Abstract
Objectives
The aim of this study was to report the prevalence of iatrogenic hypothyroidism, with or without azotaemia, based on the measurement of serum total thyroxine (T4), thyroid-stimulating hormone (TSH) and creatinine concentrations, in hyperthyroid cats undergoing radioiodine (131I) treatment where the 131I dose was calculated using a previously described scoring system. A secondary aim of the study was to determine the positive and negative predictive values of serum T4 and TSH concentrations obtained 19 days after treatment in order to predict the development of iatrogenic hypothyroidism 6–9 months after 131I treatment.
Methods
Serum T4, TSH and creatinine concentrations were measured 19 days and 6–9 months after 131I treatment. The prevalence of iatrogenic hypothyroidism was assessed with the results obtained 6–9 months after 131I treatment.
Results
The prevalence of overt and subclinical hypothyroidism 6–9 months after 131I treatment was 40.0% (22/55 cats) and 12.7% (7/55 cats). Overt hypothyroidism with azotaemia was diagnosed in 8/55 (14.5%) cats. The positive and negative predictive values for the prediction of the development of iatrogenic hypothyroidism 6–9 months after 131I treatment were 72.2% and 80.0%, respectively, for a low serum T4 concentration, and 75.0% and 44.6%, respectively, for an increased serum TSH concentration.
Conclusions and relevance
The use of an individualised scoring system is effective in determining the 131I dose for the treatment of hyperthyroid cats. However, the prevalence of overt hypothyroidism was higher in comparison with other studies using different dosing protocols. Further studies comparing the efficacy of individualised scoring systems and different fixed doses to determine which method is superior are warranted.
Keywords: Iodine isotopes, hyperthyroidism, hypothyroidism, thyroid gland, azotaemia
Introduction
Hyperthyroidism is the most common endocrinopathy seen in geriatric cats and is most commonly caused by adenomas or adenomatous hyperplasia of the thyroid gland. 1 Treatment options include medical management with oral or transdermal antithyroid drugs (methimazole or carbimazole), an iodine-restricted diet, thyroidectomy or radioiodine treatment.2–5
Radioiodine (131I) therapy is generally considered the treatment of choice for feline hyperthyroidism, whenever significant comorbidities are absent, given its simplicity, safety and efficacy.1,5–8 As with any other treatment options, there are a few disadvantages to the use of 131I in hyperthyroid cats, including cost of treatment, a limited number of centres offering this treatment modality, the need for a protracted period of hospitalisation following treatment and the risk of causing iatrogenic hypothyroidism. 8
Iatrogenic hypothyroidism is a well-recognised complication of 131I treatment, which has been reported in up to 79% of hyperthyroid cats following 131I therapy. 9 Cats that develop iatrogenic hypothyroidism are more likely to develop azotaemia than euthyroid cats. 10 Moreover, cats that become hypothyroid and also develop azotaemia have shorter survival times than non-azotaemic cats. 10 Therefore, the optimal 131I dose should ideally restore euthyroidism without inducing hypothyroidism. Different methods have been used to try to determine the optimal 131I dose including different fixed 131I dosages or individualised 131I dosing protocols using tracer kinetic studies or scoring systems.7,8 However, the optimal method to calculate the 131I dose remains controversial. 8
In 1995, Peterson and Becker described a scoring system used to calculate the 131I dose based on serum total thyroxine (T4) concentration, severity of the clinical signs and size of the palpable thyroid gland, and concluded that it was safe and effective to treat feline hyperthyroidism. 5 In this study, only 1.5% of cats were persistently hyperthyroid 6 months after 131I treatment and only 2.1% cats developed clinical features of hypothyroidism with a decreased T4 concentration, despite 11.4% cats having a low serum T4. 5
At the time of this study, a thyroid-stimulating hormone (TSH) assay was not available, thereby limiting the ability to diagnose and confirm hypothyroidism in these cats. Moreover, many cats with iatrogenic hypothyroidism do not develop overt clinical signs and other cats may only display subtle and non-specific clinical signs such as lethargy, unspecific dermatological changes, decreased appetite or weight gain, some of which could be attributed to the resolution of hyperthyroidism rather than hypothyroidism.11,12 In addition, the use of subnormal T4 or free T4 concentration alone to diagnose iatrogenic hypothyroidism can also be misleading because non-thyroidal illness can decrease serum T4 and free T4 concentrations.13–16 Instead, the measurement of serum TSH concentration appears to be more sensitive and specific than T4 and free T4 alone for the diagnosis of iatrogenic hypothyroidism and to differentiate between cats with iatrogenic hypothyroidism and those with non-thyroidal illnesses. 16
We have routinely been using the scoring system described by Peterson and Becker to determine 131I dosing for several years. 5 Therefore, the aim of this study was to report the prevalence of iatrogenic hypothyroidism, with and without azotaemia, based on the measurement of serum T4, TSH and creatinine concentrations, in hyperthyroid cats undergoing 131I treatment where the 131I dose was calculated using this scoring system. Another objective of the study was to determine the positive and negative predictive values (PPV and NPV, respectively) of serum T4 and TSH concentrations obtained prior to hospital discharge to predict the development of iatrogenic hypothyroidism 6–9 months after 131I treatment.
