Abstract
Objectives
This study aimed to determine the efficacy and safety of oral misoprostol (MIS) administration in the induction of mid-term pregnancy termination in cats.
Methods
Twenty-eight cats that were pregnant for 30–40 days were allocated to four groups. The aglepristone (AGL) group (n = 7) received 10 mg/kg SC aglepristone q24h for two consecutive days. In the AGL+MIS group (n = 7), AGL (as administered in the AGL group) and MIS (200 µg/cat PO q12h until the start of abortion) were administered. The MIS200 (n = 7) and MIS400 groups (n = 7) received MIS (200 or 400 µg/cat misoprostol, respectively) alone PO q12h until the start of abortion. Blood samples were collected at the start of treatment (d0), 4 days after the start of treatment (d4) and on the day of complete abortion/end of administration (dA/d7).
Results
The efficacy of the treatment was 71.4% in the AGL group, 100% in the AGL+MIS group, 0% in MIS200 group and 57.4% in MIS400 group (P = 0.004). No significance was found in relation to the interval from treatment to the start/end of abortion and the duration of abortion in all groups. The most observed side effect was vomiting in both groups administered MIS, particularly in the MIS400 group (56.7%). Progesterone (P4) concentrations were reduced during the abortion, but not to basal levels, in all groups. P4 concentrations were significantly lower at dA/d7 in the MIS400 group compared with the AGL and AGL+MIS groups (P = 0.002).
Conclusions and relevance
The results obtained from this study showed that low doses of MIS do not induce abortions in cats but increase the effect of AGL. Although higher doses could terminate pregnancies, this also causes intense unwanted side effects. Therefore, the use of MIS alone as an abortifacient in cats is not recommended. For mid-term pregnancy termination in cats, the combination of misoprostol and aglepristone provides a more effective abortifacient than using either of them alone.
Keywords: Pregnancy termination, PGE1, aglepristone, mid-term pregnancy, misoprostol
Introduction
Unwanted mating and pregnancy is a common problem in privately owned sexually mature cats. Methods such as spaying, keeping the cat indoors, provocation of ovulation by vaginal stimulation and hormonal practices can be applied by owners or veterinarians to prevent reproduction. Nevertheless, unwanted pregnancies may still occur. If mating is observed by the owner, the necessary attempts to prevent or terminate the pregnancy, such as spaying or hormonal treatment, can be made in the first trimester of pregnancy. However, in most cases, pregnancies are usually discovered incidentally or based on the owners’ conjecture during the second or third trimester.
Pregnancies should not be terminated during the third trimester, owing to the ethical considerations, unless the queen has a medical or life-threating problem. This is especially true for the last half of the third trimester; in this period the fetuses are almost ready for postpartum life and inducing abortions may result in exposing a live neonate that requires intensive care. In this sense, this is not inducing an abortion but labour. There is also the consideration of increased pain and discomfort for the queen resulting from the size of fetuses and the enlargement of the uterus. Therefore pregnancy terminations should be performed by the second trimester of gestation at the latest. In any case, there are two options: spaying and hormonal termination of pregnancy. Owing to the irreversible feature of spaying, some owners prefer hormonal termination.
Prostaglandin F2alpha (PGF2α), ergot derivatives, progesterone (P4) receptor (PR) antagonist, or a combination of these,1,2 can be used for this purpose. Nevertheless, these hormones have some disadvantages, such as a repeated administration requirement, vomiting, diarrhoea, salivation and abdominal cramps. 3 PGF2α is more effective in termination after day 40 of pregnancy but not before 33–38 days of gestation.4,5 The main disadvantages of ergot derivatives are their lack of efficacy before the last trimester of pregnancy and their induction of vomiting. 6 A synthetic steroid, aglepristone (AGL), binds the uterine PR, and its affinity to uterine PR is 9.6 times higher in queens than in the native P4. 7 Aglepristone has been reported to be safe and effective in inducing abortions, in preventing pregnancy and in inducing labour in bitches and cats.5,8–14 However, the success rate of AGL decreases as the gestation period progresses. 15 The cost, lack of licensing and limited availability are the main disadvantages of AGL. Therefore, there is an ongoing investigation in veterinary practice for new, safe, efficient, cheap and fast-acting hormones/methods for pregnancy termination.
Misoprostol (MIS) is a synthetic prostaglandin E1 (PGE1) analogue that was originally developed for the treatment or prevention of gastrointestinal tract ulcers because of its gastric acid antisecretory properties. 16 Unlike other prostaglandins, MIS is relatively cheap, readily available, can be stored at room temperature 17 and is orally active. 18 With its uterotonic and cervical ripening effects, MIS has been used in human gynaecology. It has been used for inducing labour, pregnancy termination, cervical ripening and surgical preparations in gynaecological practice through oral, intravaginal or intracervical administration in women. 16 While vaginal use of MIS to induce labour has not been approved by US Food and Drug Administration, it has been approved by the European Decentralized Procedure. 19 Hence, it is used in Europe, and is easily available.
After having been used in human medicine, MIS has been tested in veterinary gynaecology for pregnancy termination, induction of parturition or cervical ripening in dogs, ewes, goats, cows and mares.10,20–25 There are no reports of health risks associated with this drug, and the results have been successful in the aforementioned studies, according to the study authors. The drug can be safely used in dogs and rats in a wide dose margin without causing any acute or chronic systemic dysfunction.26,27 Previous studies utilising radiolabelled MIS conclude that its absorption rate and the duration required to reach its peak plasma concentration are the same in dogs and humans. 28 In addition, MIS has no known drug interactions. 29 While there are studies concerning the safety of MIS in veterinary gynaecology, these are scarce and there is not enough clinical information available for all relevant domestic species. Although the drug has been used as a gastric protectant in cats, 30 no studies were found regarding the use of MIS in cats for pregnancy termination. This study aimed to determine the efficacy and safety of two different doses of oral MIS administration alone or in combination with AGL for mid-term pregnancy termination in cats.
