Abstract
Objectives
The aim of this retrospective study was to describe the spinal anaesthesia (SA) technique and evaluate the incidence of perioperative complications in cats.
Methods
The anaesthetic records of cats of American Society of Anaesthesiologists physical status I, II and III, which received general and SA for different surgeries between 2012 and 2016, were examined. SA was administered through a 25 G Quincke needle, using an isobaric solution of bupivacaine and morphine at the level of either the L7–S1 interspaces (sternal recumbency) or the L5–6/L6–7 interspaces (lateral recumbency). Procedural failure rate (PFR), drugs and dose used, heart rate (HR), arterial blood pressure, incidence of bradycardia (HR <100 bpm) and hypotension (mean arterial pressure [MAP] <60 mmHg for at least 5 mins), intraoperative rescue analgesia (iRA) and any other detrimental events and their treatment until discharge were recorded. Abdominal surgery cases were excluded from the intraoperative evaluation.
Results
A total of 58 anaesthetic records met the inclusion criteria and were analysed. PFR related to the space of injection (L7–S1 vs L5–6/L6–7) was 3/11 (27%) and 1/47 (2%), respectively (P = 0.017). The total median dose of intrathecal bupivacaine and morphine was 0.8 (range 0.5–1.6 mg/kg) and 0.10 (0.05–0.18 mg/kg), respectively. Nine of 46 (20%) cats received iRA, and no iRA cases were reported with a dose of bupivacaine higher than 0.8 mg/kg. Median HR and MAP before intrathecal injection (T0) and 10 mins after (T1) were, respectively, 118 bpm (range 74–190 bpm) and 106 bpm (67–160 bpm) (P = 0.005), and 65 mmHg (range 50–94 mmHg) and 52 mmHg (range 35–85 mmHg) (P = 0.003). Bradycardia was reported in 18/46 (39%) cats and hypotension in 23/46 (50%) cats. No complications were recorded during the observation period.
Conclusions and relevance
SA was characterised by a low PFR when performed at the L5–6/L6–7 interspaces and low postoperative complications. Hypotension and bradycardia were the most common side effects.
Keywords: Spinal anaesthesia, bupivacaine, hypotension, bradycardia
Introduction
Spinal anaesthesia (SA) is a well-established regional technique, which results in altered nerve function by deposition of local anaesthetics (LAs) and/or other drugs in the subarachnoid space. Of the regional anaesthetic techniques, SA exhibits some remarkable characteristics, such as an excellent quality of nerve block, ease of performance, reliability and economical affordability compared with other regional techniques. 1
Even though SA in dogs was reported for the first time in 1901 by Cuillé and Sendrail, 2 only 2 years after the first reported SA in humans by Bier in 1899, 3 this technique has never been extensively used in veterinary clinical practice. Small animals were commonly adopted as experimental animals in the 1980s and 1990s in studies that aimed for a better understanding of the pharmacokinetics and pharmacodynamics of various drugs administered into the cerebrospinal fluid (CSF).4,5 Nevertheless, the first study that attempted to characterise the clinical use of SA in small animals was only published in 2011, evaluating the use of SA in dogs in various types of surgical procedures. 6 This and other studies that followed have confirmed in dogs what was known and accepted in humans about the effectiveness and good safety profile of this technique.7–9 SA may also have a lower procedural failure rate than epidural anaesthesia, a better quality nerve block and a faster motor function recovery time, as was shown in dogs undergoing hindlimb orthopaedic surgeries. 10
While SA has been clinically characterised in dogs, data in cats are lacking. Viscasillas et al 11 described an SA in a cat undergoing a unilateral pelvic fracture with a hypobaric solution of ropivacaine and morphine. Other authors have reported complications, relative to possible intrathecal injection of bupivacaine and/or morphine, such as pruritus and total spinal block in cat.12–14
The aim of this retrospective study was to describe the spinal technique, and evaluate the requirement for intraoperative rescue analgesia (iRA) and the perioperative complications in cats.
Materials and methods
A retrospective analysis was performed reviewing the medical and anaesthetic records of all cats in which elective SA with isobaric bupivacaine and morphine solution for caesarean section, caudal abdomen and hindlimb surgery was attempted between January 2012 and December 2016 at Centro Veterinario Fossanese and at Veterinary Teaching Hospital University of Padua. Case records with complete information (reported in Appendix 1 in the supplementary material) were included. Abdominal surgeries were excluded from the intraoperative evaluation.