Material and methods
Cats
Hyperthyroid cats referred for radioiodine treatment at the Centre for Small Animal Studies (Animal Health Trust, Newmarket, UK) between October 2013 and December 2015 were eligible for this prospective study. The diagnosis of hyperthyroidism was based on compatible clinical signs and an increased serum T4 concentration above the reference interval (RI) for the reference laboratory used. 1 Different reference laboratories with different methodologies were used, but none of the cats were diagnosed with hyperthyroidism using an in-house point-of-care analyser. The study was approved by the Animal Health Trust’s ethics committee and enrolment of the cats in the study was subject to informed owner consent.
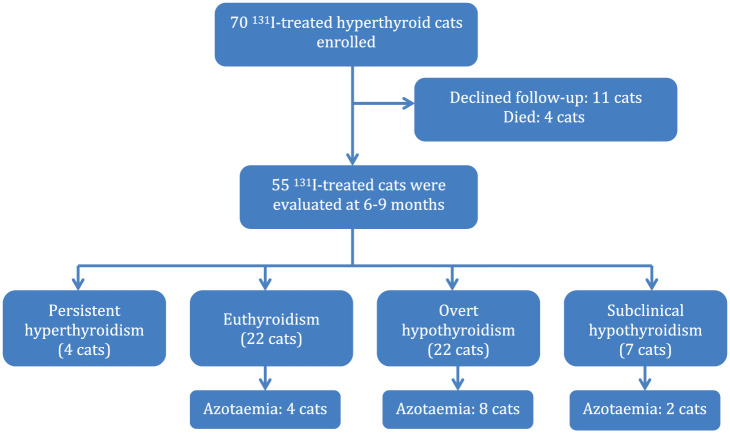
During the study period, 70 cats were prospectively enrolled, receiving 71 131I treatments, and 55 cats completed the study at 6–9 months after 131I treatment. Eleven cats were lost to follow-up and four cats died due to non-thyroidal illnesses (Figure 1). Most hyperthyroid cats (40/55) that completed the study had a pretreatment assessment performed at our hospital, during a different appointment, which included a review of the history, physical examination, systolic blood pressure measurement, laboratory testing (full haematology, comprehensive serum biochemistry, serum T4 concentration for hyperthyroidism monitoring and urinalysis) if these were not performed within 1 month prior to assessment by the referring veterinarian, along with thoracic and abdominal radiographs and abdominal ultrasound. Further investigations (eg, echocardiography, fine-needle aspirates, etc) were carried out in some cats depending on the findings during the assessment. 17 The remainder of the cats (15/55) had the above investigations performed at the referring veterinarian.
Figure 1.
Flowchart for the enrolment of hyperthyroid cats, separated into thyroid status and presence of azotaemia at the end of the study
The authors reviewed the clinical history (including the investigations performed and treatments prescribed) of all these cats, and performed a consultation with the owners and a thorough physical examination the day of hospital admission prior to 131I treatment. Treatment with antithyroid drugs or the use of a veterinary prescription iodine-restricted diet were discontinued for 2 weeks prior to 131I treatment.
Procedures
The dose of 131I was determined for each hyperthyroid cat by various internal medicine specialists and based on a scoring system that included severity of clinical signs, serum T4 concentration and palpable thyroid gland size, as previously described. 5 Every variable had a score from 1–3. Five cats without a palpable goitre received a score of 1 for this variable. Cats with a total score of 3–5 received a low 131I dose (74–130 MBq; 2.0–3.4 mCi), cats with a total score of 6 or 7 received a moderate 131I dose (130–167 MBq; 3.5–4.4 mCi) and cats with a total score of 8 or 9 received a high 131I dose (167–222 MBq; 4.5–6.0 mCi). 5 After 131I treatment, all cats were hospitalised in individual cages in a designated radiation isolation area for 3 weeks and then discharged.
