Abstract
Practical relevance:
Non-regenerative anemia, or anemia with reticulocytopenia, is a daily diagnosis in feline practice.
Clinical challenges:
The disease processes underlying non-regenerative anemia are many and diverse. A major diagnostic evaluation may be required to correctly diagnose and treat the underlying cause.
Audience:
All veterinarians caring for cats will face the diagnostic and therapeutic challenge of non-regenerative anemia. Readers will benefit from the review of diagnostic testing and therapeutic options for non-regenerative anemia.
Evidence base:
This review summarizes the currently available literature informing diagnostic and treatment recommendations related to non-regenerative anemia. The evidence available to support the recommendations in this review is graded as low and includes predominantly expert opinion, case reports and cases series, on which the authors’ interpretation/consensus is based.
Keywords: Anemia, anaemia, anemia of inflammation, anemia of chronic disease, bone marrow disease, non-regenerative, nonregenerative, pure red cell aplasia, immune-mediated hemolytic anemia, aplastic anemia
Non-regenerative anemia, or anemia with reticulocytopenia, is frequently encountered in feline practice. The reason for the high frequency of non-regenerative anemia in the cat is unknown, but is most likely because cats develop anemia more commonly than dogs, and cats suffer from a number of chronic diseases that contribute to the feline propensity for anemia (see box). 1 A wide variety of diseases may underlie feline non-regenerative anemia. Review of all causes and therapies is beyond the scope of this article and the reader is referred to other reviews in this journal for further information (see box on page 616). The two main pathologic mechanisms leading to non-regenerative anemia are decreased or ineffective erythropoiesis and decreased red blood cell (RBC) lifespan.
Pathogenesis
Non-regenerative anemia in cats can occur either at the level of the bone marrow secondarily to decreased and/or ineffective erythropoiesis or as a sequela to decreased RBC lifespan once the mature RBCs are in circulation.
Decreased and ineffective erythropoiesis
Erythropoiesis can be decreased due to an absolute or a relative lack of erythropoietin, or secondary to a decreased bone marrow response to erythropoietin. Erythropoietin is produced primarily by the kidney in the peritubular interstitial cells of the inner renal cortex and outer medulla. Renal hypoxia is the main driving factor stimulating erythropoietin synthesis. Erythropoietin production is decreased by both acute and chronic causes of kidney disease, and chronic kidney disease is known to be a common cause of non-regenerative anemia in the cat.27–29 Production of neutralizing antibodies against erythropoietin has occasionally been associated with the administration of ESAs, and infrequently results in a relative deficiency of erythropoietin and non-regenerative anemia in cats.7,8,30
Ineffective erythropoiesis can be secondary to absolute deficiencies in the nutrients essential for hemoglobin or RBC biosynthesis, such as iron. Deficiencies of vitamin B12 can also cause non-regenerative anemia in the cat due to inhibition of purine and thymidylate synthases, which impairs DNA synthesis within erythroblasts and causes erythroblast apoptosis.12–14 Ineffective erythropoiesis in the cat also occurs as a result of cytokine abnormalities seen in various states of inflammation, and is commonly designated anemia of chronic disease.
Primary bone marrow disorders, including PRCA, NRIMHA and AA, as well as myelophthisis, can cause both decreased and ineffective erythropoiesis in the cat.
Decreased RBC lifespan
Feline RBCs have an expected survival of 73 days, shorter than that of dogs which is 100–115 days. 31 In circulation, the RBC must retain the ability to deform in order to deliver oxygen to tissues. With age, RBCs accumulate cellular injuries, triggering the removal of these senescent RBCs from the circulation by the reticuloendothelial system (RES).
RBC oxidative damage is one of the principal mechanisms leading to RBC senescence. Because cats have higher numbers of oxidizable sulfhydryl groups in comparison to many other species, feline RBCs are more susceptible to oxidative injury, commonly manifested as Heinz body formation.2,32 Feline RBCs also possess a lower intrinsic antioxidant capacity than many other species, such as humans, rabbits and mice, 33 which further increases the risk of Heinz body formation. 34 Feline RBCs lack N-acetyl transferase 2 (NAT2) and its absence results in a futile metabolic pathway where the reactive compound p-aminophenol is produced, leading to perpetuation of methemoglobin production. 35 Clinically healthy, non-anemic cats may have small, single Heinz bodies found on a minority of RBCs due to closed splenic circulation, increased feline RBC sulfhydryl groups and reduced feline RBC reductive capacity.32,36,37 However, the presence of Heinz bodies on the majority of RBCs, large Heinz bodies or multiple Heinz bodies per RBC should alert the feline practitioner to underlying pathology.36,37
Oxidative stress, mechanical stress, complement-induced injury, rearrangement of membrane phospholipids, contact with cationic proteins released from activated neutrophils, Heinz body formation and hemotropic parasites all alter cytoplasmic viscosity, resulting in impaired deformability, which targets RBCs for early removal from circulation. 38 Similarly, hereditary erythrocyte defects such as membrane protein abnormalities, erythrocyte enzyme deficiencies, hemoglobinopathies and increased osmotic fragility lead to shortened RBC lifespan. Excessive activation of the RES, as seen with certain immune-mediated, infectious, inflammatory and paraneoplastic conditions, can also stimulate early removal of RBCs from the circulation. In general, disorders resulting in decreased RBC lifespan cause regenerative anemia in cats; however, this is variable and non-regenerative anemia can also be seen (Table 1).
Table 1.
‘Regenerative’ anemias that do not always play by the rules
| Disease* | Breed association | Cats reported with non-regenerative anemia |
|---|---|---|
| Porphyria | Siamese, domestic shorthair | Case reports39,40 |
| Erythrocyte osmotic fragility | Abyssinian, Somali, domestic shorthair, others | 41% 41 |
| Pyruvate kinase deficiency | Abyssinian, 42 Somali, 42 Bengal, 43 Egyptian Mau, 43 LaPerm, 43 Maine Coon, 43 Norwegian Forest Cat, 43 Savannah, 43 Siberian, 43 Singapura 43 | 11% 42 |
| Hemotrophic mycoplasmas | None reported | Up to 50% 20 |
| Immune-mediated hemolytic anemia | None reported | 57% 44 |
| Propylthiouracil-induced hemolytic anemia | None reported | 66% 3 |
While typically associated with regenerative anemia, these diseases may cause a non-regenerative anemia in a proportion of cases
Table 2.
Breeds where ‘normal’ may not be the norm
| Hematiocrit | Reticulocy | te count | ||
|---|---|---|---|---|
| Breed* | Percentage of population outside of standard RI | Suggested breed-specific RI (l/l) | Percentage of population outside of standard RI | Suggested breed-specific RI (x 1012/l) |
| Holy Birman | 30% | 0.28–0.55 53 | 30% | <0.12 53 |
| Norwegian Forest Cat | 30% | 0.22–0.55 53 | 30% | 0.01–0.08 53 |
| Siberian | 35% | 0.23–0.65 53 | 40% | <0.19 53 |
| Maine Coon | 0%† | 0.37–0.48 54 † | 35% | <0.113–0.250 54 |
Breeds that have been shown to have a reference interval (RI) that differs from other published feline RIs for hematocrit and reticulocyte count
The proposed RI for hematocrit in Maine Coons is narrower than most other published feline RIs
Diagnosis
Reticulocyte counts
A reticulocyte count is required to differentiate regenerative from non-regenerative anemia and an elevated reticulocyte count indicates a regenerative anemia. The definition of reticulocytosis in the cat ranges from >0.045 x 1012/l to >0.060 x 1012/l.45–47 However, interpreting reticulocyte counts is more complex than just assessing the absolute number of reticulocytes. Some authors advocate using a four-tiered system for assessing reticulocytes: strongly, moderately and weakly regenerative and non-regenerative based on reticulocyte counts of >0.2 x 1012/l, 0.06–0.19 x 1012/l, 0.016–0.05 x 1012/l and <0.015 x 1012/l, respectively. 48 Cats produce two types of reticulocytes: punctate and aggregate. Aggregate reticulocytes are released by the bone marrow and then mature into punctate reticulocytes while in circulation. Aggregate reticulocytes are those considered to represent the regenerative response. Punctate reticulocytes are not considered when differentiating regenerative from non-regenerative anemia.
Feline reticulocytes can be counted using manual and automated techniques. Although reticulocyte counts are slightly higher using automated techniques, results obtained from different automated hematology analyzers (Sysmex XT-2000iV, ADVIA 2120, Cell Dyn and ProCyte Dx) are similar and do correlate with manual aggregate reticulocyte counts.49–51 Reference laboratories typically report reticulocyte numbers based on automated counts unless manual counts are specifically requested or if there is sample interference, such as with autoagglutination. Because reticulocytes continue to mature ex vivo, reticulocyte counts should be performed as soon as possible after blood sample collection in order to obtain an accurate count. 49
Reticulocyte percentages
The reticulocyte percentage and corrected reticulocyte percentage are additional parameters used to quantify regeneration. These methods of assessing regeneration consider bone marrow production of reticulocytes and the peripheral RBC count by incorporating these numbers into the calculation. The formula for corrected reticulocyte percentage also considers the severity of the anemia. A recent study shows reticulocyte percentage may discriminate between regenerative and non-regenerative anemia and can be used when evaluating anemic cats. 52 Recommended cut-offs for a lack of regeneration are a reticulocyte percentage <1.70 for manual counts and <3.06 for automated counts. 52

Differential diagnosis
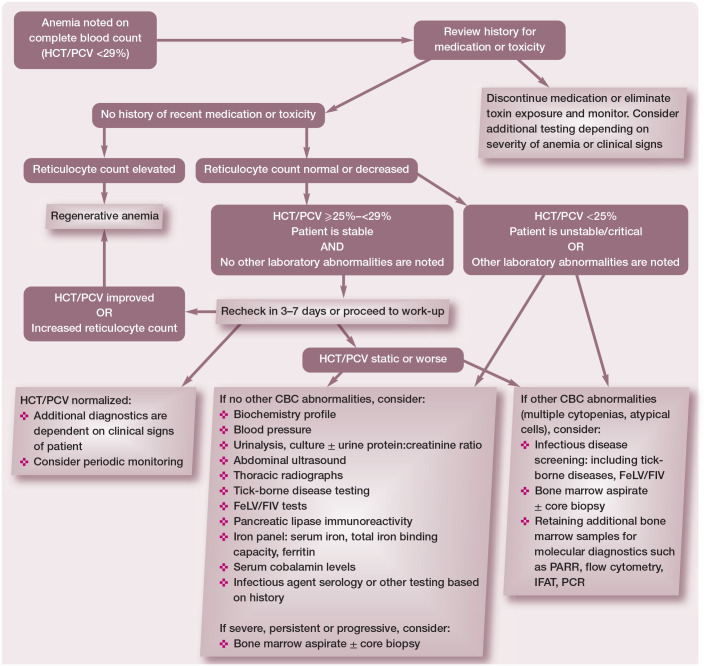
The differential diagnosis list for causes of non-regenerative anemia in cats is extensive, although some causes are more common than others (see box on page 615). Differential diagnoses should be prioritized based on patient signalment, detailed history and clinical presentation. Use of an algorithm to guide diagnostic recommendations will help to minimize unnecessary testing while simultaneously maximizing the chances of obtaining a diagnosis (Figure 1).
