Abstract
Objectives
Fertility and reproductive management were investigated, via questionnaires, in breeding establishments of Norwegian Forest Cats, Maine Coon, Persian and Bengal cats in Italy.
Methods
Six Bengal, five Maine Coon, eight Norwegian Forest Cat and seven Persian breeders responded for a total of 128 queens, 565 heats, 337 litters and 1424 kittens spanning the period 1998–2012. The mean number of queens per cattery was 4.9 ± 2.3, with primiparous queens constituting 20.5%. Of the catteries, 61.5% were indoor, with 50% of them having access to a fenced balcony. Fifteen percent of the catteries were outdoor with cat runs. No light supplementation was used in any of the catteries.
Results
Queens showed oestrous cycles throughout the year, although 67% of oestrous cycles occurred from January–June. Puberty occurred, on average, at 12.3 ± 7.4 months. Pregnancy diagnosis was performed routinely in only 30% of catteries. Both pregnancy length (average 64.7 ± 2.4 days, range 59–76 days) and litter size (average 4.2 ± 1.8 kittens, with 11.8% of kittens stillborn) showed some breed-specific differences; in Norwegian Forest Cats larger litter size was associated with shorter pregnancy length. Kitten mortality between birth and weaning was, on average, 14%. Stillborn kittens (P <0.01) and kitten mortality (0.01 <P <0.05) increased with litter size. In Maine Coons and Persians, a total of 3% of parturitions occurred via caesarean section (CS); litters born via CS were larger and characterised by a greater number of stillborn kittens and kitten mortality.
Conclusions and relevance
No previous data concerning reproductive patterns of pedigree cats raised in Southern Europe are available. Feline reproductive management should be adjusted for latitude owing to different climate and daylight patterns that may have an impact on feline reproduction.
Keywords: Queen, breeding management, fertility, oestrus cycle, pregnancy, litter size, stillborn kittens
Introduction
Cat breeding in Italy is attracting increasing interest around the world, and therefore cat breeders are paying more attention to breeding management practices in an effort to increase queen fertility and kitten survival. Most of what is known on cat reproduction is derived from studies performed in North America, Australia and Northern Europe in climatic and photoperiodic conditions typical of continental climates.1–10 Little, if anything, is known about specific reproductive patterns of these animals in temperate climates with longer photoperiods such as in Southern Europe. The purpose of our study was to investigate fertility, reproductive parameters and reproductive management of the four most important breeds of pedigree cats in Italy – Norwegian Forest Cat, Maine Coon, Persian and Bengal cats – through a questionnaire in order to ascertain whether or not there are breed peculiarities of reproductive patterns of any of these breeds that might justify breed-specific counselling of cat owners in this climate.
Materials and methods
Selection of catteries
The study was conducted in the Veneto region of North Italy (45°30’0.00” N 11°45’0.00” E). Breeders of Bengal, Maine Coon, Norwegian Forest Cats and Persian breeds registered with the Italian Cat Breeder’s Association were contacted at cat shows, or by telephone or e-mail. Following the initial contact, 110 breeders received two ‘.doc’ files, containing a questionnaire to investigate breeding management of their catteries and a table to collect the reproductive data of queens to be returned by e-mail. As it was considered a survey study where animals were not manipulated, no approval from any ethical committee was required for data collection. Catteries were managed with a natural light regime and were maintained at room temperature. Commercial diets were used in all catteries. Retrospective data were collected between January 2011 and December 2012. Prior to the survey, the breeders were informed about the aim, methodology and the voluntary nature of the study. Any personal information, as well as the survey forms, were confidential.
The survey consisted of questions that required either categorical or numeric answers and, in a few instances, comments divided into the following three sections: (1) demographic information; (2) practices of breeding management, including questions on how heat was identified and monitored, how mating was managed (how long cats were left together at each mating, how many times cats were allowed to mate, whether and how the occurrence of mating was checked), and whether and when pregnancy diagnosis was performed; (3) reproductive information, including age at first breeding, gestation length (calculated from the day of the first breeding), yearly distribution of heats and parturitions, age at first parturition, caesarean sections (CSs), number of kittens born alive, number of stillbirths, litter size, total number of kittens weaned, kitten death between parturition and weaning.
Once questionnaires were received by e-mail, they were thoroughly analysed and the breeder was contacted by phone to verify reproductive data and obtain missing information, thus ensuring a sound interpretation of the information obtained. Breeders that bred more than one breed were included in the study as just one breeder because all cats were housed in the same facility.
The age of the queens included in the study varied from puberty to 10 years old, in order to discard variations in reproductive physiology.
Puberty and heat were detected based on vocalisation, crouching with the forequarters pressed to the ground and hyperextension of the back, causing lordosis, increased affection and head rubbing.
Data analysis
Differences in reproductive patterns and performance were analysed by ANOVA using the GLM procedure (SigmaStat 2.03) and Tukey test (P <0.05). Independent variables were breed, age (<18 months, 18–36 months, >36 months), litter size (⩽3, 4–5, ⩾6), season (January–March, April–June, July–September, October–December) and type of parturition (spontaneous vs CS), while dependent variables were time intervals (intervals between heats, age at first heat and gestation length) and number of kittens (kittens born alive, stillbirths, number of kittens weaned). Significant differences obtained with ANOVA were further evaluated using the Tukey test. Correlation coefficients for all parameters among breeds, as well as within each breed, were calculated using Pearson’s test.
