Abstract
Practical relevance:
Systemic hypertension is common in older cats and can result in damage to organs with a rich arteriolar supply such as the eyes, kidneys, myocardium and brain. Hypertensive disease in these organs is known as target organ damage (TOD). Disease in the eye resulting from hypertension is the most easily identifiable form of TOD and can often be the reason the cat is presented to the veterinarian. Routine blood pressure measurement and fundic examination allows cats with hypertensive ocular lesions to be detected early in the course of the disease, when the lesions have the best chance of responding to treatment.
Clinical challenges:
Detecting early evidence of TOD in the fundus requires a veterinarian to be competent in recognising lesions associated with mild hypertensive disease, as well as the more easily recognised advanced lesions that frequently result in impaired vision and blindness.
Audience:
This review is written for all veterinarians who treat cats. It provides information and images to facilitate and guide veterinarians performing fundoscopy in cats, in particular in those over 7 years of age, with the aim of diagnosing hypertensive ocular lesions when they are present.
Equipment:
The clinical manifestations of hypertensive ocular disease can be detected non-invasively with inexpensive equipment. A summary of the equipment available for general practitioners to perform fundoscopy is provided.
Evidence base:
This is a comprehensive review of the literature on hypertensive ocular disease in cats. The author has also included images of hypertensive ocular lesions taken in general practice to highlight the variety of lesions that can be detected.
Keywords: Hypertensive ocular disease, hypertension treatment, fundic examination, fundic lesions
Pathophysiology
The pathogenesis by which systemic hypertension (SHT) occurs in cats is not well understood, and the contributing factors are multifactorial and highly complex.1-3 Blood pressure (BP), the pressure exerted on the walls of the blood vessels, is determined by cardiac output and total peripheral resistance, with many neural and hormonal factors being involved in the fine control of BP. 1
Persistent high BP leads to damage to vulnerable organs, especially the eyes, kidneys, myocardium and brain.4,5 SHT has a profound effect on different parts of the eye. 6 The retina, choroid and optic nerve head have separate blood supplies, all of which are susceptible to the effect of elevated BP.6,7
The retina in cats, and some other vertebrates, is inverted, placing photoreceptors on the outer (deepest) surface, adjacent to the retinal pigment epithelium (RPE).8,9 Thus light passes through all other layers of the neurosensory retina before it reaches the photoreceptors; there it is converted to neural activity, which passes back through the same route. To keep the photoreceptors, which have very high metabolic and nutritional demands, in tight formation, the outer retinal layers (principally the photoreceptor nuclei and cytoplasm) are avascular and supplied with nutrients by diffusion from the adjacent choroidal circulation. 9 A copious blood supply through the choroidal circulation has developed to enable the photoreceptors to be supplied with the necessary nutritional elements. 9 The retinal circulation supplies the inner retinal layers. 6 In contrast to the choroidal circulation, the velocity of blood flow through the retinal vessels is relatively low. 10
The vascular beds of the retinal and optic nerve head autoregulate, thereby ensuring constant blood flow to the tissues they supply, despite changes in perfusion pressure. 7 Since the choroidal capillaries are not located in the tissues they supply, the choroidal vascular bed does not have the ability to autoregulate in response to changes in perfusion pressure. 9 There is also a blood–retinal barrier that contributes significantly to pathological changes in the eye when its function is compromised. 7 This consists of two components: the tight barrier between retinal pigment epithelial cells, which regulates fluid movement from the choroid to the retina, and the retinal vascular endothelium, which is non-fenestrated and joined by tight junctions that regulate movement of molecules from the blood to the retina.6,7 By contrast, in the choroid, the endothelium of the choriocapillaris has large fenestrations that allow proteins and fluids to move easily into the choroidal interstitial space.6,7
There is extensive literature on the patho-physiology of SHT on each of the parts of the eye. In brief, as BP rises there is constriction of retinal vessels, which is likely a repercussion of autoregulation. 11 Persistent elevation in BP results in arteriosclerotic changes to the vessel walls. 11 Eventually the ability of the retinal vessels to autoregulate fails and the blood-retinal barrier becomes compromised with damage to the vessel walls. 11 This results in leakage of plasma and cells, which may be identified as retinal oedema, haemorrhage and possible retinal detachment. 11
Clinical appearance
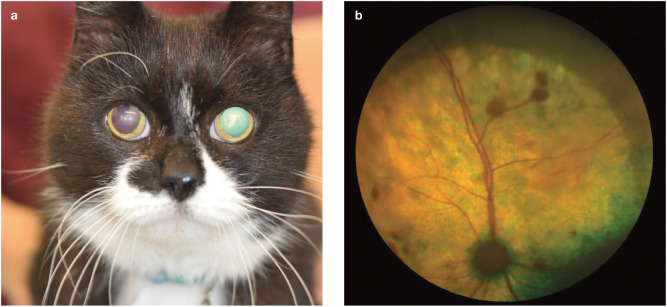
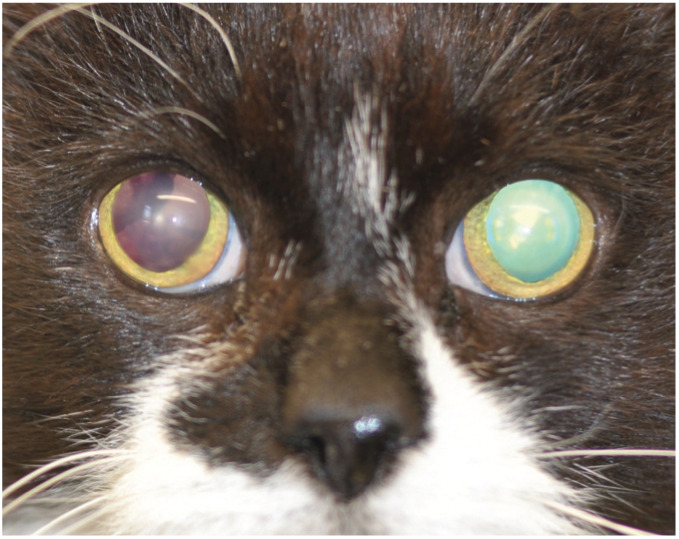
In advanced cases of hypertensive ocular disease, the owner of the cat or the veterinarian recognise there is a problem when blindness is observed or bilateral mydriasis, hyphaema or vitreal haemorrhages are seen (Figures 1-3).13-15 In one unusual case, a 14-year-old hypertensive cat presented with ocular protrusion which was thought to be associated with a retrobulbar thrombus. 17 There is a spectrum of fundic lesions secondary to SHT in cats that can be observed with ophthalmoscopy.13,19 These are usually classified into three groups: hypertensive retinopathy, hypertensive choroidopathy and hypertensive optic neuropathy. 19
Figure 1.

Bilateral mydriasis. Both pupils were excessively dilated despite the cat being in a well-lit room. Bilateral mydriasis in a patient with hypertensive fundic disease can suggest poor or lost vision
Figure 2.
Vitreal haemorrhage. Note the asymmetrical red fundic reflex in this patient, revealing unilateral vitreal haemorrhage in the right eye
Figure 3.
