesions in MMTV-erbB2 mice. Br. J. Cancer. 2008;98:1380–1388. doi: 10.1038/sj.bjc.6604320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sahin K., Yabas M., Orhan C., Tuzcu M., Sahin T.K., Ozercan I.H., Qazi S., Uckun F.M. Prevention of DMBA-induced mammary gland tumors in mice by a dual-function inhibitor of JAK3 and EGF receptor tyrosine kinases. Expert Opin. Ther. Targets. 2020;24:379–387. doi: 10.1080/14728222.2020.1737014. [DOI] [PubMed] [Google Scholar]
- 89.Chun Y.S., Bisht S., Chenna V., Pramanik D., Yoshida T., Hong S.M., de Wilde R.F., Zhang Z., Huso D.L., Zhao M., et al. Intraductal administration of a polymeric nanoparticle formulation of curcumin (NanoCurc) significantly attenuates incidence of mammary tumors in a rodent chemical carcinogenesis model: Implications for breast cancer chemoprevention in at-risk populations. Carcinogenesis. 2012;33:2242–2249. doi: 10.1093/carcin/bgs248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Okugawa H., Yamamoto D., Uemura Y., Sakaida N., Tanano A., Tanaka K., Kamiyama Y. Effect of perductal paclitaxel exposure on the development of MNU-induced mammary carcinoma in female S-D rats. Breast Cancer Res. Treat. 2005;91:29–34. doi: 10.1007/s10549-004-6455-6. [DOI] [PubMed] [Google Scholar]
- 91.Murata S., Kominsky S.L., Vali M., Zhang Z., Garrett-Mayer E., Korz D., Huso D., Baker S.D., Barber J., Jaffee E., et al. Ductal access for prevention and therapy of mammary tumors. Cancer Res. 2006;66:638–645. doi: 10.1158/0008-5472.CAN-05-4329. [DOI] [PubMed] [Google Scholar]
- 92.Stearns V., Mori T., Jacobs L.K., Khouri N.F., Gabrielson E., Yoshida T., Kominsky S.L., Huso D.L., Jeter S., Powers P., et al. Preclinical and clinical evaluation of intraductally administered agents in early breast cancer. Sci. Transl. Med. 2011;3:106ra108. doi: 10.1126/scitranslmed.3002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gao D., Liu J., Yuan J., Wu J., Kuang X., Kong D., Zheng W., Wang G., Sukumar S., Tu Y., et al. Intraductal administration of N-methyl-N-nitrosourea as a novel rodent mammary tumor model. Ann. Transl. Med. 2021;9:576. doi: 10.21037/atm-21-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Groot J.S., van Diest P.J., van Amersfoort M., Vlug E.J., Pan X., Ter Hoeve N.D., Rosing H., Beijnen J.H., Youssef S.A., de Bruin A., et al. Intraductal cisplatin treatment in a BRCA-associated breast cancer mouse model attenuates tumor development but leads to systemic tumors in aged female mice. Oncotarget. 2017;8:60750–60763. doi: 10.18632/oncotarget.18490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang G., Chen C., Pai P., Korangath P., Sun S., Merino V.F., Yuan J., Li S., Nie G., Stearns V., et al. Intraductal fulvestrant for therapy of ERα-positive ductal carcinoma in situ of the breast: A preclinical study. Carcinogenesis. 2019;40:903–913. doi: 10.1093/carcin/bgz084. [DOI] [PubMed] [Google Scholar]
- 96.Park J., Thomas S., Zhong A.Y., Wolfe A.R., Krings G., Terranova-Barberio M., Pawlowska N., Benet L.Z., Munster P.N. Local delivery of hormonal therapy with silastic tubing for prevention and treatment of breast cancer. Sci. Rep. 2018;8:92. doi: 10.1038/s41598-017-18436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sivaraman L., Gay J., Hilsenbeck S.G., Shine H.D., Conneely O.M., Medina D., O’Malley B.W. Effect of selective ablation of proliferating mammary epithelial cells on MNU induced rat mammary tumorigenesis. Breast Cancer Res. Treat. 2002;73:75–83. doi: 10.1023/A:1015227719105. [DOI] [PubMed] [Google Scholar]