Serum T4, TSH and creatinine concentrations were measured by the same reference veterinary diagnostic laboratory (Powell Torrance Diagnostic Services, UK ) 19 days (prior to hospital discharge) and 6–9 months after 131I treatment. Serum T4 concentration was measured by a chemiluminescent immunoassay (Immulite Total T4; Siemens Healthcare Diagnostics Products); serum TSH was measured similarly using a canine assay (Immulite Canine TSH; Siemens Healthcare Diagnostics Products), both validated in cats.10,18 The T4 and canine TSH assays were also validated for use in cats at the same laboratory (see Appendix A in the supplementary material). Serum creatinine was measured by a modified Jaffe picrate reaction (Werfen-Instrumentation Laboratory, Italy). In order to maintain enrolment compliance, the owners of treated cats could have the follow-ups performed by their local veterinarians, who could submit the blood samples directly to the designated reference veterinary diagnostic laboratory.
Data analysis
SPSS Statistics for Macintosh version 22.0 (IBM) was used for statistical analysis. Continuous data were assessed for normality using the Shapiro–Wilk test. Only serum creatinine was normally distributed and therefore all analyses were performed using non-parametric tests. Continuous data were expressed as median and interquartile ranges (IQR; 25th–75th percentile). Categorical variables were compared between groups by the Pearson’s χ2 test. For all analyses P values <0.05 were considered to be statistically significant.
The thyroid status of treated hyperthyroid cats was classified based on the laboratory RIs, as persistently hyperthyroid (T4: >50.0 nmol/l; TSH: <0.03 ng/ml), euthyroid (T4: <50.0 nmol/l; TSH: ⩽0.20 ng/ml), overtly hypothyroid (T4: <15.0 nmol/l; TSH: >0.20 ng/ml) and subclinically hypothyroid (T4: 15.0–50.0 nmol/l; TSH: >0.20 ng/ml), as previously defined. 7 See Appendix A in the supplementary material for details on how the RIs for serum T4 and TSH concentrations were determined. The lower limits of quantification of the T4 and TSH assays were 12.9 nmol/l and 0.03 ng/ml, respectively. Therefore, for data analysis, all undetectable serum T4 and TSH concentrations were given an arbitrary concentration of 10 nmol/l and 0.02 ng/ml, respectively. 19 The ultimate thyroid status of each cat was assessed with the results obtained 6–9 months after 131I treatment. Azotaemia was defined as a creatinine above the laboratory RI (creatinine: >180 μmol/l).
Results
Study population
Cat breeds that completed the study included domestic shorthair (n = 47), domestic longhair (n = 6), British Shorthair (n = 1) and Birman (n = 1). The median age of these cats was 12 years (IQR 11–14 years). There were 31 male and 24 female cats, all of which were neutered. Prior to radioiodine treatment, 38 cats had been treated with oral anti-thyroid drugs (methimazole [17 cats] or carbimazole [21 cats]), eight cats with transdermal methimazole, three cats with the use of an iodine-restricted diet and six cats were not receiving any treatment due to antithyroid drug side effects (five cats) or inability to medicate the cat (one cat). None of the cats were azotaemic at diagnosis. Euthyroidism had been achieved in 44 cats prior to 131I treatment, with 42 cats remaining non-azotaemic (median 110 μmol/l; IQR 91–124 μmol/l) and two cats developing a mild azotaemia (184 μmol/l and 189 μmol/l, respectively). None of the 11 cats that remained hyperthyroid prior to 131I treatment were azotaemic (median 76 μmol/l; IQR 60–94 μmol/l).
An immediate pretreatment serum T4 concentration, measured at a single laboratory (Dick White Referrals, Diagnostic Pathology, UK), and performed at least 2 weeks after discontinuing antithyroid treatment, was available in 46/55 cats. A serum T4 concentration, in cats not receiving any antithyroid treatment for at least 2 weeks, was available at a median of 3 months (IQR 2.5–4.0 months) prior to the remaining 9/55 131I treatments. The median pretreatment serum T4 concentration was 163 nmol/l (IQR 119–229 nmol/l). Based on the scoring system, 17/55 cats (30.9%) received a low 131I dose (median 100 MBq [2.7 mCi]; IQR 80–110 MBq [2.2–3.0 mCi]), 30/55 cats (54.5%) received a moderate 131I dose (median and IQR 130 MBq, 3.5 mCi) and 8/55 (14.5%) cats received a high 131I dose (median 167 MBq [4.5 mCi]; IQR 167–200 MBq [4.5–5.4 mCi]). The median 131I dose administered to all cats was 130 MBq (3.5 mCi) (IQR 110–130 MBq [2.7–3.5 mCi]).
Thyroid and renal parameters after 131I treatment
The modal serum T4 concentration 19 days after 131I treatment for the 55 cats completing the study was 10.0 nmol/l (IQR 10.0–13.6 nmol/l). Serum T4 concentration was within the RI in 14/55 (25.5%) cats and decreased in 35/55 (63.6%) cats, most of which (33/35 cats) had a serum T4 concentration below the detection limit of the assay. Six of the 55 cats (10.9%) had an increased serum T4 concentration (range 53.5–354.0 nmol/l).
