Abstract
Practical relevance:
New technologies capable of sequencing the genetic material in any given biological sample, combined with computer-based algorithms for sequence assembly and analysis, have revolutionised infectious disease research. The rate at which novel viruses are being discovered now exceeds our understanding of their clinical relevance. Novel viruses may contribute to diseases that are major causes of feline morbidity and mortality, including cancer and chronic kidney disease. The identification of new viral pathogens raises the prospect of not only improved patient outcomes through specific treatment but even disease prevention through viral control measures.
Clinical challenges:
It can be difficult to determine the role of a novel virus in disease development. Disease may be an occasional outcome, often years after infection. A high prevalence of infection in the general population can make disease associations harder to identify and almost impossible to rule out. Host cofactors such as immune dysfunction, genetic background or coinfections may be required for manifestation of disease, and one virus species may be linked to a range of pathological sequelae. Establishing causality relies on evaluating accumulating evidence from multiple investigations, which is often hard to access by practitioners.
Global importance:
The worldwide distribution of gammaherpesvirus and morbillivirus infections in domestic cats underlines the potential of these viruses to negatively impact feline health and welfare globally.
Evidence base:
This review relies on grade Ia–III evidence.
Keywords: Virus, morbillivirus, gammaherpesvirus, herpes
Gammaherpesviruses – current understanding and pathogenic potential
The herpesvirus family (Herpesviridae) is a large group of double-stranded DNA viruses comprising three subfamilies, the Alpha-, Beta- and Gammaherpesvirinae. Gammaherpesviruses (GHVs) have co-evolved with a diverse range of mammals including humans and other primates, ruminants, horses, sun bears and sea lions.

Until recently, domestic cats were identified as the natural host for a single herpesvirus, the alphaherpesvirus feline herpesvirus 1 (FHV1), a common cause of feline ocular and upper respiratory tract disease. 1 While a bovine GHV, bovine herpesvirus 4 (BHV4), has been suggested to cause disease in cats, substantiating evidence is not yet available. Experimental infection of cats with BHV4 did not result in disease 2 and molecular epidemiological studies reported divergent results: 26.9% of 104 blood samples from Michigan were found to be BHV4 positive in one study, 3 whereas none of 101 cats from California, Colorado and Florida tested PCR positive in a more recent study. 4
Virus discovery
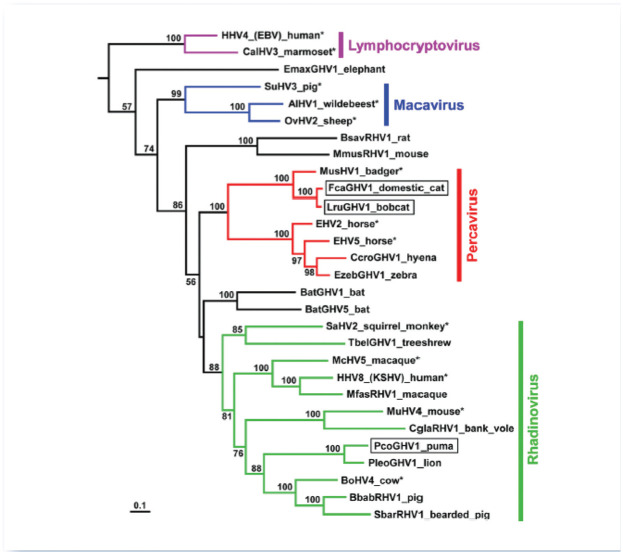
In 2014, an international collaboration identified the first GHV known to infect domestic cats, Felis catus gammaherpesvirus 1 (FcaGHV1; proposed species Felid gammaherpesvirus 1).5,6 The impetus for this targeted virus discovery programme was the clinical observation that cats develop the types of cancer that, in humans, are caused by GHVs. Specifically, many lymphomas arising in immunodeficient patients are causally linked to one or both of the GHVs that infect humans, namely Epstein-Barr virus (EBV) and Kaposi’s sarcoma associated herpesvirus. 7 Given that feline immunodeficiency virus (FIV)-infected cats have an increased risk of developing similar lymphomas, and that FIV infection alone is rarely directly lymphomagenic, the existence of a feline GHV with oncogenic potential was proposed.8–10 PCR assays that detect broadly conserved herpesvirus sequences were used to probe DNA extracted from domestic cats and two other felids, resulting in the discovery of three novel viruses: FcaGHV1 in domestic cats, Lynx rufus GHV1 (LruGHV1) in bobcats and Puma concolor GHV1 (PcoGHV1) in pumas (Figure 1). 6
Figure 1.
