Abstract
Aim:
The overarching purpose of the 2019 AAFP Feline Zoonoses Guidelines (hereafter referred to as the ‘Guidelines’) is to provide accurate information about feline zoonotic diseases to owners, physicians and veterinarians to allow logical decisions to be made concerning cat ownership.
Scope and accessibility:
The Panelists are physicians and veterinarians who worked closely together in an attempt to make these Guidelines a document that can be used to support the International One Health movement. This version of the Guidelines builds upon the first feline zoonosis panel report, published in 2003 (catvets.com/guidelines), and provides an updated reference list and recommendations. Each of the recommendations received full support from every Panelist. Primary recommendations are highlighted in a series of ‘Panelists’ advice’ boxes.
Keywords: Zoonoses, ownership, wellness, bacterial, parasitic, rickettsia
Introduction
The American Association of Feline Practitioners (AAFP) first published a feline zoonoses panel report in 2003, followed by a panel report on feline bartonellosis in 2006.1,2 Those documents were extensively referenced and this version will focus on new information published since 2003. The aim of these Guidelines is to offer practical recommendations to help physicians and veterinarians provide accurate information to owners concerning health risks associated with cat ownership. (See Panelists’ advice 1.)

The recommendations of the Panelists are based on published data when available, and recommendations of other public health affiliated groups are taken into consideration. Information from the Centers for Disease Control and Prevention (CDC; cdc.gov/healthypets/index.html), the Companion Animal Parasite Council (CAPC; capcvet.org), the World Small Animal Veterinary Association (WSAVA) One Health Committee (wsava.org/educational/one-health-committee) and the American Association of Food Safety and Public Health Veterinarians (AAFSPHV; aaphv.org) was consulted and referenced within this Guidelines document.
For each recommendation in the draft documents, the Panelists were individually asked whether they agreed or disagreed with the recommendation. All of the final recommendations were supported by each Panelist. After the Panelists had what was considered a final document, select members of the CAPC board and the WSAVA One Health Committee were asked to make comments, which were considered for inclusion in this document. Select members of the International Society of Feline Medicine (ISFM) also reviewed the document.
Zoonotic diseases are defined as being common to, shared by, or naturally transmitted between humans and other vertebrate animals. There are multiple agents that can infect cats and their owners, and these are summarized in Tables 1–5.1,3 Humans are infected with zoonot-ic agents from direct contact with infected cats, contact via contaminated food or water, from shared vectors and from the shared environment. Direct contact with feline feces (enteric zoonoses), respiratory secretions, urogenital secretions, or infected skin and exudates, as well as bites and scratches, can result in human infections. Some zoonotic agents are transmitted between cats and people by shared vectors such as fleas, ticks or mosquitoes. Anaplasma phagocytophilum (ticks), Borrelia burgdorferi (ticks), Ehrlichia species (ticks), Bartonella species (fleas, ticks), Rickettsia felis (fleas) and Dirofilaria immitis (mosquitoes) are examples of vector-borne zoonoses.4,5 With these agents, the cat potentially brings the vector of the organism into the human environment, resulting in exposure. 6 Some zoonotic agents, including Histoplasma capsulatum, Coccidioides immitis, Blastomyces dermatitidis, Cryptococcus neofor-mans and Aspergillus species, do not usually infect humans through direct contact with the infected cat but are acquired from the same environmental source. Other agents like Sporothrix species can be acquired from infected cats or the environment.7,8
Table 1.
Potential enteric zoonotic agents of cats
| Agent | Principal clinical syndromes | |
|---|---|---|
| Ancylostoma species (hookworms)* | Cats Humans |
Blood loss anemia, diarrhea, failure to thrive Cutaneous larva migrans, eosinophilic pain syndrome |
| Campylobacter jejuni and Campylobacter coli (bacteria) † | Cats Humans |
Subclinical infection or diarrhea and vomiting Diarrhea and vomiting |
| Cryptosporidium felis (coccidian) ‡ | Cats Humans |
Subclinical infection or diarrhea Immunocompetent: self-limiting diarrhea and vomiting Immunocompromised: severe protracted diarrhea |
| Escherichia coli (bacterium; some strains) | Cats Humans |
Subclinical infection or diarrhea and vomiting Diarrhea and vomiting |
| Echinococcus multilocularis (cestode) | Cats Humans |
Subclinical infection Polysystemic disease |
| Giardia species (flagellate) § | Cats Humans |
Subclinical infection or diarrhea and vomiting Diarrhea and vomiting |
| Helicobacter species (bacteria) ¶ | Cats Humans |
Vomiting Reflux disease and vomiting |
| Salmonella species (bacteria; some strains) | Cats Humans | Subclinical infection or signs of bacteremia; diarrhea and vomiting Diarrhea and vomiting |
| Strongyloides stercoralis (hookworm) | Cats Humans |
Blood loss anemia, failure to thrive Cutaneous larva migrans |
| Toxocara cati (roundworm)* | Cats Humans |
Vomiting, failure to thrive Ocular and visceral larva migrans |
| Toxoplasma gondii (coccidian) # | Cats Humans |
Subclinical infection; rarely diarrhea, polysystemic disease Congenital infection Immunocompromised: central nervous system and ocular disease |
| Uncinaria stenocephala (hookworm)* | Cats Humans |
Blood loss anemia, diarrhea, failure to thrive Cutaneous larva migrans |
| Yersinia enterocolitica (bacterium) | Cats Humans | Subclinical infection Diarrhea and vomiting, mesenteric lymphadenopathy |
Ancylostoma braziliense is the most likely to cause cutaneous larva migrans in the USA. Infective larvae develop after passage of the eggs into the environment; hence, direct transmission through contact with cats is less likely than exposure through environmental contamination
Most cats are infected by Campylobacter upsaliensis; this host-adapted species is rarely found in humans
Most cats are infected by C felis, and this host-adapted species is rarely found in humans
Host-adapted and zoonotic assemblages exist. Cats can harbor zoonotic assemblages, but whether levels of infection result in reinfection of humans is not established
Most Helicobacter species found in cats are host-adapted species. When Helicobacter pylori is detected in a cat it is likely from reverse zoonotic transmission
Sporulation of oocysts occurs after passage into the environment; hence, direct transmission by contact with cats is less likely than exposure through environmental contamination
Table 2.