Data on patients, drugs and dose used for premedication, induction and maintenance of anaesthesia, as well as any other drug administered perioperatively, any other relevant perioperative events and their management, were recorded.
Bradycardia was defined as a heart rate (HR) of <100 beats/min and hypotension as a mean arterial pressure (MAP) <60 mmHg for at least 5 mins or systolic arterial pressure (SAP) <80 mmHg for at least 5 mins. Every administration of fentanyl intraoperatively found in the anaesthesia record was recorded as an iRA.
HR and MAP values were also recorded before intrathecal injection (T0) and 10 mins after (T1), in order to describe the cardiovascular variation due to SA.
SA was performed with two techniques: a median approach at the L7–S1 intervertebral space, and a paramedian approach with cephalad angulation at the L6–7/L5–6 intervertebral spaces. The skin proximal to the intervertebral space where the needle was to be inserted was clipped and aseptically prepared.
When the median approach at L7–S1 intervertebral space was executed, the cat was positioned in sternal recumbency, with the hindlimbs pulled forward, and the spinal needle perpendicularly inserted into the skin, between the dorsal spinous process of L7 and S1. The needle was advanced until the tip was felt passing through the ligamentum flavum. If the needle hit the vertebral bone, it was withdrawn, repositioned and then advanced again.
When the paramedian approach with cephalad angulation was performed, the cat was positioned in lateral recumbency in such a way that the back, kept slightly flexed, protruded over the edge of the table. The needle was inserted on the dependent side, lateral to the caudal margin of the dorsal spinous process of the vertebra and caudal to the intervertebral space to be punctured. The needle was directed in a slightly ventral, cranial and medial direction aiming for the vertebral lamina. When the needle hit the lamina, the operator withdrew the needle and then advanced it again with a more cephalad angulation until the tip of the needle was felt passing through the ligamentum flavum.
Once the ligamentum flavum was pierced, the stylet was removed from the needle and its hub checked for the presence of CSF. With the stylet repositioned, the needle was slightly advanced, and often a sensation of piercing a sheet of paper indicated having pierced the dura and arachnoid membranes.
A room temperature solution of preservative-free isobaric bupivacaine 0.5% (Bupivacaina; Angelini) and morphine 1% (Morfina; Molteni) was injected at approximately 1 ml/min to all cats, while the operating table was maintained in a horizontal position. When CSF flow was not evident in the hub of the needle, LA solution was not injected and the case was recorded as a procedural failure.
The bladder was expressed at the end of the surgical procedure.
Postoperative pain was evaluated with the Colorado State University feline acute pain scale, as the cats were sufficiently conscious to respond to stimulations. Rescue analgesia (Buprenodal,10 μg/kg IM) was administered if the cat reached a score ≥2.
The presence of signs of nausea or vomiting, urinary retention (inability to spontaneously void urine in the presence of a distended bladder), pruritus, prolonged motor block or postoperative rescue analgesia treatment, as well as any other major complications until discharge, were extrapolated from the clinical records.
Statistical analysis
Categorical variables were reported as frequencies and percentages, and differences between groups were analysed using Fisher’s exact test. Continuous variables were checked for normal distribution by visual inspection of bar graphs and histograms and by using the Shapiro–Wilk test. Data not normally distributed are reported as the median and the range (minimum–maximum), and the differences analysed using the Mann–Whitney U-test. Odds ratios and 95% confidence intervals (CI) for univariate analysis of independent predictors were calculated using logistic regression. The significance level was set at 5% for all statistical methods (MedCalc Software for Windows version 12.5).
Results
Fifty-eight records of cats undergoing caesarean section, caudal abdomen and hindlimb surgery meeting the inclusion criteria were retrieved. However, a total of 54 anaesthesia records were analysed, as four cases were excluded from (intraoperative and postoperative) evaluations owing to procedural failure. Demographic data, area of surgery and perioperative records of the enrolled population of cats are reported in Tables 1 and 2.
Table 1.