Figure 1.
Algorithm for the identification and evaluation of a cat with non-regenerative anemia. HCT = hematocrit; PCV = packed cell volume; CBC = complete blood count; FeLV = feline leukemia virus; FIV = feline immunodeficiency virus; PARR = PCR for antigen receptor rearrangements; IFAT = immunofluorescence antibody test. Adapted from Ettinger SJ, Feldman EC and Cote E. Textbook of Veterinary Internal Medicine, Expert Consult, 8th ed, 2017, with permission of Elsevier
If initial hematology, biochemistry and infectious disease testing and diagnostic imaging do not identify the cause of non-regenerative anemia, the presence of cytopenias, abnormal blood cells identified on a blood smear, organ enlargement or diagnostic imaging abnormalities should prompt additional testing such as blood smear review (Table 3), bone marrow cytology or organ aspiration cytology (Table 4). The degree of anemia can also help to prioritize differentials. A severe anemia (HCT <14%), especially in a hemodynamically stable patient, suggests a slow onset of anemia and is more commonly associated with primary bone marrow disease, neoplasia and infectious diseases, including FIP, FeLV, FIV and coinfection with FIV and Haemoplasma species. 48
Table 3.
Blood smear evaluation for investigation of underlying causes of non-regenerative anemia in cats
| Abnormality | Possible causes | Image | Description of image |
|---|---|---|---|
| Red blood cell parasites | • Merozoites18,19 (Cytauxzoon felis)
Hemoplasmas 20 (Mycoplasma haemofelis, Mycoplasma haemominutum) |
|
Peripheral blood smear with Cytauxzoon piroplasms. Reproduced from JFMS 2015; 17: 1069–1072, with permission of Amy MacNeill |
| Circulating mast cells or other atypical cells | • Mast cell tumor55–57
• Lymphoma 58 • Leukemia • Multiple myeloma 59 |
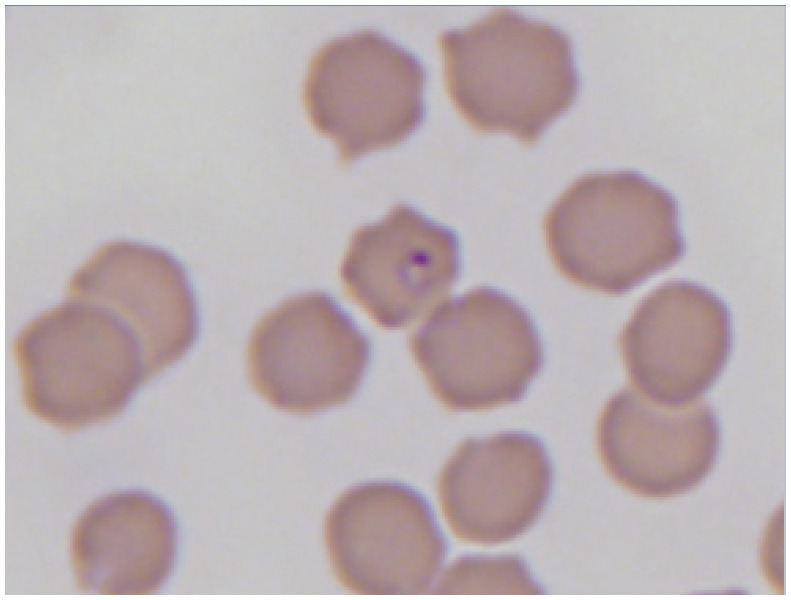
|
Erythroid precursors in a peripheral blood smear of an anemic cat. Courtesy of Andrea Siegel |
| Acanthocytes | • Neoplasia
48
• Bone marrow disorders 48 • Hepatic lipidosis and other liver disease11,60 • Doxorubicin therapy 61 |
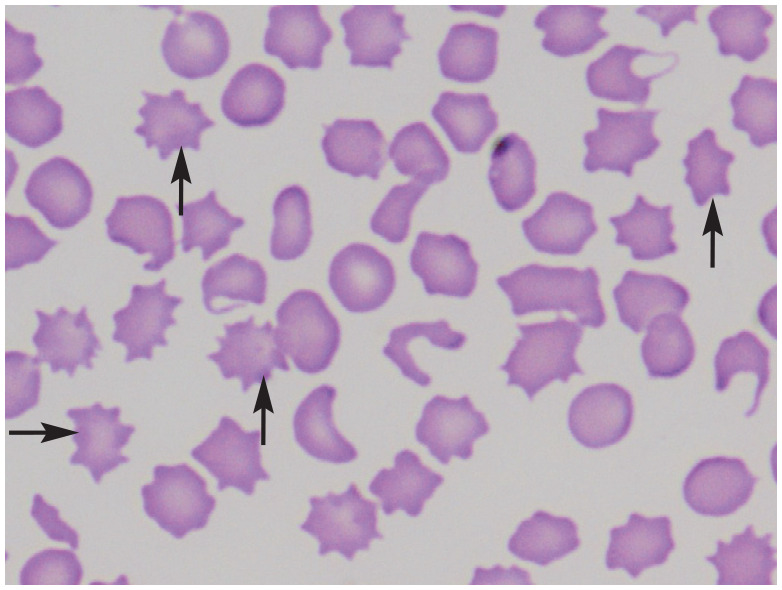
|
Acanthocytes in a peripheral blood smear of a cat with renal lymphoma. Arrows point to selected acanthocytes. Courtesy of Delphine Rivière |
| Elliptocytes/ovalocytes | • Myelofibrosis
37
• Iron deficiency 37 • Hepatic lipidosis and other liver disease 60 • Doxorubicin therapy 61 |
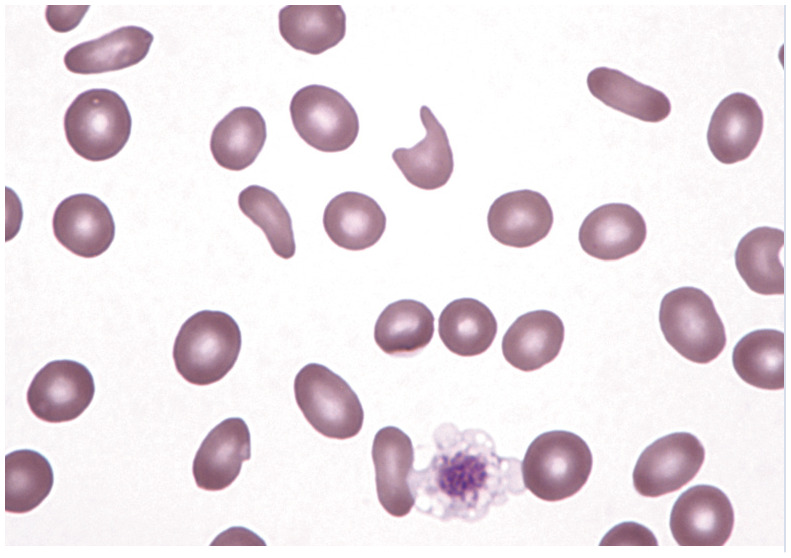
|
Multiple elliptocytes/ovalocytes in a peripheral blood smear. Reproduced from JFMS 2017; 19: 529–540, with permission of John Harvey |
| Schistocytes | • Neoplasia
37
• Liver disease37,60 • Hemophagocytic histiocytic disease37,62 • Acquired dyserythropoiesis 37 • Iron deficiency 37 • Disseminated intravascular coagulation 37 • Doxorubicin therapy 61 |
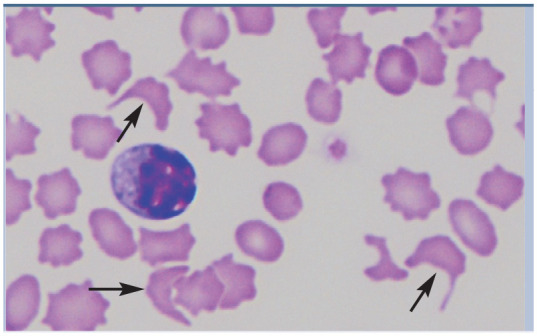
|
Schistocytes (arrows) in a peripheral blood smear of a cat with renal lymphoma. Courtesy of Delphine Rivière |
| Hemophagocytosis | • Histiocytic disease
63
• Mast cell neoplasia 56 • Mycoplasma haemofelis 64 |
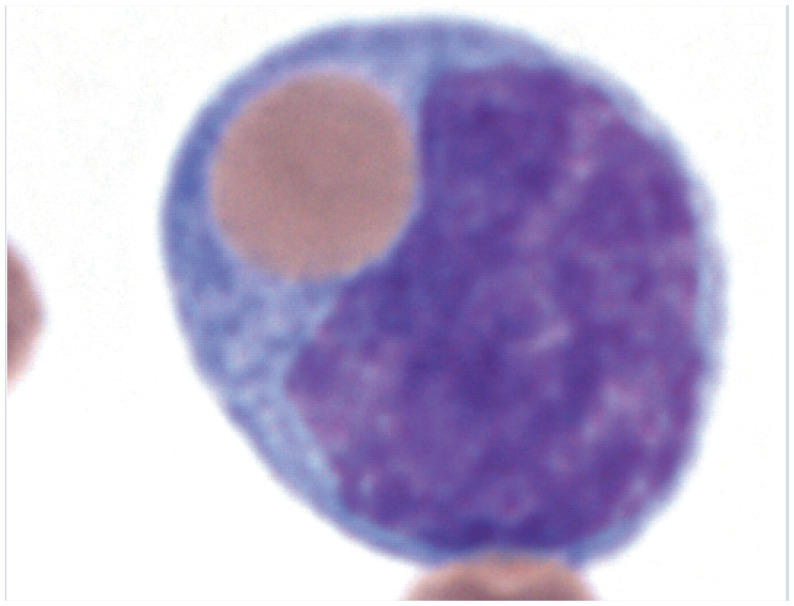
|
Peripheral blood smear with a phagocytized erythrocyte in a monocyte. Reproduced from JFMS 2017; 19: 747–757, with permission of John Harvey |
| Heinz bodies | • Acetaminophen37,64
• Onions and other Allium species37,65 • Benzocaine 37 • Propofol66,67 • Methionine 37 • Methylene blue 37 • Propylene glycol32,37,68 • Phenazopyridine 37 • Diabetes mellitus36,37,60,69 • Hyperthyroidism 36 • Lymphoma 36 • Liver disease 69 • Others 36 |
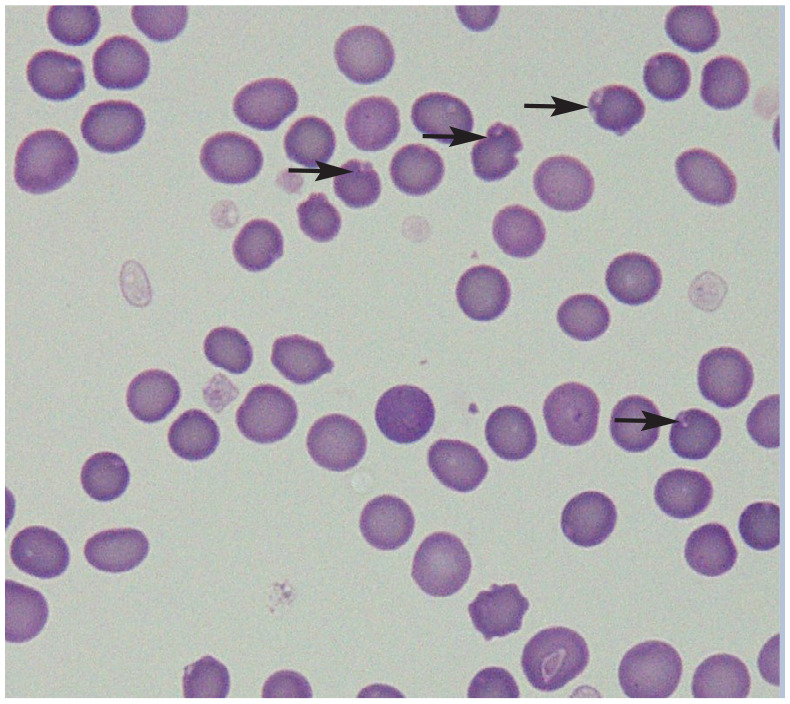
|
Red blood cells with Heinz bodies in a peripheral blood smear of a cat. Arrows point to selected Heinz bodies. Courtesy of Delphine Rivière |
Table 4.
