Abstract
Objectives
Magnesium has been ‘the forgotten ion’ for many years. Over the past decade, however, the role of magnesium in essential physiological functions and several illness conditions have been elucidated. Nevertheless, the investigation of magnesium in cats with chronic kidney disease (CKD) and nephrolithiasis is yet to be determined. The purpose of this study was to investigate whether CKD cats with nephrolithiasis have changes in total serum magnesium concentrations, and whether magnesium disorders may be associated with other electrolyte disturbances, as well as with prognosis. We also aimed to evaluate whether total serum magnesium concentration differs between CKD cats with and without nephrolithiasis.
Methods
Total serum magnesium concentrations were assessed in 42 cats with CKD with stage 1–4 nephrolithiasis. The correlation between magnesium and other electrolytes, as well as Kaplan–Meier survival analysis, were performed. We also selected 14 control cats with CKD without nephrolithiasis age-matched with 14 cats with CKD with nephrolithiasis.
Results
Hypermagnesemia was observed in 16/42 (38.1%) and hypomagnesemia in 6/42 (14.3%) cats. Serum magnesium abnormalities were observed in cats of all stages, and marked hypermagnesemia was noted in cats with stage 4 CKD with nephrolithiasis (P <0.001). There was a negative correlation between total serum magnesium and ionized calcium (r = −0.64; P <0.01), and a positive correlation between total serum magnesium and serum phosphorus (r = 0.58, P = 0.01). Cats with CKD with nephrolithiasis and hypomagnesemia or hypermagnesemia had higher mortality than those with normal total serum magnesium concentration (P <0.01), regardless of CKD stage. There was no difference in total serum magnesium concentration between CKD cats with and without nephrolithiasis.
Conclusions and relevance
Cats with CKD with nephrolithiasis have magnesium abnormalities. Hypomagnesemia and hypermagnesemia were associated with an increase in mortality, and thus total serum magnesium abnormalities may be used as prognostic factors in these cases.
Keywords: Magnesium, electrolytes, mortality, nephrolithiasis, renal insufficiency
Introduction
Magnesium plays a role in essential physiological functions as it is involved in the regulation of ion channels, acts as a cofactor in enzymatic reactions for protein synthesis, and is intrinsically related with the mineral and bone metabolism.1–3 Despite its physiological relevance, the ‘forgotten electrolyte’, as it used to be known, remained neglected for years. However, over the past decade, findings regarding the role of magnesium in several illnesses, such as diabetes, cardiovascular disease and chronic kidney disease (CKD), gained the attention of researchers worldwide to the clinical relevance of this mineral.4–8
The kidneys are the major regulators of electrolyte homeostasis through tubular excretion or reabsorption. Hence, magnesium disturbances, in addition to other electrolytes abnormalities, are expected to occur in kidney function impairment.3,9 In humans, renal insufficiency is considered the major cause of hypermagnesemia, and serum or urinary magnesium abnormalities in some tubular disorders have been associated with the occurrence of CKD, nephrocalcinosis and nephrolithiasis;8,10–12 moreover, recent studies have shown that hypomagnesemia is a risk factor for decline in kidney function and death.1,5,13–16 In addition, magnesium abnormalities have also been implicated in mineral and bone disorders in CKD (CKD-MBD), as magnesium has inhibitory effects on parathyroid hormone (PTH) levels and is closely related with calcium metabolism.2,17–20
In 2008, Schenck evaluated the serum magnesium concentrations in association with feline calcium metabolic disorders, including CKD; the occurrence of hypo- and hypermagnesemia were identified in 49% and 44% of the cases with hyperparathyroidism and hypoparathyroidism, respectively. 21 Recently, an elegant study assessed the role of magnesium in feline CKD-MBD, and it was found that low magnesium concentrations were associated with higher values of fibroblast growth factor-23 and increased risk of death. 22
In cats with kidney stones, few data are available. To the best of our knowledge, the investigation of magnesium in cats with CKD with nephrolithiasis is yet to be determined. Given the clinical relevance of these concurrent disorders in feline medical practice, the objectives of this study were: (1) to evaluate the total serum magnesium concentrations in CKD cats with nephrolithiasis; (2) to investigate whether magnesium disorders may be associated with other electrolyte disturbances, as well as with prognosis; and (3) to evaluate whether total serum magnesium concentration differs between cats with CKD with and without nephrolithiasis.
Materials and methods
This study was approved by the Animal Research Ethics Committee of the University of Sao Paulo School of Veterinary Medicine and Animal Science (Protocol number: 7919261115). Samples were collected with the informed consent of the cats’ owners. All of the cats were treated at the Clinic of the Veterinary Teaching Hospital of the University of Sao Paulo (VTH-USP), School of Veterinary Medicine and Animal Science, in the city of Sao Paulo, Brazil.