- 98.Brock A., Krause S., Li H., Kowalski M., Goldberg M.S., Collins J.J., Ingber D.E. Silencing HoxA1 by intraductal injection of siRNA lipidoid nanoparticles prevents mammary tumor progression in mice. Sci. Transl Med. 2014;6:217ra212. doi: 10.1126/scitranslmed.3007048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoshida T., Jin K., Song H., Park S., Huso D.L., Zhang Z., Liangfeng H., Zhu C., Bruchertseifer F., Morgenstern A., et al. Effective treatment of ductal carcinoma in situ with a HER-2- targeted alpha-particle emitting radionuclide in a preclinical model of human breast cancer. Oncotarget. 2016;7:33306–33315. doi: 10.18632/oncotarget.8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang G., Kumar A., Ding W., Korangath P., Bera T., Wei J., Pai P., Gabrielson K., Pastan I., Sukumar S. Intraductal administration of transferrin receptor-targeted immunotoxin clears ductal carcinoma in situ in mouse models of breast cancer-a preclinical study. Proc. Natl. Acad. Sci. USA. 2022;119:e2200200119. doi: 10.1073/pnas.2200200119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kenyon E., Westerhuis J.J., Volk M., Hix J., Chakravarty S., Claucherty E., Zaluzec E., Ramsey L., Madaj Z., Hostetter G., et al. Ductal tree ablation by local delivery of ethanol prevents tumor formation in an aggressive mouse model of breast cancer. Breast Cancer Res. 2019;21:129. doi: 10.1186/s13058-019-1217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miller W.R. Aromatase inhibitors: Mechanism of action and role in the treatment of breast cancer. Semin. Oncol. 2003;30((Suppl. 14)):3–11. doi: 10.1016/S0093-7754(03)00302-6. [DOI] [PubMed] [Google Scholar]
- 103.Maunsell E., Goss P.E., Chlebowski R.T., Ingle J.N., Alés-Martínez J.E., Sarto G.E., Fabian C.J., Pujol P., Ruiz A., Cooke A.L., et al. Quality of life in MAP.3 (Mammary Prevention 3): A randomized, placebo-controlled trial evaluating exemestane for prevention of breast cancer. J. Clin. Oncol. 2014;32:1427–1436. doi: 10.1200/JCO.2013.51.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goss P.E., Ingle J.N., Alés-Martínez J.E., Cheung A.M., Chlebowski R.T., Wactawski-Wende J., McTiernan A., Robbins J., Johnson K.C., Martin L.W., et al. Exemestane for breast-cancer prevention in postmenopausal women. N. Engl. J. Med. 2011;364:2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 105.Cuzick J., Sestak I., Forbes J.F., Dowsett M., Cawthorn S., Mansel R.E., Loibl S., Bonanni B., Evans D.G., Howell A., et al. Use of anastrozole for breast cancer prevention (IBIS-II): Long-term results of a randomised controlled trial. Lancet. 2020;395:117–122. doi: 10.1016/S0140-6736(19)32955-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Formelli F., Clerici M., Campa T., Di Mauro M.G., Magni A., Mascotti G., Moglia D., De Palo G., Costa A., Veronesi U. Five-year administration of fenretinide: Pharmacokinetics and effects on plasma retinol concentrations. J. Clin. Oncol. 1993;11:2036–2042. doi: 10.1200/JCO.1993.11.10.2036. [DOI] [PubMed] [Google Scholar]
- 107.Mehta R.G., Moon R.C., Hawthorne M., Formelli F., Costa A. Distribution of fenretinide in the mammary gland of breast cancer patients. Eur. J. Cancer. 1991;27:138–141. doi: 10.1016/0277-5379(91)90471-O. [DOI] [PubMed] [Google Scholar]
- 108.Moon R.C., Thompson H.J., Becci P.J., Grubbs C.J., Gander R.J., Newton D.L., Smith J.M., Phillips S.L., Henderson W.R., Mullen L.T., et al. N-(4-Hydroxyphenyl)retinamide, a new retinoid for prevention of breast cancer in the rat. Cancer Res. 1979;39:1339–1346. [PubMed] [Google Scholar]
