Abstract
Objectives
Despite the increasing availability of feline blood collected and stored for transfusion purposes, few studies have been performed on feline blood units. The aim of this prospective in vitro study was to evaluate haematological and morphological changes in feline blood cells in whole blood units between collection and end of storage.
Methods
Haematological examination (red blood cells [RBCs], haemoglobin, haematocrit, red cell distribution width, mean cell volume, mean cell haemoglobin concentration, mean cell haemoglobin, white blood cells [WBCs] and platelet [PLT] count) was performed on 40 non-leukoreduced feline whole blood units at the time of collection (day[D]0) and after storage (D35). The blood was collected into citrate–phosphate–dextrose–adenine anticoagulant-preservative solution using an open system in a veterinary blood bank and stored for 35 days at 4 ± 2°C. Twenty of these feline whole blood units were also analysed for blood cell morphology (normal RBCs, macrocytes, echinocytes, spherocytes, schistocytes, lysed RBCs, RBCs with Heinz bodies and recognisable WBC and PLT count). Differences between the two examination times were statistically analysed.
Results
There was a statistically significant decrease in WBC and PLT counts after storage at D35 (P <0.0001 for both). The most significant cellular morphological changes after storage were an increase in echinocyte count (P = 0.0001), and lysed RBCs (P <0.0001), and a decrease in normal RBCs (P <0.0001). Recognisable WBCs – mainly lymphocytes – were present at the end of storage.
Conclusions and relevance
This study showed that significant morphological changes occur in RBCs in feline blood units during storage for 35 days. In vivo studies are required to establish if these changes could affect the ability of stored RBCs to circulate and provide adequate oxygen delivery after transfusion.
Keywords: Whole blood unit, transfusion, storage, CBC, haematological change, morphological change
Introduction
The increasing availability of veterinary hospital blood banks and commercial sources of feline blood products means that transfusion therapy is more widely available to veterinarians, and feline stored blood products are more often used. The main indication for transfusion in cats is anaemia; therefore, whole blood (WB) and packed red blood cell (PRBC) units are the most commonly collected and stored blood products.1–4
WB is still extensively used in feline transfusion medicine, primarily reflecting the ease with which this blood component can be obtained. Although there are no legal standards for storage of feline WB (FWB) units for transfusion purposes, the length of storage is based on the criteria set by the US Food and Drug Administration (US FDA) in human transfusion medicine, which requires that 75% of transfused RBCs remain in circulation 24 h after transfusion. This corresponds to a storage length of 30 days for FWB units collected using an open system and preserved in acid–citrate–dextrose solution, 5 and 35 days of storage for FWB units collected in citrate–phosphate–dextrose–adenine (CPDA) solution. 6 Based on these data most veterinary blood banks store FWB units for 28–35 days.
During storage under standard blood bank conditions, human RBCs undergo progressive morphological alterations that affect their ability to circulate after transfusion and may cause post-transfusion complications. RBCs undergo progressive shape change, from a readily deformable biconcave disc to poorly deformable echinocytes with cell surface protrusions, and ultimately to non-deformable spheroechinocytes. 7 RBCs manifesting echinocyte shapes can return to the discocyte shape under certain conditions. In contrast, RBCs assuming spherocyte and degenerated shapes are irreversibly changed cells: these non-deformable cells will haemolyse.7,8
To date, a limited number of studies have been performed on feline blood units collected and stored for transfusion purposes; all have documented biochemical changes consistent with anaerobic metabolism in a closed system: decreased glucose, increased lactate and increased ammonia.9–12 The feline haemoglobin (Hb) P50 is only slightly reduced after FWB storage for 35 days in CPDA solution, suggesting that the storage lesion does not have a major impact on Hb-oxygen affinity in this species. 9 In addition, once collected, feline blood undergoes significant haematological changes between circulation in feline blood donors and in FWB donated units at time of collection. 13 However, to our knowledge, the haematological and morphological changes in a limited number of FWB units collected with a closed system and stored for transfusion purposes have only recently been reported. 14
The purpose of this study was to document changes in cellular blood components during storage of FWB units. We hypothesised that the FBW units undergo changes in haematological parameters and morphology after 35 days of storage.
Material and methods
The study was performed on non-leukoreduced FWB units containing 60 ml (blood and anticoagulant) produced by the Veterinary Transfusion Research Laboratory (REVLab), University of Milan, in the period 2017–2018. Suitable feline blood donors were selected and donated blood under anaesthesia, after informed owner consent was obtained and following the guidelines on veterinary transfusion from the Italian Health Minister and as previously described.15,16 All cats were feline blood type A. Blood was collected using an open system with a ratio of CPDA:blood of 1:7. Blood was collected in three 20 ml syringes, as previously described, 16 and transferred to a 150 ml empty transfer bag (Transfer Grifols 150; Grifols Italia SpA) using a spike (Combifix Adapter; B Braun Vet Care) connected to the bag. The blood in the bag was gently mixed for 2 mins and 3–5 aliquots (1 ml) of blood (segments) were isolated in the collection tubing using an electric thermal sealer (Hemoweld-B; Delcon Medical Devices). The FWB units were than stored upright at 4 ± 2°C in a dedicated blood bank refrigerator with a continuous temperature record and alarm, preventing frequent fluctuations in temperature, and manually mixed and inverted every 48 h.