Materials and methods
Animals
Twenty-eight mixed breed cats that were pregnant for 30–40 days (mean 34.5 ± 4.2 days), aged 9–24 months (mean 14.0 ± 4.7 months) and with a body weight of 2.70–4.35 kg (mean 3.5 ± 0.5 kg) were included in the study. Each queen was brought to our hospital with an unwanted pregnancy. Most of them were runaways that were recovered after a short time; however, some of them had been allowed to roam freely as they pleased. Background information on the cats was obtained from their owners. According to the patient histories, none had previously had unwanted pregnancies before and were healthy in clinical evaluations.
The queens were hospitalised freely in separate rooms, fed commercial cat food and given water ad libitum. After their abortion and the final clinical assessment, the cats were returned to their owners. A telephone survey was carried out 3 months after the return to their owners. The survey included questions about the general wellbeing of the cat in question such as normal habits, appetite, playfulness, urination/defaecation cycles and any unusual behaviour.
The study design was prospective and the pregnant queens were allocated to their groups using a predetermined random list. The groups were AGL, AGL+MIS, MIS200 and MIS400. Data on the average gestational age and the age and body weight of the cats in each group are given in Table 1.
Table 1.
Mean gestational ages, ages and body weight of the cats on the first day of treatment
| Groups | n | Gestational age (days) | Age (months) | Body weight (kg) |
|---|---|---|---|---|
| AGL | 7 | 34.1 ± 3.5 | 15.7 ± 6.3 | 3.8 ± 0.6 |
| AGL+MIS | 7 | 35.4 ± 4.2 | 10.7 ± 1.7 | 3.2 ± 0.3 |
| MIS200 | 7 | 34.3 ± 4.5 | 13.3 ± 3.4 | 3.6 ± 0.4 |
| MIS400 | 7 | 34.3 ± 5.3 | 12.6 ± 6.1 | 3.4 ± 0.4 |
| P value | 0.943 | 0.668 | 0.054 |
Data are mean ± SD
AGL = aglepristone; MIS = misoprostol
Treatments
Cats in the AGL group (n = 7) were administered aglepristone (Alizin; Virbac) at a dosage of 10 mg/kg SC q24h on two consecutive days. The AGL+MIS group (n = 7) received a combination of AGL and MIS (Cytotec; Pfizer) at a dosage of 200 μg/cat PO q12h until the start of abortion. The administration of the AGL and MIS was started at the same time. In the MIS200 group (n = 7), MIS alone was administered at the same dosage and by the same route as the MIS in the AGL+MIS group; the cats in the MIS400 group (n = 7) received 400 μg/cat misoprostol (as in the MIS200 group). With regard to ethical concerns related to the progressive gestational ages, seven administration days were accepted as a limit in the study, and those that did not start to have an abortion during this period were considered to be abortion-negative. The animals that started to have an abortion were followed until the end of pregnancy termination. All procedures involving the cats in the study were approved by the local ethics committee for animal experiments of Ondokuz Mayıs University, Turkey (2015/08).
Clinical examinations
Pregnancies were confirmed by transabdominal ultrasonographic examination using an ultrasound scanner equipped with a 5–7.5 MHz linear transducer (MyLab Five VET; Esaote). Fetal structures and the reference formulas were used to determine the cats’ gestational ages.31,32 Fetal measurements included crown–rump length, body diameter, and crown or parietal diameter, as well as the internal diameters of the gestational sac.
Starting after the first treatment, comprehensive clinical and ultrasonographic controls were performed every 12 h. However, the cats were monitored for 15 mins every 4 h for any clinical development. During these controls vaginal discharges were checked by observation, fetal viability by ultrasonography, food and water intake by observation, the injection sites by palpation, and general conditions (abdominal distension, colic, vomiting, diarrhoea and hydration status) by both observation and palpation. Clinical signs of pain were checked according to the Glasgow Composite Measure Pain Scale for cats (CMPS-Feline), and changes were recorded. Analgesic usage was not deemed necessary for any patient as none of the scores exceeded five in the CMPS-Feline. No follow-up examination was performed aside from the telephone survey after the completion of abortion.
A brown haemorrhagic vaginal discharge was considered to be the start of the abortion. The absence of a fetus or its structures during the ultrasonographical examination was accepted as a sign of pregnancy termination. The fetuses were not counted at any time during the study.
Blood sampling and hormone analysis
Blood samples were collected from the cephalic vein using vacutainer vials before the treatment (d0), 4 days after the start of treatment (d4), at the end of the abortion (dA) or the end of the administrations (d7) for abortion-negative animals. The sera were stored at −20°C until assayed. Progesterone was analysed with an electrochemiluminescence immunoassay on a Cobas Modular E170 Analyzer (Roche Diagnostics) by an accredited laboratory (Düzen Laboratories Group, Ankara, Turkey [Türkak, TS EN ISO/IEC 17025:2005]). The average intra- and inter-assay coefficients of variation were 1.4% and 2.9%, respectively.
To determine the effect of applications on the P4 concentrations, the groups were divided into two subgroups as aborted (A+) and non-aborted (A–).
Statistical analyses
The data were investigated concerning normal distribution by HOVTEST via Levene’s test. Data regarding absence of abortion were analysed using the GENMOD (Generalized Models) procedure linked with the log and binomial distribution function, and contrast statements were used to investigate the different effects on the groups. Other traits such as the start of treatment/end of abortion interval and P4 concentration were analysed using the GLM procedure with interaction effects. Except for the interaction effects, the differences in the main groups were analysed using the Duncan’s multiple range test. The differences in the interaction effects were tested using orthogonal polynomials. Data are presented as mean ± SD. SAS (2014) was executed for all statistical tests and calculations.
Results
Clinical findings
Pregnancy was terminated in all cats (100%; n = 7/7) in the AGL+MIS group, five cats (71.4%; n = 5/7) in the AGL group and four cats (57.1%; n = 4/7) in the MIS400 group. However, no pregnancy terminations (0%) were observed in the MIS200 group (P = 0.0004). No significant differences were found between the intervals from treatment to the start of abortion (T-SA), interval from the treatment to the end of abortion (T-EA) and duration of abortion (D-A: the time between T-SA and T-EA) in the groups (Table 2).
Table 2.