Cytology findings in cats with non-regenerative anemia
| Abnormality | Possible locations | Possible causes | Image | Description of image |
| Erythrophagocytosis | Bone marrow, spleen, lymph node, liver | • Immune-mediated hemolytic anemia
5
• Histiocytic disorder63,70,71 • Multiple myeloma72,73 • Lymphoma58,74 • Acute myeloblastic leukemia 75 • Leishmaniosis 21 |
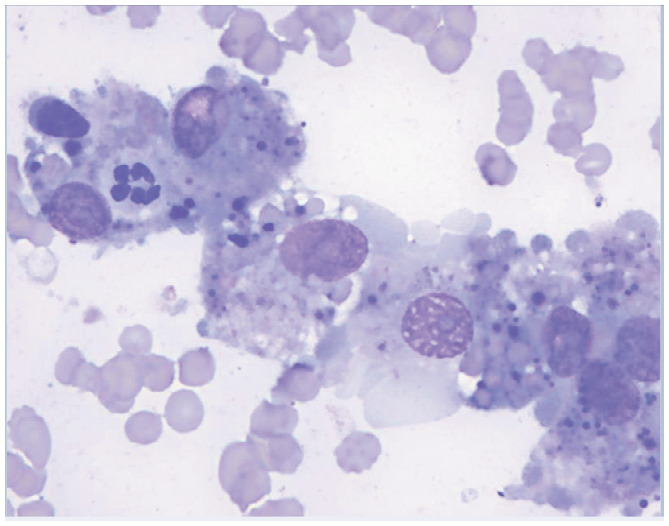
|
Erythrophagocytosis in the spleen of an anemic cat. Courtesy of Andrea Siegel |
| Increased numbers of mast cells | Bone marrow, spleen | • Mast cell neoplasia
• Histiocytic disorder 71 Leukemia 75 |
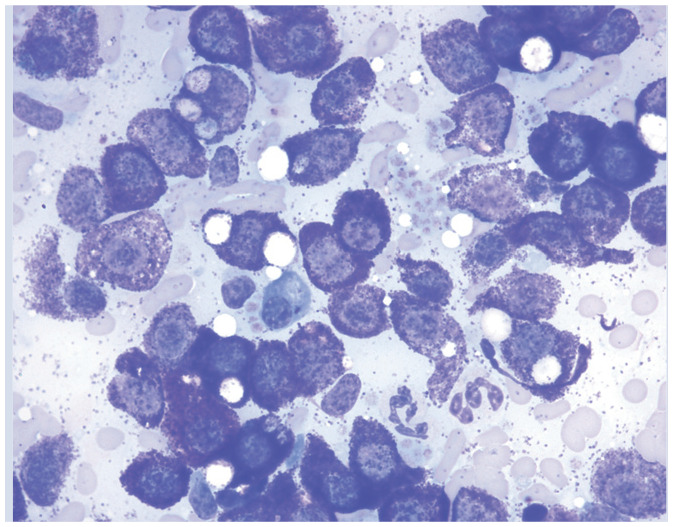
|
Aspiration cytology of a splenic mast cell tumor. Courtesy of Andrea Siegel |
| Monomorphic population of lymphoid cells | Bone marrow, spleen, lymph node, other organs | • Lymphoma |
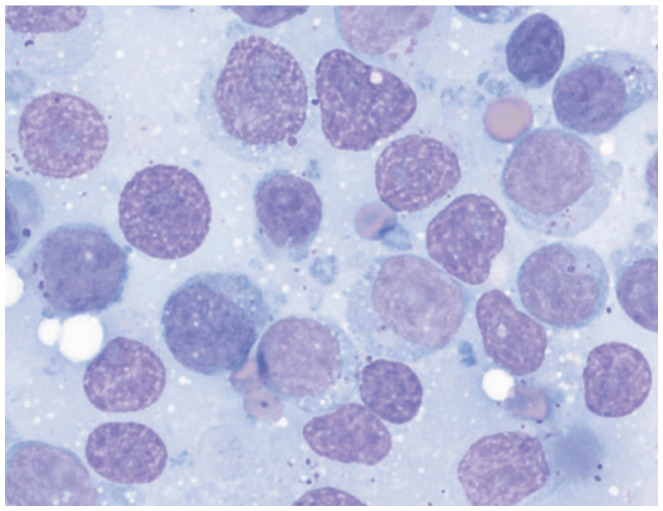
|
Renal aspiration showing large granular lymphoma. Courtesy of Andrea Siegel |
| Fungal organisms | Bone marrow, spleen, lymph node, other organs | • Systemic fungal infection |
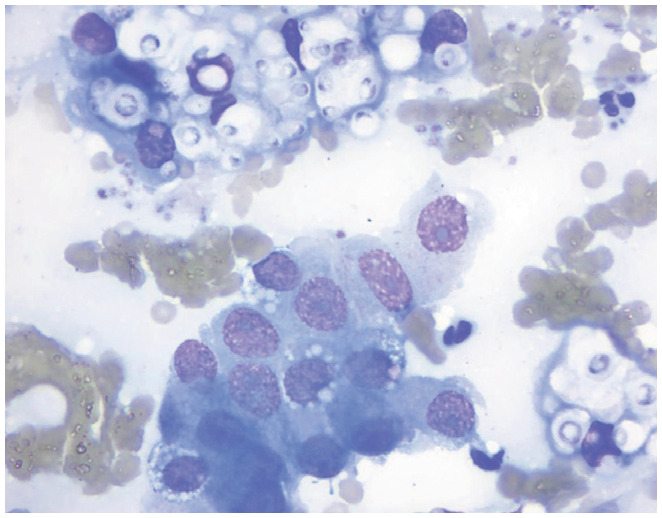
|
Transthoracic pulmonary aspiration cytology showing Cryptococcus organisms. Courtesy of Andrea Siegel |
| Mycobacterium species | Bone marrow, spleen, lymph node, other organs | • Systemic mycobacterial infection25,26 |
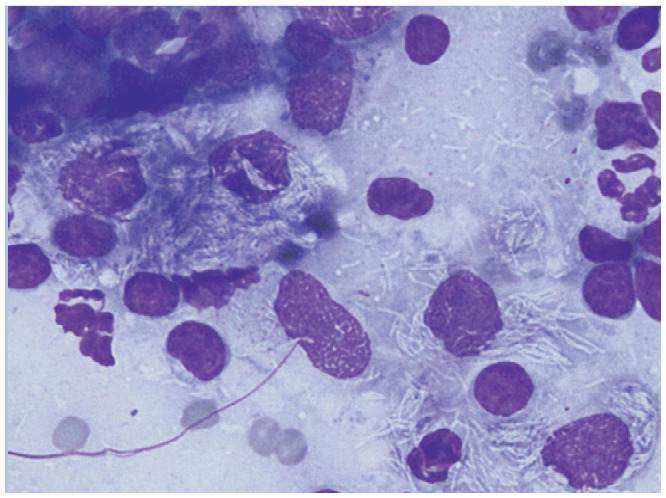
|
Aspiration cytology of mesenteric lymph nodes containing Mycobacterium species. Reproduced from JFMS 2011; 13: 125–128, with permission of Delphine Rivière |
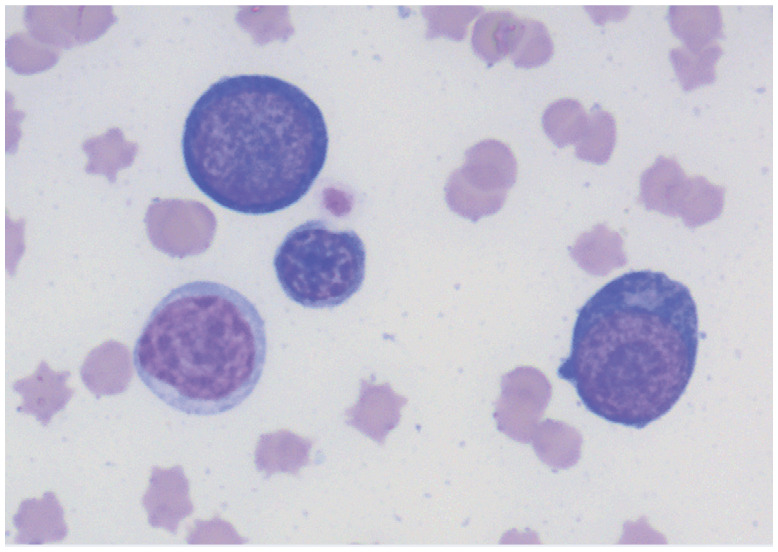
| Myelodysplasia | Bone marrow | • Primary myelodysplastic syndromes
• Secondary myelodysplasia from drug reaction or radiation therapy |
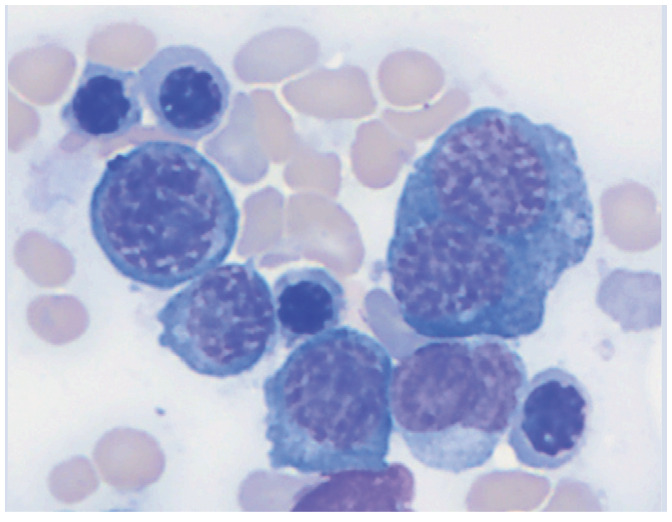
|
Bone marrow cytology showing a dysplastic, binucleate prorubricyte. Courtesy of Andrea Siegel |
Common causes of non-regenerative anemia
Infectious diseases
FeLV and FIV are the most common infectious causes of non-regenerative anemia in the cat (see box on page 615). Some infectious diseases are geographically specific and so it is important to be familiar with the local diseases and to obtain a travel history.