Demographic data and area of surgery of the analysed cases
| Data | Quantity |
|---|---|
| Number of cats | 54 |
| Breed | 53 European, 1 Sphynx |
| Sex | 28 F, 26 M |
| Median (range) age (months) | 24 (3–168) |
| Median (range) body weight (kg) | 4 (1.6–7.2) |
| Median (range) ASA status | 2 (1–3) |
| Hindlimb surgery* | 46/54 (85) |
| Cystotomy with or without urethrostomy* | 6/54 (11) |
| Caesarean section* | 2/54 (4) |
Numerosity of the group over the total number of collected cases (%)
F = female; M = male; ASA = American Society of Anesthesiologists
Table 2.
Perioperative records
| Category | Information extracted | Results |
|---|---|---|
| Pre-anaesthetic medication used (n) | Not premedicated | 41 |
| Premedicated (IM) Dexmedetomidine 5 (6–10) µg/kg + ketamine 1 (1–2 mg/kg) + methadone 0.2 mg/kg | 13 | |
| General anaesthesia | Co-inductor agent (IV) | |
| Fentanyl 3 (2–5) µg/kg | 23 | |
| Remifentanil 0.5 µg/kg/min | 2 | |
| Induction agent (IV) | ||
| Propofol to effect 4 (3–5) mg/kg | 54 | |
| Maintenance of anaesthesia | ||
| Isoflurane (et%) 0.9 (0.6–1.2) | 10 | |
| Sevoflurane (et%) 1.8 (1.7–2) | 6 | |
| CRI propofol 15 (10–20) mg/kg/h | 38 | |
| Spinal anaesthesia | Total procedural failure rate | 4/58 (7) |
| Procedural failure related to space of puncture | ||
| Procedural failure at L7–S1 | 3/11 (27) | |
| Procedural failure at L6–7/L5–6 | 1/47 (2) | |
| Dose of LA and adjuvant | ||
| Bupivacaine 0.5% (mg) | 3.0 (1.8–5.8) | |
| Bupivacaine 0.5% (mg/kg) | 0.8 (0.5–1.6) | |
| Morphine 1% (mg) | 0.3 (0.2–0.6) | |
| Morphine 1% (mg/kg) | 0.10 (0.05–0.18) | |
| Timing (mins) | Time from intrathecal injection to surgical incision | 15 (8–30) |
| Time from intrathecal injection to the end of surgery | 70 (25–190) | |
| Intraoperative complications (n) | iRA in HLS | 9/46 (20) |
| In premedicated cats | 0/13 (0) | |
| In not premedicated cats | 9/33 (27) | |
| Hypotension in HLS | 23/46 (50) | |
| In not premedicated cats | 20/33 (61) | |
| In premedicated cats | 3/13 (23) | |
| Bradycardia in HLS | 18/46 (39) | |
| Bradycardia in not premedicated cats | 12/33 (36) | |
| Bradycardia in premedicated cats | 6/13 (46) | |
| Use of vasoactive drugs | ||
| Atropine 20 g/kg | 18/46 (39) | |
| Ephedrine 50 g/kg | 6/46 (13) | |
| Noradrenaline 0.5 µg/kg/min | 1/46 (2) | |
| Postoperative complications (n) | Urinary retention | 0/54 (0) |
| Itching | 0/54 (0) | |
| Prolonged motor block | 0/54 (0) | |
| Vomiting | 0/54 (0) | |
| Other complications | 0/54 (0) | |
| Postoperative analgesia administration (n) | Preventive NSAID | 54/54 (100) |
| Preventive buprenorphine (10 µg/kg IM) at the end of surgery | 7/54 (13) | |
| Postoperative rescue analgesia | 5/54 (9) |
Data are median (range) or n (%)
CRI = continuous rate infusion; LA = local anaesthetic; iRA = intraoperative rescue analgesia; HLS = hindlimb surgery; NSAID = non-steroidal anti-inflammatory drug; et% = end-tidal concentration
General anaesthesia
Forty-one cats were not premedicated (76%), 23 of which received fentanyl IV (Fentadon; Eurovet Animal Health BV) and two received remifentanil (Ultiva; GlaxoSmithKline) as co-induction agents 3 mins before the administration of the main induction agent. Thirteen cats (24%) were premedicated with an IM mix of ketamine (Imalgene; Merial), dexmedetomidine (Dexdomitor; Orion Pharma) and methadone (Synthadon; Produlab Pharma). Induction of general anaesthesia was performed with propofol (Propovet; Zoetis) to effect and maintained either with a propofol variable rate infusion in 38/54 (70%) cats or with volatile anaesthetic agents in 16/54 (30%) cats, using isoflurane (IsoFlo; AesicaQueenborogh) or sevoflorane (Sevoflo; Aesica Queenborough), respectively, in 10 and six cases. Forty-one (76%) cats were mechanically ventilated and 13/54 (24%) were breathing spontaneously. Anaesthetic records reported the following minimum patient monitoring: electrocardiography pulse oximetry, non-invasive blood pressure (oscillometric technique), measurement of inspired–expired carbonic anhydrase, inhalant agent and oxygen concentration.