Results
Only 26/110 breeders (six Bengal, five Maine Coon, eight Norwegian Forest Cat, seven Persian) successfully passed steps 1, 2 and 3 (Table 1). The main reason for exclusion of a questionnaire was lack of accurate records, as in most catteries not all heats were recorded, or age at puberty was not recorded, and/or litter size or incidence or stillbirth were not appropriately recorded. Data included the information of 128 Norwegian Forest Cat (n = 24), Maine Coon (n = 24), Bengal (n = 28) and Persian queens (n = 52), a total of 565 heats, 337 litters and 1424 kittens over the period 1998–2012.
Table 1.
Reproductive data on four breeds of purebred cats raised in Italy
| Breed | Number of breeders | Mean number of queens per cattery | Mean age of queen (years) | % of primiparous queens | Mean number of litters per cattery | Total number of litters | % litters delivered by CS |
|---|---|---|---|---|---|---|---|
| Bengal | 6 | 5.6 | 1.9 | 25 | 10.4 | 52 | 0 |
| MC | 5 | 4.8 | 3.1 | 16.7 | 11.2 | 56 | 7.8 |
| NFC | 8 | 3.5 | 2.3 | 35.7 | 7.1 | 50 | 0 |
| Persian | 7 | 6 | 4.7 | 9.5 | 25.6 | 179 | 3.5 |
| Total ± SD | 26 | 4.9 ± 2.3 | 3.3 ± 2 | 20.5 | 13 ± 14.2 | 337 | 3 |
CS = caesarean section; MC = Maine Coon; NFC = Norwegian Forest Cat
Most catteries bred a single breed, with only three (11.5%) breeding two breeds. The mean ± SD number of queens per cattery was 4.9 ± 2.3; 20.5% of queens were primiparous. Breeders of Persian cats had more cats than those of other breeds. Most catteries (61.5%) were indoor and 50% provided free access to a fenced balcony. Four catteries (15%) were outdoor in cat runs.
Oestrous cycles
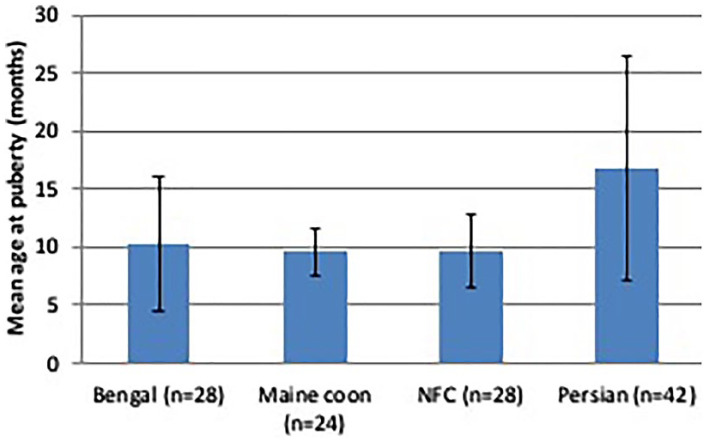
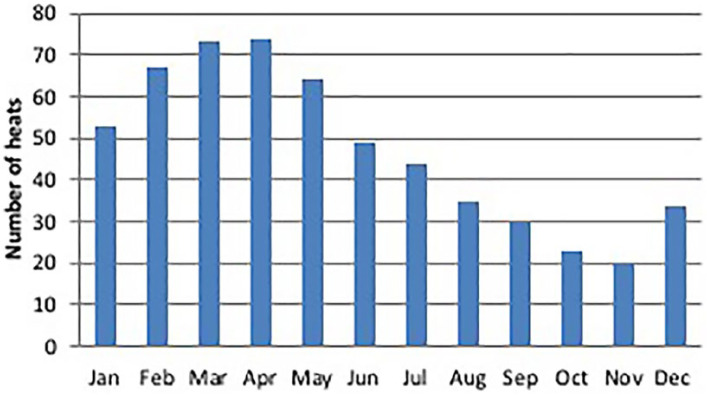
Mean age at puberty was 12.3 ± 7.4 months when all four breeds were combined, and about 10 months in Bengal, Maine Coon and Norwegian Forest Cat queens, and 16.8 months in Persians (P >0.05) (Table 2, Figure 1). There was no effect of season of birth of the queen on age at puberty. Queens were reported to be in heat every month of the year, but 67% of the oestrous cycles occurred during periods of increasing day length (January–June) (Figure 2).
Table 2.
Reproductive data on four breeds of purebred cats raised in Italy
| Breed | Mean age at puberty (months ± SD) | Gestation length (days ± SEM) | Mean age of the queen at delivery (years ± SD) |
|---|---|---|---|
| Bengal | 10.3 ± 5.8 | 64.2 ± 0.5 | 0.9 ± 0.5 |
| MC | 9.6 ± 2.1 | 64.8 ± 0.4 | 1.0 ± 0.6 |
| NFC | 9.7 ± 3.2 | 65.7 ± 0.4 | 1.0 ± 0.5 |
| Persian | 16.8 ± 9.7 | 64.6 ± 0.2 | 0.9 ± 0.5 |
| Total ± SD | 12.3 ± 7.4 | 64.7 ± 2.4 | 1.5 ± 0.5 |
MC = Maine Coon; NFC = Norwegian Forest Cat
Figure 1.