Potential scratch, bite or exudate associated zoonotic agents of cats
| Bartonella species (bacterium)* | Cats Humans |
Subclinical infection, fever, hyperglobulinemia, uveitis, lymphadenopathy, others Immunocompetent: focal lymphadenopathy, fever including fever of unknown origin, encephalopathy, osteomyelitis, polyarthritis, headaches 4 Immuncompromised: bacillary angiomatosis, bacillary peliosis, others |
| Capnocytophaga canimorsus (bacterium) | Cats Humans |
Subclinical oral colonization
Bacteremia including fulminant sepsis/purpura fulminans in asplenic individuals |
| Dermatophytes (fungi) | Cats Humans |
Superficial dermatologic disease Superficial dermatologic disease |
| Francisella tularensis (bacterium) † | Cats Humans |
Fever, lymphadenopathy, septicemia, pneumonia Ulceroglandular, oculoglandular, glandular, pneumonic or typhoidal (depending on route of inoculation) |
| Rabies (virus) | Cats Humans |
Rapidly progressive fatal encephalitis Rapidly progressive fatal encephalitis |
| Sporothrix species (fungi) | Cats Humans |
Draining cutaneous tracts Draining cutaneous tracts |
| Yersinia pestis (bacterium) | Cats Humans |
Bubonic, bacteremic or pneumonic (depending on route of inoculation) Bubonic, bacteremic or pneumonic (depending on route of inoculation) |
Bartonella henselae, Bartonella koehlerae and Bartonella clarridgeiae are transmitted among cats by Ctenocephalides felis and so are also listed under flea-borne disease (Table 5). There are other Bartonella species with zoonotic implications. Cats generally develop a higher level of bacteremia than dogs and so are epidemiologically linked more frequently to human disease. The vectors are unknown for some Bartonella species
F tularensis can be acquired by direct contact with a bacteremic cat but is also vector borne
Table 3.
Potential ocular or respiratory zoonotic agents of cats
| Agent | Principal clinical syndromes | |
|---|---|---|
| Bordetella bronchiseptica (bacterium) | Cats Humans |
Subclinical infection or sneeze and cough Prolonged cough or pneumonia, particularly in immunocompromised individuals |
| Chlamydia felis (bacterium) | Cats Humans |
Conjunctivitis, sneezing Conjunctivitis |
| Francisella tularensis (bacterium)* | Cats Humans |
Fever, lymphadenopathy, septicemia, pneumonia Ulceroglandular, oculoglandular, glandular, pneumonic or typhoidal (depending on route of inoculation) |
| Influenza (virus; H7N2, H5N1, others) | Cats Humans |
Subclinical infection or sneeze and cough Rare from cat exposure; mild and self-limited |
| Streptococcus group A (bacterium) | Cats Humans |
Subclinical infection, transient carrier ‘Strep throat’, septicemia |
| Yersinia pestis (bacterium)* | Cats Humans |
Bubonic, bacteremic or pneumonic (depending on route of inoculation) Bubonic, bacteremic or pneumonic (depending on route of inoculation) |
Also can be vector borne
Table 4.
Potential urogenital zoonotic agents of cats
| Agent | Principal clinical syndromes | |
|---|---|---|
| Coxiella burnetii (rickettsia) | Cats Humans |
Subclinical infection, abortion or stillbirth Fever, pneumonitis, lymphadenopathy, myalgia, arthritis |
| Leptospira species (spirochetes) | Cats Humans |
Subclinical infection; link to inflammatory urinary tract or hepatic disease is unclear Fever, malaise |
Table 5.
Common flea- and tick-borne zoonotic agents of cats
| Agent | Principal clinical syndromes | ||
|---|---|---|---|
| Fleas* | Bartonella species (bacteria) † | Cats Humans |
Subclinical infection, fever, hyperglobulinemia, uveitis, lymphadenopathy, others Immunocompetent: focal lymphadenopathy, fever including fever of unknown origin, encephalopathy, osteomyelitis, polyarthritis, headaches 4 Immuncompromised: bacillary angiomatosis, bacillary peliosis, others |
| Rickettsia felis (rickettsia) | Cats Humans |
Subclinical infection Fever and rash, central nervous system (CNS) disease |
|
| Yersinia pestis (bacterium) | Cats Humans |
Bubonic, bacteremic, or pneumonic (depending on route of inoculation) Bubonic, bacteremic, or pneumonic (depending on route of inoculation) |
|
| Anaplasma phagocytophilum (rickettsia) | Cats Humans |
Subclinical infection; rarely fever, lethargy, discomfort Fever, headache, muscle pain, other signs of polysystemic inflammation 5 |
|
| Ticks | Borrelia burgdorferi (spirochete) | Cats Humans |
Subclinical infection; whether fever, nephritis or polyarthritis occur in cats is unclear Rash (erythema migrans), polyarthropathy, cardiac or CNS disease |
| Ehrlichia species (rickettsia) | Cats Humans |
Subclinical infection, fever, polysystemic signs Fever, polysystemic signs |
|
| Francisella tularensis (bacterium) | Cats Humans |
Fever, lymphadenopathy, septicemia, pneumonia Ulceroglandular, oculoglandular, glandular, pneumonic or typhoidal (depending on route of infection) |
|
| Other spotted fever Rickettsia species (rickettsia) | Cats Humans |
Subclinical infection; whether fever or polysystemic signs occur in cats is unclear Fever, polysystemic signs |
|
Coxiella burnetii DNA has been amplified from Ctenocephalides felis in Cyprus, but whether this flea is a vector is unknown
Bartonella species DNA has been amplified from some ticks, but the extent of the role ticks play in the transmission of these agents has not been fully ascertained
Most of the agents discussed in these Guidelines can infect and cause disease in anyone, but disease is generally more prevalent or more severe in those with immunodeficiency-inducing disorders. 9 Humans with AIDS are discussed most frequently, but there are many more individuals with immunodeficiencies, including the very old, the very young, individuals receiving chemotherapy or glucocorti-coids for immune-mediated diseases, organ transplant recipients and cancer patients. Humans are unlikely to contract zoonotic diseases from direct contact with their healthy cats and the mental health benefits from pet ownership can be considerable.10,11 Pet ownership is known to improve general sense of wellbeing and there is some evidence that happiness influences immunological factors. 12
General recommendations to help prevent zoonotic transfer of disease for owners and veterinarians are presented in the box on page 9. In each of the subsections that follow, additional recommendations are provided based on the route that humans are exposed to feline zoonotic agents.