- 109.Howe L.R. Rexinoids and breast cancer prevention. Clin. Cancer Res. 2007;13:5983–5987. doi: 10.1158/1078-0432.CCR-07-1065. [DOI] [PubMed] [Google Scholar]
- 110.Sukumar J., Gast K., Quiroga D., Lustberg M., Williams N. Triple-negative breast cancer: Promising prognostic biomarkers currently in development. Expert Rev. Anticancer. Ther. 2021;21:135–148. doi: 10.1080/14737140.2021.1840984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Toyama T., Yamashita H., Kondo N., Okuda K., Takahashi S., Sasaki H., Sugiura H., Iwase H., Fujii Y. Frequently increased epidermal growth factor receptor (EGFR) copy numbers and decreased BRCA1 mRNA expression in Japanese triple-negative breast cancers. BMC Cancer. 2008;8:309. doi: 10.1186/1471-2407-8-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Arnes J.B., Begin L.R., Stefansson I., Brunet J.S., Nielsen T.O., Foulkes W.D., Akslen L.A. Expression of epidermal growth factor receptor in relation to BRCA1 status, basal-like markers and prognosis in breast cancer. J. Clin. Pathol. 2009;62:139–146. doi: 10.1136/jcp.2008.056291. [DOI] [PubMed] [Google Scholar]
- 113.Sarnik J., Poplawski T., Tokarz P. BET Proteins as Attractive Targets for Cancer Therapeutics. Int. J. Mol. Sci. 2021;22:11102. doi: 10.3390/ijms222011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mokbel K., Mokbel K. Chemoprevention of Breast Cancer With Vitamins and Micronutrients: A Concise Review. In Vivo. 2019;33:983–997. doi: 10.21873/invivo.11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y., Yu J., Cui R., Lin J., Ding X. Curcumin in Treating Breast Cancer: A Review. J. Lab. Autom. 2016;21:723–731. doi: 10.1177/2211068216655524. [DOI] [PubMed] [Google Scholar]
- 116.Boyce B.F., Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res. Therapy. 2007;9((Suppl. 1)):S1. doi: 10.1186/ar2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim N.S., Kim H.J., Koo B.K., Kwon M.C., Kim Y.W., Cho Y., Yokota Y., Penninger J.M., Kong Y.Y. Receptor activator of NF-kappaB ligand regulates the proliferation of mammary epithelial cells via Id2. Mol. Cell. Biol. 2006;26:1002–1013. doi: 10.1128/MCB.26.3.1002-1013.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gonzalez-Suarez E., Jacob A.P., Jones J., Miller R., Roudier-Meyer M.P., Erwert R., Pinkas J., Branstetter D., Dougall W.C. Rank ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468:103–107. doi: 10.1038/nature09495. [DOI] [PubMed] [Google Scholar]
- 119.Tuohy V.K., Johnson J.M., Mazumder S. Primary immunoprevention of adult onset cancers by vaccinating against retired tissue-specific self-proteins. Semin. Immunol. 2020;47:101392. doi: 10.1016/j.smim.2020.101392. [DOI] [PubMed] [Google Scholar]
- 120.Crews D.W., Dombroski J.A., King M.R. Prophylactic Cancer Vaccines Engineered to Elicit Specific Adaptive Immune Response. Front. Oncol. 2021;11:626463. doi: 10.3389/fonc.2021.626463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.The Artemis Project® Plan to Develop a Breast Cancer Preventive Vaccine: Identification of Targets & Immune System Variations. [(accessed on 30 December 2023)]. Available online: https://www.stopbreastcancer.org/wp-content/uploads/2011/12/artemis-project-plan-saic-1.pdf.
- 122.Artemis Project. [(accessed on 30 December 2023)]. Available online: https://www.stopbreastcancer.org/wp-content/uploads/2020/04/2015-artemis-preventive.pdf.
- 123.Artemis Project. [(accessed on 30 December 2023)]. Available online: https://www.stopbreastcancer.org/wp-content/uploads/2021/01/2020_ArtemisReport-Final-PDF-for-Website-1.pdf.
