Abstract
Practical relevance:
An understanding of the process of musculoskeletal ageing – which all senior and geriatric cats will experience – is vital to maintaining the health and welfare of our ageing cat population.
Clinical challenges:
Assessment of the feline musculoskeletal system is not always straightforward. Diagnosis of impairment relies on input from owners and veterinarians in terms of visual observation, and clinical and orthopaedic examination, in addition to diagnostic imaging
Audience:
This review is written for the primary care veterinary team.
Aims:
The goals are to raise awareness and improve clinical diagnosis of musculoskeletal impairment as a result of ageing. The article also reviews therapeutic options and considers the evidence available for the prevention/deceleration of musculoskeletal ageing and impairment.
Evidence base:
There is good evidence of a high prevalence of osteoarthritis (OA) and degenerative joint disease (DJD) in older cats. There is also good evidence to indicate that functional impairment and chronic pain are sequelae of musculoskeletal disease. However, there is a paucity of information for what is best practice for the management and treatment of musculoskeletal impairment in a clinical situation. There is also a lack of evidence on how prevention of central stimulation of the nervous system caused by musculoskeletal impairment and, in turn the development of chronic pain, can be avoided.
Keywords: Musculoskeletal, osteoarthritis, degenerative joint disease, chronic pain
Ageing is not a disease
Ageing is not a disease, but it is characterised by a progressive loss of physiological integrity leading to functional impairment. 1 The boundaries between what is normal physiological ageing of tissues and what is a ‘diseased state’ are blurred. Musculoskeletal disease can be considered present | where there is functional impairment, pain or loss of mobility. 2 In the human musculoskeletal system, bone loss, degradation of articular cartilage and degenerative, narrowed intervertebral disc spaces are considered the primary features of musculoskeletal ageing. 2
Aspects of feline musculoskeletal ageing
Bone loss as a feature of normal musculoskeletal ageing has not been determined in cats. 2 Osteoporosis following feline ovariectomy has been shown not to occur; 3 therefore, the ageing-related hormonal effects leading to bone loss seen in women 2 are unlikely to affect cats, but other causes of age-related bone loss have not been investigated.
Degradation of articular cartilage leading to osteoarthritis (OA) and degeneration of the intervertebral disc spaces are both common in ageing cats.4–11 Numerous radiographic studies have shown a high prevalence of OA and degenerative joint disease (DJD) (see box on page 1070).
Age has been shown as being the greatest risk factor for the presence of radiographic OA9,11 or DJD, 10 with the severity of changes and the number of joints affected increasing with age.

Diagnostic considerations in musculoskeletal ageing
In cats with radiographic changes consistent with OA or DJD, there is not necessarily always a corresponding clinical diagnosis of pain or functional impairment.9,12 The sensitivity and specificity of different aspects of an orthopaedic examination in cats with DJD are variable, with Lascelles et al reporting: 12
Pain response to palpation: sensitivity 0–67%, specificity 62–99%
Crepitus: sensitivity 0–56%, specificity 87–99%
Presence of effusion: sensitivity 6–38%, specificity 82–100%
Joint thickening: sensitivity 0–59%, specificity 74–99%
The higher specificity for crepitus and presence of effusion in a joint leads these findings to be more predictive of radiographic changes consistent with OA/DJD. The absence of these findings on examination is likely to predict radiographically normal joints. The low sensitivity of all aspects of the orthopaedic assessment, however, increases the likelihood of false-positive results. This study did use radiographic changes to determine a diagnosis of OA/DJD, so while some of the findings on orthopaedic assessment may be true false positives, this may not be the case with all.
Unfortunately, radiography is also poor at detecting cartilage degradation in appen-dicular joints.13,14 Cartilage damage without any radiographic evidence of joint disease has been shown to be present in the stifle, hip, elbow and tarsal joints in 30 cats, 13 and the shoulder, elbow, hip, stifle, carpus and tarsus in 58 cats. 14 Positive findings on orthopaedic assessment indicative of DJD/OA in cats with radiographically normal joints may reflect the presence of pain caused by cartilage degradation. 12 It has been shown in a feline OA model that radiographic degenerative changes can take up to 4 months to become apparent post-surgical transection of the cranial cruciate ligament. 15
Feline sarcopenia and its impact on musculoskeletal ageing
Loss of lean body mass in the absence of disease (sarcopenia) is also associated with feline ageing. 16 In people, sarcopenia is closely related to adverse health-related outcomes such as increased risk of falls, fractures and disability. 17 Sarcopenia is a complex process characterised by loss of muscle quantity (from atrophy and loss of motor units) and muscle quality (from infiltration of non-contractile material, fat and connective tissue). 18 In people, the diagnosis of sarcopenia requires the demonstration of low muscle mass in addition to either low muscle strength or low physical performance. 19
While a loss of lean tissue mass with age has been demonstrated in cats, 20 a reduction in muscle strength and physical performance has not. Further complicating the diagnosis of sarcopenia in cats is the high prevalence of DJD/OA in ageing cats, as a diagnosis of sarcopenia relies on the absence of other disease processes (otherwise the muscle loss would be considered cachexia). 21 Tumour necrosis factor-alpha (TNF-a) has been shown to be elevated in feline DJD/OA 22 and has also been shown to have a role in cachexia. 21 In addition, chronic pain associated with DJD/OA has been shown to reduce activity in cats,23,24 leading to an increased risk of disuse atrophy developing. This does raise the question of whether sarcopenia in cats is true sarcopenia or whether some of the loss of lean body mass is a result of a cachexic process secondary to DJD/OA. Cats also have a tendency to develop obesity in their mature to senior years, 25 which may exacerbate an impairment of mobility, especially if there is concurrent loss of muscle mass.
A longitudinal study of feline gait is currently being performed at the Feline Healthy Ageing Clinic (University of Liverpool, UK). 26 The researchers will be assessing the impact that ageing has on feline gait kinematics as part of a larger study into feline ageing. ‘Usual gait speed’ is included in the diagnosis of sarcopenia in humans and it has been shown to have a non-linear relationship with leg strength. 19 Changes in gait speed with age have not been studied to date in cats.
Diagnosis of feline musculoskeletal impairment
For the abovementioned reasons, diagnosis of musculoskeletal impairment and pain in cats can be challenging.11–13 Diagnostic approaches can be separated currently into subjective and objective measures. Subjective measures involve physical examination find-ings11,12 and use of clinical metrology instruments (CMIs).27–29 Objective measures can be divided into quantitative measures and diagnostic imaging of the musculoskeletal system. 30 Quantitative measures can be variously generated by force plates, pressure-sensitive walkways and activity monitors. The use of analgesic treatment trials has also been reported as a diagnostic tool in clinical settings. 31 However, a response to analgesic treatment should include improvement in both subjective and objective measures to mitigate a placebo effect, 30 and this is seldom ascertained outside of clinical trials.