Average age (± SD) at puberty for 122 purebred queens raised in Italy. NFC = Norwegian Forest Cat
Figure 2.
Distribution during the year of the oestrous cycles of 128 queens of four breeds (24 Norwegian Forest Cats, 24 Maine Coons, 28 Bengals and 52 Persians) raised in Italy. Data collected through questionnaires
A few questionnaires reported an interval between consecutive heats of 75 or more days. While going through the data review process with the breeder (step 3) it became clear that in a few of these cases a heat might have not been recorded. Therefore, it was arbitrarily decided to only consider as valid those interoestrus intervals <60 days. As a result, the calculation of the interoestrous interval was based on 18/26 questionnaires and on a total of 205 queens (58 Bengals, 51 Maine Coons, 27 Norwegian Forest Cats and 69 Persians). The mean interoestrous interval of these animals was 39.3 ± 17 days with no significant difference among breeds. There was no effect of season of birth of the queen on the duration of interoestrus.
Breeding management
Oestrous queens were placed with a tom cat as soon as signs of oestrus were observed, and given multiple chances to breed for periods of 2–10 days. The total number of breedings/oestrus varied from 2–5 to >10. The breeder was present to observe the breeding in 35% of the cases, although in the majority of cases breeding was confirmed through listening to the postcoital reaction from an adjacent room.
Pregnancies
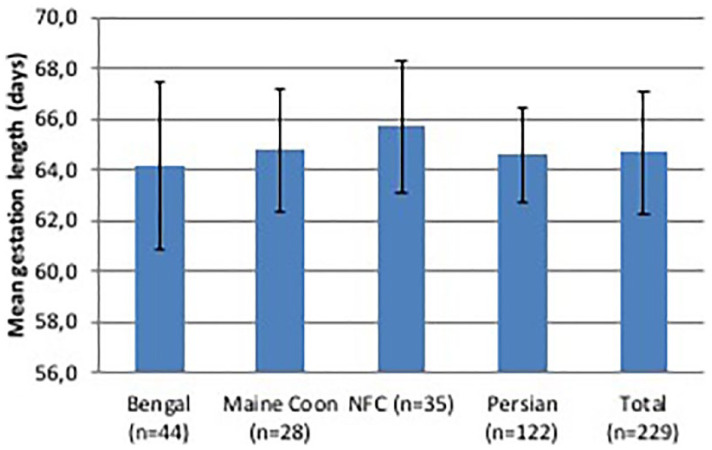
Pregnancy was diagnosed only by 31% (n = 8/26) of breeders by ultrasonography (n = 5/8) or manual palpation (n = 2/8) at 15–28 days from breeding or abdominal radiography at the end of pregnancy (n = 1/8). Six of 26 breeders (23%) requested radiography at the end of pregnancy only for primiparous queens or queens with a history of dystocia. Average gestation length was 64.7 ± 2.4 days (range 59–76 days), 57% of gestations lasted 64–66 days, while 94% were 61–69 days in duration. There were only 13 gestations ⩽60 days or ⩾70 days in length. Pregnancy was significantly longer in Norwegian Forest Cats than in Bengals, while there was no significant difference among the other breeds (Table 2, Figure 3). A moderately negative correlation (r = −0.44) between gestation length and litter size was observed in Norwegian Forest Cats (P <0.01).
Figure 3.
Average (± SD) duration of gestation in purebred queens raised in Italy. Data collected through questionnaires. NFC = Norwegian Forest Cat
Parturitions
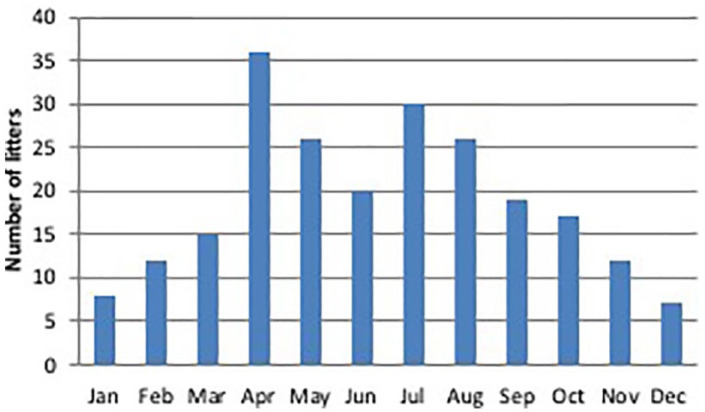
Litters were distributed throughout the year, although 67% of kittens were born between April and September (Figure 4). The average age of the queens at first parturition was 1.5 ± 0.5 years (range 0.9–3.7 years, median 1.4 years) (Table 2).
Figure 4.