Terminated pregnancy (TP) ratio, the interval from treatment to the start/end of abortion (T-SA and T-EA, respectively) and duration of abortion (D-A)
| Groups | n | TP (%) | T-SA (days) | T-EA (days) | D-A (days) |
|---|---|---|---|---|---|
| AGL | 7 | 71.4 (5/7) ab | 6.1 ± 0.6 | 7.2 ± 0.4 | 0.9 ± 0.4 |
| AGL+MIS | 7 | 100 (7/7) b | 4.7 ± 1.6 | 6.1 ± 1.6 | 2.1 ± 1.5 |
| MIS200 | 7 | 0 (0/7) c | – | – | – |
| MIS400 | 7 | 57.1 (4/7) a | 5.0 ± 0.7 | 6.0 ± 0.0 | 1.0 ± 0.7 |
| P value | 0.0004* | 0.162 | 0.207 | 0.458 |
Data are mean ± SD. Different superscript letters indicate statistical differences
Analyses Lagrange ratio statistics for type 3
AGL = aglepristone; MIS = misoprostol
When the duration of drug usage was considered, 57.1% (n = 4/7) of the AGL+MIS and all of the MIS400 (57.1%; n = 4/7) groups completed abortions on day 6; in contrast, no queens (0%) in the AGL group had an abortion at that time (P = 0.018). The AGL group’s pregnancies (n = 5/5) were terminated on days 7 and 8. The remaining pregnancies (42.9%; n = 3/7) of the AGL+MIS group were also terminated on days 7 and 8 (P = 0.03; Figure 1).
Figure 1.
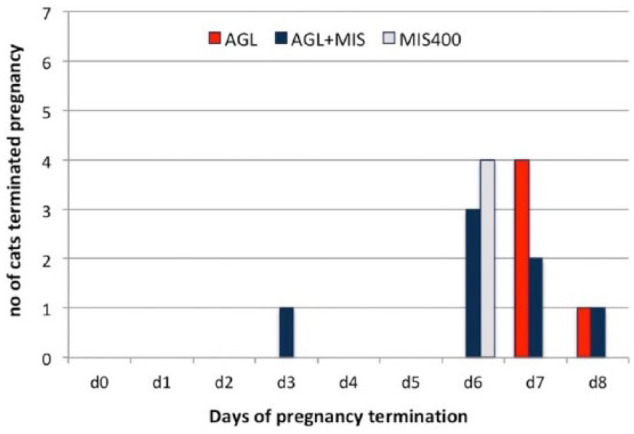
Distribution of number of days to terminate pregnancy. AGL = aglepristone; MIS = misoprostol; d = number of days after the start of treatment
Side effects
Varying rates of vomiting, abdominal distension, pain, diarrhoea and dehydration were observed during the study (Table 3). Although no significant difference was found between the side effects ratio of the AGL, AGL+MIS and MIS200 groups, this ratio was higher in the MIS400 group than in the other groups (P = 0.001).
Table 3.
Prevalance of the observed side effects of the different drug groups
| Group | Number of applications | Vomiting | Abdominal distension | Pain | Diarrhoea | Dehydration |
|---|---|---|---|---|---|---|
| AGL | 14 | 0 (0/14) a | 7.1 (1/14) a | 7.1 (1/14) a | 0 (0/14) a | 0 (0/14) a |
| AGL+MIS | 86 | 1.2 (1/86) a | 0 (0/86) a | 0 (0/86) a | 0 (0/86) a | 0 (0/86) a |
| MIS200 | 98 | 11.2 (11/98) a | 0 (0/98) a | 1.0 (1/98) a | 0 (0/98) a | 0 (0/98) a |
| MIS400 | 90 | 56.7 (51/90) b | 33.3 (30/90) b | 33.3 (30/90) b | 12.2 (11/90) b | 12.2 (11/90) b |
| P value | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
Data are % followed by, in parentheses, the number of observed side effects/number of observations. Different superscript letters indicate statistical differences
AGL = aglepristone; MIS = misoprostol
Vomiting started between drug applications 4 and 7 (5.5 ± 1.2; the time at which the cat received a dose of drug) in the MIS400 group, applications 3 and 12 (8.0 ± 4.2) in the MIS200 group and at application 6 (6.0 ± 0.0) in the AGL+MIS group. Vomiting stopped after a few hours in the MIS200 and AGL+MIS groups, but persisted until abortion completion in the MIS400 group. The frequency of vomiting was higher in the MIS400 group, and it started about 2–3 h after each MIS application. Undigested food was seen in the vomit in MIS400 group.
Abdominal distension was first seen at applications 5 and 6 (5.5 ± 0.6) in the MIS400 group, 2 days after drug application in the AGL group, and persisted to the end of abortion in both groups.
Pain was not obvious and only detectable by abdominal palpation. It was present in the MIS400 group at applications 5 and 6 (5.5 ± 0.7) and persisted to the end of abortion. In the MIS200 group the pain started between applications 3 and 12 (8.0 ± 4.2) and lasted for a few hours. Finally, the pain in the AGL group started 2 days after drug applications and lasted until the end of abortion. None of the cats had a score of five or higher in the CMPS-Feline; therefore, no pain medication was given.
Diarrhoea and mild dehydration were only seen at the MIS400 group, which started at application 6 (6.0 ± 0.0) and persisted through the abortion.
In the telephone surveys performed 3 months after the treatment, the owners reported no health problems in their cats.
Progesterone findings
No significant difference was found between groups on the first day of the treatment. In addition, no statistically significant change was observed from d0 to d4 in the groups after starting drug treatment. A significant decrease was found in serum P4 concentrations from d0 to dA/d7 in the MIS400 group (P = 0.004). On dA/d7, the P4 concentrations were lower in the MIS400 group than in the other groups (MIS400 vs AGL and AGL+MIS; P = 0.002) (Figure 2).
Figure 2.