Subjective measures
CMIs
CMIs are questionnaires designed to measure the sensory and affective effects of pain and have been used in therapeutic trials.32–35 The affective domain of pain assessment is the emotional component of the pain. 36 Diagnosing feline musculoskeletal disease requires an understanding of what is ‘normal’ for that individual cat,37,38 and this can be difficult to determine in a clinical setting. Owner questionnaires or clinical questioning are often the first step in the diagnosis of mobility issues.37,38 Owner-observed abnormalities were used in the diagnosis of OA in 94% of cats in one study, with changes in gait, jumping and stair climbing being the most commonly reported mobility-associated issues. 31
Feline musculoskeletal disease CMIs are designed to determine if pain related to mus-culoskeletal disease is present and detect any improvement following therapy, 32 and have been shown to be clinically valuable in both research and clinical settings.33–35 A number of feline OA-specific CMIs have been developed, four of which have recently been evaluated and compared. 32 The ‘Owner Behaviour Watch’ is an owner questionnaire-based tool that was developed from behavioural/lifestyle changes that feline experts expected to be induced by chronic musculoskeletal pain. 33 The ‘Zamprogno Question Bank’ was initially developed using questions chosen by the study investigators that were based on personal opinion as well as opinions expressed in the veterinary literature. These questions were then refined in owner focus groups and interviews. The questions aimed to assess the impact that DJD has on the cat’s lifestyle, environment, diet, activity and quality of life. 29 The ‘Feline Musculoskeletal Pain Index’ (FMPI) is a CMI built on the Zamprogno Question Bank, optimising the questionnaire for readability. 27 The fourth was the ‘Feline Physical Function Formula’, which was developed by the authors of the review, 32 based on the Owner Behaviour Watch; 33 to date, this CMI is not published. In addition to the four CMIs discussed in this review, there is also the ‘Montreal Instrument for Cat Arthritis Testing’. 39 Initially developed as a pain scale for veterinary surgeons,39,40 there is a version of the questionnaire for caretakers/owners. 41
Currently, the FMPI is the most developed CMI. 32 This tool has undergone further development and modification. 28 Most recently it has formed the basis of a six-question checklist that can be used as a DJD screening tool, with the complete FMPI being used on cats that score highly on the checklist. 42 It is able to distinguish between normal cats and cats with painful OA/DJD. 28 It has subsequently been used to test the effectiveness of meloxicam as an analgesic for cats with DJD.27,43 This instrument is freely available from painfreecats.org and it can be used by clinicians to aid in the diagnosis and management of painful OA/DJD. Full directions on its use in clinical practice are on the website.
A CMI that is more specific to the individual has also been developed, the ‘Client-Specific Outcome Measures’ (CSOM) CMI. This questionnaire is based on distinct activities that the owner feels are problematic for their cat. 44 The instrument has undergone some validation testing. A randomised, stratified, double-blinded, placebo-controlled, crossover clinical trial using meloxicam found no treatment effect using the CSOM tool but a significant treatment effect using the FMPI. 43 A further placebo-controlled, blinded clinical study assessing feline-specific anti-nerve growth factor antibody by the same group did find a significant treatment effect using the CSOM tool but no significant treatment effect on the FMPI tool. 45 This CSOM tool was also used in a randomised, placebo-controlled, crossover study assessing gabapentin, which found a significant treatment effect using the CMI. 46 The value of a CSOM-based CMI over a CMI that is not based on the individual has therefore yet to be determined.
CMIs have been used to evaluate responses to therapy.27,33,34,40,41,43,45,47 However, it is important to recognise that when CMIs are solely used to evaluate a cat’s response to therapy, the caregiver placebo effect may inflate the success rate. 30
Orthopaedic examination
In the clinic, orthopaedic assessment can be separated into visual assessment of the cat moving around the consulting room (if the cat is willing) and a detailed physical assessment of the musculoskeletal system.37,38 Orthopaedic examinations are not always straightforward to perform in cats,37,38 but the current authors would recommend incorporating a routine visual and physical assessment into annual health examinations to allow the clinician to determine what is normal for that individual and recognise any changes.
For the first (visual assessment) component of the examination, place the cat carrier on the floor of the consulting room and allow the cat to come out and explore the environment at its own pace. 38 Lameness in cats with OA has been reported to be low in prevalence (13–17% of cats with OA/DJD) but a ‘stiff’ gait may be noted,11,38 in addition to other subtle changes such as a ‘hip hike’ or a ‘head bob’. 38 If possible, assess how the cat is sitting or lying for asymmetry; look out for weight shifting from painful limbs or the inability to flex joints. 38 Confident cats will often be happy to explore the room, so having different objects at various heights around the room can be useful to assess the ability to jump (Figure 1). Finally, if you can, record what jumping and walking behaviour you see from the cat during the physical examination and monitor how this changes over time.37,38 Recording what you see in a systematic way that is used by all members of the clinical team will enable the rapid detection of changes over time.
Figure 1.

Examples of cats exploring different heights in the consultation room at the Feline Healthy Ageing Clinic, University of Liverpool, UK
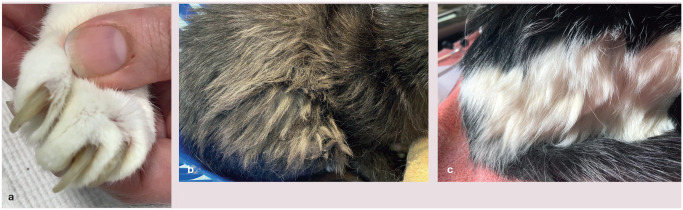
Several methods of performing an orthopaedic examination have been described.37,38 The authors use the system summarised in the box below, checking for asymmetry, and any cervical, lumbar and coccygeal pain, and then manipulating each of the large joints, assessing for pain, thickening and crepitus. 12 Finally, the overall demeanour of the cat during the examination should be recorded, 12 as well as the coat and nail condition of the cat as poor grooming may also indicate musculoskeletal pain (Figure 2). 33
Figure 2.
Examples of poor grooming and nail care showing (a) thickening of the nails and (b,c) matting of hair on the lower spine
Objective measures
Quantitative, objective measures can be generated by a range of equipment such as accelerometers,23,24,49 force plates, which enable the collection of ground reaction forces, 50 and pressure-sensitive walkways, which enable the collection of gait kinematic data as well as ground reaction forces.51–54 In addition, there are specific tests for nociception – the von Frey anaesthesiometer;40,55,56 and for the central sensitisation of pain – mechanical repetitive stimuli quantitative sensory testing 55 or thermal sensitivity. 56 These tests are not easily accessible for most clinical practitioners, as generally they are being used in research and analgesic efficacy trials.43,44,47,52,55,57,58 However, some knowledge of these modalities is useful for clinicians for interpretation of studies.
Accelerometers
Actical MiniMitter (Bend) and ActiWatch Minimitter/Respironics (Bio-Lynx Scientific) are the two most common accelerometer systems that have been employed in feline medicine studies. The equipment, which is fitted to cats’ collars, has been used to measure daily activity levels, as well as response to analgesia and veterinary diets.23,24,43,44,47,57,58 Accelerometers are often used in combination with other quantitative measures and qualitative tools such as CMIs.43–45 Note that the ActiWatch Minimitter device is no longer being produced.
Pressure and force plate analysis
Force plates and pressure-sensitive walkways (Figure 3) measure the ground reaction forces created between the paw and the ground during locomotion. 59 Peak vertical force (the point that is the highest product of the mass of the body and the net vertical acceleration of the centre of mass 53 ) can detect asymmetry 52 and distinguish between healthy and osteoarthritic cats. 23 Ground reaction forces have been used to evaluate different surgical and analgesic protocols.60,61
Figure 3.

A cat walking on a Tekscan pressure-sensitive walkway
Pressure-sensitive walkways can also collect data on spatiotemporal kinetic parameters, though these are less commonly investigated. 59 Such parameters include swing time (the amount of time each leg is off the ground), stance time (the amount of time the paw is on the ground) and stride length (the distance between two foot falls of the same limb during a gait cycle). Spatiot emporal parameters, as well as ground reaction forces, have been investigated in healthy cats51,54,62,63 and cats with cranial cruciate ligament disease. 52 Studies looking at longitudinal changes in gait kinematics in addition to pressure distribution associated with naturally occurring musculoskeletal disease in ageing cats are currently being undertaken at the Feline Healthy Ageing Clinic (University of Liverpool, UK).