Enteric zoonoses
There are multiple infectious agents of the gastrointestinal tract that can be shared between cats and humans (Table 1). Since some enteric zoonotic agents (eg, some Campylobacter species, Salmonella species, Yersinia enterocoliti-ca) are infectious when passed in feces, direct contact with infected cats can result in human infection and disease.13,14 Some enteric agents of cats that are infectious immediately in feces, like Giardia species or Cryptosporidium felis, are not considered significant zoonotic agents; when these infections occur, the strains are generally cat-specific.15–18
Other infectious agents, such as Ancylostoma species, Toxocara cati and Toxoplasma gondii, require a period of time out of the host prior to becoming infectious. Thus, many enteric zoonoses result from ingestion of the infectious agent in contaminated food, water or other environmental sources. (See Panelists’ advice 2.)

Raw meat can harbor enteric pathogenic bacteria like Campylobacter species, entero-toxigenic Escherichia coli and Salmonella species.19–21 Freezing meat does not consistently kill all bacteria and it is recognized that feeding raw meat to pets can result in amplification of potentially pathogenic bacteria in animal feces. (See Panelists’ advice 3.) The consensus statement of the American College of Veterinary Internal Medicine (ACVIM) on enteropathogenic bacteria in dogs and cats is an excellent resource for information concerning the control of bacterial zoonotic agents. 22

Some Ancylostoma species of cats are associated with cutaneous larva migrans and T cati is associated with ocular and visceral larva migrans (see Table 1). Toxocara species eggs were found on the fur of dogs and cats in one study, but were non-viable. 23 While hookworm and roundworm egg shedding is generally highest in kittens, adult cats can also shed eggs that become infectious in the human environ-ment.24–26 (See Panelists’ advice 4.)

Cats (and other felids) are the only definitive host for T gondii and shed millions of oocysts in feces after primary infection. 27 Once these oocysts have sporulated (Figure 1), infection of humans can occur. Human exposure can also occur by ingestion of T gondii tissue cysts and transplacentally if a previously uninfected mother ingests sporulated oocysts or tissue cysts during pregnancy. It is now known that humans are commonly infected by ingestion of sporulated oocysts. 28 (However, see Panelists’ advice 5.)
Figure 1.
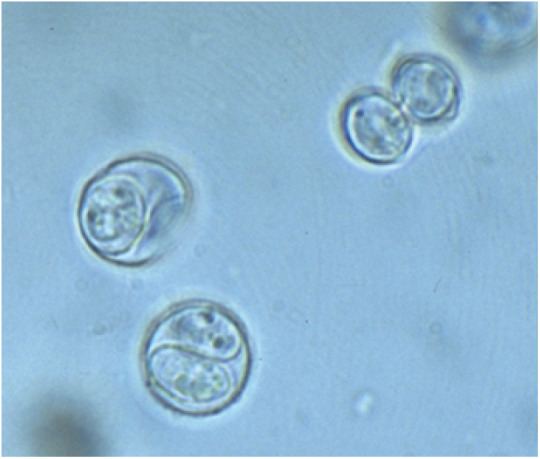
Toxoplasma gondii sporulated oocysts (8 μm × 10 μm), each containing two sporocysts. This form of the oocyst is zoonotic to humans
Most studies evaluating cat ownership as a risk factor for human toxoplasmosis have shown minimal associations, including one study of HIV-infected individuals. 29 In addition, in one study, veterinary staff members that worked frequently with cats had low sero-prevalence rates, suggesting exposure to cats did not increase their risk of acquiring T gondii infection. 30 Cats that are infected by T gondii usually do not shed or shed lower numbers of oocysts on secondary exposure, 31 and in one experimental study did not have repeat oocyst shedding after being administered ciclosporin. 32 (See Panelists’ advice 6 and 7.)

Enteric zoonotic agent prevalence rates that have been reported in several studies of cats are generally higher in young cats with diar-rhea.24–26,33 However, most of the agents can still be present even if the stool is normal. These findings emphasize that diagnostic work-ups for enteric infections are indicated due to potential human health risks. (See Panelists’ advice 8.) For cats with persistent small bowel diarrhea after treatment for giardiasis, immunofluorescent assay or PCR for Cryptosporidium species is indicated. Fecal bacterial culture should be considered if fever is present and Salmonella species or Campylobacter species are on the differential list.34,35 However, fecal bacterial culture and measurement of Clostridium species enterotox-ins had limited diagnostic value as routine tests in cats with diarrhea in one study. 33 (See Panelists’ advice 9.)

Gastrointestinal signs of enteric bacterial infections generally resolve with supportive care such as use of therapeutic diets and probiotic administration. Antibiotics should only be considered if these cats have fever or other evidence of bac-teremia or sepsis; and, if believed to be necessary, should only be administered parenterally.
Scratch, bite or exudate exposure zoonoses
Approximately 1% of emergency room visits per year in the USA are to evaluate people bitten by animals. 36 Most of the aerobic and anaerobic bacteria associated with bite or scratch wounds (eg, Pasteurella species, Staphylococcus species) cause cellulitis in immunocompetent individuals. Approximately 28–80% of cat bites become infected, and severe sequelae including meningitis, endocarditis, septic arthritis, osteoarthritis and septic shock can occur. 37 Immunodeficient humans or humans exposed to Pasteurella species, Capnocytophaga canimorsus or Capnocytophaga cynodegmi more consistently develop systemic clinical illness.38–40 Splenectomized humans, as well as those with non-functional spleens, such as in sickle cell disease, are at increased risk of developing overwhelming sepsis/purpura fulminans with Capnocyto-phaga species infection. 41 (See Panelists’ advice 10.) Techniques to lessen feline stress to help protect staff members and owners, such as the use of pheromones or appropriate sedation, should be considered for cats, as required.42,43 Primary care physicians need to be aware of the potentially serious sequelae of untreated cat bites and scratches.
Bartonella species infection of humans can be associated with bites and scratches, and these agents are also vector-associated zoonoses (Tables 2 and 5; also see box on page 9). It is known that Bartonella species (particularly Bartonella henselae), the cause of cat scratch disease, peliosis hepatis, bacillary angiomato-sis, bacterial endocarditis and a number of other human inflammatory syndromes such as polyarthritis, are present in the oral cavity, on the skin and on the claws of cats with Ctenocephalides felis infestations.4,44–46 Veterinary healthcare providers may be at greater risk of development of Bartonella species-associated syndromes from exposure to cats or infected C felis. 47 Consistent use of flea control products has been shown in a B henselae (cat scratch agent) model to block transmission of the pathogen among cats.48,49 (Thus, see Panelists’ advice 11.) Currently, no drugs can consistently eliminate the Bartonella species carrier state from healthy cats and antibiotics like azithromycin can rapidly select for resistant strains. 50 (Thus, see Panelists’ advice 12.) However, in some circumstances the veterinarian and physician may choose to test cats in contact with immunosup-pressed people in a family or those with clinical manifestations of bartonellosis.