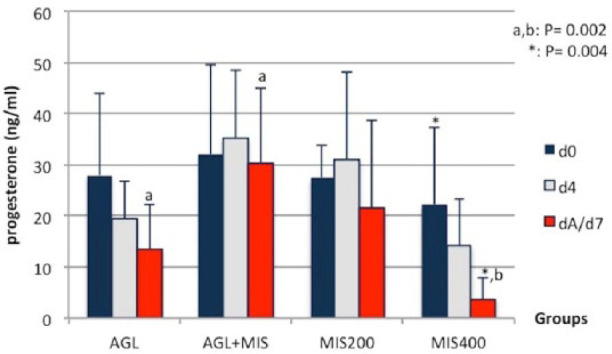
Serum progesterone concentrations (ng/ml, mean ± SD) for the different groups. AGL = aglepristone; MIS = misoprostol; d0 = start of treatment; d4 = 4 days after start of treatment; dA/d7 = day of complete abortion/end of administration
On d0, no significant difference was found in statistical analyses between the P4 concentrations of the A+ and A– cats in all groups. Varying rates of P4 decrease was also seen from d0 to dA/d7. A decrease was found in the P4 concentrations of both the A+ (P = 0.03) and the A– (P = 0.73) animals in the AGL group. Almost no decrease occurred in the P4 concentration of the AGL+MIS group (P = 0.79). A non-significant decrease was seen in the A– animals in the MIS200 group (P = 0.37). The P4 concentrations decreased in both the A+ (P = 0.07) and A– animals (P = 0.04) in the MIS400 group (Table 4).
Table 4.
Serum progesterone concentrations (ng/ml) in aborted (A+) and non-aborted (A–) cats for each of the groups
| Days | AGL |
AGL+MIS |
MIS200 |
MIS400 | ||||
|---|---|---|---|---|---|---|---|---|
| A+ | A– | A+ | A– | A+ | A– | A+ | A– | |
| d0 | 32.4 ± 20.5 a | 21.3 ± 6.2 | 31.9 ± 17.6 | – | – | 27.5 ± 6.3 | 18.3 ± 20.6 | 27.4 ± 9.9 a |
| dA/d7 | 12.2 ± 2.5 b | 17.0 ± 6.2 | 30.2 ± 14.9 | – | – | 21.5 ± 17.3 | 2.2 ± 4.0 | 6.0 ± 5.3 b |
| P value | 0.03 | 0.73 | 0.79 | – | – | 0.37 | 0.07 | 0.04 |
Data are mean ± SD. Different superscript letters indicate statistical differences
AGL = aglepristone; MIS = misoprostol; d0 = start of treatment; dA/d7 = day of complete abortion/end of administration
Discussion
To the best of our knowledge, this is the first study to determine the abortifacient traits of MIS in cats. The results of this study showed that low doses (200 μg/cat) of oral MIS do not induce abortion in cats, but they increase the abortion rate when used in conjunction with AGL. Misoprostol increases the activity of collagenases elastase, glycosaminoglycan and hyaluronic acid in the cervix and the intracellular calcium levels in the uterus,18,33 thereby triggering uterine contractions. This mechanism is well established, with previous studies examining the effects of MIS on uterine contractions.33,34
A single oral dose is sufficient to initiate uterine contractions; however, repeated doses of MIS are required to produce regular uterine contractions. 35 According to pharmacokinetic studies, MIS is rapidly absorbed into the mucosal membranes, 16 facilitating its vaginal, sublingual, buccal or oral use. 36 Its bioavailability in the vaginal route is three times stronger than the oral route. 37 After oral administration, plasma concentrations increase rapidly, peaking after 30 mins and rapidly declining within 120 mins. After vaginal administration, the plasma concentrations increase gradually and reach peak levels after 80 mins and then slowly decline. It can still be detected in the plasma after 6 h. 35 Thus, vaginal MIS administration produces intense and long-term uterine contractions, 34 and should be repeated at 3–12 h intervals. 36 In parallel with these data, clinical studies on women and bitches reported that MIS is more effective when vaginally applied.10,16,20 The effective MIS dose is reported to range from 100–800 μg for an individual dose and in various regimens in women; 36 there is no consensus on its optimal dose. Misoprostol dosages in this study were determined in accordance with previous canine and human studies,20,21,38,39 but we opted for the oral route because the vaginal size of cats is not suitable for MIS administration.
Clinical findings
The induction of an abortion in the cat by AGL has been tested at various stages of pregnancy by many studies. In these studies, AGL success rates depend on the time period in which it is used. When used on days 5 or 6 after mating, 14 the pregnancy termination rate is 100%; the success rate then gradually declines as gestation progresses, with a ratio of 87.0–88.5% to 100% between days 21 and 25,5,12,13 and finally down to a 66.7% success rate on days 45 and 46. 40 According to Scott, 41 an ovariectomy after day 45 of gestation does not disrupt pregnancies in cats, and after day 30 of gestation the placental P4 becomes more important than the luteal P4 for the maintenance of pregnancy. 42 This physiological property could explain the lower pregnancy termination rate (71.5%) in the AGL group in this study compared with the aforementioned studies,5,12–14 which were performed in the early days of gestation.
In this study, the AGL+MIS combination was found to be more effective for the induction of abortion than either AGL or MIS administration alone. This result corroborates previous studies on women and bitches,5,21,43 which reported that the combination of MIS-anti-progestogen was more efficacious for the induction of abortion than MIS alone. Anti-gestogen targets the cells of the endometrium and myometrium and sensitises the pregnant uterus to exogenous prostaglandins in women. 43 Kowalewski et al 44 showed that the prostaglandin system components are expressed in the utero/placental unit in dogs, and PGF2α inactive metabolite 13,14-dihydro-15-keto prostaglandin F2α (PGFM) concentrations are low during postimplantation and mid-gestation. AGL causes an increase in blood PGFM concentrations in canine pregnancy on day 58 and between days 40 and 45.44,45 Although there is no report showing the relationship between AGL and PGFM concentrations in cats, PGFM concentrations were found to be low during the first and second trimester of feline pregnancies. 46 Therefore, it can be speculated that the increased sensitivity of the uterus caused by the combination of AGL and MIS may account for the increased success rates vs either AGL or MIS alone.