- 124.Nicolas-Morales M.L., Luisa-Sanjuan A., Gutierrez-Torres M., Vences-Velazquez A., Ortuno-Pineda C., Espinoza-Rojo M., Navarro-Tito N., Cortes-Sarabia K. Peptide-Based Vaccines in Clinical Phases and New Potential Therapeutic Targets as a New Approach for Breast Cancer: A Review. Vaccines. 2022;10:1249. doi: 10.3390/vaccines10081249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jaini R., Rayman P., Cohen P.A., Finke J.H., Tuohy V.K. Combination of sunitinib with anti-tumor vaccination inhibits T cell priming and requires careful scheduling to achieve productive immunotherapy. Int. J. Cancer. 2014;134:1695–1705. doi: 10.1002/ijc.28488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim S.W., Goedegebuure P., Gillanders W.E. Mammaglobin-A is a target for breast cancer vaccination. Oncoimmunology. 2016;5:e1069940. doi: 10.1080/2162402X.2015.1069940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bharat A., Benshoff N., Fleming T.P., Dietz J.R., Gillanders W.E., Mohanakumar T. Characterization of the role of CD8+T cells in breast cancer immunity following mammaglobin-A DNA vaccination using HLA-class-I tetramers. Breast Cancer Res. Treat. 2008;110:453–463. doi: 10.1007/s10549-007-9741-2. [DOI] [PubMed] [Google Scholar]
- 128.Narayanan K., Jaramillo A., Benshoff N.D., Campbell L.G., Fleming T.P., Dietz J.R., Mohanakumar T. Response of established human breast tumors to vaccination with mammaglobin-A cDNA. J. Natl. Cancer Inst. 2004;96:1388–1396. doi: 10.1093/jnci/djh261. [DOI] [PubMed] [Google Scholar]
- 129.Tiriveedhi V., Tucker N., Herndon J., Li L., Sturmoski M., Ellis M., Ma C., Naughton M., Lockhart A.C., Gao F., et al. Safety and preliminary evidence of biologic efficacy of a mammaglobin-a DNA vaccine in patients with stable metastatic breast cancer. Clin. Cancer Res. 2014;20:5964–5975. doi: 10.1158/1078-0432.CCR-14-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Visvader J.E. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes. Dev. 2009;23:2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Paine I.S., Lewis M.T. The Terminal End Bud: The Little Engine that Could. J. Mammary Gland. Biol. Neoplasia. 2017;22:93–108. doi: 10.1007/s10911-017-9372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Henson D.E., Tarone R.E., Nsouli H. Lobular involution: The physiological prevention of breast cancer. J. Natl. Cancer Inst. 2006;98:1589–1590. doi: 10.1093/jnci/djj454. [DOI] [PubMed] [Google Scholar]
- 133.Radisky D.C., Visscher D.W., Frank R.D., Vierkant R.A., Winham S., Stallings-Mann M., Hoskin T.L., Nassar A., Vachon C.M., Denison L.A., et al. Natural history of age-related lobular involution and impact on breast cancer risk. Breast Cancer Res. Treat. 2016;155:423–430. doi: 10.1007/s10549-016-3691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ghosh K., Vachon C.M., Pankratz V.S., Vierkant R.A., Anderson S.S., Brandt K.R., Visscher D.W., Reynolds C., Frost M.H., Hartmann L.C. Independent association of lobular involution and mammographic breast density with breast cancer risk. J. Natl. Cancer Inst. 2010;102:1716–1723. doi: 10.1093/jnci/djq414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rasul M.F., Hussen B.M., Salihi A., Ismael B.S., Jalal P.J., Zanichelli A., Jamali E., Baniahmad A., Ghafouri-Fard S., Basiri A., et al. Strategies to overcome the main challenges of the use of CRISPR/Cas9 as a replacement for cancer therapy. Mol. Cancer. 