Maximum-likelihood phylogenetic analysis of gammaherpesviruses using concatenated DNApol and gB amino alignments. From Troyer et al. 6
© American Society for Microbiology, Journal of Virology 2014, 88: 3914–3924. DOI: 10.1128/JVI.03405-13
Epidemiology
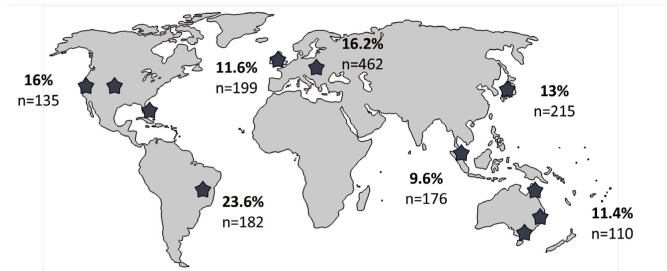
FcaGHV1 infection is widely endemic (Figure 2). A virus-specific qPCR targeting the glyco-protein B gene of FcaGHV1 DNA in blood has been used for most epidemiological studies to date. 5 Because the detection of viral DNA does not differentiate between virus-infected cells, virions or free DNA in plasma, the term DNAemia, rather than viraemia, is used. The prevalence of FcaGHV1 DNAemia is 9.6–23.6% in cats from Australia, the USA, Europe, Singapore, Japan and Brazil.5,6,11–14
Figure 2.
FcaGHV1 molecular epidemiology. Stars indicate regions from which published studies of molecular prevalence in feline whole blood are available. The percentages of samples positive for FcaGHV1 DNA using virus-specific PCRs are indicated along with the number of cats tested5,6,11–14
Molecular studies do not detect all FcaGHV1 infections. A recent serological study suggests that the true FcaGHV1 infection rate could be at least double that indicated by molecular studies. 15 Age, sex, neuter status, health status and infectious cofactors have been identified as risk factors for FcaGHV1 DNAemia, with some regional variations.5,6,11–14 Adult male cats are most likely to be infected, and FcaGHV1 DNA-emia is rare in cats under 2 years of age. Among coinfections, FIV infection increases the chance of FcaGHV1 DNA detection by five to six times, whereas detection of haemoplasma DNA (Mycoplasma haemofelis, ‘Candidatus Mycoplasma haemominutum’) is associated with a 19-fold increased risk of FcaGHV1 DNA detection compared with age- and sex-matched controls.5,12 This epidemiological picture supports horizontal transmission during territorial aggression as one possible route of FcaGHV1 infection. Recent data from a study of oronasal swabs and tissues from shelter-housed and client-owned cats demonstrate that cats can be infected with FcaGHV1 from 2 months of age and suggest that most adult cats are persistently infected with FcaGHV1. 16 The potential for FcaGHV1 to be transmitted between cats via oronasal secretions is also demonstrated in this study.