Spinal anaesthesia
The overall procedural failure rate was 4/58 (7%). SA was attempted in 47 cases at L6–7/L5–6 intervertebral spaces using the paramedian approach with cephalad angulation, and in 11 cats at the L7–S1 intervertebral space using the median approach. In 1/47 (2%) cats during paramedian approach and in 3/11 (27%) cats during the median approach, the correct execution of the technique could not be performed for up to three attempts and, consequently, SA was aborted (P = 0.017).
The median dose of bupivacaine was 3.0 mg (range 1.8–5.8 mg) in total or 0.8 mg/kg (range 0.5–1.6 mg/kg). The median dose of morphine was 0.30 mg (range 0.2–0.60 mg) in total or 0.1 mg/kg (range 0.05–0.18 mg/kg).
iRA
iRA in cats undergoing hindlimb orthopaedic surgeries was administered in 9/46 (20%) cats as a single or multiple bolus of fentanyl at 1–2 µg/kg IV. None of 13 premedicated cats received iRA, while 9/33 (27%) non-premedicated cats undergoing the same procedures required iRA (P = 0.044).
In non-premedicated cats undergoing hindlimb orthopaedic surgeries (n = 33), the bupivacaine dose per body mass (mg/kg) was not a predictor of iRA, with a median bupivacaine dose of 0.7 mg/kg (range 0.5–0.8 mg/kg) and 0.7 mg/kg (range 0.5–1.6 mg/kg) for iRA and no-iRA groups, respectively (P = 0.19). All iRA events were related to a bupivacaine dose ⩽0.8 mg/kg, and no iRA events were reported with a dose of bupivacaine >0.8 mg/kg (Figure 1).
Figure 1.
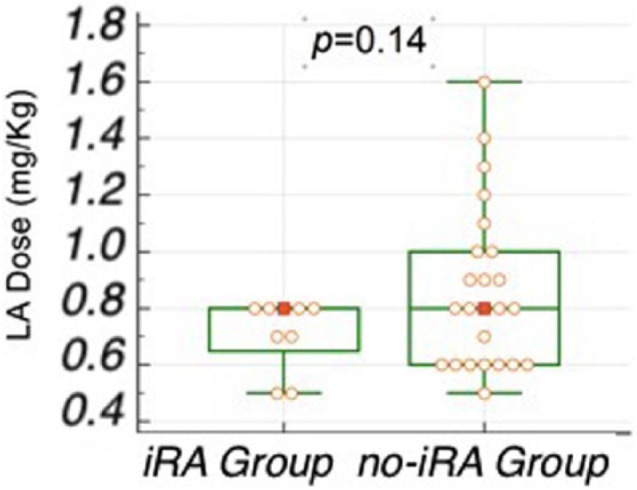
Intraoperative rescue analgesia (iRA) in cats undergoing hindlimb orthopaedic surgery not premedicated and not maintained with antinociceptive drugs (n = 33). The total dose of bupivacaine by body mass was not different between the iRA and no-iRA groups (P = 0.14). A dose of bupivacaine >0.8 mg/kg was associated with no cases of iRA.
LA = local anaesthetic
Intraoperative cardiovascular events
In cats undergoing hindlimb orthopaedic surgeries hypotension was reported in 23/46 (50%) cases: 12 cases were treated only by lightening the general anaesthetic plane and administering a bolus of Ringer’s lactated solution; five cases were treated with intravenous (IV) atropine (20 µg/kg), six with ephedrine IV (50 µg/kg) and one with noradrenaline IV (0.5 µg/kg/min).