2022;21:64. doi: 10.1186/s12943-021-01487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen Y., Zhang Y. Application of the CRISPR/Cas9 System to Drug Resistance in Breast Cancer. Adv. Sci. 2018;5:1700964. doi: 10.1002/advs.201700964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Annunziato S., Kas S.M., Nethe M., Yücel H., Del Bravo J., Pritchard C., Bin Ali R., van Gerwen B., Siteur B., Drenth A.P., et al. Modeling invasive lobular breast carcinoma by CRISPR/Cas9-mediated somatic genome editing of the mammary gland. Genes. Dev. 2016;30:1470–1480. doi: 10.1101/gad.279190.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Annunziato S., de Ruiter J.R., Henneman L., Brambillasca C.S., Lutz C., Vaillant F., Ferrante F., Drenth A.P., van der Burg E., Siteur B., et al. Comparative oncogenomics identifies combinations of driver genes and drug targets in BRCA1-mutated breast cancer. Nat. Commun. 2019;10:397. doi: 10.1038/s41467-019-08301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Annunziato S., Lutz C., Henneman L., Bhin J., Wong K., Siteur B., van Gerwen B., de Korte-Grimmerink R., Zafra M.P., Schatoff E.M., et al. In situ CRISPR-Cas9 base editing for the development of genetically engineered mouse models of breast cancer. EMBO J. 2020;39:e102169. doi: 10.15252/embj.2019102169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Heitink L., Whittle J.R., Vaillant F., Capaldo B.D., Dekkers J.F., Dawson C.A., Milevskiy M.J.G., Surgenor E., Tsai M., Chen H.R., et al. In vivo genome-editing screen identifies tumor suppressor genes that cooperate with Trp53 loss during mammary tumorigenesis. Mol. Oncol. 2022;16:1119–1131. doi: 10.1002/1878-0261.13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pujol H., Girault J., Rouanet P., Fournier S., Grenier J., Simony J., Fourtillan J.B., Pujol J.L. Phase I study of percutaneous 4-hydroxy-tamoxifen with analyses of 4-hydroxy-tamoxifen concentrations in breast cancer and normal breast tissue. Cancer Chemother. Pharmacol. 1995;36:493–498. doi: 10.1007/BF00685799. [DOI] [PubMed] [Google Scholar]
- 142.Rouanet P., Linares-Cruz G., Dravet F., Poujol S., Gourgou S., Simony-Lafontaine J., Grenier J., Kramar A., Girault J., Le Nestour E., et al. Neoadjuvant percutaneous 4-hydroxytamoxifen decreases breast tumoral cell proliferation: A prospective controlled randomized study comparing three doses of 4-hydroxytamoxifen gel to oral tamoxifen. J. Clin. Oncol. 2005;23:2980–2987. doi: 10.1200/JCO.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 143.Osborne C.K., Wakeling A., Nicholson R.I. Fulvestrant: An oestrogen receptor antagonist with a novel mechanism of action. Br. J. Cancer. 2004;90((Suppl. 1)):S2–S6. doi: 10.1038/sj.bjc.6601629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Passos J.S., Dartora V., Cassone Salata G., Draszesski Malago I., Lopes L.B. Contributions of nanotechnology to the intraductal drug delivery for local treatment and prevention of breast cancer. Int. J. Pharm. 2023;635:122681. doi: 10.1016/j.ijpharm.2023.122681. [DOI] [PubMed] [Google Scholar]
- 145.Love S.M., Zhang W., Gordon E.J., Rao J., Yang H., Li J., Zhang B., Wang X., Chen G., Zhang B. A feasibility study of the intraductal administration of chemotherapy. Cancer Prev. Res. 2013;6:51–58. doi: 10.1158/1940-6207.CAPR-12-0228. [DOI] [PubMed] [Google Scholar]
- 146.Teo W.W., Sukumar S. Combining the strength of genomics, nanoparticle technology, and direct intraductal delivery for breast cancer treatment. Breast Cancer Res. 2014;16:306. doi: 10.1186/bcr3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Havaei S.M., Aucoin M.G., Jahanian-Najafabadi A. Pseudomonas Exotoxin-Based Immunotoxins: Over Three Decades of Efforts on Targeting Cancer Cells With the Toxin. Front. Oncol. 