Pathogenesis
It is not yet known whether FcaGHV1 has any pathogenic role in cats. Persistent infection is a common feature of herpesviruses and, in other species, most GHV infections quickly become latent in their natural host. However, comparative evidence suggests that while GHV infections typically remain subclinical, in certain circumstances, often after many years of infection, GHVs can cause severe diseases that are frequently fatal. For example, EBV infects over 90% of adult humans and is usually innocuous. Occasionally, however, EBV causes lymphomas, carcinomas and other cancers. Because EBV infection is common, these cancers together comprise 2% of the global cancer burden. 17 Risk factors including loss of T cell immunity and genetic predisposition are defined for some, but not all, EBV-associated malignancies. EBV infection is also linked to several respiratory, neurological, dermatological and other conditions, where the role of the virus, if any, remains to be defined. 18
Among veterinary species, malignant catarrhal fever is recognised as an acute fatal lymphoproliferative disease where one of several ruminant GHVs infects a non-adapted, but susceptible host. As with EBV, most cattle that are infected by GHVs do not develop clinical disease and the factors that result in the development of disease are poorly understood. Horses harbour two endemic GHVs, equine herpesvirus (EHV)2 and EHV5. Pharyngitis, lymphadenopathy and lymphocytosis in foals have been associated with EHV2 infection and are suggested to have an immune-mediated pathogenesis. EHV5 has been linked to equine multinodular pulmonary fibrosis and a role for GHVs in some cases of idiopathic pulmonary fibrosis in humans is postulated. 19
If FcaGHV1 infection is pathogenic, it is likely that disease would be only a rare outcome of chronic infection, with most infected animals remaining asymptomatic. On the other hand, given how widely distributed FcaGHV1 is, the total number of potential disease-affected animals could be sizeable. Deciphering the impact of FcaGHV1 in cats will require multiple lines of investigation. In a retrospective study of over 200 cats from Australia and Singapore, animals infected with FcaGHV1 were 2.8 times more likely to be classified as sick than healthy on physical examination by a veterinarian blinded to the cat’s infection status, lending indirect support for a pathological role for FcaGHV1. 5 The relationship between FIV and FcaGHV1 is particularly interesting; independent studies report significantly higher FcaGHV1 DNAemia in FIV-infected cats compared with matched controls (Figure 3).5,11 However, neither ciclosporin treatment nor progressive feline leukaemia virus infection had an effect on FcaGHV1 DNAemia, suggesting that the relationship between FIV and FcaGHV1 may not be solely a consequence of immunodeficiency. 20
Figure 3.

A significant proportion (40–55%) of FIV-infected cats are FcaGHV1 DNAemic.5,11,13 Big Kev, an adult male entire domestic shorthair rescued from a shelter in Sydney, Australia was found to be coinfected with FIV and FcaGHV1. After neutering and rehoming he remained disease-free for 3.5 years. Sadly, he went on to develop high grade B cell lymphoma presenting as a large transmural jejunal mass with splenic, renal and lymph node (cervical, thoracic, mesenteric) involvement. While lymphoma is a common presentation in cats, the cause of most lymphomas is unknown. The role of FcaGHV1 as a potential copathogen in FIV infection requires further investigation. Courtesy of Dr Jelena Vukcevic
A recent study found no association between the detection of FcaGHV1 and the development of high grade or other clinically aggressive lymphomas. 21 However, survival time from diagnosis was significantly shorter in cats with FcaGHV1 DNAemia compared with FcaGHV1-negative cats. 21 A large prospective investigation would assist in understanding whether FcaGHV1 DNAemia could be a clinically useful negative prognostic indicator for cats with lymphoma.
Diagnosis, prevention and zoonotic potential
Currently, diagnosis of FcaGHV1 infection is limited to a small number of research laboratories. Should the diagnosis of FcaGHV1 be found to have prognostic significance then it is likely that commercial tests would become available. FcaGHV1 is not known or suspected to infect humans. Most GHVs are highly host-specific. However, limited transmission of GHVs between felids is possible; infection of critically endangered Tsushima leopard cats with FcaGHV1 has recently been identified in Japan 14 and LruGHV1 infects bobcats and pumas. 6
Morbilliviruses – current understanding and pathogenic potential
Feline morbillivirus (FeMV) was named the seventh species in the genus Morbillivirus, family Paramyxoviridae, by the International Committee on the Taxonomy of Viruses in 2016. 22 Members of the Morbillivirus genus are important pathogens of humans and animals, causing significant morbidity and mortality. The other recognised morbilliviruses are measles virus, canine distemper virus, the now eradicated rinderpest virus, peste des petits ruminants virus, phocine distemper virus and cetacean morbillivirus. 23 Morbilliviruses are negative-sense, single-stranded, non-segmented RNA viruses. 23
Virus discovery
FeMV was first reported in domestic cats in Hong Kong and China in 2012. 24 In the 7 years since its discovery, FeMV has been detected in Japan, 25 Europe (Germany, 26 Italy, 27 Turkey, 28 UK 29 ) and the Americas (USA, 30 Brazil 31 ). Despite the apparent widespread distribution of this virus, whether or not FeMV causes disease in cats remains unclear.