Iron deficiency
Iron deficiency is typically related to chronic blood loss in the cat (gastrointestinal, parasitic from severe flea infestation, or anecdotally reported in cases of chronic hematuria). Iron stores are depleted over time as the bone marrow compensates for ongoing losses, resulting in a progressive non-regenerative anemia. In many species, anemia secondary to iron deficiency results in microcytosis and hypochromasia.
Differentiating between iron deficiency anemia and anemia of inflammation can be difficult and, in some cases, both conditions may coexist. In cats, because conventional evaluation of mean cell volume and mean cell hemoglobin concentration for microcytosis and hypochromasia are insensitive markers of iron deficiency, reticulocyte indices are better indicators of iron status. 76 Decreased reticulocyte hemoglobin content (CHR <0.88 fmol) has a 93.8% sensitivity and 76.9% specificity for identifying iron deficiency in cats; 77 however, reticulocyte hemoglobin is not uniformly available.
Measurement of iron parameters may further differentiate iron deficiency anemia from anemia of inflammation, although the distinction can still be difficult. Serum iron levels are low in both conditions. Ferritin, the soluble storage form of iron in tissues, is typically decreased in iron deficiency anemia and increased in anemia of inflammation. Transferrin, the main protein that binds and transports iron in blood, is measured indirectly as total iron binding capacity (TIBC), and TIBC levels are typically normal to increased in cases of iron deficiency, although concurrent inflammation can decrease TIBC and hinder interpretation.78 Iron saturation percentages (serum iron/TIBC) <20% can be suggestive of iron deficiency.78 While iron assays may help to provide insight, in some cases these results may not change therapeutic recommendations.
Bone marrow disorders
Multiple primary (medullary) and secondary (extramedullary) bone marrow disorders cause ineffective erythropoiesis, producing a non-regenerative anemia. These include: PRCA, NRIMHA, AA/pancytopenia, primary and secondary MDSs, myelofibrosis and primary and secondary myelophthisis. While bone marrow disorders as a general category are common causes of non-regenerative anemia in the cat, individual specific diagnoses are uncommon in cats. Bone marrow aspirate and cytology and/or bone marrow biopsy may be required to distinguish between these bone marrow disorders.
PRCA, NRIMHA and AA present to the feline practitioner with a similar clinical picture: a profoundly anemic cat without obvious blood loss and often in need of an urgent RBC transfusion. Each may be idiopathic/immune-mediated or triggered by an underlying cause, such as FeLV infection. These three forms of non-regenerative anemia are differentiated by complete blood count (CBC), and bone marrow cytology and histopathology findings. Both PRCA and NRIMHA, but not AA, may produce increased numbers of lymphocytes in the peripheral blood, lymphocyte aggregates in bone marrow samples and appear to occur in younger cats.44,79–81 Recently an association has been made between IMHA and pancreatitis. 82
PRCA The CBC in cats diagnosed with PRCA is characterized by a normocytic, normochromic, non-regenerative anemia in the absence of other cytopenias. Bone marrow cytology and biopsy demonstrate decreased to absent RBC precursors. 80
NRIMHA The CBC in cats diagnosed with NRIMHA is characterized by either a normocytic or macrocytic anemia, and often neutropenia or thrombocytopenia. 83 The macrocytosis is spurious and due to agglutination as NRIMHA lacks reticulocytes, which are the typical cause of macrocytosis. 44 In addition to the lack of reticulocytes, cats diagnosed with NRIMHA should have at least one of the following laboratory findings: persistent agglutination following saline washing of RBCs, positive direct antiglobulin test, or the presence of ghost cells on a fresh blood smear.44,81 Bone marrow cytology in cats diagnosed with NRIMHA demonstrates marked erythroid hyperplasia with a low myeloid:erythroid ratio. There may be a maturation arrest at a certain stage of erythropoiesis (eg, a reduction in proportion of metarubricytes relative to rubricytes). Sometimes erythrophagocytosis is seen. Commonly dysplasia, fibrosis, necrosis and evidence of inflammation are seen in the bone marrow of cats with NRIMHA and these abnormalities contribute to the non-regenerative anemia. 83
AA The CBC in cats diagnosed with AA is characterized by pancytopenia, although some authors add bicytopenia to the definition. 4 Bone marrow cytology and biopsy reveal a marked reduction in, or absence of, normal hematopoietic tissue, which is replaced by fat. 4
MDS The CBC in cats diagnosed with MDS shows bicytopenia or pancytopenia as a result of ineffective hematopoiesis.84,85 Macrocytosis is common. 86 Bone marrow samples from these cats are normocellular to hypercellular, with blast cell counts less than 30% of nucleated cells. 87 Blast cells can be either rubriblasts or myeloblasts. Dysplastic change is seen in all three cell lines: RBCs, white blood cells and platelets. MDS is considered a preleukemic state since acute myelogenous leukemia commonly develops.84,88 FeLV infection is common in cats with MDS. 85 Secondary MDS results from chemotherapy or radiation therapy.
Secondary dysmyelopoiesis The CBC in cats diagnosed with secondary dysmyelopoiesis shows a non-regenerative anemia without macrocytosis. 86 Bone marrow specimens have normal to increased cellularity, with dysplastic changes most prominent in the red cell line. Blast cell numbers are not increased. Underlying disorders associated with secondary dysmyelopoiesis include immune-mediated hematologic disorders, glomerulonephritis and FIP. 86 Secondary dysmelopoeisis is very uncommon in cats.
Myelofibrosis The CBC and bone marrow sample from rare cases in cats diagnosed with myelofibrosis may reflect the underlying etiology, which includes MDS, acute myelogenous leukemia, NRIMHA and PRCA; however, often the changes are so severe that the underlying cause cannot be determined.83,88,89 Confirmation of myelofibrosis requires histopathology with special staining to identify an excess of reticulin fibrosis, which is absent in normal feline bone marrow. 88
Myelophthisis Infiltration and replacement of normal bone marrow space by abnormal cells results in non-regenerative anemia. In primary myelophthisis, the infiltrating cells originate in the bone marrow. In secondary myelophthisis, the neoplastic cells originate from neoplasia outside the medullary space. The CBC in cats diagnosed with either primary or secondary myelophthisis would be expected to be similar, with variable cytopenias. In rare cases, neoplastic cells may also be noted on the CBC. Bone marrow cytology or histopathology would reveal infiltration with neoplastic hematopoietic cells.
Therapeutic options
Identifying the underlying cause of non-regenerative anemia is important for tailoring the patient’s treatment plan. Treatment of non-regenerative anemia includes supportive therapy such as increasing the oxygen-carrying capacity of the blood via RBC transfusion or with ESAs. Specific therapies should target the underlying cause and are not all covered within the scope of this article.
Supportive therapy
Transfusion
RBC transfusion can be used to rapidly increase oxygen-carrying capacity in non-regenerative anemia of any etiology. Approximately one-third of RBC transfusions in cats are for non-regenerative anemia;90,91 this may reflect the high prevalence of chronic kidney disease in cats.
All cats should be AB blood typed prior to the first transfusion and undergo crossmatching for subsequent transfusions. 92 Crossmatching in transfusion-naive cats has been recommended, but may not be required for a safe transfusion.93,94
Erythropoiesis-stimulating agents
In cats, ESAs are used commonly to treat non-regenerative anemia secondary to chronic kidney disease with good response.8,95 Use of ESAs for other primary medullary causes of non-regenerative anemia in the cat is mentioned sporadically in the literature without clear consensus regarding their utility.
An increase in HCT is reported with ESA use in cats with non-regenerative anemia due to AA, FIV and FIP.4,96,97 No increase in viral load was reported in cats with FIV. 96 In humans, ESAs are in widespread clinical use and are the most commonly used therapy for anemia secondary to MDSs, despite not being approved by the Food and Drug Administration for this specific use. 98 In one case series there appeared to be no clinical benefit in 2/4 cats with PRCA that received ESAs; however, interpretation of response, or lack thereof, is hindered as the cats were also on immunosuppressive medications, and durations of therapy and response were not reported. 99 The use of ESAs in cats with PRCA secondary to FeLV and in some cases with primary PRCA is not expected to be beneficial as endogenous erythropoietin levels are already elevated. Testing erythropoietin levels in cases of PRCA prior to administration of ESAs may be warranted.99,100
A number of recombinant human ESA products are available on the market including epoetin alfa, epoetin beta and darbepoetin alfa. Significant homology (>80%) between human erythropoietin and feline erythropoietin allows recombinant human products to bind to and interact with erythropoietin receptors in cats.101,102 Darbepoetin is hyperglycosylated compared with epoetin, resulting in a circulating half-life that is three times longer and a reduction in mean clearance rate by over 70% (ml/kg x h). 103 Both products may be associated with secondary PRCA, a refractory anemia after initial response to therapy as a sequela to the formation of anti-erythropoietin antibodies. Literature supports that this may occur in up to 8% of cats receiving darbepoetin or 25–45% cats receiving epoetin.7,8 With cessation of therapy, resolution of the anemia over months, associated with a concurrent reduction in antibody levels, has been reported; however, transfusions may be necessary in the interim. 7 The risk of PRCA, at least with epoetin, seems to increase with duration of therapy. 7 No longer produced, recombinant feline erythropoietin has historically been evaluated and was also shown to induce neutralizing antibodies, causing red cell aplasia in 30% of cats. 30 The choice of which agent to use should be based on numerous factors (see box).