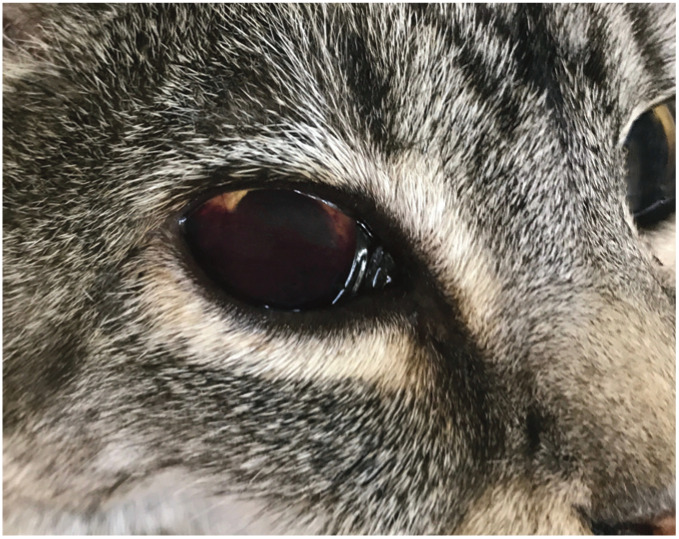
The presence of blood within the aqueous fluid of the anterior chamber indicates hyphaema. The blood tends to pool ventrally
Hypertensive retinopathy in cats most frequently manifests as retinal haemorrhages, which can range markedly in size and number and are typically easily visible with an ophthalmoscope.13,19 For most veterinarians, ocular haemorrhage is one of the most recognised manifestations of hypertensive disease in the cat.13,14 However, SHT is not the only cause of hyphaema and vitreal, choroidal or retinal haemorrhages in cats, and so consideration must be given to other potential causes, such as coagulopathies, trauma, anterior uveitis and chorioretinitis.16,20
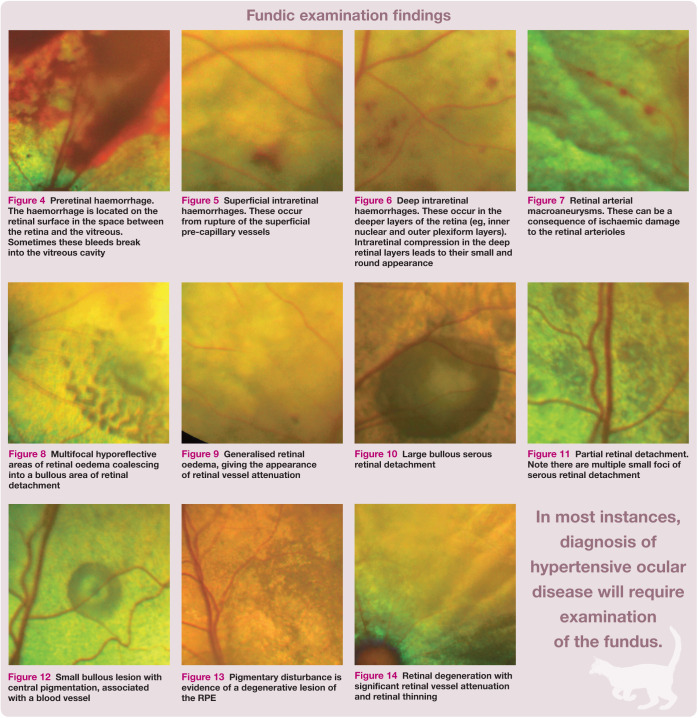
Retinal haemorrhage can have a varied clinical appearance, depending on its location within the retina.19,21 Preretinal haemorrhages, which can be quite large, are located on the retinal surface, consequently concealing the underlying retinal structures such as retinal blood vessels (Figure 4). 21 Superficial (inner) intraretinal haemorrhages located within the nerve fibre layer are described as ‘flame-shaped’ (Figure 5). 21 Deeper intraretinal haemorrhages are more confined by retinal anatomy and therefore generally smaller, darker and round (Figure 6). 21 Subretinal haemorrhages are located between the retina and the choroid; they are dark and the overlying retinal vessels are visible. 21 A prospective study of feline hypertensive ocular disease 18 revealed a lower incidence of ocular haemorrhage than earlier retrospective studies,13,14 suggesting that haemorrhage may be less prevalent as an early sign of hypertensive ocular disease. Focal retinal arteriolar dilations have been seen in cats with SHT.18,19 The associated ophthalmoscopic finding is retinal arterial macroaneurysms (Figure 7). 22
Figure 4.
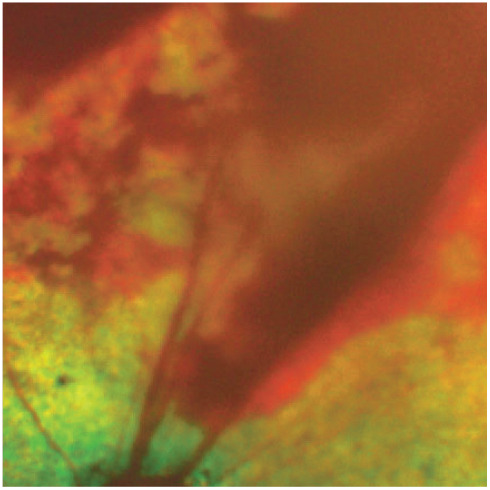
Preretinal haemorrhage. The haemorrhage is located on the retinal surface in the space between the retina and the vitreous. Sometimes these bleeds break into the vitreous cavity
Figure 5.
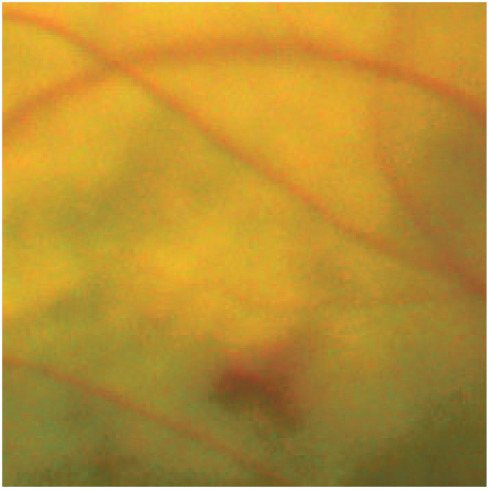
Superficial intraretinal haemorrhages. These occur from rupture of the superficial pre-capillary vessels
Figure 6.
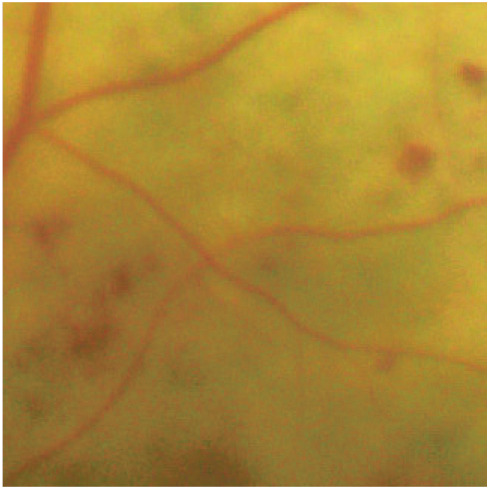
Deep intraretinal haemorrhages. These occur in the deeper layers of the retina (eg, inner nuclear and outer plexiform layers). Intraretinal compression in the deep retinal layers leads to their small and round appearance
Figure 7.
