Abstract
Practical relevance:
Chronic pain is a feline health and welfare issue. It has a negative impact on quality of life and impairs the owner–cat bond. Chronic pain can exist by itself or may be associated with disease and/or injury, including osteoarthritis (OA), cancer, and oral and periodontal disease, among others.
Clinical challenges:
Chronic pain assessment is a fundamental part of feline practice, but can be challenging due to differences in pain mechanisms underlying different conditions, and the cat’s natural behavior. It relies mostly on owner-assessed behavioral changes and time-consuming veterinary consultations. Beyond OA – for which disease-specific clinical signs have been described – little is known regarding other feline conditions that produce chronic pain.
Recent advances:
Knowledge of the subject has, however, greatly improved in the past few years, informed by study of the mechanisms of pain in cats with OA and the development of pain scales that can be used by owners or veterinarians. Pain scales may facilitate the diagnosis and follow-up evaluation of chronic painful conditions, providing a basis for therapeutic decision-making. Assessment of quality of life is also recommended in cats with chronic pain, and its improvement can be used as a positive outcome in response to therapy.
Aims:
This article reviews recent advances and presents the challenges and some future perspectives on clinical chronic pain assessment. The most common feline chronic conditions associated with pain are also described.
Keywords: Analgesia, animal welfare, pain assessment, behavior, chronic, osteoarthritis, pain scale, quality of life
Negative impacts of chronic pain
Domestic animals may now have a long life expectancy, given advances in veterinary healthcare; as a consequence, there is an increased prevalence of chronic conditions associated with pain. Chronic pain affects feline health and welfare. It has a negative impact on quality of life (QoL) and impairs the owner–cat bond. Nowadays, chronic pain assessment should be considered a fundamental part of feline practice. Indeed, lack of knowledge on the subject and the use of appropriate tools for pain recognition are some of the reasons why analgesic administration is commonly neglected in cats. 1
In chronic pain, changes in behavior are subtle and slow, and may only be evident in the home environment. Thus, owners are always involved in chronic pain assessment. Their participation helps to empower them as part of the healthcare team and they will often ‘reconnect’ with their cats during the treatment process.
Knowledge about chronic pain in cats has greatly improved in the past few years. There have been significant advances in research, including the development of pain and health-related quality of life (HRQoL) scales that are completed by owners,2–6 with the exception of one pain scale that is completed by the veterinarian. 7
When does acute become chronic?
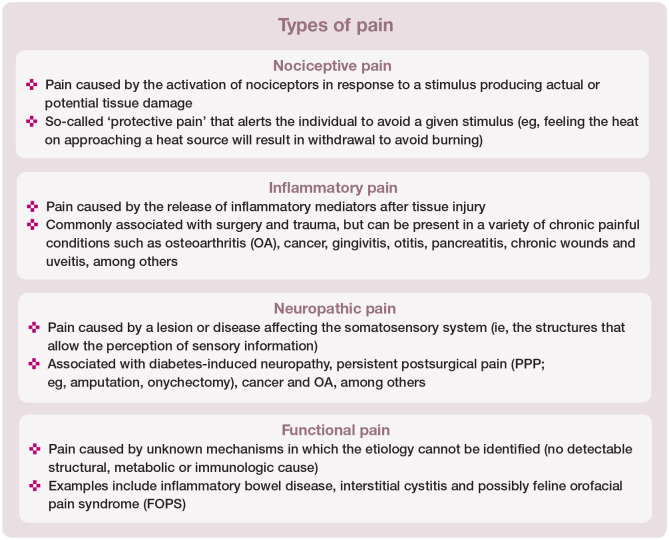
Pain is a complex phenomenon, with pathophysiological changes affecting behavior and QoL. It affects each individual uniquely. It is impossible to pinpoint when acute pain becomes chronic, particularly in animals and given the neuroplasticity of the nervous system. The word chronic commonly implies pain of long-lasting duration. However, it is much more important to understand that chronic pain is characterized by mixed nociceptive input (ie, chronic inflammatory, neuropathic and/or functional pain) that can be caused by disease or injury or can occur by itself (see box below). For example, OA can cause a combination of inflammatory and neuropathic pain, while neuropathic pain per se can be produced in part by inflammation. 8
Unlike acute or ‘adaptive’ pain, chronic pain has no clear end point or physiological function. For these reasons chronic pain has often been termed ‘maladaptive’ pain since it can persist beyond the expected course of an acute disease process. 9 Indeed, persistent postsurgical pain (PPP) is defined as pain that develops after surgical intervention and lasts at least 2 months, when other causes of pain have been excluded. Central sensitization is a feature of PPP which is caused, at least in part, by nerve injury during surgery. Injured nerves result in spontaneous ectopic discharges and consequent increased nociceptive input into the spinal cord. Complex mechanisms of pain facilitation and impairment of normal inhibitory control systems contribute to the central sensitization phenomenon. The clinical manifestations include hyperalgesia, allodynia and spontaneous pain (see definitions box).10,11
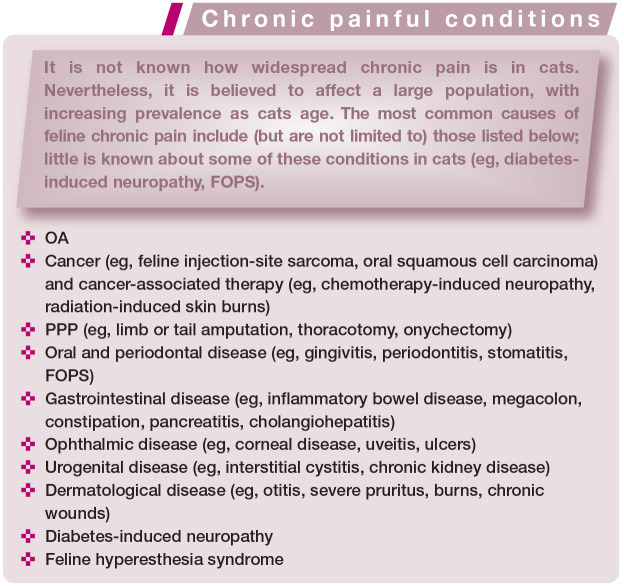
The conditions most commonly associated with chronic pain in cats are summarized in the box on page 603.
Same disease, different presentations
Joint pain secondary to OA is one of the most prevalent chronic painful conditions in cats. It primarily comprises inflammatory pain but can also involve components of neuropathic pain. A multitude of factors contribute to OA-related pain, and these can be categorized into three main groups:
Pathological changes in the osteoarthritic joint (see box on page 609);
Neuroplastic changes affecting the nervous system;
General factors including obesity, metabolic disease, genetic factors, environment and psychological factors, among others.
