Abstract
Practical relevance:
Feline triaditis describes concurrent pancreatitis, cholangitis and inflammatory bowel disease (IBD). The reported prevalence is 17–39% in ill referral patients. While the aetiology is poorly understood, it is known to include infectious, autoimmune and physical components. What is not known is whether different organs are affected by different diseases, or the same process; indeed, triaditis may be part of a multiorgan inflammatory disease. Feline gastrointestinal tract anatomy plays its role too. Specifically, the short small intestine, high bacterial load and anatomic feature whereby the pancreatic duct joins the common bile duct before entering the duodenal papilla all increase the risk of bacterial reflux and parenchymal inflammation. Inflammation may also be a sequela of bowel bacterial translocation and systemic bacteraemia.
Diagnostic challenges:
Cholangitis, pancreatitis and IBD manifest with overlapping, vague and non-specific clinical signs. Cholangitis may be accompanied by increased serum liver enzymes, total bilirubin and bile acid concentrations, and variable ultrasonographic changes. A presumptive diagnosis of pancreatitis is based on increased serum pancreatic lipase immunoreactivity or feline pancreas-specific lipase, and/or abnormal pancreatic changes on ultrasonography, though these tests have low sensitivity. Diagnosis of IBD is challenging without histopathology; ultrasound findings vary from normal to mucosal thickening or loss of layering. Triaditis may cause decreased serum folate or cobalamin (B12) concentrations due to intestinal disease and/or pancreatitis. Triaditis can only be confirmed with histopathology; hence, it remains a presumptive diagnosis in most cases.
Evidence base:
The literature on feline triaditis, pancreatitis, cholangitis and IBD is reviewed, focusing on histopathology, clinical significance and diagnostic challenges. Current management recommendations are provided. Further studies are needed to understand the complex pathophysiology, and in turn improve diagnosis and treatment.
Keywords: Triaditis, pancreatitis, inflammatory bowel disease, IBD, cholangitis
Introduction
In human medicine, triaditis refers to inflammation of the portal triads within the liver. In contrast, triaditis in cats has widespread acceptance in the veterinary field as referring to concurrent pancreatitis, cholangitis and inflammatory bowel disease (IBD). Despite being reported relatively fre-quently,1–3robust literature regarding the true prevalence of triaditis and its aetiopathogenesis is still lacking. Case reports investigating this condition started to emerge in the mid-1990s 1 and, to date, it has been reported in 17–39% of cats in ill referral populations.1–3While no sex, age or breed predisposition has been convincingly documented, cats with concurrent acute neutrophilic cholangitis (NC) tend to be younger than those with chronic lymphocytic cholangitis (LC), 4 and Siamese cats are over-represented in some studies of pancreatitis.5,6
This article reviews the literature on feline triaditis, focusing initially on pancreatitis, cholangitis and IBD. Histopathological descriptions, relative clinical significance and difficulties inherent in making these diagnoses are discussed, and recommendations given on how best to manage these conditions individually and collectively.
Component inflammatory disorders
Pancreatitis
In most cases, pancreatitis is considered idiopathic; however, some underlying causes such as viral infection, toxoplasmosis, fluke infection, trauma and organophosphate poisoning have been reported.6–8In humans, autoimmune and genetic components have been associated with pancreatitis;9–11and the role of the microbiome is currently gaining more interest as a study area. However, none of these have been recognised in feline pancreatitis as yet.
Pancreatitis can be divided into acute and chronic inflammation on the basis of histological findings.12,13The published scoring system is based on histopathological characteristics in humans, and recognises two main forms of feline pancreatitis. 14 Acute pancreatitis (AP) is characterised by neutrophilic inflammation, with associated interstitial oedema and necrosis of mesenteric fat, while chronic pancreatitis (CP) is characterised by lymphocytic inflammation, fibrosis and acinar atrophy. 14 However, a consensus scoring system for feline pancreatitis is still lacking. Since cats with CP are more likely to have concurrent diseases (eg, hepatobiliary disease) than cats with AP, 15 CP may be more common in triadi-tis. Pancreatic inflammation can extend to the pancreatic duct and even the sphincter of Oddi (see later), going on to cause cholangitis and, potentially, extrahepatic biliary obstruction (EHBO). Cholangitis occurring as a primary disease can cause inflammation extending to the sphincter of Oddi and pancreatic duct, and so, in turn, is a risk factor for pancreatitis. 16

Cholangitis
Cholangitis describes inflammation of the biliary duct; when the inflammation extends into the hepatic parenchyma, a diagnosis of cholangiohepatitis is made. 17 Cholangitis is a common form of feline hepatic disease: in a UK study of almost 1500 feline liver biopsy samples, 18 the most common histopathological diagnoses were NC and reactive hepatitis, while LC was ranked fourth (after reversible hepatocellular injury). Hepatic lipidosis is the most common form of liver disease in the USA. 19 It is important to remember that this can occur as a primary condition or secondarily to other diseases, particularly cholangitis and pancreatitis; as such, it is often associated with triaditis. 20
The World Small Animal Veterinary Association (WSAVA) Liver Standardization Group has revised its histopathological classification of feline liver disease, and recognises three distinct forms of cholangitis in cats (Table 1): neutrophilic, lympho-cytic and chronic cholangitis associated with liver fluke infection. 17 Acute and chronic forms of NC are no longer considered as separate diseases, as is now generally accepted.
Table 1.
WSAVA histopathological classification of feline cholangitis
| Classification | Features |
|---|---|
| Neutrophilic cholangitis | Characterised by the presence of neutrophils in the lumen and/or epithelium of the bile ducts. In the acute stage, the lesion is often associated with oedema and neutrophils in the portal areas. The neutrophilic inflammation may extend to the hepatic parenchyma and may even result in hepatic abscesses. The chronic stage is often associated with a mixed inflammatory infiltrate in the portal areas consisting of neutrophils, lymphocytes and plasma cells, plus possibly fibrosis and bile duct proliferation |
| Lymphocytic cholangitis | Consistent infiltration of small lymphocytes is restricted to the portal areas, often associated with variable portal fibrosis and bile duct proliferation. Lymphocytes may also be seen centring on the bile ducts or within the biliary epithelium. Solitary plasma cells and eosinophils may additionally be present. Lymphocytic cholangitis can be difficult to distinguish from well-differentiated lymphocytic lymphoma |
| Chronic cholangitis associated with liver fluke infection | Characterised by dilated larger bile ducts with papillary projections and marked periductal and portal fibrosis. A slight-to-moderate inflammation may be seen both within the ducts (neutrophils and macrophages) as well as in the portal areas (neutrophils, lymphocytes and plasma cells). Eosinophils are usually limited in number. The number of liver flukes and eggs within the dilated bile ducts varies markedly |
Adapted from WSAVA standards for clinical and histological diagnosis of canine and feline liver diseases 17

The most common type of cholangitis in cats is NC, which is characterised by infiltration of large numbers of neutrophils into portal areas of the liver and bile ducts. 17 It is believed to result from bacterial infection ascending from the intestine,19,21,22an assumption that is supported by the involvement of common enteric species, including Escherichia coli, Streptococcusspecies, Clostridiumspecies and Salmonella typhimurium. 23 NC can be further divided into acute and chronic forms, which are distinguished by their histopatho-logical appearance. Acute neutrophilic cholangitis (ANC) is characterised by neu-trophilic inflammation alone, while chronic neutrophilic cholangitis (CNC) is thought to be a progression of ANC, and is characterised by variable infiltrates of neutrophils, lymphocytes and plasma cells (Table 1). Fibrosis and bile duct proliferation depend on the chronic-ity of disease. 17
EHBO is often associated with NC. One study identified EHBO in 64% of cats with cholangitis, 24 with another study reporting EHBO in 76% of cats with CNC and 40% of cats with ANC. 25 Separately, in an investigation where EHBO was confirmed at surgery or necropsy in 22 cats, 15 (68%) had at least one inflammatory disease, including pancreatitis, cholangiohepatitis, cholelithiasis and chole-cystitis. 26 These inflammatory conditions, together with biliary or pancreatic neoplasia, were the most common causes of EHBO in that study. 26
LC is characterised by infiltration of lymphocytes and plasma cells confined around portal areas, with variable degrees of fibrosis and biliary hyperplasia. 27 The nature of the inflammatory infiltrate suggests an immune-mediated pathogenesis.28,29A study of 51 cases of feline LC found no strong evidence to implicate in situ bacterial colonisation as an aetiopathogenesis using standardised histo-pathology, immunophenotyping (B and T cells), PCR for T-cell receptor gene rearrangement, and fluorescence in situ hybridisation (FISH) for eubacteria. 29 LC typically has a chronic progressive clinical course, extending over months or years.28,30Affected cats may be young or old, and Persian cats have been reported to be over-represented, suggesting a possible genetic predisposition. 31
Chronic cholangitis associated with infection by liver flukes (family Opisihorchiidae) - the third distinct type of feline cholangitis - can be seen in cats from tropical environments. Cats, as the definitive host for liver flukes, acquire infection by ingestion of raw fish, and the young flukes migrate from the intestine to the liver via the bile ducts, causing thickening and cystic dilatation of the bile ducts.32,33In cats (and dogs), chronic cholangitis due to liver fluke infection has been associated with intra-hepatic and extrahepatic cholangiocellular car-cinomas. 32 Slight to moderate inflammation may be seen both within the ducts (neutrophils and macrophages) as well as in the portal areas (neutrophils, lymphocytes and plasma cells). 17 There is little to implicate chronic cholangitis as having a role to play in triaditis.
IBD
The exact cause of IBD remains unknown. However, it is believed that (as in people and dogs) the pathogenesis of IBD in cats involves the intestinal microbiome, dietary antigens, genetics and dysregulation of the intestinal immune system.34,35Both quantitative and qualitative alterations in intestinal microbiota are closely linked to the aetiopathogenesis of feline intestinal diseases, and a balanced intestinal microbiota ecosystem is crucial to ensure feline intestinal health. 36
In a study by Janeczko et al, 35 the number of mucosa-associated Enterobacteriaceae was higher in cats with signs of gastrointestinal (GI) disease than in healthy cats. The number of Enterobacteriaceae and Clostridiumspecies has been correlated with abnormalities in mucosal architecture (principally atrophy and fusion), upregulation of cytokine mRNA (particularly interleukin [IL]-1, IL-8 and IL-12), the density of cellular infiltrates, particularly macrophages and CD3+ lymphocytes, and the number of clinical signs exhibited by affected cats.30,35The composition and number of mucosa-associated bacteria correlate with the presence and severity of IBD in cats, raising the possibility that abnormal mucosal flora are involved in the pathogenesis of feline IBD and, in turn, that therapeutic intervention directed at the mucosal flora may abrogate the mucosal inflammatory response. 35
Another study found that cats with IBD had lower FISH counts on faecal samples for total bacteria, Bacteroidesspecies and Bifidobacteriumspecies, but higher counts of Desulfovibriospecies, compared with healthy cats. 29 Desulfovibriospecies are able to produce hydrogen sulphide, which may be associated with the pathogenesis of feline IBD. 36 However, in contrast, another study failed to identify significant differences in FISH counts between cats with IBD and controls, despite targeting the same bacterial groups. 37 Since cats with IBD have lower numbers of faecal Bifidobacteriumspecies compared with healthy cats, 34 bifidobacteria may be helpful in promoting an anti-inflammatory environment and decreased numbers of bifidobacteria might be harmful to cats at risk for primary GI disease. 38 While this area of research is expanding rapidly, there is still much to learn about the role of the microbiome in the patho-genesis and treatment of IBD.
Feline IBD predominantly affects middle-aged animals, although it is also seen in cats of 2 years of age or less. 39 Compared with cats with IBD or alimentary lymphoma, cats with food-responsive enteropathy tend to be younger and present with diarrhoea, while muscle wasting is infrequent. 40 Certain breeds are predisposed to IBD (eg, Siamese), but all breeds may be affected. 41
The WSAVA Gastrointestinal Standardization Group developed ‘Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat’ and established a grading system for the severity of GI inflammation with a simple four-point scale: normal (0), mild (1), moderate (2) or marked (3). 42 However, it is important to note that the number of intraepithelial lymphocytes (IELs) is much greater in healthy cats compared with dogs.43,44In addition, feline IELs are more frequently seen within the villus than crypt epithelium, and within the villus the number of IELs increases from the duodenum (where they number ~50% of epithelial cells) to the ileum (~80%).43,44This can lead to the over-diagnosis of IBD, as seen in a study where 42% of cats diagnosed with inflammatory lesions in their small intestine had no clinical signs suggestive of enteropa-thy. 3 More studies are needed to explore how this grading system for feline GI inflammation helps in the understanding and management of our feline patients.

Triaditis: insights on prevalence, aetiology and manifestations
Prevalence
Several studies have looked at the prevalence of triaditis (Table 2). In an early investigation, Weiss et al 1 evaluated sections of liver, intestine and pancreas from 78 cats at necropsy; 46% had lymphocytic portal hepatitis, 23% had cholangitis and 31% had no inflammatory cholangiohepatic disease. This study revealed that the prevalence of IBD (28%) and pancreatitis (14%) in cats with lymphocytic portal hepatitis was not significantly different from that of cats with no inflammatory cholangiohepatic disease. However, the researchers found that 39% of cats with cholangitis also had IBD and pancreatitis (ie, triaditis), and the prevalence of IBD and pancreatitis was greater in cats with cholangitis (83% and 50%, respectively) compared with cats with non-inflammatory hepatic disease. 1 Evidence of IBD in association with cholangitis was characterised by cholangio-hepatic infiltration of lymphocytes and plasma cells; however, neutrophilic infiltrates were also found in more than 40% of cats with concurrent IBD. Pancreatitis was histopathologi-cally mild in all cats. 1
Table 2.