At the time of sampling, one of the segments was removed from the bag, and the blood from this transferred to an empty tube for analysis. Sampling and analyses occurred on day 0 (D0; the date of blood collection) and on day 35 (D35; the date of final storage/expiration of the FWB units based on the study of Bücheler and Cotter). 17
Haematological parameters included red blood cells (RBCs), haemoglobin (Hb), haematocrit (Hct), red cell distribution width (RDW), mean cell volume (MCV), mean cell Hb concentration (MCHC), mean cell Hb (MCH), white blood cells (WBCs) and platelet (PLT) count, and were assessed using an automated multi-parameter haematology analyser with software for animal samples (Cell-Dyn 3700; Abbott Diagnostics Laboratories).
RBC morphology analysis, recognisable WBC count and PLT evaluation were performed on May–Grünwald–Giemsa-stained slides. Blood smears were examined by LB (DVM, professional veterinary technician, master in veterinary laboratory medicine) first at lower power (× 20 objective) to assess for the presence of erythrocyte autoagglutination, leukocyte aggregates, large platelet aggregates and abnormal cells, and then using high-power magnification (× 100) to assess RBC morphology, and WBC and PLT number and morphology.18–21
The different RBC shapes evaluated at D0 and D35 were normal RBCs, macrocytes, echinocytes, spherocytes, schistocytes, lysed RBCs (ghost cells) and RBCs with Heinz bodies. Counts were performed over 10 microscopic fields and results expressed as mean count per high-power field (hpf) examined using a × 100 objective (× 1000 magnification). One hundred WBCs were counted and classified according to their recognisability; the final value was expressed as a percentage.
All analysis was conducted using a protocol approved by the University of Milan Animal Welfare Bioethical Committee.
Data were statistically analysed using computer software (MedCalc version 16.4.3). Determination of data distribution was established using the Shapiro–Wilk test. Comparison of differences between D0 and D35 for data with normal distribution was performed using a paired t-test. Comparison for non-normally distributed data was performed with Wilcoxon test for paired samples. Echinocytes and normal RBCs were counted as a percentage of 200 RBCs counted in two adjacent hpf to allow for statistical comparison. For all statistical tests, the significance threshold was set at P <0.05.
Results
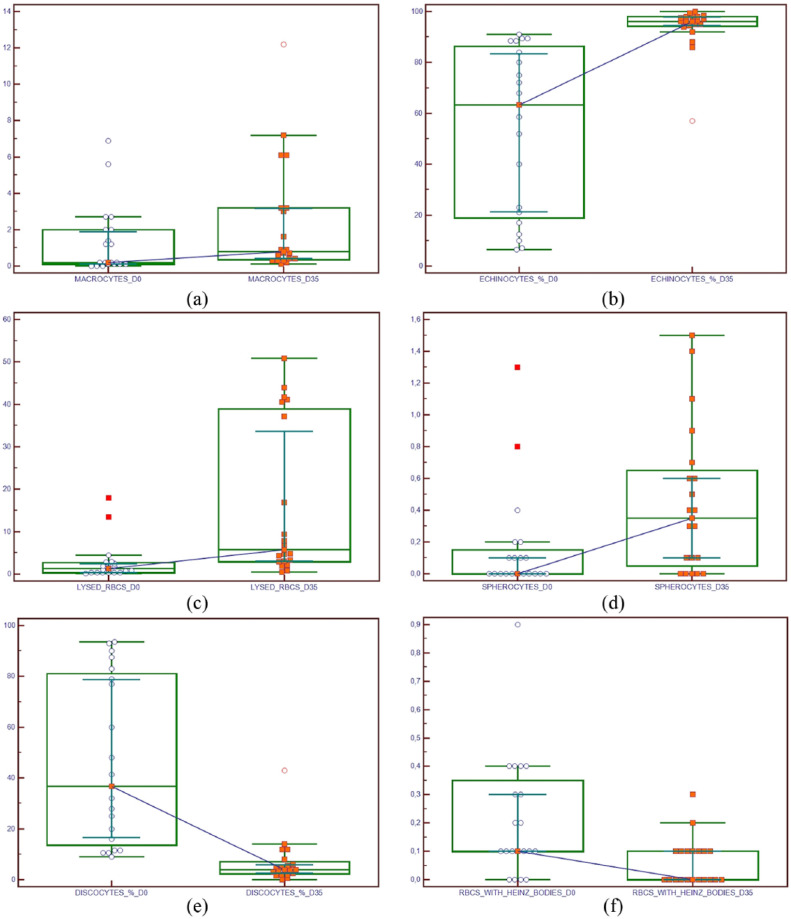
A complete blood cell count was performed on 40 FWB units at D0 and at D35. Summary statistics are presented in Table 1. There was a decrease in most haematological parameters after storage for 35 days, but statistically significant decreases were only seen in WBC and PLT counts (Figure 1). Only RDW showed a slight non-statistically significant increase at D35 with respect to D0.
Table 1.