Given the interplay of so many factors, it becomes clear that the pain profile of patients will be different. As with other chronic conditions, OA may produce varying degrees of peripheral and central sensitization, resulting in a range of clinical presentations.12,13 For example, osteoarthritic cats are less active,2,14 have abnormal kinetics (ie, gait abnormalities), 13 experience difficulty in performing routine activities, 15 and show changes in demeanor and grooming habits. 16 There may also be demonstrable changes in the brain observed with positron emission tomography 17 consistent with chronic pain.
Moreover, it is now known that a subpopulation of cats with OA (probably close to 30%) experience allodynia, which, as discussed above, is a feature of central sensitization. 12 Clinically, patients with central sensitization generally have widespread increased sensory sensitivity and may not respond promptly to analgesics that act primarily in the periphery, such as nonsteroidal anti-inflammatory drugs. 12 In these cases, multimodal analgesia is recommended, incorporating other agents that act at the spinal and supraspinal levels such as tramadol and gabapentin. 13
Thus the same disease/condition (eg, OA) may have different clinical presentations with substantial individual variability that impacts the outcomes of treatment. While treatment of chronic pain is beyond the scope of this article, clearly there is no ‘one size fits all’ approach in these scenarios.
The challenge of chronic pain assessment
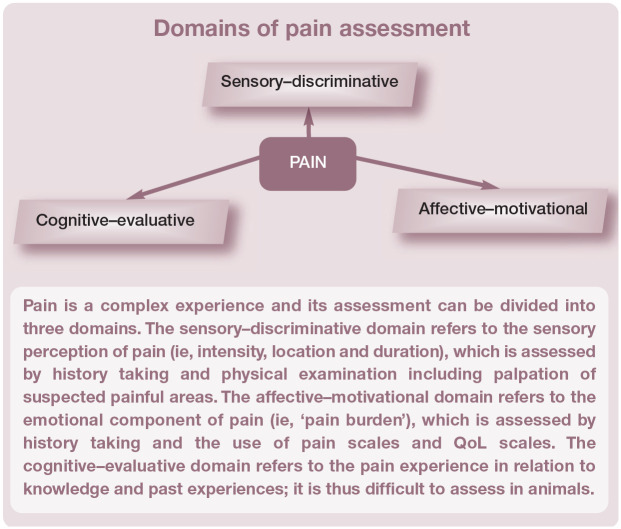
Pain results in a mixture of negative sensory and emotional experiences and these two dimensions have considerable importance in chronic pain assessment (see box below).
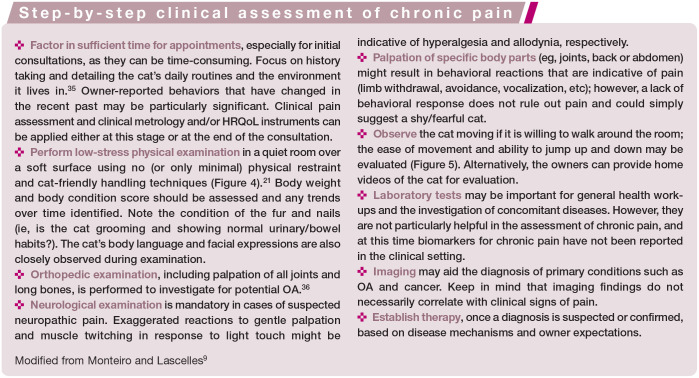
The sensory component is mostly assessed via physical examination and palpation of suspected areas of pain (Figure 1; see also the accompanying video in the supplementary material). As such, pain assessment involving the sensory component can be subjective as it depends on the amount of force used for palpation, the behavioral reaction of the cat (which is usually less evident outside its home environment) and the veterinarian’s interpretation. Imaging (ie, radiography and MRI) might corroborate clinical findings and contribute further information on sensory changes. However, abnormal imaging findings are not always associated with clinical pain, 18 and vice versa (a cat can be painful despite normal imaging findings). In research settings, quantitative sensory testing is a non invasive method of assessing sensory information that is used to provide objective information on the somatosensory profile of healthy cats and those with chronic pain (Figure 2).
Figure 1.
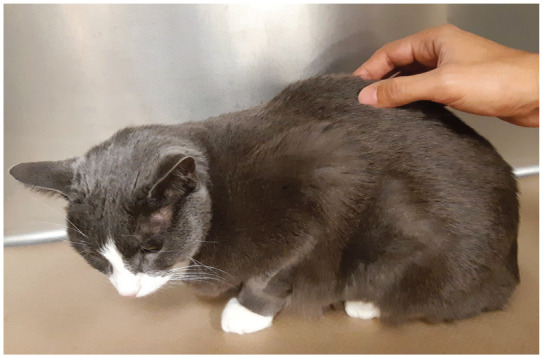
Examination of a cat with suspected back pain. Palpation of the back is performed in a craniocaudal direction while the cat is closely observed for any behavioral reaction. Gentle pressure is used initially. If no response is observed, palpation can be repeated with increased pressure. (See also the accompanying video in the supplementary material)
Figure 2.

Illustration of quantitative sensory testing. With the cat temporarily confined within a cage with a meshed floor, the evaluator uses a digital pressure algometer to apply increasing pressure to the metacarpal and metatarsal pads. The stimulus is stopped immediately after a behavioral reaction (eg, paw withdrawal) is observed, and the amount of force required for this reaction is recorded (grams or Newtons). Research indicates that cats with OA withdraw their paws earlier compared with healthy individuals, indicating increased somatosensory sensitivity in osteoarthritic cats 12

Domains of pain assessment
Pain is a complex experience and its assessment can be divided into three domains. The sensory-discriminative domain refers to the sensory perception of pain (ie, intensity, location and duration), which is assessed by history taking and physical examination including palpation of suspected painful areas. The affective-motivational domain refers to the emotional component of pain (ie, ‘pain burden’), which is assessed by history taking and the use of pain scales and QoL scales. The cognitive-evaluative domain refers to the pain experience in relation to knowledge and past experiences; it is thus difficult to assess in animals.
The emotional component of pain (pain burden) is generally assessed using pain scoring systems (ie, tools, questionnaires or scales) that help to determine how much pain is affecting an individual and its ability to perform activities and enjoy positive experiences. Pain scales rely on the detection of behavioral changes, which are often subtle in cats and also potentially influenced by demeanor. 19 For example, dogs with OA might present with lameness, whereas lameness is not particularly obvious in osteoarthritic cats. Changes in mobility are less evident generally in cats due to their small body size and normal behavioral repertoire, in particular their tendency to withdraw and hide away. In research, one method of assessing mobility and level of activity is with the use of collar-attached activity monitors (Figure 3), which provide objective measures of motor activity.12,13,20
Figure 3.