Testing for nociception/allodynia/central stimulation
von Frey anaesthesiometers, thermal sensitivity and mechanical repetitive stimuli quantitative sensory testing have been used to demonstrate central stimulation in naturally occurring OA and to assess the response to therapeutics in cats.40,55,56,58 The von Frey anaesthesiometer uses a filament at increasing forces applied to the skin to determine what force the cat reacts to. These studies have demonstrated that cats with OA have a lower paw withdrawal threshold upon von Frey anaesthesiometry than non-osteoarthritic cats.40,55,56 It has been shown that mechanical repetitive stimuli quantitative sensory testing evokes temporal summation of pain faster in cats with OA compared with non-osteoarthritic cats. 55 Temporal summation refers to an increased perception of pain in response to a repetitive stimulus. The faster temporal summation demonstrated indicates that central sensitisation is involved in chronic pain associated with OA. 55 Gabapentin therapy in cats with OA can increase the paw withdrawal threshold determined by von Frey anaesthesiometry. 40
Imaging
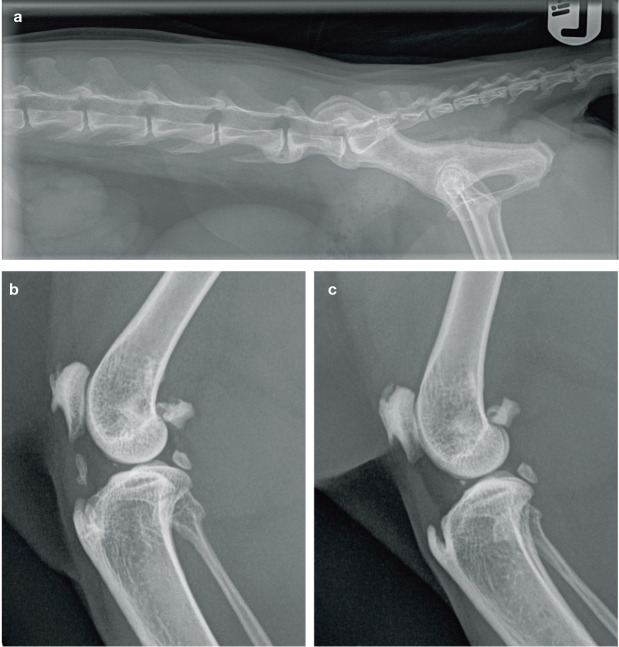
To date, radiography has been the diagnostic tool of choice in most studies of feline musculoskeletal disease.10–12 CT and MRI have also been used,5,49 but radiography is the most readily available imaging modality for clinical practitioners in the diagnosis of OA/DJD, and also the most affordable for clients (Figure 4).
Figure 4.
Radiographs of a mature cat with spondylosis and degenerative joint disease. (a) Lateromedial view showing spondylosis in the lower spine at L6-L7. (b,c) Lateromedial views of the (b) right and (c) left stifle joints. Intrameniscal mineralisation is present in both stifle joints of this cat. An enthesiophyte is also present in both joints where the patellar ligament attaches to the tibial tuberosity and there is new bone formation on the patella and fabella. Note the mineralisation within the synovium in the right stifle joint. L = lumbar. Images courtesy of University Veterinary Practice, The University of Liverpool, UK
Radiographic changes consistent with OA/DJD do not always equate with clinically evident musculoskeletal impairment.9,11,12 Conversely, detection of musculoskeletal impairment through physical examination/CMIs may be low, especially with early or mild disease.11,12 As discussed earlier, radiographs are also not sensitive for detecting cartilage degradation.13,14 In other words, clinically significant lesions may not create radiographic changes that are observable with current technology. CT is considered superior to radiography for examining and assessing mineralised tissue 5 and MRI may allow assessment of structural changes related to OA and a more global assessment of osteo-arthritic joints. 49 However, large studies using these advanced forms of imaging for feline OA/DJD are lacking.
Other diagnostics aids
Treatment trial
A response to analgesics or other treatments is used as a supporting diagnostic tool in cases where owner-perceived signs and/or physical examination findings are consistent with feline OA/DJD but where the diagnosis has not been definitively confirmed. 31 One study found that treatment response was used in 24% of cases of feline OA to support a diagnosis, in conjunction with owner-reported signs, orthopaedic examination findings and radiographic changes. 31
Use of CMIs or a health-related quality of life tool for OA/DJD 64 can help to standardise treatment responses observed by the owner if applied before, during and after the treatment. It has been suggested that the time point of day 15 of treatment may serve as a better baseline than a pre-treatment assessment to allow a learning period for the owner and reduce any placebo effect. 43
A review of six placebo-controlled studies looking at therapy for feline DJD showed success rates of 54–75% using a CSOM CMI 30 compared with success rates of 10–63% for objectively measured activity scores using an accelerometer. CMIs may, however, be overestimating treatment success by up to 63% in some studies. 30 So, ideally, an objective measurement, such as accelerometry data, should also be used in any trial to assess the efficacy of an analgesic treatment and reduce the potential placebo effect of CMIs. Currently objective measures are not commonly used in a clinical situation, though this may change in the future with the increased availability of fitness trackers for pets.
Biomarkers
To date, no biomarkers have been identified for diagnosing feline DJD/OA. Cytokines and chemokines have been reported,22,65 as have higher serum concentrations of interleukin (IL)-4 and IL-8 in cats with higher radiograph-ic DJD scores, and higher serum concentrations of IL-2, IL-8 and TNF-a in cats with higher pain scores associated with DJD. 65 IL-8, however, has also been found to increase with age and a lack of age matches in the study in question makes it difficult to determine if the increase in IL-8 is more strongly associated with pain/DJD or age. 22
Treatment of feline musculoskeletal impairment and disease
A large number of different treatments are prescribed for feline chronic musculoskeletal disease, often with little evidence to back up their effectiveness. 66 A multimodal approach involving therapeutics, environmental management and other complementary therapies has been described to manage feline chronic pain, 67 and is the focus of the following discussion.
The effect of obesity on musculoskeletal disease is discussed later in the prevention section; suffice to say at this point that controlled weight loss should be part of the treatment programme for obese cats with established musculoskeletal disease. 67
Therapeutics
A recent survey of predominantly North American veterinarians recorded the top five therapeutics prescribed for chronic muscu-loskeletal pain as being gabapentin (75%), joint supplement (67.8%), meloxicam (64%), opioids (62.6%) and fish oil (62.1%). 66 Therapeutics can be divided into analgesics and disease-modifying osteoarthritis (DMOA) drugs. Note that therapeutic licensing laws vary around the world.
Analgesics
Analgesics are often the mainstay of therapy for feline musculoskeletal disease, which is the principal cause of chronic pain in cats. 67
Non-steroidal anti-inflammatory drugs (NSAIDSs) Meloxicam and robenacoxib are the two most commonly prescribed NSAIDs for musculoskeletal disease. 66 In the UK, meloxicam is licensed for long-term use for chronic musculoskeletal disorders; robenacoxib is also licensed for use in chronic mus-culoskeletal disorders for up to 12 weeks. 68 Both have been shown to be effective in treating pain associated with musculoskeletal disease.23,33,34,40,69–71 Long-term use of NSAIDs in older cats can be a cause for concern, particularly in relation to renal disease. Consensus guidelines on NSAID use have been produced, 72 and it has been shown that long-term NSAIDs are suitable for cats with concurrent renal disease if used appropriately.70,73,74 NSAIDs, however, may not be an appropriate analgesic if maladaptive pain is present. 75
Grapiprant is a new class of NSAID, which has recently been licensed for dogs with OA (Galliprant; Elanco). It is a prostaglandin E receptor 4 (EP4) antagonist and works by blocking prostaglandin E2 (PGE2) receptors. Safety and toxicity of this drug has been investigated in cats, and administration at doses of up to 15 mg/kg q24h for 28 days was shown to have no adverse effects. 76 There was a large variation in serum half-life in this study (2–15 h), so further work on formulation and dosing frequency is required in cats. In addition, grapiprant’s pharmacokinetics have been investigated in a small group of healthy adult cats, using a dose rate of 2 mg/kg, and no adverse effects were reported. 77 Further work to establish the effectiveness of grapiprant for feline OA is required before treatment recommendations can be made.