Distribution during the year of 337 litters of 128 queens of four breeds (24 Norwegian Forest Cats, 24 Maine Coons, 28 Bengals and 52 Persians) raised in Italy. Data collected through questionnaires
Litter size
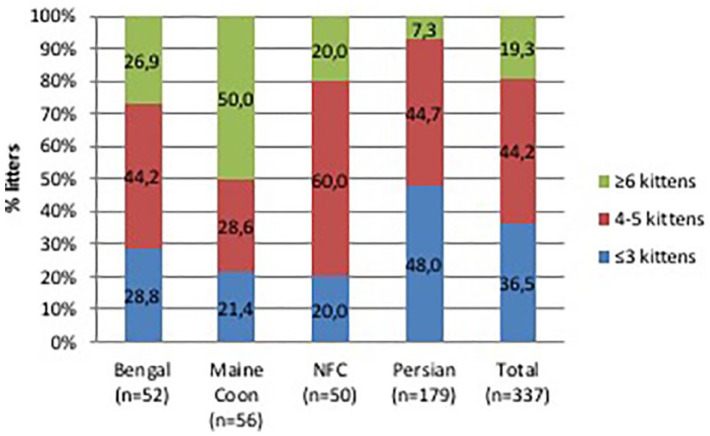
Mean litter size for Bengal and Norwegian Forest Cats was 4.2 ± 1.8 kittens, while the mean litter size of Maine Coons (5.5 ± 2.3) was significantly higher than in other breeds (P <0.01) (Table 3, Figure 5). Litter size distribution in Bengals and Norwegian Forest Cats was quite regular, with litters of 4–5 kittens reported in 44% of Bengal pregnancies and 50% of Norwegian Forest Cat pregnancies. Litter size was higher in Maine Coons (with at least 50% having six-kitten litters) and lower in Persians (with 48% of litters having a maximum of three kittens) (Figure 6). In breeds with an incidence of CS, the average size of litters born naturally was 4.1 ± 1.8, while the average size of litters born through CS was 5.9 ± 2.4.
Table 3.
Reproductive data for four breeds of purebred cats raised in Italy
| Breed | Mean litter size | Mean % of stillborn kittens | Mean number of weaned kittens | % pre-weaning kitten mortality |
|---|---|---|---|---|
| Bengal | 4.3 | 9.2 | 3.2 | 19 |
| MC | 5.5 | 18.8 | 4.3 | 8 |
| NFC | 4.5 | 5.2 | 4 | 6 |
| Persian | 3.7 | 12.1 | 2.7 | 20 |
| Total ± SD | 4.2 ± 1.8 | 11.8 | 3.2 ± 1.7 | 14 |
MC = Maine Coon; NFC = Norwegian Forest Cat
Figure 5.
Distribution of litters size within each of four breeds of cats raised in Italy. Data collected through questionnaires. NFC = Norwegian Forest Cat
Figure 6.
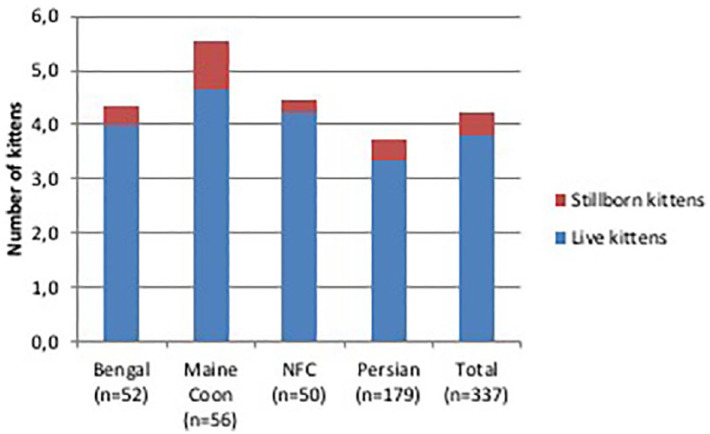
Number of live born vs stillborn kittens in 337 litters of four breeds of cats raised in Italy. Data collected through questionnaires. NFC = Norwegian Forest Cat
Stillbirths
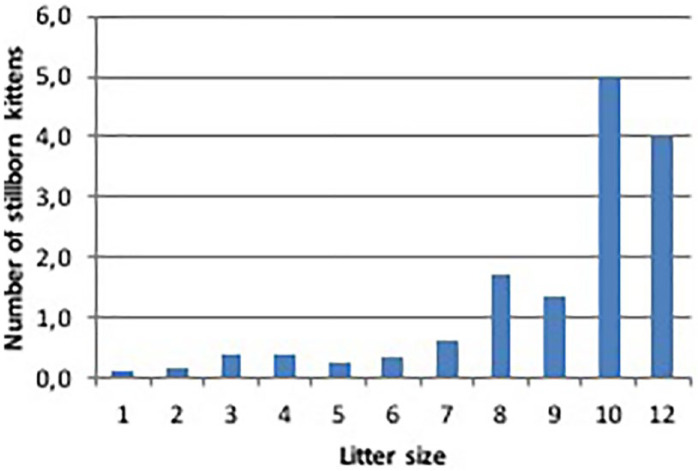
Stillbirths averaged 11.8% across the four breeds: their incidence in Maine Coon (0.87 ± 0.2) was significantly higher than in other breeds (P <0.05). Approximately 74% of the litters had no report of stillbirth, while at least three stillbirths were reported in 3.3% of the litters (Table 2). The number of stillborn kittens was positively correlated (r = 0.32) with litter size (P <0.01): 9% of stillborn kittens were reported in litters with <seven kittens, while an incidence of 22% of stillborn kittens was reported in litters with >eight kittens (Figure 7). Litters born through CS were characterised by a greater number of stillborn kittens (32%) than in natural queenings (11%) (Table 4).