Francisella tularensis (tick-borne agent) and Yersinia pestis (rodent fleas) infections can also be associated with bite wounds, but are not as common in humans as Bartonella species-associated infections (Tables 2 and 5, also see box on page 9).51–53
Of the many fungal agents that infect both humans and animals, Sporothrix species (Figure 2) and the dermatophytes (Figure 3) appear to be the most common to infect humans upon direct exposure.7,8,54 Histoplasma, Blastomyces, Coccidioides, Aspergillus and Cryptococcus species infections of humans and animals can occur in the same household, but infection of humans generally results from a common environmental source rather than direct contact with an infected animal. (See Panelists’ advice 13.)
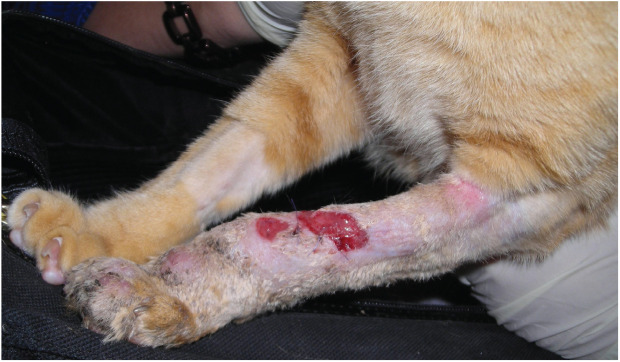
Figure 2.
Cutaneous sporotrichosis in a young adult cat
Figure 3.
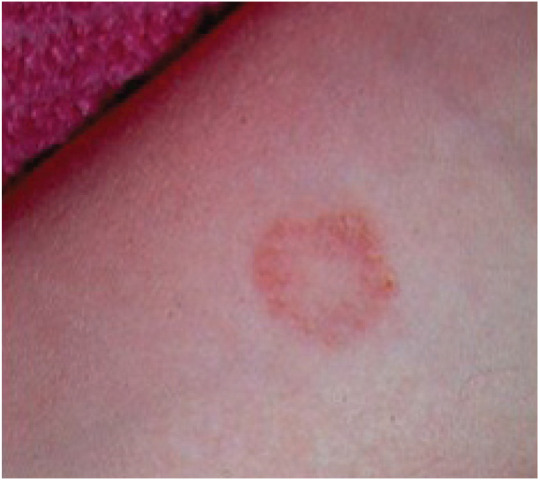
Characteristic cutaneous ringworm lesion on the forearm of a veterinarian
Cats can harbor meticillin-resistant Staphylococcus aureus (MRSA) and Staphylococcus pseudintermedius, and transmission between animals and humans is likely to occur within households.55–57 Unlike other infections discussed in these Guidelines, MRSA in cats in generally acquired from humans and is considered a ‘reverse zoonosis’ or ‘humanosis’. (See Panelists’ advice 14.)

Although uncommon in cats, rabies is still the only significant small animal viral zoono-sis in the USA and is associated with bite wounds.59,60 (See Panelists’ advice 15.) While the feline retroviruses can be transmitted among cats by direct contact, including bites and scratches, one study of veterinarians showed no evidence of transmission. 61
Ocular or respiratory zoonoses
Bordetella bronchiseptica and Chlamydia felis cause mild respiratory disease in cats (Table 3). 62 Cough is most common with B bron-chiseptica infection and conjunctivitis with C felis infection. It is believed that C felis may be associated with conjunctivitis in people.63,64 Most people with Bordetella species infections are infected by Bordetella pertussis, but some individuals, particularly immunocompro-mised people, can be infected with B bronchi-septica.65,66
Cats with cough and systemic evidence of bacterial infection such as fever might occasionally be infected with Y pestis and/or F tularensis, if living in endemic areas; these agents can be transmitted from cats to humans in respiratory secretions.53,67,68 (See Panelists’ advice 16.)

Humans are the principal natural hosts for Streptococcus group A (Streptococcus pyogenes) bacteria, which cause ‘strep throat’ in people. Cats in close contact with infected humans can develop transient, subclinical colonization of pharyngeal tissues and can transmit the infection to other humans. Older data suggested this was a common occurrence; however, with improved diagnostics it has been shown to be an uncommon event. Early studies used crude antimicrobial data on bacitracin susceptibilities rather than genetic subtyping.69,70 Later, when Lancefield typing was performed, the true prevalence in household pets was found to be only 0–3% and not correlated with the presence of infection in the owner, and hence the risk for transfer of infection from pet cats to humans is considered low. 71
Avian influenza viruses occasionally infect cats.72,73 Rarely, cats infected with an influenza virus are associated with clinical disease in humans. 74 Recently, an H3N2 vaccine for dogs marketed in the United States was approved for use in cats (Merck Animal Health, New Jersey, USA) and a different canine vaccine was shown to induce H3N2 immune responses in cats. 75 Whether these vaccines are indicated for use in cats to lessen human exposure in the USA is currently unknown. (See Panelists’ advice 17.)

Urogenital tract zoonoses
Coxiella burnetii (Table 4) is a rickettsial agent found throughout the world that is associated with Q fever.76,77 Many ticks, including Rhipicephalus sanguineus, are naturally infected with C burnetii and so this agent is considered to be a shared vector zoonosis. While abortion can occur, cats infected by C burnetii usually do not show clinical signs of disease. C burnetii DNA was amplified from 8.5% of uterine biopsies taken after elective ovario-hysterectomy in a small study of cats in Colorado, USA. 78 Human illness associated with close contact with infected cats occurs after aerosol exposure to the organism passed by parturient or aborting cats; clinical signs develop 4–30 days after contact. 79 Humans commonly develop acute clinical signs similar to those associated with other rickettsial diseases, including fever, malaise, headache, pneumonitis, myalgia and arthralgia. (See Panelists’ advice 18.)

The major agents associated with urine that could be a direct feline zoonosis are the Leptospira species. 80 These spirochetes can be transmitted in urine to humans and result in clinical disease. While Leptospira species antibodies and DNA have been detected in cats, the role these agents play in leptospirosis of people is unclear.81–83 (See Panelists’ advice 19.)