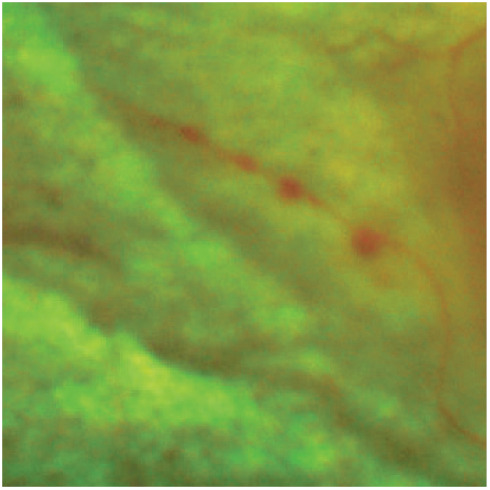
Retinal arterial macroaneurysms. These can be a consequence of ischaemic damage to the retinal arterioles
Maggio et al 13 suggested that the choroid, not the retina, may be the most frequently affected vascular bed within the eyes of cats with SHT. Histological examination of eyes from hypertensive cats demonstrated the significance of hypertensive choroidopathy – with subretinal fluid accumulation, originating from the choriocapillaris, being a very common feature. 19 The subretinal fluid can diffuse into the retinal tissue to produce retinal oedema (see box on page 36), which can be localised or generalised. 12 Localised retinal oedema is considered to be a mild retinal lesion. 13 Larger areas of localised oedema can progress to cause serous retinal detachment (Figure 8). 18 Retinal oedema can create the impression that the vessels, particularly the arterioles, are narrowed, as the oedema can obscure the edges of the vessels when examined by ophthalmoscopy (Figure 9).12,19 Genuine narrowing of the arterioles may result from arteriosclerosis due to prolonged SHT. 13
Figure 8.
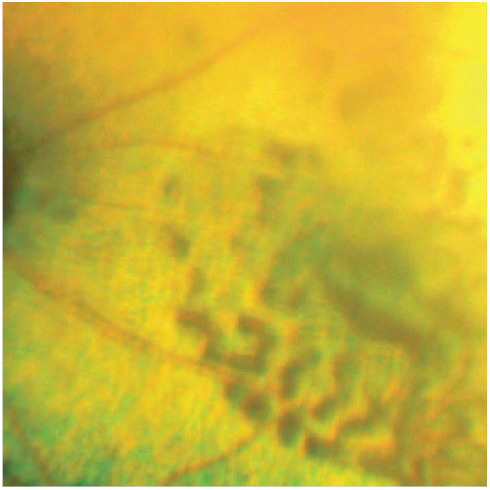
Multifocal hyporeflective areas of retinal oedema coalescing into a bullous area of retinal detachment
Figure 9.
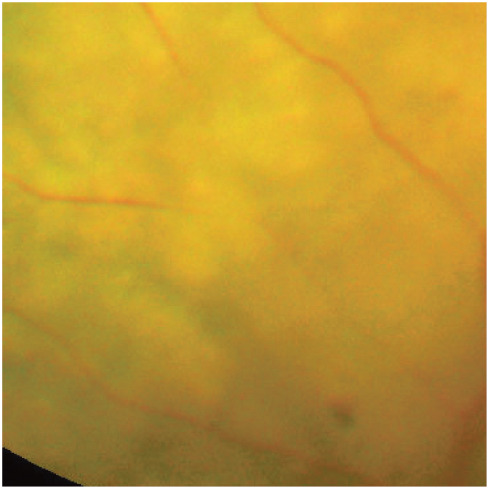
Generalised retinal oedema, giving the appearance of retinal vessel attenuation
Retinal detachments in cats with SHT can comprise bullous or flat partial detachments (Figures 10 and 11) or may involve the whole retina. 19 Discrete bullous detachments can, at times, be the only type of hypertensive ocular lesion detected, indicating they are likely an early sign of hypertensive ocular disease (Figure 12). 18 Retinal reattachment (which can occur with blood pressure fluctuations before hypertension is controlled or with successful hypertension treatment) can be uneven, which can lead to the retina appearing to be folded. 19 When the retina detaches, the overlying retinal arterioles often appear more tortuous than normal. 19 Note, however, that retinal arterial tortuosity is unlikely to occur as the only manifestation of hypertensive ocular disease. 13
Figure 10.
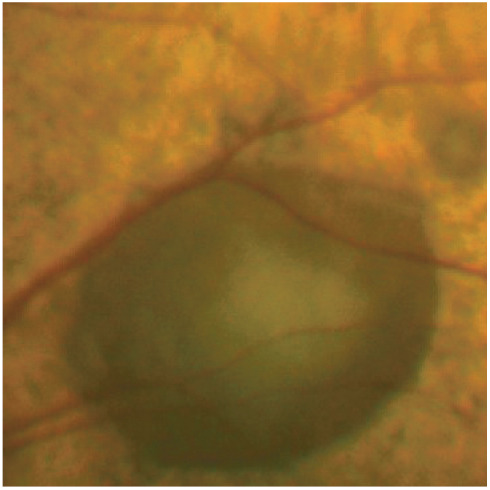
Large bullous serous retinal detachment
Figure 11.
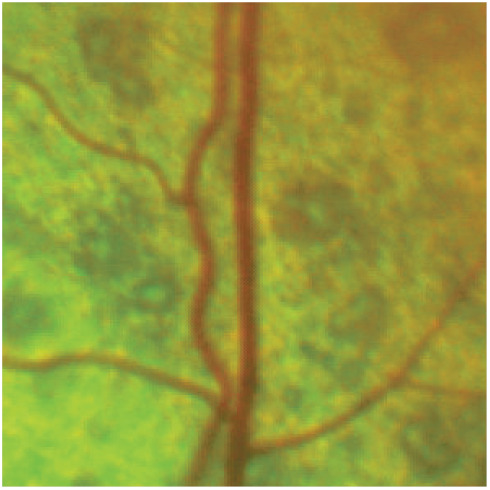
Partial retinal detachment. Note there are multiple small foci of serous retinal detachment
Figure 12.
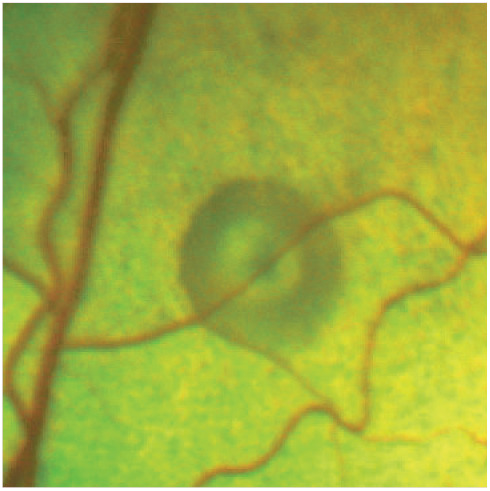
Small bullous lesion with central pigmentation, associated with a blood vessel
Histological examination reveals that disturbance to, and consequent damage of, the RPE is a common sequela of hypertensive damage to the choroid in cats. 19 Evidence of degenerative retinal pigment epithelial lesions is, however, not easily observed on ophthalmoscopy, but pigmentary disturbance may sometimes be seen (Figure 13). 19
Figure 13.