Bradycardia was recorded in 18/46 (39%) cases, and five of these were associated with hypotension. All of these cases were treated with atropine IV (20 µg/kg). The incidence of hypotension in non-premedicated subjects was 20/33 (61%), while in premedicated cats it was 3/13 (23%) (P = 0.047). The incidence of bradycardia was 12/33 (36%) and 6/13 (46%) (P = 0.73), respectively, in non-premedicated and in premedicated cats.
HR and MAP were analysed at T0 and T1, only in 33 non-premedicated cats, maintained under anaesthesia with total IV anaesthesia of propofol and undergoing orthopaedic procedures on the hindlimbs. Median HR at T0 and T1 was 118 bpm (range 74–190 bpm) and 106 bpm (range 67–160 bpm), respectively (P = 0.005); median MAP at T0 and T1 was 65 mmHg (range 50–94 mmHg) and 52 mmHg (35–85 mmHg), respectively (P = 0.003) (Figure 2).
Figure 2.
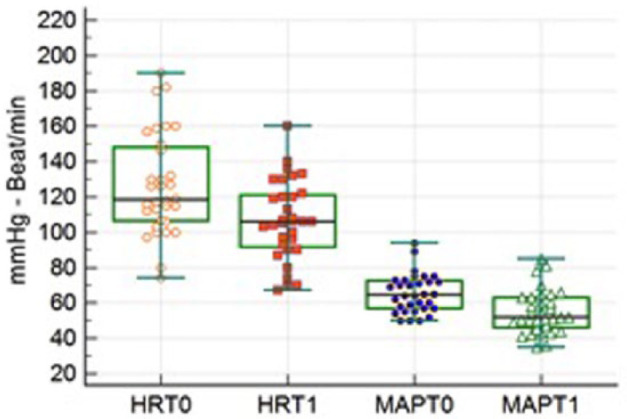
Box plot of mean arterial pressure (MAP) and heart rate (HR) before (T0) and 10 mins after (T1) intrathecal injection. HR and MAP decreased after intrathecal anaesthesia (P = 0.005 and P = 0.003, respectively); dots refer to the value of individual cases. The whiskers show the range values, the width of the box shows the interquartile range and the bar in the box is median value
Postoperative complications and management
Patients were kept under observation for a median time of 7 h (range 5–36 h) after surgery. At the end of the procedure, all cats received 0.1 mg/kg of meloxicam (IV). Seven of nine cats that needed iRA received preventive buprenorphine 10 µg/kg IM at the end of surgery, regardless the pain scale, and five cats received 10 µg/kg IM of buprenorphine as rescue analgesia in the postoperative observation period.
No postoperative complications such as pruritus, urinary retention, vomiting, prolonged residual nervous block or any other neurological complications were recorded in the postoperative observation period. No signs of such complications were reported from the owner in the following days.
Discussion
SA performed in 54 cats caused neither major complications nor mortality. In the limited observation period minor side effects such as pruritus, urinary retention, vomiting and prolonged residual nervous block were similar to previously published data on SA and in other species.6,15 However, considering the limited observation time in this study, less noticeable complications, such as urinary retention, may have gone unobserved by the owner after hospital discharge.
The overall technical failure rate was similar to published data in dogs. 10 However, excluding the data from attempts on L7–S1, with a median approach the technical failure rate was much lower in cats (2%) than previously reported in dogs (ranging from 7–26%).6,10 In our opinion, this interesting finding may be due to several anatomical differences between the two species, making SA easier to perform in cats. Cats have less variability in spinal morphology, a greater ratio between the spinal cord and the vertebral canal at the site of injection, a smaller distance between the skin and dura mater, and a greater flexibility of the spine. These are substantial differences that may contribute to the discrepancy between cat and dog SA. 16
A greater procedural failure rate was related to the L7–S1 space of injection. Using this technique, the angle of the needle entering the vertebral canal is less predictable than the paramedian technique. When well executed, the paramedian technique allows the needle to enter the vertebral canal with a repeatable angulation. 17 Moreover, the median approach as described above requires the CSF to have enough pressure to reach the hub of the needle. The paramedian approach, by inserting the needle in the dependent side of the subject, may favour CSF outflow, even when low pressure is present. According to the data presented in this study, the paramedian approach with cephalad angulation, as described above, reducing SA procedural failures should be taken into account when performing SA in cats.