Results of complete blood count performed in 40 feline whole blood units at collection (D0) and after storage for 35 days (D35)
| Parameter | Time | Mean | 95% CI | SD | Median | 95% CI | Minimum | Maximum | P value for paired differences |
|---|---|---|---|---|---|---|---|---|---|
| RBCs (× 103/µl) | D0 | 7008.2 | 6544.0–7472.4 | 1451.5 | 6585.0 | 6196.7–7465.9 | 4580.0 | 10700.0 | 0.3971 |
| D35 | 6777.0 | 6298.5–7255.4 | 1496.1 | 6560.0 | 6234.0–6712.8 | 4270.0 | 12000.0 | ||
| Hb (g/dl) | D0 | 9.7 | 9.0–10.3 | 2.0 | 9.5 | 8.7–10.1 | 6.2 | 14.9 | 0.1288 |
| D35 | 9.3 | 8.6–10.0 | 2.1 | 8.8 | 8.6–9.4 | 6.0 | 15.3 | ||
| Hct (%) | D0 | 27.8 | 25.9–29.6 | 5.6 | 27.1 | 25.0–29.6 | 18.7 | 44.6 | 0.6502 |
| D35 | 26.8 | 25.0–28.5 | 5.4 | 25.8 | 25.1–27.3 | 18.1 | 43.5 | ||
| MCV (fl) | D0 | 40.0 | 38.8–41.1 | 3.5 | 39.4 | 38.2–40.2 | 34.7 | 51.8 | 0.9625 |
| D35 | 39.7 | 38.8–40.6 | 2.8 | 39.2 | 38.7–40.6 | 33.5 | 45.5 | ||
| MCH (pg) | D0 | 13.9 | 13.5–14.4 | 1.3 | 13.9 | 13.6–14.2 | 10.9 | 16.6 | 0.1650 |
| D35 | 13.7 | 13.3–14.2 | 1.3 | 13.5 | 13.2–14.2 | 11.2 | 16.9 | ||
| MCHC (g/dl) | D0 | 34.9 | 34.3–35.6 | 2.1 | 35.3 | 33.5–36.2 | 30.5 | 38.8 | 0.5106 |
| D35 | 34.6 | 33.9–35.4 | 2.4 | 34.7 | 34.0–35.7 | 29.2 | 40.0 | ||
| RDW (%) | D0 | 17.3 | 16.8–17.9 | 1.6 | 17.4 | 16.7–18.0 | 13.3 | 20.8 | 0.0596 |
| D35 | 17.9 | 17.3–18.5 | 1.9 | 17.6 | 17.1–18.1 | 15.2 | 23.9 | ||
| WBCs (/µl) | D0 | 6716.5 | 5799.7–7633.2 | 2866.4 | 6435.0 | 5678.2–7422.0 | 1560.0 | 13400.0 | <0.0001 |
| D35 | 1001.4 | 727.4–1275.5 | 856.8 | 762.0 | 559.3–1012.2 | 123.0 | 4030.0 | ||
| PLTs (× 10³/µl) | D0 | 366.7 | 307.6–425.8 | 184.8 | 315.5 | 271.0–404.8 | 84.0 | 1087.5 | <0.0001 |
| D35 | 204.9 | 163.1–246.6 | 130.5 | 157.0 | 123.5–228.4 | 58.0 | 527.0 |
Statistically significant P values (<0.05) are in bold
CI = confidence interval; RBCs = red blood cells; Hb = haemoglobin; Hct = haematocrit; MCV = mean cell volume; MCH = mean cell haemoglobin; MCHC = mean cell haemoglobin concentration; RDW = red blood cell distribution width; WBCs = white blood cells; PLTs = platelets
Figure 1.
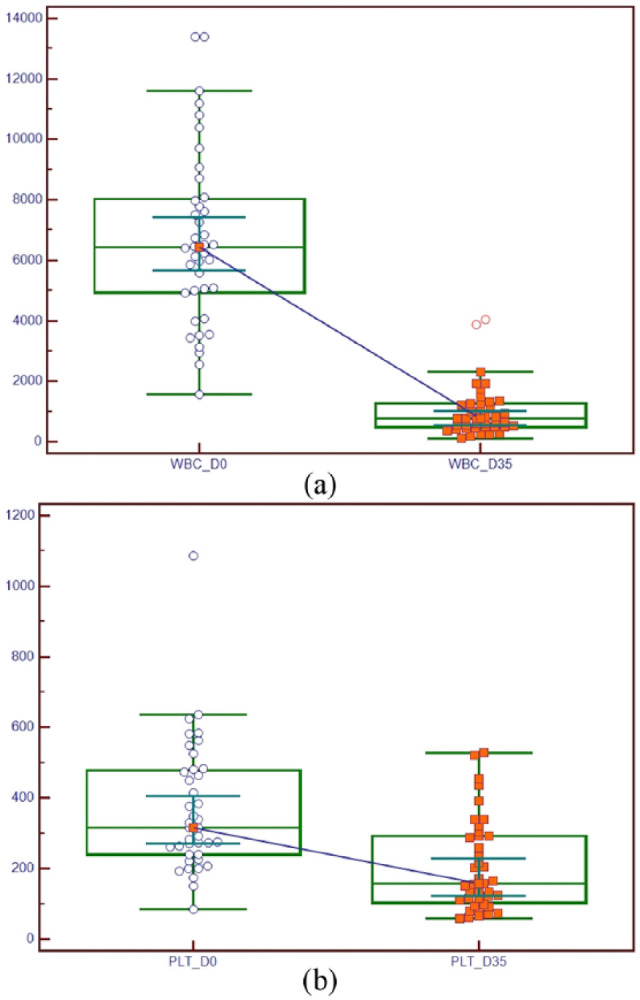
Graph displaying the statistically significant (P <0.0001, Wilcoxon test) decrease in (a) white blood cells (WBCs) and (b) platelets (PLTs) between counts at D0 (date of blood collection) and D35 (date of final storage/expiration of the feline whole blood units) in 40 feline whole blood units collected and stored for transfusion purposes. Values were expressed as unit/µl for WBCs and unit × 103/µl for PLTs. The central box represents the values from the lower to upper quartile (25–75 percentile). The middle line represents the median. A line extends from the minimum to the maximum value, excluding ‘outside’ and ‘outlying’ values, which are displayed as separate points
On microscopic examination, RBC morphology and the number of recognisable WBCs at D0 and D35 were evaluated in 20 pairs of blood smears relative to 20 randomly selected samples from 40 FWB units evaluated haematologically (Table 2). There was a statistically significant increase in macrocytes, echinocytes, spherocytes and lysed RBC count, and a significant decrease in normal RBCs, schistocytes, RBCs with Heinz bodies and percentage of recognisable WBCs (predominantly lymphocytes) (Figure 2). At D35 the echinocyte count was assigned a value of >350 units/hpf as almost all the cells in every field had this morphology. With respect to D0 the PLTs at D35 showed a lower colour intensity (Figures 3 and 4).