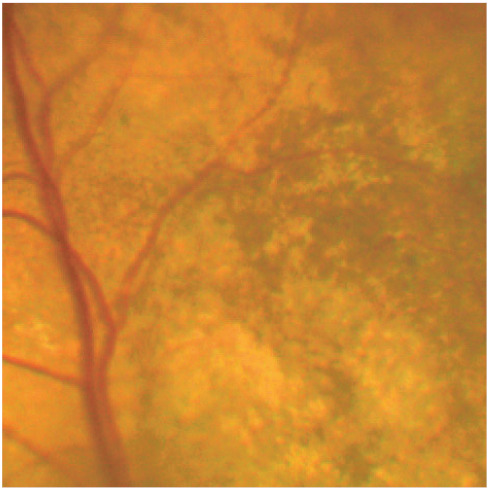
Pigmentary disturbance is evidence of a degenerative lesion of the RPE
Focal areas of advanced retinal degeneration are attributed to hypertensive ocular disease when elevated BP is measured and there are other lesions associated with hypertensive ocular disease present concurrently (Figure 14). 13 Bilateral generalised retinal degeneration in cats results from a variety of different causes, including previous enrofloxacin administration,23,24 and a genetic predisposition in certain purebred cats, 23 in addition, potentially, to hypertensive ocular disease following detachment and reattachment of the retina. 13 Hypertensive ocular disease is not, however, a common cause of bilateral generalised retinal degeneration in cats. 24
Figure 14.
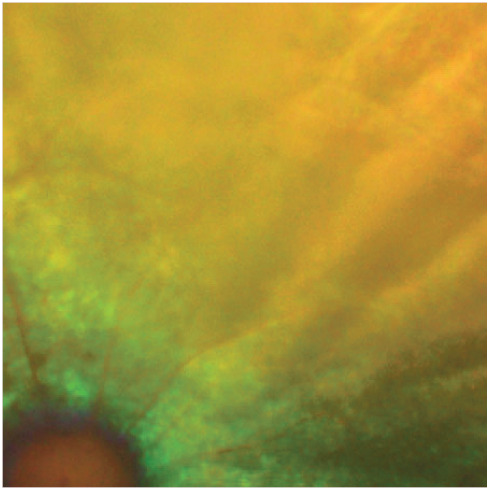
Retinal degeneration with significant retinal vessel attenuation and retinal thinning
Lesions of optic neuropathy recognised in cats with SHT include optic disc oedema or atrophy, though these are difficult to observe, possibly because the feline optic nerve head is not myelinated (unlike that of primates and dogs). 19
Diagnosis
Diagnosis of hypertensive ocular disease is based on observing characteristic clinical findings in one or both eyes, in association with measurement of elevated BP in a relaxed cat in which standardised protocols for BP measurement are employed.
Some manifestations of hypertensive ocular disease such as hyphaema and vitreal haemorrhage are easily seen when examining the cat without special instrumentation, but in most instances the diagnosis will require examination of the fundus.19,25 The key to successful fundic examination is pupil dilation, correct use of the ophthalmoscope, practice and a systematic approach. The examination should be performed in a darkened room to limit reflections. 26 Pupil dilation allows a more thorough examination of the fundus to be performed. 25 Mydriasis is achieved in approximately 15–20 mins following topical application of one drop of 1% tropicamide. 27
Ophthalmoscopy
Choice of technique
Examination with a direct ophthalmoscope provides an upright image in normal orientation, but one that is highly magnified and so there is only a small field of view (Table 1). 25 Therefore, direct ophthalmoscopy is not as valuable as indirect ophthalmoscopy for screening the entire fundus for pathology. Rather, it is useful for examining certain areas of the fundus in greater detail. 28 By contrast, indirect ophthalmoscopy provides a wider field of view and is, therefore, a more efficient way to perform a thorough fundic examination. 25 However, indirect ophthalmoscopy produces an upside down, reversed image, 25 making interpretation confusing without practice. The indirect ophthalmoscope allows more light penetration than the direct ophthalmoscope, and therefore a better view of the fundus in cats with particularly opaque media as a result of corneal, anterior chamber or lens pathology (Table 1). 29
Table 1.
Equipment for fundic examination
| Equipment | Advantages | Disadvantages | Notes |
|---|---|---|---|
| Direct ophthalmoscope | |||

|
• Inexpensive
• Direct, upright image • Provides greater magnification of a lesion once a screening view of the fundus has been obtained with another type of ophthalmoscope |
• Small field of view (focal lesions can easily be missed); therefore, not as useful for routine screening for hypertensive lesions
• Examiner is close to the cat’s head • Poorer view of the fundus than that afforded by indirect methods when there is opacification of the ocular media • No stereopsis |
• Helpful to examine the animal’s left eye with examiner’s left eye, and vice versa
• Use the optic nerve as a reference point |
| Monocular indirect ophthalmoscope | |||
|
• Inexpensive
• Large field of view allows a rapid and thorough examination of the fundus • Examiner is not as close to the cat’s head |
• Inverted, reversed image
• No stereopsis |
• Needs only a bright focal light source such as a penlight or a transilluminator and a 20 or 28 dioptre condensing lens
• An assistant is required to hold the patient’s head and open the eyelids |
| Binocular indirect ophthalmoscope | |||

|
• Stereopsis
• Examiner has two hands free and so can hold the condensing lens in one hand and the patient’s head/eyelids with the other • Examiner is not as close to the cat’s head |
• Expensive
• Inverted, reversed image |
• Similar technique to that used for monocular indirect ophthalmoscopy. The ophthalmoscope is head-mounted and then a condensing lens is placed close to the patient’s eye |
| Optibrand ClearView retinal camera | |||
|
• Allows photographic documentation of findings
• Excellent image quality |
• Expensive
• Needs to be used in conjunction with a computer • No stereopsis • May be difficult in some cases to differentiate between artefact and a lesion when a photo is viewed later (eg, hyperreflectivity of retina vs overexposure of image) |
|
| Welch Allyn iExaminer | |||

|
• Inexpensive if you already have a Welch Allyn Panoptic ophthalmoscope and a spare iPhone (6, 6s or 6 Plus)
• Allows lesions to be documented with a photo or video |
• Image quality is not as good as that obtained with a specialised fundic camera
• No stereopsis • May be difficult in some cases to differentiate between artefact and a lesion when an image is viewed later (eg, hyperreflectivity of retina vs overexposure of image) |
• Other optical devices that attach to smartphones are available for imaging the fundus of cats, such as the D-Eye Portable Ophthalmoscope. 30 Also fundic images can be captured using a smartphone app and lenses that may already be available in a practice31,32 |
Equipment
The equipment needed to perform monocular indirect ophthalmoscopy is inexpensive. Binocular indirect ophthalmoscopes are more expensive but have the added advantages of leaving both of the examiner’s hands free, placing the examiner at a greater distance from the patient, and providing stereopsis to better evaluate lesions that protrude or are recessed (Table 1). 29
Blood pressure measurement
Most cats with hypertensive ocular lesions will have an SBP reading ≥160 mmHg.13,14,33,34 While obtaining a BP reading in a clinic is quite possible, the information can only be considered useful if the result measured is representative of the average BP of that patient while at home. Elevated BP readings in cats can be recorded as a consequence of the anxiety associated with a clinic visit, the restraint needed for the BP measurement, or the procedure itself, and this is known as situational hypertension.4,35 The effect of hospital visits on BP values is recognised in humans as well, and often referred to as white coat hypertension. 36 The recent consensus guidelines on hypertension from the International Society of Feline Medicine (ISFM) 37 have outlined procedures that increase the likelihood of obtaining a representative and useful reading.