Vector-borne zoonoses
There are multiple infectious agents potentially transferred from cats to humans by fleas and ticks (Table 5). C felis from cats contain multiple pathogens, with Bartonella species and R felis being the most common.46,84,85 (See the scratch, bite or exudate exposure section on pages 4–6 for more information about Bartonella species.) R felis can cause mild clinical signs of disease including fever and malaise. 86 While C felis and ticks collected from cats or dogs are commonly positive for R felis DNA, it appears the dog is the better reservoir. 87 It is possible that C burnetii and Y pestis could be transmitted to humans from cats with fleas. Humans can also be infected by Dipylidium caninum by accidental ingestion of infected fleas, which typically is reported in young children that spend time on the carpet where fleas usually reside when not on the host. 88 (See Panelists’ advice 20.)

Recently, it was documented that pet ownership increases the risks for humans of exposure to ticks. 6 Cats have been shown to be infected experimentally and naturally with many tick-borne agents that could infect humans including B burgdorferi and A phagocy-tophilum.89–92 (Thus, see Panelists’ advice 21.)

There are many more vector-borne diseases of potential significance in cats of the USA, including West Nile virus (mosquitoes) and Leishmania species.93,94 However, in contrast to the flea- and tick-associated zoonoses, cats are unlikely to influence exposure of these agents to owners in the USA. However, travel history from other countries should always be considered. For example, cats can harbor both Old World and New World Leishmania species. International guidelines (leishvet.org/fact-sheet-feline-leishmaniosis) are available to provide information about leishmaniosis and control for cats imported from endemic areas.
Summary Points
While humans are rarely infected with a zoonotic agent by exposure to a healthy cat, there are many potential infections that can occur.
Disease is generally more prevalent or more severe in people with immunodeficiency-inducing disorders, the very old, very young, individuals receiving chemotherapy or glucocorticoids for immune-mediated diseases, organ transplant recipients and cancer patients.
Cats should have consistent deworming and should be prescribed vector control.
Cats with clinical signs of disease should be assessed by a veterinarian to determine the risk of zoonotic disease transmission and to have the clinical abnormalities treated.
Supplemental Material
Supplemental material, Supplementary_Material_Feline_Zoonoses_Client_Brochure for 2019 AAFP Feline Zoonoses Guidelines by Michael R Lappin, Tom Elston, Lisanne Evans, Carol Glaser, Lorraine Jarboe, Peter Karczmar, Cathy Lund and Michael Ray in Journal of Feline Medicine and Surgery
Acknowledgments
The Panelists thank Ms Kimberley Kern for helping with literature reviews. The final draft of the document was reviewed by the Companion Animal Parasite Council Board (capcvet.org). The members of the WSAVA One Health Committee (wsava.org) provided comments on the document.
Appendix: Client brochure
Footnotes
The Panelists have no conflicts of interest to declare.
Funding: The Panelists received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article.
Contributor Information
Michael R Lappin, Department of Clinical Sciences, Colorado State University, 300 West Drake Road, Fort Collins, CO 80523, USA.
Tom Elston, The Cat Hospital, 3069 Edinger Avenue, Tustin, CA, USA.
Lisanne Evans, All Pets Veterinary Hospital, Rancho Palos Verdes, CA, USA.
Carol Glaser, Pediatric Infectious Diseases, Kaiser Permanente, CA, USA.
Lorraine Jarboe, Jarboe Veterinary Services, Fort Walton Beach, FL, USA.
Peter Karczmar, Coastal Medical, East Providence, RI, USA.
Cathy Lund, City Kitty, Providence, RI, USA.
Michael Ray, The Cat Clinic of Roswell, Roswell, GA, USA.
References
- 1. Brown RR, Elston TH, Evans L, et al. Feline zoonoses guidelines from the American Association of Feline Practitioners. Comp Cont Educ Pract Vet 2003; 25: 936–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brunt J, Guptill L, Kordick DL, et al. Association of Feline Practitioners 2006 Panel report on diagnosis, treatment, and prevention of Bartonella spp. infections. J Feline Med Surg 2006; 8: 213–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Halsby KD, Walsh AL, Campbell C, et al. Healthy animals, healthy people: zoonosis risk from animal contact in pet shops, a systematic review of the literature. PLoS One 2014; 9: e89309. DOI: 10.1371/journal.pone.0089309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Breitschwerdt EB, Maggi RG, Chomel BB, et al. Bartonellosis: an emerging infectious disease of zoonotic importance to animals and human beings. J Vet Emerg Crit Care (San Antonio) 2010; 20: 8–30. [DOI] [PubMed] [Google Scholar]
- 5. McFee RB. Tick borne illness – anaplasmosis. Dis Mon 2018; 64: 181–184. [DOI] [PubMed] [Google Scholar]
- 6. Jones EH, Hinckley AF, Hook SA, et al. Pet ownership increases human risk of encountering ticks. Zoonoses Public Health 2018; 65: 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Delia Terra PP, Rodrigues AM, Fernandes GF, et al. Exploring virulence and immunogenicity in the emerging pathogen Sporothrix brasiliensis. PLoS Negl Trop Dis 2017; 11: e0005903. DOI: 10.1371/journal.pntd.0005903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dunston RW, Langham RF, Reimann KA, et al. Feline sporotrichosis: a report of five cases with transmission to humans. J Am Acad Dermatol 1986; 15: 37. [DOI] [PubMed] [Google Scholar]
- 9. Kaplan JE, Benson C, Holmes KK, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 2009; 58 (RR4): 1–207. [PubMed] [Google Scholar]
- 10. Angulo FJ, Glaser CA, Juranek DD, et al. Caring for pets of immunocompromised persons. J Am Vet Med Assoc 1994; 205:1711–1718. [PubMed] [Google Scholar]
- 11. McConnell AR, Brown CM, Shoda TM, et al. Friends with benefits: on the positive consequences of pet ownership. J Pers Soc Psychol 2011; 101: 1239–1252. [DOI] [PubMed] [Google Scholar]
- 12. Marchant J. Immunology: the pursuit of happiness. Nature 2013; 503: 458–460. [DOI] [PubMed] [Google Scholar]