Table 2.
Results of morphological evaluations at day 0 (D0) and day 35 (D35) in 20 feline whole blood units collected and stored for transfusion purposes
| Parameters | Time | Mean | 95% CI | SD | Median | 95% CI | Minimum | Maximum | P value for paired differences |
|---|---|---|---|---|---|---|---|---|---|
| Macrocytes (n/hpf) |
D0 | 1.3 | 0.4–2.2 | 1.9 | 0.2 | 0.1–1.8 | 0.0 | 6.9 | 0.0007 |
| D35 | 2.4 | 0.9–3.9 | 3.1 | 0.8 | 0.4–3.1 | 0.1 | 12.2 | ||
| Echinocytes (n/hpf) |
D0 | 197.5 | 141.7–253.4 | 119.2 | 183.1 | 109.3–295.0 | 10.9 | 350.0 | 0.0001 |
| D35 | 340.0 | 319.2–360.8 | 44.4 | 350.0 | 350.0–350.0 | 151.1 | 350.0 | ||
| Echinocytes (%) |
D0 | 53.6 | 38.2–69.0 | 32.9 | 63.2 | 21.3–83.3 | 6.5 | 91.0 | <0.0001 |
| D35 | 93.6 | 89.2–97.9 | 9.3 | 96.0 | 94.5–97.9 | 57.0 | 100.0 | ||
| Schistocytes (n/hpf) |
D0 | 0.5 | 0.08–1.0 | 1.0 | 0.2 | 0.1–0.4 | 0.0 | 4.6 | 0.1297 |
| D35 | 0.2 | 0.08–0.4 | 0.4 | 0.1 | 0.1–0.3 | 0.0 | 1.8 | ||
| Spherocytes (n/hpf) |
D0 | 0.1 | 0.01–0.3 | 0.3 | 0.0 | 0.0–0.1 | 0.0 | 1.3 | 0.0139 |
| D35 | 0.4 | 0.2–0.6 | 0.4 | 0.3 | 0.1–0.6 | 0.0 | 1.5 | ||
| Lysed RBCs (n/hpf) |
D0 | 2.8 | 0.6–4.9 | 4.6 | 1.3 | 0.4–2.5 | 0.1 | 17.9 | <0.0001 |
| D35 | 16.2 | 7.7–24.7 | 18.1 | 5.7 | 3.0–33.6 | 0.5 | 50.9 | ||
| RBCs with Heinz bodies (n/hpf) |
D0 | 0.2 | 0.1–0.3 | 0.2 | 0.1 | 0.1–0.3 | 0.0 | 0.9 | 0.0110 |
| D35 | 0.06 | 0.02–0.09 | 0.08 | 0.0 | 0.0–0.1 | 0.0 | 0.3 | ||
| Normal RBCs (%) | D0 | 46.3 | 30.9–61.7 | 32.9 | 36.7 | 16.6–78.6 | 9.0 | 93.5 | <0.0001 |
| D35 | 6.8 | 2.5–11.2 | 9.3 | 4.0 | 2.5–5.9 | 0.0 | 43.0 | ||
| Recognisable WBCs (%) |
D0 | 88.4 | 84.2–92.6 | 8.9 | 88.5 | 85.3–92.4 | 64.0 | 100.0 | <0.0001 |
| D35 | 9.0 | 5.1–12.9 | 8.2 | 6.0 | 3.1–11.8 | 1.0 | 31.0 |
Echinocytes were assigned a value of >350 units/high-power field (hpf) at D35 as almost all the cells showed this morphology. Statistically significant differences are shown in bold (P <0.05)
CI = confidence interval; RBCs = red blood cells; WBCs = white blood cells
Figure 2.
Graph displaying the increase in (a) macrocyte count (mean/10 high-power field [hpf]), (b) echinocyte count (%), (c) lysed red blood cells (RBCs; mean/10 hpf) and (d) spherocyte count (mean/10 hpf), and the decrease in (e) normal RBC count (%) and (f) RBCs with Heinz bodies (mean/10 hpf) in 20 feline whole blood units collected and stored for transfusion purposes. There is a statistically significant difference (P = 0.0007; P <0.0001; P <0.0001; P = 0.0139; P <0.0001; P = 0.0110, respectively; Wilcoxon test) between counts at D0 (date of blood collection) and D35 (final date of storage/expiration of the feline whole blood unit). The central box represents the values from the lower to upper quartile (25–75 percentile). The middle line represents the median. A line extends from the minimum to the maximum value, excluding ‘outside’ and ‘outlier’ values, which are displayed as separate points
Figure 3.