Even if it is not possible to find a relationship between the dose of LA and an iRA event in our data, we can speculate that a bupivacaine dose >0.8 mg/kg and morphine of 0.1 mg/kg could provide an effective anaesthetic block in a cat undergoing hindlimb surgery. At the beginning, the doses of LA and morphine were decided upon after looking at what the medium range published for dogs was (0.2–0.8 mg/kg for bupivacaine). 8 Then, in order to decrease the incidence of iRA the dose of LA was progressively increased towards the upper limit and beyond. Of note, premedicated cats did not require iRA; this finding may be a result of the extra analgesic effect of the drugs used in premedication, such as an alpha2-agonist and methadone.18,19 However, the small number of premedicated subjects analysed in this study does not allow further speculation.
Hypotension and bradycardia were the most common complications affecting cats undergoing SA. The overall incidence of hypotension due to the combined effect of general and SA in our study was 50%. The most likely cause of hypotension after SA is sympathetic blockade, which, in turn, decreases cardiac preload and causes vasodilation. 20 General anaesthesia with inhalational agents may substantially reduce the compensatory vasoconstriction found in awake human patients, where the sympathetic supply has not been blocked by the spinal anaesthesia. 21 The retrospective evaluation of HR before and 10 mins after spinal injection suggests that cats undergoing SA have a reduction in HR, which can contribute to hypotension, as reported in human patients. 1 Although arbitrary, 10 mins after subarachnoid injection can be considered an ideal time for observing the rapid cardiovascular changes induced by SA, because the subject, once repositioned after the injection, is then left unstimulated until the beginning of the surgery.
The abovementioned incidence of hypotension was similar to that reported in cats (55%) undergoing peripheral nerve block on hindlimbs under general anaesthesia, 22 even if the different anaesthetic protocols of the two studies cannot be overlooked. Indeed, approximately 70% of cats in our study were anaesthetised and maintained with propofol, while all cats in the above-cited study, were maintained with halogenated agents. Monitoring of arterial blood pressure was performed with a non-invasive oscillometric system. As widely reported in the literature, this method of blood pressure monitoring is not accurate, particularly in small and/or hypotensive subjects, and underestimation of blood pressure is a common finding. 23
From the retrospective analysis of the anaesthetic records, the intrathecal dose of morphine administered to cats ranged from 50–180 µg/kg. As already mentioned, pruritus has been described in cats as a complication of the administration of intrathecal morphine.12,13,24 However, no signs of pruritus or other clinical signs possibly related to morphine administration (such as dysphoria or hypersalivation) were recorded in the postoperative period of the cases involved in this retrospective study. Nevertheless, 70% of cats included in this study were anaesthetised and maintained with propofol, which may have had an antipruritic effect during recovery. This injectable anaesthetic is well known as a treatment for pruritus induced by spinal morphine administration.13,25
Postoperative pain was evaluated until discharge by experienced veterinarians. Only 12/54 (22%) cats received one dose of rescue analgesia in the postoperative period. The prolonged analgesic effect showed in this study by spinal analgesia in cats has been similarly published in dogs. 26 The profound nerve block at the time of surgery, which may limit central sensitisation, 27 and the prolonged analgesic effect of spinal morphine can explain this finding. 28 However, definitive conclusions on the postoperative analgesic effect of SA, as executed in this study, cannot be drawn because the vast majority of our surgical procedures were carried out in a day surgery regime, limiting the postoperative observation time. Moreover, the postoperative use of buprenorphine, as rescue analgesia, may have interfered with the morphine administered intrathecally, reducing its long-lasting analgesic effect. 29
This study also has other limitations. Its retrospective nature and the low number of cases do not allow for a thorough assessment of the safety of this technique in cats. An experienced clinician, familiar with the paramedian technique and the use of small-calibre spinal needles, performed all the punctures. A less experienced operator could encounter more difficulties and obtain different results. Different general anaesthesia protocols used in the cases analysed in this study represent a confounding factor.
