Abstract
Objectives
The aim of this study was to design and carry out a preliminary evaluation of a urine point-of-care test for kidney injury molecule 1 (KIM-1) in healthy and diseased cats.
Methods
Part of the feline KIM-1 gene was amplified, ligated into a plasmid with a signal peptide and monomeric human IgGFc, and transfected into a mammalian cell line. Supernatant was purified and tested for the fusion protein by gel electrophoresis and Western blot. Mice were immunized three times with purified proteins, and hybridomas were generated from splenocytes. Antibodies were tested by ELISA for detection of recombinant KIM-1 and naturally occurring KIM-1 in disease-state urine. Next, a lateral flow assay (LFA) with capture and detection antibodies was constructed, and tested with 34 urine samples from healthy and diseased cats. Antibodies were also tested for reactivity with formalin-fixed paraffin-embedded kidney tissue.
Results
Three antibodies were assessed. Antibodies detected between 0.4 and 60 ng/ml feline KIM-1 fusion protein in the LFA. Urine samples from healthy cats yielded faint bands in the LFA corresponding to optical density (OD) values of 4.8–8.8. Samples from cats with suspected or confirmed acute kidney injury (AKI) had OD values ranging from 1.6–20.5. Urine KIM-1 varied over multiple days in cats with sepsis or urethral obstruction despite normalizing serum creatinine concentration. In tissue sections, KIM-1 antibodies labeled tubular cells with morphological features of injury.
Conclusions and relevance
A practical patient-side assay for detection of KIM-1 in feline urine has been developed. Preliminary results show marked though transient increases in cats with sepsis and urethral obstruction-associated AKI, and expression in injured tubules. Although initial data indicating that the LFA is sensitive and specific for KIM-1 in cats with AKI are promising, values associated with different types of injury, urine collection, urine storage and specific gravity need to be investigated.
Keywords: Acute kidney injury, chronic kidney disease, creatinine, ELISA, lateral flow assay, urine
Introduction
In humans and rodents, kidney injury molecule 1 (KIM-1) is a sensitive marker of acute kidney injury (AKI). KIM-1 is a proximal tubular transmembrane glycoprotein thought to function in cell-to-cell or cell-to-matrix adhesion, and to mediate phagocytosis and clearance of injured or apoptotic proximal tubule cells.1–3 There is nominal detection of KIM-1 protein in kidneys and urine of healthy humans and rodents, but with injury of proximal tubular cells KIM-1 is increased three- to 30-fold.2,4–7 KIM-1 is measurable in urine and kidneys of humans and rodents with ischemic, toxic, septic, prerenal azotemic and transplant-related kidney injury.1,4,5,8–11 The extracellular portion of KIM-1 is cleaved by metalloproteinases in injured proximal tubular cells, and then released into urine, enabling non-invasive detection and monitoring of kidney injury.12,13 In humans, urine KIM-1 concentration correlated with severity of AKI and decreased as kidney repair proceeded. 14
The development of urinary markers (such as KIM-1) and scoring systems for AKI have fundamentally changed the understanding of the bidirectional relationship between AKI and chronic kidney disease (CKD) in humans.15,16 Epidemiological data showed that even mild AKI is a risk factor for subsequent development of CKD, and that conditions previously considered inconsequential, such as prerenal azotemia, may be associated with AKI.9,15,17–21 Recognition and greater emphasis on even small intra-individual changes in serum creatinine concentration (SCr), and evaluation of urine biomarkers such as KIM-1, substantiated that in critically ill patients AKI precedes increases in SCr.17,18,22–25
Recently, the feline KIM-1 gene (fKIM-1) and protein were characterized; using a cross-reactive antibody, KIM-1 was detected in urine and tissue of cats with critical illness and suspected AKI.26,27 In cats, as in other species, KIM-1 localized to the proximal tubule. More specifically, in an injury model of 45 mins of ischemia followed by reperfusion, KIM-1 was transiently expressed in S1 and S2, and persistently in S3 segments of proximal tubules. 27 In addition, using immunohistochemistry (IHC), injured proximal tubular cells shed into lumens were KIM-1 positive and formed casts detectable in distal tubules. 27 In that study, SCr was frequently within the reference interval (RI), which would have impeded diagnosis of AKI.
A method to confidently identify and monitor sub-lethal or subtle AKI in cats is unavailable. Fulminate kidney injury due to urinary tract obstruction, and Lilium species, ethylene glycol or drug toxicity, is readily recognized, but there is a large unmet need to detect less severe and potentially reversible injury.28–33 Less severe injury may result from subclinical septic episodes, drug-related nephrotoxicity, episodes of hemoconcentration and other conditions. It is also increasingly recognized that in cats, as in humans, AKI predisposes to eventual CKD.34,35 CKD is extremely common in older cats but causes remain largely unknown.36–38 In this study we report development of a patient-side assay for non-invasive detection of fKIM-1 in urine.
Materials and methods
Production of recombinant fKIM-1
A construct consisting of partial sequences for the interleukin (IL)-2 signal peptide, monomeric human IgGFc and nucleotides 1–261 of fKIM-1 (GenBank KF540034.1) was cloned into a pUC57 plasmid. Correct orientation was verified by sequencing (GenScript), and the purified plasmid was transfected into 293-6E cells grown in serum-free expression media (Life Technologies). Day 6 cell culture supernatants were centrifuged, and the recombinant protein was purified first by Protein A chromatography and then gel filtration chromatography (HiLoad 26/600 Superdex column; GE). The eluted fractions were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot under reducing and non-reducing conditions. A prominent band of 40–44 kD was present, and detected as a single band in Western blot with antibody against human IgG (goat anti-human IgG–horseradish peroxidase [HRP]; GenScript). Approximately 10 mg 95% pure recombinant fusion protein containing fKIM-1 (rfKIM-1) was obtained. Negative control samples consisted of the fusion protein lacking the fKIM-1 insert.
Generation of monoclonal antibodies
Four Balb/c mice were each immunized intraperitoneally (IP) with 100 μg purified rfKIM-1 in Freund’s complete adjuvant on day 1, followed by immunizations with 50 µg on days 21 and 42 (BluePoint Bioscience). Mice were boosted with 20 µg at 48–72 h prior to fusions. Titers to rfKIM-1 and control fusion protein determined by primary ELISA of serum samples 2 weeks after last immunization indicated hyperimmunity. Splenocytes from one mouse were fused with a myeloma cell line, and the resulting hybridoma was propagated in vitro. Fusion outgrowths were tested by screening ELISA for production of specific antibody prior to cloning by limiting dilution. Subsequent clones were tested by Western blot with antibody 219211 directed to human KIM-1 (RnD). Three clones were chosen for expansion and cryopreservation; all were of the IgG1κ isotype. Supernatant was purified through protein A columns, dialyzed, biotinylated and tested for detection of serial dilutions of rfKIM-1 in ELISA.