Tramadol Tramadol is not licensed in the UK for use in cats but it is licensed for dogs. 68 Recent work has started to explore longer term use of tramadol for feline OA/DJD at a dose of 2–3 mg/kg.40,57,58 Mydriasis, sedation, hypersalivation and vomiting were reported side effects. 57 An additional study looked at doses of 1, 2 and 4 mg/kg in cats; side effects reported were euphoria, dysphoria, sedation, decreased appetite and diarrhoea. 78 Adverse effects were dose dependent, with 2 mg/kg giving a significant improvement in activity levels and owner-assessed improvement using a CSOM CMI. 78 There are also indications that tramadol may be useful in cats with OA causing chronic maladaptive pain. 58
Gabapentin Gabapentin is an analogue of the neurotransmitter gamma (y)-aminobutyric acid (GABA) and is used in people for the treatment of neuropathic pain. 79 There is no licensed formulation in the UK. 68 If chronic maladaptive pain is established, then gabapentin may be appropriate to use in cats with OA. 75 A dose rate of 10 mg/kg q8h administered for a period of 30 days in six and four cats with and without OA, respectively, produced an improvement in night-time activity scores and von Frey anaesthesiometer-induced paw withdrawal thresholds in the cats that had OA. 40 A 14-day trial using gabapentin at 10 mg/kg q12h showed an improvement as determined by a CSOM CMI, but a decrease in activity levels was noted, with sedation being the most common side effect. 46
Opioids While it has been reported that opioids are commonly prescribed for chronic musculoskeletal pain in cats by North American veterinarians, 66 they are often only prescribed for short periods (8–10 days), which likely reflects the lack of a licensed opi-oid that can be easily administered at home. In addition, in the UK some opioids are a class B drug, restricting their availability and use. In the authors’ observation, sublingual buprenorphine at 0.01–0.03 mg/kg q6–12h would be the most commonly prescribed opioid for musculoskeletal pain in the UK. Sublingual administration has been shown to be effective and a practical option in a home environment.80,81
Amantadine The pharmacokinetics of amantadine in cats have been investigated but not its precise metabolism within this species. 82 One study looking at its effectiveness as an analgesic in cats, determined its dose-sparing effect on oxymorphone using a thermal nociception model. 83 However, the results were not conclusive due to a larger than expected individual variation between cats (n = 6), leading to the study being underpowered. In addition, a nociceptive model for trialling amantadine use as a therapeutic for chronic pain in cats may not be suitable. Amantadine is an N-methyl-D-aspartate receptor antagonist and its action may be more due to prevention of central stimulation by a noxious stimulus, with a minimal effect on nociception. 83 These characteristics may make it a potentially good candidate for cases where OA is leading to chronic maladaptive pain, 75 although clinical data are lacking. Dosages of 3–5 mg/kg q12h PO are currently recommended. 66
Amitriptyline Amitriptyline is a tricyclic antidepressant that inhibits reuptake of serotonin, norepinephrine and dopamine. 75 This medication is occasionally prescribed by veterinarians for chronic musculoskeletal pain in cats. 66 However, there is no evidence of its effectiveness for feline musculoskeletal pain, although it may be beneficial if maladaptive pain is present. 75
Anti-nerve growth factor The release of nerve growth factor (NGF) following tissue injury stimulates sensory neurons, leading to the sensation of pain; anti-NGF antibodies block this neuronal stimulation. 84 Tanezumab (Pfizer), a human anti-NGF antibody, has been in development for a number of years. It is currently in phase 3 trials but the adverse effect profile, especially rapidly progressing OA in a small percentage of patients that were treated concurrently with NSAIDs, may limit its clinical use. 84
A neutralising antibody against feline NGF has been created for the treatment of pain in cats. 85 A placebo-controlled, blinded, clinical pilot study has been performed using felinised anti-NGF (NV-02) in 34 cats. 45 NV-02 had analgesic effects in cats with DJD-associated pain and a single injection of NV-02 resulted in an increase in objectively measured activity for 2–6 weeks after treatment, as well as an improvement in CSOM CMI assessment. 45 Further studies are required into feline anti-NGF but its use looks promising, and work is ongoing in this area.
Further studies investigating feline anti-nerve growth factor for the treatment of pain are required, but its use looks promising
DMOA therapies
DMOA therapies inhibit structural disease progression and may improve the clinical signs of disease. Currently there are no licensed DMOA drugs for cats but there are nutraceuticals that may fall into this category. Some early work using gene therapy to reduce IL-1 expression has been performed in OA models with promising results, 86 though it may take some time before these types of therapy become available for companion animals.
Glucosamine–chondroitin There is a paucity of good quality evidence to support Glucosamine–chondroitin supplementation in cats. 87 One double-blinded, placebo-controlled trial has been published comparing the efficacy of meloxicam and Glucosamine–chondroitin in the management of cats with OA. 34 The product used in this study (glucosamine 225 mg; chondroitin sulphate 175 mg; N acetyl glucosamine 25 mg; ascorbic acid 2 mg; zinc sulphate 15 mg) was administered q12h for 6 weeks and then reduced to q24h for 4 weeks. 34 This study was underpowered (n = 30) so significant conclusions could not be made about the results, but there was a trend for owner assessment scores to be maintained after the cats were started on the placebo and the Glucosamine–chondroitin supplement was stopped. The cats that were in the meloxicam treatment group showed a more rapid decrease in owner assessment scores. However, given the potential caregiver placebo effects 30 and the lack of an objective measure in this study, the significance of this trend is even more difficult to determine.
A systematic review of studies using chron-droprotective treatments for human knee OA found that Glucosamine–chondroitin may serve as a cartilage protector and slow the progression of OA. 88 Veterinary Glucosamine–chondroitin products are available, as are diets that contain Glucosamine–chondroitin and are high in eicosapentaenoic acid (EPA)/docosahexaenoic acid (DHA), and these are marketed as helping to maintain cartilage health. To date, the evidence is not conclusive as to their benefits.
Polysulfated glycosaminoglycans (PSGAGs) PSGAGs are commonly prescribed for chronic musculoskeletal pain in cats. 66 However, while there is a licensed injectable formulation of pentosan polysulphate for dogs for the treatment of pain associated with DJD/OA, 68 there is no licensed product for cats.
The use of PSGAGs has been investigated in cats with idiopathic lower urinary tract disease, 89 but there are no studies investigating their use for feline musculoskeletal pain. There is some indication that PSGAGs may have disease-modifying effects in carti-lage, 67 but currently there is no clear evidence for this in cats. There is better evidence for their benefit in dogs, showing the reduction of cartilage destruction in synovial joints with OA. 90
Omega-3 fatty acid supplementation There are two studies reporting omega-3 supplementation in cats with DJD/OA.47,91 The first used a diet high in EPA and DHA, as well as green-lipped mussel extract and glucosamine chondroitin, in a randomised, controlled, blinded, parallel group, prospective design. 47 The study was completed by 40 cats, all with DJD/OA, 20 in each group. A significant increase in activity levels assessed by accelerometer in the group on the therapeutic diet compared with the control group was determined after regression analysis to account for weight loss. 47
The second study reported the sole use of omega-3 fatty acid supplementation. 91 This was a randomised, double-blinded, placebo-controlled, crossover design, with 16 cats with OA/DJD completing the study. Significant differences were shown between the omega-3 and the placebo in the reported CMI. 91 The CMI used in this study was based on questionnaires created for two previous studies;11,29 however, as mentioned earlier, caregiver placebo effect may inflate the outcome when a CMI is the sole outcome measure.
Both studies are small but suggest some benefit for the use of omega-3 fatty acid supplementation in cats with DJD/OA. In vivo cellular models of arthritis have shown that EPA can reduce inflammation.92,93 A canine chondrocyte OA model showed a negligible effect with DHA, but EPA and arachidonic acid decreased inflammatory markers. 93 There is, at this time, greater evidence for the benefit of EPA supplementation compared with glucosamine-chon-droitin products in companion animals.87,94,95
Complementary therapy
Complementary therapies can also be considered as non-pharmacological treatments to aid in the management of musculoskeletal disease and are becoming increasingly important in pain management. 67
Acupuncture
Acupuncture has become an accepted treatment modality for pain in animals; it is minimally invasive and generally well tolerated. 67 To date there are no controlled clinical trials to show the effectiveness of acupuncture for chronic pain associated with feline muscu-loskeletal disease, though there is some evidence to support the use of acupuncture in cats with musculoskeletal pain, mostly in the form of case reports/series that indicate some benefit.96,97
Hydrotherapy
Canine hydrotherapy is readily available and is used for orthopaedic conditions as well as for weight loss and fitness. 98 Feline hydro-therapy is far less common but may be beneficial in patients that will tolerate it.99,100 To date there are no formally published case reports on feline hydrotherapy.