Figure 7.
Pearson’s correlation between litter size and stillbirths in 337 litters of four breeds of cats raised in Italy. Data collected through questionnaires
Table 4.
Parturition, litter size and kitten mortality in natural vs caesarean section (CS) delivery on four breeds of purebred cats raised in Italy
| Breed | Mean age of the queen at delivery (years) |
Litter size |
% stillborn kittens |
Pre-weaning kitten mortality (%) |
||||
|---|---|---|---|---|---|---|---|---|
| Natural delivery | CS | Natural delivery | CS | Natural delivery | CS | Natural delivery | CS | |
| MC | 2.2 | 3.5 | 5.4 | 7 | 13 | 43 | 6 | 11 |
| Persian | 3.5 | 2.4 | 3.7 | 5.2 | 10 | 23 | 17 | 39 |
| Total ± SD | 3.2 ± 1.8 | 3.2 ± 1.7 | 4.1 ± 1.8 | 5.9 ± 2.4 | 11 | 32 | 14 | 25 |
MC = Maine Coon. Caesarian section was not reported by owners of the Norwegian Forest Cat and Bengal
Pre-weaning mortality
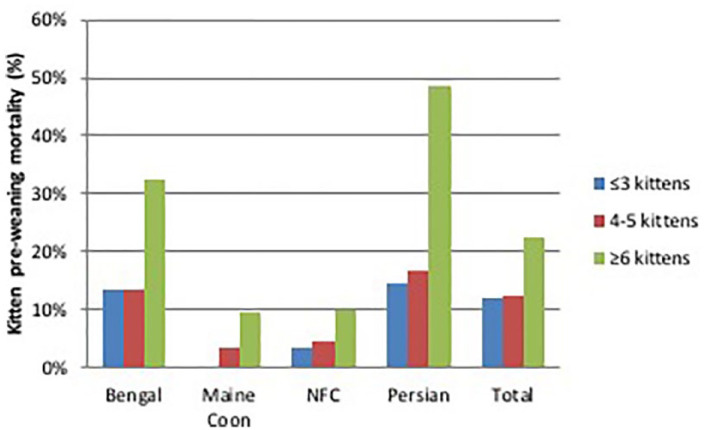
Mortality between birth and weaning averaged 14%: it was lowest in Norwegian Forest Cats (6%) and highest in Persians (20%) (Table 2). It was positively correlated (r = 0.14) with increasing litter size (P <0.05), being 12% in litters with <five kittens, and 21% in litters with >six kittens (Figure 8).
Figure 8.
Pearson’s correlation between litter size and pre-weaning mortality in 337 litters of four breeds of cats raised in Italy. Data collected through questionnaires. NFC = Norwegian Forest Cat
Caesarean sections
Ten of 337 births (3%) occurred via CS. They were reported in eight queens: four Maine Coons and four Persians; one of these gave birth via CS three times. Data about CSs and the comparison between CS and natural deliveries are shown in Table 4.
Statistics
Significant differences obtained with ANOVA and further confirmed with the Tukey’s test are reported in Table 5 (all queens), Table 6 (Bengal and Norwegian Forest Cat breeds) and Table 7 (Maine Coon and Persian breeds).
Table 5.
Correlations between all the reproductive indexes in the breedings examined
| Queen’s weight | Queen’s age | Parity | Gestation length | Kittens born | Kittens born alive | Stillborn kittens | Weaned kittens | Pre-weaning kitten mortality | CS | |
|---|---|---|---|---|---|---|---|---|---|---|
| Queen’s weight | – | −0.122 ‡ | NS | NS | 0.32* | 0.1* | 0.242* | 0.26* | −0.163 † | NS |
| Queen’s age | – | – | 0.825* | NS | −0.187* | −0.206* | NS | −0.181* | NS | NS |
| Parity | – | – | – | NS | −0.145* | −0.15* | NS | −0.126 † | NS | NS |
| Gestation length | – | – | – | – | NS | NS | NS | 0.161 † | −0.271* | NS |
| Kittens born | – | – | – | – | – | 0.857* | 0.318* | 0.628* | 0.136 † | 0.165* |
| Kittens born alive | – | – | – | – | – | – | −0.217* | 0.808* | NS | NS |
| Stillborn kittens | – | – | – | – | – | – | – | −0.298* | 0.12 † | 0.271* |
| Weaned kittens | – | – | – | – | – | – | – | – | −0.462* | NS |
| Pre-weaning kitten mortality | – | – | – | – | – | – | – | – | – | 0.116 † |
| CS | – | – | – | – | – | – | – | – | – | – |
P <0.01
0.01< P <0.05
0.05< P <0.1
CS = caesarean section; NS = not significant
Table 6.