2021;11:781800. doi: 10.3389/fonc.2021.781800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Poty S., Francesconi L.C., McDevitt M.R., Morris M.J., Lewis J.S. Alpha-Emitters for Radiotherapy: From Basic Radiochemistry to Clinical Studies-Part 1. J. Nucl. Med. 2018;59:878–884. doi: 10.2967/jnumed.116.186338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xie L., Zhang L., Hu K., Hanyu M., Zhang Y., Fujinaga M., Minegishi K., Ohkubo T., Nagatsu K., Jiang C., et al. A (211)At-labelled mGluR1 inhibitor induces cancer senescence to elicit long-lasting anti-tumor efficacy. Cell Rep. Med. 2023;4:100960. doi: 10.1016/j.xcrm.2023.100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mark C., Lee J.S., Cui X., Yuan Y. Antibody-Drug Conjugates in Breast Cancer: Current Status and Future Directions. Int. J. Mol. Sci. 2023;24:13726. doi: 10.3390/ijms241813726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chakravarty S., Hix J.M.L., Wiewiora K.A., Volk M.C., Kenyon E., Shuboni-Mulligan D.D., Blanco-Fernandez B., Kiupel M., Thomas J., Sempere L.F., et al. Tantalum oxide nanoparticles as versatile contrast agents for X-ray computed tomography. Nanoscale. 2020;12:7720–7734. doi: 10.1039/D0NR01234C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kenyon E., Zaluzec E., Powell K., Volk M., Chakravarty S., Hix J., Kiupel M., Shapiro E.M., Sempere L.F. X-Ray Visualization of Intraductal Ethanol-Based Ablative Treatment for Prevention of Breast Cancer in Rat Models. J. Vis. Exp. 2022;190:e64042. doi: 10.3791/64042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kenyon E., Zaluzec E.K., Powell K., Volk M., Chakravarty S., Hix J., Arora R., Westerhuis J.J., Kiupel M., Shapiro E.M., et al. Intraductal Delivery and X-ray Visualization of Ethanol-Based Ablative Solution for Prevention and Local Treatment of Breast Cancer in Mouse Models. J. Vis. Exp. 2022;182:e63457. doi: 10.3791/63457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Robertson N., Sempere L., Kenyon E., Mallet C., Smith K., Hix J., Halim A., Fan J., Moore A. Omniparticle Contrast Agent for Multimodal Imaging: Synthesis and Characterization in an Animal Model. Mol. Imaging Biol. 2023;25:401–412. doi: 10.1007/s11307-022-01770-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Metcalfe K.A., Birenbaum-Carmeli D., Lubinski J., Gronwald J., Lynch H., Moller P., Ghadirian P., Foulkes W.D., Klijn J., Friedman E., et al. International variation in rates of uptake of preventive options in BRCA1 and BRCA2 mutation carriers. Int. J. Cancer. 2008;122:2017–2022. doi: 10.1002/ijc.23340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kuang M., Lu M.D., Xie X.Y., Xu H.X., Xu Z.F., Liu G.J., Yin X.Y., Huang J.F., Lencioni R. Ethanol ablation of hepatocellular carcinoma Up to 5.0 cm by using a multipronged injection needle with high-dose strategy. Radiology. 2009;253:552–561. doi: 10.1148/radiol.2532082021. [DOI] [PubMed] [Google Scholar]
- 157.Ansari D., Andersson R. Radiofrequency ablation or percutaneous ethanol injection for the treatment of liver tumors. World J. Gastroenterol. 2012;18:1003–1008. doi: 10.3748/wjg.v18.i10.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang W.Y., Li Z.S., Jin Z.D. Endoscopic ultrasound-guided ethanol ablation therapy for tumors. World J. Gastroenterol. 2013;19:3397–3403. doi: 10.3748/wjg.v19.i22.