We recruited 42 cats with CKD with nephrolithiasis: 19 males and 23 females, all neutered (mean ± SD age 9.2 ± 4.6 years) and of all types of breed. The cats were divided into four groups by CKD stage (stage 1, n = 7; stage 2, n = 10; stage 3, n = 15; stage 4, n = 10). The International Renal Interest Society recommendations were used for the diagnostic and staging of CKD. 23 Cats with stage 1 CKD were non-azotemic but showed some combination of abnormal renal ultrasound findings, isosthenuria or proteinuria (urine to protein creatine ratio >0.4). Cats with stage 2 CKD had mild renal azotemia and ultrasound findings indicative of chronic nephropathy, isosthenuria or proteinuria. Cats with stage 3 CKD cats showed moderate renal azotemia, together with mild-to-moderate clinical signs, such as polyuria and polydipsia, and abnormal renal ultrasound findings, isosthenuria or proteinuria. Cats with stage 4 CKD had severe renal azotemia, ultrasound findings indicative of chronic nephropathy, isosthenuria or proteinuria, and renal and extra-renal clinical signs, such as great weight loss, decreased appetite and gastrointestinal disturbance. Abdominal ultrasound was performed by board-certified radiologists or radiology residents and the diagnosis of nephrolithiasis was made based on previously published criteria. 24
Serum samples of 21 clinically normal cats were obtained in order to establish the normal reference interval (RI) of total serum magnesium concentrations (mean ± SD, 1.87 ± 0.29 mg/dl; range 1.58–2.16 mg/dl), in accordance with the clinical pathology laboratory/VTH-USP. Healthy cats were recruited based on normal findings from history and physical examination, as well as in the complete blood count and normal serum biochemical profiles.
In order to compare the mean total serum magnesium concentrations between kidney stone formers and non-stone formers, we also selected 14 control cats with CKD without nephrolithiasis that were stage (stage 2, n = 7; stage 3, n = 7) and age-matched with 14 cats with CKD with nephrolithiasis. We used cats from a multi-cat household, in order to obtain similar environmental and dietary conditions. CKD cats without nephrolithiasis ranged from 1.7–19.0 years in age, and cats with CKD with nephrolithiasis were aged 1.3–18.4 years old.
Magnesium is minimally affected by albumin. 25 Herein, we used the total serum magnesium concentrations to assess the magnesium status of cats with CKD with nephrolithiasis, regardless of albumin levels.
The exclusion criteria of the study were the use of magnesium supplementation or magnesium binders, as well as infectious, endocrine, hepatic or neoplastic diseases.
Blood samples were obtained via jugular venepuncture and placed in both gel tubes and lithium heparin (Blood Gas Monovette; Sarstedt), for serum and electrolyte profiles, respectively. Total serum magnesium (Mg 3880; Randox), creatinine (Creatinina K 96; Labtest), urea (Urea FS 1 3101; DiaSys) and phosphorus (Phosphorus 12-200; Labtest) were performed by routine automated techniques (Labmax 240; Labtest). Ionized calcium, sodium, potassium and chloride were performed by ion-selective electrode method (Cobas b 121; Roche).
Depending on the results obtained on the Kolmogorov–Smirnov normality test, we used either one-way ANOVA or the Kruskal–Wallis test. For the comparison of two means, Student’s t-test or the Mann–Whitney test was applied. For multiple comparison tests, the Tukey–Kramer test or Dunn’s test was performed. Pearson or Spearman correlation tests were also performed. Survival among cats with CKD with nephrolithiasis and normal, low or high total serum magnesium concentrations were determined by Kaplan–Meier analysis, based on the days of survival since the diagnostic of magnesium abnormalities. The log-rank (Mantel–Cox) method was performed to test differences among the survival curves. P values <0.05 were considered statistically significant. The data in the text and graphs are presented as mean ± SD. Statistical analysis was performed with GraphPad Prism software, version 6.
Results
Hypermagnesemia was observed in 16/42 (38.1%) and hypomagnesemia in 6/42 (14.3%) cats. The total serum magnesium concentration of all CKD cats with nephrolithiasis ranged from 0.91–4.50 mg/dl, with a mean of 2.13 ± 0.74 mg/dl; healthy cats ranged from 1.27–2.43 mg/dl, with a mean of 1.87 ± 0.29 mg/dl. There were no differences between the mean total serum magnesium levels of cats with CKD with nephrolithiasis and healthy cats (Figure 1).
Figure 1.
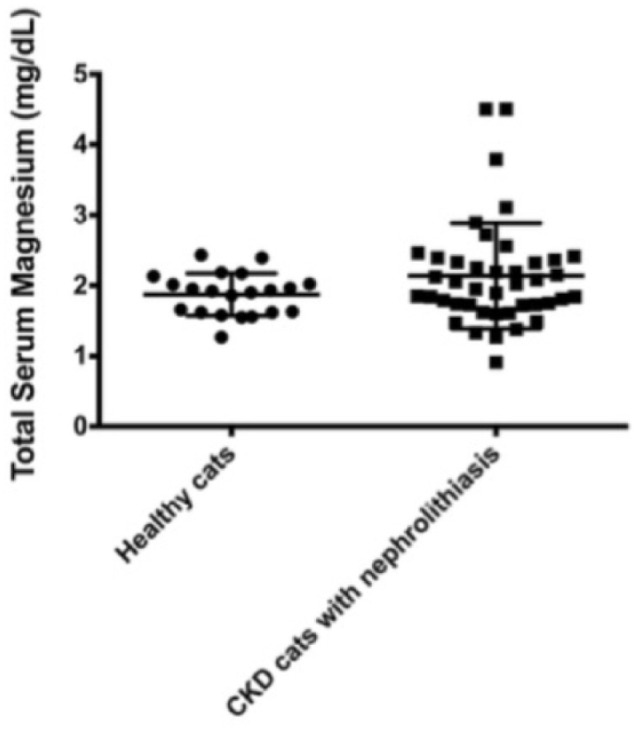
Total serum magnesium concentrations (mean ± SD) of healthy cats and cats with chronic kidney disease (CKD) with nephrolithiasis
Cats with stage 1 CKD with nephrolithiasis had 1.94 ± 0.32 mg/dl (1.60–2.56 mg/dl) total serum magnesium concentrations, and two of them (2/7) showed hypermagnesemia. Cats with stage 2 CKD with nephrolithiasis had 1.72 ± 0.32 mg/dl (1.27–2.32 mg/dl) total serum magnesium, and both hypomagnesemia (3/10) and hypermagnesemia (2/10) were observed. Cats with stage 3 CKD with nephrolithiasis had a level of 1.98 ± 0.31 mg/dl (range 1.33–2.39 mg/dl), five of them (5/15) had hypermagnesemia and two (2/15) hypomagnesemia. Cats with stage 4 CKD with nephrolithiasis had a level of 2.90 ± 1.14 mg/dl (range 0.91–4.50 mg/dl) total serum magnesium concentrations, and one of them (1/10) showed hypomagnesemia, whereas 8/10 had hypermagnesemia. There were differences of total serum magnesium concentration among cats with CKD with stage 4 and those with stage 1 (P <0.05), 2 (P< 0.001) and 3 CKD (P <0.01) (Table 1). Total serum magnesium was positively correlated with serum creatinine concentrations (r = 0.47; P <0.001).