Conclusions
SA was an easy-to-perform regional technique in cats, characterised by a low PFR when a paramedian approach at the L5–6/L6–7 interspaces was used. No iRA was recorded with dosing of bupivacaine >0.8 mg/kg along with morphine of 0.1 mg/kg in cats undergoing hindlimb surgeries. Hypotension and bradycardia were the most common intraoperative side effects. Future studies are required to better investigate the duration and the quality of postoperative analgesia.
Supplemental Material
Supplemental material, Supplementary_Appendix_1 for Combined spinal and general anaesthesia in 58 cats undergoing various surgical procedures: description of technique and retrospective perioperative evaluation by Diego Sarotti, Andrea Cattai and Paolo Franci in Journal of Feline Medicine and Surgery
Footnotes
Accepted: 25 November 2018
Supplementary material: The following file is available online: Appendix 1: Case records with complete information.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Diego Sarotti  https://orcid.org/0000-0003-3839-0334
https://orcid.org/0000-0003-3839-0334
References
- 1. Di Cianni S, Rossi M, Baradari AG, et al. Spinal anesthesia: an evergreen technique. Acta Biomed 2008; 79: 9–17. [PubMed] [Google Scholar]
- 2. Cuillé J, Sendrail M. Analgesiecocainique par voierachidienne. Rev Vet 1901; 26: 98–103. [Google Scholar]
- 3. Marx GF. The first spinal anesthesia. Who deserves the laurels? Reg Anesth 1994; 19: 429–430. [PubMed] [Google Scholar]
- 4. Mensink FJ, Kozody R, Wade JG, et al. Dose-response relationship of clonidine in tetracaine spinal anesthesia. Anesthesiology 1987; 67: 717–721. [DOI] [PubMed] [Google Scholar]
- 5. Jordan DA, Miller ED. Subarachnoid blockade alters homeostasis by modifying compensatory splanchnic responses to haemorrhagic hypotension. Anesthesiology 1991; 75: 654–661. [DOI] [PubMed] [Google Scholar]
- 6. Sarotti D, Rabozzi R, Corletto F. Efficacy and side effects of intraoperative analgesia with intrathecal bupivacaine and levobupivacaine: a retrospective study in 82 dogs. Vet Anaesth Analg 2011; 38: 240–251. [DOI] [PubMed] [Google Scholar]
- 7. Sarotti D, Rabozzi R, Franci P. A retrospective study of efficacy and side effects of intrathecal administration of hyperbaric bupivacaine and morphine solution in 39 dogs undergoing hind limb orthopaedic surgery. Vet Anaesth Analg 2013; 40: 220–224. [DOI] [PubMed] [Google Scholar]
- 8. Adami C, Casoni D, Spadavecchia C, et al. Addition of magnesium sulphate to ropivacaine for spinal analgesia in dogs undergoing tibial plateau levelling osteotomy. Vet J 2016; 209: 163–168. [DOI] [PubMed] [Google Scholar]
- 9. Romano M, Portela DA, Otero PE, et al. Stress-related biomarkers in dogs administered regional anaesthesia or fentanyl for analgesia during stifle surgery. Vet Anaesth Analg 2016; 43: 44–54. [DOI] [PubMed] [Google Scholar]
- 10. Sarotti D, Rabozzi R, Franci P. Comparison of epidural versus intrathecal anaesthesia in dogs undergoing pelvic limb orthopaedic surgery. Vet Anaesth Analg 2015; 42: 405–413. [DOI] [PubMed] [Google Scholar]
- 11. Viscasillas J, Sanchis-Mora S, Lafuente P, et al. Spinal anesthesia with hypobaric ropivacaine and morphine in a cat with a unilateral pelvic fracture. J Vet Sci Med Diagn 2015; 4: 5. DOI: 10.4172/2325-9590.1000178. [DOI] [Google Scholar]
- 12. Bauquier SH. Hypotension and pruritus induced by neuraxial anaesthesia in a cat. Aust Vet J 2012; 90: 402–403. [DOI] [PubMed] [Google Scholar]
- 13. Gent T, Iff I, Bettschart-Wolfensberger R, et al. Neuraxial morphine induced pruritus in two cats and treatment with sub anaesthetic doses of propofol. Vet Anaesth Analg 2013; 40: 517–520. [DOI] [PubMed] [Google Scholar]