Correlations between all the reproductive indexes in Bengals and Norwegian Forest Cat queens
| ← | Bengals | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Queen’s weight | Queen’s age | Parity | Gestation length | Stillborn kittens | Kittens born alive | Stillborn kittens | Weaned kittens | Pre-weaning kitten mortality | ↓ | ||
| Queen’s weight | – | 0.395 † | NS | NS | NS | NS | NS | NS | −0.454 † | ||
| Queen’s age | −0.316 ‡ | – | 0.7* | 0.314 ‡ | NS | NS | NS | NS | NS | ||
| Parity | NS | 0.701* | – | NS | NS | NS | NS | 0.238 ‡ | NS | ||
| Gestation length | NS | NS | NS | – | NS | NS | NS | 0.344 † | −0.456* | ||
| Kittens born | NS | NS | NS | −0.436* | – | 0.879* | 0.355* | 0.571* | NS | ||
| Kittens born alive | NS | NS | −0.298* | −0.365* | 0.886* | – | NS | 0.719* | NS | ||
| Stillborn kittens | NS | NS | NS | NS | NS | −0.506* | – | NS | NS | ||
| Weaned kittens | NS | NS | −0.334 † | NS | 0.784* | 0.906* | −0.493* | – | −0.579* | ||
| ↑ | Pre-weaning kitten mortality | NS | NS | NS | NS | NS | NS | NS | −0.247 ‡ | – | |
| NFC → | |||||||||||
P <0.01
0.01< P <0.05
0.05< P <0.1
CS = caesarean section; NS = not significant
Table 7.
Correlations between all the reproductive indexes in Persian and Maine Coon queens
| ← | Persian | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Queen’s weight | Queen’s age | Parity | Gestation length | Kittens born | Kittens born alive | Stillborn kittens | Weaned kittens | Pre-weaning kittens mortality | CS | ↓ | ||
| Queen’s weight | – | 0.199 † | NS | NS | 0.45* | 0.302* | 0.307* | 0.236 † | NS | NS | ||
| Queen’s age | NS | – | 0.839* | 0.161 ‡ | −0.149 † | NS | NS | NS | NS | NS | ||
| Parity | NS | 0.762* | – | NS | NS | NS | NS | NS | NS | NS | ||
| Gestation length | NS | 0.388 † | 0.464 † | – | NS | NS | NS | 0.164 ‡ | −0.183 † | NS | ||
| Kittens born | NS | NS | NS | NS | – | 0.838* | 0.346* | 0.416 * | 0.272 * | 0.192 † | ||
| Kittens born alive | −0.322 † | NS | −0.271 † | NS | 0.825* | – | −0.223* | 0.653 * | 0.195 * | NS | ||
| Stillborn kittens | 0.344 † | 0.237 ‡ | 0.388* | NS | 0.269 † | −0.323 † | – | −0.378 * | 0.15 † | 0.182 † | ||
| Weaned kittens | −0.316 † | NS | NS | NS | 0.702* | 0.939* | −0.424* | – | −0.544 * | NS | ||
| Pre-weaning kitten mortality | NS | NS | −0.247 ‡ | NS | 0.404* | 0.267 † | 0.223 ‡ | NS | – | 0.16 † | ||
| ↑ | CS | NS | NS | 0.233 ‡ | NS | NS | NS | 0.428 * | NS | NS | – | |
| Maine Coon → | ||||||||||||
P <0.01
0.01< P <0.05
0.05< P <0.1
CS = caesarean section; NS = not significant
Discussion
This is the first retrospective study documenting reproductive patterns of pedigree cats in Southern Europe. Although the number of questionnaires that could be used was only 26 (23.6%), and we used a convenience sampling technique, the total number of queens (n = 128), heats (n = 565), litters (n = 337) and kittens (n = 1424) and the statistical significance of our results justifies the drawing of some considerations on reproductive patterns of Norwegian Forest Cats, Maine Coon, Bengal and Persian cats in a temperate Mediterranean climate (45° latitude north) and a comparison with feline reproductive physiology as it occurs at different climates.
The mean ± SD age of all queens was 3.3 ± 2.0 years, but was higher for Persian queens (4.7 ± 2.1 years). The average age of Persian queens reported in our study was 10 months older than that of Persian queens reported in a Canadian study, 3 and 18 months older than the age of Persian queens reported in a UK study. 9 A similar difference was observed for our Maine Coon queens, for which the average age was approximately 1 year older with respect to the age of Maine Coon queens bred in the UK. 9 Although age at puberty may play a role in this (see later), a higher average age might indicate a longer breeding career for the queens in our study, an observation that appears to be confirmed by the difference in percentage of our primiparous queens (20.5%) when compared with the UK study (36.9%). 9
Reasons for increased breeding longevity in the queens of our study are unclear. In production animals, breeding longevity is influenced by various factors such as age at first parturition, nutrition, general management or degree of sanitation of breeding premises. In cat breeding factors such as frequency of queening and incidence of spontaneous ovulation may have an effect. The role of climate and photoperiod in breeding longevity in cats has not been studied. Breeding longevity is also an important trait for cats: careful reproductive management may allow cat breeders to continue to exploit the fertility of their breeding queens beyond the young and adult age.