Aside from PRCA, other side effects of these agents include hypertension, seizures and iron deficiency. 9 Concurrent monthly parenteral iron supplementation is recommended (see below). For epoetin specifically, additional adverse events of local reactions at injection sites (which may be predictive of antibody formation), fever, arthralgia and transient cutaneous or mucocutaneous reactions are also possible.7,9
Specific therapies
Targeted therapies to control blood loss
If chronic blood loss is suspected to be the cause of iron deficiency resulting in anemia, treatments to control bleeding and prevent further loss are indicated. Treatment options vary depending on the underlying disease and the source of the bleeding. These may include gastroprotective agents for ulcers, ectoparasite control and environmental decontamination for flea infestation or antiparasitics for chronic intestinal parasitism.
Iron supplementation
Cats being treated with ESAs or for iron deficiency anemia require iron supplementation. Parenteral iron supplementation is preferable given its more reliable absorption, especially if iron deficiency is secondary to malabsorption. Parenteral supplementation includes use of iron dextran, iron gluconate and iron sucrose. Iron dextran is the most common choice in veterinary medicine. The recommended dosage in cats is 10 mg/kg administered every 3–4 weeks.9,104 To decrease the risk of anaphylactic reaction, which occurs with intravenous iron dextran, intramuscular administration is recommended. Iron is absorbed slowly via the lymphatic system after injection, with approximately 60% absorption within 3 days and up to 90% after 1–3 weeks. 9
Oral iron supplementation, however, is the least expensive form of supplementation and is also considered the safest. Ferrous sulfate is the most common of the oral supplements, with varied dosing reported. The recommended dosage is 50–100 mg/cat/day PO for a total of 8.8–25 mg of elemental iron.9,104 Side effects are typically mild but include gastrointestinal upset. Concurrent administration with food or with calcium may reduce absorption; so will medications that increase gastric pH. 9 Administration of iron orally reduces absorption of antibiotics such as fluoroquinolones and tetracyclines, and dose spacing is recommended. 104 Other oral iron supplements are available including ferrous gluconate dosed at 16.25 mg/kg/day and ferrous fumarate dosed at 2–4 mg/kg/day in the cat. 104
Vitamin B12 supplementation
Anemia as a consequence of hypocobalaminemia requires vitamin B12 supplementation. As with iron, parenteral administration is preferred if the hypocobalaminemia is secondary to malabsorption. In cats with inflammatory bowel disease, the half-life of serum cobalamin is shortened from 12.75 days to 5 days, prompting the recommendation for weekly administration at least initially. 105
For parenteral supplementation vitamin B12 is administered as cyanocobalamin. Dosing protocols vary, with the most recent recommendation being to administer 250 μg SC once weekly for 6 weeks, followed by an additional dose 30 days later; serum cobalamin is reassessed 30 days after the final injection.9,106 Data supports that weekly injections increase serum cobalamin levels to supranormal levels. However, a recent publication demonstrated that following completion of a 6 week course of 250 μg SC once weekly, 95% of cats with hypocobalaminemia and enteropathy experienced significant decreases in serum cobalamin levels at 4 weeks after completion of the protocol, and by 10 weeks 55% of the cats again had levels below the reference interval. 107 Given the recent data, chronic intermittent parenteral supplementation may be required to maintain serum cobalamin levels within the reference interval after the initial weekly regimen. Additional studies are required to determine an appropriate dosing protocol. Anecdotally, for cats where cyanocobalamin does not succeed in increasing serum cobalamin levels, hydroxocobalamin can be used at the same dose and frequency.106,108
Data supports that oral supplementation with cyanocobalamin can also achieve supranormal serum cobalamin levels in cats that are hypocobalaminemic. The dosing recommendation is 250 μg PO q24h with continuous supplementation. 109 Tapering protocols for oral cyanocobalamin administration have not been evaluated in the cat. The efficacy of oral supplementation in comparison to parenteral administration has not yet been established.
Immunosuppressive treatment of primary medullary causes of non-regenerative anemia
Because single case reports and case series predominate in the literature, evidence-based treatments for primary medullary disease are lacking in feline medicine. Multiple classification schemes exist for primary bone marrow diseases, making comparison of treatment outcomes difficult.
Dosages of immunosuppressive agents used in non-regenerative anemia are provided in Table 5.
Table 5.
Dosages for immunosuppressive drugs used to treat non-regenerative anemia
| Drug | Dose | Frequency | Reference |
|---|---|---|---|
| Prednisone/prednisolone | 2–4 mg/kg PO | q24h | 5 |
| 1–2 mg/kg PO | q12h | 44 | |
| Ciclosporin | 5–20 mg/kg PO | q24h | 80 |
| Chlorambucil | 2 mg/cat PO | q48h | 5 |
| Mycophenolate mofetil | 10 mg/kg PO | q12h | 110 |
| 10 mg/kg IV | q12h x 3 days | 111 | |
| 15 mg/kg PO | q12h x 7 days | 111 | |
| Cytosine arabinoside | 0.7–1.4 mg/kg SC | q24h | 84 |
| 20 mg/m2 SC | q24h | 112 |
Glucocorticoids Because glucocorticoids are anti-inflammatory, immunosuppressive and, in lymphoid malignancies, antineoplastic, nearly all cats with primary medullary disease receive treatment with glucocorticoids. A significant proportion of cases of PRCA and NRIMHA respond to glucocorticoid monotherapy.5,44,81 In other bone marrow disorders, response is more variable.
Ciclosporin The most well studied and widely used adjuvant to or substitute for glucocorticoids is ciclosporin, and this drug has been used in cats with NRIMHA, PRCA and MDS.5,80,81,84
Ciclosporin is available for intravenous and oral administration. Reports of ciclosporin use in cats include administration of the feline oral microemulsion solution as well as human oral solution, ciclosporin in oil and generic products. 80 Compounded transdermal ciclosporin in pluronic lecithin organogel is inconsistently absorbed and is not recommended for use in cats. 113 The feline oral microemulsion solution of ciclosporin administered at the label dosage reaches steady state in 7 days; however, doses needed to achieve clinical remission for PRCA cases have tended to be higher than the labeled dose, with 2–7 weeks required to achieve clinical remission.80,114 The optimal blood level of ciclosporin is unknown, but in a group of cats with PRCA, whole blood trough levels of 96–368 ng/ml were associated with clinical remission. 80
Gastrointestinal signs are among the most common adverse effects associated with ciclosporin administration. These include vomiting, hypersalivation, diarrhea, weight loss, regurgitation and lethargy.115,116 Effects on appetite are variable, with both increases and decreases reported, and anorexia only occurring rarely.114,117 Secondary infections, including toxoplasmosis and salmonellosis, have been reported.80,118–120 Less common adverse reactions include anaphylaxis during intravenous infusion of ciclosporin and hepatotoxicity. 80 Malignancy, most commonly lymphoma, has been reported in cats receiving long-term ciclosporin.114,121,122 Cats receiving chronic ciclosporin therapy following renal transplantation are at six times higher risk of both developing a malignant tumor and of lymphoma. 122
Chlorambucil A limited number of cats with NRIMHA have responded to treatment with chlorambucil as an adjunct to glucocorticoid therapy.5,81 Adverse effects reported with chlorambucil use include myelosuppression, hepatopathy, Fanconi syndrome and myoclonus.123–125 Compounded chlorambucil suspension has limited stability (7 days at 5°C), 126 which is not practical for routine use.
Mycophenolate mofetil Successful treatment of two cats with regenerative IMHA with a compounded oral suspension of mycophenolate has been described, and thus mycophenolate could be considered for the treatment of NRIMHA, PRCA or AA. 110 Despite pharmacokinetic studies showing biotransformation of mycophenolate to its active metabolite in cats, blood levels are variable and unpredictable and, without drug monitoring, there is the risk of over- or under-dosing. 127 Gastrointestinal side effects are dose-related and appear to be self-limiting. 111
Azathioprine The use of azathioprine is not recommended in feline patients and a safe dose has not been determined. 128 Cats have been shown to have lower activity of RBC thiopurine methyltransferase, the enzyme important in the metabolism of azathioprine, than dogs or humans. 129 Low levels of thiopurine methyltransferase are associated with increased adverse effects in humans and may play a role in the severe neutropenia reported in cats treated with azathioprine. 128
Cytosine arabinoside (cytarabine) Although cytosine arabinoside is a chemotherapy agent most commonly used in feline renal lymphoma, it has been used to treat feline MDS and myelofibrosis.84,112 The dose administered to treat these bone marrow diseases is lower and administration is daily by subcutaneous injection. Side effects with this dosing regimen have not been reported.
Vitamin K2 Data available only in abstract form indicates a vitamin K2 analog (menatetrenone) was beneficial in feline MDSs when given at a dose of 2 mg/kg, but optimal dosing in the cat has not been determined. 130 Menatetrenone promotes cellular differentiation in vitro.
New directions in therapy
Management of non-regenerative anemia of inflammation using iron and ESAs is ineffective for some patients and also there are risks, driving the search for novel therapies to stimulate erythropoiesis. Many of these therapies target the hepcidin–ferroportin axis. Direct antagonists inhibit hepcidin function.131–133 Hepcidin production inhibitors (including IL-6 pathway inhibitors and vitamin D) prevent hepcidin transcription.131–133
Ferroportin agonists and stabilizers promote ferroportin resistance to hepcidin action.131–133 Prolyl hydroxylase inhibitors reduce hepcidin levels and also stabilize hypoxia-inducible factors to promote endogenous erythropoietin production.131,133,134 Other targets include erythropoietin gene therapy.131,134
Many different drugs in these categories are under commercial development and in clinical trials for use in humans. 133 As their efficacy and safety profiles are established, these novel agents will likely also be considered in feline patients to expand our options for long-term management of these challenging cases.
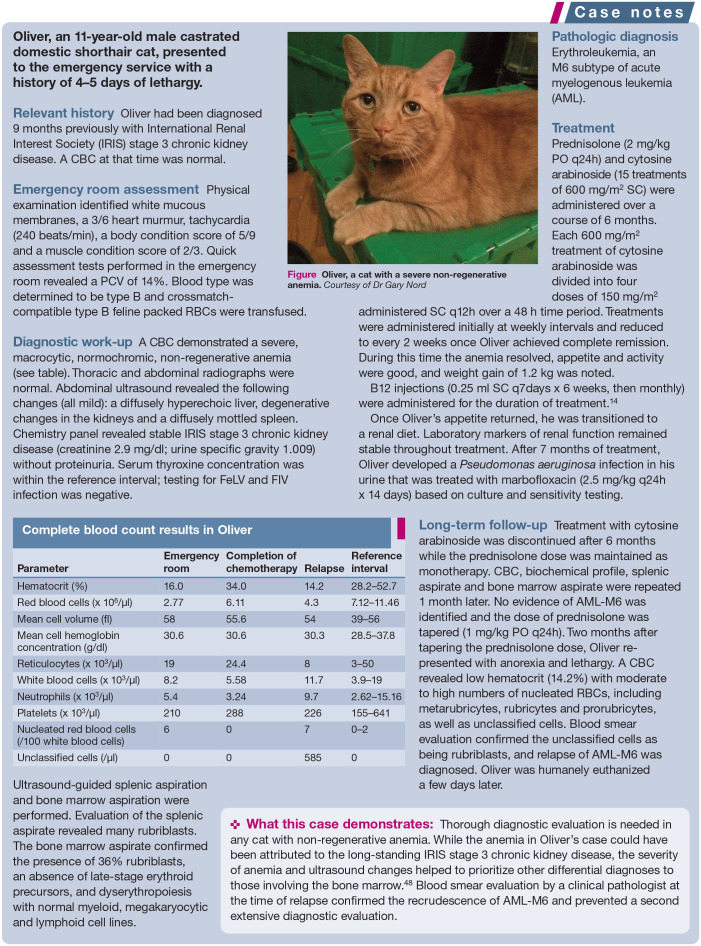
Figure 2.