Lateral flow assay
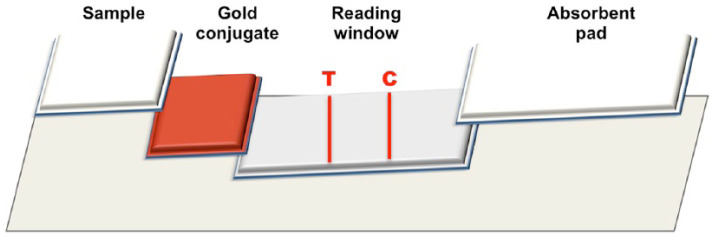
KIM-1 lateral flow strips consist of a sample pad, a gold-conjugate pad, a membrane and an absorbent pad. The strip components were sequentially layered onto an adhesive backing card (Figure 1). All three KIM-1 antibodies were screened using nine different detection/capture combinations on lateral flow assays (LFAs). For detection, the monoclonal antibodies were coated on NakedGold 40 nm gold nanoparticles (BioAssay Works) at 2 µg/OD (optical density, 450 nm) in a final 20 OD/ml solution. For capture, a cellulose ester membrane was sprayed at a rate of 0.1 µl/mm with a test line solution containing 2.0 mg/ml monoclonal antibody and a proprietary control line solution. Membranes were allowed to dry at 37 ± 2°C in a roll-in incubator (Bellco Glass) from 16 to 24 h. After assembly of the lateral flow cards, strips were cut to 5.1 mm using a guillotine cutter (Index-Cut II; A-Point Technologies). The first and last strip of each card was systematically tested (positive and negative samples) to evaluate assay performance, as per internal ‘in-process’ quality control procedures (BioAssay Works). Testing of LFAs included a range of pH, antigen and antibody dilution and incubation times. Once approved, the strips were manually inserted into plastic cassettes and pressed using a friction feeder apparatus (Closure-I; A-Point Technologies). Each LFA was packaged in labeled foil pouches with desiccant. All LFA components were kept in a low humidity environment (relative humidity ~20%) and a final functional test was performed on randomly selected pouches in a lot-size-dependent manner (minimum of three assays per testing condition) to ensure conformance to manufacturing specifications.
Figure 1.
Schematic of the feline kidney injury molecule 1 lateral flow assay assembly
ELISA
All antibodies were tested for detection of serial dilutions of rfKIM-1 following a standard sandwich ELISA protocol. Briefly, wells of a 96-well plate were coated overnight at 4°C with antibody to rfKIM-1. The next day, unbound antibody was washed off and 100 µl test sample at two-fold dilutions ranging from neat to 1:5120 were added to duplicate wells. Samples were incubated for 30 mins, the wells were washed and anti-fKIM-1 antibody conjugated to biotin was added. After 30 mins unbound antibody was washed off, streptavidin–HRP was added, wells were washed, bound antibody was visualized by incubation with tetramethylbenzidine enzyme substrate and OD at 450 nm was measured.
Test procedure
Each LFA was allowed to reach room temperature, removed from its pouch and placed on a flat, horizontal surface. Frozen feline urine samples were thawed and allowed to reach room temperature. Each urine sample was tested in triplicate with the observer blinded to clinical information regarding the origin of the sample. Five microliters of urine was added to a 95 µl sample dilution buffer (BioAssay Works). Test samples (100 µl) were deposited in the sample well of each lateral flow cassette. The sample solution subsequently flowed across the different zones of the strip through capillary action, from the sample pad to the absorbent pad. Excess sample, buffer and gold particles were collected in the absorbent pad, allowing for constant flow and clearing of the reading window by capillary movement of the fluid. Strongly positive samples were first observed at ~5 mins. Each test was read with an optical reader (OpTrilyzer; OpTricon) at 15 mins, to allow clearing of the reading window. Well-defined red lines in the test (T) and control (C) region indicated positive results, and a single line for the control reagent indicated appropriate assay performance with a KIM-1-negative sample (Figure 2).
Figure 2.
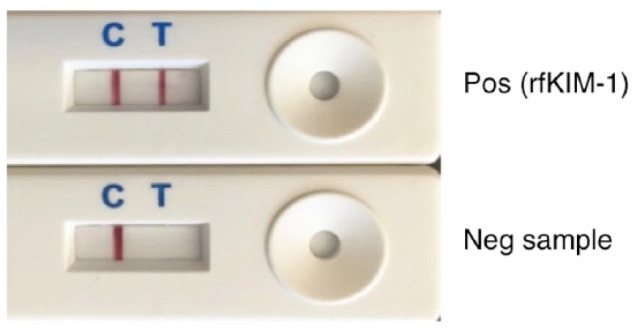
Feline kidney injury molecule 1 (fKIM-1) lateral flow assay cassettes. Top: control (C; internal positive [pos] control sample of recombinant fKIM-1 [rfKIM-1]) and positive test (T) sample. Bottom: internal positive control sample and negative (neg) test urine sample
Urine samples
Urine was obtained from 13 male cats (seven sexually intact cats <12 months of age and six neutered cats aged 2–4 years), prior to their inclusion in a blood donor program (Institutional Animal Care and Use Committee Protocol no. 3668). The cats lacked historical, clinical and laboratory evidence of kidney disease, and had a SCr <160 μmol/l, urine specific gravity (USG) >1.035 and unremarkable urine chemistry and sediment (Table 1). All cats were in good body condition with adequate muscling, had free access to dry food and water, and were housed individually with daily group socialization. Blood for biochemistry and urine for KIM-1 assay were obtained without sedation by venipuncture and ultrasound-guided cystocentesis, respectively. Urine samples were assessed for KIM-1 concentration in LFAs subjected to quality assurance protocols as described above. Other samples originated from cats with known or suspected AKI in the intensive care unit (ICU) of the Ontario Veterinary College Health Sciences Center (OVC-HSC). Urine samples remaining after concurrent complete blood cell count, serum biochemical analysis and urinalysis, as part of standard of care, were selected. The urine samples were collected immediately after voiding into non-absorbent litter or collected by catheter or cystocentesis, as per preference of the attending clinician. Samples were refrigerated at 4°C before analysis. When available, leftover urine samples from subsequent days were also collected.
Table 1.