The direct method of BP measurement gives the most accurate estimation of arterial BP and is the ‘gold standard’. 37 However, direct measurement techniques are technically difficult to undertake, potentially painful, and adverse effects such as haemorrhage at the site of arterial puncture can occur. 38 Obtaining direct measurements is not practical for cats examined in veterinary clinics. Therefore, in veterinary practice, BP is usually measured indirectly using technology such as Doppler sphygmomanometry and high definition oscillometry.4,37 These techniques are easily learned, and the procedures are safe for the patient. However, concerns have been raised about the accuracy of some of the equipment used. 4
Investigation of underlying disease
Cats with hypertensive ocular lesions need to be investigated for underlying disease. While up to 20% of hypertensive cats have idiopathic (or primary) hypertension, 40 the majority have an identifiable underlying disease and are classified as having secondary hypertension.4,37
Chronic kidney disease SHT in cats is most commonly associated with chronic kidney disease (CKD),13,14,34 and SHT secondary to CKD is frequently implicated in causing hypertensive ocular lesions.13,14
Hyperthyroidism Hyperthyroidism has been identified as a cause of secondary SHT in cats, but hypertensive ocular lesions occur infrequently in hyperthyroid cats unless there is concurrent CKD. 13
Diabetes mellitus Hypertensive ocular lesions in diabetic cats without concurrent CKD have been reported; therefore, diabetes mellitus should be considered as a possible cause of secondary hypertension in cats. 13
Adrenal tumours and hyperaldosteronism Adrenal tumours and non-neoplastic primary hyperaldosteronism need to be considered as possible underlying causes in cats with hypertensive ocular lesions; in particular cats that also present with hindlimb weakness or cervical ventroflexion and where the serum potassium measures below, or in the low end of, the normal reference interval.39,41
Treatment
Once TOD of the eye is recognised, it is vital that treatment which provides the best prognosis for maintaining or regaining vision is instituted. Early hypertensive lesions may be reversed with treatment; however, prolonged retinal detachment makes visual recovery less likely. 13
Amlodipine
The calcium channel blocker amlodipine besylate is recommended as a first-line anti-hypertensive treatment in cats with ocular lesions and is frequently efficacious as a single agent.13,37 Hypertensive cats treated with amlodipine generally experience a 40–70 mmHg reduction in SBP,4,33,42 and the decrease can be achieved in as little as 7 days. 42
The influence of amlodipine on BP persists for at least 24 h in most cats, which allows for once daily dosing. 43 Many cats are treated effectively on 0.625 mg/cat q24h. The dose is typically doubled if the target BP has not been achieved within 7 days (Table 2).37,44 The dose can be doubled again if need be at a subsequent visit in another 1–2 weeks, as long as the veterinarian is happy that compliance is adequate. The maximum recommended dose is 2.5 mg/cat q24h.37,44 Bijsmans et al 44 reported that cats with an SBP ≥200 mmHg needed a greater dose of amlodipine to achieve control than cats with an SBP <200 mmHg, and proposed that cats with an SBP ≥200 mmHg at the initial visit could benefit from commencing amlodipine therapy at 1.25 mg/cat q24h (Table 2).
Table 2.
Drugs used for management of cats with hypertensive ocular lesions
| Concurrent clinical findings | Drugs to consider | Notes | Drugs and doses |
|---|---|---|---|
| SBP <200 mmHg | Amlodipine | • Amlodipine: 0.625 mg/cat q24h PO37,44 | |
| SBP ≥200 mmHg | Amlodipine | • Amlodipine: 1.25 mg/cat q24h PO 44 | |
| Azotaemia and proteinuria (UPC ≥0.4) 45 | ACE inhibitor or angiotensin II receptor blocker + amlodipine | Antihypertensive effect of telmisartan may be dose dependent. One small clinical trial showed telmisartan at 3 mg/kg may outperform benazapril as an antihypertensive agent 46 | • Benazapril: 0.5-1.0 mg/kg q24h PO
47
• Enalapril: 0.5 mg/kg q12-24h PO 37 • Ramipril: 0.125-0.25 mg/kg q24h PO 37 • Telmisartan: 1 mg/kg q24h PO 47 • Amlodipine: 0.625-1.25 mg/cat q24h PO37,44 |
| HR repeatedly >180 bpm (as measured by the owner with cat resting at home) | Atenolol + amlodipine | Can be useful for newly diagnosed hyperthyroid cats starting treatment. May be possible to taper and discontinue atenolol after a few weeks | • Atenolol: 1-2 mg/kg q12h PO
48
• Amlodipine: 0.625-1.25 mg/cat q24h PO37,44 |
| Blindness with bilateral retinal detachments | Amlodipine | Monitor BP closely | • Amlodipine: 0.625-1.25 mg/cat repeated up to q4h, to a maximum of 2.5 mg/cat for a 24 h period 37 |
| Hyphaema | Amlodipine + corticosteroid eye drops ± mydriatic | To reduce the risk of glaucoma, atropine should be tapered once pupil dilation is achieved
16
Discontinue atropine if intraocular pressure increases 16 |
• Amlodipine: 0.625-1.25 mg/cat q24h PO37,44
• Prednisolone acetate suspension 1% or Dexamethasone solution 0.1% One drop q4-6h 16 • Topical atropine 1% – one drop to dilate the pupil, can be repeated after 12-24 h if needed to achieve dilation 16 |
ACE = angiotensin-converting enzyme; BP = blood pressure; bpm = beats per minute; HR = heart rate; PO = oral; SBP = systolic blood pressure; UPC = urine protein/creatinine ratio
A cat that is blind due to bilateral retinal detachments at the time of presentation may not regain vision, despite appropriate treatment being instituted. 13 The dose of amlodipine can be repeated up to 4–8 hourly if needed, with close monitoring of BP, until the daily maximum dose of 2.5 mg/cat is reached (Table 2). 37
The long-term aim of antihypertensive treatment is to protect important organs from damage caused by sustained high BP. 37 The recent ISFM consensus guidelines on hypertension recommend a target SBP of <160 mmHg but suggest that an SBP <150 mmHg may be even more desirable to provide the least possible risk of TOD. 37
Transdermal amlodipine may be preferred for patients that prove difficult to medicate orally with tablets (Table 3). 42 However, the BP-lowering effects of transdermal amlodipine may be less pronounced than those of oral amlodipine at equivalent doses; hence, an increased dose of transdermal amlodipine may be required. 42
Table 3.