Oliver, a cat with a severe non-regenerative anemia. Courtesy of Dr Gary Nord
Table 6.
Complete blood count results in Oliver
| Parameter | Emergency room | Completion of chemotherapy | Relapse | Reference interval |
|---|---|---|---|---|
| Hematocrit (%) | 16.0 | 34.0 | 14.2 | 28.2–52.7 |
| Red blood cells (x 106/μl) | 2.77 | 6.11 | 4.3 | 7.12–11.46 |
| Mean cell volume (fl) | 58 | 55.6 | 54 | 39–56 |
| Mean cell hemoglobin concentration (g/dl) | 30.6 | 30.6 | 30.3 | 28.5–37.8 |
| Reticulocytes (x 103/μl) | 19 | 24.4 | 8 | 3–50 |
| White blood cells (x 103/μl) | 8.2 | 5.58 | 11.7 | 3.9–19 |
| Neutrophils (x 103/μl) | 5.4 | 3.24 | 9.7 | 2.62–15.16 |
| Platelets (x 103/μl) | 210 | 288 | 226 | 155–641 |
| Nucleated red blood cells (/100 white blood cells) | 6 | 0 | 7 | 0–2 |
| Unclassified cells (/μl) | 0 | 0 | 585 | 0 |
Key Points
Obtain an aggregate reticulocyte count for every cat diagnosed with anemia.
Use all components of the CBC to direct the diagnostic plan.
Review the results of a minimum database, CBC, biochemical profile and urinalysis to direct further diagnostic testing.
A blood film should be reviewed by a clinical pathologist or trained technician for clues to the underlying cause of anemia.
Anemia of inflammation is a diagnosis of exclusion.
Once extramedullary causes of anemia are ruled out, a bone marrow aspirate/biopsy is appropriate.
Some diseases are geographically specific – know your local diseases and do not forget to obtain a travel history.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Lynch AM, Respess M, Boll AE, et al. Hospital-acquired anemia in critically ill dogs and cats: a multi-institutional study. J Vet Intern Med 2016; 30: 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balakrishnan A, Drobatz KJ, Reineke EL. Development of anemia, phlebotomy practices, and blood transfusion requirements in 45 critically ill cats (2009-2011). J Vet Emerg Crit Care 2016; 26: 406–411. [DOI] [PubMed] [Google Scholar]
- 3. Peterson ME, Hurvitz AI, Leib MS, et al. Propylthiouracil-associated hemolytic anemia, thrombocytopenia, and anti-nuclear antibodies in cats with hyperthyroidism. J Am Vet Med Assoc 1984; 184: 806–808. [PubMed] [Google Scholar]
- 4. Weiss DJ. Aplastic anemia in cats - clinicopathological features and associated disease conditions 1996-2004. J Feline Med Surg 2006; 8: 203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Black V, Adamantos S, Barfield D, et al. Feline non-regenerative immune-mediated anaemia: features and outcome in 15 cases. J Feline Med Surg 2016; 18: 597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stokol T, Randolph JF, Nachbar S, et al. Development of bone marrow toxicosis after albendazole administration in a dog and cat. J Am Vet Med Assoc 1997; 210: 1753–1756. [PubMed] [Google Scholar]
- 7. Cowgill LD, James KM, Levy JK, et al. Use of recombinant human erythropoietin for management of anemia in dogs and cats with renal failure. J Am Vet Med Assoc 1998; 212: 521–528. [PubMed] [Google Scholar]
- 8. Chalhoub S, Langston CE, Farrelly J. The use of darbepoetin to stimulate erythropoiesis in anemia of chronic kidney disease in cats: 25 cases. J Vet Intern Med 2012; 26: 363–369. [DOI] [PubMed] [Google Scholar]
- 9. Plumb DC. Veterinary drug handbook 8th ed. Stockholm, PharmaVet, 2015. Accessed via www.vin.com on December 6, 2017. [Google Scholar]
- 10. Boothe DM. Phenicols. Merck veterinary manual. http://www.merckvetmanual.com/pharmacology/antibac-terial-agents/phenicols (2017, accessed December 6, 2017).
- 11. Center SA, Crawford MA, Guida L, et al. A retrospective study of 77 cats with severe hepatic lipidosis: 1975-1990. J Vet Intern Med 1993; 7: 349–359. [DOI] [PubMed] [Google Scholar]
- 12. Vaden SL, Wood PA, Ledley FD, et al. Cobalamin deficiency associated with methylmalonic acidemia in a cat. J Am Vet Med Assoc 1992; 200: 1101–1103. [PubMed] [Google Scholar]
- 13. Koury MJ, Ponka P. New insights into erythropoiesis: the roles of folate, vitamin B12, and iron. Annu Rev Nutr 2004; 24: 105–131. [DOI] [PubMed] [Google Scholar]
- 14. Stanley EL, Eatroff AE. Hypocobalaminaemia as a cause of bone marrow failure and pancytopenia in a cat. Aust Vet J 2017; 95: 156–160. [DOI] [PubMed] [Google Scholar]
- 15. Gleich S, Hartmann K. Hematology and serum biochemistry of feline immunodeficiency virus-infected and feline leukemia virus-infected cats. J Vet Intern Med 2009; 23: 552–558. [DOI] [PubMed] [Google Scholar]
- 16. Shelton GH, Linenberger ML, Grant CK, et al. Hematologic manifestations of feline immunodeficiency virus infection. Blood 1990; 76: 1104–1191. [PubMed] [Google Scholar]
- 17. Paltrinieri S, Grieco V, Comazzi S, et al. Laboratory profiles in cats with different pathological and immunohistochemical findings due to feline infectious peritonitis (FIP). J Feline Med Surg 2001; 3: 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. MacNeill AL, Barger AM, Skowronski MC, et al. Identification of Cytauxzoon felis infection in domestic cats from southern Illinois. J Feline Med Surg 2015; 17: 1069–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carli E, Trotta M, Bianchi E, et al. Cytauxzoon sp. infection in two free ranging young cats: clinicopathological findings, therapy and follow up. Turkiye Parazitol Derg 2014; 38: 185–189. [DOI] [PubMed] [Google Scholar]
- 20. Weingart C, Tasker S, Kohn B. Infection with haemoplas-ma species in 22 cats with anaemia. J Feline Med Surg 2016; 18: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marcos R, Santos M, Malhao F, et al. Pancytopenia in a cat with visceral leishmaniasis. Vet Clin Pathol 2009; 38: 201–205. [DOI] [PubMed] [Google Scholar]
- 22. Pennisi MG, Cardoso L, Baneth G, et al. LeishVet update and recommendations on feline leishmaniosis. Parasit Vectors 2015; 8: 302. DOI: 10.1186/s13071-015-0909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Breitschwerdt EB, Abrams-Ogg AC, Lappin MR, et al. Molecular evidence supporting Ehrlichia canis-like infection in cats. J Vet Intern Med 2002; 16: 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Braga IA, dos Santos LG, Melo AL, et al. Hematological values associated to the serological and molecular diagnostic in cats suspected of Ehrlichia canis infection. Rev Bras Parasitol Vet 2013; 22: 470–474. [DOI] [PubMed] [Google Scholar]
- 25. Jordan HL, Cohn, Armstrong PJ. Disseminated Mycobacterium avium complex infection in three Siamese cats. J Am Vet Med Assoc 1994; 204: 90–93. [PubMed] [Google Scholar]
- 26. Hughes MS, Ball NW, Love DN, et al. Disseminated Mycobacterium genavense infection in a FIV-positive cat. J Feline Med Surg 1999; 1: 23–29. [DOI] [PubMed] [Google Scholar]
- 27. Maxwell PH, Ferguson DJ, Nicholls LG, et al. Sites of erythro-poietin production. Kidney Int 1997; 51: 393–401. [DOI] [PubMed] [Google Scholar]
- 28. Dibartola SP, Rutgers HC, Zack PM, et al. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973-1984). J Am Vet Med Assoc 1987; 190: 1196–1202. [PubMed] [Google Scholar]
- 29. Furman E, Leidinger E, Hooijberg EJ, et al. A retrospective study of 1098 blood samples with anemia from adult cats: frequency, classification and association with serum creatinine concentration. J Vet Intern Med 2014; 28: 1391–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Randolph JE, Scarlett JM, Stokol T, et al. Expression, bioactiv-ity, and clinical assessment of recombinant feline erythro-poietin. Am J Vet Res 2004; 65: 1355–1366. [DOI] [PubMed] [Google Scholar]
- 31. Smith JE. Erythrocytes. Adv Vet Sci Comp Med 1991; 36: 9–55. [DOI] [PubMed] [Google Scholar]
- 32. Christopher MM, White JG, Eaton JW. Erythrocyte pathology and mechanisms of Heinz body-mediated hemolysis in cats. Vet Pathol 1990; 27: 299–310. [DOI] [PubMed] [Google Scholar]
- 33. Trepanier LA, Cribb AE, Spielberg SP, et al. Deficiency of cytosolic arylamine N-acetylation in the domestic cat and wild felids caused by the presence of a single NAT1-like gene. Pharmacogenetics 1998; 8: 169–179. [DOI] [PubMed] [Google Scholar]
- 34. Court MH. Feline drug metabolism and disposition: pharma-cokinetic evidence for species differences and molecular mechanisms. Vet Clin North Am Small Anim Pract 2013; 43: 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McConkey SE, Grant DM, Cribb AE. The role of paraaminophenol in acetaminophen-induced methemoglobine-mia in dogs and cats. J Vet Pharmacol Ther 2009; 32: 585–595. [DOI] [PubMed] [Google Scholar]
- 36. Christopher MM. Relation of endogenous Heinz bodies to disease and anemia in cats: 120 cases (1978-1987). J Am Vet Med Assoc 1989; 194: 1089–1095. [PubMed] [Google Scholar]
- 37. Stockham SL, Scott MA. Erythrocytes. In: Stockham SL, Scott MA. (eds). Fundamentals of veterinary clinical pathology. 2nd ed. Ames, Iowa: Blackwell Publishing; 2008, pp 141–187. [Google Scholar]
- 38. Stuart J, Nash GB. Red cell deformability and haematological disorders. Blood Rev 1990; 4: 141–147. [DOI] [PubMed] [Google Scholar]
- 39. Clavero S, Bishop DF, Haskins ME, et al. Feline acute intermittent porphyria: a phenocopy masquerading as an ery-thropoietic porphyria due to dominant and recessive hydroxymethylbilane synthase mutations. Hum Mol Genet 2010; 19: 584–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schnier JJ, Hanna P. Feline porphyria associated with anemia, severe hepatic disease, and renal calculi. Can Vet J 2010; 51: 1146–1151. [PMC free article] [PubMed] [Google Scholar]
- 41. Tritschler C, Mizukami K, Raj K, et al. Increased erythrocytic osmotic fragility in anemic domestic shorthair and purebred cats. J Feline Med Surg 2016; 18: 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kohn B, Fumi C. Clinical course of pyruvate kinase deficiency in Abyssinian and Somali cats. J Feline Med Surg 2008; 10: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grahn RA, Grahn JC, Penedo MCT, et al. Erythrocyte pyru-vate kinase deficiency mutation identified in multiple breeds of domestic cats. BMC Vet Research 2012; 8: 207. DOI: 10.1186/1746-6148-8-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kohn B, Weingart C, Eckmann V, et al. Primary immune-mediated hemolytic anemia in 19 cats: diagnosis, therapy and outcome (1998-2004). J Vet Intern Med 2006; 20: 159–166. [DOI] [PubMed] [Google Scholar]
- 45. Fielder SE. Hematology reference ranges. Merck veterinary manual. http://www.merckvetmanual.com/appendixes/reference-guides/hematologic-reference-ranges (2017, accessed November 19, 2017).