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mueller J.L., Morhard R., DeSoto M., Chelales E., Yang J., Nief C., Crouch B., Everitt J., Previs R., Katz D., et al. Optimizing ethyl cellulose-ethanol delivery towards enabling ablation of cervical dysplasia. Sci. Rep. 2021;11:16869. doi: 10.1038/s41598-021-96223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nief C., Morhard R., Chelales E., Adrianzen Alvarez D., Bourla Bs I., Lam C.T., Sag A.A., Crouch B.T., Mueller J.L., Katz D., et al. Polymer-assisted intratumoral delivery of ethanol: Preclinical investigation of safety and efficacy in a murine breast cancer model. PLoS ONE. 2021;16:e0234535. doi: 10.1371/journal.pone.0234535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chelales E., Morhard R., Nief C., Crouch B., Everitt J.I., Sag A.A., Ramanujam N. Radiologic-pathologic analysis of increased ethanol localization and ablative extent achieved by ethyl cellulose. Sci. Rep. 2021;11:20700. doi: 10.1038/s41598-021-99985-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Morhard R., Nief C., Barrero Castedo C., Hu F., Madonna M., Mueller J.L., Dewhirst M.W., Katz D.F., Ramanujam N. Development of enhanced ethanol ablation as an alternative to surgery in treatment of superficial solid tumors. Sci. Rep. 2017;7:8750. doi: 10.1038/s41598-017-09371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Morhard R., Mueller J.L., Tang Q., Nief C., Chelales E., Lam C.T., Alvarez D.A., Rubinstein M., Katz D.F., Ramanujam N. Understanding Factors Governing Distribution Volume of Ethyl Cellulose-Ethanol to Optimize Ablative Therapy in the Liver. IEEE Trans. Biomed. Eng. 2020;67:2337–2348. doi: 10.1109/TBME.2019.2960049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sannier K., Dompmartin A., Theron J., Labbe D., Barrellier M.T., Leroyer R., Toure P., Leroy D. A new sclerosing agent in the treatment of venous malformations. Study on 23 cases. Interv. Neuroradiol. 2004;10:113–127. doi: 10.1177/159101990401000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dompmartin A., Blaizot X., Theron J., Hammer F., Chene Y., Labbe D., Barrellier M.T., Gaillard C., Leroyer R., Chedru V., et al. Radio-opaque ethylcellulose-ethanol is a safe and efficient sclerosing agent for venous malformations. Eur. Radiol. 2011;21:2647–2656. doi: 10.1007/s00330-011-2213-4. [DOI] [PubMed] [Google Scholar]
- 166.Zhang J., Li H.B., Zhou S.Y., Chen K.S., Niu C.Q., Tan X.Y., Jiang Y.Z., Lin Q.Q. Comparison between absolute ethanol and bleomycin for the treatment of venous malformation in children. Exp. Ther. Med. 2013;6:305–309. doi: 10.3892/etm.2013.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wohlgemuth W.A., Muller-Wille R., Teusch V., Hammer S., Wildgruber M., Uller W. Ethanolgel sclerotherapy of venous malformations improves health-related quality-of-life in adults and children-results of a prospective study. Eur. Radiol. 2017;27:2482–2488. doi: 10.1007/s00330-016-4603-0. [DOI] [PubMed] [Google Scholar]
- 168.Steiner F., FitzJohn T., Tan S.T. Ethanol sclerotherapy for venous malformation. ANZ J. Surg. 2016;86:790–795. doi: 10.1111/ans.12833. [DOI] [PubMed] [Google Scholar]
- 169.Chin M., Chen C.L., Chang K., Lee J., Samarasena J. Ethanol Ablation of a Peripheral Nerve Sheath Tumor Presenting as a Small Bowel Obstruction. ACG Case Rep. J. 2015;3:31–32. doi: 10.14309/crj.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gueng M.K., Chou Y.H., Tiu C.M., Chiou S.Y., Cheng Y.F. Pseudoaneurysm of the Breast Treated with Percutaneous Ethanol Injection. J. Med. Ultrasound. 2014;22:114–116. doi: 10.1016/j.jmu.2014.04.006. [DOI] [Google Scholar]
- 171.Chun Y.S., Yoshida T., Mori T., Huso D.L., Zhang Z., Stearns V., Perkins B., Jones R.J., Sukumar S. Intraductally administered pegylated liposomal doxorubicin reduces mammary stem cell function in the mammary gland but in the long term, induces malignant tumors. Breast Cancer Res. Treat. 2012;135:201–208. doi: 10.1007/s10549-012-2138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Consumption of Alcoholic Beverages. [(accessed on 30 December 2023)]. Available online: https://monographs.iarc.fr/wp-content/uploads/2018/06/mono100E-11.pdf.