Cat wearing a collar-attached activity monitoring device. The device continuously collects and stores activity data (objective outcome). These measures can help to differentiate pain-free from painful animals, and also help in the monitoring of treatment effects
Several studies have set out to identify changes in behaviors (ie, new behaviors that appear and old behaviors that disappear) in cats with OA.14–16 Based on these, researchers have developed and validated pain scales to help veterinarians with the assessment of chronic pain.
Common behaviors associated with chronic pain in cats are described in Table 1.
Table 1.
Behavioral changes and clinical signs commonly observed in cats with chronic pain
| Behavioral and clinical signs | Description |
|---|---|
| Decreased activity levels | • Horizontal movement appears stiff rather than fluid
• Cat spends less time roaming, playing or exploring and more time resting • Intensity and vitality of movements is decreased (exercise intolerance) |
| Decreased ability to perform activities | • Difficulty jumping up or down (ie, cat jumps in stages by using an intermediate surface instead of jumping directly on to or down from a high surface)
• Difficulty performing routine activities such as grooming, use of litter box, hunting, scratching, etc |
| Change in appetite | • Decreased or increased |
| Inappropriate elimination | • Difficulty getting into or out of the litter box, resulting in urination or defecation outside of the litter box |
| Unkempt haircoat and nails | • Haircoat may be greasy and tangled and the nails can be dirty due to difficulty in positioning for grooming or reduced scratching behavior |
| Areas of alopecia | • Excessive grooming due to pain or abnormal sensory sensitivity (eg, numbness or tingling) can result in self-induced alopecia |
| Skin rippling | • Muscle spasms or skin twitches along the back after being stroked could indicate hypersensitivity |
| Decreased socialization | • Cat is less willing to interact with owners and other pets, resulting in isolation
Cat seems ‘grumpy’ and resents being stroked or handled |
| General loss of interest | • Cat appears bored and/or to have lost interest in the environment and/or things that used to please it (eg, playing, going outside, greeting owners and other pets, jumping onto or between elevated surfaces, sniffing objects, looking under furniture) |
| Sudden vocalization or agitation | • Sudden vocalizations or running away (escape reaction); this behavior can occur spontaneously or in response to attempts to pet or stroke the cat
• Cat suddenly becomes fixated on a particular part of its body and starts licking it intensively without an obvious cause • Normal behavior resumes shortly after these episodes (possibly indicating abnormal sensitivity such as numbness or tingling) |
Modified from Monteiro and Lascelles 9
Feline chronic pain scales: clinical metrology instruments
Pain scales are the result of rigorous research to identify and validate key behaviors that are indicative of pain. Like all scientifically robust measures, these instruments undergo several steps of validation to ensure that they measure what it is intended to measure (validity), produce consistent results (reliability), and can detect clinically relevant changes after administration of analgesics (responsiveness), among other criteria. Indeed, health measurement scales are considered ‘living documents’, subject to ongoing studies that help to further validate them by removing and/or adding new items, for example.
It is well recognized that a simple visit to the veterinarian can be stressful for cats (Figure 4). They usually hide their pain-related behaviors in the clinical setting due to stress, which might involve, at least in part, an element of stress-induced analgesia. Cats can become ‘paralyzed’ in the clinic, which will further add to the challenge of chronic pain assessment. For these reasons, most pain scales rely on owner assessment of changes in behavior and the cat’s daily routine.2–4,7,14,15,21,22
Figure 4.

A visit to the veterinarian can be a stressful event for a cat. Guidelines on how to transport cats and reduce fear and stress in the clinic using feline-friendly handling techniques are detailed elsewhere. 21 (a) Upon arrival in the exam room, this female cat is hesitant and is left to come out of the carrier on her own. (b) A few minutes later, she builds confidence and slowly emerges and begins to explore the room
There are currently three owner-based and one veterinarian-based clinical metrology instruments for use in cats with OA. Although none of them have been thoroughly validated, they have been used in several studies in cats with OA. Veterinary technicians/nurses are an excellent source of help in the use of owner-based pain scales. They will be primarily involved with owner interviews, providing information on the practical implementation of these scales.
Feline Musculoskeletal Pain Index
The Feline Musculoskeletal Pain Index (FMPI) involves owner assessment of the severity and impact of chronic pain in their cat. Following initial study identifying specific behaviors, 15 the FMPI has been used in several investigations for its ongoing validation.3,20,22–25 The most recent version consists of 17 items pertaining to mobility, ability to perform daily activities (eg, jumping up and down, playing with toys, grooming, using the litter box) and interaction with other pets and people. Each item can be scored from ‘normal’ to ‘not at all’ with respect to the cat’s ability or willingness to perform a given activity.
The FMPI differentiates pain-free from painful cats with OA, and helps with assessing the response to treatment. However, it is not clear if it can discriminate severity of pain in cats with OA at this time.
Client Specific Outcome Measures
The Client Specific Outcome Measures (CSOM) is an instrument that was originally developed for dogs and subsequently applied in cats. The CSOM has been used in some studies for its ongoing validation.2,20,22,23 It is based on owner assessment of the impact of chronic pain on a cat’s ability to perform specific activities that are particular to the individual animal. This means that there are no set items to be scored. Instead, the owner, with the help of the veterinarian or technician, will choose three activities that are observed in the home environment (an example is provided in Table 2).2,22 The CSOM can be time-consuming to implement because of its bespoke nature, requiring clear understanding by owners.
Table 2.
Example of the use of Client Specific Outcome Measures
| Activity* | No problem Mild difficulty | Moderate difficulty | Severe difficulty Impossible to perform |
|---|---|---|---|
| 1. Jumping onto the kitchen counter for her/his breakfast without using the bench to aid the jump | X | ||
| 2. Playing with the string at night in the living room without losing interest after 2 mins | X | ||
| 3. Appropriately using the litter box in the basement in the morning | X | ||
Three activities that are particular to the individual cat, including time and place, are carefully chosen by the owner after thorough discussion with the veterinarian or veterinary technician. Each activity is then scored from ‘no problem’ to ‘impossible to perform’, and the scores are followed over the course of treatment with repeated evaluations
Montreal Instrument for Cat Arthritis Testing (MI-CAT[C] and MI-CAT[V])
The Montreal Instrument for Cat Arthritis Testing for Use by Caretaker (MI-CAT[C]) and the Montreal Instrument for Cat Arthritis Testing for Use by Veterinarian (MI-CAT[V]) are instruments to be completed by the owner and the veterinarian, respectively. The MI-CAT(C), similar to the FMPI, was developed based on owner-perceived clinical signs for cats with OA, 14 and some preliminary validation has been performed. 4 The most recent version, MI-CAT(C)-v2, consists of 18 items in subscale 1 with three categories (agility; social, play and exploratory behaviours; self-maintenance), and 20 items in subscale 2, also with three categories (agility; self-maintenance; physical condition). Each item is scored as ‘yes’ or ‘no’ relating to the cat’s ability to perform a certain activity, and a final score is calculated.