The modal serum TSH concentration 19 days after 131I treatment was 0.02 ng/ml (IQR 0.02–0.09 ng/ml). Serum TSH concentration was within the RI in 12/55 (21.8%) cats and below the detection limit of the assay in 39/55 (70.9%) cats, including all cats with increased serum T4 concentration. Serum TSH concentration was increased in 4/55 (7.3%) cats (range 0.24–0.60 ng/ml) and the serum T4 concentration was below the detection limit of the assay in these four cats.
The median serum creatinine concentration was 130 μmol/l (IQR 107–158 μmol/l). Five of the 55 cats (9.1%) had an increased creatinine concentration (range 186–198 μmol/l) and all these cats had a decreased serum T4 concentration, four had a decreased serum TSH concentration and one cat had a normal (0.10 ng/ml) serum TSH concentration. Four cats with decreased serum T4 and increased serum TSH concentrations had a serum creatinine concentration that ranged from 110–179 μmol/l. The serum creatinine concentration in two cats that developed azotaemia after achieving a euthyroid state prior to 131I treatment remained stable (⩽16% variation) at 19 days after 131I treatment. 20
Fifty-five cats completed the study at the 6–9 month re-check (median 206 days; IQR 198–238 days) after 131I treatment. Of the six cats with an increased serum T4 concentration at 19 days, prior to hospital discharge, four cats remained persistently hyperthyroid, giving a treatment failure rate of 5.6% (4/71 131I treatments). Three of these four cats had received a moderate 131I dose and one cat a low 131I dose. One of these four cats received a successful second 131I treatment and the other three cats were managed with antithyroid drugs because the owners declined a second 131I treatment.
Median serum T4 concentration at the 6–9 month follow-up, excluding the persistently hyperthyroid cats as three of them were already receiving antithyroid drugs, was 13.0 nmol/l (IQR 10.0–21.5 nmol/l) (Figure 2). The serum T4 concentration was within the RI in 22/51 (43.1%) cats and decreased in 29/51 (56.9%) cats. The serum T4 concentration was below the detection limit of the assay in 25/29 of these cats. Median serum TSH concentration 6–9 months after 131I treatment was 0.30 ng/ml (IQR 0.10–1.63 ng/ml). Serum TSH concentration was within the RI in 14/51 (27.4%) cats, below the detection limit of the assay in 8/51 (15.7%) cats and increased in 29/51 (56.9%) cats. The serum creatinine concentration was within the RI in 37/51 (72.5%) cats (median 144 μmol/l; IQR 130–160 μmol/l) and increased in 14/51 (27.5%) cats (median 204 μmol/l; IQR 192–221 μmol/l).
Figure 2.
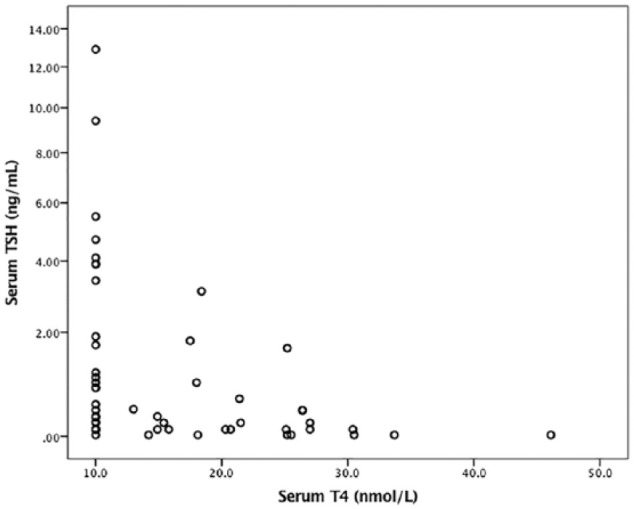
Scatter plots comparing serum thyroid-stimulating hormone (TSH) and total thyroxine (T4) concentrations in 51 cats after radioiodine treatment
Two cats that were azotaemic after achieving euthyroidism prior to 131I treatment were euthyroid and subclinically hypothyroid 6–9 months after 131I treatment, but in both cases the serum creatinine concentrations remained stable throughout the study (<4% variation between samples taken at 19 days and 6–9 months).
Thyroid status and development of azotaemia after 131I treatment
The prevalence of euthyroidism, overt hypothyroidism and subclinical hypothyroidism 6–9 months after 131I treatment was 40.0% (22/55 cats), 40.0% (22/55 cats) and 12.7 % (7/55 cats), respectively. There was no statistically significant association between remaining persistently hyperthyroid or the development of either overt or subclinical hypothyroidism and the 131I dose given (low, moderate or high). Fifteen of 22 euthyroid cats had serum T4 concentrations within the RI, with normal or below the detection limit of the assay serum TSH concentrations in nine and six cats, respectively. Seven of 22 euthyroid cats had serum T4 concentrations below the RI, with normal or below the detection limit of the assay serum TSH concentrations in five and two cats, respectively. Both cats with decreased serum T4 and TSH concentrations were suspected to have non-thyroidal illness.