History and laboratory findings in cats with and without acute kidney injury
| Cat | Sex | Age (years) | Clinical status | SCr* | USG | Urine KIM-1 † |
|---|---|---|---|---|---|---|
| B1 | M | <1 | Blood donor | 61 | 1.047 | 4.8 |
| B2 | M | <1 | Blood donor | 81 | 1.060 | 7.4 |
| B3 | M | <1 | Blood donor | 80 | 1.068 | 7.4 |
| B4 | M | <1 | Blood donor | 91 | 1.037 | 5.2 |
| B5 | M | <1 | Blood donor | 66 | 1.047 | 5.6 |
| B6 | M | <1 | Blood donor | 94 | 1.042 | 6.7 |
| B7 | M | <1 | Blood donor | 93 | 1.065 | 8.1 |
| B8 | MN | 2–4 | Blood donor | 118 | 1.065 | 6.1 |
| B9 | MN | 2–4 | Blood donor | 101 | 1.056 | 5.0 |
| B10 | MN | 2–4 | Blood donor | 136 | 1.058 | 7.1 |
| B11 | MN | 2–4 | Blood donor | 78 | 1.057 | 8.8 |
| B12 | MN | 2–4 | Blood donor | 140 | 1.058 | 7.4 |
| B13 | MN | 2–4 | Blood donor | 154 | 1.056 | 7.5 |
| 1 | MN | 9.5 | Gastric perforation, peritonitis, AKI | 246 | 1.024 | 13.0 |
| 2 | MN | 2.1 | Day 3, AKI, unknown cause | 1500 | 1.010 | 1.6 |
| 2 | Day 4, AKI, unknown cause | 641 | 1.015 | 6.8 | ||
| 2 | Day 7, AKI, unknown cause | 131 | 1.015 | 4.1 | ||
| 3 | FN | 7.2 | Day 2, trauma and sepsis | 149 | 1.035 | 6.1 |
| 3 | Day 3, trauma and sepsis | 129 | 1.005 | 3.0 | ||
| 4 | MN | 9.0 | CKD, exacerbation, day 1 | 1134 | 1.015 | 2.9 |
| 4 | CKD, exacerbation, day 3 | 548 | 1.015 | 3.6 | ||
| 5 | MN | 12.9 | Congestive heart failure | 180 | 1.012 | 5.0 |
| 6 | MN | 7.1 | Day 1, urethral obstruction | 786 | 1.019 | 10.9 |
| 6 | Day 2, urethral obstruction | 158 | 1.015 | 20.5 | ||
| 6 | Day 4, urethral obstruction | 158 | 1.010 | 5.6 | ||
| 7 | MN | 8.8 | Day −180, healthy | 104 | 1.046 | 5.7 |
| 7 | Day 1, pyrexia | 102 | 1.021 | 6.1 | ||
| 7 | Day 5, pyrexia, post-anesthesia | 115 | 1.019 | 19.5 | ||
| 8 | MN | 0.9 | Day 5, AKI | 709 | 1.010 | 7.1 |
| 9 | MN | 5.5 | Day 2, urethral obstruction | 560 | 1.019 | 8.0 |
| 10 | MN | 1.4 | Day 2, urethral obstruction | 619 | 1.036 | 9.4 |
| 11 | FN | 9.4 | Hyperthyroid, >6 months | 126 | 1.015 | 8.0 |
| 12 | MN | 11.9 | Hyperthyroid, >3 months | 50 | 1.065 | 7.3 |
| 13 | FN | 13.0 | Hyperthyroid, >3 months | 81 | 1.046 | 11.0 |
Serum creatinine (SCr) concentration (μmol/l)
Urine lateral flow assay
USG = urine specific gravity; KIM-1 = kidney injury molecule 1; M = male; MN = male neutered; AKI = acute kidney injury; FN = female neutered; CKD = chronic kidney disease
Urinalysis was performed using an automated dipstick reader (CLINITEK Status+ Analyzer; Siemens), refractometer (Vet 360 Refractometer; Reichert Technologies) and semiquantitative microscopic sediment analysis. Serum assays were performed on a Cobas 4800 biochemistry analyzer (Roche). Urine samples were stored in polyethylene terephthalate tubes without additives at 4°C for up to 24 h before freezing at −80°C.
Statistical methods
Data were analyzed using GraphPad Prism (7.0d). Standard descriptive statistics were reported as mean ± 1 SD and range, if appropriate. Correlation coefficients (Spearman or Pearson) for OD results on ELISA and LFA relative to fusion protein concentration were calculated.
IHC
In order to assess antibody cross-reactivity with formalin-fixed epitopes, kidney sections from two cats euthanized due to sepsis or urethral obstruction, respectively, were assessed by IHC. Briefly, tissues were fixed in 10% neutral buffered formalin for 24–48 h, embedded in paraffin and sectioned at 4 µm. Sections were de-paraffinized, and incubated with a 1:5 dilution of fKIM-1 antibody overnight at 4 °C. The following day, sections were rinsed with Tris-buffered saline, incubated with goat anti-mouse secondary antibody (DakoCytomation), rinsed again and bound antibodies were detected with NovaRED chromogen and counterstained with hematoxylin.
Results
Recombinant fKIM-1
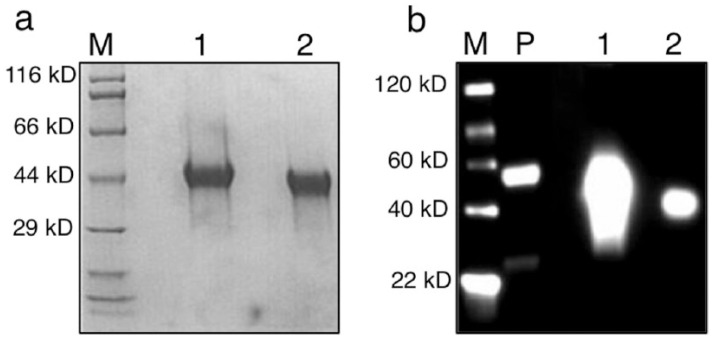
Recombinant fKIM-1 was generated as a fusion protein (monoFc_IgGFc_KIM-1) to facilitate initial immunodetection with antibody to human IgG. The predicted molecular weight of the fKIM-1 sequence was 14.9 kD, and as a recombinant with FC_IgGFc and the IL-2 signal peptide the predicted weight was 40.8 kD. The recombinant protein was expressed with high efficiency in 293-6E cells, and purified and concentrated from supernatant to yield a single protein of 40–44 kD on SDS-PAGE. Western blot analysis of purified cell culture supernatant showed a dispersed band under reducing conditions, consistent with disruption of disulfide linkages, and a more discrete band around 42 kD under non-reducing conditions (Figure 3).
Figure 3.
(a) Sodium dodecyl sulfate polyacrylamide gel electrophoresis and (b) Western blot analysis of purified IgGFc–recombinant feline kidney injury molecule 1. Primary antibody is goat anti-human IgG-HRP. M = molecular weight marker; 1 = reducing conditions; 2 = non-reducing conditions; P = positive control (human IgG1)
ELISA
Among hybridoma fusion outgrowths, supernatants from three clones (1H7, 11B3 and 14G2) were purified and tested at multiple dilutions for detection of rfKIM-1 in ELISA. With the ELISA, triplicate serial dilutions of purified rfKIM-1 fusion protein ranging from 0–1.25 ng/ml were detected in an approximately linear fashion at an antibody dilution of 1:40 with a Pearson correlation coefficient of 0.9648 (Figure 4). Each antibody detected the rfKIM-1 fusion protein in a similar fashion with slightly variable sensitivity (data not shown). Purified supernatant of the transfectant lacking the fKIM-1 insert gave OD readings <0.2.