Environmental modification
While environmental modification is not medical care, it is an important part of a multimodal approach to managing chronic musculoskeletal pain in cats.67,101 Examples of environmental modifications that can be considered are presented in the box below and Figure 5.
Figure 5.
Examples of environmental modifications: (a) steps to allow access to the window sill; (b) elevated water bowl; (c) elevated feeding bowl; (d) flat feeding mat; (e) large, high-sided litter tray with easy access; and (f) sleeping places next to a heat source for the cats of one of the authors (ND). (a) Courtesy of Jeanette Bradshaw; (b,c) courtesy of Lucie Allcut; (d,e) courtesy of Kelly Eyre
Physiotherapy
Physiotherapy can play a role in the rehabilitation of cats after injury to prevent the development of musculoskeletal disease, and in cases of established musculoskeletal disease it can help to maintain and improve the range of motion in joints and improve muscle strength and tone 100 Techniques appropriate in cats with musculoskeletal disease include joint mobilisations and manipulations, and soft tissue techniques such as massage and stretches, as well as forms of therapeutic exercise. 100 In addition, therapeutic programmes can also consider the chronic pain element of musculoskeletal disease and use heat/cold therapy and electrophysical therapies to help address this. 101 There are a number of review articles about feline physiotherapy101-102 and some case studies showing its benefit post-surgery following femoral head and neck excision.102,103
Prevention strategies
Maintenance of a healthy weight
The cause of the high prevalence of DJD/OA in ageing cats is poorly understood, which makes it difficult to offer clear preventive advice.37,104,105 It has been shown that activity levels are lower in overweight adult cats, 106 albeit the cats in this study, aged between 3.9 and 5 years, were not screened for musculoskeletal disease. While the prevalence of OA/DJD is usually considered low in young adult cats, one study has reported prevalence rates of 80% for appendicular and 16% for axial radiographic OA/DJD in cats aged 6 months to 5 years. 10 Hence, musculoskeletal disease as a confounder for the reduced activity levels in the above-mentioned study cannot be ruled out. 106
Body condition scores of over 6/9 have been shown to be associated with an increased incidence of musculoskeletal disease in an Australian cat population; 107 although the retrospective nature of this study means a causal relationship between obesity and muscu-loskeletal disease could not be established. In addition to the mechanical load on joints associated with obesity, the metabolic effects of obesity may create a pro-inflammatory state, with elevations in C-reactive protein, IL-6 and TNF-a reported in obese humans. 108 As the prevalence of obesity peaks in middle-aged cats, 109 maintaining a healthy weight and preventing obesity is important in cats of all ages for health and longevity.107,110
Prevention of muscle loss
Muscle wastage is not uncommon in older cats and sarcopenia, as discussed earlier, is recognised as the loss of lean body mass in the absence of another disease process. 16 Sarcopenia in people increases their risk of physical disability, poor quality of life and death. 19 Loss of muscle strength is likely to play a role in functional impairment of the feline musculoskeletal system. Preventing muscle loss needs to include early diagnosis and treatment of any underlying disease process that may lead to reduced food intake or absorption. 16 Some environmental modifications may help to improve food intake (see box on page 1078 and Figure 5). In addition, dietary intervention has been shown to extend longevity and preserve lean body mass. 111 Obesity is common in older cats, and many senior diets are formulated to reflect this; so, for some cats, changing to a senior formulation diet that is more calorie dense is appropriate to help preserve muscle mass. 16 An exercise programme may also be beneficial in maintaining muscle strength and tone. Developing an enriched environment for the cat is important in its own right, in addition to facilitating appropriate play and interaction between the cat and the owner. 101
Slowing of disease progression
The prevention or slowing of the development of OA is focused on the reduction of inflammation within the joint and preserving cartilage integrity.88,112 As discussed earlier in the section on DMOA therapies, there is some evidence that some therapeutics may aid with slowing disease progression.
Avoidance of chronic maladaptive pain
Finally, preventing central stimulation/maladaptive pain is an important consideration, given that chronic pain in cats with DJD/OA can lead to maladaptive pain.40,55,56 Early identification and appropriate treatment are likely to be key in preventing chronic maladaptive pain; however, the difficulty in diagnosing early musculoskeletal pain in cats makes this a challenge. An increased awareness of musculoskeletal pain by both the owner and the veterinary team, alongside a consistent annual veterinary assessment including a CMI and an orthopaedic examination, may improve the clinician’s ability to detect early changes in musculoskeletal health.
Key Points
There is a large deficit in our knowledge as to what healthy ageing of the feline musculoskeletal system is and how it can be identified and managed.
Currently, age-related musculoskeletal disease in cats is predominantly diagnosed by recognition of pain and loss of mobility, which is subjective and unreliable. 31
Early recognition of ageing in the musculoskeletal system and appropriate intervention will improve the cat’s quality of life and welfare in their advancing years. As part of this, it is important to:
– Remember that all cats are individuals and will age differently.
– Develop a consistent approach for assessing the musculoskeletal health of feline patients through regular clinical assessment.
– Raise awareness of musculoskeletal impairment among cat owners.
– Consider using a regular CMI or questionnaire with cat owners to help diagnose musculoskeletal impairment.
– Aim to identify and treat musculoskeletal pain early in the disease process to prevent/reduce central stimulation.
– Use a multimodal approach to treating musculoskeletal impairment, including appropriate analgesia, nutraceuticals, exercise, environmental modification and additional complementary therapies in appropriate cases.
– Try to preserve lean body mass and prevent obesity in cats of all ages.
– Encourage activity in cats of all ages.
– Encourage appropriate weight loss in cats that are obese.
Footnotes
Author note: At the time of writing, Nathalie Dowgray was based at the University of Liverpool, UK, while studying for a PhD, where she also remains as a research assistant/post doc on a part-time basis.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Nathalie Dowgray’s PhD and work with the Feline Healthy Ageing Clinic is funded by Royal Canin.
Ethical approval: This work did not involve the use of animals and therefore ethical approval was not necessarily required.
Informed consent: This work did not involve the use of animals and therefore informed consent was not required. For any animals or humans individually identifiable within this publication, informed consent (either verbal or written) for their use in the publication was obtained from the people involved.
Contributor Information
Nathalie Dowgray, International Cat Care, Tisbury, Salisbury, UK.
Eithne Comerford, Institute of Life Course and Medical Sciences and School of Veterinary Science, University of Liverpool, UK.