- 46. IDEXX. Reticulocyte panel. Idexx laboratory test directory. https://www.idexx.com/small-animal-health/directory-tests-services.html (accessed November 25, 2017). [Google Scholar]
- 47. Antech Diagnostics. Reticulocyte count. https://online.antechdiagnostics.com/#/results/labresults (accessed November 29, 2017).
- 48. Korman RM, Hetzel N, Knowles TG, et al. A retrospective study of 180 anaemic cats: features, aetiologies and survival data. J Feline Med Surg 2013; 15: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bauer N, Nakagawa J, Dunker C, et al. Evaluation of the automated hematology analyzer Sysmex XT-2000iV compared to the ADVIA 2120 for its use in dogs, cats, and horses. Part II: Accuracy of leukocyte differential and retic-ulocyte count, impact of anticoagulant and sample aging. J Vet Diagn Invest 2012; 24: 74–89. [DOI] [PubMed] [Google Scholar]
- 50. Fujino Y, Nakamura Y, Matsumoto H, et al. Development and evaluation of a novel in-clinic automated hematology analyzer, ProCyte Dx, for canine erythrocyte indices, leuko-gram, platelet counts and reticulocyte counts. J Vet Med Sci 2013; 75: 1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weissenbacher S, Riond B, Hofmann-Lehmann R, et al. Evaluation of a novel haematology analyser for use with feline blood. Vet J 2011; 187: 381–387. [DOI] [PubMed] [Google Scholar]
- 52. Paltrinieri S, Fossati M, Menaballi V. Diagnostic performances of manual and automated reticulocyte parameters in anaemic cats. J Feline Med Surg 2018; 20: 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Paltrinieri S, Ibba F, Rossi G. Haematological and biochemical reference intervals of four feline breeds. J Feline Med Surg 2014; 16: 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Spada E, Antognoni MT, Proverbio D, et al. Haematological and biochemical reference intervals in adult Maine Coon cat blood donors. J Feline Med Surg 2015; 17: 1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Madewell BR, Gunn C, Gribble DH. Mast cell phagocytosis of red blood cells in a cat. Vet Pathol 1983; 20: 638–640. [DOI] [PubMed] [Google Scholar]
- 56. Tachikawa S, Tachikawa T. Hemophagocytic mastocy-toma with multiple cutaneous tumors in a cat. Jpn J Vet Derm 2009; 25: 75–78. [Google Scholar]
- 57. Harvey JW. The feline blood film: 2. leukocyte and platelet morphology. J Feline Med Surg 2017; 19: 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Darbes J, Majzoub M, Breuer W, et al. Large granular lymphocyte leukemia/lymphoma in six cats. Vet Pathol 1998; 35: 370 -379. [DOI] [PubMed] [Google Scholar]
- 59. Patel RT, Caceres A, French AF, et al. Multiple myeloma in 16 cats: a retrospective study. Vet Clin Pathol 2005; 34: 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Christopher MM, Lee SE. Red cell morphologic alterations in cats with hepatic disease. Vet Clin Pathol 1994; 23: 7–12. [DOI] [PubMed] [Google Scholar]
- 61. O’Keefe DA, Schaeffer DJ. Hematologic toxicosis associated with doxorubicin administration in cats. J Vet Intern Med 1992; 6: 276–282. [DOI] [PubMed] [Google Scholar]
- 62. Walton RM, Modiano JF, Thrall MA, et al. Bone marrow cyto-logical findings in 4 dogs and a cat with hemophagocytic syndrome. J Vet Intern Med 1996; 10: 7–14. [DOI] [PubMed] [Google Scholar]
- 63. Mylonakis ME, Soubasis N, Kritsepi-Konstantinou M, et al. Presumptive histiocytic neoplasm with unusual immuno-phenotype in a cat. Comp Clin Pathol 2012, 21: 231–236. [Google Scholar]
- 64. Harvey JW. The feline blood film: 1. Leukocyte and platelet morphology. J Feline Med Surg 2017; 19: 529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Robertson JE, Christopher MM, Rogers QR. Heinz body formation in cats fed baby food containing onion powder. J Am Vet Med Assoc 1998; 212: 1260–1266. [PubMed] [Google Scholar]
- 66. Andress JL, Day TK, Day D. The effects of consecutive day propofol anesthesia on feline red blood cells. Vet Surg 1995; 24: 277–282. [DOI] [PubMed] [Google Scholar]
- 67. Matthews NS, Brown RM, Barling KS, et al. Repetitive propo-fol administration in dogs and cats. J Am Anim Hosp Assoc 2004; 40: 255–260. [DOI] [PubMed] [Google Scholar]
- 68. Christopher MM, Perman V, Eaton JW. Contribution of propylene glycol-induced Heinz body formation to anemia in cats. J Am Vet Med Assoc 1989; 194: 1045–1056. [PubMed] [Google Scholar]
- 69. Adams LG, Hardy RM, Weiss DJ, et al. Hypophosphatemia and hemolytic anemia associated with diabetes mellitus and hepatic lipidosis in cats. J Vet Intern Med 1993; 7: 266–271. [DOI] [PubMed] [Google Scholar]
- 70. Court EA, Earnest-Koons KA, Barr SC, et al. Malignant histiocytosis in a cat. J Am Vet Med Assoc 1993; 203: 1300–1302. [PubMed] [Google Scholar]
- 71. Walton RM, Brown DE, Burkhard MJ, et al. Malignant histiocytosis in a domestic cat: cytomorphologic and immunohistochemical features. Vet Clin Pathol 1997; 26: 56–60. [DOI] [PubMed] [Google Scholar]
- 72. Webb J, Chary P, Northrup N, et al. Erythrophagocytic multiple myeloma in a cat. Vet Clin Pathol 2008; 37: 302–307. [DOI] [PubMed] [Google Scholar]
- 73. Dunbar MD, Lyles S. Hemophagocytic syndrome in a cat with multiple myeloma. Vet Clin Pathol 2013; 42: 55–60. [DOI] [PubMed] [Google Scholar]
- 74. Carter JE, Tarigo JL, Vernau W, et al. Erythrophagocytic low-grade extranodal T-cell lymphoma in a cat. Vet Clin Pathol 2008; 37: 416–421. [DOI] [PubMed] [Google Scholar]
- 75. Jain NC. Classification of myeloproliferative disorders in cats using criteria proposed by the animal leukaemia study group: a retrospective study of 181 cases (1969-1992). Comp Haematol Int 1993; 3: 125–134. [Google Scholar]
- 76. Fry MM, Kirk CA. Reticulocyte indices in a canine model of nutritional iron deficiency. Vet Clin Pathol 2006; 35: 172–181. [DOI] [PubMed] [Google Scholar]
- 77. Prins M, van Leeuwen MW, Teske E. Stability and reproducibility of ADVIA 120-measured red blood cell and platelet parameters in dogs, cats, and horses, and the use of reticulocyte haemoglobin content (CH(R)) in the diagnosis of iron deficiency. Tijdschr Diergeneeskd 2009; 134: 272–278. [PubMed] [Google Scholar]
- 78. Bohn AA. Diagnosis of disorders of iron metabolism in dogs and cats. Vet Clin North Am Small Anim Pract 2013: 43: 1319–1330. [DOI] [PubMed] [Google Scholar]
- 79. Weiss DJ. Differentiating benign and malignant causes of lymphocytosis in feline bone marrow. J Vet Intern Med 2005; 19: 855–859. [DOI] [PubMed] [Google Scholar]
- 80. Viviano KR, Webb JL. Clinical use of cyclosporine as an adjunctive therapy in the management of feline idio-pathic pure red cell aplasia. J Feline Med Surg 2011; 13: 885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Swann JW, Szladovits B, Glanemann B. Demographic characteristics, survival and prognostic factors for mortality in cats with primary immune-mediated hemolytic anemia. J Vet Intern Med 2016; 30: 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zoia A, Drigo M. Association between pancreatitis and immune-mediated haemolytic anaemia in cats: a cross sectional study. J Comp Pathol 2017; 156: 384–388. [DOI] [PubMed] [Google Scholar]
- 83. Weiss DJ. Bone marrow pathology in dogs and cats with non-regenerative immune-mediated haemolytic anaemia and pure red cell aplasia. J Comp Pathol 2008; 138: 46–53. [DOI] [PubMed] [Google Scholar]
- 84. Hisasue M, Okayama H, Okayama T, et al. Hematologic abnormalities and outcome of 16 cats with myelodysplastic syndromes. J Vet Intern Med 2000; 15: 471–477. [DOI] [PubMed] [Google Scholar]
- 85. Shimoda T, Shiranaga N, Mashita T, et al. Chronic myelomonocytic leukemia in a cat. J Vet Med Sci 2000; 62: 195–197. [DOI] [PubMed] [Google Scholar]
- 86. Weiss DJ. Evaluation of dysmyelopoiesis in cats: 34 cases (1996-2005). J Am Vet Med Assoc 2006; 228: 893–897. [DOI] [PubMed] [Google Scholar]
- 87. Jain NC, Blue JT, Grindem CB, et al. Proposed criteria for classification of acute myeloid leukemia in dogs and cats. Vet Clin Pathol 1991; 20: 63–82. [DOI] [PubMed] [Google Scholar]
- 88. Blue JT. Myelofibrosis in cats with myelodysplastic syndrome and acute myelogenous leukemia. Vet Pathol 1988; 25: 154–160. [DOI] [PubMed] [Google Scholar]
- 89. Weiss DJ. Feline myelonecrosis and myelofibrosis: 22 cases 1996-2006. Comp Clin Pathol 2007; 16: 181–185. [Google Scholar]
- 90. Castellanos I, Couto CG, Gray TL. Clinical use of blood products in cats: a retrospective study (1997-2000). J Vet Intern Med 2004; 18: 529–532. [DOI] [PubMed] [Google Scholar]
- 91. Klaser DA, Reine NJ, Hohenhaus AE. Red blood cell transfusions in cats: 126 cases (1999). J Am Vet Med Assoc 2005; 226: 920–923. [DOI] [PubMed] [Google Scholar]