- 13. Campagnolo ER, Philipp LM, Long JM, et al. Pet-associated campylobacteriosis: a persisting public health concern. Zoonoses Public Health 2018; 65: 304–311. [DOI] [PubMed] [Google Scholar]
- 14. Stamm I, Hailer M, Depner B, et al. Yersinia enterocolitica in diagnostic fecal samples from European dogs and cats: identification by Fourier transform infrared spectroscopy and matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 2013; 51: 887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Lucio A, Bailo B, Aguilera M, et al. No molecular epidemio-logical evidence supporting household transmission of zoonot-ic Giardia duodenalis and Cryptosporidium spp. from pet dogs and cats in the province of Álava, Northern Spain. Acta Trop 2017; 170: 48–56. [DOI] [PubMed] [Google Scholar]
- 16. Scorza AV, Ballweber LR, Tangtrongsup S, et al. Comparisons of mammalian Giardia duodenalis assemblages based on the β-giardin, glutamate dehydrogenase and triose phosphate isomerase genes. Vet Parasitol 2012; 189: 182–188. [DOI] [PubMed] [Google Scholar]
- 17. Scorza V, Willmott A, Gunn-Moore D, et al. Cryptosporidium felis in faeces from cats in the UK. Vet Rec 2014; 174: 609. DOI: 10.1136/vr.102205. [DOI] [PubMed] [Google Scholar]
- 18. De Santis-Kerr AC, Raghavan M, Glickman NW, et al. Prevalence and risk factors for Giardia and coccidia species of pet cats in 2003-2004. J Feline Med Surg 2006; 8: 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giacometti F, Magarotto J, Serraino A, et al. Highly suspected cases of salmonellosis in two cats fed with a commercial raw meat-based diet: health risks to animals and zoonotic implications. BMC Vet Res 2017; 13: 224. DOI: 10.1186/s12917-017-1143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bojanić K, Midwinter AC, Marshall JC, et al. Isolation of Campylobacter spp. from client-owned dogs and cats, and retail raw meat pet food in the Manawatu, New Zealand. Zoonoses Public Health 2017; 64: 438–449. [DOI] [PubMed] [Google Scholar]
- 21. Strohmeyer RA, Morley PS, Hyatt DR, et al. Evaluation of bacterial and protozoal contamination of commercially available raw meat diets for dogs. J Am Vet Med Assoc 2006; 228: 537–542. [DOI] [PubMed] [Google Scholar]
- 22. Marks SL, Rankin SC, Byrne BA, et al. Enteropathogenic bacteria in dogs and cats: diagnosis, epidemiology, treatment, and control. J Vet Intern Med 2011; 25: 1195–1208. [DOI] [PubMed] [Google Scholar]
- 23. Overgaauw PA, van Zutphen L, Hoek D, et al. Zoonotic parasites in fecal samples and fur from dogs and cats in The Netherlands. Vet Parasitol 2009; 163: 115–122. [DOI] [PubMed] [Google Scholar]
- 24. Hill S, Cheney JM, Taton-Allen GF, et al. Prevalence of enteric zoonotic agents in cats. J Am Vet Med Assoc 2000; 216: 687–692. [DOI] [PubMed] [Google Scholar]
- 25. Spain CV, Scarlett JM, Wade SE, et al. Prevalence of enteric zoonotic agents in cats less than 1 year old in central New York State. J Vet Intern Med 2001; 15: 33–38. [DOI] [PubMed] [Google Scholar]
- 26. De Santis AC, Raghavan M, Caldanaro RJ, et al. Estimated prevalence of nematode parasitism among pet cats in the United States. J Am Vet Med Assoc 2006; 228: 885–892. [DOI] [PubMed] [Google Scholar]
- 27. Lappin MR. Update on the diagnosis and management of Toxoplasma gondii infection in cats. Top Companion Anim Med 2010; 25: 136–141. [DOI] [PubMed] [Google Scholar]
- 28. Mangiavacchi BM, Vieira FP, Bahia-Oliveira LM, et al. Salivary IgA against sporozoite-specific embryogenesis-related protein (TgERP) in the study of horizontally transmitted toxoplasmosis via T. gondii oocysts in endemic settings. Epidemiol Infect 2016; 144: 2568–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wallace MR, Rossetti RJ, Olson PE. Cats and toxoplasmosis risk in HIV-infected adults. JAMA 1993; 269: 76–77. [PubMed] [Google Scholar]
- 30. Shuhaiber S, Koren G, Boskovic R, et al. Seroprevalence of Toxoplasma gondii infection among veterinary staff in Ontario, Canada (2002): implications for teratogenic risk. BMC Infect Dis 2003; 3: 8. DOI: 10.1186/1471-2334-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zulpo DL, Sammi AS, Dos Santos JR, et al. Toxoplasma gondii: a study of oocyst re-shedding in domestic cats. Vet Parasitol 2018; 249: 17–20. [DOI] [PubMed] [Google Scholar]
- 32. Lappin MR, VanLare KA, Seewald W, et al. Effect of oral administration of cyclosporine on Toxoplasma gondii infection status of cats. Am J Vet Res 2015; 764: 351–357. [DOI] [PubMed] [Google Scholar]
- 33. Queen EV, Marks SL, Farver TB. Prevalence of selected bacterial and parasitic agents in feces from diarrheic and healthy control cats from Northern California. J Vet Intern Med 2012; 26: 54–60. [DOI] [PubMed] [Google Scholar]
- 34. Tauni MA, Osterlund A. Outbreak of Salmonella typhimurium in cats and humans associated with infection in wild birds. J Small Anim Pract 2000; 41: 339–341. [DOI] [PubMed] [Google Scholar]
- 35. Taylor DJ, Philbey AW. Salmonella infections in garden birds and cats in a domestic environment. Vet Rec 2010; 167; 26–27. [DOI] [PubMed] [Google Scholar]
- 36. Ellis R, Ellis C. Dog and cat bites. Am Fam Physician 2014; 90:239–243. [PubMed] [Google Scholar]
- 37. Talan DA, Citron DM, Abrahamian FM, et al. Bacteriologic analysis of infected dog and cat bites. N Engl J Med 1999; 340: 85–92. [DOI] [PubMed] [Google Scholar]
- 38. Valtonen M, Lauhio A, Carlson P, et al. Capnocytophaga canimor-sus septicemia: fifth report of a cat-associated infection and five other cases. Eur J Clin Microbiol Infect Dis 1995; 14: 520–523. [DOI] [PubMed] [Google Scholar]
- 39. Lloret A, Egberink H, Addie D, et al. ; European Advisory Board on Cat Diseases. Capnocytophaga canimorsus infection in cats: ABCD guidelines on prevention and management. J Feline Med Surg 2013; 15: 588–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zajkowska J, Król M, Falkowski D, et al. Capnocytophaga cani-morsus – an underestimated danger after dog or cat bite – review of literature. Przegl Epidemiol 2016; 70: 289–295. [PubMed] [Google Scholar]
- 41. Dragomir M, Petrescu DGE, Manga GE, et al. Patients after splenectomy: old risks and new perspectives. Chirurgia (Bucur) 2016; 111: 393–399. [DOI] [PubMed] [Google Scholar]