Figure 4.
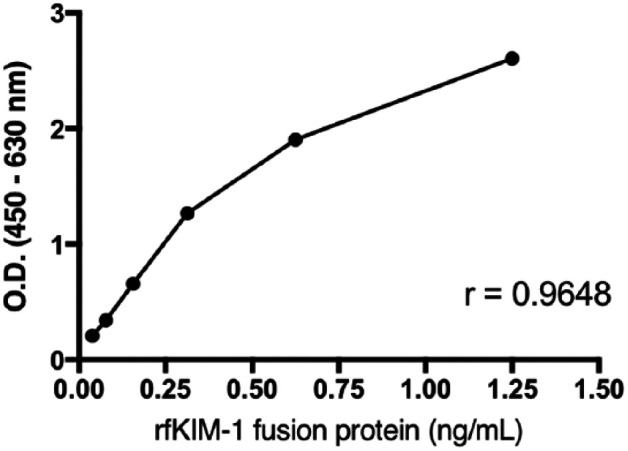
Serial dilutions of recombinant feline kidney injury molecule 1 (rfKIM-1) detected by ELISA. OD = optical density
Lateral flow assay
Serial dilutions of purified rfKIM-1 protein were applied to the LFA cassettes, as described above, to determine the range of detection. An optimal line intensity signal was detected after 15 mins of incubation at room temperature. The ratio of the control to the test line OD signal was calculated and plotted against rfKIM-1 concentrations (Figure 5). The LFA detected an expanded range of rfKIM-1 fusion protein concentrations in a curvilinear fashion relative to the ELISA (1:40 dilution). Pearson correlation coefficient was 0.9237. Measurement of KIM-1 in triplicate by LFA in urine samples from cats with and without AKI, including the 13 healthy blood donor cats, eight cats with suspected AKI (described in this study) and 7 samples from cats with other conditions, indicated SDs ranging from 0.1–3.5 (see supplementary material).
Figure 5.
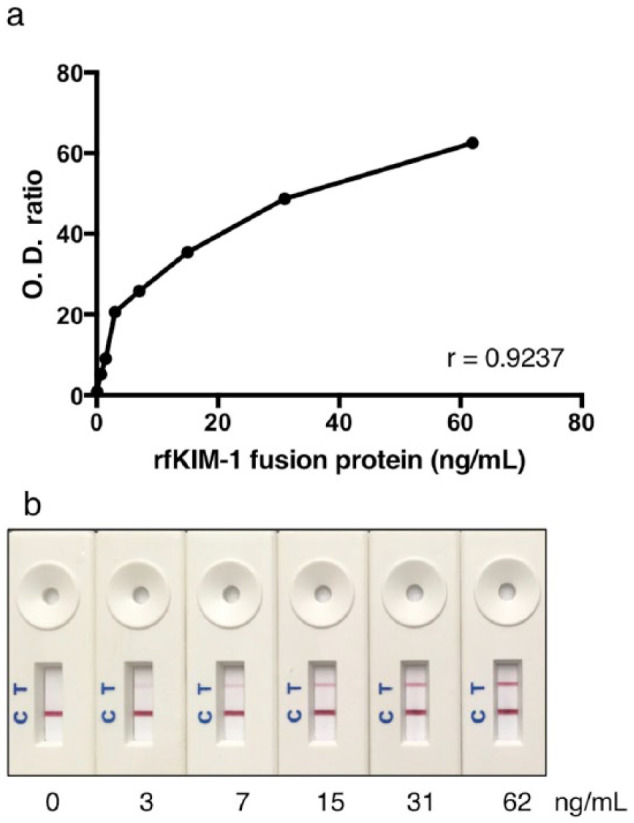
Serial dilutions of recombinant feline kidney injury molecule 1 (rfKIM-1) detected in the lateral flow assay with an optical reader (a) and by visual inspection (b). Optical density (OD) in (a) is expressed as the color intensity of the ‘internal positive control’ band relative to that of the ‘test’ band
Assessment of cat urine samples
Urine samples were analyzed from 13 healthy control blood donor cats and 13 cats hospitalized in the ICU for various conditions (Table 1). Mean SCr for control cats was 99.5 µmol/l (range 61–154 µmol/l) and mean USG was 1.057 (range 1.037–1.068; see table in the supplementary material). Results of urine KIM-1 LFA showed OD ratios from 4.8–8.8 with a mean, median and SD of 6.7, 7.1 and 1.26, respectively (Figure 6a). KIM-1 results from intact and neutered cats were not significantly different (Mann–Whitney test, P = 0.531).
Figure 6.
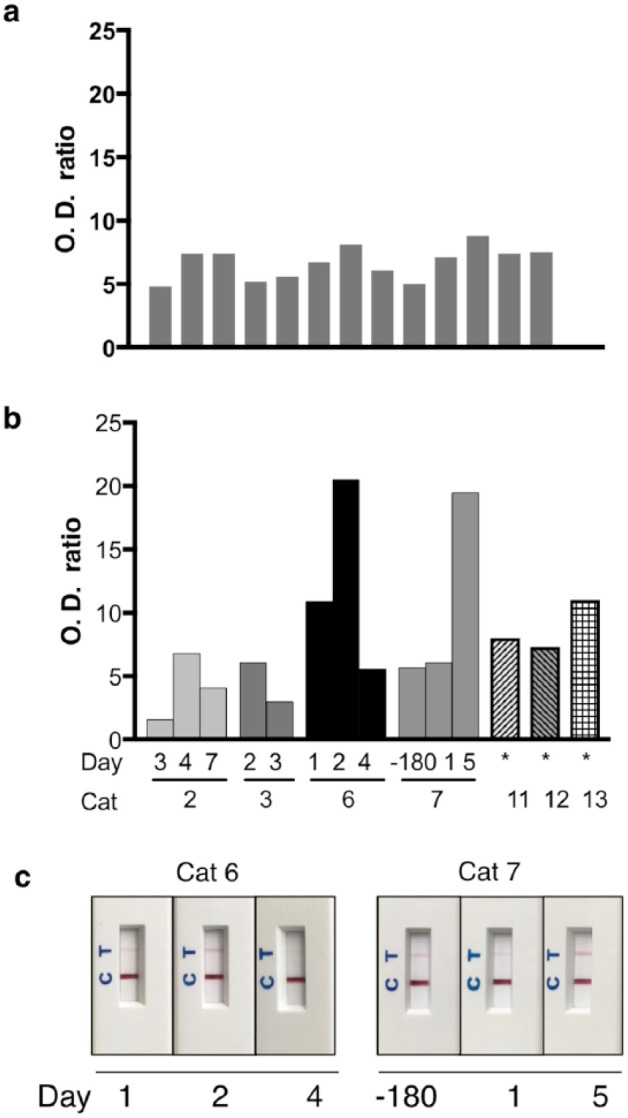
(a) Kidney injury molecule 1 (KIM-1) measured by lateral flow assay (LFA) in urine from 13 healthy blood donors has a mean (SD) optical density (OD) ratio of 6.7 ± 1.257. (b) KIM-1 concentration in urine from cats with clinical disease is highly variable. See case descriptions in Table 1. (c) LFA cassettes from cats 6 and 7. *Hyperthyroid for >3 months. OD = optical density
Thirteen cats with suspected or confirmed AKI consisted of neutered males and females ranging in age from 0.9–13.0 years. Mean SCr was 392 µmol/l (range 50–1500 µmol/l); USG ranged from 1.005–1.065 (Table 1; see also table in the supplementary material). Diseased cats had AKI due to sepsis secondary to trauma or perforating gastrointestinal neoplasm, urethral obstruction, hyperthyroidism, congestive heart failure or unknown cause. Urine KIM-1 was highly variable in this group of clinically ill cats, and included values below and well above the median of healthy cats (Figure 6).