The MI-CAT(V) was developed for use by veterinarians in the context of OA. 26 This scale has undergone preliminary validation which determined that cats with and without OA could be distinguished. However, it was unable to discriminate an effect of treatment. 7 The most recent version, MI-CAT(V)-v5, consists of 25 items related to body posture, gait, willingness and ease of horizontal movements, jumping and a general lameness score (Figure 5).
Figure 5.

Cat undergoing assessment using the Montreal Instrument for Cat Arthritis Testing for Use by Veterinarian (MI-CAT[V]). The cat is encouraged to walk around the room using positive reinforcement (eg, food, gentle brushing) and is observed from a distance. The evaluator scores the cat’s mobility by carefully assessing the conformation and gait of the (a) thoracic and (b) pelvic limbs, as well as (c) the cat’s ability to perform certain activities such as jumping down from a height (ie, whether it hesitates and how it lands on the floor)
Feline chronic pain scales: health-related quality of life instruments
The assessment of HRQoL is paramount in the context of chronic pain, and an improvement in HRQoL can be used as one of the main treatment outcomes for patients. The definition of QoL from the World Health Organization (WHO) reflects the view that QoL refers to a subjective evaluation which is ‘embedded in a cultural, social and environmental context’. 27 Hence, the focus is on an individual’s perception of their life and the WHO definition may not be applicable in veterinary medicine. Some authors suggest that QoL assessment in pets should take into consideration all aspects of an animal’s life, 28 whereas HRQoL refers more specifically to the effect of a medical condition on the physical and emotional health of the individual. 29 The latter is applicable in the context of chronic pain. In fact, HRQoL might usefully be defined as ‘the subjective evaluation of circumstances that include an altered health state and related interventions’. 30
Moreover, there seems to exist an overlap between the assessment of QoL and animal welfare. The WHO’s assessment of QoL includes four domains (physical health, psychological, social relationships and environment), 27 similar to the ‘Five Domains’ model for animal welfare assessment that includes nutrition, environment, health, behavior and mental state (positive and negative). 31 Despite the lack of consensus in this area, an assessment of HRQoL is always recommended in cats with chronic pain.
Feline-specific HRQoL questionnaires have been developed and partially validated. Some are focused on the impact of a particular condition (eg, diabetes mellitus 32 or skin disease 33 ), whereas others are generic and can be applied to any chronic health condition, including chronic pain.
A web-based generic HRQoL questionnaire (VetMetrica) measures the affective–motivational impact of chronic disease in cats (see ‘domains of pain assessment’ box on page 603) and has been shown to differentiate healthy from sick cats. 6 There is initial evidence to support its use in cats with OA since it was also able to differentiate between healthy cats, cats with mild OA and cats with moderate/severe OA. 34 It consists of 20 items divided into three domains (vitality, comfort and emotional wellbeing).6,34 The VetMetrica HRQoL for cats is available for use in clinical practice and research based on paid subscription. A series of webinars explaining the validation process of this and other instruments, as well as their use, is available at http://www.newmetrica.com/demo-videos/.
An owner-based HRQoL instrument (Feline QoL measure) with strong cross-sectional psychometric properties has also been developed, and its evaluation in healthy cats reported. It consists of 16 items divided into two domains (healthy behaviours and clinical signs). 5 This instrument is not yet available for download.
A third instrument, a generic paper-based HRQoL questionnaire entitled Cat HEalth and Wellbeing (CHEW), has undergone preliminary validation; however, it has yet to demonstrate discriminatory ability (ie, differentiating healthy from sick cats). It consists of 33 items in eight domains including physical (mobility, eyes, coat, fitness and appetite), mental and emotional (emotions and energy), and social functioning (engagement) domains. 29 This instrument is available for download as supplementary material from Freeman et al. 29
These HRQoL tools are not yet freely available (ie, as a discrete document for open access download). Nevertheless, an assessment of QoL must somehow be incorporated into the clinical recognition of chronic pain and response to analgesic therapy. Some of these QoL features are encompassed in the behavioral and clinical signs of chronic pain outlined in Table 1.
Examples of chronic painful conditions in cats
Osteoarthritic pain
OA is a progressive degenerative disease of synovial joints leading to structural and functional dysfunction and deleterious effects on mobility and QoL. The pathogenesis is complex and multifactorial, involving inflammatory (‘inflammaging’; ie, systemic low-grade inflammation due to aging), metabolic (eg, obesity) and biomechanical components. 39 Thus, the risk factors associated with OA include aging, obesity, inactive/sedentary lifestyle and metabolic disease. Where present, these risk factors can guide diagnosis, 39 as these patients are considered an ‘at risk’ population for the development of OA (Figure 6).
Figure 6.

A 14-year-old female spayed cat is presented with an owner complaint of decreased activity levels and difficulty jumping onto heights. (a) On presentation, the cat is friendly and willing to interact with the clinician. (b) Physical examination reveals a body condition score of 7 out 9, a stiff gait, and reluctance to jump up and down. OA is suspected and a CSOM instrument is created and completed with the owners (Table 2)
While the prevalence of OA-related pain in cats is unknown, the radiographic evidence of OA can be as high as 61% in cats >6 years old and 90% in cats >12 years.40–42 The most commonly affected joints and regions (elbow, hip, stifle, tarsus, lumbar, lumbosacral) should possibly receive extra attention during examination.41,42 Several factors can contribute to the generation and maintenance of pain in OA (see box on page 609). 43 Clinical signs can, therefore, vary greatly among patients. Characteristic clinical manifestations include decreased mobility and ability to perform daily activities, altered grooming, decreased socialization and/or abnormal use of the litter box.
Clinical metrology and HRQoL instruments should be used in osteoarthritic cats with the aim of creating a ‘reference point’ against which to monitor pain, disease progression and treatment outcomes over time. Radiographic imaging can be performed to confirm and document the presence of OA. However, the severity of OA on radiographs does not correlate with the presence and/or severity of clinical pain. Thus, pain must be assessed regardless of the radiographic signs in these patients and radiographs should not dictate therapy.