Epidemiology
The majority of the FeMV literature focuses on its prevalence in domestic cat populations around the world. Most investigators have used RT-PCR to detect FeMV in urine samples, using primers targeting the FeMV L gene or consensus pan-paramyxovirus primers.24–37 A small number of investigators have performed RT-PCR for FeMV on kidney tissue, among other samples and tissues.28,35 Of note, few investigators have successfully isolated the virus from clinical samples, and thus the significance of a positive RT-PCR result has been debated. False-positive RT-PCR results are possible, and there is the additional possibility of amplifying non-viable nucleic acid. Nonetheless, current evidence suggests that FeMV has a global distribution, and that the presence of FeMV RNA in urine is not uncommon in domestic cats.

The prevalence of FeMV, as determined by RT-PCR in cat urine and kidney tissues, is summarised in Table 1. Although these studies vary considerably in size and the demographic of the cats enrolled, they document that FeMV sequences can be found in both healthy and sick cats, with a prevalence ranging from 3% 30 to 52.9%. 31 Interestingly, studies that included multi-cat environments had the highest rate of positive RT-PCR tests from urine: 22/72 (30.6%) cats in a colony in Italy, 36 and 9/17 (52.9%) cats that had contact with a colony of 23 stray cats in Brazil. 31 These findings suggest that close contact may be necessary for FeMV to be transmitted between cats. However, the mode of FeMV transmission is currently unknown.
Table 1.
Molecular prevalence of FeMV sequences in cat urine or kidney tissues in published studies
| Region/country | Prevalence (by RT-PCR) |
|---|---|
| Asia | |
| Hong Kong/China 24 | 53/427 = 12.4% |
| Japan25,32–35 | 53/383 = 13.8% |
| Europe | |
| Germany 26 | 0/86 = 0%, healthy control cats 5/120 = 4.2%, diseased cats |
| Turkey 28 | 6/110 = 5.5% overall 3/15 kidney samples of deceased cats 3/68 unhealthy cat urine samples |
| Italy 36 | 16/156 = 10.3%, stray cats 22/72 = 30.6%, colony cats |
| UK 29 | 1/16 = 6.3%, azotaemic CKD cats 4/24 = 16.7%, non-azotaemic cats |
| Americas | |
| USA 30 | 10/327 = 3% |
| Brazil 31 | 9/17 = 52.9%, healthy cats in a multi-cat household 3/35 = 8.6%, ‘diseased’ cats |
FeMV = feline morbillivirus, CKD = chronic kidney disease
Studies evaluating FeMV seroprevalence are summarised in Table 2. Published seroprevalence varies widely depending on the population tested and the assay used. Various serological methods have been used, but virus neutralisation assays, the gold standard for diagnostic serology, have not been developed. Therefore, it is important to view percentage seropositivity data with caution, particularly since the range is wide (Table 2). Combining the results of urine RT-PCR and serology identifies four groups of cats: those that are RT-PCR negative and seronegative, RT-PCR negative but seropositive, RT-PCR positive and seronegative, and both RT-PCR positive and seropositive (see box below).
Table 2.
Seroprevalence of anti-FeMV antibodies in cats in published studies
| Country | Seropositivity in RT-PCR-positive cats | Seropositivity in RT-PCR-negative cats | Overall seroprevalence |
|---|---|---|---|
| Hong Kong/ China 24 | 54/56 = 96.4% | 78/401 =19.5% | 132/457 = 28.9% |
| Japan31,34 | 15/25 = 60% | 9/88 = 10.2% | 24/113 = 21.2% |
| Italy 36 | 18/24 = 75% | 16/38 = 42.1 % | 34/62 = 54.8% |
| UK 29 * | 5/5 = 100% † | 12/26 = 46.2% ‡ | 46/69 = 66.7% ‡ |
FeMV = feline morbillivirus
Not all cats had both urine RT-PCR and serology performed. Seropositive includes all results reported as positive (strong positive, positive, weak positive) in this study. Serology results reported as ‘high background’ are excluded
Includes 1/1 azotaemic and 4/4 non-azotaemic cats
Includes cats that did not have RT-PCR performed
Persistence of FeMV RT-PCR positivity for up to 15 months has been documented in a healthy pet cat. 30 Chronic infection is a feature of FeMV that warrants further investigation. Persistent morbillivirus infection is recognised in other species; for example, measles virus causing subacute sclerosing panencephalitis and measles inclusion body encephalitis in humans. 37
Pathogenesis
Morbilliviruses are highly lymphotropic and immunosuppressive, with infection of epithelial cells occurring in the later stages of disease.38–42 The other recognised morbilliviruses spread systemically and clinical signs can manifest in the skin, respiratory and gastrointestinal tracts and nervous system.38–42 Whether or not FeMV results in a similar spectrum of disease remains to be determined.