Azotaemia developed in 4/22 (18.2%) euthyroid cats, 8/22 (36.4%) overtly hypothyroid cats and in 2/7 (28.6%) subclinically hypothyroid cats. There was no significant difference in the development of azotaemia between euthyroid cats or cats with either overt or subclinical hypothyroidism.
The PPV and NPV for the prediction of the development of iatrogenic hypothyroidism 6–9 months after 131I treatment were 72.2% and 80.0%, respectively, for a low serum T4 concentration; and 75.0% and 44.6%, respectively, for an increased serum TSH concentration, obtained at 19 days after 131I treatment (prior to hospital discharge).
Discussion
In this study, 40% of cats treated with 131I, where the dose was calculated based on an individualised scoring system, were overtly hypothyroid 6–9 months after treatment. Although the prevalence of iatrogenic hypothyroidism defined as cats with a decreased T4 concentration ⩾3 months after 131I treatment has been reported to be as high as 79%, most studies report a prevalence of ⩽20%, which is significantly lower than that reported in this study.7,8,21–23 Persistent hyperthyroidism was diagnosed in 5.6% of treated cats, which is comparable to previous studies where the prevalence is usually ⩽5%.5,7,16,21–23
Successful treatment of hyperthyroidism in cats results in a decrease in glomerular filtration rate, which can lead to the development of azotaemia if underlying chronic kidney disease is present. In cats with iatrogenic hypothyroidism the glomerular filtration rate may decrease even further leading to an additional decline in renal function. 24 One study identified that cats that developed iatrogenic hypothyroidism were more likely to develop azotaemia in comparison to cats that remained euthyroid, and those cats with iatrogenic hypothyroidism that also developed azotaemia had a shorter survival. 10
As iatrogenic hypothyroidism appears to contribute to the progression of renal dysfunction, treatment of hyperthyroid cats with 131I should be aimed at restoring euthyroidism without inducing iatrogenic hypothyroidism. However, the optimal method to calculate the 131I dose to achieve this aim remains controversial. 8 The dose employed to treat feline hyperthyroidism in veterinary medicine is determined by either individualised protocols or the use of a fixed 131I dose, with a trend towards the use of fixed 131I doses.5–7,21,22 However, no method has been demonstrated to be superior. In a previous and larger study evaluating the efficacy of the scoring system used in this study, <2% of treated cats were persistently hyperthyroid and 11% of treated cats had a low serum T4 concentration 6–12 months after 131I treatment. 5 The median 131I dose used was lower than in the current study (110 MBq [3.0 mCi] vs 130 MBq [3.5 mCi]) and almost twice as many cats in the former study received a low 131I dose (59.2% vs 29.6%), which possibly accounts for the lower prevalence of iatrogenic hypothyroidism. 5
One recent retrospective study using a fixed dose of 124 MBq (3.35 mCi), identified a prevalence of persistent hyperthyroidism and iatrogenic hypothyroidism of 2%. 22 Cats were classified as hypothyroid if they had a subnormal serum T4 concentration and clinical signs consistent with the disease. However, serum TSH concentrations were not measured and cats with iatrogenic hypothyroidism uncommonly develop overt clinical signs.11,12
Moreover, the use of low serum T4 concentration without TSH to diagnose iatrogenic hypothyroidism is suboptimal as non-thyroidal illness can lower T4 concentration.16,25 Therefore, it is possible that the study underestimated the prevalence of iatrogenic hypothyroidism. 12
While the use of lower 131I doses could decrease the prevalence of iatrogenic hypothyroidism in our cohort of cats, the prevalence of persistent hyperthyroidism could increase above the commonly reported prevalence of ⩽5%.5,7,16,21–23 Additionally, only one of the four persistent hyperthyroidism cats underwent a second 131I, highlighting that many owners decline a second 131I treatment for various reasons. 26 In the light of this, and as 131I therapy is generally considered superior to other treatment options, many clinicians may try to use higher dosages of 131I to minimise the risk of treatment failure. 6 However, a recent study comparing the efficacy of a lower (74 MBq [2.0 mCi]) or higher 131I dose (148 MBq [4.0 mCi]) for the treatment of cats with mild-to-moderate hyperthyroidism identified a lower incidence of overt and subclinical hypothyroidism in the former group without a significant difference in the rate of persistent hyperthyroidism between both groups. 7 Therefore, an increase in the incidence of treatment failure may not occur, even when lower 131I dosages are given.