Studies performed on ewes, goats and cows reported that the administration of MIS alone might cause cervical relaxations.22,23,24 The success of MIS in women ranged from 13–96% depending on different indications (cervix relaxation, and abortion/parturition induction), dosage, application route and gestational age. 47 Higher MIS doses are more effective at achieving the desired purpose than lower doses. 36 For example, during the first trimester of gestation in women, the abortion rates were 9% and 11% after 200 μg and 400 μg of MIS, respectively; 48 64% after 600 μg of MIS; 49 and 88–93% after 800 μg or 1000 μg of MIS.17,38,39 In the present study, higher doses of MIS induced abortion in >50% of the cats, which corroborates the aforementioned studies.
Vaginal discharge starts in 2 days and fetal death happens between days 4 and 7 of the termination of mid-term pregnancies in dogs. 15 No other protocol tried previously had a more meaningful improvement than single AGL use in this pregnancy period of dogs.10,11,20 In the present study, no significant difference was found in the T-SA and the T-EA between groups. These parameters were in parallel with previous studies that reported vaginal discharge starting in 4–7 days, 13 and fetal expulsion occurring in 5–9 days, 13 in 8 days 12 and in an average of 4.4 days after the first AGL injection. 5 In addition, the D-A parameter in the groups was similar to the previous studies reporting that the abortions were generally completed within 24 h of initiation. 13 However, in the present study, more than half of the pregnancies in the AGL+MIS group and all pregnancies in the MIS400 group were terminated within 6 days; the pregnancies in the AGL group did not terminate during this period. It can be concluded that the combination of AGL and MIS terminates pregnancies earlier than AGL alone, at least in 57.1% of cats. Similar results were obtained in the two studies conducted on bitches.10,20
Side effects
No intense, severe or life-threating side effects were observed in cats after the AGL treatment. The rate of side effects in the AGL group was negligible, as found in previous studies.11,12
The finding of temporary vomiting in the AGL+MIS group was in line with previous studies conducted using AGL combinations.10,11,20 The use of MIS combined with anti-progestogens showed a decrease in the incidence and severity of the side effects in women. 50 The aforementioned studies reported fewer side effects in combined treatment, and this study supports those findings, as the AGL+MIS group had fewer and less intense side effects than the other groups.
The side effects in this study were mostly related to the function of the gastrointestinal system. The effects of prostaglandins on the gastrointestinal system are well known, and include cytoprotection, tropic action, enteral pooling and gut motility. 51 Generally, MIS decreases acid secretion in the stomach and increases bicarbonate and mucus secretion. 52 Nevertheless, vomiting was the most common side effect in the cats that received MIS in this study. A high dose of cumulative PGE1 (1.650 μg/kg) in human neonates may trigger foveolar hyperplasia of gastric mucosa and submucosa, and lead to pyloric stenosis and difficulty in the passage of stomach contents to the intestines. In these instances, feeding intolerance, abdominal distension, regurgitation and vomiting may occur. 53 Despite being uncommon, diarrhoea may also be a side effect in addition to increased secretory activity and smooth muscle activity in intestines. 54 In the present study, the cumulative dose in the MIS400 group was found to be around 1.270 μg/kg. PGE1 is known to abolish the pyloric sphincter contractions in cats. 55
Human gynaecologists indicate that the intensity, severity and diversity of side effects depend on the dosage and administration route, and these effects increase in response to the oral administration of MIS. 56 Therefore, the findings of this study related to the side effects, particularly in the groups administered higher doses, were in line with the aforementioned studies. We believe the prominent gastrointestinal side effects in this study occurred because MIS was administered orally. As in humans, it is well known that vaginal application of MIS causes milder/fewer side effects than oral application.57,58 Therefore, if the drug can be applied to cats vaginally, the expectancy of side effects should be lower.
The higher efficacy and relatively lower side effects of the combined group may be attributed to a cumulative drug effect. It is a well-known fact that using several drugs in lower doses is much more efficacious than using one drug alone. 59 The gastrointestinal side effects of the combination group may also be lower because the start of abortion happened faster, so the application of the drugs could be discontinued sooner.
Previous studies performed on dogs indicate that MIS has no effect on platelet aggregation and has no antagonistic or agonistic effects on oestrogenic, progestogenic or androgenic processes; also, even if the dosage is increased by a multiple of 30, renal cardiovascular and endocrine systems remain unaffected. 26 Preclinical rat and dog studies document no signs of acute or chronic toxicity when 500–1000 times the normal dosage of MIS was administered. 27 According to the owner telephone reports in this study, the cats were healthy 3 months after drug application. Based on this it may be suggested that there are no long-term effects, although more detailed follow-up studies are necessary to ascertain this assumption.
Progesterone
In cats, as in other animals, high serum P4 concentrations are the dominant factors in sustaining and determining the length of pregnancy, as well as maintaining uterine and cervical functions. 60 Therefore, the dramatic decrease in serum P4 concentrations could be an indicator to detect spontaneous or induced parturition/abortion in dogs and cats. This is not true for AGL use; the pregnancy is terminated while P4 concentrations remain high. Previous studies showed a non-significant decrease in serum P4 concentrations, which remained high after AGL administration until the end of abortion in dogs. 20 It has been reported that AGL has no effect on luteal function but rather inhibits the biological effects of P4 by blocking access to the P4 receptors.8,60 However, Baan et al and Kowalewski 61 reported that AGL causes an incomplete luteolytic effects in dogs.45,61
The effect of MIS on P4 is controversial. PGE1 prevented luteolysis, and acute PGE1 treatment increased P4 concentration in ewes compared with prostaglandin E2 (PGE2). PGE1 causes these effects via two mechanisms: it increases the binding capacity of luteal receptors and increases luteal P4 secretion by stimulating cyclic adenosine monophospate. 62 In addition, PGE2 has been shown to have a luteotropic effect in cattle, dogs and sheep.62–65 Honkanen reported an increase in P4 concentrations after MIS treatment. 66 In contrast, it has been reported that MIS treatment caused a decrease in P4 concentrations during the abortion period. 10 It also caused a distinct decrease in P4 concentrations after MIS treatment. 21 Studies in women showed that P4 concentrations dropped after 4 h of gemeprost, a PGE1 analogue, 67 and increased 2 days after a high dose of MIS treatment. 47
In this study, serum P4 concentrations were higher in all groups at dA/d7 than the basal P4 levels (Figure 2), which was in parallel with previous studies. 68 However, the P4 results examined as A+ and A– (Table 4) showed that AGL caused a significant decrease in P4 concentrations in the A+ animals in the AGL group and a slight reduction in the AGL+MIS group. In addition to the slight increases in P4 concentrations in the MIS group on the d4 (Figure 2), the PGE1 and PGE2 showed similar luteotropic effects as reported in the aforementioned studies. Therefore, it can be speculated that AGL causes incomplete luteolysis in cats.