A single study evaluating FeMV cell tropism in vitro documented viral replication in cat fibroblasts, lymphoid cells and glial cells. 43 No other morbilliviruses have been linked to kidney disease (see box on page 8), although they can infect epithelial cells of the urogenital tract. In addition to the hypothesis that FeMV may cause kidney disease, one study has suggested a potential association with liver disease, 28 although strong evidence is lacking. It would be surprising if FeMV caused significant acute mortality in domestic cats, since it appears to be a prevalent infection, yet few cat deaths go unexplained.
Diagnosis, prevention and zoonotic potential
Until there is more definitive evidence of a link between FeMV and feline disease, the development of diagnostic tools beyond the research setting may be premature. There is a need to unite molecular virology and veterinary medicine to attain a comprehensive understanding of the basic biology of the virus, the lack of which represents a major deficit in the field. Future studies focusing on acute and chronic pathogenesis in a natural animal model of disease will help elucidate the route of infection, assess modes of transmission and characterise the immune responses to FeMV.
Evidence from other animal species suggests that FeMV is extremely unlikely to infect humans. The potential for infection of other felids is yet to be determined.
Role of practising veterinarians
The range of feline diseases that are linked to known or novel infectious agents is expanding and, with increased availability of advanced molecular techniques, more novel infectious agents of cats will be identified. The role of practising veterinarians in progressing clinical research, as experts at diagnosing disease and collecting appropriate samples, should not be underestimated – veterinary clinicians together with pathologists are as important a part of the research team as laboratory-based investigators. While the pathogenic potential of novel viruses can be difficult to determine, recognition of these agents may offer the opportunity to improve the diagnosis, treatment and prevention of significant causes of suffering in cats.

Key Points
FcaGHV1 was first reported in 2014 and FeMV in 2012. Evidence to date suggests that both viruses commonly infect domestic cats worldwide.
An understanding of the pathogenic potential of FcaGHV1 and FeMV is important for feline health and welfare globally.
Disease prevention through vaccination and improved patient outcomes from treatments targeting any new viruses are key goals of novel pathogen discovery.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Gould D. Feline herpesvirus-1: ocular manifestations, diagnosis and treatment options. J Feline Med Surg 2011; 13: 333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kruger JM, Osborne CA, Goyal SM, et al. Clinicopathologic and pathologic findings of herpesvirus-induced urinary tract infection in conventionally reared cats. Am J Vet Res 1990; 51: 1649–1655. [PubMed] [Google Scholar]
- 3. Kruger JM, Venta PJ, Swenson CL, et al. Prevalence of bovine herpesvirus-4 infection in cats in Central Michigan. J Vet Intern Med 2000; 14: 593–597. [DOI] [PubMed] [Google Scholar]
- 4. Chiu E, Troyer RM, Lappin MR, et al. Bovine herpesvirus 4 DNA is not detected in free-ranging domestic cats from California, Colorado or Florida. J Feline Med Surg 2015; 19: 235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beatty JA, Troyer RM, Carver S, et al. Fells catus gammaher-pesvirus 1; a widely endemic potential pathogen of domestic cats. Virology 2014; 460: 100–107. [DOI] [PubMed] [Google Scholar]