References
- 1. Lopez-Otin C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell 2013; 153: 1194–1217. DOI: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roberts S, Colombier P, Sowman A, et al. Ageing in the musculoskeletal system: cellular function and dysfunction throughout life. Acta Orthop 2016; 87: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ugmak M, Yilmaz OT, Gunduz MC, et al. Osteoporotic risk and physeal closure in prepubertal ovariohysterectomized cats. Anim Reprod Sci 2015; 161: 146–151. [DOI] [PubMed] [Google Scholar]
- 4. Ryan J, Lascelles B, Benito J, et al. Histological and molecular characterisation of feline humeral condylar osteoarthritis. BMC Vet Res 2013; 9: 110. DOI: 10.1186/1746-6148-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leijon A, Ley CJ, Corin A, et al. Cartilage lesions in feline stifle joints – associations with articular mineralizations and implications for osteoarthritis. Res Vet Sci 2017; 114: 186–193. [DOI] [PubMed] [Google Scholar]
- 6. Freire M, Meuten D, Lascelles D. Pathology of articular cartilage and synovial membrane from elbow joints with and without degenerative joint disease in domestic cats. Vet Pathol 2014; 51: 968–978. [DOI] [PubMed] [Google Scholar]
- 7. Hardie EM, Roe SC, Martin FR. Radiographic evidence of degenerative joint disease in geriatric cats: 100 cases (1994-1997). J Am Vet Med Assoc 2002; 220: 628–632. [DOI] [PubMed] [Google Scholar]
- 8. Clarke SP, Mellor D, Clements DN, et al. Prevalence of radio-graphic signs of degenerative joint disease in a hospital population of cats. Vet Rec 2005; 157: 793–799. [DOI] [PubMed] [Google Scholar]
- 9. Godfrey DR. Osteoarthritis in cats: a retrospective radiological study. J Small Anim Pract 2005; 46: 425–429. [DOI] [PubMed] [Google Scholar]
- 10. Lascelles BDX, Henry JB, Brown J, et al. Cross-sectional study of the prevalence of radiographic degenerative joint disease in domesticated cats. Vet Surg 2010; 39: 535–544. [DOI] [PubMed] [Google Scholar]
- 11. Slingerland LI, Hazewinkel HAW, Meij BP, et al. Cross-sectional study of the prevalence and clinical features of osteoarthritis in 100 cats. Vet J 2011; 187: 304–309. [DOI] [PubMed] [Google Scholar]
- 12. Lascelles BDX, Dong Y-H, Marcellin-Little DJ, et al. Relationship of orthopedic examination, goniometric measurements, and radiographic signs of degenerative joint disease in cats. BMC Vet Res 2012; 8: 10. DOI: 10.1186/1746-6148-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freire M, Robertson I, Bondell HD, et al. Radiographic evaluation of feline appendicular degenerative joint disease vs. Macroscopic appearance of articular cartilage. Vet Radiol Ultrasound 2011; 52: 239–247. [DOI] [PubMed] [Google Scholar]
- 14. Ariffin SMZ. Radiographic and pathologic studies of feline appendicular osteoarthritis. PhD thesis, University of Glasgow, 2014. [Google Scholar]
- 15. Leumann A, Leonard T, Nuesch C, et al. The natural initiation and progression of osteoarthritis in the anterior cruciate ligament deficient feline knee. Osteoarthr Cartil 2019; 27: 687–693. [DOI] [PubMed] [Google Scholar]
- 16. Laflamme DP. Sarcopenia and weight loss in the geriatric cat. In: Little S. (ed). August’s consultations in feline internal medicine. 7th ed. St Louis, MO: Elsevier, 2015, pp 951–956. [Google Scholar]
- 17. Veronese N, Demurtas J, Soysal P, et al. Sarcopenia and health-related outcomes: an umbrella review of observational studies. Eur Geriatr Med 2019; 10: 853–862. [DOI] [PubMed] [Google Scholar]
- 18. Ryall JG, Schertzer JD, Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology 2008; 9: 213–228. [DOI] [PubMed] [Google Scholar]
- 19. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010; 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perez-Camargo G. Feline decline in key physiological reserves: implictions for mortality. In: Proceedings of the Nestle Purina Companion Animal Nutrition Summit: Fous on gerontology. St Louis, MO: 2010, pp 6–13. [Google Scholar]
- 21. Freeman LM. Cachexia and sarcopenia: emerging syndromes of importance in dogs and cats. J Vet Intern Med 2012; 26: 3–17. [DOI] [PubMed] [Google Scholar]
- 22. Gruen ME, Messenger KM, Thomson AE, et al. Evaluation of serum cytokines in cats with and without degenerative joint disease and associated pain. Vet Immunol Immunopathol 2017; 183: 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guillot M, Moreau M, Heit M, et al. Characterization of osteoarthritis in cats and meloxicam efficacy using objective chronic pain evaluation tools. Vet J 2013; 196: 360–367. [DOI] [PubMed] [Google Scholar]
- 24. Gruen ME, Alfaro-Cordoba M, Thomson AE, et al. The use of functional data analysis to evaluate activity in a spontaneous model of degenerative joint disease associated pain in cats. PLoS One 2017; 12. DOI: 10.1371/journal.pone.0169576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohlund M, Palmgren M, Holst BS. Overweight in adult cats: a cross-sectional study. Acta Vet Scand 2018; 60: 5. DOI: 10.1186/s13028-018-0359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dowgray NJ, Bates KT, Comerford EJ, et al. Feline gait kinematics: what changes with ageing? Proceedings of the Gordon Conference: Biology of Ageing, 2019. [Google Scholar]
- 27. Benito J, Hansen B, Depuy V, et al. Feline musculoskeletal pain index: responsiveness and testing of criterion validity. J Vet Intern Med 2013; 27: 474–482. [DOI] [PubMed] [Google Scholar]
- 28. Benito J, DePuy V, Hardie E, et al. Reliability and discriminatory testing of a client-based metrology instrument, feline muscu-loskeletal pain index (FMPI) for the evaluation of degenerative joint disease-associated pain in cats. Vet J 2013; 196: 368–373. [DOI] [PubMed] [Google Scholar]
- 29. Zamprogno H, Hansen BD, Bondell HD, et al. Item generation and design testing of a questionnaire to assess degenerative joint disease-associated pain in cats. Am J Vet Res 2010; 71: 1417–1424. [DOI] [PubMed] [Google Scholar]
- 30. Gruen ME, Dorman DC, Lascelles BDX. Caregiver placebo effect in analgesic clinical trials for cats with naturally occurring degenerative joint disease-associated pain. Vet Rec 2017; 180: 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klinck MP, Frank D, Guillot M, et al. Owner-perceived signs and veterinary diagnosis in 50 cases of feline osteoarthritis. Can Vet J 2012; 53: 1181–1186. [PMC free article] [PubMed] [Google Scholar]
- 32. Stadig S, Lascelles DBX, Nyman G, et al. Evaluation and comparison of pain questionnaires for clinical screening of osteoarthritis in cats. Vet Rec 2019; 185: 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bennett D, Morton C. A study of owner observed behavioural and lifestyle changes in cats with muscu-loskeletal disease before and after analgesic therapy. J Feline Med Surg 2009; 11: 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sul RM, Chase D, Parkin T, et al. Comparison of meloxicam and a Glucosamine–chondroitin supplement in management of feline osteoarthritis: a double-blind randomised, placebo-controlled, prospective trial. Vet Comp Orthop Traumatol 2014; 27: 20–26. [DOI] [PubMed] [Google Scholar]