Studies investigating the association between gastrointestinal, hepatic and pancreatic inflammatory disorders in cats
| Study type | Authors | Aim | Conclusions |
|---|---|---|---|
| Retrospective study of 78 cats at necropsy | Weiss et al 1996 1 | Assess the association of feline inflammatory hepatic disease with inflammation in other organs | Prevalence of IBD (28%) and pancreatitis (14%) in cats with lymphocytic portal hepatitis was not significantly different from cats without inflammatory hepatic disease
Prevalence of IBD (83%) and pancreatitis (50%) was greater for cats with cholangitis compared with cats without inflammatory hepatic disease; 39% of cats diagnosed with cholangitis also had IBD and pancreatitis (ie, triaditis) Concurrent nephritis was found in 33% of cats with cholangitis |
| Retrospective study of 30 cats with AP and 33 cats with CP | Ferreri et al 2003 15 | Characterise clinical, clinicopathological, radiographic and ultrasonographic findings in cats with histologically confirmed AP or CP | Concurrent disease was found in 83% of cats with AP and in 100% of cats with CP
Hepatobiliary disease was found in 17% cats with AP and 48% of cats with CP GI disease was found in 13% of cats with AP and 15% of cats with CP Renal disease was found in 27% of cats with AP and 15% of cats with CP |
| Retrospective study of 44 cats at necropsy | Callahan et al 2011 2 | Describe the clinical, laboratory, imaging and necropsy findings of a group of cats diagnosed with moderate-to-severe cholangitis | Pancreatitis (60%), IBD (50%) or both (32%) commonly accompanied cholangitis
Most cats that succumbed to cholangitis had ANC or CNC, and concurrent disease may have contributed to death in many cases Concurrent nephritis was found in 81% of cats with cholangitis |
| Retrospective study of 26 cats with cholangitis | Marolf et al 2012 45 | Evaluate the ultrasonographic changes within the liver, biliary system, pancreas and small intestine of cats with known cholangitis | Pancreas was enlarged in 39%, hypoechoic in 26% and hyperechoic in 11% of cats with cholangitis
Abnormal duodenal layering was reported in 12% of cats with cholangitis |
|
Prospective study
of 27 symptomatic, 20 asymptomatic and eight normal cats |
Fragkou et al 2016 3 | Investigate the frequency of IBD, cholangitis, pancreatitis, or combinations of these, in symptomatic and asymptomatic cats | A total of 34% of cats had concurrent IBD and cholangitis, 17% had triaditis, and 6% had IBD and pancreatitis
A mild, positive correlation was detected between the severity (score) of IBD lesions and the number of comorbidities |
IBD = inflammatory bowel disease; AP = acute pancreatitis; CP = chronic pancreatitis; ANC = acute neutrophilic cholangitis; CNC = chronic neutrophilic cholangitis
In a study of 44 cats diagnosed with moderate to severe cholangitis at necropsy, the majority had CNC (n = 33) or ANC (n = 7); 60% also had pancreatitis, 50% also had IBD and 32% had both (ie, triaditis). 2 However, necropsy studies may overestimate the prevalence of chronic disease as, unless patients die during the acute stage of the disease, the histopathology may only show signs of chronic changes due to the ongoing disease. Also, cats are notoriously good at hiding pain and illness, so some diseases, such as pancreatitis, can be apparently subclinical. In a study of 115 cats, 45% of apparently healthy cats had evidence of pancreatic lesions on post-mortem examination, 14 which underlines the challenges of diagnosing acute disease.
A later prospective study set out to investigate the frequency of IBD, cholangitis, pancreatitis, or a combination of these conditions, in symptomatic and asymptomatic cats by comparing clinicopathological features with histopathological and laboratory findings. 3 Inflammatory changes were detected in 47 cats (27 symptomatic, 20 asymptomatic); 28% of the cats had histopathology consistent with IBD alone, 13% with cholangitis alone and 2% with pancreatitis alone. 3 However, 57% of the cats had inflammation involving more than one organ: 34% had concurrent IBD and cholangitis; 17% were diagnosed with triaditis; and 6% had IBD and pancreatitis. 3 Importantly, triaditis was identified only in symptomatic cats (20%), and a mild, positive correlation was detected between the severity (score) of IBD lesions and the number of comorbidities. 3
Aetiology
The aetiology of feline triaditis is poorly understood. The disease may occur because of an infectious or an autoimmune process, or a physical problem such as duct obstruction. It is also not certain if cats suffer from different diseases in each organ or if the disease process in each organ has the same aetiology. The anatomy of the feline GI tract may play a role: the small intestine is comparatively shorter than in the dog; 46 there is a higher concentration of bacteria in the duodenum;47–49and the pancreatic duct joins the common bile duct before entering the duodenum at the papilla (Figure 1). 46 These adaptations increase the risk of bacteria ascending from the duodenum into the liver and pancreas, resulting in parenchymal inflam mation. 50 In support of this, the bacteria isolated from the pancreas and liver of cats with pancreatitis and hepatic diseases are common enteric species, such as E coli. 51 Vomiting can cause reflux of duodenal juice into the pancreatic and/or bile duct, spreading enteric bacteria into the pancreas and biliary tree. 52
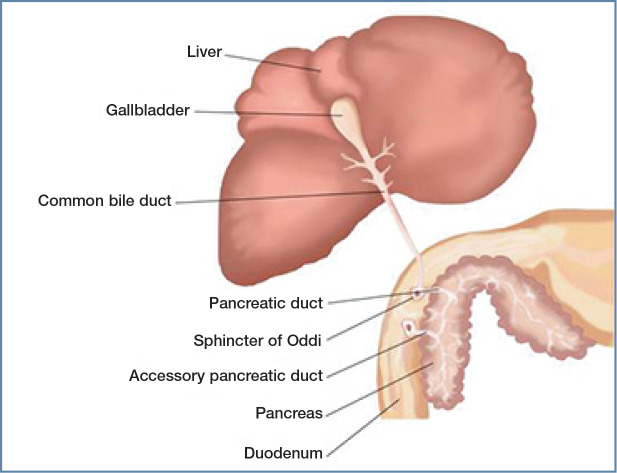
Figure 1.
Illustration showing the close proximity and anatomical relationship between the liver, pancreas and duodenum of the cat. The pancreatic duct joins the bile duct before entering the duodenum at the papilla, which may predispose to ascending infections of both the liver and pancreas, and potentially multiorgan inflammation. Of note, the accessory pancreatic duct is present in only around 20% of cats. Courtesy of Ceva
A study of cats with AP demonstrated that bacterial colonisation of the pancreas can occur by reflux from the intestinal tract into the pancreatic duct, and also by haematoge-nous spread. 53 Additionally, bacteria may pass, by translocation, across the mucosal barrier into the portal blood circulation and then enter the liver, rather than ascending via the bile duct. A FISH study has provided supporting evidence, showing intrahepatic bacterial colonisation of venous sinuses, portal vessels and the hepatic parenchyma, rather than colonisation within the bile ducts. 54 Inflammation or infection could also spread as a local effect due to the close proximity of the pancreas, bile duct and small intestine in cats. Intestinal inflammation may promote the translocation of enteric bacteria into the liver and pancreas. 51
Obstruction to the flow of bile or pancreatic juices may also play a role in triaditis. The sphincter of Oddi is a muscular valve that controls the flow of bile and pancreatic juices and is located within the wall of the duodenum at the terminal end of the common bile duct. 55 Blockage or dysfunction of the sphincter can predispose cats to pancreatitis and cholangitis. 55 In humans, gallstones can cause a blockage of the duct near the sphincter of Oddi, but this is very rare in cats.55–57Dysfunction or spasm of the sphincter has been described in humans and cats, causing blockage of both pancreatic and bile ducts and, in turn, obstruction.55–57In humans, this is often diagnosed in association with IBD -with inflammation in the intestinal wall around the sphincter predisposing it to spasm. 58 A case series study reported six cats with obstructive processes within their sphincter of Oddi, and three of these cats had concurrent IBD. 55 Also of note, only around 20% of cats have an accessory pancreatic duct; and, when present, it is most commonly rudi-mentary. 59 Hence, when pancreatitis does occur, digestive enzymes are unable to leave the inflamed pancreas, exacerbating the activation of digestive enzymes within the acinar cells, leading to pancreatic autodigestion. 60
The pathophysiology of triaditis may also have an immune component. 51 Memory T lymphocytes that arise as a consequence of IBD have been shown to express homing receptors that lead them to migrate to the liver and pancreas. These memory lymphocytes can then be activated within these organs, producing inflammation and tissue damage. This, in turn, stimulates the recruitment of more lymphocytes and causes a persistent inflammatory state. 51 Moreover, it has been suggested that altered mucosal integrity, secondary to IBD, may lead to inflammatory mediators, endo-toxins and microbes accessing the portal circulation. This can lead to immune complex deposition within the liver, complement activation and hepatocellular necrosis.50,61Changes to intestinal permeability that can occur with IBD also allow passage of bacterial and colonic epithelial cell antigens, promoting production of autoreactive antibodies. 50
It is possible that the three organs involved in triaditis are all affected by the same process concurrently. For example, humans with primary sclerosing cholangitis commonly have concurrent IBD, which is thought to be part of the same disease process as they have the same unique disease phenotype. The pathogenesis of this comorbidity in humans is still unclear, but the disease phenotype is different when these conditions occur separately and an immune-mediated component has been suggested. 62
Clinical signs
The clinical signs associated with feline pancreatitis are vague and non-specific (Table 3). Anorexia has been reported in 63-97% of cases and lethargy in 28–100%, while weight loss, dehydration, pallor and icterus are frequently noted on physical examination.2,5,8,15In contrast to dogs, fever is less common with feline pancreatitis. In one study, only 25% of feline patients with pancreatitis presented with pyrex-ia, whereas hypothermia was noted in almost 50% of cases. 8 A study evaluating systolic blood pressure in feline patients in an intensive care unit found that pancreatitis and/or hep-atopathy were associated with systemic hypotension, and reported that a Doppler systolic blood pressure lower than 124 mmHg was a negative prognostic indicator for survival. 70
Table 3.
Clinical signs that can be seen in cats with triaditis
| Pancreatitis | Cholangitis | Inflammatory bowel disease | |
|---|---|---|---|
| Hyporexia/anorexia | |||
| Polyphagia | ✓ | ||
| Vomiting | ✓* | ||
| Diarrhoea | ✓ | ✓* | |
| Weight loss | |||
| Lethargy | ✓* | ✓* | |
| Dehydration | ✓* | ||
| Jaundice | ✓* | ✓* | |
| Pyrexia | |||
| Hypothermia | ✓ | ||
| Abdominal pain | ✓ | ✓ |
Clinical signs marked with an asterisk are common. EPI = exocrine pancreatic insufficiency
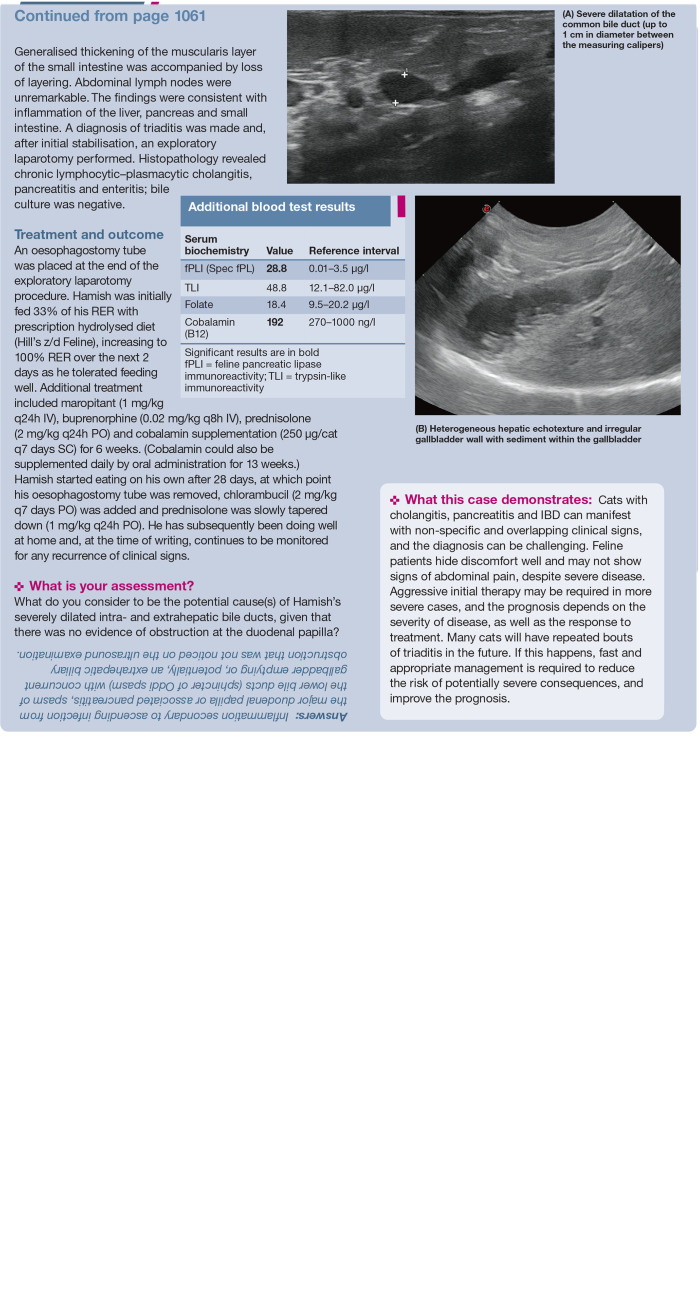
Anecdotally, the authors have seen many cases of severe triaditis develop vasculitis (Figure 2). Vasculitis markers used in people (antineutrophil cytoplasmic antibodies) are variably increased in cats with pancreatitis, but have also been seen in healthy cats, so studies are needed to understand the importance of these antibodies in cats (K Rolph, unpublished data). Also, it is difficult to distinguish between vasculitis and bruising due to other causes. Ideally, platelet number and function should be evaluated by blood smear and buccal mucosal bleeding time and clotting times (prothrombin time [PT] and activated partial thromboplastin time [APTT]) measured; where available, thromboelastography can be used to diagnose possible platelet and clotting disorders or to detect disseminated intravascular coagulation. These are life-threatening conditions and require immediate treatment. More studies, possibly involving thromboelastography, are needed to investigate the exact cause of coagulation problems in these cases to inform treatment decision-making.