- 92. Barfield D, Adamantos S. Feline blood transfusions: a pinker shade of pale. J Feline Med Surg 2011; 13: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Weltman JG, Fletcher DJ, Rogers C. Influence of cross-match on posttransfusion packed cell volume in feline packed red blood cell transfusion. J Vet Emerg Crit Care 2014; 24: 429–436. [DOI] [PubMed] [Google Scholar]
- 94. Sylvane B, Prittie J, Hohenhaus AE, et al. Effect of cross-match on packed cell volume after transfusion of packed red blood cells in transfusion-naive anemic cats. J Vet Intern Med 2018; 32: 1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chalhoub S, Langston C, Eatroff A. Anemia of renal disease: what it is, what to do and what’s new. J Feline Med Surg 2011; 13: 629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Arai M, Darman J, Lewis A, et al. The use of human hematopoietic growth factors (rhGM-CSF and rhEPO) as a supportive therapy for FIV-infected cats. Vet Immunol Immunopathol 2000; 77: 71–92. [DOI] [PubMed] [Google Scholar]
- 97. Tanaka Y, Sato Y, Takahashi D, et al. Treatment of a case of feline infectious peritonitis with cyclosporin A. Vet Rec Case Rep 2015; 3: 41. DOI: 10.1136/vetreccr-2014-000134. [DOI] [Google Scholar]
- 98. Casadevall N, Durieux P, Dubois S, et al. Health, economic, and quality-of-life effects of erythropoietin and granulocyte colony-stimulating factor for the treatment of myelodys-plastic syndromes: a randomized, controlled trial. Blood 2004; 104: 321–327. [DOI] [PubMed] [Google Scholar]
- 99. Stokol T, Blue JT. Pure red cell aplasia in cats: 9 cases (1989-1997). J Am Vet Med Assoc 1999; 214: 75–79. [PubMed] [Google Scholar]
- 100. Kociba GJ, Lange RD, Dunn CD, et al. Serum erythropoietin changes in cats with feline leukemia virus-induced ery-throid aplasia. Vet Pathol 1983; 20: 548–552. [DOI] [PubMed] [Google Scholar]
- 101. MacLeod JN, Tetreault JW, Lorschy KAS, et al. Expression and bioactivity of recombinant canine erythropoietin. Am J Vet Res 1998; 59: 1144–1148. [PubMed] [Google Scholar]
- 102. MacLeod JN. Species-specific recombinant erythropoietin preparations for companion animals. Proceedings of 2001 ACVIM Veterinary Medical Forum; May 23-26, 2001; Denver, CO, pp 578–579. [Google Scholar]
- 103. Macdougall IC. Darbepoetin alfa: a new therapeutic agent for renal anemia. Kidney Int Suppl 2002; May: 55–61. [DOI] [PubMed] [Google Scholar]
- 104. Langston CE. Clinical use of erythropoietin in feline medicine. In: August JR. (ed). Consultations in feline internal medicine. 6th ed. St Louis, MO: Elsevier, 2010, pp 684–693. [Google Scholar]
- 105. Simpson KW, Fyfe J, Cornetta A, et al. Subnormal concentrations of serum cobalamin (vitamin B12) in cats with gastrointestinal disease. J Vet Intern Med 2001; 15: 26–32. [DOI] [PubMed] [Google Scholar]
- 106. Steiner JM. Cobalamin: diagnostic use and therapeutic considerations. http://vetmed.tamu.edu/gilab/research/cobalamin-information#-dosing (accessed December 12, 2017).
- 107. Kempf J, Hersberger M, Melliger RH, et al. Effects of 6 weeks of parenteral cobalamin supplementation on clinical and biochemical variables in cats with gastrointestinal disease. J Vet Intern Med 2017; 31: 1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kuehn N. North American companion animal formulary. 11th ed. Port Huron, Michigan, North American Compendiums. www.vin.com (2015, accessed December 6, 2017). [Google Scholar]
- 109. Toresson L, Steiner JM, Olmedal G, et al. Oral cobalamin supplementation in cats with hypocobalaminaemia: a retrospective study. J Feline Med Surg 2017; 19: 1302–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bacek LM, Macintire DK. Treatment of primary immune-mediated hemolytic anemia with mycophenolate mofetil in two cats. J Vet Emerg Crit Care 2011; 21: 45–49. [DOI] [PubMed] [Google Scholar]
- 111. Slovak JE, Villarino NF. Safety of oral and intravenous mycophenolate mofetil in healthy cats. J Feline Med Surg 2017; 31: 1827–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Iwanaga T, Miura N, Miyoshi N, et al. Abnormal erythroid cell proliferation and myelofibrosis in a cat. J Vet Med Sci 2012; 74: 909–912. [DOI] [PubMed] [Google Scholar]
- 113. Miller R, Schick AE, Boothe DM, et al. Absorption of transdermal and oral cyclosporine in six healthy cats. J Am Anim Hosp Assoc 2014; 50: 36–41. [DOI] [PubMed] [Google Scholar]
- 114. Roberts ES, Vanlare KA, Strehlau G, et al. Safety, tolerability, and pharmacokinetics of 6-month daily dosing of an oral formulation of cyclosporine (Atopica for cats) in cats. J Vet Pharmacol Therap 2014; 37: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Roberts ES, VanLare KA, Roycroft LM, et al. Effect of highdose ciclosporin on the immune response to primary and booster vaccination in immunocompetent cats. J Feline Med Surg 2015; 17: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Roberts ES, Speranza C, Friberg C, et al. Confirmatory field study for the evaluation of ciclosporin at a target dose of 7.0 mg/kg (3.2 mg/lb) in the control of feline hypersensi-tivity dermatitis. J Feline Med Surg 2016; 18: 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Heinrich NA, McKeever PJ, Eisenschenk MC. Adverse events in 50 cats with allergic dermatitis receiving ciclosporin. Vet Dermatol 2011; 22: 511–520. [DOI] [PubMed] [Google Scholar]
- 118. Barrs VR, Martin P, Beatty JA. Antemortem diagnosis and treatment of toxoplasmosis in two cats on cyclosporine therapy. Aust Vet J 2006; 84: 30–35. [DOI] [PubMed] [Google Scholar]
- 119. Callegari C, Palermo G, Greco MF, et al. Pneumonia associated with Salmonella spp. infection in a cat receiving cyclosporine. Schweiz Arch Tierheilkd 2014; 156: 499–503. [DOI] [PubMed] [Google Scholar]
- 120. Lappin MR, vanLare KA, Seewald W, et al. Effect of oral administration of cyclosporine on Toxoplasma gondii infections status of cats. Am J Vet Res 2015; 76: 351–357. [DOI] [PubMed] [Google Scholar]
- 121. Schmiedt CW, Grimes JA, Holzman G, et al. Incidence and risk factors for development of malignant neoplasia after feline renal transplantation and cyclosporine-based immunosuppression. Vet Comp Oncol 2009; 7: 45–53. [DOI] [PubMed] [Google Scholar]
- 122. Wormser C, Mariano A, Holmes ES, et al. Post-transplant malignant neoplasia associated with cyclosporine-based immunotherapy: prevalence, risk factors and survival in feline renal transplant recipients. Vet Comp Oncol 2016; 14: e126–e134. DOI: 10.1111/vco.12120. [DOI] [PubMed] [Google Scholar]
- 123. Benitah N, de Lorimier LP, Gaspar M. Chlorambucil-induced myoclonus in a cat with lymphoma. J Am Anim Hosp Assoc 2003; 39: 283–287. [DOI] [PubMed] [Google Scholar]
- 124. Pope KV, Tun A, McNeill C, et al. Outcome and toxicity assessment of feline small cell lymphoma: 56 cases (2000-2010). Vet Med Sci 2015; 1: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Reinert NC, Feldman DG. Acquired Fanconi syndrome in four cats treated with chlorambucil. J Feline Med Surg 2016; 18: 1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Dressman JB, Poust RI. Stability of allopurinol and of five antineoplastics in suspension. Am J Hosp Pharm 1983; 40: 616–618. [PubMed] [Google Scholar]
- 127. Slovak JE, Rivera SM, Hwang JK, et al. Pharmacokinetics of mycophenolic acid after intravenous administration of mycophenolate mofetil to healthy cats. J Vet Intern Med 2017; 31: 1827–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Beale KM, Altman D, Clemmons RR, et al. Systemic toxicosis associated with azathioprine administration in domestic cats. Am J Vet Res 1992; 53: 1236–1240. [PubMed] [Google Scholar]
- 129. Foster AP, Shaw SE, Duley JA, et al. Demonstration of thiopurine methyltransferase activity in the erythrocytes of cats. J Vet Intern Med 2000; 14: 552–554. [DOI] [PubMed] [Google Scholar]
- 130. Hisasue M, Neo S, Tuchiya R, et al. Successful therapy with a vitamin K2 analog (menatetrenone) in feline myelodysplastic syndromes [abstract]. J Vet Intern Med 2004; 18: 440. [Google Scholar]
- 131. Macdougall IC. New anemia therapies: translating novel strategies from bench to bedside. Am J Kidney Dis 2012; 59: 444–451. [DOI] [PubMed] [Google Scholar]
- 132. Sun CC, Vaja V, Babitt JL, et al. Targeting the hepcidin-ferroportin axis to develop new treatment strategies for anemia of chronic disease and anemia of inflammation. Am J Hematol 2012; 87: 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Crielaard BJ, Lammers T, Rivella S. Targeting iron metabolism in drug discovery and delivery. Nat Rev Drug Discov 2017; 16: 400–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sankaran VG, Weiss MJ. Anemia: progress in molecular mechanisms and therapies. Nat Med 2015; 21: 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]