Table 1.
Total serum magnesium concentrations and magnesium abnormalities of cats with International Renal Interest Society (IRIS) stage 1–4 chronic kidney disease (CKD) and nephrolithiasis
| CKD cats with nephrolithiasis | IRIS 1 (n = 7) |
IRIS 2 (n = 10) |
IRIS 3 (n = 15) |
IRIS 4 (n = 10) |
|---|---|---|---|---|
| Mean ± SD (range) total serum magnesium concentrations (mg/dl) | 1.94 ± 0.32 (1.60–2.56)* | 1.72 ± 0.32 (1.27–2.32) † | 1.98 ± 0.31 (1.33–2.39) ‡ | 2.90 ± 1.14 (0.91–4.50) ‡ |
| Magnesium abnormalities | 0/7 (0%) hypomagnesemia 2/7 (28%) hypermagnesemia |
3/10 (30%) hypomagnesemia 2/10 (20%) hypermagnesemia |
2/15 (13%) hypomagnesemia 5/15 (33%) hypermagnesemia |
1/10 (10%) hypomagnesemia 8/10 (80%) hypermagnesemia |
P <0.05
P <0.001
P <0.01
Hypo- and hypermagnesemia did not show correlation with other electrolyte disorders; however, potassium, sodium, chloride, phosphorus and ionized calcium abnormalities were found in cats with CKD with nephrolithiasis. The mean of ionized calcium was different between cats with low and high, and normal and high total serum magnesium concentrations (Figure 2). Serum phosphorus concentrations were also different between hypermagnesemic and normomagnesemic cats (Table 2).
Figure 2.
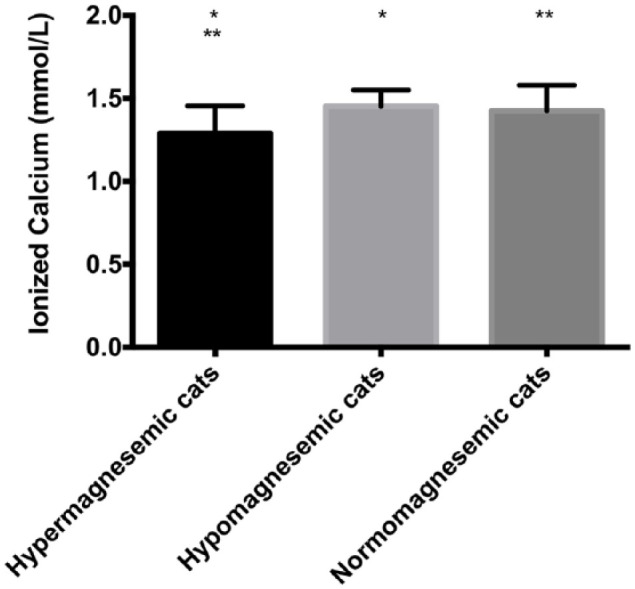
Blood ionized calcium levels of cats chronic kidney disease with nephrolithiasis and normal, hypo- and hypermagnesemia reference intervals (Veterinary Teaching Hospital of the University of Sao Paulo): total serum magnesium 1.58–2.16 mg/dl; ionized calcium 1.1–1.4 (mmol/l). *P <0.05; **P <0.01
Table 2.