Options if target BP is not achieved
| Check compliance | Consider transdermal amlodipine if giving oral medication is too difficult |
|---|---|
| Check for an undiagnosed underlying disease | Ensure testing for CKD and hyperthyroidism is current and complete. Consider abdominal ultrasound to assess the adrenal glands and, if necessary, measure an aldosterone concentration |
| Double amlodipine dose | Up to a maximum of 2.5 mg/cat for a 24 h period 37 |
| Add ACE inhibitor
or atenolol or spironolactone |
If proteinuric If tachycardic
If primary hyperaldosteronism is diagnosed |
| Switch from amlodipine to oral hydralazine | Start at 0.5-1 mg/kg q12h and titrate upwards to a maximum of 2 mg/kg q12h 49 |
ACE = angiotensin-converting enzyme; BP = blood pressure; CKD = chronic kidney disease
Other, concurrent therapies
In cats with hyphaema, topical therapy with a penetrating corticosteroid, such as prednisolone acetate, sometimes along with a mydriatic agent (eg, atropine) can be used to reduce complications such as anterior and posterior synechiae. 16 These medications should be used in addition to amlodipine, and only after establishing that corneal ulceration is not present. If hyphaema is noted, it is imperative to monitor intraocular pressure since glaucoma may develop. 16 Appropriate therapy to treat elevated intraocular pressure will be needed in cats that develop glaucoma. 50
Drugs that inhibit the renin-angiotensin-aldosterone system, such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers, have not proven effective enough to be a first-line anti-hypertensive treatment in cats with hypertensive ocular lesions.33,51 However, they may be required for cats with renal disease and proteinuria (Table 3). 45 Atenolol is useful for reducing heart rate in hyperthyroid cats, but the majority of hypertensive hyperthyroid cats will not achieve sufficient reduction in BP when it is used as a sole agent (Table 3). 48
In cats with hypertensive ocular lesions and where target BP cannot be achieved on amlodipine alone, the addition of an ACE inhibitor could be considered, as there is some evidence that this will provide a further reduction in BP. 52 In cats refractory to amlodipine, oral hydralazine can be used for long-term treatment of SHT (Table 3). 49 Doses start at 0.5 mg/kg q12h and are titrated up to 2 mg/kg q12h. 49
Prognosis
The ocular prognosis varies depending on the severity of the lesions at diagnosis and how quickly BP control can be achieved.
Lesions in eyes with advanced hypertensive disease may improve with antihypertensive treatment. However, there may be permanent sequelae that prevent the return of normal vision. 13 In eyes with retinal detachment, the duration of the detachment before diagnosis is influential, as recovery of function is optimal if detachment has been present for less than 1 week. 53 Maggio et al 13 observed that amlodipine treatment improved the morphological appearance of the fundus in four blind cats’ eyes with complete bullous retinal detachment, and these eyes regained vision. However, the prognosis for restoration of vision is guarded, as reattached retinas are often degenerate. 13 When vitreal haemorrhage resolves with BP control, the resultant traction on the vitreal clots can result in rhegmatogenous retinal detachment if a tear in the retina develops, leading to fluid accumulation within the retinal layers; an affected eye may never regain vision. 54 Preretinal, intraretinal and subretinal haemorrhages can resolve completely with BP control, but long-term consequences such as retinal degeneration can remain (Figure 15). In the case of subretinal haemorrhages, iron in the blood is involved in photoreceptor toxicity and clot retraction can irreversibly damage the photoreceptors.55-57
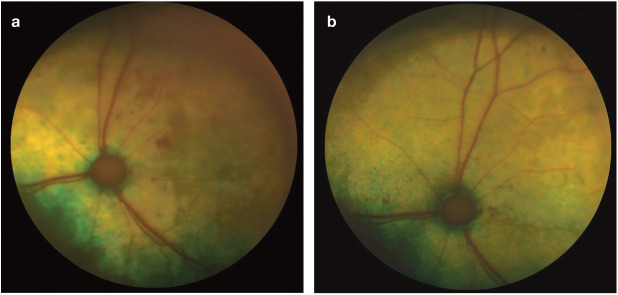
Figure 15.
(a) A 17-year-old domestic shorthair (DSH) cat with an SBP of 195 mmHg (same cat as in Figure 2). Vitreal haemorrhages were present in the right eye and the fundus could not be visualised. (b) Fundus of the right eye of the cat at reassessment following 2 months of treatment with amlodipine at 1.25 mg q24h. SBP = 162 mmHg. The vitreal haemorrhage has resolved and the fundus is visible. Note the scattered intraretinal haemorrhages, suspected aneurysmal dilations of retinal vessels, and retinal degeneration over much of the fundus. There was no menace response in this eye and it was considered blind
Early hypertensive ocular disease can be effectively treated, and apparently normal vision returned. 13 Small intraretinal haemorrhages can resolve within 1–2 months, leaving behind no permanent retinal scars (Figure 16). 55 Significant improvement in multifocal retinal oedema can be seen in just 2 weeks. 13 When partial retinal detachments are present, there can be improvement in the size and number of lesions within 1–2 months (Figures 17 and 18).
Figure 16.
(a) Fundus of the left eye of a 16-year-old DSH cat with an SBP of 174 mmHg. Note the generalised retinal oedema and intraretinal haemorrhages. (b) Same eye following 3 weeks of treatment with amlodipine at 0.625 mmHg q24h. SBP = 140 mmHg. The retinal oedema has improved and there are fewer intraretinal haemorrhages
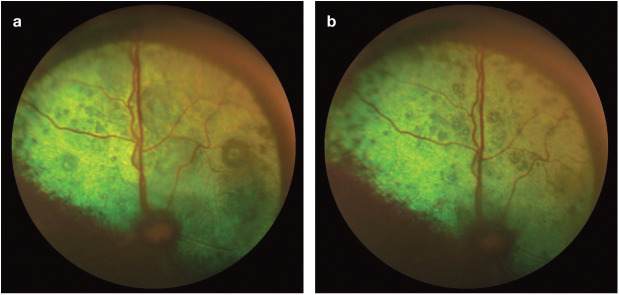
Figure 17.
(a) Fundus of the left eye of a 16-year-old DSH cat with an SBP of 185 mmHg. Note the multiple small bullous retinal detachments throughout the tapetal fundus. (b) Same eye following 6 weeks of amlodipine treatment at 0.625 mg q24h. SBP = 138 mmHg. The multiple bullous retinal detachments persist but are reduced in size
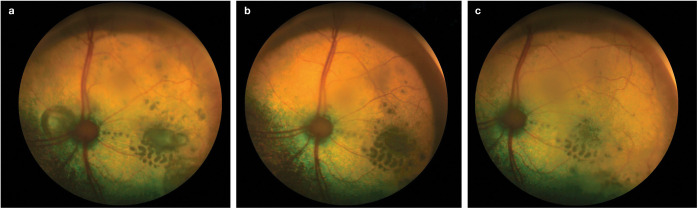
Figure 18.
(a) Fundus of the left eye of an 18-year-old DSH cat with an SBP of 180 mmHg. There is a large bullous retinal detachment medial to the optic nerve head and focal areas of retinal oedema lateral to the optic nerve head. Centrally, within the oedema, there is a serous retinal detachment. (b) Same eye following 4 weeks of amlodipine treatment at 1.25 mg q24h. SBP = 152 mmHg. The bullous detachment has resolved, and the serous retinal detachment has improved, leaving a hyporeflective area of retina. (c) Following 10 weeks of amlodipine treatment at 1.25 mg q24h, the SBP is 148 mmHg and the focal areas of retinal oedema have reduced in size
Monitoring
Cats with hypertensive ocular lesions that are overtly affecting vision should be rechecked within 24–72 h of first being diagnosed.4,37 Cats with more mild lesions should be rechecked 7–10 days after initial diagnosis. 37 Each recheck should include BP measurement and an ophthalmic examination including fundoscopy.