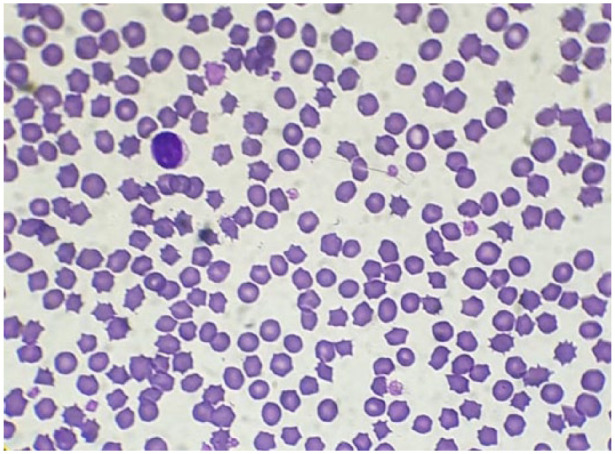
Blood films performed at the time of collection from a feline whole blood unit collected for transfusion purposes. Most cells were normal erythrocytes (original magnification × 100, May–Grünwald–Giemsa stain)
Figure 4.
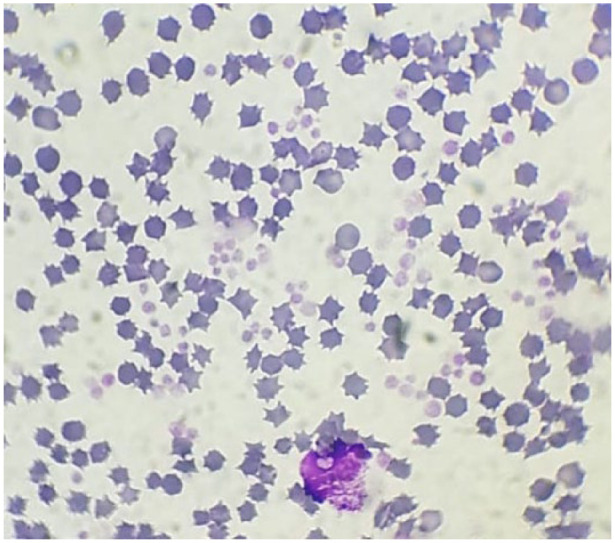
Blood film performed at end of storage, 35 days (D35) after blood collection, from feline whole blood units collected for transfusion purposes. Most cells were echinocytes, platelets had a lower intensity of colouration, there was a lysed unrecognisable white blood cell, some lysed red blood cells and few normal erythrocytes (original magnification × 100, May–Grünwald–Giemsa stain)
Discussion
Studies of canine RBC products have shown that increasing storage time is associated with an increased risk of transfusion-related haemolysis, 22 inflammation 23 and risk of infection in septic dogs with experimental pneumonia. 24 To date, storage-related transfusion reactions have not been documented in cats and no experimental or clinical studies have evaluated storage-related changes in feline blood products or the effects of transfusion of blood products of varying age. In addition, despite the increasing availability of stored feline blood units, analysis of blood parameters in feline blood collected for transfusion purposes has rarely been reported in the scientific literature.9,11–14 For these reasons we studied the haematological and cell morphological changes at the end of storage.
Our results were in agreement with a recent study that investigated feline blood collected with a closed system and stored in a similar manner to the blood units in the present study, 14 which found that RBC parameters (RBC count, MCV, MCHC, MCH, RDW, Hct, Hb) did not change significantly during the 35 days of storage, reflecting the situation in equine PRBC units and in canine PRBC units for PCV.25,26 These results were in contrast to analysis of ovine WB units in which a significant decline in RBC count and an increase in MCV was demonstrated, 27 and in WB units in ferrets where an increase in Hct was noted in the early phase of storage. 28 Species-specific differences, type of blood collection systems and storage conditions could explain these findings.
The absence of a significant increase in Hb at D35 was in contrast with the results of the morphological analysis, where a significant increase in lysed RBCs was seen. Lysis would be expected to result in a significant increase in Hb content after 35 days, but this apparent inconsistency can be explained by the methodology of the automated analyser used in the study for haematological analysis. This analyser uses a laser-impedance technique that requires lysis of RBCs to determine the Hb content and therefore cannot distinguish between the Hb present in the supernatant derived from natural haemolysis and the Hb contained within the cells. Obviously, the Hb present outside the erythrocytes is not able to transport oxygen and is therefore not useful in predicting the effectiveness of the transfusion. Evaluation of the percentage of haemolysis in blood units could help clarify this result, but, unfortunately, we did not evaluate this parameter during storage.
Another apparent incongruent result was the concomitant increase in the macrocyte count and decrease in MCV at D35. This could be explained by the fact that the number of macrocytes counted at the end of storage was too low in number to influence the MCV index. In addition, even if not statistically significant, RDW increased at D35 because RBCs showed morphologies with varied dimensions. However the predominant RBCs at D35 were small RBCs, and this explains the low MCV index value at D35.
There was a significant decrease in WBC counts at the end of storage in accordance with previous literature reports. It is known that the very short lifespan of granulocytes does not allow the preservation and transfusion of these cells. 29 Although significantly decreased in number, WBCs and PLTs were present at the end of storage. WBCs and PLTs in non-leukoreduced human and canine stored RBC units are immunologically active and the release of pro-inflammatory, pro-thrombotic compounds and vascular endothelial growth factor increases with storage time. Leukoreduction involves passing WB or a blood component through a filter to remove donor WBCs and PLTs. Removal of these cells mitigates some of the effects associated with stored blood transfusion, including febrile non-haemolytic transfusion reactions, and infection.23,30,31 A pilot study showed that prestorage leukoreduction of FWB units is possible using an in-line neonatal filter. 32 However, the main limitation of this technique is the reduction in blood volume, as the residual filter volume is 8 ml. For this reason, leukoreduction is not routinely performed in feline transfusion medicine.