Cancer pain
Cancer pain can be considered a specific type of pain, distinct from inflammatory and neuropathic pain. 44 It involves complex mechanisms including the release of pain-enhancing mediators by the cancer itself.
As well as originating from the primary tumor, pain in patients with cancer can originate from diagnostic procedures, cancer therapies, metastatic disease and concomitant diseases such as OA or dental disease. Based on its multidimensional nature, the risk factors for cancer pain include the obvious progression of the cancer, as well as its therapies (chemotherapy-induced neuropathy, radiotherapy-induced burns, PPP after invasive surgery) and the type and location of the tumor; for example, tumors affecting the oral cavity, bone, urogenital tract, eyes, nose, nerve roots, gastrointestinal tract and skin tend to be more painful. 45 Some types of cancer will impact food intake and have an important effect on the nutritional status of patients (Figure 7).
Figure 7.
(a,b) Large oral mass in a geriatric male cat that was showing signs of dysphagia and progressive weight loss. The owners also reported that the cat had become reclusive and was no longer willing to play with toys, which he had enjoyed doing in the past
The prevalence of cancer pain in cats is unknown. In people, it can affect up to 50% of patients at the time of diagnosis and up to 90% of those with advanced-stage cancer. 46 Based on biologic similarities between cancer in people and animals, 47 it is reasonable to assume that cats are similarly affected. Breakthrough pain (ie, episodes of extreme pain that arise spontaneously or induced by movement) can occur. Clinical signs tend to progress over time and patients become severely affected, with altered function and QoL.
Dental pain
Periodontal disease, such as gingivitis and periodontitis, causes pain, inflammation, dysphagia, weight loss and hemorrhage (Figure 8); pawing at the mouth after eating may be observed. It is the most commonly reported disorder in companion animals presented in private practice, 48 and as such affects a large population of cats. Cats with severe oral disease and periodontitis have decreased food intake, increased pain scores and present distinct behaviors in the hospital setting (eg, reduced attention to surroundings, spending more time lying down) compared with cats with minimal periodontal disease.49,50 Clinical experience suggests that these clinical signs normally disappear and cats are reported to become friendlier following dental treatment.
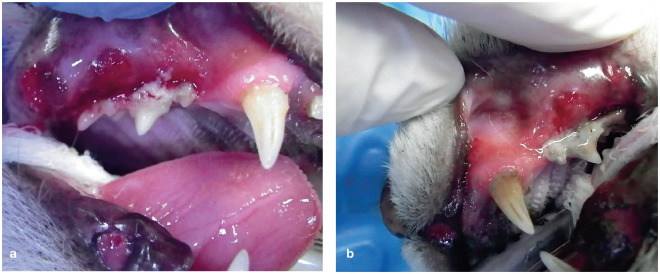
Figure 8.
(a,b) Advanced periodontal disease in an 11-year-old female cat. Severe gingivitis – a source of inflammatory pain – is evident and the cat would be observed pawing at the mouth while eating. Following dental treatment under general anesthesia, including tooth extractions, the owners reported that the cat’s clinical signs had subsided, and she was once again displaying friendly behaviors
Persistent postsurgical pain
PPP has been discussed earlier in this article in the context of the ‘chronification’ of pain. Although the mechanisms involved in the transition from acute to chronic pain are not yet well elucidated, it is known that the severity of acute postoperative pain correlates with the risk of developing chronic postoperative pain. 51 Thus, invasive surgery including onychectomy, thoracotomy, mastectomy and limb amputation are likely risk factors for the development of PPP in cats (Figures 9 and 10). 52
Figure 9.

PPP in a 7-year-old female cat after onychectomy. The cat persistently keeps the left paw lifted while sitting, to avoid bearing weight on it. She also resents being touched on that paw. Courtesy of Dr André Desrochers
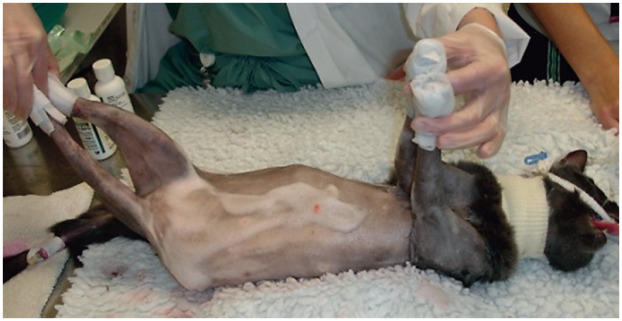
Figure 10.
Male cat anesthetized for wound debridement of caustic burns affecting all four paws. The injuries were sustained after the cat stepped in accidentally spilled drain-cleaning product. Necrotic skin, tendons and multiple phalanges were removed during surgery. Procedures with the potential to cause severe pain require aggressive perioperative analgesic management to avoid the risk of PPP
The prevalence of PPP in cats is unknown. Clinical signs of PPP can be observed in onychectomized cats and include lameness, back pain, inappropriate elimination habits, licking and chewing at the digits, aversion to the feet being touched, and altered weightbearing, among others. 53 These signs lead to suffering, disability and reduced QoL, and are a major burden on these cats. Veterinary authorities in regions where onychectomy is performed (including the USA and Canada) should be calling for legislation to prohibit this non-therapeutic surgical procedure.
Neuropathic pain
Neuropathic pain is caused by a lesion or disease of the somatosensory system. It is considered a maladaptive phenomenon and involves a variety of mechanisms including aberrant ectopic nociceptive discharge, neuroplasticity, impaired endogenous inhibitory modulation and activation of microglia. 54 Because of these changes in the somatosensory system, people who suffer from neuropathic pain describe abnormal sensations, and cats may be similarly affected.
FOPS, diabetes-induced neuropathy and feline hyperesthesia syndrome are possible examples of conditions producing neuropathic pain in cats.
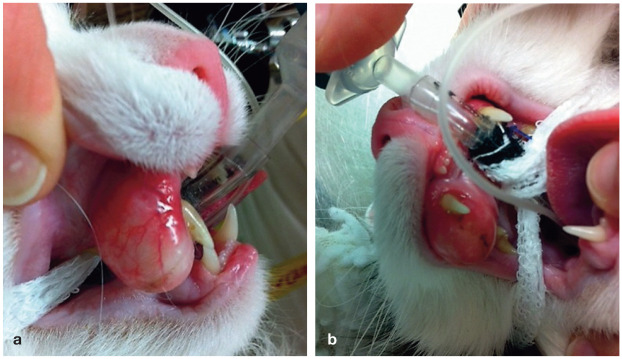
FOPS could be analogous to trigeminal neuralgia in humans. The pathogenesis of the disease involves stress and hereditary components, with Burmese and older cats showing a higher prevalence. These cats should undergo dental treatment to exclude other sources of oral pain. 55 Clinical signs include pawing at the mouth, exaggerated licking and chewing movements, and vocalization followed by escape reaction, all of which can manifest spontaneously or may be triggered by eating (Figure 11). In addition, cats with FOPS may be anorexic or show decreased appetite.