- 35. Gruen ME, Griffith E, Thomson A, et al. Detection of clinically relevant pain relief in cats with degenerative joint disease associated pain. J Vet Intern Med 2014; 28: 346–350. DOI: 10.1111/jvim.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Monteiro BP, Steagall PV. Chronic pain in cats: recent advances in clinical assessment. J Feline Med Surg 2019; 21: 601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bennett D, Ariffin SMZ, Johnston P. Osteoarthritis in the cat: 1. how common is it and how easy to recognise? J Feline Med Surg 2012; 14: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kerwin S. Orthopedic examination in the cat: clinical tips for ruling in/out common musculoskeletal disease. J Feline Med Surg 2012; 14: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klinck MP, Rialland P, Guillot M, et al. Preliminary validation and reliability testing of the Montreal Instrument for Cat Arthritis Testing, for Use by Veterinarians, in a colony of laboratory cats. Animals 2015; 5: 1252–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Klinck MP, Monteiro BP, Lussier B, et al. Refinement of the Montreal Instrument for Cat Arthritis Testing, for Use by Veterinarians: detection of naturally occurring osteoarthritis in laboratory cats. J Feline Med Surg 2018; 20: 728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Klinck MP, Gruen ME, del Castillo JRE, et al. Development and preliminary validity and reliability of the montreal instrument for cat arthritis testing, for use by caretaker/owner, MI-CAT(C), via a randomised clinical trial. Appl Anim Behav Sci 2018; 200: 96–105. [Google Scholar]
- 42. Enomoto M, Lascelles BDX, Gruen ME. Development of a checklist for the detection of degenerative joint disease-associated pain in cats. J Feline Med Surg. Epub ahead of print 3 March 2020. DOI: 10.1177/1098612X20907424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gruen ME, Griffith EH, Thomson AE, et al. Criterion validation testing of clinical metrology instruments for measuring degenerative joint disease associated mobility impairment in cats. PLoS One 2015; 10. DOI: 10.1371/journal.pone.0131839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lascelles BDX, Hansen BD, Roe S, et al. Evaluation of client-specific outcome measures and activity monitoring to measure pain relief in cats with osteoarthritis. J Vet Emerg Crit Care 2007; 21: 410–416. [DOI] [PubMed] [Google Scholar]
- 45. Gruen ME, Thomson AE, Griffith EH, et al. A feline-specific anti-nerve growth factor antibody improves mobility in cats with degenerative joint disease-associated pain: a pilot proof of concept study. J Vet Intern Med 2016; 30: 1138–1148. DOI: 10.1111/jvim.13972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guedes AGP, Meadows JM, Pypendop BH, et al. Assessment of the effects of gabapentin on activity levels and owner-perceived mobility impairment and quality of life in osteo-arthritic geriatric cats. J Am Vet Med Assoc 2018; 253: 579–585. [DOI] [PubMed] [Google Scholar]
- 47. Lascelles BD, Depuy V, Thomson A, et al. Evaluation of a therapeutic diet for feline degenerative joint disease. J Vet Intern Med 2010; 24: 487–495. [DOI] [PubMed] [Google Scholar]
- 48. Clarke SP, Bennett D. Feline osteoarthritis: a prospective study of 28 cases. J Small Anim Pract 2006; 47: 439–445. [DOI] [PubMed] [Google Scholar]
- 49. Guillot M, Moreau M, D’Anjou MA, et al. Evaluation of osteoarthritis in cats: novel information from a pilot study. Vet Surg 2012; 41: 328–335. [DOI] [PubMed] [Google Scholar]
- 50. Corbee RJ, Maas H, Doornenbal A, et al. Forelimb and hindlimb ground reaction forces of walking cats: assessment and comparison with walking dogs. Vet J 2014; 202: 116–127. [DOI] [PubMed] [Google Scholar]
- 51. Stadig SM, Bergh AK. Gait and jump analysis in healthy cats using a pressure mat system. J Feline Med Surg 2015; 17: 523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stadig S, Lascelles BDX, Bergh A. Do cats with a cranial cruciate ligament injury and osteoarthritis demonstrate a different gait pattern and behaviour compared to sound cats? Acta Vet Scand 2016; 58: 70. DOI: 10.1186/s13028-016-0248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moreau M, Lussier B, Ballaz L, et al. Kinetic measurements of gait for osteoarthritis research in dogs and cats. Can Vet J 2014; 55: 1057–1065. [PMC free article] [PubMed] [Google Scholar]
- 54. Schnabl-Feichter E, Tichy A, Gumpenberger M, et al. Comparison of ground reaction force measurements in a population of domestic shorthair and Maine Coon cats. PLoS One 2018; 13. DOI: 10.1371/journal.pone.0208085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guillot M, Taylor PM, Rialland P, et al. Evoked temporal summation in cats to highlight central sensitization related to osteoarthritis-associated chronic pain: a preliminary study. PLoS One 2014; 9. DOI: 10.1371/journal.pone.0097347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Addison ES, Clements DN. Repeatability of quantitative sensory testing in healthy cats in a clinical setting with comparison to cats with osteoarthritis. J Feline Med Surg 2017; 19: 1274–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Monteiro BP, Klinck MP, Moreau M, et al. Analgesic efficacy of an oral transmucosal spray formulation of meloxicam alone or in combination with tramadol in cats with naturally occurring osteoarthritis. Vet Anaesth Analg 2016; 43: 643–651. [DOI] [PubMed] [Google Scholar]
- 58. Monteiro BP, Klinck MP, Moreau M, et al. Analgesic efficacy of tramadol in cats with naturally occurring osteoarthritis. PLoS One 2017; 12. DOI: 10.1371/journal.pone.0175565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schnabl E, Bockstahler B. Systematic review of ground reaction force measurements in cats. Vet J 2015; 206: 83–90. [DOI] [PubMed] [Google Scholar]
- 60. Robinson DA, Romans CW, Gordon-evans WJ, et al. Evaluation of short-term limb function following unilateral carbon dioxide laser or scalpel onychectomy in cats. J Am Vet Med Assoc 2007; 230: 353–358. [DOI] [PubMed] [Google Scholar]
- 61. Romans CW, Gordon WJ, Robinson DA, et al. Effect of postoperative analgesic protocol on limb function following onychectomy in cats. J Am Vet Med Assoc 2005; 227: 89–93. [DOI] [PubMed] [Google Scholar]
- 62. Lascelles BDX, Findley K, Correa M, et al. Kinetic evaluation of normal walking and jumping in cats, using a pressure-sensitive walkway. Vet Rec 2007; 160: 512–516. [DOI] [PubMed] [Google Scholar]
- 63. Verdugo MR, Rahal SC, Agostinho FS, et al. Kinetic and temporospatial parameters in male and female cats walking over a pressure sensing walkway. BMC Vet Res 2013; 9: 129. DOI: 10.1186/1746-6148-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Noble CE, Wiseman-Orr LM, Scott ME, et al. Development, initial validation and reliability testing of a web-based, generic feline health-related quality-of-life instrument. J Feline Med Surg 2019; 21: 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gruen ME, Messenger KM, Thomson AE, et al. A comparison of serum and plasma cytokine values using a multiplexed assay in cats. Vet Immunol Immunopathol 2016; 182: 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Adrian DE, Rishniw M, Scherk M, et al. Prescribing practices of veterinarians in the treatment of chronic musculoskeletal pain in cats. J Feline Med Surg 2019; 21: 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Epstein ME, Rodanm I, Griffenhagen G, et al. 2015 AAHA/AAFP pain management guidelines for dogs and cats. J Feline Med Surg 2015; 17: 251–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. National Office of Animal Health. www.noahcompendium.co.uk/home (2020, accessed August 17, 2020).