Once the patient is normotensive and the eyes are starting to recover from hypertensive ocular disease the patient should be rechecked at least every 3 months to include BP assessment and repeat fundic examinations. 37 In patients where BP is difficult to stabilise, or the hypertensive ocular disease is not improving as expected, more frequent checks will be required.
As discussed, since subclinical or early hypertensive fundic lesions respond better to antihypertensive medication than do advanced lesions, it is highly desirable to detect lesions before vision loss occurs.13,18 By the time the disease is sufficiently advanced to cause visual disturbance that the owner notices, the findings on fundic examination are usually severe (eg, retinal detachment and significant retinal haemorrhages), and it is unlikely these patients can have their vision restored, even with prompt, appropriate treatment.5,13,34 The most effective way to detect subclinical fundic lesions is to examine the fundus of all cats that require a BP measurement.
Routine BP monitoring is recommended at least annually in healthy senior cats (>7 years of age), and every 6–12 months in healthy geriatric cats (≥11 years). 37 All cats with known risk factors for the development of SHT, such as CKD, should have their BP measured and their fundus examined even more frequently. 37 If lesions consistent with hypertensive ocular disease are identified and elevated BP readings have been obtained, a diagnosis of SHT is confirmed, and white coat hypertension as a cause of the elevated BP readings is ruled out. In some patients, hypertensive lesions may be detected when BP readings are normal (see ‘Diagnostic dilemma’ box on page 40), highlighting the benefit of fundic examinations accompanying BP measurement. 18
Key Points
Ophthalmoscopic examination can be undertaken quickly with basic equipment. Proficiency in interpretation of whether or not the fundus is normal can be gained with experience.
Fundic examination findings can add weight to the finding of elevated BP values, and provide additional evidence that a cat has clinically significant SHT.
Subclinical and early hypertensive fundic lesions are more amenable to antihypertensive medication than advanced lesions.
Detection of subclinical fundic lesions requires that an ocular examination is performed in all cats at risk of developing such lesions, not just those that present with vision loss.
Acknowledgments
The binocular indirect ophthalmoscopy image in Table 1 was provided courtesy of Dr Craig Irving.
Footnotes
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Jepson RE. Feline systemic hypertension: classification and pathogenesis. J Feline Med Surg 2011; 13: 25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Syme H. Hypertension in small animal kidney disease. Vet Clin North Am Small Anim Pract 2011; 41: 63-89. [DOI] [PubMed] [Google Scholar]
- 3. Taylor SS, Sparkes AH, Briscoe K, et al. ISFM consensus guidelines on the diagnosis and management of hypertension in cats. J Feline Med Surg 2017; 19: 288-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Acierno MJ, Brown S, Coleman AE, et al. ACVIM consensus statement: Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med. Epub ahead of print 24 October 2018. DOI: 10.1111/jvim.15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Littman MP. Spontaneous systemic hypertension in 24 cats. J Vet Intern Med 1994; 8: 79-86. [DOI] [PubMed] [Google Scholar]
- 6. Bill A, Nilsson SF. Control of ocular blood flow. J Cardiovasc Pharmacol 1985; 7 Suppl 3: S96-102. [DOI] [PubMed] [Google Scholar]
- 7. Hayreh SS. Systemic arterial blood pressure and the eye. Eye 1996; 10: 5-28. [DOI] [PubMed] [Google Scholar]
- 8. Kroger RHH, Biehlmaier O. Space-saving advantage of an inverted retina. Vision Res 2009; 49: 2318-2321. [DOI] [PubMed] [Google Scholar]
- 9. Stone J, Maslim J, Valter-Kocsi K, et al. Mechanisms of photoreceptor death and survival in mammalian retina. Prog Retin Eye Res 1999; 18: 689-735. [DOI] [PubMed] [Google Scholar]
- 10. Linsenmeier RA, Padnick-Silver L. Metabolic dependence of photoreceptors on the choroid in the normal and detached retina. Invest Ophthalmol Vis Sci 2000; 41: 3117-3123. [PubMed] [Google Scholar]
- 11. Garner A, Ashton N, Tripathi R, et al. Pathogenesis of hypertensive retinopathy. An experimental study in the monkey. Brit J Ophthalmol 1975; 59: 3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayreh SS, Servais GE, Virdi PS. Fundus lesions in malignant hypertension. VI: hypertensive choroidopathy. Ophthalmology 1986; 93: 1383-1400. [DOI] [PubMed] [Google Scholar]
- 13. Maggio F, DeFrancesco TC, Atkins CE, et al. Ocular lesions associated with systemic hypertension in cats: 69 cases (1985-1998). J Am Vet Med Assoc 2000; 217: 695-702. [DOI] [PubMed] [Google Scholar]
- 14. Sansom J, Barnett KC, Dunn KA, et al. Ocular disease associated with hypertension in 16 cats. J Small Anim Pract 1994; 35: 604-611. [Google Scholar]
- 15. Syme HM, Barber PJ, Markwell PJ, et al. Prevalence of systolic hypertension in cats with chronic renal failure at initial evaluation. J Am Vet Med Assoc 2002; 220: 1799-1804. [DOI] [PubMed] [Google Scholar]
- 16. Telle RM, Betbeze C. Hyphema: considerations in the small animal patient. Top Companion Anim Med 2015; 30: 97-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Norsworthy GD, de Faria VP. Retrobulbar thrombus in a cat with systemic hypertension. J Feline Med Surg 2010; 13: 144-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carter JM, Irving AC, Bridges JP, et al. The prevalence of ocular lesions associated with hypertension in a population of geriatric cats in Auckland, New Zealand. NZ Vet J 2014; 62: 21-29. [DOI] [PubMed] [Google Scholar]
- 19. Crispin SM, Mould JR. Systemic hypertensive disease and the feline fundus. Vet Ophthalmol 2001; 4: 131-140. [DOI] [PubMed] [Google Scholar]
- 20. La Croix NC. Ocular manifestations of systemic disease in cats. Clin Tech Small Anim Pract 2005; 20: 121-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ofri R. Retina. In: Maggs D, Millar P, Ofri R. (eds). Slatter’s fundamentals of veterinary ophthalmology. 4th ed. St Louis, MO: Elsevier, 2008, p 302. [Google Scholar]
- 22. Panton RW, Goldberg MF, Farber MD. Retinal arterial macroaneurysms: risk factors and natural history. Brit J Ophthalmol 1990; 74: 595-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gelatt KN, van der Woerdt A, Ketring KL, et al. Enrofloxacin-associated retinal degeneration in cats. Vet Ophthalmol 2001; 4: 99-106. [DOI] [PubMed] [Google Scholar]