The normal mean PLT survival time is 31 h in healthy cats; 33 therefore, the PLTs in blood units at the end of storage were not viable. This could explain why these PLTs had a lower intensity of colouration on microscopic evaluation. Cat platelets are prone to activation and clumping when blood samples are collected.18–21 At the end of storage (D35) PLTs were no longer viable and so the PLTs originally aggregating at D0 were unable to aggregate at D35. This could be the reason why a relatively high mean PLT count, in comparison to PLT D0 count, was recorded at the end of storage.
Transfusion efficacy depends not only on the quantity of cells provided, but also their quality. At D35 significant morphological changes were detected, predominantly the formation of echinocytes, and presence of lysed RBCs. The most obvious change was an increase in echinocyte count, as previously shown in feline blood collected with a closed system in which adenosine triphosphate (ATP) depletion was also demonstrated during feline blood unit storage, 14 which may explain the echinocytic transformation of normal RBCs. 18 A gradual echinocytic shape transformation was also observed for canine RBCs in PRBC units. 34 Human studies have demonstrated that echinocytes are less deformable than discocytes and increase blood viscosity because of entanglement of their cell spicules and intercellular interference during flow. 35 However, echinocyte formation might be reversible and these cells are capable of returning to a discocyte shape under certain conditions. In fact, transfusion itself may encourage some normalisation of the echinocytic shape of stored RBCs. During storage, lysophosphatidylcholine is produced from phosphatidylcholine, which accumulates in cell membranes and is a potent echinocytogenic stimulant. 36 When incubated in fresh autologous plasma, such echinocytic compounds may equilibrate with the low concentrations in fresh plasma, explaining the shape reversibility towards the normal discocytic shape.37,38
Heinz body development was also considered a possible consequence of oxidative damage to the RBCs. Oxidative processes in the RBC membrane are considered to be one of the main causes of decreased erythrocyte deformability and increased rigidity during storage in human medicine. 39 In this study we found a decrease in number of RBCs with Heinz bodies during storage; however, these were present in very low numbers at D0, as occasionally reported in healthy cats. 40 By D35 it was hard to read the blood slides accurately due to the numerous echinocytes and some Heinz body-containing cells may have been missed. We cannot conclude that Heinz body numbers reduce following storage, but rather that their identification at the end of storage is very difficult under light microscopy.
In this study we looked for erythrocyte morphologies, such as spherocytes, that are difficult to recognise with certainty in cats because of the small size and limited central pallor of feline erythrocytes, and schistocytes, which are less consistently seen in cats.18–21 However, we wanted to verify if collection and storage of blood could result in RBC morphology not commonly seen in normal feline blood films. The number of these cells, both spherocytes and schistocytes changed significantly during storage; however, they were present in very low numbers at collection and the end of storage. This confirms that feline RBC shape abnormalities were not common in blood collected and stored for transfusion purposes, in accordance with results of a previous study. 14
This study has some limitations. The first is that all the analyses were performed using blood from the line segments. During creation of blood unit tube segments turbulent blood flow may be generated. This turbulent flow may result in RBC shape abnormalities such as schistocytes, which are not present in healthy cats, being associated with disseminated intravascular coagulation, severe iron deficiency and other conditions, 18 but were found in low numbers in feline blood units at D0. Tubing segments, generated during RBC component production, have been evaluated to determine their suitability as a sample source for quality testing in human PRBC units. That study demonstrated that segments from RBC units should not be used for quality testing. 41 However, for some other parameters, such as cytokine measurement, the blood unit segments were considered to be representative of the entire donor unit. 42 It is not known whether the blood sample in the unit segment could serve as a surrogate sample in veterinary transfusion medicine as no studies have been performed. This is a potential limitation of the current study and should be addressed in future studies. The non-destructive testing of blood units by analysis of blood segments offers several advantages, such as in-process quality control to facilitate screening of blood donor units, continued storage of the units after testing without a shortened expiry time, and reduction in discarded samples. These factors are of particular importance in feline transfusion medicine.
No percentage of haemolysis was calculated, and this is an important parameter in human transfusion medicine. US FDA rules in human transfusion medicine require that haemolysis affects <1% of stored cells at the end of the approved storage period. While this standard has been adopted in veterinary studies, acceptable limits for feline transfusion blood have not yet been established. Percentage haemolysis was not measured in this study because determining the longevity of these samples was not our focus.
Feline RBC morphology was assessed only by light microscopy, and there were some difficulties in reading slides at D35. The difficulties encountered in the morphological evaluation of cells have been overcome in human medicine, thanks to the use of scanning electron microscopy. This provides sharper images and therefore easier and better reading and recognition of the different shape changes.7,43
Only blood products collected with a closed system should be stored for >24 h in the refrigerator. However, closed systems are less readily available for feline transfusion medicine and blood is frequently collected with an open system, as in our study. Blood units and blood products collected using open systems have previously been stored successfully without microbial growth when all blood banking was done by experienced staff. 2 In addition, our previous studies confirmed that feline blood units collected using an open system were negative for aerobic bacterial growth.10,44
Conclusions
The study adds important information on quality of stored RBCs in FWB units. The parameters considered (ie, the number of erythrocytes, erythrocyte indexes and cell morphology) seem to characterise in vitro a product that is effective for transfusion purposes, even at the end of storage. However, this conclusion is based on in vitro evaluations. In vivo studies are required to establish if these changes could affect the ability of stored RBCs to circulate and be effective in delivering oxygen effectively after transfusion.