A significant decrease was observed in P4 concentrations of the MIS400 group from d0 to dA/d7 (Figure 2), which corroborates the aforementioned studies. The increase in the dose of MIS caused a decrease in the concentration of P4 in the MIS200 and MIS400 groups, indicating that a lower dose of MIS has a luteotropic effect. However, a higher dose had a luteolytic effect. Further studies are needed to determine the luteolytic potency of AGL in relation to MIS dosage.
Conclusions
In this study higher doses of MIS had a higher efficacy in inducing abortions than lower doses of MIS but with the disadvantage of markedly increased side effects. Hence, with the current knowledge, the use of the higher dose is not recommended. While increasing MIS dosage or prolonging its usage may increase the success rate of abortions, it is inadvisable owing to the possibility of severe and intense side effects. Therefore, according to the hypothesis of this study, a higher dose of misoprostol is not safe or practical in terminating mid-term pregnancies in cats. The results of this study indicate that the combination of low-dose MIS and AGL is a more efficacious abortifacient regimen than the use of these drugs alone for mid-term pregnancy termination in cats. The results of this study also show that the side effects seen in the combined group (AGL+MIS) are manageable and this regimen could be suggested for clinical application in cats.
Footnotes
Accepted: 3 August 2018
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Serhan Ay  https://orcid.org/0000-0003-2116-5149
https://orcid.org/0000-0003-2116-5149
Murat Fındık  https://orcid.org/0000-0003-1408-2548
https://orcid.org/0000-0003-1408-2548
References
- 1. Erünal-Maral N, Aslan S, Findik M, et al. Induction of abortion in queens by administration of cabergoline (Galastop) solely or in combination with the PGF2alpha analogue Alfaprostol (Gabbrostim). Theriogenology 2004; 61: 1471–1475. [DOI] [PubMed] [Google Scholar]
- 2. Verstegen JP, Onclin K, Silva LD, et al. Abortion induction in the cat using prostaglandin F2 alpha and a new anti-prolactinic agent, cabergoline. J Reprod Fertil Supp 1993; 47: 411–417. [PubMed] [Google Scholar]
- 3. Romagnoli SE, Camillo F, Cela M, et al. Clinical use of prostaglandin F2 alpha to induce early abortion in bitches: serum progesterone, treatment outcome and interval to subsequent oestrus. J R Fertil Supp 1993; 47: 425–431. [PubMed] [Google Scholar]
- 4. Nachreiner RF, Marple DN. Termination of pregnancy in cats with prostaglandin F2 alpha. Prostaglandins 1974; 7: 303–308. [DOI] [PubMed] [Google Scholar]
- 5. Garcia Mitacek MC, Stornelli MC, Praderio R, et al. Efficacy of cloprostenol or aglepristone at 21–22 and 35–38 days of gestation for pregnancy termination in queens. Reprod Domest Anim 2012; 47 Suppl 6: 200–203. [DOI] [PubMed] [Google Scholar]
- 6. Jochle W, Arbeiter K, Post K, et al. Effects on pseudopregnancy, pregnancy and interoestrous intervals of pharmacological suppression of prolactin secretion in female dogs and cats. J Reprod Fertil Supp 1989; 39: 199–207. [PubMed] [Google Scholar]
- 7. Jurka P, Max A, Hawrynska K, et al. Age-related pregnancy results and further examination of bitches after aglepristone treatment of pyometra. Reprod Domest Anim 2010; 45: 525–529. [DOI] [PubMed] [Google Scholar]
- 8. Galac S, Kooistra HS, Butinar J, et al. Termination of mid-gestation pregnancy in bitches with aglepristone, a progesterone receptor antagonist. Theriogenology 2000; 53: 941–950. [DOI] [PubMed] [Google Scholar]
- 9. Baan M, Taverne MA, Kooistra HS, et al. Induction of parturition in the bitch with the progesterone-receptor blocker aglepristone. Theriogenology 2005; 63: 1958–1972. [DOI] [PubMed] [Google Scholar]
- 10. Agaoglu AR, Aslan S, Emre B, et al. Clinical evaluation of different applications of misoprostol and aglepristone for induction of abortion in bitches. Theriogenology 2014; 81: 947–951. [DOI] [PubMed] [Google Scholar]
- 11. Kaya D, Kucukaslan I, Agaoglu AR, et al. The effects of aglepristone alone and in combination with cloprostenol on hormonal values during termination of mid-term pregnancy in bitches. Anim Reprod Sci 2014; 146: 210–217. [DOI] [PubMed] [Google Scholar]
- 12. Fieni F, Martal J, Marnet PG, et al. Clinical, biological and hormonal study of mid-pregnancy termination in cats with aglepristone. Theriogenology 2006; 66: 1721–1728. [DOI] [PubMed] [Google Scholar]
- 13. Georgiev P, Wehrend A. Mid-gestation pregnancy termination by the progesterone antagonist aglepristone in queens. Theriogenology 2006; 65: 1401–1406. [DOI] [PubMed] [Google Scholar]
- 14. Goericke-Pesch S, Georgiev P, Wehrend A. Prevention of pregnancy in cats using aglepristone on days 5 and 6 after mating. Theriogenology 2010; 74: 304–310. [DOI] [PubMed] [Google Scholar]
- 15. Gogny A, Fieni F. Aglepristone: a review on its clinical use in animals. Theriogenology 2016; 85: 555–566. [DOI] [PubMed] [Google Scholar]
- 16. Chong YS, Su LL, Arulkumaran S. Misoprostol: a quarter century of use, abuse, and creative misuse. Obstet Gynecol Surve 2004; 59: 128–140. [DOI] [PubMed] [Google Scholar]
- 17. Carbonell JL, Rodriguez J, Aragon S, et al. Vaginal misoprostol 1000 μg for early abortion. Contraception 2001; 63: 131–136. [DOI] [PubMed] [Google Scholar]
- 18. Alfirevic Z, Aflaifel N, Weeks A. Oral misoprostol for induction of labour. Cochrane Database Syst Rev 2014; 6: CD001338. DOI: 10.1002/14651858.CD001338.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pharmaceuticals F. Misodel, Ferring’s removable misoprostol vaginal delivery system, approved for labour induction in European decentralised procedure. http://www.businesswire.com/news/home/20131017005499/en/MISODEL%AA-Ferring%D5s-Rempvable-Misoprostol-Vaginal-Delivery-System (2013, accessed March 14, 2018).