- 6. Troyer RM, Beatty JA, Stutzman-Rodriguez KR, et al. Novel gammaherpesviruses in north American domestic cats, bobcats, and pumas: identification, prevalence, and risk factors. J Virol 2014; 88: 3914–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cesarman E. Gammaherpesvirus and lymphoproliferative disorders in immunocompromised patients. Cancer Lett 2011; 305: 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shelton GH, Grant CK, Cotter SM, et al. Feline immunodeficiency virus and feline leukemia virus infections and their relationships to lymphoid malignancies in cats: a retrospective study (1968-1988). J Acquir Immune Defic Syndr 1990; 3: 623–630. [PubMed] [Google Scholar]
- 9. Beatty J, Terry A, MacDonald J, et al. Feline immunodeficiency virus integration in B-cell lymphoma identifies a candidate tumor suppressor gene on human chromosome 15q15. Cancer Res 2002; 62: 7175–7180. [PubMed] [Google Scholar]
- 10. Beatty JA, Troyer RM, Brewster C, et al. Feline immunodeficiency virus (FlV)-associated lymphoma. Is a gammaherpesvirus involved? Proceedings of the 2nd International Society for Companion Animal Infectious Diseases symposium [abstract 03]. San Francisco, CA, USA: ISCAID, 2012, p 27. [Google Scholar]
- 11. Ertl R, Korb M, Langbein-Detsch I, et al. Prevalence and risk factors of gammaherpesvirus infection in domestic cats in Central Europe. Virol J 2015; 12: 146. DOI: 10.1186/s12985-015-0381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McLuckie A, Tasker S, Dhand NK, et al. High prevalence of Fells catus gammaherpesvirus 1 infection in haemoplasma-infected cats supports co-transmission. Vet J 2016; 214: 117–121. [DOI] [PubMed] [Google Scholar]
- 13. Kurissio JK, Rodrigues MV, Taniwaki SA, et al. Fells catus gammaherpesvirus 1 (FcaGHV1) and coinfections with feline viral pathogens in domestic cats in Brazil. Ciencia Rural 2018; 48. DOI: 10.1590/0103-8478cr20170480. [DOI] [Google Scholar]
- 14. Makundi I, Koshida Y, Endo Y, et al. Identification of Fells catus Gammaherpesvirus 1 in Tsushima Leopard Cats (Prionallurus bengalensls euptllurus) on Tsushima Island, Japan. Viruses 2018; 10: pii E378. DOI: 10.3390/v10070378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stutzman-Rodriguez K, Rovnak J, VandeWoude S, et al. Domestic cats seropositive for Fells catus gammaherpesvirus 1 are often qPCR negative. Virology 2016; 498: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tse T, Pesavento P, Oates A, et al. Detection of Fells catus gammaherpesvirus1 in DNA in oronasal swabs and tissues from domestic cats. Proceedings of the ISCAID/IFRRS symposium [poster P-15]. 2018 Sept 30 to Oct 3; Portland, Oregon, USA. ISCAID, 2018. [Google Scholar]
- 17. Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006; 118: 3030–3044. [DOI] [PubMed] [Google Scholar]
- 18. Kutok JL, Wang F. Spectrum of Epstein-Barr virus-associated diseases. Annu Rev Pathol Mech 2006; 1: 375–404. [DOI] [PubMed] [Google Scholar]
- 19. Williams KJ. Gammaherpesviruses and pulmonary fibrosis: evidence from humans, horses, and rodents. Vet Pathol 2014; 51: 372–384. [DOI] [PubMed] [Google Scholar]
- 20. McLuckie AJ, Barrs VR, Wilson B, et al. Fells catus gamma-herpesvirus 1 DNAemia in whole blood from therapeutically immunosuppressed or retrovirus-infected cats. Vet Sci 2017; 4: pii, E16. DOI: 10.3390/vetsci4010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McLuckie AJ, Barrs VR, Lindsay S, et al. Molecular diagnosis of Fells catus gammaherpesvirus 1 (FcaGHV1) infection in cats of known retrovirus status with and without lymphoma. Viruses 2018; 10: pii, E128. DOI: 10.3390/v10030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Afonso CL, Amarasinghe GK, Banyai K, et al. Taxonomy of the order Mononegavirales: update 2016. Arch Virol 2016; 161: 2351–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nambulli S, Sharp CR, Acciardo AS, et al. Mapping the evolutionary trajectories of morbilliviruses: what, where and whither. Curr Opin Virol 2016; 16: 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Woo PC, Lau SK, Wong BH, et al. Feline morbillivirus, a previously undescribed paramyxovirus associated with tubulo-interstitial nephritis in domestic cats. Proc Natl Acad Sci USA 2012; 109: 5435–5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Furuya T, Sassa Y, Omatsu T, et al. Existence of feline morbil-livirus infection in Japanese cat populations. Arch Virol 2014; 159: 371–373. [DOI] [PubMed] [Google Scholar]