From five cats, serial urine samples were available (Table 1). Cat 2 had AKI of unknown cause and had been ill for 3 days at initial presentation. Urine assessment on day 3 after admission showed low KIM-1 concentration vs control cats, but concentration increased subsequently on day 4 (with concurrent casts in the urine; see table in the supplementary material) and then decreased again on day 7 (Figure 6b). This cat had very high SCr on day 3, which decreased on day 4 and returned to within the RI on day 7 (Table 1). Cat 3 (Figure 6b) had severe trauma from dog bites resulting in abdominal external herniation, liver fracture, pancreatic avulsion and splenic tear and septic peritonitis. The cat was euthanized owing to intractable hypotension after attempted surgical repair, and was receiving fluid therapy throughout. Acute tubular necrosis (in addition to numerous other lesions) was diagnosed postmortem. Urine KIM-1 was detected on both days 2 and 3 with values similar to those in control cats, while SCr remained within the RI.
Cat 6 had urethral obstruction that was relieved approximately 24 h prior to admission, and urine samples were then obtained 1, 2 and 4 days after admission to the OVC-HSC (Figure 6b,c). On day 1, urine KIM-1 concentration was higher than that of the mean in control cats, on day 2 it was approximately three times the concentration of the mean in controls cats and on day 4 it decreased to a level similar to that in control cats. Only on day 1 was SCr increased in this cat. Cat 7 (Figure 6b,c) had urine KIM-1 values similar to those of control cats during a health check 6 months (when a urine sample was collected and stored at −80°C) prior to illness, and on day 1 of an episode of pyrexia due to unknown cause. On day 5, following general anesthesia and rhinoscopy for presumed Cuterebra species infection, urine KIM-1 was markedly higher. Urinalysis on day 5 showed marked hematuria and numerous cellular and erythrocyte casts, but SCr remained within the RI throughout with a small increase between days 1 and 5 (Table 1; see also table in the supplementary material). Additional single samples from cats with confirmed or suspected AKI due to urethral obstruction or hyperthyroidism yielded KIM-1 values at the upper end or above those from control cats.
IHC
Kidney sections obtained at autopsy from cats with AKI due to sepsis and urethral obstruction were assessed by IHC for expression of KIM-1. In cat 1 with AKI associated with peritonitis and sepsis, the urine KIM-1 LFA yielded a value of 13.0 at 24 h before euthanasia (Table 1). With IHC, KIM-1 was detected in tubular cells of the inner cortex, the outer stripe of the outer medulla and the inner stripe of the outer medulla (Figure 7). Intense staining was apparent in tubular cells and luminal debris. In one cat with AKI associated with urethral obstruction and anuria, specific staining for KIM-1 was also noted in tubular cells with morphological features of injury (data not shown). Pre-immune serum did not label tubular cells, and interstitial cells, glomeruli and leukocytes also were negative.
Figure 7.
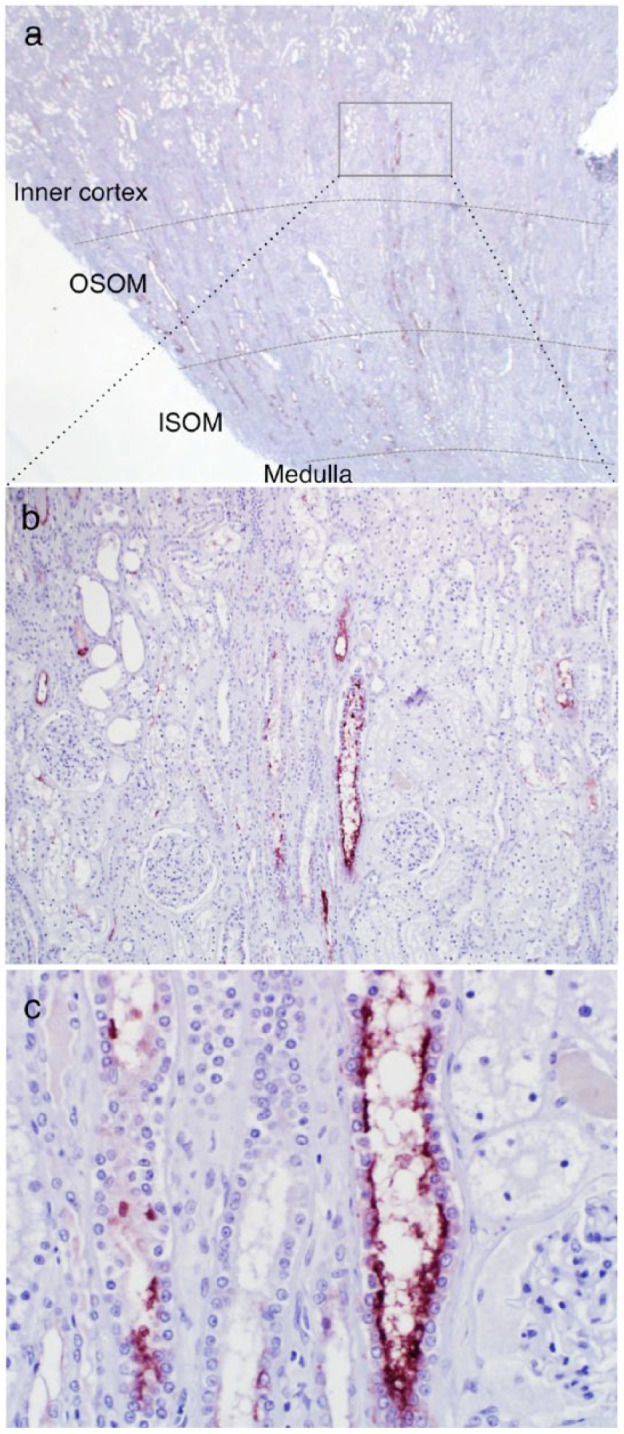
Immunohistochemical staining of kidney injury molecule 1 (KIM-1) in formalin-fixed, paraffin-embedded kidney sections from cat 1. The cat had peritonitis and sepsis secondary to a perforated gastric lymphoma, developed acute kidney injury and acutely decompensated over 2 days. Acute myocardial necrosis, and renal and pulmonary thrombosis were diagnosed on autopsy. (a) KIM-1 (red–brown color) is expressed in tubules in the inner cortex, the outer stripe of the outer medulla (OSOM) and the inner stripe of the outer medulla (ISOM), × 1.25 objective. (b) On closer view, predominant expression is in tubules in the inner cortex and OSOM, × 10 objective. (c) At high magnification (× 40 objective) proximal tubule cells in the inner cortex and luminal debris are KIM-1 positive
Discussion
The aim of this study was to develop a non-invasive assay to detect AKI in cats. KIM-1 is among the most promising markers to detect injury of proximal tubules in humans and rats, and we previously identified KIM-1 in kidney tissue of cats with AKI due to ischemia/reperfusion (I/R) injury.26,27 Attempts to use the human and rat Renastick (BioAssay Works) to detect feline urine KIM-1 resulted in inconsistent or no results, despite histologic evidence of kidney injury.26,27 Therefore, feline-specific KIM-1 monoclonal antibodies were generated and configured into an LFA. The LFA was then used to measure KIM-1 in urine of healthy and diseased cats.