Figure 2.
Siamese cat with triaditis that developed vasculitis. Courtesy of Kerry Rolph
Most cases of mild CP remain subclinical. 14 In one study, 45% of apparently healthy cats showed evidence of pancreatic lesions on post-mortem examination. 9 Ante-mortem diagnosis can be challenging, and the reported prevalence, based on histopathological findings, ranges between 0.6% and 67%.14,71,72Pancreatitis is often seen in cats with diabetes mellitus and can be a factor that contributes to deterioration of this disease and/or leads to the development of diabetic ketoacidosis.15,73–75
Cats with NC are often young (3–5 years old) and present with an acute illness that usually lasts for about a week. 76 The clinical signs are often nonspecific (Table 3), including anorexia, lethargy, vomiting and weight loss, with icterus, dehydration, fever, hepatomegaly and abdominal pain being observed in fewer than half of all cases. 71 Cats with LC may show clinical signs of anorexia, lethargy, vomiting and weight loss. The most common physical findings include icterus (Figure 3), 3 ascites and hepatomegaly. As with NC, severe cases may develop signs of hepatic encephalopathy 77 (Figure 4) or coagu-lopathy.78,79However, hepatic encephalopathy appears to be relatively uncommon in cats with acquired liver diseases and most commonly develops with severe chronic disease and/or secondary hepatic lipidosis. Its most frequent manifestations are ptyalism (excessive salivation) and altered mentation. Acquired hypocobalaminaemic encephalopathy has also been reported, and measurement of serum cobalamin concentration in patients with encephalopathy is recommended. 80
Figure 3.
Burmese cat diagnosed with cholangitis. Jaundice is usually seen after a five- to-10-fold increase in serum bilirubin concentration
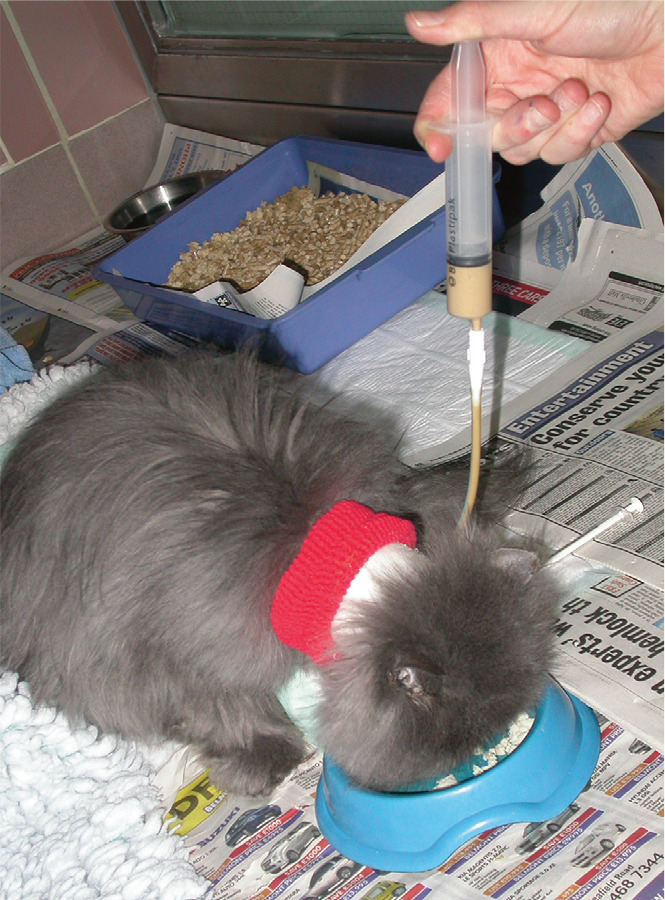
Figure 4.
Same cat as in Figure 3, receiving a lactulose enema for treatment of hepatic encephalopathy that developed secondarily to neutrophilic cholangitis. Lactulose therapy is considered a first-line treatment and can be administered orally and/or rectally. Note, however, that there are high risks associated with oral administration in cats with decreased mentation (eg, aspiration pneumonia), so rectal administration is preferred in these cases
In cats, IBD is a diagnosis of exclusion, and clinical signs resemble those of other chronic GI diseases (eg, alimentary tract lymphoma), including diarrhoea, vomiting, hyporexia and/or weight loss (Table 3). 81 However, clinical signs can be subtle, and many cats found to have IBD show only hyporexia.
Diagnosing triaditis
Haematology and serum biochemistry abnormalities
Haematology, serum biochemistry (including serum total thyroxine), serum feline trypsin-like immunoreactivity (fTLI) and urine analysis are all useful in excluding common systemic and metabolic disorders such as renal disease, hepatopathy, hyperthyroidism (a frequent cause of increased liver enzymes and GI signs in elderly cats), 82 exocrine pancreatic insufficiency or other diseases causing chronic intestinal signs in cats. The most common haematological and serum biochemistry findings are listed in Table 4.
Table 4.
Haematology and serum biochemistry abnormalities in cats with triaditis
| Pancreatitis | Cholangitis | Inflammatory bowel disease | |
|---|---|---|---|
| Haematocrit | N or ↓ | N or ↓ | N or ↓ |
| Leukocytes | N or ↓ | N or ↑ | N or ↑ |
| Neutrophils | N or ↑ or ↓ | N or ↑ | N or ↑ |
| Lymphocytes | N or ↑ or ↓ | N or ↑ or ↓ | N or ↑ or ↓ |
| ALT | N or ↑ | N or ↑ | N |
| ALP | N or ↑ | N or ↑ | N |
| Bilirubin | N or ↑ | N or ↑ | N |
| Bile acids | N or ↑ | N or ↑ | N |
| Glucose | N or ↑ or ↓ | N | N or ↑ |
N = normal; ↑ = increased; ↓ = decreased; ALT = alanine aminotransferase; ALP = alkaline phosphatase
Changes in haematology and serum biochemistry in feline pancreatitis are nonspecific. Regenerative or non-regenerative anaemia can be seen with AP; however, this is often masked by dehydration.8,15Leukopenia may be associated with a poorer prognosis. 8 Non-immune-mediated haemolytic anaemia may be seen, likely associated with oxidative damage. 83 Changes in serum biochemistry may reflect concurrent disease. Increased serum concentrations of alanine amino-transferase (ALT; 24–68%) and alkaline phosphatase (ALP; 50%) are commonly report-ed.5,8,15In one study, liver enzymes increased more in cats with CP than AP, possibly owing to concomitant hepatobiliary inflammation. 15 Other non-specific abnormalities include both hyperglycaemia and hypoglycaemia, as well as hypokalaemia.5,8,70Where available, ionised calcium should be measured in cats with pancreatitis, as ionised hypocalcaemia has been reported in many cats with AP (32–61% of cases) and, according to several studies, is associated with a poorer outcome.5,8,15,25
Haematology findings in cats with cholan-gitis may include neutrophilia (with or without a left shift) and serum biochemistry may reveal increased concentrations of ALT, aspar-tate aminotransferase (AST) and ALP, and mild to severe bilirubinaemia.84,85Serum bile acid concentrations may be increased if there is obstruction to bile flow. While increased liver enzymes may give information about liver damage, the bile acid stimulation test gives more information about liver function. 86 Note, however, that measuring bile acids in a jaundiced cat does not provide additional information on liver function in the case of cholestasis, as biliary stasis increases both bile acids and bilirubin. Clotting times may be extended and should be measured in cats with suspected liver disease prior to any sampling (eg, fine-needle aspiration).78,79
Additional tests
A number of assays have been assessed for their utility in the diagnosis of feline pancreatitis. Serum lipase and amylase activities are of limited value as they are insensitive and can be affected by liver, renal or GI dis-eases;5,72,87,88however, the DGGR-lipase assay has been shown to be useful, especially when compared with histology as the gold stan-dard. 25 Seroassays specifically measuring pancreatic lipase include feline pancreatic lipase immunoreactivity (fPLI), which is available as Spec fPL and SNAP fPL. Spec fPL is a quantitative test, for which concentrations >5.3 ug/l are consistent with pancreatitis and concentrations between 3.5 and 5.3 ug/l are in a grey zone. 89 Unlike Spec fPL, which is not available as an in-house assay, SNAP fPL is available as an in-house semiquantitative test. A positive SNAP fPL result indicates an fPLI value >3.5 Ug/l; it does not differentiate patients in the grey zone from patients with fPLI values considered consistent with pancreatitis. The DGGR-lipase test has similar sensitivity and specificity to the Spec fPL and, where available, may be a cost-effective alternative. 90 Serum fTLI is a species-specific immunoassay that has a relatively low sensitivity for the diagnosis of feline pancreatitis27,84,88and is more commonly used for diagnosing exocrine pancreatic insufficiency.
Evaluation of serum cobalamin and folate concentrations may aid in the diagnosis of IBD and chronic pancreatitis, and be important for guiding treatment in cats with both IBD and chronic pancreatitis. Low serum cobalamin concentration can indicate diffuse distal small intestinal (more specifically ileal) and/or pancreatic disease, while low serum folate concentration can be seen with diffuse proximal small intestinal disease. 77 Dietary cobalamin in cats is absorbed in the ileum and the uptake of the cobalamin-intrinsic factor complex is a receptor-mediated process.91,92
This can be affected by diseases of the intestinal mucosa as well as pancreatic disease, as the carrier protein, intrinsic factor, only comes from the pancreas in cats. Repeated bouts of pancreatitis can result in exocrine pancreatic insufficiency, with a concurrent deficiency in cobalamin, probably due to a lack of pancreatic intrinsic factor in these cats. Cats with GI disease may also have abnormalities in amino acid metabolism consistent with cobalamin deficiency. 93 Subnormal serum cobalamin concentrations may be found in 60% of cats with GI disease, and suggest that this test is a useful indirect indicator of feline enteric and pancreatic disease. 94
Other possible causes of chronic intestinal inflammation need to be ruled out through faecal examination for parasites (Giardia, Cryptosporidiumand Cystoisosporaspecies); culture for potential pathogenic bacteria; and/or a PCR panel including, for example, Tritrichomonas foetus, Giardiaspecies, Cryptosporidiumspecies, Toxoplasma gondii, Salmonellaspecies, Clostridium perfringensenterotoxin A gene, feline coronavirus and feline panleukopenia virus.
Diagnostic imaging
Imaging may be useful in the diagnosis of feline pancreatitis; however, abdominal ultrasound has been reported to have low sensitivity (11–35%).15,27,76,95Moreover, a study that evaluated ultrasonographic findings in cats with clinical, gross pathological and histo-pathological evidence of acute pancreatic necrosis found that the results of ultrasonog-raphy were consistent with a diagnosis of pancreatitis in only 7/20 (35%) cats. 96 Nonetheless, it has been suggested that a thick left limb of the pancreas, severely irregular pancreatic margins, and hyperechoic peripancreatic fat in cats with appropriate clinical signs and increased serum fPLI are highly supportive of pancreatitis. 96 The sensitivity of abdominal ultrasound is further influenced by difficulties in detecting the pancreas in some patients and is highly dependent on the skills of the imager and quality of the equipment.27,76
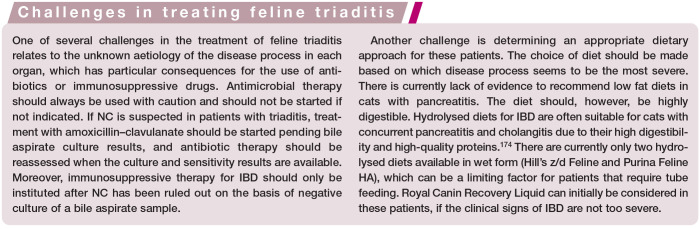
Abdominal radiography and/or abdominal ultrasound examination is used to confirm hepatomegaly and free abdominal fluid, if present. Ultrasonography may demonstrate diffuse heterogeneous (typically hyperechoic) liver parenchyma, sometimes with segmental dilatations in intrahepatic and extrahepatic bile ducts suggestive of repeated obstructions. There may also be hyperechoic contents. 45 However, in one retrospective study of cats with cholangitis, most of the cats had ultra-sonographically normal liver size, echogenici-ty and normal biliary systems. 45 In cases of EHBO, abdominal ultrasound may reveal diffuse heterogeneous change in the hepatic parenchyma, 97 plus distension of the common bile duct and gallbladder, with or without increased bile sediment. 25
Abdominal radiography is rarely useful in the diagnosis of IBD. Abdominal ultrasonog-raphy can be normal in many cases of IBD and low-grade alimentary lymphoma, but is important in ruling out other causes of chronic GI clinical signs. 98 Nonetheless, abdominal ultrasound is superior to radiology in defining focal vs diffuse mucosal disease, loss of intestinal layering, thickening of the muscu-laris layer, generalised intestinal thickening and mesenteric lymphadenopathy, which may be seen with IBD, as well as other infiltra-tive disorders (eg, lymphoma). 98 The significance of finding a thickened muscularis layer is debated. One study found this more commonly in cats with intestinal lymphoma or IBD compared with healthy cats; 99 in another study, a thickened muscularis layer in older cats was found more commonly in cats with lymphoma than those with IBD. 100 However, in a study of 56 cats - 22 with food-responsive enteropathy, 17 with IBD and 17 with alimentary lymphoma - there were no significant differences in the ultrasonographic findings between the three groups. 40
Ultrasonography can aid in the recognition of concurrent disease, providing useful information on the presence of multiorgan inflammatory disease, particularly involving the liver, pancreas and/or alimentary tract. A retrospective study by Marolf et al, looking at 26 cats with cholangitis, reported concurrent changes in the pancreas (enlarged in 39% of cats, hypoechoic in 26% and hyperechoic in 11%) as well as abnormal layering of the duodenum in 12% of cats. 45 In a necropsy study by Callahan Clark et al, looking at cats that had moderate to severe cholangitis diagnosed by abdominal ultrasound, liver abnormalities were described in 81% of cats (17/21), biliary tract abnormalities in 64% (14/22), pancreatic abnormalities in 52% (11/21) and GI tract abnormalities in 65% (13/20). 2 However, in another study, 58% (26/45) of cats with triaditis had no detectable abnormalities on abdominal ultrasound. 3
Practical guidance on performing abdominal ultrasonography in cats, and interpreting the resulting images, is provided in a virtual special issue series in JFMS, available at cpsi.jfms.com.