Diabetes-induced neuropathy is a complication of chronic diabetes mellitus, risk factors for which include indoor confinement and inactivity/sedentary lifestyle. 56 People with diabetes-induced neuropathy self-report numbness and tingling, varying degrees of pain including allodynia, lethargy and poor QoL. 57 It is likely that similar sensory abnormalities affect cats. Indeed, the following behaviors have been observed in cats with diabetes: aversion to touching of the distal limbs, licking of the feet leading to discoloration of the fur, and impaired ability to jump. 57 These cats can present with a plantigrade stance, reduced patellar reflexes, hindlimb weakness, muscle atrophy, poor postural reactions and electrophysiologic dysfunction. 57
Feline hyperesthesia syndrome is a condition with an unclear etiology. It is believed that dermatological (eg, hypersensitivity dermatitis), neurological (eg, focal epileptic seizures), neuropathic (eg, neuropathic itch or pain) and behavioral components play a role in the pathogenesis of the disease. Its diagnosis is one of exclusion once other conditions have been ruled out (eg, allergic dermatitis, food allergy, compulsive disorder, displacement behavior). It may sometimes be seen concomitantly with spinal disease (eg, disc disease, neoplasia, myelitis, OA), myositis or myopathy. Clinical signs reflect abnormal sensory sensitivities and include skin rippling (muscle spasms) along the spine, pain on palpation, self-mutilation, excessive grooming and/or areas of alopecia. 58 These behaviors may occur spontaneously or be induced by petting or stroking the cat (Figure 12).
Figure 11.

Cat with suspected FOPS. Clinical signs included spontaneous vocalization and pawing at the mouth for no apparent reason
Figure 12.
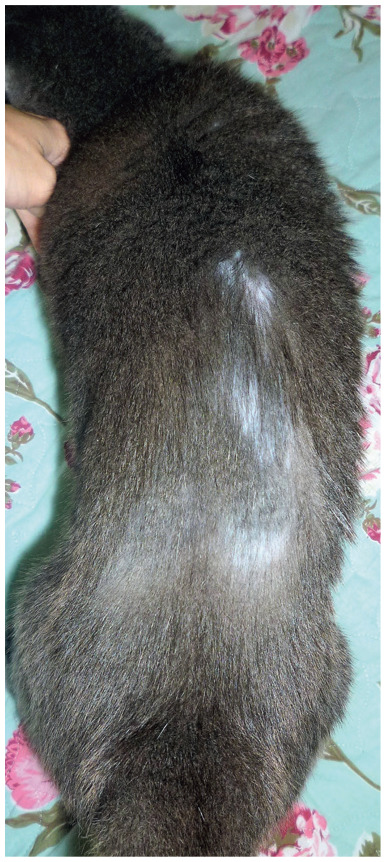
Self-induced alopecia along the thoracolumbar back of a 15-year-old male cat with suspected feline hyperesthesia syndrome
Future perspectives
A recent companion article (see right) described the future for feline acute pain assessment as looking promising and exciting. The future of chronic pain assessment in cats is likewise promising. In this regards, a few particular perspectives are worth highlighting:
Translation of knowledge from the research setting into clinical practice Quick and simple sensory testing (eg, quantitative sensory testing) could become a tool for the assessment of increased sensory sensitivity in cats with chronic pain.12,13,59 Accerelometer-based activity monitors (Figure 3) have been used both in research and clinical studies and are useful to detect the effect of analgesics as cats become more active after treatment.2,12,13,20,23 These might be applicable in the clinical scenario in the future. Thermographic imaging has also shown promising results in preliminary studies in terms of its applicability in detecting areas of the body with increased temperature due to inflammation. 60
Clinical metrology and HRQoL instruments The continuing development and validation of short and practical clinical metrology and HRQoL instruments will certainly contribute to the advancement of feline chronic pain assessment.
Education of veterinarians There is a need for appropriate continuing education of veterinarians on the subject to reduce animal suffering. Teaching of chronic pain management as part of the veterinary medicine curriculum is fundamental.
Support from stakeholders Future research would benefit from support from a range of interested parties, or stakeholders, such as cat-dedicated organizations, owners, industry and grant funding agencies.
Improved rates of analgesia Ultimately, better chronic pain assessment should translate into better treatment outcomes for cats, with an improvement in their health and QoL. It may increase rates of analgesic administration in this species.

Conclusions
Chronic pain can exist as an entity in itself and can also be associated with disease and/or injury. It induces behavioral changes that affect the cat’s health and QoL, and the owner–cat bond. Chronic pain-related changes in behavior are subtle and likely to be suppressed in the clinical setting. Thus, evaluation of chronic pain relies mostly on owner-assessed behavioral changes and time-consuming veterinary consultations. Chronic painful conditions can have disease-specific clinical signs, and there is a critical need for research on feline chronic pain conditions other than OA.
Key Points
Assessment of chronic pain can be challenging due to differences in pain mechanisms involved in different conditions, and the cat’s natural behavior.
Chronic pain assessment in cats is mostly based on owner-reported changes in behavior. Owners’ involvement as part of the healthcare team is crucial.
Key behavioral indicators of chronic pain in cats with OA include decreased activity and difficulty performing daily activities (eg, jumping, scratching, using the litter box, grooming), changes in demeanor and appetite, and decreased socialization with people and other pets.
Cats with chronic pain may present with widespread sensory sensitivity (ie, hyperalgesia throughout the body) as a result of central sensitization.
Currently, there is one veterinarian-based and three owner-based pain scales that have been evaluated for use in cats with OA. They provide a basis for therapeutic decision-making, dosage regimen changes and analgesic challenge. One of these (the CSOM) may be used for the diagnosis and follow-up evaluation of painful chronic conditions other than OA.
Assessment of chronic pain includes a general assessment of QoL. This can be performed using HQoL questionnaires. Improvement in QoL can be used as a positive outcome in response to therapy.