The LFA worked in a practical and simple manner similar to other patient-side immunoassays. Five microliters of urine were diluted 1:20 with buffer prior to application, and cassettes were read after 15 mins. Such a small urine volume is easily obtained even from oliguric cats. Results from the LFA of urine are not directly comparable to those of serial dilutions of rfKIM-1 as the latter is a fusion protein not exclusively composed of KIM-1. Nevertheless, dilutions of rfKIM-1 yielded OD values and color reactions similar to those of many clinical samples. Although both ELISA and LFA formats were tested, for clinical use at the point of care the LFA is much more practical. Results can be assessed visually or with a hand-held reader, with the latter providing numerical results that can be accurately compared over time. The ELISA format would be of value for analysis of a large number of samples, and has the advantage of being readily run in duplicate or triplicate and over multiple dilutions.
Following development of the immunoassays, we assessed a preliminary set of urine samples from healthy young cats free of historical illness and conditions that could potentially impact renal function, as well as urine samples from cats with AKI or at risk of developing AKI. This limited number of samples is insufficient to determine sensitivity and specificity of urine KIM-1 in relation to other markers of AKI or outcome. In addition, many variables pertaining to type, severity and timing of kidney injury resulting in increased shedding of KIM-1, and individual variation, remain to be determined. Furthermore, it is unknown whether urinary KIM-1 increases with age in cats. However, single samples from older cats without kidney disease (ie, cat 7, day −180, health check) had levels of KIM-1 similar to those of control cats. At this point, stability of the molecule, potential interferents with the assay and urinary excretion in conditions other than AKI also remain to be fully characterized. Results in humans and rodents have indicated that KIM-1 is stable at 4 °C for 5 days and for >1 year at −80°C, and that substances such as hemoglobin, glucose, albumin, bilirubin and a variety of medications did not interfere with KIM-1 measurements. 7 fKIM-1 may also be stable frozen (unpublished observations), and as the assay is based on monoclonal antibodies with high specificity, interferents are not expected but were not expressly evaluated in this study. 7
A prototypic assay for cats has now been designed and manufactured, and initial assessment with clinical samples enables several conclusions. Healthy blood donor cats had low but consistently detectable KIM-1 in urine. This suggests that there is a constitutive, albeit low, level of excretion by kidneys, which is similar to that in humans and rodents.5,8,12 In healthy humans, KIM-1 in urine increases very slightly with age. 39 Urine KIM-1 in health may result from normal turnover of proximal tubular cells, and is not necessarily the same in cats as in humans or rodents. Detection of KIM-1 in urine of healthy young cats indicates that an optimal cut-off value to identify renal injury has to be carefully calibrated. Determination of such a cut-off is challenging using clinical samples as it is rare that pre-injury urine KIM-1 concentration or the exact time and extent of nephric injury are known, and urine composition and specific gravity are affected by fluid and other therapy. Conversely, measuring urine KIM-1 in experimental I/R injury may be informative regarding that type of injury but may not reflect cell-specific induction of KIM-1 expression, cell death and reduced or selective urine flow from toxic or obstructive injury, both of which are considered common causes of naturally occurring AKI. 32
Urine KIM-1 concentration in cats with suspected AKI overlapped with that in control cats (Figure 6), and therefore analysis of serial samples was far more informative than analysis of single samples. For example, cat 2 (Table 1) had an episode of AKI of unknown cause thought to have occurred 3 days prior to admission. The first urine KIM-1 concentration (day 3) was very low compared with that in control cats. When this cat was examined by the referring veterinarian 3 days prior, SCr was within the RI, but on admission to the OVC-HSC ICU, SCr was 1500 µmol/l, urine production was <0.5 ml/kg and the cat was overhydrated. With therapy, urine production increased and SCr decreased, but the urine sample from day 4 had higher KIM-1 concentration and numerous granular casts, indicating ongoing tubular injury. It is conceivable that in this cat most proximal tubules were injured and sloughed prior to analysis of the first urine sample, removing pre-existing or de novo produced KIM-1. Subsequent slightly increased urine KIM-1 may reflect regeneration of proximal tubules with ensuing KIM-1 re-excretion into urine. Injury with this magnitude of SCr change is rarely assessed in studies of humans with AKI; therefore, comparable information on the dynamics of KIM-1 excretion in humans is sparse. Within 1 year of this episode of AKI, cat 2 was classified as having IRIS stage 2 CKD with persistent poorly concentrated to isosthenuric urine.