Biopsies
The gold standard for the ante-mortem diagnosis of inflammation in the pancreas, liver and GI tract in cats remains histopatho-logical analysis of biopsies. However, these patients are not always stable enough for surgical procedures.
Pancreatic biopsy (Figure 5) is invasive in nature and, because of the risk of further pancreatic inflammation, is not generally recommended in clinical practice for evaluating the presence of feline pancreatitis. 14 , 25 , 72 The biopsy procedure is often complicated by the sensitivity of the pancreas to hypoxaemia, whether induced by hypotension during anaesthesia or by pancreatic blood flow impairment following manipulation of other organs during surgery. Moreover, most cats with severe pancreatitis are poor candidates for anaesthesia. That said, pancreatic biopsy should be considered if laparotomy or laparoscopy are being performed for other reasons.89,101
Figure 5.
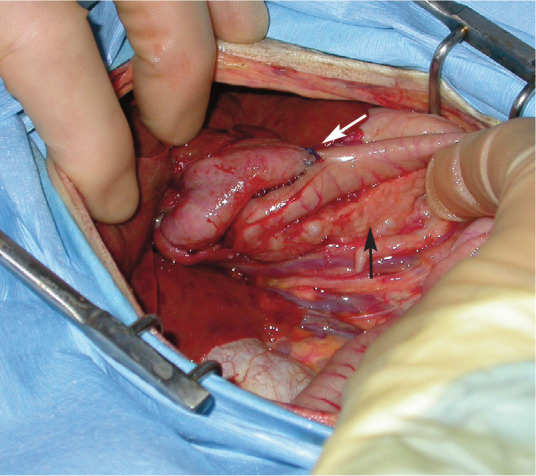
Biliary bypass surgery in a 7-year-old cat with a biliary obstruction and acute pancreatitis. This image shows the right limb of the pancreas (black arrow) and the biliary bypass in situ (the gallbladder has been sutured to the duodenum [white arrow]). Pancreatic biopsies were also acquired. Courtesy of Donald Yool
In the literature, it was previously recommended that, in the absence of grossly identifiable abnormalities in the pancreas, a single biopsy at the distal aspect of the right limb of the pancreas should be taken because of its distance from the pancreatic duct system and comparatively reduced vascular supply to the other organs. 24 A later study reported that this may not be a reliable method of whole organ assessment; 102 however, taking multiple pancreatic biopsies increases the risks referred to above and is not recommended. A small study evaluating laparoscopic pancreatic punch biopsy of the left pancreatic limb in healthy cats reported this to be a safe technique. 103 Certainly, more studies are needed to evaluate the safety of pancreatic biopsies in cats with pancreatitis.
Intestinal biopsies should be considered in cats with normal intestinal layering on ultrasound or focal intestinal mass effects.98,100Intestinal biopsies can be performed via endoscopy, laparoscopy or laparotomy to confirm histopathological inflammation and to determine the extent of mucosal disease. 81 A study looking at techniques for taking intestinal biopsies in cats found endoscopic biopsies to be useful for the diagnosis of gastric lym-phoma, but inadequate for differentiating between IBD and lymphoma in the small intestine; this is because the common locations for lymphoma in cats are the jejunum and ileum, which can be challenging to reach by endoscopy in feline patients. 104 Full-thickness biopsies obtained via laparotomy or laparoscopy are better for more accurate evaluation of the jejunum and ileum, and for diagnosing inflammation in the pancreas and liver at the same time. However, these are invasive procedures, and laparos-copy may offer a minimally invasive alternative to laparoto-my for obtaining biopsy samples of all three organs, where available.
In cases of IBD, histology typically reveals lymphoplasma-cytic inflammation in the small and / or large intestine; 81 note that neutrophilic / suppurative, granulomatous and rarely eosinophilic scle-rosing fibroplasia can also be detected. 104 Full-thickness biopsies were useful in differentiating between intestinal lymphoma and chronic enteritis in one study, where ultrasonography or clinicopathological characteristics alone were not helpful for this differentiation. 105 Triaditis is most commonly associated with lymphoplasmacytic IBD; it can also occur as a suppurative syndrome, but this is much less common. 106
Treatment of triaditis
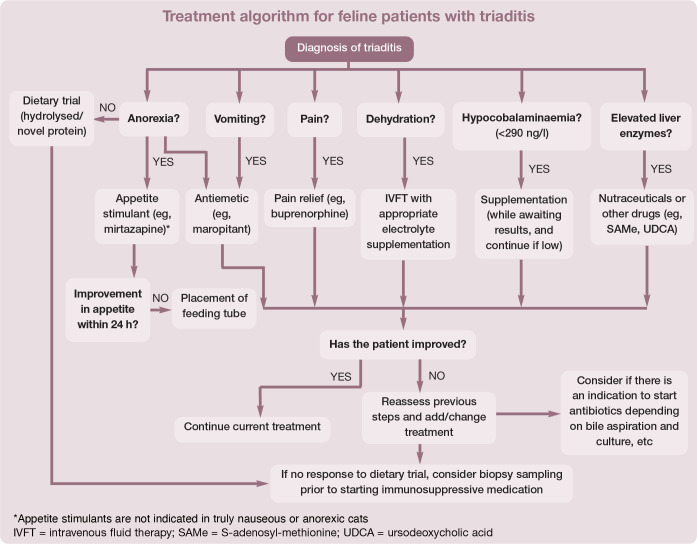
Since triaditis describes concurrent pancreatitis, cholangitis and IBD, and the aetiology of these comorbidities is unclear, treatment should be focused on the specific type and severity of the disease present in each of the organs involved in the individual cat (Table 5). 20 The authors recommend following a treatment algorithm for feline patients with triaditis, as shown on page 1058.
Table 5.
Recommended dosages of medications commonly used for the treatment of triaditis in cats
| Specific agents and dosages | |
|---|---|
| Maropitant*1 mg/kg SC/IV/PO q24h Ondansetron*0.5–1 mg/kg PO q12–24h Metoclopramidet0.2–0.5 mg/kg IV/SC/PO q12h or 1–2 mg/kg/day CRI †Should be avoided in patients with pancreatitis |
|
| Analgesics | Buprenorphinet 0.02–0.03 mg/kg IV/IM/SC q6–12h †Can also be given transmucosally Methadone 0.1–0.3 mg/kg IV q4–6h Gabapentin*5–10 mg/kg PO q8–12h Maropitant*1 mg/kg SC/IV/PO q24h (visceral analgesia) Fentanyl*5 ug/kg IV bolus or 2–4 ug/kg/h CRI |
| Antibiotics* | Amoxicillin clavulanate 10–20 mg/kg SC/IV/PO q8–12h Tylosin*7–15 mg/kg PO q12–24h Metronidazolet 7.5–15 mg/kg PO q12–24h †/n severe liver disease: 7.5 mg/kg PO q12–24h |
| Immunosuppressive drugs | Prednisolone 1–2 mg/kg PO q24h Chlorambucil*2 mg PO q2–7 days Ciclosporin 5 mg/kg PO q24h |
| Vitamins | Cobalamin 250 ug/cat SC/IM q7 days for 6 weeks, then monthly (re-evaluate serum concentration 1 month after the last injection) 250 ug/cat po q24h for 12 weeks (re-evaluate serum concentration 1 week after stopping the treatment) Folate 400 ug-1 mg/cat PO q24h Vitamin K†0.5–1.5 mg/kg SC/IM q12h for 2–3 days (acute supplementation, particularly prior to biopsy sampling) 0.5–1 mg/kg SC/IM q7 days (chronic cholangitis) †Check coagulation factors |
| Nutraceuticals | SAMe 100 mg/cat PO q24h - for hepatitis and as an antioxidant that may help with pancreatitis |
| Appetite stimulants | Mirtazapine 1.9 mg/cat PO or percutaneously q48h (can be double dosed if needed or increased in frequency to q24h but not both) Capromorelin*2 mg/kg PO q24h |
| Other drugs | Ursodeoxycholic acid*10–15 mg/kg q24h - to aid bile flow and potentially reduce biliary inflammation |
Not licensed for cats
See text for further discussion on when antimicrobial therapy is justified, and practical considerations
SC = subcutaneously; IV = intravenously; PO = orally; CRI = constant rate infusion; IM = intramuscularly; SAMe = S-adenosyl-methionine
Cats with mild clinical signs that are haemo-dynamically stable may be treated on an outpatient basis, but cats with severe clinical signs require hospitalisation and aggressive therapy including fluid therapy, analgesics, antiemetics and assisted feeding to prevent the development of hepatic lipidosis. Intravenous fluid therapy helps to ensure that the patient is well hydrated and the organs (eg, pancreas) are adequately perfused. At first, fluids should correct dehydration over the first 12–24 h, while also meeting maintenance needs and replacing ongoing losses from vomiting, diarrhoea and third-space losses such as peritoneal and/or pleural effusions secondary to pancreatitis. Acid-base and electrolyte abnormalities, such as hypo-kalaemia and hypocalcaemia, should be monitored closely and addressed if present.8,25Hypokalaemia can be corrected parenterally by adding potassium chloride to intravenous fluids for hospitalised patients or orally by supplementing with potassium gluconate (initial dose is 2.2 mmol/l per 4.5 kg q12h). The dose should be adjusted based on clinical improvement and serial measurements of serum potassium concentration.
Recommendations regarding the use of synthetic colloids, such as hydroxyethyl starch, have changed in recent years, with newer evidence questioning their efficacy and safety. 107 The use of fresh frozen plasma (FFP) in pancreatitis is questionable and one study reported higher mortality in dogs with pancreatitis that were receiving FFP compared with those that were not. 108 There is little specific information on the use of FFP in feline pancreatitis. However, the use of FFP may be beneficial in cats with severe liver disease and/or AP that have developed coagulopathy, 109 as active pancreatic enzymes can cause damage to cellular membranes, endothelium and adipose tissue, and interfere with the coagulation cascade; this can result in tissue damage, necrosis, disseminated intravascular coagulation, thrombosis, oedema and hypoxia.110,111
The fact that cats can hide pain and illness very well (a protective mechanism linked to predator avoidance in the wild 112 ) means they may appear healthy to their owners until their condition is very serious. Therefore, although cats do not always show abdominal pain, analgesia is important in the management of feline pancreatitis. Opioids such as buprenor-phine, methadone or fentanyl provide a good level of analgesia in cats.87,113Fentanyl trans-dermal patches have become popular as they provide a longer duration of analgesia; however, there is great individual variability in drug absorption and the risk of toxicity if the patient ingests the patch, so their reliability and safety have been questioned.114,115
Patients must be pain-scored frequently (eg, using the Glasgow Feline Composite Measure Pain Scale) to ensure they are receiving adequate pain relief.114–118Analgesia is also an important part of the management of cats with CP, and sublingual buprenorphine has been reported to provide a good level of analgesia in these cases.113,119Moreover, neuromodulatory analgesic drugs, such as gabapentin, have been reported to be useful for providing analgesia against neuropathic pain in humans and dogs,120,121and are a relatively well tolerated, easy-to-administer and potentially effective pain medication for cats, 122 especially the chicken-flavoured liquid version (where available).
Antiemetic therapy plays an important role in the treatment of triaditis. Although vomiting may only be intermittent in cats, patients may still be nauseous, which can contribute to the frequent anorexia seen in these patients. Maropitant citrate is a very effective antiemetic in cats and acts on the neurokinin (NK-1) receptors in the vomiting centre in the medulla oblongata. 123 Maropitant is also beneficial in reducing visceral pain in cats, which further supports its use in feline pancreatitis. 124 Ondansetron acts on the serotonin 5-HT3 receptors in the chemoreceptor trigger zone (CRTZ) 125 and is a very effective antiemetic in cats. 126 Metoclopramide is a dopamine antagonist and inhibits vomiting by blocking the central nervous system dopamine receptors in the CRTZ; however, cats are reported to have few dopamine receptors in the CRTZ, questioning the efficacy of this drug in this species.127,128In addition, as the effect of metoclopramide on splanchnic perfusion in cats is unclear, it should be avoided in patients with pancreatitis.