- 42. Rodan I, Sundahl E, Carney H, et al. AAFP and ISFM feline-friendly handling guidelines. J Feline Med Surg 2011; 13: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pereira JS, Fragoso S, Beck A, et al. Improving the feline veterinary consultation: the usefulness of Feliway spray in reducing cats’ stress. J Feline Med Surg 2016; 18: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Breitschwerdt EB, Maggi RG, Duncan AW, et al. Bartonella species in blood of immunocompetent persons with animal and arthropod contact. Emerg Infect Dis 2007; 13: 938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Breitschwerdt EB, Mascarelli PE, Schweickert LA, et al. Hallucinations, sensory neuropathy, and peripheral visual deficits in a young woman infected with Bartonella koehlerae. J Clin Microbiol 2011; 49: 3415–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lappin MR, Hawley J. Presence of Bartonella species and Rickettsia species DNA in the blood, oral cavity, skin and claw beds of cats in the United States. Vet Dermatol 2009; 20: 509–514. [DOI] [PubMed] [Google Scholar]
- 47. Lantos PM, Maggi RG, Ferguson B, et al. Detection of Bartonella species in the blood of veterinarians and veterinary technicians: a newly recognized occupational hazard? Vector Borne Zoonotic Dis 2014; 14: 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bradbury CA, Lappin MR. Evaluation of topical application of 10% imidacloprid–1% moxidectin to prevent Bartonella henselae transmission from cat fleas. J Am Vet Med Assoc 2010; 236: 869–873. [DOI] [PubMed] [Google Scholar]
- 49. Lappin MR, Davis WL, Hawley JR, et al. A flea and tick collar containing 10% imidacloprid and 4.5% flumethrin prevents flea transmission of Bartonella henselae in cats. Parasit Vectors 2013; 6: 26. DOI: 10.1186/1756-3305-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Biswas S, Maggi RG, Papich MG, et al. Comparative activity of pradofloxacin, enrofloxacin, and azithromycin against Bartonella henselae isolates collected from cats and a human. J Clin Microbiol 2010; 48: 617–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rimawi RH, Shah KB, Chowdhary RA, et al. Hunting for tularaemia – a review of cases in North Carolina. Zoonoses Public Health 2015; 62: 159–164. [DOI] [PubMed] [Google Scholar]
- 52. Magnarelli L, Levy S, Koski R. Detection of antibodies to Francisella tularensis in cats. Res Vet Sci 2007; 82: 22–26. [DOI] [PubMed] [Google Scholar]
- 53. Gage KL, Dennis DT, Orloski KA, et al. Cases of cat-associated human plague in the Western US, 1977–1998. Clin Infect Dis 2000;30: 893–900. [DOI] [PubMed] [Google Scholar]
- 54. Moriello KA, Coyner K, Paterson S, et al. Diagnosis and treatment of dermatophytosis in dogs and cats. Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet Dermatol 2017; 28: 266–268. [DOI] [PubMed] [Google Scholar]
- 55. Feßler AT, Schuenemann R, Kadlec K, et al. Methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant Staph ylococcus pseudintermedius (MRSP) among employees and in the environment of a small animal hospital. Vet Microbiol 2018; 221: 153–158. [DOI] [PubMed] [Google Scholar]
- 56. Gingrich EN, Kurt T, Hyatt DR, et al. Prevalence of methicillin-resistant staphylococci in northern Colorado shelter animals. J Vet Diagn Invest 2011; 23: 947–950. [DOI] [PubMed] [Google Scholar]
- 57. Weese JS, Dick H, Willey BM, et al. Suspected transmission of methicillin-resistant Staph ylococcus aureus between domestic pets and humans in veterinary clinics and in the household. Vet Microbiol 2006; 114: 148–155. [DOI] [PubMed] [Google Scholar]
- 58. Morris DO, Loeffler A, Davis MF, et al. Recommendations for approaches to meticillin-resistant staphylococcal infections of small animals: diagnosis, therapeutic considerations and preventative measures. Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet Dermatol 2017; 28: 304–e69. DOI: 10.1111/vde.l2444. [DOI] [PubMed] [Google Scholar]
- 59. Scherk MA, Ford RB, Gaskell RM, et al. 2013 AAFP Feline Vaccination Advisory Panel Report. J Feline Med Surg 2013; 15: 785–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brown CM, Slavinski S, Ettestad P, et al. Compendium of animal rabies prevention and control, 2016. National Association of State Public Health Veterinarians; Compendium of Animal Rabies Prevention and Control Committee. J Am Vet Med Assoc 2016; 248: 505–517. [DOI] [PubMed] [Google Scholar]
- 61. Butera ST, Brown J, Callahan ME, et al. Survey of veterinary conference attendees for evidence of zoonotic infection by feline retroviruses. J Am Vet Med Assoc 2000; 217: 1475–1479. [DOI] [PubMed] [Google Scholar]
- 62. Lappin MR, Blondeau J, Boothe D, et al. Antimicrobial use guidelines for treatment of respiratory tract disease in dogs and cats: Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases. J Vet Intern Med 2017; 31: 279–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yan C, Fukushi H, Matsudate H, et al. Seroepidemiological investigation of feline chlamydiosis in cats and humans in Japan. Microbiol Immunol 2000; 44: 155–160. [DOI] [PubMed] [Google Scholar]
- 64. Hartley JC, Stevenson S, Robinson AJ, et al. Conjunctivitis due to Chlamydophila felis (Chlamydia psittaci feline pneumonitis agent) acquired from a cat: case report with molecular characterization of isolates from the patient and cat. J Infect 2001; 43: 7–11. [DOI] [PubMed] [Google Scholar]
- 65. Wernli D, Emonet S, Schrenzel J, et al. Evaluation of eight cases of confirmed Bordetella bronchiseptica infection and colonization over a 15-year period. Clin Microbiol Infect 2011; 17: 201–203. [DOI] [PubMed] [Google Scholar]
- 66. Dworkin MS, Sullivan PS, Buskin SE, et al. Bordetella bronchisep-tica infection in human immunodeficiency virus-infected patients. Clin Infect Dis 1999; 28: 1095–1099. [DOI] [PubMed] [Google Scholar]
- 67. Kassem AM, Tengelsen L, Atkins B, et al. Notes from the field: plague in domestic cats – Idaho, 2016. MMWR Morb Mortal Wkly Rep 2016; 65: 1378–1379. [DOI] [PubMed] [Google Scholar]