- 14. Casoni D, Rohrbach H, Spadavecchia C. Total spinal anaesthesia following spinal lumbosacral injection of bupivacaine 0.5 per cent in a cat. Vet Rec Case Rep 2014; 2. DOI: 10.1136/vetreccr-2014-000117. [DOI] [Google Scholar]
- 15. Carpenter RL, Caplan RA, Wu R, et al. Incident and risk factors for side effects for spinal anaesthesia. Anesthesiology 1992; 76: 906–916. [DOI] [PubMed] [Google Scholar]
- 16. Otero PE, Campoy L. Epidural and spinal anesthesia. In Campoy L, Read M. (eds). Small animals and regional anesthesia and analgesia. New York: Wiley-Blackwell, 2013, pp 227–231. [Google Scholar]
- 17. Blomberg RG. Technical advantages of the paramedian approach for lumbar epidural puncture and catheter introduction. Anesthesiology 1988; 43: 837–843. [DOI] [PubMed] [Google Scholar]
- 18. Warne LN, Beths T, Bauquier SH, et al. Comparison of perioperative analgesic efficacy between methadone and butorphanol in cats. J Am Vet Med Assoc 2013; 243: 844–850. [DOI] [PubMed] [Google Scholar]
- 19. Porters N, Bosmans T, Polis I, et al. Sedative and antinociceptive effects of dexmedetomidine and buprenorphine after oral transmucosal or intramuscular administration in cats. Vet Anaesth Analg 2014; 41: 90–96. [DOI] [PubMed] [Google Scholar]
- 20. Sevarino F. Management of hypotension in obstetric anesthesia: is it time to rewrite the textbooks? Curr Opin Anaesthesiol 2003; 16: 249–251. [DOI] [PubMed] [Google Scholar]
- 21. Neumann C, Foster AD, Rovestine EA. The importance of compensating vasoconstriction in unanesthetized areas for the maintenance of blood pressure during spinal anaesthesia. J Clin Invest 1945; 24: 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vettorato E, Corletto F. Retrospective assessment of peripheral nerve block techniques used in cats undergoing hindlimb orthopaedic surgery. J Feline Med Surg 2016; 18: 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caulkett NA, Cantwell SL, Houston DM. A comparison of indirect blood pressure monitoring techniques in the anesthetized cat. Vet Surg 1998; 27: 370–377. [DOI] [PubMed] [Google Scholar]
- 24. McFadzean WJ, Holopherne-Doran D. Myoclonus and hypersensitivity of the tail following intrathecal administration of morphine and bupivacaine in a cat. Vet AnaesthAnalg 2018; 45: 238–239. [DOI] [PubMed] [Google Scholar]
- 25. Borgeat A, Wilder-Smith OH, Rifat K, et al. Subhypnotic doses of propofolrelieve pruritus induced by epidural and intrathecal morphine. Anesthesiology 1992; 76: 510–522. [DOI] [PubMed] [Google Scholar]
- 26. De Gennaro C, Vettorato E, Corletto F. Retrospective clinical evaluation of hypobaric spinal anaesthesia in dogs undergoing pelvic limb orthopaedic surgery. J Small Anim Pract 2014; 55: 497–503. [DOI] [PubMed] [Google Scholar]
- 27. Curatolo M, Petersen-Felix S, Zbinden AM, et al. Spinal anaesthesia inhibits central temporal summation. Br J Anaesth 1997; 78: 88–89. [DOI] [PubMed] [Google Scholar]
- 28. Kaczocha M, Azim S, Benveniste H, et al. Intrathecal morphine administration reduces postoperative pain and peripheral endocannabinoid levels in total knee arthroplasty patients: a randomized clinical trial. BMC Anesthesiol 2018; 18: 27. DOI: 10.1186/s12871-018-0489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zamani N, Hassanian-Moghaddam H, Assar N, et al. Reversal of opioid overdose syndrome in morphine-dependent rats using buprenorphine. Toxicol Lett 2014; 232: 590–594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Appendix_1 for Combined spinal and general anaesthesia in 58 cats undergoing various surgical procedures: description of technique and retrospective perioperative evaluation by Diego Sarotti, Andrea Cattai and Paolo Franci in Journal of Feline Medicine and Surgery