- 20. Agaoglu AR, Schafer-Somi S, Kaya D, et al. The intravaginal application of misoprostol improves induction of abortion with aglepristone. Theriogenology 2011; 76: 74–82. [DOI] [PubMed] [Google Scholar]
- 21. Cetin Y, Macun HC, Beceriklisoy HB, et al. Intravaginal application of misoprostol improves pregnancy termination with cabergoline and alfaprostol in dogs. Berl Munch Tierarztl Wochensc 2010; 123: 236–242. [PubMed] [Google Scholar]
- 22. Leethongdee S, Khalid M, Bhatti A, et al. The effects of the prostaglandin E analogue Misoprostol and follicle-stimulating hormone on cervical penetrability in ewes during the peri-ovulatory period. Theriogenology 2007; 67: 767–777. [DOI] [PubMed] [Google Scholar]
- 23. Alan M, Tasal I. Efficacy of prostaglandin F2alpha and misoprostol in the induction of parturition in goats. Vet Rec 2002; 150: 788–789. [DOI] [PubMed] [Google Scholar]
- 24. Yıldız A. Induction of parturition in cows with misoprostol. J Anim Vet Adv 2009; 8: 876–879. [Google Scholar]
- 25. McNaughten J, Pozor M, Macpherson M, et al. Effects of topical application of misoprostol on cervical relaxation in mares. Reprod Domest Anim 2014; 49: 1057–1062. [DOI] [PubMed] [Google Scholar]
- 26. Bauer RF. Misoprostol preclinical pharmacology. Dig Dis Sci 1985; 30: 118–125. [DOI] [PubMed] [Google Scholar]
- 27. Kotsonis FN, Dodd DC, Regnier B, et al. Preclinical toxicology profile of misoprostol. Dig Dis Sci 1985; 30: 142–146. [DOI] [PubMed] [Google Scholar]
- 28. Schoenhard G, Oppermann J, Kohn FE. Metabolism and pharmacokinetic studies of misoprostol. Dig Dis Sci 1985; 30: 126–128. [DOI] [PubMed] [Google Scholar]
- 29. Monograph P. Misoprostol. http://www.aapharma.ca/downloads/en/PIL/2016/Misoprostol-PM.pdf (2010, accessed March 16, 2018).
- 30. Villar D, Buck WB, Gonzalez JM. Ibuprofen, aspirin and acetaminophen toxicosis and treatment in dogs and cats. Vet Hum Toxicol 1998; 40: 156–162. [PubMed] [Google Scholar]
- 31. Davidson AP, Nyland TG, Tsutsui T. Pregnancy diagnosis with ultrasound in the domestic cat. Vet Radiol 1986; 27: 109–114. [Google Scholar]
- 32. Zambelli D, Castagnetti C, Belluzzi S, et al. Correlation between the age of the conceptus and various ultrasonographic measurements during the first 30 days of pregnancy in domestic cats (Felis catus). Theriogenology 2002; 57: 1981–1987. [DOI] [PubMed] [Google Scholar]
- 33. Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod 2004; 19: 81–84. [DOI] [PubMed] [Google Scholar]
- 34. Danielsson KG, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol 1999; 93: 275–280. [DOI] [PubMed] [Google Scholar]
- 35. Tang OS, Gemzell-Danielsson K, Ho PC. Misoprostol: pharmacokinetic profiles, effects on the uterus and side-effects. Int J Gynaecol Obstet 2007; 99 Suppl 2: 160–167. [DOI] [PubMed] [Google Scholar]
- 36. Borgatta L, Kapp N. Clinical guidelines. Labor induction abortion in the second trimester. Contraception 2011; 84: 4–18. [DOI] [PubMed] [Google Scholar]
- 37. Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol 1997; 90: 88–92. [DOI] [PubMed] [Google Scholar]
- 38. Carbonell JL, Rodriguez J, Velazco A, et al. Oral and vaginal misoprostol 800 μg every 8 h for early abortion. Contraception 2003; 67: 457–462. [DOI] [PubMed] [Google Scholar]
- 39. Creinin MD, Moyer R, Guido R. Misoprostol for medical evacuation of early pregnancy failure. Obstet Gynecol 1997; 89: 768–772. [DOI] [PubMed] [Google Scholar]
- 40. Georgiev P, Bostedt H, Goericke-Pesch S, et al. Induction of abortion with aglepristone in cats on day 45 and 46 after mating. Reprod Domest Anim 2010; 45: 161–167. [DOI] [PubMed] [Google Scholar]
- 41. Scott PP. Cats. In: Hafez ESE. (ed). Reproduction and breeding techniques for laboratory animals. Philadelphia: Lea and Febiger, 1970, pp 192–208. [Google Scholar]
- 42. Braun BC, Zschockelt L, Dehnhard M, et al. Progesterone and estradiol in cat placenta – biosynthesis and tissue concentration. J Steroid Biochem Mol Biol 2012; 132: 295–302. [DOI] [PubMed] [Google Scholar]
- 43. Goh SE, Thong KJ. Induction of second trimester abortion (12–20 weeks) with mifepristone and misoprostol: a review of 386 consecutive cases. Contraception 2006; 73: 516–519. [DOI] [PubMed] [Google Scholar]
- 44. Kowalewski MP, Beceriklisoy HB, Pfarrer C, et al. Canine placenta: a source of prepartal prostaglandins during normal and antiprogestin-induced parturition. Reproduction 2010; 139: 655–664. [DOI] [PubMed] [Google Scholar]
- 45. Baan M, Taverne MA, de Gier J, et al. Hormonal changes in spontaneous and aglepristone-induced parturition in dogs. Theriogenology 2008; 69: 399–407. [DOI] [PubMed] [Google Scholar]