Cat 4 had established stable CKD with acute exacerbation of unknown cause. Admission and subsequent urine KIM-1 results were lower than those in control cats, but the initial urine sample was obtained several days after exacerbation and initiation of therapy at the referring clinic. This cat was eventually euthanized owing to lack of resolution of azotemia. Histopathology confirmed severe CKD consisting of bilateral renal fibrosis with lymphocytic and plasmacytic interstitial inflammation, as well as bilateral tubular necrosis, glomerular sclerosis and lack of cortical tubules in >60% of the sections. Therefore, this cat with chronic CKD may have had superimposed severe AKI, which injured the few remaining proximal tubular cells but did not increase urine KIM-1 at the time of testing. Scenarios such as this, where cats with relatively stable CKD suffer an additional nephrotoxic insult that becomes fatal, are not uncommon; determining the value of urine KIM-1 measurement in this context will require analysis of multiple samples during stable CKD and immediately during and following onset of illness. It has been suggested in other species that monitoring urine KIM-1 concentration is useful to assess transition from AKI to CKD, and during progression of CKD. 19
Cat 6 arrived at the OVC-HSC approximately 24 h after relief of urethral obstruction at the referring clinic. The first urine sample after admission to the OVC-HSC (day 1) had higher KIM-1 concentration than that of control cats, and the cat at that time had extremely high SCr. The next day, urine KIM-1 had nearly doubled although SCr had decreased by >75% (Table 1). These findings suggest that urine KIM-1 concentration does not parallel SCr but is likely influenced by factors such as urine flow, degree and type of tubular cell injury, and renal perfusion. On day 4, urine KIM-1 was at a level similar to that in control cats, which could indicate that a large proportion of the tubular cells capable of synthesizing KIM-1 were sloughed, that KIM-1 production was only transiently increased or that homeostasis was re-established. KIM-1 is a phosphatidylserine receptor expressed and upregulated on renal epithelium that mediates recognition and phagocytosis of apoptotic cells. 3 In a large multi-center rodent study, renal injury from antimicrobial, antineoplastic and other drugs was detected with higher sensitivity and specificity by KIM-1 urine assay than by SCr, serum urea concentration or urinary N-acetyl-beta-d-glucosaminidase assay and had very high correlation with histopathologic assessment. 13 Urinary KIM-1 increased by 3 h after bilateral I/R injury, reached a maximum between 12 and 48 h and then decreased slightly until 120 h. 13 These findings from rats cannot be readily translated to cats as in clinical cases neither the time nor the nature or severity of injury are known; however, based on the limited data analyzed here, maximal urine KIM-1 increases may occur sooner than measured in clinical cases in this study, and urine KIM-1 can be increased when SCr is normal and when there is some concentrating ability. Findings in cat 7 parallel those in cats 2 and 6, and the stark increase in KIM-1 on day 5 may be because this sample was collected immediately after presumed AKI in association with sepsis and anesthesia.
Hyperthyroidism results in tachycardia, increased cardiac output, decreased vascular resistance and increased renal perfusion, with the degree of change likely influenced by the severity and duration of hyperthyroidism.40–42 Up to 50% of cats with hyperthyroidism have CKD that becomes apparent once euthyroidism has been re-established. 43 In the light of recognition that CKD predisposes to AKI, it is not surprising that the hyperthyroid cats in this study had urine KIM-1 concentration at the high end or above that of control cats. 15 Assessment of urine KIM-1 during hyperthyroidism and after treatment may yield useful insight into kidney disease in this condition, and help to determine whether higher urine KIM-1 is due to the increased metabolic demand or ongoing low-grade AKI.
The nature of patient-side rapid LFAs does not preclude concurrent assessment of USG and other urine parameters. Therefore, the KIM-1 LFA is not a replacement for urinalysis but may be an adjunct tool for monitoring tubular injury. In this preliminary study, urine KIM-1 was consistently detected in healthy young cats, and had limited variability. Specific gravity of urine samples from these healthy cats was much higher than that of cats with suspected or proven AKI. It remains to be determined whether high urine solute concentrations affect urinary KIM-1. For example, on day 3, cat 3 had a marked decrease in USG and a slight decrease in KIM-1 relative to day 2, which might reflect a dilution effect and/or altered shedding of KIM-1 from proximal tubular cells. Fluid and other therapy and obstruction of specific nephron segments might contribute to some low KIM-1 values in cats with AKI, but additional studies are required to characterize such potential relationships.
Finally, availability of antibodies reactive with formalin-exposed epitopes, and therefore suitable for routine immunohistochemical detection of KIM-1, will be very advantageous in efforts to characterize different types of feline renal disease in biopsies or post-mortem tissue. Detailed assessment of the tissue distribution of KIM-1 expression in specific types of AKI was beyond the scope of this investigation, but availability of a non-invasive patient-side test for this biomarker will hopefully promote understanding, recognition and therapy of kidney disease in cats.
Conclusions
A patient-side assay for detection of urine KIM-1 in cats was developed. KIM-1 was present at a low level in healthy cats and increased up to three-fold in cats with AKI of variable degree and duration. The range, magnitude and timing of kidney injuries that result in increased excretion of KIM-1 in urine over time remain to be determined, but a tool for such inquiry has now been generated.
Supplemental Material
This shows measurement of kidney injury molecule 1 in triplicate by lateral flow assay in urine samples from 28 cats. Samples were from cats with and without acute kidney injury (AKI), including 13 healthy blood donor cats, eight cats with suspected AKI (described in this study) and 7 samples from cats with other conditions. Mean and SD are shown. For several samples (samples 2–7, 9–11, 17, 18, 25, 26) the SD was too small to be visualized in the graph.
Complete urinalysis results for all urine samples used in this study.
Acknowledgments
We thank Drs S Blois, A Abrams-Ogg, S Bateman, A Bersenas and A Defarges from the Department of Clinical Studies at the Ontario Veterinary College for identifying samples from cats with and without suspected kidney disease.
Footnotes
Accepted: 22 October 2018
Olivier Côté is the principal scientist at BioAssay Works.
Funding: The PetTrust Foundation at the University of Guelph supported initial investigation of feline KIM.
ORCID iD: Dorothee Bienzle  https://orcid.org/0000-0002-2301-2931
https://orcid.org/0000-0002-2301-2931
Supplementary material: The following files are available online:
Supplementary table: complete urinalysis results for all urine samples used in this study.
Supplementary figure: this shows measurement of KIM-1 in triplicate by LFA in urine samples from 28 cats. Samples were from cats with and without AKI, including 13 healthy blood donor cats, eight cats with suspected AKI (described in this study) and seven samples from cats with other conditions. Mean and SD are shown. For several samples (samples 2–7, 9–11, 17, 18, 25, 26) the SD was too small to be visualized in the graph.