Nutritional support is crucial in the management of feline triaditis. The historical recommendation of nil per os for animals with pancreatitis is no longer accepted as this can cause intestinal mucosal atrophy and increased infectious complications due to bacterial translocation from the gut; in humans with severe acute pancreatitis, early initiation of feeding has resulted in reduced mortality.101,128–130This is especially important in cats as they often develop hepatic lipidosis with inadequate calorie intake.8,71,131Enteral nutrition stabilises the GI barrier, improves enterocyte health and immune function, improves GI motili-ty, prevents catabolism, and decreases morbidity and mortality.132–134
Appetite stimulants can help to support caloric intake, may reduce the need for feeding tube placement, decrease dependency on the feeding tube over time, and support the earlier removal of feeding tubes.
However, appetite stimulants are not indicated in truly nauseous and anorexic cats, and should not be used as an alternative to tube feeding. In cats that are not eating a significant amount of their daily requirements, feeding tube placement is preferred.
Mirtazapine is a commonly used appetite stimulant in cats and, although its mechanism of action is not fully understood, it most likely antagonises the 5HT2c and H1 receptors, which inhibit the appetite and regulate the appetite, respectively.135,136As mirtazapine undergoes hepatic metabolism, liver disease is reported to delay the clearance of the drug in humans and cats and, therefore, it is recommended that oral (or percutaneous) mirtazapine q24–72h only be given with caution to cats with significant liver disease. 137
Cats can be tempted with small portions of food, which can be warmed to enhance the smell. Note that syringe feeding should not be used as this can be stressful for cats and may create food aversion.
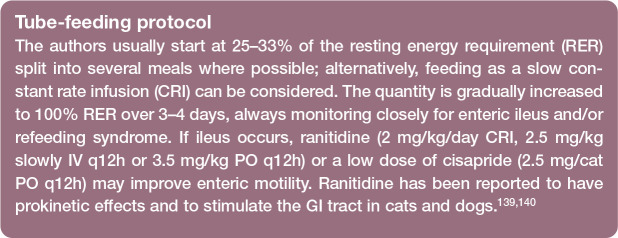
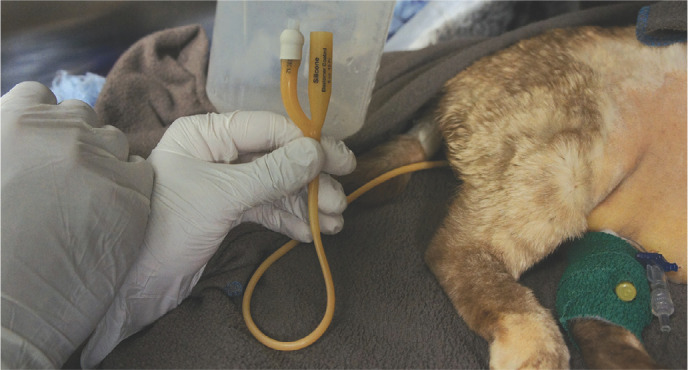
Assisted feeding may be provided through a naso-oesophageal, oesophagostomy (Figure 6) or gastrotomy tube. Typically, naso-oesophageal feeding is used on presentation, as cats with severe AP are often unstable, and have increased anaesthetic risk and severe hepatopathy (typically hepatic lipidosis), which can result in coagulopathy, thereby precluding surgical tube placement. 138 Once coagulation parameters (PT and APTT) have normalised (usually within 1-2 days of vitamin K treatment) and pancreatitis has stabilised, an oesophagostomy tube can be placed. Gradual introduction of food (see box) with continued monitoring for refeeding syndrome is recommended. 131
Figure 6.
Assisted feeding through an oesophagostomy tube may be highly beneficial in the management of feline triaditis. Cats should always be offered food before tube feeding. This cat is eating warm chicken while also being fed through its oesophagostomy tube. There is an empty syringe beside the cat as the tube was also used to administer medications
If vomiting cannot be controlled, parenteral nutrition (with total parenteral nutrition solutions) can be used to meet some or all of the patient’s caloric needs. However, although parenteral nutrition supports the patient’s caloric needs, it has significant side effect risks, and it does not nourish the enterocytes.131,141In human medicine, early initiation of enteral nutrition in the management of AP has been reported, with decreased mortality.101,128–130Feeding tubes can also be used to give extra fluids and facilitate drug administration.
Pancreatitis
Cats with pancreatitis should be monitored for the development of exocrine pancreatic insufficiency and hypocobalaminaemia 142 and, where necessary, cobalamin should be supplemented either parenterally or orally.94,143A recent study looking at the use of cortico-steroids in dogs with AP, reported an earlier reduction in C-reactive protein concentration and earlier improvement of clinical signs after initial treatment with prednisolone (1 mg/kg/day) compared with the non-prednisolone group; 144 however, more (longer term) studies are needed before this can be recommended in dogs. 144 Studies are also needed to see if an anti-inflammatory dose of cortico-steroids improves clinical signs in cats with AP. At present, there is not enough evidence to support the use of corticosteroids in this way.
Cholangitis
The management of cholangitis is ideally based on bile culture results and the histopathological diagnosis; however, symptomatic therapies can also help. As NC is often associated with bacterial infection, antibiotics are warranted. When possible, culture and sensitivity testing of a bile aspirate or liver biopsy/aspirate should be used to determine the most suitable antibiotics. In general, a 4-week course of antibiotics is indicated, with acute cases usually responding more quickly than chronic cases.
The use of antibiotics may also be supported by the finding of positive FISH staining for bacteria. 54 (The pathology laboratory can be requested to send the histopathology block to special laboratories in the University of Bristol, UK or Cornell University, USA, for FISH testing.) Gram-negative and Gram-positive bacteria can be involved in hepatobiliary infections: E coli, Enterococcusspecies and anaerobes (Bacteroidesspecies and Clostridiumspecies, among others) have all been reported, with a prevalence of 7–56%.145,146Empirical treatment is with amoxicillin-clavulanate; however, this is only appropriate while pending results of culture (both aerobic and anaerobic) and sensitivity testing of a bile aspirate or liver biopsy/aspirate, as resistance is an important concern, especially for E coli.146,147
The duration of antibiotic therapy should be adjusted according to the clinical signs and liver enzyme values, and treatments lasting several weeks are often necessary. Caution with antibiotics that are metabolised in the liver (eg, metronidazole) is required in animals with jaundice or significant hepatic damage, making an adjustment in dose or administration interval, as necessary. 148
Symptomatic adjunct therapies may include ursodeoxycholic acid (UDCA), S-adenosyl-methionine (SAMe), silybin (milk thistle)149,150or other antioxidants such as N-acetylcysteine (NAC) or vitamin E (tocopherol). Cats with liver disease or severe IBD have a high prevalence of vitamin K-responsive coagulopathies, so treatment with vitamin K is recommend-ed. 151 In one study, 98% of cats with liver disease had one or more abnormalities of the coagulation parameters (APTT, PT, thrombin time [TT]), most consistently prolonged APTT. 79 Measurement of coagulation factors is crucial in patients undergoing surgery (eg, surgical biopsies) or oesophagostomy tube placement.
LC is thought to be immune mediated and many cats will require immunosuppressive therapy with drugs such as prednisolone, ciclosporin or chlorambucil. 152 A retrospective study comparing UDCA alone vs cortico steroids alone in the treatment of LC found UDCA to be inferior based on improved survival time in the prednisolone group. 153 Prospective studies are needed to evaluate the benefit of using UDCA and corticosteroids together for these patients.
IBD
Lymphocytic-plasmacytic enteritis, the most prevalent form of IBD, frequently responds to dietary modification with an antigen-restricted (potentially novel protein) or a hydrolysed diet, as multiple dietary components are recognised as foreign antigens by the enteric immune system. 154 A novel protein is a protein source that the cat has never been exposed to before, such as rabbit or kangaroo, while the protein in hydrolysed protein diets has been broken down by enzymes into small peptides that are less allergenic than entire proteins. 155
One study of cats with chronic idiopathic enteric signs reported improvement in clinical signs in 50% of cases, usually within 4 days of eating only a commercially available chicken-or venison-based single protein diet (Whiskas Feline Selected Protein Diet [chicken and rice] or Waltham Veterinary Diet Feline Selected Protein Diet [venison and rice]). Clinical signs did not recur after challenge with the animal’s previous diet in 20% of these cases, indicating that this was not an allergic response in these cats, merely a temporary enteric imbalance. 156 In a study of eight cats with IBD, a hydrolysed protein diet (Royal Canin Hypoallergenic) resulted in resolution of clinical signs within 4–8 days in all cases; however, in these cats, a challenge with the previous diet did result in recurrence of clinical signs. 157
The importance of clients feeding only the single diet, and not letting the cat eat or drink anything other than that diet and water, must be emphasised and understood. In stable patients, if one dietary trial fails to reduce clinical signs in 14 days, the authors recommend instituting a trial with a different diet for another 2 weeks before considering drug therapy.
Immunosuppression plays an important role in the treatment of IBD, and prednisolone should be considered in patients that do not respond to at least one strict dietary trial. 81 In the event of clinical signs recurring when prednisolone is tapered, therapy with additional immunosuppressive drugs such as chloram-bucil or ciclosporin can be initiated.81,158Chlorambucil or ciclosporin should be used as a first-choice treatment over prednisolone in cats with concurrent diabetes, and pred-nisolone should be used with caution in Burmese cats, as they are predisposed to diabetes.159–163It is important to note that chlorambucil is a chemotherapeutic agent and owners need to take precautions when handling this drug, such as wearing gloves when administering the medication and when cleaning the litter tray; particular caution is required in homes with young children and pregnant women.
Other medications that may also be useful include probiotics or prebiotics, which have been shown to have a beneficial effect in some cases of IBD.164–166If a cobalamin deficiency is documented, it should be supplemented; initially by parenteral injection, and then either by parenteral injection or orally.143,167
Antimicrobial therapy using tylosin or metronidazole, among other antibiotic agents, can be justified in the presence of intestinal infiltration with macrophages or neutrophils, or when a suspected infectious process has been confirmed by culture, special stains and/or FISH.78,168As mentioned earlier, metronidazole should be given at a lower dose in patients with severe hepatic disease because it is metabolised by the liver. 41 Where culture and sensitivity testing for intestinal disease is available, antibiotic selection should be based on this.
Faecal microbiota transplantations are now being used in cases of intractable diarrhoea in people and dogs,169–173and the authors have had some success with using them in feline cases too.
Prognosis
The prognosis for cats with triaditis depends on the severity of their disease. Cats with only mild disease can be treated on an outpatient basis and have a good prognosis. However, cats with acute severe disease, especially with systemic complications (systemic hypotension, hepatic encephalopathy, coagulopathy, vasculitis, disseminated intravascular coagulation, etc), may have a poor prognosis and more aggressive therapy is warranted.
Several risk factors have been reported in patients with triaditis. Hypoalbuminaemia and hypoglycaemia are strong indicators of severe and life-threatening disease 15 and systemic hypotension has been reported as a negative prognostic indicator in feline patients within an intensive care setting. 69 The outcome for cats with triaditis will depend on the successful management of all concurrent conditions, which can be challenging. Many affected cats develop chronic signs of illness, with recurrent episodes of triaditis over time, and appropriate management is important for decreasing the risk of potentially severe consequences. Chronic pancreatitis can predispose to exocrine pancreatic insufficiency and/or diabetes;71,110,175chronic/severe cholangitis can predispose to cholangiohepatitis, gallstones, chronic biliary cirrhosis and possibly cholangiocarcino-ma;176,177and chronic lymphoplasmacytic IBD can predispose to enteric lymphoma.178,179
Summary
Undoubtedly there is still a lot to learn about the natural history and aetiology of triaditis. More studies are needed to impart better understanding of which organs can be affected by this inflammatory process, how best to diagnose all component parts, which treatments are most effective and what factors are important prognostically.
Key Points
Triaditis is the concurrent presence of pancreatitis, cholangitis and IBD; the exact aetiology and prevalence of triaditis in cats has yet to be determined.
Triaditis may occur as a result of an infectious or autoimmune process, or potentially a physical problem, such as duct obstruction.
The anatomy of the feline GI tract may play its role in the aetiology of triaditis, due to the increased risk of ascending bacterial infections of the liver and pancreas.
It is important to recognise that feline patients with cholangitis, pancreatitis and/or IBD can manifest with non-specific and overlapping clinical signs and the ante-mortem diagnosis of triaditis can be challenging. Definitive diagnosis involves assessing histopathology from each organ; hence triaditis remains a presumptive diagnosis in many cases.
Early and appropriate medical therapy and nutritional support are crucial in the management of feline triaditis.
The treatment of triaditis should be focused on the specific type and severity of disease in each of the affected organs; however, it is often symptomatic.
Acknowledgments
The authors thank Camilla Tørnqvist-Johnsen for help with developing the treatment algorithm that appears on page 1058.
Footnotes
Author note: At the time of writing, Petra Černá was based at the Royal (Dick) School of Veterinary Studies, Edinburgh, UK.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work did not involve the use of animals and therefore ethical approval was not necessarily required.
Informed consent: This work did not involve the use of animals and therefore informed consent was not required. For any animals or humans individually identifiable within this publication, informed consent (either verbal or written) for their use in the publication was obtained from the people involved.