The mean ± SD age at puberty in our study was 12.3 ± 7.4 months, slightly older than the age of 9–10 months reported previously.2–4 Heats were observed throughout the year, although 67% of cycles (n = 383/569) occurred between January and June, a feature already reported for queens from North and South America.4,11 The considerable percentage of cycling queens between July and December in our study (33%) might be due to the small difference (7 h) between the amount of daylight during increasing and decreasing daylight periods during the year: at our latitude, 7 h of difference are probably not enough to cause a block in cyclicity but may increase the duration of anoestrus during the months of November and December with respect to the rest of the year, as observed. 11
Mean ± SD duration of interoestrus was 39.3 ± 17 days, which was longer than the 22.1 ± 21.6 days reported by Root et al. 8 Our criterion to discard from this calculation intervals ⩾60 days was based on the observation that queens may ovulate spontaneously,12,13 after which they may maintain a luteal phase of approximately 40 days’ duration:8,14–16 the longest normal feline interoestrus (considering the maximum duration of oestrus and non-pregnant luteal phase of 19 days8,17,18 and 40 days,12,13 respectively) should not exceed 60 days. A long interoestrus interval may also be due to a short phase of anoestrus occurring during the breeding season.
Spontaneous ovulation has been reported to occur in 35–87% cats, depending on how frequently and with what intensity queens are petted or exposed to a tom cat or other visual, tactile or olfactory stimuli.12,13,19 This phenomenon has only been studied in domestic shorthair queens and potential relationships with seasonality have not been investigated. No information is available on the incidence of spontaneous ovulation in any of the breeds investigated in our study. The long duration of interoestrus in the queens of our study may indicate that some of these queens might ovulate spontaneously during certain seasons of the year. Spontaneous ovulation and anoestrus during the breeding season are worth exploring in purebred queens, as a high incidence of these conditions may influence the development of uterine disease due to the prolonged endometrial stimulation, similarly to what occurs in the bitch.
Gestation length was calculated based on the interval between the first day of breeding and the day of parturition. Mean ± SD duration of pregnancy (64.7 ± 2.4 days) and range of gestation length (59–76 days) in the queens in our study were similar to what has previously been reported for domestic cat queens.1,2,5,7–9,11 Gestation length was slightly longer for Norwegian Forest Cats (66 days) when compared with cats of the other three breeds (64 days, P <0.05), and shorter with increasing litter size. Our findings on correlations of the duration of pregnancy with breed and litter size are in agreement with what has been reported in larger studies from Northern Europe.9,10 Parturitions occur throughout the year and are concentrated during the months with a longer photoperiod (April–September), similarly to what has been reported in Sweden, 10 where queenings occur mainly from March to July. In an Australian study, Persian cat litters were not recorded during the month of June (equivalent to December in the Northern Hemisphere). 5
The mean ± SD litter size in our study of 4.2 ±1.8 kittens was in agreement with previous reports of 4.6 9 and 3.7 kittens.2,8,10 Breed had a significant effect on litter size, with Maine Coons producing more kittens (5.5 ± 2.3) than Persians (3.7 ± 1.4).2,5,8–10 Litter size was negatively correlated with the age of the queen, with females younger than 36 months producing larger litters than older queens.
The incidence of stillbirth was positively correlated with litter size and was, on average, 11.8%, which is in agreement with previously reported values of 4.7–11.6%.2,3,8–10 Maine Coons had the highest incidence of stillbirths (18.8%, n = 49/207) and not Persian, as previously reported.3,9 The percentage of kittens weaned in our study of 85% (n = 1084/1276) is in line with previous reports (71–92%).2,3,8–10 Also our recorded percentage of neonatal death was in agreement with what has already been reported, with slightly lower values in Persian (20%, n = 120/596) and Maine Coon (8%, n = 20/261) cats when compared with studies in the UK.3,9 Similarly to stillbirth, postnatal mortality in our study was positively correlated with litter size, with a significant increase in litters composed of >6 kittens.
CSs were reported only in 3% (n = 10/337) of parturitions, which is slightly lower than the 7–8% reported in the UK or Swedish studies.9,10 Unlike British cats in which CSs were in one study reported to be performed only in Persians (incidence of 6.6%), 9 in our study CSs were reported in Persians (3.4%, n = 6/179 litters) and also Maine Coons (7.1%, n = 4/56 litters).
Conclusions
This study documents the fertility parameters of breeding cats raised at a latitude of 45° north. The queen is a seasonal animal and, as such, its reproductive cyclicity is influenced by photoperiod, which varies with season. Therefore, feline reproductive management should probably be adjusted for latitude. From this perspective, it is important to have information on fertility parameters from different parts of the globe as latitude may have an impact on certain aspects of feline reproduction. 20 In addition, knowing the reproductive characteristics of every breed at a different latitude can determine the choice of breeds for cat breeders.