- 69. Lascelles BD, Henderson AJ, Hackett IJ. Evaluation of the clinical efficacy of meloxicam in cats with painful locomotor disorders. J Small Anim Pract 2001; 42: 587–593. [DOI] [PubMed] [Google Scholar]
- 70. King JN, King S, Budsberg SC, et al. Clinical safety of robenacoxib in feline osteoarthritis: results of a randomized, blinded, placebo-controlled clinical trial. J Feline Med Surg 2016; 18: 632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Giraudel JM, Gruet P, Alexander DG, et al. Evaluation of orally administered robenacoxib versus ketoprofen for treatment of acute pain and inflammation associated with musculoskeletal disorders in cats. Am J Vet Res 2010; 71: 710–719. [DOI] [PubMed] [Google Scholar]
- 72. Sparkes AH, Heiene R, Lascelles DX, et al. ISFM and AAFP consensus guildlines: long-term use of NSAIDs in cats. J Feline Med Surg 2010; 12: 521–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gowan RA, Lingard AE, Johnston L, et al. Retrospective case-control study of the effects of long-term dosing with meloxicam on renal function in aged cats with degenerative joint disease. J Feline Med Surg 2011; 13: 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Monteiro B, Steagall PVM, Lascelles BDX, et al. Long-term use of non-steroidal anti-inflammatory drugs in cats with chronic kidney disease: from controversy to optimism. J Small Anim Pract 2019; 60: 459–462. [DOI] [PubMed] [Google Scholar]
- 75. Adrian D, Papich M, Baynes R, et al. Chronic maladaptive pain in cats: a review of current and future drug treatment options. Vet J 2017; 230: 52–61. [DOI] [PubMed] [Google Scholar]
- 76. Rausch-Derra L, Rhodes L. Safety and toxicokinetic profiles associated with daily oral administration of grapiprant, a selective antagonist of the prostaglandin E2 EP4 receptor, to cats. Am J Vet Res 2016; 77: 688–692. [DOI] [PubMed] [Google Scholar]
- 77. Lebkowska-Wieruszewska B, De Vito V, Owen H, et al. Pharmacokinetics of grapiprant, a selective EP4 pros-taglandin PGE2 receptor antagonist, after 2 mg/kg oral and i.v. Administrations in cats. J Vet Pharmacol Ther 2017; 40: e11–e15. [DOI] [PubMed] [Google Scholar]
- 78. Guedes AGP, Meadows JM, Pypendop BH, et al. Evaluation of tramadol for treatment of osteoarthritis in geriatric cats. J Am Vet Med Assoc 2018; 252: 565–571. [DOI] [PubMed] [Google Scholar]
- 79. Adrian D, Papich MG, Baynes R, et al. The pharmacokinetics of gabapentin in cats. J Vet Intern Med 2018; 32: 1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Robertson SA, Taylor M, Sear JW. Systemic uptake of buprenorphine by cats after oral mucosal administration. Vet Rec 2003; 152: 675–678. [DOI] [PubMed] [Google Scholar]
- 81. Gulledge BM, Messenger KM, Cornell KK, et al. Pharmacokinetic comparison of two buprenorphine formulations after buccal administration in healthy male cats. J Feline Med Surg 2018; 20: 312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Siao KT, Pypendop BH, Stanley SD, et al. Pharmacokinetics of amantadine in cats. J Vet Pharmacol Ther 2011; 34: 599–604. [DOI] [PubMed] [Google Scholar]
- 83. Siao KT, Pypendop BH, Escobar A, et al. Effect of amantadine on oxymorphone-induced thermal antinociception in cats. J Vet Pharmacol Ther 2012; 35: 169–174. [DOI] [PubMed] [Google Scholar]
- 84. Hefti F. Pharmacology of nerve growth factor and discovery of tanezumab, an anti-nerve growth factor antibody and pain therapeutic. Pharmacol Res 2020; 154: 104240. [DOI] [PubMed] [Google Scholar]
- 85. Gearing DP, Huebner M, Virtue ER, et al. In vitro and in vivo characterization of a fully felinized therapeutic anti-nerve growth factor monoclonal antibody for the treatment of pain in cats. J Vet Intern Med 2016; 30: 1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nixon AJ, Grol MW, Lang HM, et al. Disease-modifying osteoarthritis treatment with interleukin-1 receptor antagonist gene therapy in small and large animal models. Arthritis Rheumatol 2018; 70: 1757–1768. [DOI] [PubMed] [Google Scholar]
- 87. Vandeweerd J, Coisnon C, Clegg P, et al. Systematic review of efficacy of nutraceuticals to alleviate clinical signs of osteoarthritis. J Vet Intern Med 2012; 26: 448–456. [DOI] [PubMed] [Google Scholar]
- 88. Gallagher B, Tjoumakaris FP, Harwood MI, et al. Chondroprotection and the prevention of osteoarthritis progression of the knee: a systematic review of treatment agents. Am J Sports Med 2015; 43: 734–744. [DOI] [PubMed] [Google Scholar]
- 89. Wallius BM, Tidholm AE. Use of pentosan polysulphate in cats with idiopathic, non-obstructive lower urinary tract disease: a double-blind, randomised, placebo-controlled trial. J Feline Med Surg 2009; 11: 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fujiki M, Shineha J, Yamanokuchi K, et al. Effects of treatment with polysulfated glycosaminoglycan on serum cartilage oligomeric matrix protein and C-reactive protein concentrations, serum matrix metalloproteinase-2 and -9 activities, and lameness in dogs with osteoarthritis. Am J Vet Res 2007; 68: 827–833. [DOI] [PubMed] [Google Scholar]
- 91. Corbee RJ, Barnier MMC, van de Lest CHA, et al. The effect of dietary long-chain omega-3 fatty acid supplementation on owner’s perception of behaviour and locomotion in cats with naturally occurring osteoarthritis. J Anim Physiol Anim Nutr (Berl) 2013; 97: 846–853. [DOI] [PubMed] [Google Scholar]
- 92. Hurst S, Rees SG, Randerson PF, et al. Contrasting effects of n-3 and n-6 fatty acids on cyclooxygenase-2 in model systems for arthritis. Lipids 2009; 44: 889–896. [DOI] [PubMed] [Google Scholar]
- 93. Adler N, Schoeniger A, Fuhrmann H. Polyunsaturated fatty acids influence inflammatory markers in a cellular model for canine osteoarthritis. J Anim Physiol Anim Nutr (Berl) 2018; 102: e623–e632. [DOI] [PubMed] [Google Scholar]
- 94. Bhathal A, Spryszak M, Louizos C, et al. Glucosamine and chondroitin use in canines for osteoarthritis: a review. Open Vet J 2017; 7: 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Moreau M, Troncy E, del Castillo JRE, et al. Effects of feeding a high omega-3 fatty acids diet in dogs with naturally occurring osteoarthritis. J Anim Physiol Anim Nutr (Berl) 2013; 97: 830–837. [DOI] [PubMed] [Google Scholar]
- 96. Rose WJ, Sargeant JM, Hanna WJB, et al. A scoping review of the evidence for efficacy of acupuncture in companion animals. Anim Heal Res Rev 2017; 18: 177–185. [DOI] [PubMed] [Google Scholar]
- 97. Choi KH, Hill SA. Acupuncture treatment for feline multifocal intervertebral disc disease. J Feline Med Surg 2009; 11: 706–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. McCormick W, Oxley JA, Spencer N. Details of canine hydrotherapy pools and treadmills in 22 hydrotherapy centres in the United Kingdom. Vet Rec 2018; 183: 128. [DOI] [PubMed] [Google Scholar]
- 99. Lindley S, Watson P. BSAVA manual of canine and feline rehabilitation, supportive and palliative care: case studies in patient management. BSAVA, 2010. [Google Scholar]
- 100. Sharp B. Feline physiotherapy and rehabilitation: 1. principles and potential. J Feline Med Surg 2012; 14: 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sharp B. Feline physiotherapy and rehabilitation: 2. clinical application. J Feline Med Surg 2012; 14: 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Price H. Feline physiotherapy. Companion Anim 2014; 19: 374–378. [Google Scholar]
- 103. Sabiza S, Ronagh A, Khajeh A. Effective medical management and physiotherapy program of femoral head and neck ostectomy in 24 dogs and cats; clinical report. Iran J Vet Surg 2019; 14: 78–84. [Google Scholar]
- 104. Lascelles BDX. Feline degenerative joint disease. Vet Surg 2010; 39: 2–13. [DOI] [PubMed] [Google Scholar]
- 105. Bennett D, Ariffin SMZ, Johnston P. Osteoarthritis in the cat: 2. how should it be managed and treated? J Feline Med Surg 2012; 14: 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. de Godoy MRC, Shoveller AK. Overweight adult cats have significantly lower voluntary physical activity than adult lean cats. J Feline Med Surg 2017; 19: 1267–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Teng KT, McGreevy PD, Toribio JALML, et al. Associations of body condition score with health conditions related to overweight and obesity in cats. J Small Anim Pract 2018; 59: 603–615. [DOI] [PubMed] [Google Scholar]
- 108. German AJ, Ryan VH, German AC, et al. Obesity, its associated disorders and the role of inflammatory adipokines in companion animals. Vet J 2010; 185: 4–9. [DOI] [PubMed] [Google Scholar]
- 109. Tarkosova D, Story M, Rand J, et al. Feline obesity – prevalence, risk factors, pathogenesis, associated conditions and assessment: a review. Vet Med (Praha) 2016; 61: 295–307. [Google Scholar]
- 110. Teng KT, McGreevy PD, Toribio J-AL, et al. Strong associations of nine-point body condition scoring with survival and lifespan in cats. J Feline Med Surg 2018; 20: 1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cupp CJ, Kerr WW, Jean-Philippe C, et al. The role of nutritional interventions in the longevity and maintenance of long-term health in aging cats. Int J Appliled Res Vertin Med 2008; 6: 69–81. [Google Scholar]
- 112. Guan VX, Mobasheri A, Probst YC. A systematic review of osteoarthritis prevention and management with dietary phytochemicals from foods. Maturitas 2019; 122: 35–43. [DOI] [PubMed] [Google Scholar]