Contributor Information
Petra Černá, Department of Clinical Sciences, Colorado State University, Fort Collins, Colorado, and Small Animal Clinic, The University of Veterinary and Pharmaceutical Sciences, Brno, Czech Republic.
Scott Kilpatrick, Veterinary Thought Exchange, East Ayrshire, UK.
Danielle A Gunn-Moore, The Royal (Dick) School of Veterinary Studies, and The Roslin Institute, University of Edinburgh, UK.
References
- 1. Weiss DJ, Gagne JM, Armstrong PJ. Relationship between inflammatory hepatic disease and inflammatory bowel disease, pancreatitis, and nephritis in cats. J Am Vet Med Assoc 1996; 209: 1114–1116. [PubMed] [Google Scholar]
- 2. Callahan Clark JE, Haddad JL, Brown DC, et al. Feline cholangitis: a necropsy study of 44 cats (1986-2008). J Feline Med Surg 2011; 13: 570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fragkou FC, Adamama-Moraitou KK, Poutahidis T, et al. Prevalence and clinicopathological features of triaditis in a prospective case series of symptomatic and asymptomatic cats. J Vet Intern Med 2016; 30: 1031–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weiss DJ, Armstrong PJ, Gagne JM. Feline cholangiohepatitis. In: Bonagura JD. (ed). Kirk’s current veterinary therapy XIII. 13th ed.Philadelphia: WB Saunders, 2000, pp 672–674. [Google Scholar]
- 5. Hill RC, Van Winkle TJ. Acute necrotizing pancreatitis and acute suppurative pancreatitis in the cat. A retrospective study of 40 cases (1976-1989). J Vet Intern Med 1993; 7: 25–33. [DOI] [PubMed] [Google Scholar]
- 6. Nivy R, Kaplanov A, Kuzi S, et al. A retrospective study of 157 hospitalized cats with pancreatitis in a tertiary care center: clinical, imaging and laboratory findings, potential prognostic markers and outcome. J Vet Intern Med 2018; 32: 1874–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smart ME, Downey RS, Stockdale PH. Toxoplasmosis in a cat associated with cholangitis and progressive pancreatitis. Can Vet J 1973; 14: 313–316. [PMC free article] [PubMed] [Google Scholar]
- 8. Koster LS, Shell L, Ketzis J, et al. Diagnosis of pancreatic disease in feline platynosomosis. J Feline Med Surg 2017; 19: 1192–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Habtezion A. Inflammation in acute and chronic pancreatitis. Curr Opin Gastroenterol 2015; 31: 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mayerle J, Sendler M, Hegyi E, et al. Genetics, cell biology, and pathophysiology of pancreatitis. Gastroenterology 2019; 156: 1951–1968.e1. DOI: 10.1053/j.gastro.2018.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okazaki K, Uchida K. Current perspectives on autoimmune pancreatitis and IgG4-related disease. Proc Jpn Acad Ser B Phys Biol Sci 2018; 94: 412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williams DA. The pancreas. In: Guilford WG, Center SA, Strombeck DR, et al. (eds). Strombeck’s small animal gastro-enterology. Philadelphia: WB Saunders, 1996, pp 381–410. [Google Scholar]
- 13. Washabau RJ. Necrosis and inflammation: feline. In: Washabau RJ, Day MJ. (eds). Canine and feline gastroenterology. St Louis, MO: Saunders Elsevier, 2013, pp 821–848. [Google Scholar]
- 14. De Cock HEV, Forman MA, Farver TB, et al. Prevalence and histopathologic characteristics of pancreatitis in cats. Vet Pathol 2007; 44: 39–49. [DOI] [PubMed] [Google Scholar]
- 15. Ferreri JA, Hardam E, Kimmel SE, et al. Clinical differentiation of acute necrotizing from chronic non-suppurative pancreatitis in cats: 63 cases (1996-2001). J Am Vet Med Assoc 2003; 223: 469–474. [DOI] [PubMed] [Google Scholar]
- 16. McLoughlin MT, Mitchell RM. Sphincter of Oddi dysfunction and pancreatitis. World J Gastroenterol 2007; 13: 6333–6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van den Ingh TS, Cullen JM, Twedt DC, et al. Morphological classification of biliary disorders of the canine and feline liver. In: WSAVA standards for clinical and histological diagnosis of canine and feline liver diseases. St Louis, MO: WB Saunders, 2006, pp 61–76. [Google Scholar]
- 18. Bayton WA, Westgarth C, Scase T, et al. Histopathological frequency of feline hepatobiliary disease in the UK. J Small Anim Pract 2018; 59: 404–410. [DOI] [PubMed] [Google Scholar]
- 19. Gagne JM, Weiss DJ, Armstrong PJ. Histopathologic evaluation of feline inflammatory liver disease. Vet Pathol 1996; 33: 521–526. [DOI] [PubMed] [Google Scholar]
- 20. Simpson KW. Pancreatitis and triaditis in cats: causes and treatment. J Small Anim Pract 2015; 56: 40–49. [DOI] [PubMed] [Google Scholar]
- 21. Day DG. Feline cholangiohepatitis complex. Vet Clin North Am Small Anim Pract 1995; 25: 375–385. [DOI] [PubMed] [Google Scholar]
- 22. Weiss DJ, Armstrong PJ, Gagne J. Inflammatory liver disease. Semin Vet Med Surg (Small Anim) 1997; 12: 22–27. [DOI] [PubMed] [Google Scholar]
- 23. Brain PH, Barrs VR, Martin P, et al. Feline cholecystitis and acute neutrophilic cholangitis: clinical findings, bacterial isolates and response to treatment in six cases. J Feline Med Surg 2006; 8: 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cornell K. Pancreas. In: Tobias K, Johnston SA. (eds). Veterinary surgery small animal. Vol 2. St Louis, MO: Elsevier Saunders, 2012, pp 1659–1673. [Google Scholar]
- 25. Kimmel SE, Washabau RJ, Drobatz KJ. Incidence and prognostic value of low plasma ionized calcium concentration in cats with acute pancreatitis: 46 cases (1996-1998). J Am Vet Med Assoc 2001; 219: 1105–1109. [DOI] [PubMed] [Google Scholar]
- 26. Mayhew PD, Holt DE, McLear RC, et al. Pathogenesis and outcome of extrahepatic biliary obstruction in cats. J Small Anim Pract 2002; 43: 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Swift NC, Marks SL, MacLachlan NJ, et al. Evaluation of serum feline trypsin-like immunoreactivity for the diagnosis of pancreatitis in cats. J Am Vet Med Assoc 2000; 217: 37–42. [DOI] [PubMed] [Google Scholar]
- 28. Day MJ. Immunohistochemical characterization of the lesions of feline progressive lymphocytic cholangitis/cholangiohepati-tis. J Comp Pathol 1998; 119: 135–147. [DOI] [PubMed] [Google Scholar]
- 29. Warren A, Center S, McDonough S, et al. Histopathologic features, immunophenotyping, clonality, and eubacterial fluorescence in situ hybridization in cats with lymphocytic cholangitis/cholangiohepatitis. Vet Pathol 2011; 48: 627–641. [DOI] [PubMed] [Google Scholar]
- 30. Lucke VM, Davies JD. Progressive lymphocytic cholangitis in the cat. J Small Anim Pract 1984; 25: 249–260. [Google Scholar]
- 31. Harvey A. Feline inflammatory liver disease: diagnosis and management. In Practice 2009; 31: 414–422. [Google Scholar]
- 32. Wetzel R. Parasitare Erkrankungen der Leber und der Gallenwege. In: Dobberstein J, Pallaske G, Stunzi H. (eds). Joest-Handbuch der Speziellen Pathologischen Anatomie der Haustiere. 3rd ed. Berlin: Paul Parey Verlag, 1967, pp 209–299. [Google Scholar]
- 33. Bowman DD, Hendrix CM, Lindsay DS, et al. The trematodes. In: Feline clinical parasitology. Ames: Iowa State University Press, 2002, pp 144–150. [Google Scholar]
- 34. Inness VL, McCartney AL, Khoo C, et al. Molecular characterization of the gut microflora of healthy and inflammatory bowel disease cats using fluorescence in situ by hybridi zation with special reference to Desulfovibriospp. J Anim Physiol Anim Nutr 2007; 91: 48–53. [DOI] [PubMed] [Google Scholar]
- 35. Janeczko S, Atwater D, Bogel E, et al. The relationship of mucos-al bacteria to duodenal histopathology, cytokine mRNA, and clinical disease activity in cats with inflammatory bowel disease. Vet Microbiol 2008; 128: 178–193. [DOI] [PubMed] [Google Scholar]
- 36. Minamoto Y, Hooda S, Swanson KS, et al. Feline gastrointestinal microbiota. Anim Health Res Rev 2012; 13: 64–77. [DOI] [PubMed] [Google Scholar]
- 37. Abecia LH, Hoyles L, Khoo C, et al. Effects of a novel galacto-oligosaccharide on the faecal microbiota of healthy and inflammatory bowel disease cats during a randomized, double-blind, cross-over feeding study. Int J Probiotics Prebiotics 2010; 5: 61–68. [Google Scholar]
- 38. Schmid SM, Suchodolski JS, Price JM, et al. Omeprazole minimally alters the fecal microbial community in six cats: a pilot study. Front Vet Sci 2018; 5: 79. DOI: 10.3389/fvets.2018.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jergens AE, Moore FM, Haynes JS, et al. Idiopathic inflammatory bowel disease in dogs and cats: 84 cases (1987-1990). J Am Vet Med Assoc 1992; 201: 1603–1608. [PubMed] [Google Scholar]
- 40. Gianella P, Pietra M, Crisi PE, et al. Evaluation of clinicopatho-logical features in cats with chronic gastrointestinal signs. Pol J Vet Sci 2017; 20: 403–410. [DOI] [PubMed] [Google Scholar]
- 41. Guilford WG. Idiopathic inflammatory bowel diseases. In: Guilford WG.(ed). Strombeck’s small animal gastroenterology. 3rd ed.Philadelphia: WB Saunders, 1996, pp 451–486. [Google Scholar]
- 42. Day MJ, Bilzer T, Mansell J, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol 2008; 138 Suppl 1: S1–S43. [DOI] [PubMed] [Google Scholar]
- 43. Waly N, Gruffydd-Jones TJ, Stokes CR, et al. The distribution of leucocyte subsets in the small intestine of healthy cats. J Comp Pathol 2001; 124: 172–182. [DOI] [PubMed] [Google Scholar]
- 44. Waly NE, Stokes CR, Gruffydd-Jones TJ, et al. Immune cell populations in the duodenal mucosa of cats with inflammatory bowel disease. J Vet Intern Med 2004; 18: 816–825. [DOI] [PubMed] [Google Scholar]
- 45. Marolf AJ, Leach L, Gibbons DS, et al. Ultrasonographic findings of feline cholangitis. J Am Anim Hosp Assoc 2012; 48: 36–42. [DOI] [PubMed] [Google Scholar]
- 46. Sutton SC. Companion animal physiology and dosage form performance. Adv Drug Deliv Rev 2004; 56: 1383–1398. [DOI] [PubMed] [Google Scholar]
- 47. Johnston K, Lamport A, Batt RM. An unexpected bacterial flora in the proximal small intestine of normal cats. Vet Rec 1993; 132: 362–363. [DOI] [PubMed] [Google Scholar]
- 48. Johnston KL, Swift NC, Forster-van Hijfte M, et al. Comparison of the bacterial flora of the duodenum in healthy cats and cats with signs of gastrointestinal tract disease. J Am Vet Med Assoc 2001; 218: 48–51. [DOI] [PubMed] [Google Scholar]
- 49. Papasouliotis K, Sparkes AH, Werrett G, et al. Assessment of the bacterial flora of the proximal part of the small intestine in healthy cats, and the effect of sample collection method. Am J Vet Res 1998; 59: 48–51. [PubMed] [Google Scholar]
- 50. Jergens AE. Feline enteric triaditis - is it real? Proceedings of the 79th Western Veterinary Conference; Las Vegas, February18-22, 2007. [Google Scholar]
- 51. Simpson KW. Is there a direct link between IBD, cholangitis, and pancreatitis in cats? Proceedings of the 22nd ECVIM-CA Congress; Maastrich, September6-8, 2012. [Google Scholar]
- 52. Ishida T. Feline triaditis: inflammatory diseases of the liver, pancreas and small intestine. Proceedings of the 36th World Small Animal Veterinary Association World Congress; Korea, October14-17, 2011. [Google Scholar]
- 53. Widdison AL, Karanjia ND, Reber HA. Routes of spread of pathogens into the pancreas in a feline model of acute pancreatitis. Gut 1994; 35: 1306–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Twedt DC, Cullen J, McCord K, et al. Evaluation of fluorescence in situ hybridization for the detection of bacteria in feline inflammatory liver disease. J Feline Med Surg 2014; 16: 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Furneaux RW. A series of six cases of sphincter of Oddi pathology in the cat (2008-2009). J Feline Med Surg 2010; 12: 794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen JWC, Saccone GTP, Toouli J.Sphincter of Oddi dysfunction and acute pancreatitis. Gut 1998; 43: 305–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shaffer EA, Hershfield NB, Logan K, et al. Cholescintigraphic detection of functional obstruction of the sphincter of Oddi. Effect of papillotomy. Gastroenterology 1986; 90: 728–733. [DOI] [PubMed] [Google Scholar]
- 58. Evans PR, Dowsett JF, Bak YT, et al. Abnormal sphincter of Oddi response to cholecystokinin in postcholecystectomy syndrome patients with irritable bowel syndrome. The irritable sphincter. Dig Dis Sci 1995; 40: 1149–1156. [DOI] [PubMed] [Google Scholar]