Footnotes
Supplementary material: Video Examination of a cat with suspected back pain.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Simon BT, Scallan EM, Carroll G, et al. The lack of analgesic use (oligoanalgesia) in small animal practice. J Small Anim Pract 2017; 58: 543–554. [DOI] [PubMed] [Google Scholar]
- 2. Lascelles BDX, Hansen BD, Roe S, et al. Evaluation of client-specific outcome measures and activity monitoring to measure pain relief in cats with osteoarthritis. J Vet Intern Med 2007; 21: 410–416. [DOI] [PubMed] [Google Scholar]
- 3. Benito J, Hansen B, Depuy V, et al. Feline musculoskeletal pain index: responsiveness and testing of criterion validity. J Vet Intern Med 2013; 27: 474–482. [DOI] [PubMed] [Google Scholar]
- 4. Klinck MP, Gruen ME, del Castillo JRE, et al. Development and preliminary validity and reliability of the Montreal Instrument for Cat Arthritis Testing, for Use by Caretaker/Owner, MI-CAT(C), via a randomised clinical trial. Appl Anim Behav Sci 2018; 200: 96–105. [Google Scholar]
- 5. Tatlock S, Gober M, Williamson N, et al. Development and preliminary psychometric evaluation of an owner-completed measure of feline quality of life. Vet J 2017; 228: 22–32. [DOI] [PubMed] [Google Scholar]
- 6. Noble CE, Wiseman-Orr LM, Scott ME, et al. Development, initial validation and reliability testing of a web-based, generic feline health-related quality-of-life instrument. J Feline Med Surg 2019; 21: 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klinck MP, Monteiro BP, Lussier B, et al. Refinement of the Montreal Instrument for Cat Arthritis Testing, for Use by Veterinarians: detection of naturally occurring osteoarthritis in laboratory cats. J Feline Med Surg 2018; 20: 728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xanthos DN, Sandkuhler J. Neurogenic neuroinflamma-tion: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci 2014; 15: 43–53. [DOI] [PubMed] [Google Scholar]
- 9. Monteiro B, Lascelles BDX. Assessment and recognition of chronic (maladaptive) pain. In: Steagall PVM, Robertson SA, Taylor PM. (eds). Feline anesthesia and pain management. Hoboken, NJ: Wiley/Blackwell, 2017, pp 241–256. [Google Scholar]
- 10. Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010; 9: 807–819. [DOI] [PubMed] [Google Scholar]
- 11. von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 2012; 73: 638–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guillot M, Moreau M, Heit M, et al. Characterization of osteoarthritis in cats and meloxicam efficacy using objective chronic pain evaluation tools. Vet J 2013; 196: 360–367. [DOI] [PubMed] [Google Scholar]
- 13. Monteiro BP, Klinck MP, Moreau M, et al. Analgesic efficacy of tramadol in cats with naturally occurring osteoarthritis. PLoS One 2017; 12: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klinck MP, Frank D, Guillot M, et al. Owner-perceived signs and veterinary diagnosis in 50 cases of feline osteoarthritis. Can Vet J 2012; 53: 1181–1186. [PMC free article] [PubMed] [Google Scholar]
- 15. Zamprogno H, Hansen BD, Bondell HD, et al. Item generation and design testing of a questionnaire to assess degenerative joint disease-associated pain in cats. Am J Vet Res 2010; 71: 1417–1424. [DOI] [PubMed] [Google Scholar]
- 16. Bennett D, Morton C. A study of owner observed behavioural and lifestyle changes in cats with muscu-loskeletal disease before and after analgesic therapy. J Feline Med Surg 2009; 11: 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guillot M, Chartrand G, Chav R, et al. [18F]-fluorodeoxyglucose positron emission tomography of the cat brain: a feasibility study to investigate osteoarthritis-associated pain. Vet J 2015; 204: 299–303. [DOI] [PubMed] [Google Scholar]
- 18. Finan PH, Buenaver LF, Bounds SC, et al. Discordance between pain and radiographic severity in knee osteoarthri-tis: findings from quantitative sensory testing of central sensitization. Arthritis Rheum 2013; 65: 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bennett PC, Rutter NJ, Woodhead JK, et al. Assessment of domestic cat personality, as perceived by 416 owners, suggests six dimensions. Behav Processes 2017; 141: 273–283. [DOI] [PubMed] [Google Scholar]
- 20. Gruen ME, Griffith EH, Thomson AE, et al. Criterion validation testing of clinical metrology instruments for measuring degenerative joint disease associated mobility impairment in cats. PLoS One 2015; 10: e0131839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodan I, Sundahl E, Carney H, et al. AAFP and ISFM feline-friendly handling guidelines. J Feline Med Surg 2011; 13: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gruen ME, Griffith E, Thomson A, et al. Detection of clinically relevant pain relief in cats with degenerative joint disease associated pain. J Vet Intern Med 2014; 28: 346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gruen ME, Thomson AE, Griffith EH, et al. A feline-specific anti-nerve growth factor antibody improves mobility in cats with degenerative joint disease-associated pain: a pilot proof of concept study. J Vet Intern Med 2016; 30: 1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benito J, Depuy V, Hardie E, et al. Reliability and discriminatory testing of a client-based metrology instrument, feline musculoskeletal pain index (FMPI) for the evaluation of degenerative joint disease-associated pain in cats. Vet J 2013; 196: 368–373. [DOI] [PubMed] [Google Scholar]
- 25. Gruen ME, Dorman DC, Lascelles BDX. Caregiver placebo effect in analgesic clinical trials for cats with naturally occurring degenerative joint disease-associated pain. Vet Rec 2017; 180: 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klinck M, Rialland P, Guillot M, et al. Preliminary validation and reliability testing of the Montreal Instrument for Cat Arthritis Testing, for Use by Veterinarians, in a colony of laboratory cats. Animals 2015; 5: 1252–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization. Division of Mental Health. WHOQOL-BREF: introduction, administration, scoring and generic version of the assessment. Geneva, https://www.who.int/mental_health/media/en/76.pdf (1996, accessed March 6, 2019). [Google Scholar]
- 28. McMillan FD. Quality of life in animals. J Am Vet Med Assoc 2000; 216: 1904–1910. [DOI] [PubMed] [Google Scholar]