- 46. Siemieniuch MJ, Jursza E, Szostek AZ, et al. Placental origin of prostaglandin F2alpha in the domestic cat. Mediators Inflamm 2014; 2014: 364787. DOI 10.1155/2014/364787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barnhart KT, Bader T, Huang X, et al. Hormone pattern after misoprostol administration for a nonviable first-trimester gestation. Fertil Steril 2004; 81: 1099–1105. [DOI] [PubMed] [Google Scholar]
- 48. Rabe T, Basse H, Thuro H, et al. Effect of the PGE1 methyl analog misoprostol on the pregnant uterus in the first trimester. Geburtshilfe Frauenheilkd 1987; 47: 324–331. [DOI] [PubMed] [Google Scholar]
- 49. Velazco A, Varela L, Tanda R, et al. Misoprostol for abortion up to 9 weeks’ gestation in adolescents. Eur J Contracept Reprod Health Care 2000; 5: 227–333. [DOI] [PubMed] [Google Scholar]
- 50. Pymar HC, Creinin MD, Schwartz JL. Mifepristone followed on the same day by vaginal misoprostol for early abortion. Contraception 2001; 64: 87–92. [DOI] [PubMed] [Google Scholar]
- 51. Shinohara K, Shimizu T, Igarashi J, et al. Correlation of prostaglandin E2 production and gastric acid secretion in infants with hypertrophic pyloric stenosis. J Pediatric Surg 1998; 33: 1483–1485. [DOI] [PubMed] [Google Scholar]
- 52. Lindstrom E, Hakanson R. Prostaglandins inhibit secretion of histamine and pancreastatin from isolated rat stomach ECL cells. Br J Pharmacol 1998; 124: 1307–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kosiak W, Swieton D, Fryze I, et al. Gastric outlet obstruction due to an iatrogenic cause in a neonatal period – report of two cases. Ultraschall Med 2009; 30: 401–403. [DOI] [PubMed] [Google Scholar]
- 54. Hawkey CJ, Rampton DS. Prostaglandins and the gastrointestinal mucosa: are they important in its function, disease, or treatment? Gastroenterology 1985; 89: 1162–1188. [DOI] [PubMed] [Google Scholar]
- 55. Velkova V, Papasova M, Radomirov R. Effects of PGE1 and PGF2 alpha on the responses of gastrointestinal sphincters to field stimulation. Eur Pharmacol 1981; 75: 297–303. [DOI] [PubMed] [Google Scholar]
- 56. Allen R, O’Brien BM. Uses of misoprostol in obstetrics and gynecology. Rev Obstet Gynecol 2009; 2: 159–168. [PMC free article] [PubMed] [Google Scholar]
- 57. Carbonell JL, Varela L, Velazco A, et al. The use of misoprostol for termination of early pregnancy. Contraception 1997; 55: 165–168. [DOI] [PubMed] [Google Scholar]
- 58. Gemzell-Danielsson K, Lalitkumar S. Second trimester medical abortion with mifepristone-misoprostol and misoprostol alone: a review of methods and management. Reprod Health Matters 2008; 16: 162–172. [DOI] [PubMed] [Google Scholar]
- 59. Toews ML, Bylund DB. Pharmacologic principles for combination therapy. Proc Am Thorac Soc 2005; 2: 282–289. [DOI] [PubMed] [Google Scholar]
- 60. Hoffmann B, Schuler G. Receptor blockers – general aspects with respect to their use in domestic animal reproduction. Anim Reprod Sci 2000; 60–61: 295–312. [DOI] [PubMed] [Google Scholar]
- 61. Kowalewski MP. Luteal regression vs. prepartum luteolysis: regulatory mechanisms governing canine corpus luteum function. Reprod Biol 2014; 14: 89–102. [DOI] [PubMed] [Google Scholar]
- 62. Weems YS, Nett TM, Rispoli LA, et al. Prostaglandin E1 (PGE1), but not prostaglandin E2 (PGE2), alters luteal and endometrial luteinizing hormone (LH) occupied and unoccupied LH receptors and mRNA for LH receptors in ovine luteal tissue to prevent luteolysis. Prostaglandins Other Lipid Mediat 2010; 91: 42–50. [DOI] [PubMed] [Google Scholar]
- 63. Kotwica J, Skarzynski D, Mlynarczuk J, et al. Role of prostaglandin E2 in basal and noradrenaline-induced progesterone secretion by the bovine corpus luteum. Prostaglandins Other Lipid Mediat 2003; 70: 351–359. [DOI] [PubMed] [Google Scholar]
- 64. Kowalewski MP, Fox B, Gram A, et al. Prostaglandin E2 functions as a luteotrophic factor in the dog. Reproduction 2013; 145: 213–226. [DOI] [PubMed] [Google Scholar]
- 65. Weems YS, Raney A, Pang J, et al. Prostaglandin E1 or E2 (PGE1, PGE2) prevents premature luteolysis induced by progesterone given early in the estrous cycle in ewes. Theriogenology 2013; 80: 507–512. [DOI] [PubMed] [Google Scholar]
- 66. Honkanen H. Medical abortion with mifepristone and misoprostol in the first trimester: efficacy, side effects, women’s perceptions and endocrine effects. PhD Thesis, University of Helsinki, 2004. [Google Scholar]
- 67. Howell RJ, Olajide F, Teisner B, et al. Circulating levels of placental protein 14 and progesterone following Mifepristone (RU38486) and Gemeprost for termination of first trimester pregnancy. Fertil Steril 1989; 52: 66–68. [DOI] [PubMed] [Google Scholar]
- 68. Honkanen H, Ranta S, Ylikorkala O, et al. The kinetics of serum hCG and progesterone in response to oral and vaginal administration of misoprostol during medical termination of early pregnancy. Human Reprod 2002; 17: 2315–2319. [DOI] [PubMed] [Google Scholar]