- 68. Pennisi MG, Egberink H, Hartmann K, et al. ; European Advisory Board on Cat Diseases. Yersinia pestis infection in cats: ABCD guidelines on prevention and management. J Feline Med Surg 2013; 15: 582–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Crowder HR, Dorn CR, Smith RE. Group A Streptococcus in pets and group A streptococcal disease in man. Int J Zoonoses 1978; 5: 45–54. [PubMed] [Google Scholar]
- 70. Copperman SM. Cherchez le chien: household pets as reservoirs of persistent or recurrent streptococcal sore throats in children. N Y State J Med 1982: 82; 1685–1687. [PubMed] [Google Scholar]
- 71. Wilson KS, Maroney SA, Gander RM. The family pet as an unlikely source of group A beta-hemolytic streptococcal infection in humans. Pediatr Infect Dis J 1995; 14: 372–375. [DOI] [PubMed] [Google Scholar]
- 72. Leschnik M, Weikel J, Möstl K, et al. Subclinical infection with avian influenza A (H5N1) virus in cats. Emerg Infect Dis 2007; 13: 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Thiry E, Zicola A, Addie D, et al. Highly pathogenic avian influenza H5N1 virus in cats and other carnivores. Vet Microbiol 2007; 122: 25–31. [DOI] [PubMed] [Google Scholar]
- 74. Lee CT, Slavinski S, Schiff C, et al. ; Influenza A(H7N2) Response Team. Outbreak of influenza A (H7N2) among cats in an animal shelter with cat-to-human transmission – New York City, 2016. Clin Infect Dis 2017; 65: 1927–1929. [DOI] [PubMed] [Google Scholar]
- 75. Velineni S, Hainer N, Conlee D, et al. Vaccination with an inactivated canine influenza H3N2 virus vaccine is safe and elicits immune response in cats. J Feline Med Surg. Epub ahead of print 14 March 2019. DOI: 10.1177/1098612X19833261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kopecny L, Bosward KL, Shapiro A, et al. Investigating Coxiella burnetii infection in a breeding cattery at the centre of a Q fever outbreak. J Feline Med Surg 2013; 15: 1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shapiro AJ, Norris JM, Bosward KL, et al. Q Fever (Coxiella burnetii) knowledge and attitudes of Australian cat breeders and their husbandry practices. Zoonoses Public Health 2017; 64: 252–261. [DOI] [PubMed] [Google Scholar]
- 78. Cairns K, Brewer M, Lappin MR. Prevalence of Coxiella bur-netii DNA in vaginal and uterine samples from healthy cats of north-central Colorado. J Feline Med Surg 2007; 9: 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Marrie TJ, MacDonald A, Durant H, et al. An outbreak of Q fever probably due to contact with a parturient cat. Chest 1988; 93: 98–103. [DOI] [PubMed] [Google Scholar]
- 80. Sykes JE, Hartmann K, Lunn KF, et al. ACVIM small animal consensus statement on leptospirosis: diagnosis, epidemiology, treatment, and prevention. J Vet Intern Med 2011; 25: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rodriguez J, Blais MC, Lapointe C, et al. Serologic and urinary PCR survey of leptospirosis in healthy cats and in cats with kidney disease. J Vet Intern Med 2014; 28: 284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shropshire SB, Veir JK, Morris AK, et al. Evaluation of the Leptospira species microscopic agglutination test in experimentally vaccinated cats and Leptospira species seropositivity in aged azotemic client-owned cats. J Feline Med Surg 2016; 18: 768–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Weis S, Rettinger A, Bergmann M, et al. Detection of Leptospira DNA in urine and presence of specific antibodies in outdoor cats in Germany. J Feline Med Surg 2017; 19: 470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. McElroy KM, Blagburn BL, Breitschwerdt EB, et al. Flea-associated zoonotic diseases of cats in the USA: bartonellosis, flea-borne rickettsioses, and plague. Trends Parasitol 2010; 26: 197–204. [DOI] [PubMed] [Google Scholar]
- 85. Hawley JR, Shaw SE, Lappin MR. Prevalence of Rickettsia felis DNA in the blood of cats and their fleas in the United States. J Feline Med Surg 2007; 9: 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Angelakis E, Mediannikov O, Parola P, et al. Rickettsia felis: the complex journey of an emergent human pathogen. Trends Parasitol 2016; 32: 554–564. [DOI] [PubMed] [Google Scholar]
- 87. Hii SF, Kopp SR, Abdad MY, et al. Molecular evidence supports the role of dogs as potential reservoirs for Rickettsia felis. Vector Borne Zoonotic Dis 2011; 11: 1007–1012. [DOI] [PubMed] [Google Scholar]
- 88. Jiang P, Zhang X, Liu RD, et al. A human case of zoonotic dog tapeworm, Dipylidium caninum (Eucestoda: Dilepidiidae), in China. Korean J Parasitol 2017; 55: 61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hoyt K, Chandrashekar R, Beall M, et al. Evidence for clinical anaplasmosis and borreliosis in cats in Maine. Top Companion Anim Med 2018; 33: 40–44. [DOI] [PubMed] [Google Scholar]
- 90. Savidge C, Ewing P, Andrews J, et al. Anaplasma phagocy-tophilum infection of domestic cats: 16 cases from the northeastern USA. J Feline Med Surg 2016; 18: 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pantchev N, Vrhovec MG, Pluta S, et al. Seropositivity of Borrelia burgdorferi in a cohort of symptomatic cats from Europe based on a C6-peptide assay with discussion of implications in disease aetiology. Berl Munch Tierarztl Wochenschr 2016; 129: 333–339. [PubMed] [Google Scholar]
- 92. Lappin MR, Chandrashekar R, Stillman B, et al. Evidence of Anaplasma phagocytophilum and Borrelia burgdorferi infection in cats after exposure to wild-caught adult Ixodes scapularis. J Vet Diagn Invest 2015; 27: 522–555. [DOI] [PubMed] [Google Scholar]
- 93. Egberink H, Addie DD, Boucraut-Baralon C, et al. ; European Advisory Board on Cat Diseases. West Nile virus infection in cats: ABCD guidelines on prevention and management. J Feline Med Surg 2015; 17: 617–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Trainor KE, Porter BF, Logan KS, et al. Eight cases of feline cutaneous leishmaniasis in Texas. Vet Pathol 2010; 47: 1076–1081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Material_Feline_Zoonoses_Client_Brochure for 2019 AAFP Feline Zoonoses Guidelines by Michael R Lappin, Tom Elston, Lisanne Evans, Carol Glaser, Lorraine Jarboe, Peter Karczmar, Cathy Lund and Michael Ray in Journal of Feline Medicine and Surgery