Footnotes
Accepted: 12 August 2018
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was funded in part by Piano di Sostegno alla Ricerca 2017, Linea 2, University of Milan, Milan, Italy.
ORCID iD: Eva Spada  https://orcid.org/0000-0003-3898-6955
https://orcid.org/0000-0003-3898-6955
References
- 1. Castellanos I, Couto CG, Gray TL. Clinical use of blood products in cats: a retrospective study (1997–2000). J Vet Intern Med 2004; 18: 529–532. [DOI] [PubMed] [Google Scholar]
- 2. Weingart C, Giger U, Kohn B. Whole blood transfusions in 91 cats: a clinical evaluation. J Feline Med Surg 2004; 6: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klaser DA, Reine NJ, Hohenhaus AE. Red blood cell transfusions in cats: 126 cases (1999). J Am Vet Med Assoc 2005; 226: 920–923. [DOI] [PubMed] [Google Scholar]
- 4. Langston C, Cook A, Eatroff A, et al. Blood transfusions in dogs and cats receiving hemodialysis: 230 cases (June 1997–September 2012). J Vet Intern Med 2017; 31: 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marion RS, Smith JE. Posttransfusion viability of feline erythrocytes stored in acid-citrate-dextrose solution. J Am Vet Med Assoc 1983; 183: 1459–1460. [PubMed] [Google Scholar]
- 6. Bücheler J. Storage of feline and canine whole blood in CPDA-1 and determination of the posttransfusion viability. Research Abstract Program of the 12th Annual ACVIM Forum; 1994. Jun 2; San Francisco, CA, p 120. [Google Scholar]
- 7. Berezina TL, Zaets SB, Morgan C, et al. Influence of storage on red blood cell rheological properties. J Surg Res 2002; 102: 6–12. [DOI] [PubMed] [Google Scholar]
- 8. Obrador R, Musulin S, Hansen B. Red blood cell storage lesion. J Vet Emerg Crit Care (San Antonio) 2015; 25: 187–199. [DOI] [PubMed] [Google Scholar]
- 9. Wong C, Haskins SC. The effect of storage on the P50 of feline blood: original study. J Vet Emerg Crit Care 2007; 17: 32–36. [Google Scholar]
- 10. Spada E, Proverbio D, Martino PA, et al. Ammonia concentration and bacterial evaluation of feline whole blood and packed red blood cell units stored for transfusion. Int J Health Anim Sci Food Safety 2014; 1: 15–23. [Google Scholar]
- 11. Cummings KA, Abelson AL, Rozanski EA, et al. The effect of storage on ammonia, cytokine, and chemokine concentrations in feline whole blood. J Vet Emerg Crit Care 2016; 26: 639–645. [DOI] [PubMed] [Google Scholar]
- 12. Heinz JA, Pashmakova MB, Wilson CR, et al. Biochemical evaluation of the effects of storage on feline erythrocytes. J Small Anim Pract 2016; 57: 637–643. [DOI] [PubMed] [Google Scholar]
- 13. Spada E, Proverbio D, Baggiani L, et al. Change in haematological and selected biochemical parameters measured in feline blood donors and feline whole blood donated units. J Feline Med Surg 2017; 19: 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crestani C, Stefani A, Carminato A, et al. In vitro assessment of quality of citrate-phosphate-dextrose-adenine-1 preserved feline blood collected by a commercial closed system. J Vet Intern Med 2018; 32: 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Italian Health Minister. Guide line relating to the exercise of the health activity concerning the transfusion medicine in the veterinary field. http://www.salute.gov.it (2016, accessed March 9, 2018).