Blood sodium, potassium, chloride and ionized calcium, and serum phosphorus concentrations (mean ± SD [range]) of cats with chronic kidney disease (CKD) with nephrolithiasis and magnesium abnormalities
| CKD cats with nephrolithiasis | Sodium concentrations (mEq/l) | Potassium concentrations (mEq/l) |
Chloride concentrations (mEq/l) |
Phosphorus concentrations (mg/dl) |
Ionized calcium concentrations (mmol/l) |
|---|---|---|---|---|---|
| Low total serum magnesium concentrations (n = 6) a | 154 ± 4.15 (148.6–161.4) |
3.95 ± 0.45 (3.22–4.55) |
119.5 ± 5.66 (113.3–129.9) |
5.65 ± 3.10 (3.78–11.90) |
1.45 ± 0.09
b
(1.38–1.64) |
| High total serum magnesium concentrations (n = 16) b | 152.7 ± 6.21 (142.8–167) |
4.06 ± 0.70 (3.14–5.98) |
117.5 ± 6.01 (104.6–127) |
10.37 ± 6.25
c
(3.23–24.80) |
1.28 ± 0.16a,c (0.86–1.45) |
| Normal total serum magnesium concentrations (n = 20) c | 153.5 ± 2.23 (148.9–158) |
3.61 ± 0.69 (2.25–5.11) |
118.8 ± 2.25 (113.8–123) |
5.06 ± 1.48
b
(2.69–7.82) |
1.42 ± 0.15
b
(1.19–1.85) |
Reference intervals (Veterinary Teaching Hospital of the University of Sao Paulo): total serum magnesium 1.58–2.16 mg/dl; sodium 147–156 mEq/l; potassium 3.5–5.5 mEq/l; chloride 111–123 mEq/l; phosphorus 2.7–5.0 (mg/dl); ionized calcium 1.1–1.4 (mmol/l)
P <0.01
P = 0.03
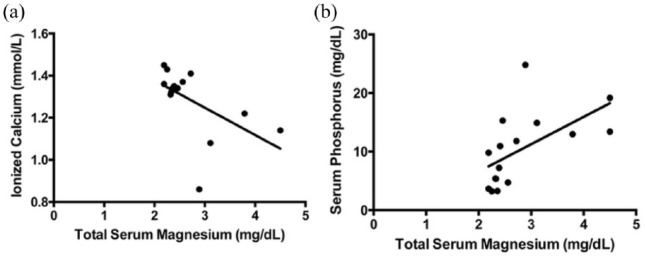
There was a negative correlation between hypermagnesemia and ionized calcium (r = −0.64; P <0.01), and a positive correlation between hypermagnesemia and serum phosphorus concentration in cats with CKD with nephrolithiasis (r = 0.58; P = 0.01) (Figure 3).
Figure 3.
(a) Spearman correlation between total serum magnesium concentrations and blood ionized calcium levels (r = −0.64; P <0.01). (b) Pearson correlation between total serum magnesium and serum phosphorus concentrations (r = 0.58, P = 0.01)
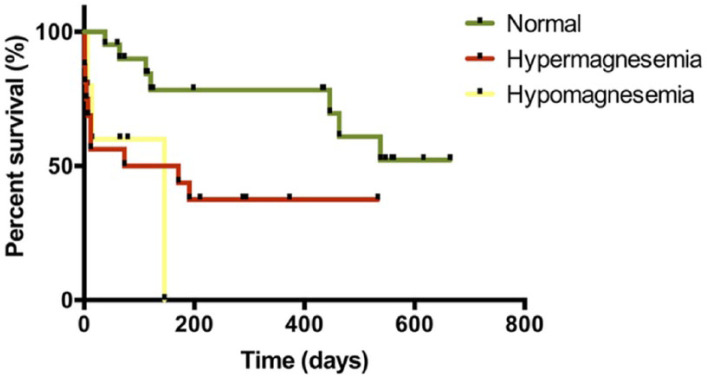
The median survival of cats with CKD with nephrolithiasis and normomagnesemia was 434 days (range 60–655 days). The median survival of cats with CKD with nephrolithiasis and hypomagnesemia was 122 days, and for those cats with CKD with nephrolithiasis and hypermagnesemia it was 146 days. There were differences among survival curves of CKD cats with nephrolithiasis and magnesium abnormalities vs CKD cats with nephrolithiasis and normal magnesium concentrations (P <0.01) (Figure 4).
Figure 4.
Kaplan–Meier curves of cats with chronic kidney disease with nephrolithiasis and normal, high and low total serum magnesium concentrations
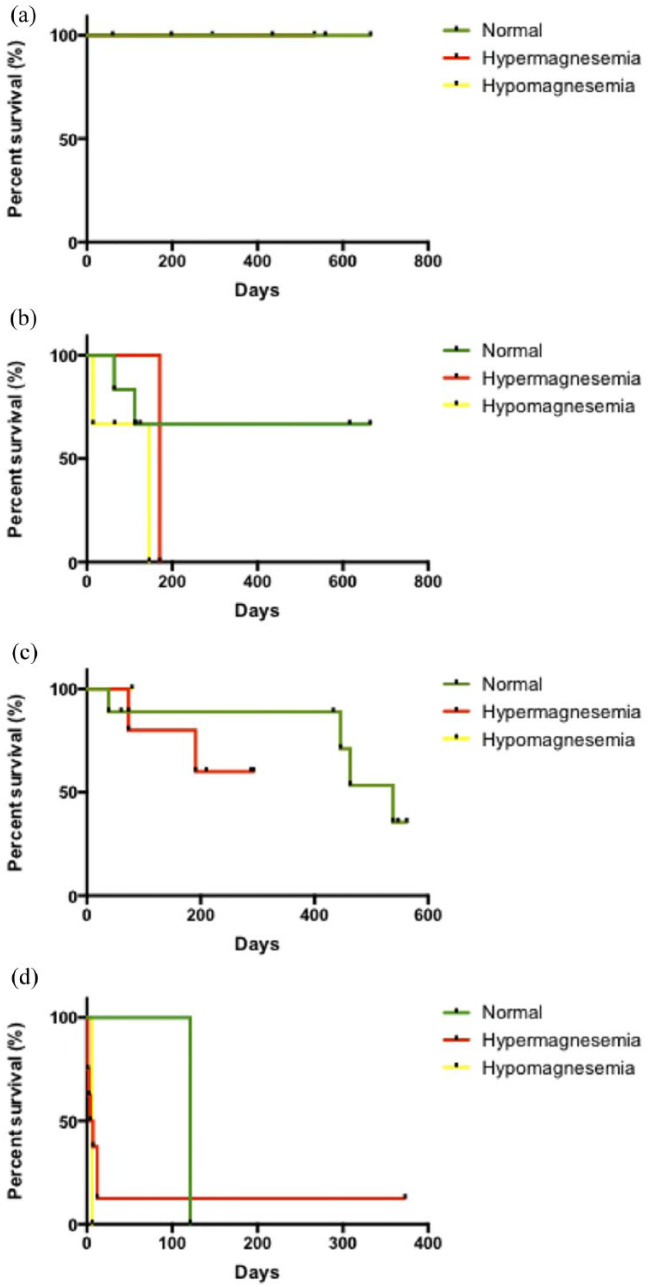
Regarding CKD stage, none of the cats with stage 1 CKD and nephrolithiasis showed magnesium abnormalities, and survival was 100%. Cats with stage 2 CKD with nephrolithiasis and hypo- or hypermagnesemia had lower survival rates (median 146 days and 171 days of survival, respectively) than cats with normal magnesium, but there were no differences among the curves. Similar results were observed in cats with stage 3 CKD, but it was not possible to determine the median survival of cats with magnesium abnormalities due to the small sample size; those with normal magnesium had 538 days. One cat with stage 4 CKD had hypomagnesemia and another had normal magnesium concentrations; both died (6 and 121 days of median of survival); all the cats with hypermagnesemia died too, except one, which had the data censored (373 days of survival at the time of the analysis). The median survival in cats with stage 4 CKD was 5 days, and there were no differences among the curves (Figure 5).