- 173.Garaycoechea J.I., Crossan G.P., Langevin F., Mulderrig L., Louzada S., Yang F., Guilbaud G., Park N., Roerink S., Nik-Zainal S., et al. Alcohol and endogenous aldehydes damage chromosomes and mutate stem cells. Nature. 2018;553:171–177. doi: 10.1038/nature25154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Patel S., Sparman N.Z.R., Arneson D., Alvarsson A., Santos L.C., Duesman S.J., Centonze A., Hathaway E., Ahn I.S., Diamante G., et al. Mammary duct luminal epithelium controls adipocyte thermogenic programme. Nature. 2023;620:192–199. doi: 10.1038/s41586-023-06361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Markiewicz E., Fan X., Mustafi D., Zamora M., Conzen S.D., Karczmar G.S. MRI ductography of contrast agent distribution and leakage in normal mouse mammary ducts and ducts with in situ cancer. Magn. Reson. Imaging. 2017;40:48–52. doi: 10.1016/j.mri.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Markiewicz E., Fan X., Mustafi D., Zamora M., Roman B.B., Jansen S.A., Macleod K., Conzen S.D., Karczmar G.S. High resolution 3D MRI of mouse mammary glands with intra-ductal injection of contrast media. Magn. Reson. Imaging. 2015;33:161–165. doi: 10.1016/j.mri.2014.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Clark A., Bird N.K., Brock A. Intraductal Delivery to the Rabbit Mammary Gland. J. Vis. Exp. 2017;121:e55209. doi: 10.3791/55209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lai Y.E., Morhard R., Ramanujam N., Nolan M.W. Minimally invasive ethyl cellulose ethanol ablation in domesticated cats with naturally occurring head and neck cancers: Six cats. Vet Comp. Oncol. 2021;19:492–500. doi: 10.1111/vco.12687. [DOI] [PubMed] [Google Scholar]
- 179.Zhao Z., Wu F. Minimally-invasive thermal ablation of early-stage breast cancer: A systemic review. Eur. J. Surg. Oncol. 2010;36:1149–1155. doi: 10.1016/j.ejso.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 180.Yu M.H., Lee J.Y., Kim H.R., Kim B.R., Park E.J., Kim H.S., Han J.K., Choi B.I. Therapeutic Effects of Microbubbles Added to Combined High-Intensity Focused Ultrasound and Chemotherapy in a Pancreatic Cancer Xenograft Model. Korean J. Radiol. 2016;17:779–788. doi: 10.3348/kjr.2016.17.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ashikbayeva Z., Tosi D., Balmassov D., Schena E., Saccomandi P., Inglezakis V. Application of Nanoparticles and Nanomaterials in Thermal Ablation Therapy of Cancer. Nanomaterials. 2019;9:1195. doi: 10.3390/nano9091195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wild C.P. The global cancer burden: Necessity is the mother of prevention. Nat. Rev. Cancer. 2019;19:123–124. doi: 10.1038/s41568-019-0110-3. [DOI] [PubMed] [Google Scholar]
- 183.Hughes K. Comparative mammary gland postnatal development and tumourigenesis in the sheep, cow, cat and rabbit: Exploring the menagerie. Semin. Cell Dev. Biol. 2021;114:186–195. doi: 10.1016/j.semcdb.2020.09.010. [DOI] [PubMed] [Google Scholar]
- 184.Schoniger S., Degner S., Jasani B., Schoon H.A. A Review on Mammary Tumors in Rabbits: Translation of Pathology into Medical Care. Animals. 2019;9:762. doi: 10.3390/ani9100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bourne R.A., Bryant J.A., Falconer I.R. Stimulation of DNA synthesis by prolactin in rabbit mammary tissue. J. Cell Sci. 1974;14:105–111. doi: 10.1242/jcs.14.1.105. [DOI] [PubMed] [Google Scholar]
- 186.Falconer I.R. The distribution of 131 I- or 125 I-labelled prolactin in rabbit mammary tissue after intravenous or intraductal injection. J. Endocrinol. 1972;53:58–59. [PubMed] [Google Scholar]
- 187.Fiddler T.J., Birkinshaw M., Falconer I.R. Effects of intraductal prolactin on some aspects of the ultrastructure and biochemistry of mammary tissue in the pseudopregnant rabbit. J. Endocrinol. 1971;49:459–469. doi: 10.1677/joe.0.0490459. [DOI] [PubMed] [Google Scholar]
- 188.Fiddler T.J., Falconer I.R. The effect of intraductal prolactin on protein and nucleic acid biosynthesis in the rabbit mammary gland. Biochem. J. 1969;115:58P–59P. doi: 10.1042/bj1150058P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chadwick A. Detection and Assay of Prolactin by the Local Lactogenic Response in the Rabbit. J. Endocrinol. 1963;27:253–263. doi: 10.1677/joe.0.0270253. [DOI] [PubMed] [Google Scholar]
- 190.Slawson S.H., Johnson B.A. Ductography: How to and what if? Radiographics. 2001;21:133–150. doi: 10.1148/radiographics.21.1.g01ja15133. [DOI] [PubMed] [Google Scholar]
- 191.Sheiman L.S., Levesque P.H. The In’s and Out’s of Ductography: A Comprehensive Review. Curr. Probl. Diagn. Radiol. 2016;45:61–70. doi: 10.1067/j.cpradiol.2015.05.007. [DOI] [PubMed] [Google Scholar]