- 16. Spada E, Proverbio D, Bagnagatti De, Giorgi G, et al. Clinical and haematological responses of feline blood donors anaesthetised with a tiletamine and zolazepam combination. J Feline Med Surg 2015; 17: 338–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bücheler J, Cotter SM. Storage of feline and canine whole blood in CPDA-1 and determination of the posttransfusion viability. Research Abstract Program of the 12th Annual ACVIM Forum; 1994. Jun 2; San Francisco, CA, p 172. [Google Scholar]
- 18. Barger AM. Erythrocyte morphology. In: Douglas JW, Wardrop KJ. (eds). Schalm’s veterinary hematology. 6th ed. Ames, IA: Wiley-Blackwell, 2010, pp 144–151. [Google Scholar]
- 19. Harvey JW. Hematology procedures. In: Harvey JW. (ed). Veterinary hematology. A diagnostic guide and color atlas. St Louis, MO: Elsevier Saunders, 2012, pp 11–32. [Google Scholar]
- 20. Harvey JW. Evaluation of erythrocytes. In: Harvey JW. (ed). Veterinary hematology. A diagnostic guide and color atlas. St Louis, MO: Elsevier Saunders, 2012, pp 49–121. [Google Scholar]
- 21. Zabolotzky SM, Walker DB. Peripheral Blood Smears. In: Valenciano AC, Cowell RL. (eds). Cowell and Tyler’s diagnostic cytology and hematology of the dog and cat. 4th ed. St Louis, MO: Elsevier, 2014, pp 457–488. [Google Scholar]
- 22. Maglaras CH, Koenig A, Bedard DL, et al. Retrospective evaluation of the effect of red blood cell product age on occurrence of acute transfusion-related complications in dogs: 210 cases (2010–2012). J Vet Emerg Crit Care 2017; 27: 108–120. [DOI] [PubMed] [Google Scholar]
- 23. Callan MB, Patel RT, Rux AH, et al. Transfusion of 28-day-old leucoreduced or non-leucoreduced stored red blood cells induces an inflammatory response in healthy dogs. Vox Sang 2013; 105: 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang D, Cortes-Puch I, Sun J, et al. Transfusion of older stored blood worsens outcomes in canines depending on the presence and severity of pneumonia. Transfusion 2014; 54: 1712–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Niinistö K, Raekallio M, Sankari S. Storage of equine red blood cells as a concentrate. Vet J 2008; 176: 227–231. [DOI] [PubMed] [Google Scholar]
- 26. Wardrop KJ, Tucker RL, Mugnai K. Evaluation of canine red blood cells stored in a saline, adenine, and glucose solution for 35 days. J Vet Intern Med 1997; 11: 5–8. [DOI] [PubMed] [Google Scholar]
- 27. Sousa RS, Barrêto-Júnior RA, Sousa IKF, et al. Evaluation of hematologic, blood gas, and select biochemical variables in ovine whole blood stored in CPDA-1 bags. Vet Clin Pathol 2013; 42: 27–30. [DOI] [PubMed] [Google Scholar]
- 28. Pignon C, Donnelly TM, Todeschini C, et al. Assessment of a blood preservation protocol for use in ferrets before transfusion. Vet Rec 2014; 174: 277. [DOI] [PubMed] [Google Scholar]
- 29. Kohn B, Weingart C. Feline transfusion medicine. In: Day MJ, Kohn B. (eds). BSAVA manual of canine and feline haematology and transfusion medicine. 2nd ed. Gloucester: BSAVA, 2012, pp 308–318. [Google Scholar]
- 30. Graf C, Raila J, Schweigert FJ, et al. Effect of leukoreduction treatment on vascular endothelial growth factor concentration in stored canine blood transfusion products. Am J Vet Res 2012; 73: 2001–2006. [DOI] [PubMed] [Google Scholar]
- 31. Notomi MK, de Gopegui RR, Escodro PB. Haematologic effects of leukoreduction on canine whole blood post-filtration and post-storage. Comp Clin Path 2016; 25: 145–149. [Google Scholar]
- 32. Schavone J, Rozanski E, Schaeffer J, et al. Leukoreduction of feline whole blood using a neonatal leukocyte reduction filter: a pilot study. 2012 ACVIM Forum Research Abstracts Program; 2012 Jun 2; New Orleans, LA, p 777. [Google Scholar]
- 33. Jacobs RM, Boyce JT, Kociba GJ. Flow cytometric and radioisotopic determinations of platelet survival time in normal cats and feline leukemia virus-infected cats. Cytometry 1986; 7: 64–69. [DOI] [PubMed] [Google Scholar]
- 34. Ergül Ekiz E, Arslan M, Akyazi Í, et al. The effects of prestorage leukoreduction and storage duration on the in vitro quality of canine packed red blood cells. Turkish J Vet Anim Sci 2012; 36: 711–777. [Google Scholar]
- 35. Chasis J, Schrier S. Membrane deformability and the capacity for shape change in the erythrocyte. Blood 1989; 74: 2562–2568. [PubMed] [Google Scholar]
- 36. Feo C. The role of lysolecithin formed in plasma on the discocyte-echinocyte transformation. A commentary. Nouv Rev Fr Hematol 1972; 12: 757–760. [PubMed] [Google Scholar]
- 37. Laczko J, Feo C, Phillips W. Discocyte-echinocyte reversibility in blood stored in CPD over a period of 56 days. Transfusion 1979; 19: 379–388. [DOI] [PubMed] [Google Scholar]
- 38. Sollberger T, Walter R, Brand B, et al. Influence of prestorage leucocyte depletion and storage time on rheologic properties of erythrocyte concentrates. Vox Sang 2002; 82: 191–197. [DOI] [PubMed] [Google Scholar]
- 39. Wolfe L. Oxidative injuries to the red cell membrane during conventional blood preservation. Semin Hematol 1989; 26: 307–312. [PubMed] [Google Scholar]
- 40. Christopher MM. Relation of endogenous Heinz bodies to disease and anemia in cats: 120 cases (1978–1987). J Am Vet Med Assoc 1989; 194: 1089–1095. [PubMed] [Google Scholar]
- 41. Kurach JDR, Hansen AL, Turner TR, et al. Segments from red blood cell units should not be used for quality testing. Transfusion 2014; 54: 451–455. [DOI] [PubMed] [Google Scholar]
- 42. Weiskopf RB, Yau R, Sanchez R, et al. Microarray kit analysis of cytokines in blood product units and segments. Transfusion 2009; 49: 2269–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Blasi B, D’Alessandro A, Ramundo N, et al. Red blood cell storage and cell morphology. Transfus Med 2012; 22: 90–96. [DOI] [PubMed] [Google Scholar]
- 44. Spada E, Baretta S, Martino PA, et al. Valutazione microbiologica di unità di sangue felino prelevate con sistema aperto. La Rassegna di Medicina Felina 2013; 1: 6–11. [Google Scholar]