Figure 5.
Survival of cats with stage 1–4 chronic kidney disease (CKD) with nephrolithiasis, and high, low or normal total serum magnesium concentrations. (a) Cats with stage 1 CKD with nephrolithiasis; (b) cats with stage 2 CKD with nephrolithiasis; (c) cats with stage 3 CKD with nephrolithiasis; (d) cats with stage 4 CKD with nephrolithiasis
There was no difference in total serum magnesium concentrations between the matched CKD groups with and without nephrolithiasis. Cats with stage 2 and stage 3 CKD without nephrolithiasis showed a total serum magnesium concentration of 1.97 ± 0.42 mg/dl, whereas for those with nephrolithiasis it was 1.83 ± 0.34 mg/dl (Figure 6).
Figure 6.
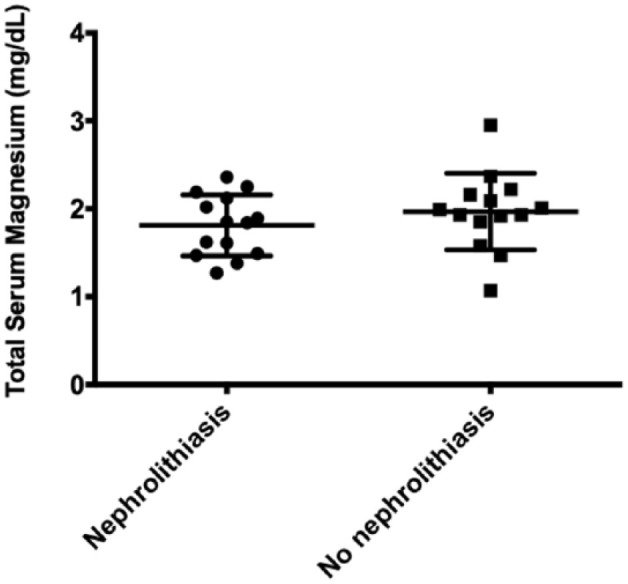
Total serum magnesium concentrations (mg/dl) of stage and age-matched cats with chronic kidney disease with and without nephrolithiasis
Discussion
The results showed that cats with CKD with nephrolithiasis have magnesium abnormalities. Both low and high total serum magnesium concentrations were observed, but hypermagnesemia was more frequent in cats with stage 4 CKD. Regardless of CKD stage, magnesium disorders were associated with increased mortality.
These findings are in accordance with previous studies that investigated the role of magnesium in several illnesses, including CKD. A study performed in hospitalized cats showed that hypermagnesemia occurred in 50% of cats with end-stage renal disease. 9 In feline renal transplant recipients, total serum magnesium concentrations were found to be higher in 10% of cases in the postoperative period. 26 Comparatively, magnesium abnormalities were also reported in dogs with CKD. In a previous prospective study, our group assessed total serum magnesium concentrations in dogs with stage 2 and 3 CKD, and we found that hypermagnesemia occurred from stage 2. Furthermore, 80% of dogs with stage 4 CKD had increased levels of total serum magnesium. 27
In humans, the occurrence of hypermagnesemia has also been reported in patients with end-stage renal disease.18,28,29 A recent study showed that almost 53% of the people under dialysis therapy had increased concentrations of total serum magnesium, and it was related to higher levels of serum inflammatory markers. 16
The kidneys play an essential role in magnesium balance, as it is mainly regulated by renal excretion. 30 In early CKD, the urinary fractional excretion of magnesium may be normal or even increased, thereby working as a compensatory mechanism in order to avoid the onset of hypermagnesemia. However, as the glomerular filtration rate (GFR) falls below 30 ml/min, this mechanism becomes insufficient, and the content of serum and total body magnesium tends to increase as a result of renal retention. Thus, higher levels of total serum magnesium are expected in later stages of CKD. 13 The positive correlation between creatinine and magnesium concentrations found in our study supports this evidence.