Interestingly, the prevalence of overt hypothyroidism in those cats treated with a higher 131I dose (148 MBq [4.0 mCi]) in the abovementioned study was less than half than in our study (18.0% vs 40.0%), where only 14.5% of cats were treated with a 131I dose of ⩾148 MBq (4.0 mCi), although the prevalence of subclinical hypothyroidism was significantly lower in our study (46% vs 12.7%). 7 The reason for the higher prevalence of overt hypothyroidism in our study is unclear. Possible causes for these results, given that there was no association between the development of iatrogenic hypothyroidism and the 131I dose given, include random variability due to the low number of cases included in both studies, patients’ variables (eg, individual variability in 131I uptake by the different thyroid follicles, population differences between American and European hyperthyroid cats), analytical variables (eg, a higher lower limit of the RI for serum T4 concentration was used in this study) and/or human errors such as injecting a higher 131I dose than the calculated or overestimation of the 131I dose (eg, assigning a cat with mild clinical signs a moderate category or overestimation of goitre size) given the degree of subjectivity of the scoring system. 5
A serum T4 concentration was not available immediately prior to 9/55 131I treatments and in these cases the most recent T4 concentration, when the cat was not receiving any antithyroid treatment, was used to calculate the 131I dose. It is unknown whether the prevalence of iatrogenic hypothyroidism would change if the 131I dose calculation included a serum T4 concentration obtained just prior to treatment in all cats. However, as T4 concentration increases over time, some cats could have received higher 131I doses, which could have increased and decreased the prevalence of iatrogenic hypothyroidism and persistent hyperthyroidism, respectively. 27
Seven of 55 (12.7%) cats were classified as subclinically hypothyroid at 6–9 months after 131I treatment. Subclinical hypothyroidism has been used to describe, in human medicine and more recently in veterinary medicine, the finding of a normal (usually low–normal) T4 concentration and an increased serum TSH concentration.7,12,16,23,28 Subclinical hypothyroidism appears to be a common consequence after 131I treatment in human patients with Graves’ disease or toxic nodular goitre, and has been correlated with a relative increase in all-cause mortality when compared with euthyroid controls.28,29 The clinical significance of subclinical hypothyroidism in cats is not completely understood and it can be self-limiting in about a third of cats with a mild TSH elevation.12,16,30 However, in a recent study cats with (overt or subclinical) iatrogenic hypothyroidism not given levothyroxine supplementation had a shorter median survival than hypothyroid cats receiving supplementation, suggesting a beneficial effect of thyroid hormone replacement on survival. 16 Overt hypothyroidism and azotaemia were identified in 8/55 (14.5%) cats, 6–9 months after 131I treatment, which has been associated with a shorter survival and therefore could also benefit from levothyroxine supplementation. 10
The PPV and NPV of decreased serum T4 concentration obtained prior to hospital discharge to predict the development of iatrogenic hypothyroidism 6–9 months after 131I treatment were 72.2% and 80.0%, respectively. In other words, while 72.2% of cats with a decreased serum T4 concentration prior to hospital discharge were subsequently confirmed to have developed either overt or subclinical hypothyroidism, only 20.0% of cats with a normal serum T4 concentration became overtly or subclinically hypothyroid, suggesting the need for a closer monitoring of cats with a low serum T4 concentration prior to hospital discharge given the higher probability of them developing iatrogenic hypothyroidism.
An increased serum TSH concentration obtained prior to hospital discharge had a PPV of 75.0% to predict the development of iatrogenic hypothyroidism 6–9 months after 131I treatment. However, this value was based on only four cats with an increased TSH at 19 days and a larger number of cases would be needed to validate this finding. The NPV of an increased TSH at 19 days after treatment was low (44.6%) as most cats had an undetectable serum TSH concentration. Therefore, three-quarters of cats with increased serum TSH concentration prior to hospital discharge developed iatrogenic hypothyroidism 6–9 months after 131I treatment. However, the presence of a normal or undetectable TSH was not useful to discriminate between which cats would become hypothyroid and which cats would become euthyroid 6–9 months after 131I therapy.
Two cats in the present study had decreased serum T4 and TSH concentrations 6–9 months after 131I treatment. These cats were reported to be clinically well by the referring veterinarian and had not become azotaemic, although the creatinine was in the upper limit of the RI in both cases. This finding was attributed to non-thyroidal illness. However, in contrast to T4, TSH concentrations in cats with non-thyroidal illnesses has been reported to decrease only in severe illness. 25 Additionally, the RI for serum T4 concentration was calculated based on 30 cats, not age-matched (see Appendix A in the supplementary material). Therefore, the lower serum T4 concentration in these two cats may have been normal if more clinically healthy, age-matched cats were initially included and a lower RI for serum T4 concentration was obtained. Unfortunately, the owners of these cats declined further investigations and the cats were lost to follow-up.