- 26. Sieg M, Heenemann K, Ruckner A, et al. Discovery of new feline paramyxoviruses in domestic cats with chronic kidney disease. Virus Genes 2015; 51: 294–297. [DOI] [PubMed] [Google Scholar]
- 27. Lorusso A, Di Tommaso M, Di Felice E, et al. First report of feline morbillivirus in Europe. Vet Ital 2015; 51: 235–237. [DOI] [PubMed] [Google Scholar]
- 28. Yilmaz H, Tekelioglu BK, Gurel A, et al. Frequency, clinicopatho-logical features and phylogenetic analysis of feline morbillivirus in cats in Istanbul, Turkey. J Feline Med Surg 2017; 19: 1206–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCallum KE, Stubbs S, Hope N, et al. Detection and seropreva-lence of morbillivirus and other paramyxoviruses in geriatric cats with and without evidence of azotemic chronic kidney disease. J Vet Intern Med 2018; 32: 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sharp CR, Nambulli S, Acciardo AS, et al. Chronic infection of domestic cats with feline morbillivirus, United States. Emerg Infect Dis 2016; 22: 760–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Darold GM, Alfieri AA, Muraro LS, et al. First report of feline morbillivirus in South America. Arch Virol 2017; 162: 469–475. [DOI] [PubMed] [Google Scholar]
- 32. Sakaguchi S, Nakagawa S, Yoshikawa R, et al. Genetic diversity of feline morbilliviruses isolated in Japan. J Gen Virol 2014; 95: 1464–1468. [DOI] [PubMed] [Google Scholar]
- 33. Park ES, Suzuki M, Kimura M, et al. Identification of a natural recombination in the F and H genes of feline morbillivirus. Virology 2014; 468: 524–531. [DOI] [PubMed] [Google Scholar]
- 34. Furuya T, Wachi A, Sassa Y, et al. Quantitative PCR detection of feline morbillivirus in cat urine samples. J Vet Med Sci 2016; 77: 1701–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Park ES, Suzuki M, Kimura M, et al. Epidemiological and pathological study of feline morbillivirus infection in domestic cats in Japan. BMC Vet Res 2016; 12: 228. DOI: 10.1186/s12917-016-0853-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De Luca E, Crisi PE, Febo E, et al. Feline morbillivirus infection in domestic cats in Italy: epidemiological and pathological aspects. Proceedings of the European College of Veterinary Internal Medicine companion animal congress. St Julian’s, Malta: Veterinary Information Network, 2017, p 301. [Google Scholar]
- 37. Rima BK, Duprex WP. Molecular mechanisms of measles virus persistence. Virus Res 2005; 111: 132–147. [DOI] [PubMed] [Google Scholar]
- 38. Laksono BM, de Vries RD, McQuaid S, et al. Measles virus host invasion and pathogenesis. Viruses 2016; 8: pii: E210. DOI: 10.3390/v8080210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ludlow M, McQuaid S, Milner D, et al. Pathological consequences of systemic measles virus infection. J Pathol 2015; 235: 253–265. [DOI] [PubMed] [Google Scholar]
- 40. Beineke A, Puff C, Seehusen F, et al. Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet Immunol Immunopathol 2009; 127: 1–18. [DOI] [PubMed] [Google Scholar]
- 41. Sykes JE. Canine distemper virus infection. In: Canine and feline infectious diseases. St Louis, MO: Elsevier, 2014, pp 152–165. [Google Scholar]
- 42. Martella V, Elia G, Buonavoglia C. Canine distemper virus. Vet Clin North Am Small Anim Pract 2008; 38: 787–797. [DOI] [PubMed] [Google Scholar]
- 43. Sakaguchi S, Koide R, Miyazawa T. In vitro host range of feline morbillivirus. J Vet Med Sci 2015; 77: 1485–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]