Interestingly, one of the cats with stage 4 CKD with nephrolithiasis had hypomagnesemia. Low total serum concentration of magnesium has also been reported in humans with late CKD, and it is usually related to malnutrition, chronic diarrhea, polyuria and concurrent electrolyte disorders, such as hypokalemia and hypercalcemia. 25
However, we did not find a correlation between hypomagnesemia and hypokalemia; a few cats with stage 4 CKD with nephrolithiasis had concurrent magnesium and potassium disturbances. Magnesium modulates the cellular potassium permeability; thus, hypomagnesemia could lead to the efflux of potassium from the cell, thereby causing potassium loss from the body in the urine.31,32 Concurrent hypomagnesemia and hypokalemia is well known in humans,33–35 but our study, and others, have failed to demonstrate such an association in cats. Further studies performed with larger sample sizes are needed to verify the relationship between potassium and magnesium abnormalities in cats.
However, we found a negative correlation between total serum magnesium and ionized calcium, and a positive correlation between total serum magnesium and serum phosphorus in all cats with CKD with nephrolithiasis. These results were not unexpected, as magnesium is intrinsically related to mineral and bone metabolism.2,29,30 Several studies in humans with end-stage renal disease have shown that there is an inverse relation between serum magnesium and PTH, which, in turn, plays a pivotal role in the development of renal hyperparathyroidism and CKD progression.2,16,18,36
The interaction between magnesium and PTH is complex. PTH increases intestinal and renal absorption of magnesium, as well as bone resorption; conversely, magnesium inhibits PTH synthesis and secretion.17,36 The magnesium mediated-PTH suppression could be a possible reason to explain the low levels of ionized calcium observed among cats with CKD with nephrolithiasis and hypermagnesemia. However, it is likely that they have increased levels of PTH, mainly those with stage 4.37,38 A more reasonable explanation is that CKD cats with nephrolithiasis and hypermagnesemia usually have a marked decrease in GFR, which leads to phosphate retention. Phosphate binds to calcium, thereby reducing its biologically active fraction – ionized calcium. 39 The finding that CKD cats with nephrolithiasis and hypermagnesemia showed higher serum phosphorus concentrations and a lower mean of ionized calcium than those with hypomagnesemia may support this evidence.
There were no correlations among sodium, chloride and magnesium abnormalities. Concurrent disorders involving these electrolytes are expected in kidney disease. 40 In humans, some renal tubular disorders associated with the development of nephrocalcinosis and/or nephrolithiasis (eg, familial hypomagnesemia with hypercalciuria and nephrocalcinosis, distal renal tubular acidosis) may cause changes in serum levels or urinary fractional excretion of magnesium along other electrolyte disturbances.10–12,41 However, to the best of our knowledge, magnesium and concurrent electrolytes abnormalities in association with such tubular disorders were not reported in cats. Further investigations are needed to elucidate whether cats may also have similar illness.
The survival curves of cats with normal, low or high magnesium values did not differ in each stage of CKD, probably owing to the small sample size. Thus, it was not possible to evaluate the effect of CKD stage in the survival of cats with magnesium abnormalities.
Regarding all CKD stages, the survival of cats with CKD with nephrolithiasis and hypomagnesemia or hypermagnesemia was lower than those with normal total serum magnesium concentrations. Hypomagnesemia is a well-established predictor of mortality in critically ill humans,42–44 and similar studies in hospitalized dogs and cats have shown similar results.45,46 Low total serum magnesium concentrations are usually associated with cardiovascular events, such as heart failure and cardiac arrhythmias.4,6 However, in humans with CKD, studies have shown that hypomagnesemia is an independent predictor of mortality.1,47 Our findings suggested that hypomagnesemia may also be implicated in higher mortality in cats with CKD with nephrolithiasis; however, we did not adjust our model for potential confounding factors such as age and creatinine values.
Studies performed in humans with CKD showed that increased total serum magnesium concentrations were related to a lower mortality risk,5,14 which was attributed to the protective effect of magnesium on vascular calcification, thus preventing cardiovascular death. Conversely, we found that cats with CKD with hypermagnesemia showed reduced survival; similar results were also reported in a previous study performed in cats. 9 It is important to note that the most cats in both reports had end-stage renal disease, which might have influenced the survival analysis. Species-specific mechanisms associated with CKD progression should be considered for the interpretation of these controversial results, as vascular calcification and cardiovascular death are not commonly observed in cats with renal failure.
Lastly, we did not find differences in mean total serum magnesium concentrations between clinically normal cats and CKD cats with nephrolithiasis; however, the magnesium values were higher in the diseased group. The clinical significance of magnesium abnormalities remains to be elucidated. In addition, there was no difference in serum magnesium concentrations between cats with CKD with and without nephrolithiasis; however, cats with kidney stones showed lower values. Previous studies demonstrated the role of magnesium depletion in urinary citrate deficiency in rats. 48 In people, the restoration of magnesium status after supplementation improved the renal excretion of citrate in patients with nephrolithiasis.3,49 Citrate, as well as magnesium, are stone inhibitors, thus increased urinary concentrations are beneficial in order to avoid urolith formation. More studies of a proper design are needed to evaluate whether body magnesium content is associated with kidney stones in cats.
The major limitations of this study were the cross-sectional design and the small sample size. In addition, it was not possible to perform ultrasonographic evaluation in all clinically normal cats, which could have led to an accidental inclusion of cats with stage 1 CKD in the healthy control group. Stage and age-matched groups of cats with CKD with and without nephrolithiasis were not randomly assigned, which could introduce some bias. We did not consider the diet content of magnesium in our analysis. We also used the total serum magnesium concentrations rather than the ionized fraction. The use of ionized magnesium may be considered as a next step in elucidating magnesium abnormalities in CKD cats with nephrolithiasis.