References
- 1. Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem 1998; 273: 4135–4142. [DOI] [PubMed] [Google Scholar]
- 2. Bailly V, Zhang Z, Meier W, et al. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem 2002; 277: 39739–39748. [DOI] [PubMed] [Google Scholar]
- 3. Ichimura T, Asseldonk EJPV, Humphreys BD, et al. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest 2008; 118: 1657–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ichimura T, Hung CC, Yang SA, et al. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol 2004; 286: F552–F563. [DOI] [PubMed] [Google Scholar]
- 5. Han WK, Bailly V, Abichandani R, et al. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 2002; 62: 237–244. [DOI] [PubMed] [Google Scholar]
- 6. Vaidya VS, Ramirez V, Ichimura T, et al. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol 2006; 290: F517–F529. [DOI] [PubMed] [Google Scholar]
- 7. Sabbisetti VS, Ito K, Wang C, et al. Novel assays for detection of urinary KIM-1 in mouse models of kidney injury. Toxicol Sci 2013; 131: 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Timmeren MM, van den, Heuvel MC, Bailly V, et al. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol 2007; 212: 209–217. [DOI] [PubMed] [Google Scholar]
- 9. Nejat M, Pickering JW, Devarajan P, et al. Some biomarkers of acute kidney injury are increased in pre-renal acute injury. Kidney Int 2012; 81: 1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bank JR, van der Pol P, Vreeken D, et al. Kidney injury molecule-1 staining in renal allograft biopsies 10 days after transplantation is inversely correlated with functioning proximal tubular epithelial cells. Nephrol Dial Transplant 2017; 32: 2132–2141. [DOI] [PubMed] [Google Scholar]
- 11. Tonomura Y, Tsuchiya N, Torii M, et al. Evaluation of the usefulness of urinary biomarkers for nephrotoxicity in rats. Toxicology 2010; 273: 53–59. [DOI] [PubMed] [Google Scholar]
- 12. Vaidya VS, Ford GM, Waikar SS, et al. A rapid urine test for early detection of kidney injury. Kidney Int 2009; 76: 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vaidya VS, Ozer JS, Dieterle F, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 2010; 28: 478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonventre JV, Yang L. Kidney injury molecule-1. Curr Opin Crit Care 2010; 16: 556–561. [DOI] [PubMed] [Google Scholar]
- 15. Ferenbach DA, Bonventre JV. Acute kidney injury and chronic kidney disease: from the laboratory to the clinic. Nephrol Ther 2016; 12: S41–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu J, Kumar S, Dolzhenko E, et al. Molecular characterization of the transition from acute to chronic kidney injury following ischemia/reperfusion. JCI Insight 2017; 2. DOI: 10.1172/jci.insight.94716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8: R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 2012; 82: 516–524. [DOI] [PubMed] [Google Scholar]
- 19. Chawla LS, Amdur RL, Amodeo S, et al. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 2011; 79: 1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Timmeren MM, Bakker SJL, Vaidya VS, et al. Tubular kidney injury molecule-1 in protein-overload nephropathy. Am J Physiol Renal Physiol 2006; 291: F456–F464. [DOI] [PubMed] [Google Scholar]
- 21. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 2012; 81: 442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol 2007; 18: 1292–1298. [DOI] [PubMed] [Google Scholar]
- 23. Kellum JA, Levin N, Bouman C, et al. Developing a consensus classification system for acute renal failure. Curr Opin Crit Care 2002; 8: 509–514. [DOI] [PubMed] [Google Scholar]
- 24. Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11; R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int 2008; 73: 538–546. [DOI] [PubMed] [Google Scholar]
- 26. Bland SK, Côté O, Clark ME, et al. Characterization of kidney injury molecule-1 in cats. J Vet Intern Med 2014; 28: 1454–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bland SK, Schmiedt CW, Clark ME, et al. Expression of kidney injury molecule-1 in healthy and diseased feline kidney tissue. Vet Pathol 2017; 54: 490–510. [DOI] [PubMed] [Google Scholar]
- 28. Cooper RL, Labato MA. Peritoneal dialysis in cats with acute kidney injury: 22 cases (2001–2006). J Vet Intern Med 2011; 25: 14–19. [DOI] [PubMed] [Google Scholar]
- 29. Dorval P, Boysen SR. Management of acute renal failure in cats using peritoneal dialysis: a retrospective study of six cases (2003–2007). J Feline Med Surg 2009; 11: 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eatroff AE, Langston CE, Chalhoub S, et al. Long-term outcome of cats and dogs with acute kidney injury treated with intermittent hemodialysis: 135 cases (1997–2010). J Am Vet Med Assoc 2012; 241: 1471–1478. [DOI] [PubMed] [Google Scholar]
- 31. Lee Y-J, Chan JP-W, Hsu W-L, et al. Prognostic factors and a prognostic index for cats with acute kidney injury. J Vet Intern Med 2012; 26: 500–505. [DOI] [PubMed] [Google Scholar]
- 32. Segev G, Nivy R, Kass PH, et al. A retrospective study of acute kidney injury in cats and development of a novel clinical scoring system for predicting outcome for cats managed by hemodialysis. J Vet Intern Med 2013; 27: 830–839. [DOI] [PubMed] [Google Scholar]
- 33. Segev G, Livne H, Ranen E, et al. Urethral obstruction in cats: predisposing factors, clinical, clinicopathological characteristics and prognosis. J Feline Med Surg 2011; 13: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dickerson VM, Rissi DR, Brown CA, et al. Assessment of acute kidney injury and renal fibrosis after renal ischemia protocols in cats. Comp Med 2017; 67: 56–66. [PMC free article] [PubMed] [Google Scholar]
- 35. Schmiedt CW, Brainard BM, Hinson W, et al. Unilateral renal ischemia as a model of acute kidney injury and renal fibrosis in cats. Vet Pathol 2016; 53: 87–101. [DOI] [PubMed] [Google Scholar]
- 36. Jepson RE, Brodbelt D, Vallance C, et al. Evaluation of predictors of the development of azotemia in cats. J Vet Intern Med 2009; 23: 806–813. [DOI] [PubMed] [Google Scholar]
- 37. Chakrabarti S, Syme HM, Elliott J. Clinicopathological variables predicting progression of azotemia in cats with chronic kidney disease. J Vet Intern Med 2012; 26: 275–281. [DOI] [PubMed] [Google Scholar]
- 38. McLeland SM, Cianciolo RE, Duncan CG, et al. A comparison of biochemical and histopathologic staging in cats with chronic kidney disease. Vet Pathol 2015; 52: 524–534. [DOI] [PubMed] [Google Scholar]
- 39. Pennemans V, Rigo J-M, Faes C, et al. Establishment of reference values for novel urinary biomarkers for renal damage in the healthy population: are age and gender an issue? Clin Chem Lab Med 2013; 51: 1795–1802. [DOI] [PubMed] [Google Scholar]
- 40. Boag AK, Neiger R, Slater L, et al. Changes in the glomerular filtration rate of 27 cats with hyperthyroidism after treatment with radioactive iodine. Vet Rec 2007; 161: 711–715. [DOI] [PubMed] [Google Scholar]
- 41. Stock E, Daminet S, Paepe D, et al. Evaluation of renal perfusion in hyperthyroid cats before and after radioiodine treatment. J Vet Intern Med 2017; 31: 1658–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Adams WH, Daniel GB, Legendre AM. Investigation of the effects of hyperthyroidism on renal function in the cat. Can J Vet Res 1997; 61: 53–56. [PMC free article] [PubMed] [Google Scholar]
- 43. Williams TL, Peak KJ, Brodbelt D, et al. Survival and the development of azotemia after treatment of hyperthyroid cats. J Vet Intern Med 2010; 24: 863–869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This shows measurement of kidney injury molecule 1 in triplicate by lateral flow assay in urine samples from 28 cats. Samples were from cats with and without acute kidney injury (AKI), including 13 healthy blood donor cats, eight cats with suspected AKI (described in this study) and 7 samples from cats with other conditions. Mean and SD are shown. For several samples (samples 2–7, 9–11, 17, 18, 25, 26) the SD was too small to be visualized in the graph.
Complete urinalysis results for all urine samples used in this study.