Up to 49% of hyperthyroid cats develop azotaemia within 6 months of treatment of hyperthyroidism and three recent studies have shown that cats that develop iatrogenic hypothyroidism are more likely to become azotaemic than those cats that remain euthyroid.10,16,23 Although in our study the percentage of azotaemia in overtly hypothyroid cats was double (36.4% vs 18.2%) that of euthyroid cats, the difference was not statistically significant. Given the current literature this likely represents a type II error due to a low number of cases. As the majority of follow-ups were performed by referring veterinarians, the body weight and muscle condition scores of treated cats was not usually available, and repeated serum creatinine or urine specific gravity measurements that could have helped to differentiate cats with pre-renal instead of renal azotaemia, or identify cats with non-azotaemic chronic kidney disease (eg, IRIS stage 1 or early stage 2), were not consistently performed.
Conclusions
This study reaffirms that the use of an individualised scoring system previously described by Peterson and Becker in 1995 is effective to determine the dose of 131I for the treatment of hyperthyroid cats, with rates of treatment failure comparable to other described methods. 5 However, the prevalence of iatrogenic hypothyroidism was higher in comparison with other studies using different dosing protocols. Further studies comparing the efficacy of an individualised scoring system and different dosing regimens are warranted to determine which method is superior to induce euthyroidism while minimising the prevalence of persistent hyperthyroidism and iatrogenic hypothyroidism.
Supplemental Material
Hormone validation information and determination of reference intervals.
Acknowledgments
We would like to thank the referring veterinarians and staff involved in the care of these cats.
Footnotes
Accepted: 7 December 2018
Author note: Part of this work was presented in abstract form at BSAVA Congress 2017.
Supplementary material: The following file is available online: Appendix A: Hormone validation information and determination of reference intervals.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The study was partially financed by the Animal Health Trust.
ORCID iD: Yordan Fernandez  https://orcid.org/0000-0002-9287-8931
https://orcid.org/0000-0002-9287-8931
References
- 1. Mooney CT, Peterson ME. Feline hyperthyroidism. In: Mooney CT, Peterson ME. (eds). Manual of canine and feline endocrinology. 4th ed. Quedgeley: British Small Animal Veterinary Association, 2012, pp 199–203. [Google Scholar]
- 2. Daminet S, Kooistra HS, Fracassi F, et al. Best practice for the pharmacological management of hyperthyroid cats with antithyroid drugs. J Small Anim Pract 2014; 55: 4–13. [DOI] [PubMed] [Google Scholar]
- 3. Hui TY, Bruyette DS, Moore GE, et al. Effect of feeding an iodine-restricted diet in cats with spontaneous hyperthyroidism. J Vet Intern Med 2015; 29: 1063–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Naan EC, Kirpensteijn J, Kooistra HS, et al. Results of thyroidectomy in 101 cats with hyperthyroidism. Vet Surg 2006; 35: 287–293. [DOI] [PubMed] [Google Scholar]
- 5. Peterson ME, Becker DV. Radioiodine treatment of 524 cats with hyperthyroidism. J Am Vet Med Assoc 1995; 207: 1422–1428. [PubMed] [Google Scholar]
- 6. Milner RJ, Channell CD, Levy JK, et al. Survival times for cats with hyperthyroidism treated with iodine 131, methimazole, or both: 167 cases (1996–2003). J Am Vet Med Assoc 2006; 228: 559–563. [DOI] [PubMed] [Google Scholar]
- 7. Lucy J, Peterson ME, Randolph JF, et al. Efficacy of low-dose (2 millicurie) versus standard-dose (4 millicurie) radioiodine treatment for cats with mild-to-moderate hyperthyroidism. J Vet Intern Med 2017; 31: 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peterson ME. Radioiodine treatment of hyperthyroidism. Clin Tech Small Anim Pract 2006; 21: 34–39. [DOI] [PubMed] [Google Scholar]
- 9. Boag AK, Neiger R, Slater L, et al. Changes in the glomerular filtration rate of 27 cats with hyperthyroidism after treatment with radioactive iodine. Vet Rec 2007; 161: 711–715. [DOI] [PubMed] [Google Scholar]
- 10. Williams TL, Elliott J, Syme HM. Association of iatrogenic hypothyroidism with azotemia and reduced survival time in cats treated for hyperthyroidism. J Vet Intern Med 2010; 24: 1086–1092. [DOI] [PubMed] [Google Scholar]