- 24. Giuliano EA. Feline retinal degeneration: clinical experience and new findings (1994-1997). J Am Anim Hosp Assoc 1999; 35: 511-514. [DOI] [PubMed] [Google Scholar]
- 25. Stiles J, Kimmitt B. Eye examination in the cat. J Feline Med Surg 2016; 18: 702-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crispin S. Notes on veterinary ophthalmology. Oxford: Blackwell Publishing, 2005, p 180. [Google Scholar]
- 27. Maggs D. Ocular pharmacology and therapeutics. In: Maggs D, Millar P, Ofri R. (eds). Slatter’s fundamentals of veterinary ophthalmology. 4th ed. St Louis, MO: Elsevier, 2008, p 53. [Google Scholar]
- 28. Maggs D. Basic diagnostic techniques. In: Maggs D, Millar P, Ofri R. (eds). Slatter’s fundamentals of veterinary ophthalmology. 4th ed. St Louis, MO: Elsevier, 2008, p 89. [Google Scholar]
- 29. Crispin S. Examination of the feline eye and adnexa. Eur J Comp Anim Pract 2007; 17: 1-15. [Google Scholar]
- 30. Balland O, Russo A, Isard PF, et al. Assessment of a smartphone-based camera for fundus imaging in animals. Vet Ophthalmol 2017; 20: 89-94. [DOI] [PubMed] [Google Scholar]
- 31. Haddock LJ, Kim DY, Mukai S. Simple, inexpensive technique for high-quality smartphone fundus photography in human and animal eyes. J Ophthalmol 2013; 518479. DOI: 10.1155/2013/518479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kanemaki N, Inaniwa M, Terakado K, et al. Fundus photography with a smartphone in indirect ophthalmoscopy in dogs and cats. Vet Ophthalmol 2017; 20: 280-284. [DOI] [PubMed] [Google Scholar]
- 33. Elliott J, Barber PJ, Syme HM, et al. Feline hypertension: clinical findings and response to antihypertensive treatment in 30 cases. J Small Anim Pract 2001; 42: 122-129. [DOI] [PubMed] [Google Scholar]
- 34. Sansom J, Rogers K, Wood JL. Blood pressure assessment in healthy cats and cats with hypertensive retinopathy. Am J Vet Res 2004; 65: 245-252. [DOI] [PubMed] [Google Scholar]
- 35. Belew AM, Bartlett T, Brown SA. Evaluation of the white-coat effect in cats. J Vet Intern Med 1999; 13: 134-142. [DOI] [PubMed] [Google Scholar]
- 36. Bangash F, Agarwal R. Masked hypertension and white-coat hypertension in chronic kidney disease: a meta-analy-sis. Clin J Am Soc Nephrol 2009; 4: 656-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taylor SS, Sparkes AH, Briscoe K, et al. ISFM consensus guidelines on the diagnosis and management of hypertension in cats. J Feline Med Surg 2017; 3: 288-303. Available at: guidelines.jfms.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Slingerland LI, Robben JH, Schaafsma I, et al. Response of cats to familiar and unfamiliar human contact using continuous direct arterial blood pressure measurement. Res Vet Sci 2008; 85: 575-582. [DOI] [PubMed] [Google Scholar]
- 39. Daniel G, Mahony O, Markovich J, et al. Clinical findings, diagnostics and outcome in 33 cats with adrenal neoplasia (2002-2013). J Feline Med Surg 2016; 18: 77-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Elliott J, Fletcher M, Syme HM. Idiopathic feline hypertension: epidemiological study [abstract]. J Vet Intern Med 2003; 17: 754. [Google Scholar]
- 41. Lo AJ, Holt DE, Brown DC, et al. Treatment of aldosterone-secreting adrenocortical tumors in cats by unilateral adrenalec-tomy: 10 cases (2002-2012). J Vet Intern Med 2014; 28: 137-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Helms SR. Treatment of feline hypertension with transdermal amlodipine: a pilot study. J Am Anim Hosp Assoc 2007; 43: 149-156. [DOI] [PubMed] [Google Scholar]
- 43. Snyder PS. Amlodipine: a randomised, blinded clinical trial in 9 cats with systemic hypertension. J Vet Intern Med 1998; 12: 157-162. [DOI] [PubMed] [Google Scholar]
- 44. Bijsmans ES, Doig M, Jepson RE, et al. Factors influencing the relationship between the dose of amlodipine required for blood pressure control and change in blood pressure in hypertensive cats. J Vet Intern Med 2016; 30: 1630-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Littman MP. Protein-losing nephropathy in small animals. Vet Clin North Am Small Anim Pract 2011; 41: 31-62. [DOI] [PubMed] [Google Scholar]
- 46. Jenkins TL, Coleman AE, Schmiedt CW, et al. Attenuation of the pressor response to exogenous angiotensin by angiotensin receptor blockers and benazepril hydrochloride in clinically normal cats. Am J Vet Res 2015; 76: 807-813. [DOI] [PubMed] [Google Scholar]
- 47. Sent U, Gossl R, Elliott J, et al. Comparison of efficacy of long-term oral treatment with telmisartan and benazepril in cats with chronic kidney disease. J Vet Intern Med 2015; 29: 1479-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Henik RA, Stepien RL, Wenholz LJ, et al. Efficacy of atenolol as a single antihypertensive agent in hyperthyroid cats. J Feline Med Surg 2008; 10: 577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smith FWK, Jr. Hypertension in 2011: diagnosis and treatment. Proceedings of the Western Veterinary Conference; 2011 Feb 20-24; Las Vegas, USA. Accessed from www.vin.com. [Google Scholar]
- 50. McLellan GJ, Teixeira LBC. Feline glaucoma. Vet Clin North Am Small Anim Pract 2015; 45: 1307-1333. [DOI] [PubMed] [Google Scholar]
- 51. Steele J, Henik R, Stepien R, et al. Effects of angiotensin-converting enzyme inhibition on plasma aldosterone concentration, plasma renin activity, and blood pressure in spontaneously hypertensive cats with chronic renal disease. Vet Ther 2002; 3: 157-166. [PubMed] [Google Scholar]
- 52. Elliott J, Fletcher MG, Souttar K, et al. Effect of concomitant amlodipine and benazepril therapy in the management of feline hypertension [abstract]. J Vet Intern Med 2004; 18: 788. [Google Scholar]
- 53. Anderson DH, Guerin CJ, Erikson PA, et al. Morphological recovery in the reattached retina. Invest Ophthalmol Vis Sci 1986; 27: 168-183. [PubMed] [Google Scholar]
- 54. Turner SM. Hypertensive retinopathy. In: Nind F. (ed). Saunders solutions in veterinary practice: small animal ophthalmology E-book: Elsevier, 2008, p 306. [Google Scholar]
- 55. Gillies A, Lahav M. Absorption of retinal and subretinal hemorrhages. Ann Ophthalmol 1983; 15: 1068-1074. [PubMed] [Google Scholar]
- 56. Glatt H, Machemer R. Experimental subretinal hemorrhage in rabbits. Am J Ophthalmol 1982; 94: 762-763. [DOI] [PubMed] [Google Scholar]
- 57. Toth CA, Morse LS, Hjelmeland LM, et al. Fibrin directs early retinal damage after experimental subretinal hemorrhage. Arch Ophthalmol 1991; 723-729. [DOI] [PubMed] [Google Scholar]