When comparing reproductive data of queens raised in Northern Italy to Canadian and UK queens, the main differences in our queens were an older age at puberty and a longer duration of interoestrus, while other parameters did not differ greatly. Duration of interoestrus is influenced by the occurrence of spontaneous ovulation, as well as short periods of anoestrus. Spontaneous ovulation has been studied in domestic shorthair queens but not in different breeds, while the occurrence of short periods of anoestrus during the breeding season has never been investigated in domestic queens. Whether or not both events can occur and how frequently these may happen in different breeds is worth investigating as repeated progesterone stimulations may have an effect from a reproductive health perspective on the uterus and/or mammary glands. Reproductive management might be improved through the identification of a spontaneous luteal phase using serum progesterone assay, thus improving fertility in individual queens.
Acknowledgments
The authors would like to thank the breeders who participated in this study
Footnotes
Accepted: 18 December 2018
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Lluis Ferré Dolcet  https://orcid.org/0000-0002-4994-768X
https://orcid.org/0000-0002-4994-768X
References
- 1. Prescott CW. Reproductive patterns in the domestic cat. Aust Vet J 1973; 49: 126–129. [DOI] [PubMed] [Google Scholar]
- 2. Jemmet JE, Evans JM. A survey of sexual behavior and reproduction of female cats. J Small Anim Pract 1977; 18: 31–37. [DOI] [PubMed] [Google Scholar]
- 3. Povey RC. Reproduction in the pedigree female cat. A survey of breeders. Can Vet J 1978; 19: 207–213. [PMC free article] [PubMed] [Google Scholar]
- 4. Lofstedt RM. The estrous cycle of the domestic cat. Compend Contin Educ Pract Vet 1982; 4: 52–58. [Google Scholar]
- 5. Johnstone I. Reproductive patterns of pedigree cats. Aust Vet J 1978; 64: 197–200. [DOI] [PubMed] [Google Scholar]
- 6. Gruffydd-Jones TJ. Some aspects of reproduction in cats. Adv Small Anim Pract 1988; 7: 68–77. [Google Scholar]
- 7. Munday HS, Davidson HPB. Normal gestation lengths in the domestic shorthair cat (Felis domesticus). J Reprod Fertil 1993; Suppl 47: 559.8410825 [Google Scholar]
- 8. Root MV, Johnston SD, Olson PN. Estrous length, pregnancy rate, gestation and parturition lengths, litter size, and juvenile mortality in the domestic cat. J Am Anim Hosp Assoc 1995; 31: 429–433. [DOI] [PubMed] [Google Scholar]
- 9. Sparkes AH, Rogers K, Henley WE, et al. A questionnaire-based study of gestation, parturition and neonatal mortality in pedigree breeding cat in the UK. J Feline Med Surg 2006; 8: 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ström Holst BS, Frössling J. The Swedish breeding cat: population description, infectious disease and reproductive performance evaluated by a questionnaire. J Feline Med Surg 2009; 11: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faya M, Carranza A, Priotto M, et al. Domestic queens under natural temperate photoperiod do not manifest seasonal anestrus. Anim Reprod Sci 2011; 129: 78–81. [DOI] [PubMed] [Google Scholar]
- 12. Lawler DF, Johnston SD, Hegstad RL, et al. Ovulation without cervical stimulation in domestic cats. J Reprod Fertil Suppl 1993; 47: 57–61. [PubMed] [Google Scholar]
- 13. Gudermuth DF, Newton L, Daels P, et al. Incidence of spontaneous ovulation in young, group-housed cats based on serum and faecal concentrations of progesterone. J Reprod Fertil Suppl 1997; 51: 177–184. [PubMed] [Google Scholar]
- 14. Verhage HG, Beamer NB, Brenner RM. Plasma levels of estradiol and progesterone in the cat during polyestrus, pregnancy and pseudopregnancy. Biol Reprod 1976; 14: 579–585. [DOI] [PubMed] [Google Scholar]
- 15. Paape SR, Shille VM, Seto H, et al. Luteal activity in the pseudopregnant cat. Biol Reprod 1975; 13: 470–474. [DOI] [PubMed] [Google Scholar]
- 16. Shille VM, Stabenfeldt GH. Luteal function in the domestic cat during presudopregnancy and after treatment with prostaglandin F2alpha. Biol Reprod 1979; 21: 1217–1223. [DOI] [PubMed] [Google Scholar]
- 17. Wildt DE, Chan SYW, Seager SWJ, et al. Ovarian activity, circulating hormones, and sexual behavior in the cat. I. Relationships during the coitus-induced luteal phase and the estrous period without mating. Biol Reprod 1981; 25: 15–28. [DOI] [PubMed] [Google Scholar]
- 18. Schmidt PM, Chakraborty PK, Wildt DE. Ovarian activity, circulating hormones, and sexual behavior in the cat. II. Relationships during pregnancy, parturition, lactation and the postpartum estrus. Biol Reprod 1983; 28: 657–671. [DOI] [PubMed] [Google Scholar]
- 19. Pelican KM, Brown JL, Wildt DE, et al. Short term suppression of follicular recruitment and spontaneous ovulation in the cat using levonorgestrel versus a GnRH antagonist. Gen Comp Endocrin 2005; 144: 110–121. [DOI] [PubMed] [Google Scholar]
- 20. Pereira da, Silva TF, Machado da, Silva LD, Couto Ochoa D, et al. Sexual characteristics of domestic queens kept in a natural equatorial photoperiod. Theriogenology 2006; 66: 1476–1481. [DOI] [PubMed] [Google Scholar]