- 59. Shummer A, Vollmerhaus B. Bauchspeicheldruse, Pankreas. In: Nickel R, Schummer A, Seiferle E.(eds). Lehrbuch der Anatomie der Haustiere. Berlin, Hamburg: Verlag Paul Parey, 1987, pp 128–131. [Google Scholar]
- 60. Williams DA. Diagnosis and management of pancreatitis. J Small Anim Pract 1994; 35: 445–454. [Google Scholar]
- 61. Marks SL. Feline triaditis - current concepts. Proceedings of the 38th World Small Animal Veterinary Association World Congress; Aukland, New Zealand, March6-9, 2013. [Google Scholar]
- 62. Mertz A, Nguyen NA, Katsanos KH, et al. Primary sclerosing cholangitis and inflammatory bowel disease comorbidity: an update of the evidence. Ann Gastroenterol 2019; 32: 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Simpson K. Does feline triaditis exist? BSAVA Companion 2015; 11: 4–7. [Google Scholar]
- 64. Zawie DA, Garvey MS. Feline hepatic disease. Vet Clin North Am Small Anim Pract 1984; 14: 1201–1230. [DOI] [PubMed] [Google Scholar]
- 65. Freiche V, Faucher MR, German AJ. Can clinical signs, clinicopathological findings and abdominal ultrasonography predict the site of histopathological abnormalities of the alimentary tract in cats? J Feline Med Surg 2016; 18: 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lopez A, Roman S, Mufioz F. Comorbidity in inflammatory bowel disease. World J Gastroenterol 2011; 17: 2723–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Divatia M, Kim SA, Ro JY. IgG4-related sclerosing disease, an emerging entity: a review of a multi-system disease. Yonsei Med J 2012; 53: 15–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Okazaki K, Uchida K, Ikeura T, et al. Current concept and diagnosis of IgG4-related disease in the hepato-bilio-pancreatic system. J Gastroenterol 2013; 48: 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Strietzel CJ, Bergeron LM, Oliphant T, et al. In vitro functional characterization of feline IgGs. Vet Immunol Immunopathol 2014; 158: 214–223. [DOI] [PubMed] [Google Scholar]
- 70. Simpson KE, McCann TM, Bommer NX, et al. Retrospective analysis of selected predictors of mortality within a veterinary intensive care unit. J Feline Med Surg 2007; 9: 364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Owens JM, Drazner FH, Gilbertson SR. Pancreatic disease in the cat. J Am Anim Hosp Assoc 1975; 11: 83–89. [Google Scholar]
- 72. Williams DA. Feline exocrine pancreatic disease. In: Bonagura JD, Twedt DC. (eds). Kirk’s current veterinary therapy. Vol XIV. St Louis, MO: Saunders Elsevier, 2009, pp 538–543. [Google Scholar]
- 73. Simpson KW, Shiroma JT, Biller DS, et al. Ante mortem diagnosis of pancreatitis in four cats. J Small Anim Pract 1994; 35: 93–99. [Google Scholar]
- 74. Akol KG, Washabau RJ, Saunders HM, et al. Acute pancreatitis in cats with hepatic lipidosis. J Vet Intern Med 1993; 7: 205–209. [DOI] [PubMed] [Google Scholar]
- 75. Forcada Y, German AJ, Noble PJM, et al. Determination of serum fPLI concentrations in cats with diabetes mellitus. J Feline Med Surg 2008; 10: 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Saunders HM, VanWinkle TJ, Drobatz K, et al. Ultrasonographic findings in cats with clinical, gross pathologic, and histologic evidence of acute pancreatic necrosis: 20 cases (1994-2001). J Am Vet Med Assoc 2002; 221: 1724–1730. [DOI] [PubMed] [Google Scholar]
- 77. Jergens AE, Allenspach K. Feline inflammatory gastrointestinal disease. In: Little S. (ed). August’s consultations in feline internal medicine. Vol 7. St Louis: WB Saunders, 2016, pp 129–137. [Google Scholar]
- 78. Lisciandro SC, Hohenhaus A, Brooks M. Coagulation abnormalities in 22 cats with naturally occurring liver disease. J Vet Intern Med 1998; 12: 71–75. [DOI] [PubMed] [Google Scholar]
- 79. Dircks B, Nolte I, Mischke R. Haemostatic abnormalities in cats with naturally occurring liver diseases. Vet J 2012; 193: 103–108. [DOI] [PubMed] [Google Scholar]
- 80. Simpson K, Battersby I, Lowrie M. Suspected acquired hypocobalaminaemic encephalopathy in a cat: resolution of encephalopathic signs and MRI lesions subsequent to cobal-amin supplementation. J Feline Med Surg 2012; 14: 350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jergens AE. Feline idiopathic inflammatory bowel disease: what we know and what remains to be unraveled. J Feline Med Surg 2012; 14: 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Foster DJ, Thoday KL. Tissue sources of serum alkaline phosphatase in 34 hyperthyroid cats: a qualitative and quantitative study. Res Vet Sci 2000; 68: 89–94. [DOI] [PubMed] [Google Scholar]
- 83. Fibach E. Involvement of oxidative stress in hemolytic anemia. In: Laher I.(ed). Systems biology of free radicals and antioxidants. Berlin, Heidelberg: Springer, 2014, pp 2499–2516. [Google Scholar]
- 84. Forman MA, Marks SL, De Cock HE, et al. Evaluation of serum feline pancreatic lipase immunoreactivity and helical computed tomography versus conventional testing for the diagnosis of feline pancreatitis. J Vet Intern Med 2004; 18: 807–815. [DOI] [PubMed] [Google Scholar]
- 85. Gagne JM, Armstrong PJ, Weiss DJ, et al. Clinical features of inflammatory liver disease in cats: 41 cases (1983-1993). J Am Vet Med Assoc 1999; 214: 513–516. [PubMed] [Google Scholar]
- 86. Schlesinger DP, Rubin SI. Serum bile acids and the assessment of hepatic function in dogs and cats. Can Vet J 1993; 34: 215–220. [PMC free article] [PubMed] [Google Scholar]
- 87. Williams DA. The pancreas. In: Guilford WG, Center SA, Strombeck DR, et al. (eds). Strombeck’s small animal gastro-enterology. 3rd ed.Philadelphia: Saunders, 1996, pp 381–410. [Google Scholar]
- 88. Steiner JM. Diagnosis of pancreatitis. Vet Clin North Am Small Anim Pract 2003; 33: 1181–1195. [DOI] [PubMed] [Google Scholar]
- 89. Bazelle J, Watson P. Pancreatitis in cats: is it acute, is it chronic, is it significant? J Feline Med Surg 2014; 16: 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Oppliger S, Hartnack S, Riond B, et al. Agreement of the serum Spec fPLTM and 1,2-o-dilauryl-rac-glycero-3-glutaric acid-(6’-methylresorufin) ester lipase assay for the determination of serum lipase in cats with suspicion of pancreatitis. J Vet Intern Med 2013; 27: 1077–1082. [DOI] [PubMed] [Google Scholar]
- 91. Fyfe JC. Feline intrinsic factor (IF) is pancreatic in origin and mediates ileal cobalamin (CBL) absorption [abstract]. J Vet Intern Med 1993; 7: 133. [Google Scholar]
- 92. Markle HV. Cobalamin. Crit Rev Clin Lab Sci 1996; 33: 247–356. [DOI] [PubMed] [Google Scholar]
- 93. Berghoff N, Parnell NK, Hill SL, et al. Serum cobalamin and methylmalonic acid concentrations in dogs with chronic gastrointestinal disease. Am J Vet Res 2013; 74: 84–89. [DOI] [PubMed] [Google Scholar]
- 94. Simpson KW, Fyfe J, Cornetta A, et al. Subnormal concentrations of serum cobalamin (vitamin B12) in cats with gastrointestinal disease. J Vet Intern Med 2001; 15: 26–32. [DOI] [PubMed] [Google Scholar]
- 95. Gerhardt A, Steiner JM, Williams DA, et al. Comparison of the sensitivity of different diagnostic tests for pancreatitis in cats. J Vet Intern Med 2001; 15: 329–333. [PubMed] [Google Scholar]
- 96. Williams JM, Panciera DL, Larson MM, et al. Ultrasonographic findings of the pancreas in cats with elevated serum pancreatic lipase immunoreactivity. J Vet Intern Med 2013; 27: 913–918. [DOI] [PubMed] [Google Scholar]
- 97. Penninck D, Berry C. Liver imaging in the cat. Semin Vet Med Surg (Small Anim) 1997; 12: 10–21. [DOI] [PubMed] [Google Scholar]
- 98. Gaschen L. Ultrasonography of small intestinal inflammatory and neoplastic diseases in dogs and cats. Vet Clin North Am Small Anim Pract 2011; 41: 329–344. [DOI] [PubMed] [Google Scholar]
- 99. Daniaux LA, Laurenson MP, Marks SL, et al. Ultrasonographic thickening of the muscularis propria in feline small intestinal small cell T-cell lymphoma and inflammatory bowel disease. J Feline Med Surg 2014; 16: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zwingenberger AL, Marks SL, Baker TW, et al. Ultrasonographic evaluation of the muscularis propria in cats with diffuse small intestinal lymphoma or inflammatory bowel disease. J Vet Intern Med 2010; 24: 289–292. [DOI] [PubMed] [Google Scholar]
- 101. Armstrong PJ, Williams DA. Pancreatitis in cats. Top Companion Anim Med 2012; 27: 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pratschke KM, Ryan J, McAlinden A, et al. Pancreatic surgical biopsy in 24 dogs and 19 cats: postoperative complications and clinical relevance of histological findings. J Small Anim Pract 2015; 56: 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cosford K, Shmon C, Myers S, et al. Prospective evaluation of laparoscopic pancreatic biopsies in 11 healthy cats. J Vet Intern Med 2010; 24: 104–113. [DOI] [PubMed] [Google Scholar]
- 104. Linton M, Nimmo JS, Norris JM, et al. Feline gastrointestinal eosinophilic sclerosing fibroplasia: 13 cases and review of an emerging clinical entity. J Feline Med Surg 2015; 17: 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Norsworthy GD, Estep S, Hollinger C, et al. Prevalence and underlying causes of histologic abnormalities in cats suspected to have chronic small bowel disease: 300 cases (2008-2013). J Am Vet Med Assoc 2015; 247: 629–635. [DOI] [PubMed] [Google Scholar]
- 106. Leib MS, Sponenberg DP, Wilcke JR, et al. Suppurative colitis in a cat. J Am Vet Med Assoc 1986; 188: 739–741. [PubMed] [Google Scholar]
- 107. Yozova ID, Howard J, Sigrist NE, et al. Current trends in volume replacement therapy and the use of synthetic colloids in small animals - an internet-based survey. Front Vet Sci 2017; 4: 140. DOI: 10.3389/fvets.2017.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Weatherton LK, Streeter EM. Evaluation of fresh frozen plasma administration in dogs with pancreatitis: 77 cases (1995-2005). J Vet Emerg Crit Care 2009; 19: 617–622. [DOI] [PubMed] [Google Scholar]
- 109. Castellanos I, Couto CG, Gray TL. Clinical use of blood products in cats: a retrospective study (1997-2000). J Vet Intern Med 2004; 18: 529–532. [DOI] [PubMed] [Google Scholar]
- 110. Watson P. Pancreatitis in dogs and cats: definitions and patho-physiology. J Small Anim Pract 2015; 56: 3–12. [DOI] [PubMed] [Google Scholar]
- 111. Dumnicka P, Maduzia D, Ceranowicz P, et al. The interplay between inflammation, coagulation and endothelial injury in the early phase of acute pancreatitis: clinical implications. Int JMol Sci 2017; 18: 354. DOI: 10.3390/ijms 18020354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Rodan I, Sparkes AH. Preventive health care for cats. The Cat 2012; 151–180. [Google Scholar]
- 113. Robertson S, Taylor P, Bloomfield M, et al. Systemic uptake of buprenorphine after buccal administration in cats. Vet Anaesth Analg 2002; 29: 97–112. [DOI] [PubMed] [Google Scholar]
- 114. Lee DD, Papich MG, Hardie EM. Comparison of pharma-cokinetics of fentanyl after intravenous and transdermal administration in cats. Am J Vet Res 2000; 61: 672–677. [DOI] [PubMed] [Google Scholar]
- 115. Yackey M, Ilkiw JE, Pascoe PJ, et al. Effect of transdermally administered fentanyl on the minimum alveolar concentration of isoflurane in cats. Anaesth Analg 2004; 31: 183–189. [DOI] [PubMed] [Google Scholar]
- 116. Reid J, Scott EM, Calvo G, et al. Definitive Glasgow acute pain scale for cats: validation and intervention level. Vet Rec 2017; 180: 449. DOI: 10.1136/vr.104208. [DOI] [PubMed] [Google Scholar]
- 117. Egger CM, Glerum LE, Allen SW, et al. Plasma fentanyl concentrations in awake cats and cats undergoing anesthesia and ovariohysterectomy using transdermal administration. Vet Anaesth Analg 2003; 30: 229–236. [DOI] [PubMed] [Google Scholar]
- 118. Davidson CD, Pettifer GR, Henry JD. Plasma fentanyl concentrations and analgesic effects during full or partial exposure to transdermal fentanyl patches in cats. J Am Vet Med Assoc 2004; 224: 700–705. [DOI] [PubMed] [Google Scholar]
- 119. Giordano T, Steagall PVM, Ferreira TH, et al. Postoperative analgesic effects of intravenous, intramuscular, subcutaneous or oral transmucosal buprenorphine administered to cats undergoing ovariohysterectomy. Vet Anaesth Analg 2010; 37: 357–366 [DOI] [PubMed] [Google Scholar]