- 29. Freeman LM, Rodenberg C, Narayanan A, et al. Development and initial validation of the Cat HEalth and Wellbeing (CHEW) Questionnaire: a generic health-related quality of life instrument for cats. J Feline Med Surg 2016; 18: 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wiseman-Orr ML, Scott EM, Reid J, et al. Validation of a structured questionnaire as an instrument to measeure chronic pain in dogs on the basis of effects on health-related quality of life. Am J Vet Res 2006; 67: 1826–1836. [DOI] [PubMed] [Google Scholar]
- 31. Mellor DJ, Beausoleil N. Extending the ‘Five Domains’ model for animal welfare assessment to incorporate positive welfare states. Anim Welf 2015; 24: 241–253. [Google Scholar]
- 32. Niessen SJM, Powney S, Guitian J, et al. Evaluation of a quality-of-life tool for cats with diabetes mellitus. J Vet Intern Med 2010; 24: 1098–1105. [DOI] [PubMed] [Google Scholar]
- 33. Noli C, Borio S, Varina A, et al. Development and validation of a questionnaire to evaluate the quality of life of cats with skin disease and their owners, and its use in 185 cats with skin disease. Vet Dermatol 2016; 27: 247–e58. [DOI] [PubMed] [Google Scholar]
- 34. Noble C, Scott EM, Nolan AM, et al. Initial evidence to support the use of a generic health-related quality of life instrument to measure chronic pain in cats with osteoarthritis [abstract]. Vet Comp Orthop Traumatol 2018; 31(S 01): A1–A6. DOI: 10.1055/s-0038-1660887.30060271 [DOI] [Google Scholar]
- 35. Ellis SLH, Rodan I, Carney HC, et al. AAFP and ISFM feline environmental needs guidelines. J Feline Med Surg 2013; 15: 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kerwin S. Orthopedic examination in the cat: clinical tips for ruling in/out common musculoskeletal disease. J Feline Med Surg 2012; 14: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steagall PV, Monteiro BP, Ruel HLM, et al. Perceptions and opinions of Canadian pet owners about anaesthesia, pain and surgery in small animals. J Small Anim Pract 2017; 58: 380–388. [DOI] [PubMed] [Google Scholar]
- 38. Simon BT, Scallan EM, Von Pfeil DJF, et al. Perceptions and opinions of pet owners in the United States about surgery, pain management, and anesthesia in dogs and cats. Vet Surg 2018; 47: 277–284. [DOI] [PubMed] [Google Scholar]
- 39. Mobasheri A, Batt M. An update on the pathophysiology of osteoarthritis. Ann Phys Rehabil Med 2016; 59: 333–339. [DOI] [PubMed] [Google Scholar]
- 40. Slingerland LI, Hazewinkel HAW, Meij BP, et al. Cross-sectional study of the prevalence and clinical features of osteoarthritis in 100 cats. Vet J 2011; 187: 304–309. [DOI] [PubMed] [Google Scholar]
- 41. Hardie EM, Roe SC, Martin FR. Radiographic evidence of degenerative joint disease in geriatric cats: 100 cases (1994-1997). J Am Vet Med Assoc 2002; 220: 628–632. [DOI] [PubMed] [Google Scholar]
- 42. Godfrey DR. Osteoarthritis in cats: a retrospective radiological study. J Small Anim Pract 2005; 46: 425–429. [DOI] [PubMed] [Google Scholar]
- 43. Eitner A, Hofmann GO, Schaible H-G. Mechanisms of osteoarthritic pain. Studies in humans and experimental models. Front Mol Neurosci 2017; 10: 349. DOI: 10.3389/fnmol.2017.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brown MRD, Ramirez JD. Neuroimmune mechanisms in cancer pain. Curr Opin Support Palliat Care 2015; 9: 103–111. [DOI] [PubMed] [Google Scholar]
- 45. Lascelles BDX. Management of chronic cancer pain. In: Withrow S, Vail D, Page R. (eds). Small animal clinical oncology. St Louis, MO: Elsevier, 2013, pp 245–259. [Google Scholar]
- 46. Marcus DA. Epidemiology of cancer pain. Curr Pain Headache Rep 2011; 15: 231–234. [DOI] [PubMed] [Google Scholar]
- 47. Ranieri G, Pantaleo M, Piccinno M, et al. Tyrosine kinase inhibitors (TKIs) in human and pet tumours with special reference to breast cancer: a comparative review. Crit Rev Oncol Hematol 2013; 88: 293–308. [DOI] [PubMed] [Google Scholar]
- 48. Lund EM, Armstrong PJ, Kirk CA, et al. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J Am Vet Med Assoc 1999; 214: 1336–1341. [PubMed] [Google Scholar]
- 49. Watanabe R, Doodnaught G, Proulx C, et al. A multidisci-plinary study of pain in cats undergoing dental extractions: a prospective, blinded, clinical trial. PLoS One 2019; 14: e0213195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Watanabe R, Frank D, Steagall P. Pain behaviors before and after treatment of oral disease: preliminary results. In: 8th Annual Congress of Asian Society of Veterinary Surgery. Taichung, Taiwan, 2018. [Google Scholar]
- 51. Hanley MA, Jensen MP, Smith DG, et al. Preamputation pain and acute pain predict chronic pain after lower extremity amputation. J Pain 2007; 8: 102–109. [DOI] [PubMed] [Google Scholar]
- 52. Steagall PVM, Monteiro-Steagall BP. Multimodal analgesia for perioperative pain in three cats. J Feline Med Surg 2013; 15: 737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Martell-Moran N, Solano M, Townsend H. Pain and adverse behavior in declawed cats. J Feline Med Surg 2018; 20: 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gilron I, Baron R, Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc 2015; 90: 532–545. [DOI] [PubMed] [Google Scholar]
- 55. Rusbridge C, Heath S, Gunn-Moore D, et al. Feline orofacial pain syndrome (FOPS): a retrospective study of 113 cases. J Feline Med Surg 2010; 12: 498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sparkes AH, Church D, Fleeman L, et al. ISFM consensus guidelines on the practical management of diabetes mellitus in cats. J Feline Med Surg 2015; 17: 235–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mizisin AP, Shelton GD, Burgers ML, et al. Neurological complications associated with spontaneously occurring feline diabetes mellitus. J Neuropathol Exp Neurol 2002; 61: 872–884. [DOI] [PubMed] [Google Scholar]
- 58. Batle P, Rusbridge C, Nuttall T, et al. Feline hyperaesthesia syndrome with self-trauma to the tail: retrospective study of seven cases and proposal for integrated multidisciplinary diagnostic approach. J Feline Med Surg 2019; 21: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Addison ES, Clements DN. Repeatability of quantitative sensory testing in healthy cats in a clinical setting with comparison to cats with osteoarthritis. J Feline Med Surg 2017; 19: 1274–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vainionpaa M, Raekallio M, Junnila J, et al. A comparison of thermographic imaging, physical examination and modified questionnaire as an instrument to assess painful conditions in cats. J Feline Med Surg 2013; 15: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]