Conclusions
CKD cats with nephrolithiasis have magnesium abnormalities. Hypo- and hypermagnesemia were associated with an increase in mortality, and thus total serum magnesium abnormalities may be used as prognostic factors in these cases.
Acknowledgments
We thank Clara Mori and Creide Costa for laboratory technical support.
Footnotes
Accepted: 13 December 2018
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Fernanda Chacar  https://orcid.org/0000-0002-8683-9960
https://orcid.org/0000-0002-8683-9960
References
- 1. Ishimura E, Okuno S, Yamakawa T, et al. Serum magnesium concentration is a significant predictor of mortality in maintenance hemodialysis patients. Magnes Res 2007; 20: 237–244. [PubMed] [Google Scholar]
- 2. Rude RK, Gruber HE, Wei LY, et al. Magnesium deficiency: effect on bone and mineral metabolism in the mouse. Calcif Tissue Int 2003; 72: 32–41. [DOI] [PubMed] [Google Scholar]
- 3. Musso CG. Magnesium metabolism in health and disease. Magnesium 2009; 41: 357–362. [DOI] [PubMed] [Google Scholar]
- 4. Eichorn EJ, Tandon PK, DiBianco R, et al. Clinical and prognostic significance failure: the PROMISE study of serum magnesium concentration in patients with severe chronic congestive heart (II) results. JACC Heart Fail 1993; 21: 634–640. [DOI] [PubMed] [Google Scholar]
- 5. Lacson E, Jr, Wang W, Ma L, et al. Serum magnesium and mortality in hemodialysis patients in the United States: a cohort study. Am J Kidney Dis 2015; 66: 1056–1066. [DOI] [PubMed] [Google Scholar]
- 6. Gottlieb SS, Baruch L, Kukin ML, et al. Prognostic importance of the serum magnesium concentration in patients with congestive heart failure. JACC Heart Fail 1990; 16: 827–831. [DOI] [PubMed] [Google Scholar]
- 7. Alhosaini M, Leehey DJ. Magnesium and dialysis: the neglected cation. Am J Kidney Dis 2015; 66: 523–531. [DOI] [PubMed] [Google Scholar]
- 8. Vormann J. Magnesium and kidney health – more on the ‘forgotten electrolyte’. Am J Nephrol 2016; 44: 379–380. [DOI] [PubMed] [Google Scholar]
- 9. Toll J, Erb H, Birnbaum N, et al. Prevalence and incidence of serum magnesium abnormalities in hospitalized cats. J Vet Intern Med 2002; 16: 217–221. [DOI] [PubMed] [Google Scholar]
- 10. Praga M, Vara J, Gonzalez-parra E, et al. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis. Kidney Int 1995; 47: 1419–1425. [DOI] [PubMed] [Google Scholar]
- 11. Izzedine H, Benalia H, Arzouk N, et al. Nephrolithiasis with hypomagnesemia: what is the cause. Am J Kidney Dis 2007; 49: 862–864. [DOI] [PubMed] [Google Scholar]
- 12. Kang JH, Choi HJ, Cho HY, et al. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis associated with CLDN16 mutations. Pediatr Nephrol 2005; 20: 1490–1493. [DOI] [PubMed] [Google Scholar]
- 13. Kanbay M, Goldsmith D, Uyar ME, et al. Magnesium in chronic kidney disease: challenges and opportunities. Blood Purif 2010; 29: 280–292. [DOI] [PubMed] [Google Scholar]
- 14. Laecke SV, Nagler EV, Verbeke F, et al. Hypomagnesemia and the risk of death and GFR decline in chronic kidney disease. Am J Med 2013; 126: 825–831. [DOI] [PubMed] [Google Scholar]
- 15. Fein PA, Weiss S, Ramos F, et al. Serum magnesium concentration is a significant predictor of mortality in peritoneal dialysis patients. Adv Perit Dial 2014; 30: 90–93. [PubMed] [Google Scholar]
- 16. Yu L, Li H, Wang S. Serum magnesium and mortality in maintenance hemodialysis patients. Blood Purif 2017; 43: 31–36. [DOI] [PubMed] [Google Scholar]
- 17. Navarro JF, Mora C, Macia M, et al. Serum magnesium concentration is an independent predictor of parathyroid hormone levels in peritoneal dialysis patients. Perit Dial Int 1999; 19: 455–461. [PubMed] [Google Scholar]
- 18. Messa P. Magnesium in chronic kidney disease stages 3 and 4 and in dialysis patients. Magnesium 2012; 5 Suppl 1: i39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Misra PS, Nessim SJ. Clinical aspects of magnesium physiology in patients on dialysis. Semin Dial 2017; 30: 438–445. [DOI] [PubMed] [Google Scholar]
- 20. Diaz-tocados JM, Peralta-Ramirez A, Lopez I, et al. Dietary magnesium supplementation prevents and reverses vascular and soft tissue calcifications in uremic rats. Kidney Int 2017; 92: 1084–1099. [DOI] [PubMed] [Google Scholar]
- 21. Schenck P. Serum magnesium concentrations in association with feline calcium metabolic disorders. J Vet Intern Med 2008; 22: 796–797. [Google Scholar]
- 22. Van den Broek DHN, Chang YM, Elliott J, et al. Prognostic importance of plasma total magnesium in a cohort of cats with azotemic chronic kidney disease. J Vet Intern Med 2018; 32: 1359–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. International Renal Interest Society. IRIS staging of chronic kidney disease. http://www.iris-kidney.com/guidelines/staging.html (2016, accessed December 19, 2017).