- 120. Gilron I, Bailey JM, Tu D, et al. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet 2009; 374: 1252–1261. [DOI] [PubMed] [Google Scholar]
- 121. Cashmore RG, Harcourt-Brown TR, Freeman PM, et al. Clinical diagnosis and treatment of suspected neuropathic pain in three dogs. Aust Vet J 2009; 87: 45–50. [DOI] [PubMed] [Google Scholar]
- 122. Rusbridge C, Heath S, Gunn-Moore DA, et al. Feline orofacial pain syndrome (FOPS): a retrospective study of 113 cases. J Feline Med Surg 2010; 12: 498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Trepanier L. Acute vomiting in cats: rational treatment selection. J Vet Intern Med 2010; 12: 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Nioym S, Boscan SNP, Twedt DC, et al. Effect of maropitant, a neurokinin 1 receptor antagonist, on the minimum alveolar concentration of sevoflurane during stimulation of the ovarian ligament in cats. Vet Anaesth Analg 2013; 40: 425–431. [DOI] [PubMed] [Google Scholar]
- 125. Machu TK. Therapeutics of 5-HT3 receptor antagonists: current uses and future directions. Pharmacol Ther 2011; 130: 338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Quimby JM, Lake RC, Hansen RJ, et al. Oral, subcutaneous, and intravenous pharmacokinetics of ondansetron in healthy cats. J Vet Pharmacol Ther 2014; 37: 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Jovanovic-Micic D, Samardzic R, Beleslin DB. The role of alpha-adrenergic mechanisms within the area postrema in dopamine-induced emesis. Eur J Pharmacol 1995; 272: 21–30. [DOI] [PubMed] [Google Scholar]
- 128. King GL. Animal models in the study of vomiting. Can J Physiol Pharmacol 1990; 68: 260–268. [DOI] [PubMed] [Google Scholar]
- 129. Tao Y, Tang C, Feng W, et al. Early nasogastric feeding versus parenteral nutrition in severe acute pancreatitis: a retrospective study. Pak J Med Sci 2016; 32: 1517–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Petrov MS, Van Santvoort HC, Besselink MG, et al. Enteral nutrition and the risk of mortality and infectious complications in patients with severe acure pancreatitis: a meta analysis of randomized trials. Arch Surg 2008; 143: 1111–1117. [DOI] [PubMed] [Google Scholar]
- 131. Mansfield CS, James FE, Steiner JM, et al. A pilot study to assess tolerability of early enteral nutrition via esophagostomy tube feeding in dogs with severe acute pancreatitis. J Vet Intern Med 2011; 25: 419–425. [DOI] [PubMed] [Google Scholar]
- 132. Nathens AB, Curtis JR, Beale RL, et al. Management of the critically ill patient with severe acute pancreatitis. Crit Care Med 2004; 32: 2524–2536. [DOI] [PubMed] [Google Scholar]
- 133. DeWitt RC, Kudsk KA. The gut’s role in metabolism, mucos-al barrier function, and gut immunology. Infect Dis Clin North Am 1999; 13: 465–481, x. [DOI] [PubMed] [Google Scholar]
- 134. Ioannidis O, Lavrentieva A, Botsios D. Nutrition support in acute pancreatitis. J Pancreas 2008; 9: 375–390. [PubMed] [Google Scholar]
- 135. He M, Deng C, Huang XF. The role of hypothalamic H1 receptor antagonism in antipsychotic-induced weight gain. CNS Drugs 2013; 27: 423–434. [DOI] [PubMed] [Google Scholar]
- 136. Schellekens H, De Francesco PN, Kandil D, et al. Ghrelin’s orexi-genic effect is modulated via a serotonin 2C receptor interaction. ACS Chem Neurosci 2015; 6: 1186–1197. [DOI] [PubMed] [Google Scholar]
- 137. Fitzpatrick RL, Quimby JM, Benson KK, et al. In vivo and in vitro assessment of mirtazapine pharmacokinetics in cats with liver disease. J Vet Intern Med 2018; 32: 1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Klaus JA, Rudloff E, Kirby R. Nasogastric tube feeding in cats with suspected acute pancreatitis: 55 cases (2001-2006). J Vet Emerg Crit Care 2009; 19: 337–346. [DOI] [PubMed] [Google Scholar]
- 139. Favarato E, Souza M, Costa P, et al. Evaluation of metoclopramide and ranitidine on the prevention of gastroesophageal reflux episodes in anesthetized dogs. Res Vet Sci 2012; 93: 466–467. [DOI] [PubMed] [Google Scholar]
- 140. Fioramonti J, Soldani G, Honde C, et al. Effects of ranitidine and oxmetidine on gastrointestinal motility in conscious dog. Agents Actions 1984; 15: 260–263. [DOI] [PubMed] [Google Scholar]
- 141. Chan DL. The inappetent hospitalised cat: clinical approach to maximising nutritional support. J Feline Med Surg 2009; 11: 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Xenoulis PG, Suchodolski JS, Steiner JM. Chronic pancreatitis in dogs and cats. Compend Contin Educ Vet 2008; 30: 166–180. [PubMed] [Google Scholar]
- 143. Toresson L, Steiner JM, Olmedal G, et al. Oral cobalamin supplementation in cats with hypocobalaminaemia: a retrospective study. J Feline Med Surg 2017; 19: 1302–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Okanishi H, Nagata T, Nakane S, et al. Comparison of initial treatment with and without corticosteroids for suspected acute pancreatitis in dogs. J Small Anim Pract 2019; 60: 298–304. [DOI] [PubMed] [Google Scholar]
- 145. Policelli Smith R, Gookin J, Smolski W, et al. Association between gallbladder ultrasound findings and bacterial culture of bile in 70 cats and 202 dogs. J Vet Intern Med 2007; 31: 1451–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Wagner KA, Hartmann FA, Trepanier LA. Bacterial culture results from liver, gallbladder, or bile in 248 dogs and cats evaluated for hepatobiliary disease: 1998-2003. J Vet Intern Med 2007; 21: 417–424. [DOI] [PubMed] [Google Scholar]
- 147. Walker A, Jang SS, Hirsch DC. Bacteria associated with pyothorax of dogs and cats: 98 cases (1989-1998). J Am Vet Med Assoc 2009; 216: 359–363. [DOI] [PubMed] [Google Scholar]
- 148. Brissot H, Cervantes S, Guardabassi L, et al. In: GRAM: Guidance for the rational use of antimicrobials: recommendations for dogs and cats. 2nd ed. France: Ceva Sante Animale, 2016, pp 210–214. [Google Scholar]
- 149. Center SA, Warner KL, Erb HN. Liver glutathione concentrations in dogs and cats with naturally occurring liver disease. Am J Vet Res 2002; 63: 1187–1197. [DOI] [PubMed] [Google Scholar]
- 150. Center SA, Randolph JF, Warner KL, et al. The effects of S-adenosylmethionine on clinical pathology and redox potential in the red blood cell, liver, and bile of clinically normal cats. J Vet Intern Med 2005; 19: 303–314. [DOI] [PubMed] [Google Scholar]
- 151. Center SA, Warner K, Corbett J, et al. Proteins invoked by vitamin K absence and clotting times in clinically ill cats. J Vet Intern Med 2000; 14: 292–297. [DOI] [PubMed] [Google Scholar]
- 152. Twedt DC, Armstrong PJ, Simpson KW. Feline cholangitis. In: Bonagura JD, Twedt DC.(eds). Kirk’s current veterinary therapy XV. St Louis, MO: Elsevier Saunders, 2014, pp 614–619. [Google Scholar]
- 153. Otte CM, Penning LC, Rothuizen J, et al. Retrospective comparison of prednisolone and ursodeoxycholic acid for the treatment of feline lymphocytic cholangitis. Vet J 2013; 195: 205–209. [DOI] [PubMed] [Google Scholar]
- 154. Raditic DM, Remillard RL, Tater KC. ELISA testing for common food antigens in four dry dog foods used in dietary elimination trials. J Anim Physiol Anim Nutr 2011; 95: 90–97. [DOI] [PubMed] [Google Scholar]
- 155. Cave NJ. Hydrolyzed protein diets for dogs and cats. Vet Clin North Am Small Anim Pract 2006; 36: 1251–1268. [DOI] [PubMed] [Google Scholar]
- 156. Guilford WG, Jones BR, Markwell PJ, et al. Food sensitivity in cats with chronic idiopathic gastrointestinal problems. J Vet Intern Med 2001; 15: 7–13. [DOI] [PubMed] [Google Scholar]
- 157. Mandigers PJ, Biourge V, German AJ. Efficacy of a commercial hydrolysate diet in eight cats suffering from inflammatory bowel disease or adverse reaction to food. Tijdschr Diergeneeskd 2010; 135: 668–672. [PubMed] [Google Scholar]
- 158. Batt RM, Scott J. Response of the small intestinal mucosa to oral glucocorticoids. Scand J Gastroenterol 1982; 74: 75–88. [PubMed] [Google Scholar]
- 159. Rand JS, Bobbermien LM, Hendrikz JK, et al. Over representation of Burmese cats with diabetes mellitus. Aust Vet J 1997; 75: 402–405. [DOI] [PubMed] [Google Scholar]
- 160. Ohlund M, Fall T, Strom Holst B, et al. Incidence of diabetes mellitus in insured Swedish cats in relation to age, breed and sex. J Vet Intern Med 2015; 29: 1342–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. McCann TM, Simpson KE, Shaw DJ, et al. Feline diabetes melli-tus in the UK: the prevalence within an insured cat population and a questionnaire-based putative risk factor analysis. J Feline Med Surg 2007; 9: 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Lederer R, Rand JS, Jonsson NN, et al. Frequency of feline diabetes mellitus and breed predisposition in domestic cats in Australia. Vet J 2009; 179: 254–258. [DOI] [PubMed] [Google Scholar]
- 163. O’Leary C, Duffy D, Gething M, et al. Investigation of diabetes mellitus in Burmese cats as an inherited trait: a preliminary study. N Z Vet J 2013; 61: 354–358. [DOI] [PubMed] [Google Scholar]
- 164. Matsumoto S, Hara T, Hori T, et al. Probiotic Lactobacillus-induced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of pro-inflammatory cytokines in lamina propria mononuclear cells. Clin Exp Immunol 2005; 140: 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Duchmann R. The role of probiotics and antibiotics in regulating mucosal inflammation. In: Blumberg RS, Neurath MF. (eds). Immune mechanisms in inflammatory bowel disease. Advances in experimental medicine and biology. Vol 579. New York: Springer, 2006, pp 35–54. [DOI] [PubMed] [Google Scholar]
- 166. Packey CD, Sartor RB. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr Opin Infect Dis 2009; 22: 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Ruaux CG. Cobalamin and gastrointestinal disease. Proceedings of the American College of Veterinary Internal Medicine 20th Annual Forum; 2002 May 29-June 1, Dallas. [Google Scholar]
- 168. Simpson KW. Managing chronic enteropathies in dogs, small animal and exotics. Proceedings of the North American Veterinary Conference; Jan 15-19, 2011; Orlando, Florida, USA, pp 650–656. [Google Scholar]
- 169. Bottero E, Benvenuti E, Ruggiero P. Faecal microbiota transplantation in 16 dogs with idiopathic inflammatory bowel disease. Veterinaria 2017; 31: 1–12. [Google Scholar]
- 170. Chaitman J, Jergens AE, Gaschen F, et al. Commentary on key aspects of fecal microbiota transplantation in small animal practice. Vet Med Res Rep 2016; 7: 71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Burton EN, O’Connor E, Ericsson AC, et al. Evaluation of fecal microbiota transfer as treatment for postweaning diarrhea in research-colony puppies. J Am Assoc Lab Anim Sci 2016; 55: 582–587. [PMC free article] [PubMed] [Google Scholar]
- 172. Weese JS, Costa MC, Webb JA. Preliminary clinical and microbiome assessment of stool transplantation in the dog and cat [abstract]. J Vet Intern Med 2013; 27: 705. [Google Scholar]
- 173. Pereira GQ, Gomes LA, Santos IS, et al. Fecal microbiota transplantation in puppies with canine parvovirus infection. J Vet Intern Med 2018; 32: 707–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Collins S. Nutritional support of cats with triaditis. Vet Nursing J 2017; 32: 158–160. [Google Scholar]
- 175. Steiner JM. Exocrine pancreatic insufficiency. In August JR. (ed). Consultations in feline internal medicine. 6th ed.St Louis, MO: Saunders Elsevier, 2010, p 225. [Google Scholar]
- 176. Gibson KS. Cholelithiasis and choledolithiasis in a cat. J Am Vet Med Assoc 1952; 121: 288–289. [PubMed] [Google Scholar]
- 177. Hirsh VM, Doige CE. Suppurative cholangitis in cats. J Am Vet Med Assoc 1983; 182: 1223–1226. [PubMed] [Google Scholar]
- 178. Evans SE, Bonczynski JJ, Broussard JD, et al. Comparison of endoscopic and full-thickness biopsy specimens for diagnosis of inflammatory bowel disease and alimentary tract lymphoma in cats. J Am Vet Med Assoc 2006; 229: 1447–1450. [DOI] [PubMed] [Google Scholar]
- 179. Mahony OM, Moore AS, Cotter SM, et al. Alimentary lymphoma in cats: 28 cases (1988–1993). J Am Vet Med Assoc 1995; 207: 1593–1598. [PubMed] [Google Scholar]