- 11. Peterson ME. Diagnosis and management of iatrogenic hypothyroidism. In: Little SE. (ed). August’s consultations in feline internal medicine. 7th ed. St Louis, MO: Elsevier, 2016, pp 260–269. [Google Scholar]
- 12. Peterson ME, Gutherl JN. Overt or subclinical iatrogenic hypothyroidism in cats: clinical, laboratory, and thyroid scintigraphy findings in 35 cases [abstract]. J Vet Intern Med 2015; 29: 447–448. [Google Scholar]
- 13. Peterson ME, Gamble DA. Effect of nonthyroidal illness on serum thyroxine concentrations in cats: 494 cases (1988). J Am Vet Med Assoc 1990; 197: 1203–1208. [PubMed] [Google Scholar]
- 14. Mooney CT, Little CJ, Macrae AW. Effect of illness not associated with the thyroid gland on serum total and free thyroxine concentrations in cats. J Am Vet Med Assoc 1996; 208: 2004–2008. [PubMed] [Google Scholar]
- 15. Peterson ME, Melian C, Nichols R. Measurement of serum concentrations of free thyroxine, total thyroxine, and total triiodothyronine in cats with hyperthyroidism and cats with nonthyroidal disease. J Am Vet Med Assoc 2001; 218: 529–536. [DOI] [PubMed] [Google Scholar]
- 16. Peterson ME, Nichols R, Rishniw M. Serum thyroxine and thyroid-stimulating hormone concentration in hyperthyroid cats that develop azotaemia after radioiodine therapy. J Small Anim Pract 2017; 58: 519–530. [DOI] [PubMed] [Google Scholar]
- 17. Puig J, Cattin I, Seth M. Concurrent diseases in hyperthyroid cats undergoing assessment prior to radioiodine treatment. J Feline Med Surg 2015; 17: 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peterson ME, Guterl JN, Nichols R, et al. Evaluation of serum thyroid-stimulating hormone concentration as a diagnostic test for hyperthyroidism in cats. J Vet Intern Med 2015; 29: 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wakeling J, Elliott J, Syme H. Evaluation of predictors for the diagnosis of hyperthyroidism in cats. J Vet Intern Med 2011; 25: 1057–1065. [DOI] [PubMed] [Google Scholar]
- 20. Baral RM, Dhand NK, Freeman KP, et al. Biological variation and reference change values of feline plasma biochemistry analytes. J Feline Med Surg 2014; 16: 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chun R, Garrett LD, Sargeant J, et al. Predictors of response to radioiodine therapy in hyperthyroid cats. Vet Radiol Ultrasound 2002; 43: 587–591. [DOI] [PubMed] [Google Scholar]
- 22. Vagney M, Desquilbet L, Reyes-Gomez E, et al. Survival times for cats with hyperthyroidism treated with a 3.35 mCi iodine-131 dose: a retrospective study of 96 cases. J Feline Med Surg 2018; 20: 528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peterson ME, Varela FV, Rishniw M, et al. Evaluation of serum symmetric dimethylarginine concentration as a marker for masked chronic kidney disease in cats with hyperthyroidism. J Vet Intern Med 2018; 32: 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Hoek I, Daminet S. Interactions between thyroid and kidney function in pathological conditions of these organ systems: a review. Gen Comp Endocrinol 2009; 160: 205–215. [DOI] [PubMed] [Google Scholar]
- 25. Davignon D, Lucy J, Randolph J, et al. Effect of non-thyroidal illness on serum concentrations of T4, free T4 and thyroid stimulating hormone in cats [abstract]. J Vet Intern Med 2015; 29: 1174–1175. [Google Scholar]
- 26. Boland LA, Murray JK, Bovens CP, et al. A survey of owners’ perceptions and experiences of radioiodine treatment of feline hyperthyroidism in the UK. J Feline Med Surg 2014; 16: 663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peterson ME, Broome MR, Rishniw M. Prevalence and degree of thyroid pathology in hyperthyroid cats increases with disease duration: a cross-sectional analysis of 2096 cats referred for radioiodine therapy. J Feline Med Surg 2016; 18: 92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vanderpump MP, Tunbridge WM. Epidemiology and prevention of clinical and subclinical hypothyroidism. Thyroid 2002; 12: 839–847. [DOI] [PubMed] [Google Scholar]
- 29. Haentjens P, Van Meerhaeghe A, Poppe K, et al. Subclinical thyroid dysfunction and mortality: an estimate of relative and absolute excess all-cause mortality based on time-to-event data from cohort studies. Eur J Endocrinol 2008; 159: 329–341. [DOI] [PubMed] [Google Scholar]
- 30. Peterson ME, Rishniw M. Hyperthyroid cats develop transient or persistent subclinical hypothyroidism after successful radioiodine treatment [abstract]. J Vet Intern Med 2017; 31: 222. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hormone validation information and determination of reference intervals.