- 24. Cléroux A, Alexander K, Beauchamp G, et al. Evaluation for association between urolithiasis and chronic kidney disease in cats. J Am Vet Med Assoc 2017; 250: 770–774. [DOI] [PubMed] [Google Scholar]
- 25. Bateman SW. A quick reference on magnesium magnesium veterinary dog cat critical care. Magnesium 2017; 47: 235–239. [DOI] [PubMed] [Google Scholar]
- 26. Wooldridge JD, Gregory CR. Ionized and total serum magnesium concentrations in feline renal transplant recipients. Vet Surg 1999; 28: 31–37. [DOI] [PubMed] [Google Scholar]
- 27. Martorelli C, Kogika M, Giovaninni L, et al. Serum magnesium concentration in dogs with naturally occurring chronic kidney disease. J Vet Intern Med 2015; 29: 1122–1256. [Google Scholar]
- 28. Randal RE, Cohen D, Spray CC, Jr, et al. Hypermagnesemia in renal failure: etiology and toxic manifestations. Ann Intern Med 1964; 61: 74–88. [DOI] [PubMed] [Google Scholar]
- 29. Hutchison AJ, Were AJ, Boulton HF, et al. Hypercalcaemia, hypermagnesaemia, hyperphosphataemia and hyperaluminaemia in CAPD: improvement in serum biochemistry by reduction in dialysate calcium and magnesium concentrations. Nephron 1996; 72: 52–58. [DOI] [PubMed] [Google Scholar]
- 30. Blaine J, Chonchol M, Levi M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol 2015; 10: 1257–1272. DOI: 10.2215/CJN.09750913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gilroy CV, Horney BS, Burton SA, et al. Evaluation of ionized and total serum magnesium concentrations in hyperthyroid cats. Can J Vet Res 2006; 70: 137–142. [PMC free article] [PubMed] [Google Scholar]
- 32. Urso C, Brucculeri S, Caimi G. Acid–base and electrolyte abnormalities in heart failure: pathophysiology and implications. Heart Fail Rev 2015; 20: 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Warnock DG. Renal genetic disorders related to K+ and Mg2+. Annu Rev Physiol 2002; 64: 845–876. [DOI] [PubMed] [Google Scholar]
- 34. Huang C-long, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18: 2649–2652. [DOI] [PubMed] [Google Scholar]
- 35. Naljayan M, Kumar S, Steinman T, et al. Hypomagnesemia and hypokalemia: a successful oral therapeutic approach after 16 years of potassium and magnesium intravenous replacement therapy. Clin Kidney J 2014; 7: 214–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martin KJ, Gonza EA. Clinical consequences and management of hypomagnesemia. J Am Soc Nephrol 2009; 20: 2291–2295. [DOI] [PubMed] [Google Scholar]
- 37. Barber PJ, Elliott J. Feline chronic renal failure: calcium homeostasis in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract 1998; 39: 108–116. [DOI] [PubMed] [Google Scholar]
- 38. Finch NC, Geddes RF, Syme HM, et al. Fibroblast growth factor 23 (FGF-23) concentrations in cats with early nonazotemic chronic kidney disease (CKD) and in healthy geriatric cats. J Vet Intern Med 2013; 23: 227–233. [DOI] [PubMed] [Google Scholar]
- 39. McCarron DA. Protecting calcium and phosphate balance in chronic renal disease. J Am Soc Nephrol 2005; 16: S93–94. [DOI] [PubMed] [Google Scholar]
- 40. Schaer M. Disorders of serum potassium, sodium, magnesium and chloride. J Vet Emerg Crit Care 1999; 9: 209–217. [Google Scholar]
- 41. Kari JA, Farouq M, Alshaya HO. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis. Eur J Pediatr 2003; 18: 506–510. [DOI] [PubMed] [Google Scholar]
- 42. Soliman HM, Mercan D, Lobo SSM, et al. Development of ionized hypomagnesemia is associated with higher mortality rates. J Crit Care Med 2003; 31: 1082–1087. [DOI] [PubMed] [Google Scholar]
- 43. Escuela MP, García-jalón A. Total and ionized serum magnesium in critically ill patients. Intensive Care Med 2005; 31: 151–156. [DOI] [PubMed] [Google Scholar]
- 44. Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20: 3–17. [DOI] [PubMed] [Google Scholar]
- 45. Khanna C, Lund EM, Raffe M, et al. Hypomagnesemia in 188 dogs: a hospital population-based prevalence study. J Vet Intern Med 1998; 12: 304–309. [DOI] [PubMed] [Google Scholar]
- 46. Martin LG, Matterson VL, Wingfield WE, et al. Abnormalities of serum magnesium in critically ill dogs: incidence and implications. J Vet Emerg Crit Care 1996; 4: 15–20. [Google Scholar]
- 47. Sakaguchi Y, Fujii N, Shoji T, et al. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int 2013; 85: 174–181. [DOI] [PubMed] [Google Scholar]
- 48. Lifshitz F, Harrison HC, Bull EC. Citrate metabolism and the mechanism of renal calcification induced by magnesium depletion. Metab Clin Exp 1967; 16: 345–357. [DOI] [PubMed] [Google Scholar]
- 49. Reungjui S, Prasongwatana V, Jirakulsomchok S, et al. Magnesium status of patients with renal stones and its effect on urinary citrate excretion. BJU Int 2002; 90: 635–639. [DOI] [PubMed] [Google Scholar]