Abstract
Objectives
The aim of this study was to evaluate the effects of dipyrone and tramadol, used for 5 days, on postoperative pain, hematological and biochemical parameters, and oxidative markers on erythrocytes.
Methods
Twenty-eight healthy cats underwent ovariohysterectomy and were randomly allocated to four groups (each n = 7), according to the postoperative treatment administered intravenously: control (saline 1 ml q8h), DIP1 (dipyrone 25 mg/kg q24h), DIP2 (dipyrone 25 mg/kg q12h) and DIP3 (dipyrone 25 mg/kg q8h). All animals received tramadol (2 mg/kg q8h). Pain was assessed by visual analog (VAS), multidimensional UNESP and Glasgow pain scales for cats preoperatively and at 3, 6, 12, 24, 36 and 48 h after extubation. Venous blood was collected daily for 5 days, and on day 10, to perform a complete blood count (CBC) and determine the percentage of Heinz bodies (HBs). Serum biochemistry was evaluated preoperatively and on days 5 and 10; superoxide dismutase (SOD), catalase (CAT), myeloperoxidase (MPO) and lipoperoxidation were evaluated preoperatively and on days 3, 5 and 10.
Results
Control cats had higher pain scores than DIP3 cats by UNESP (P = 0.0065), and DIP2 (P = 0.0035) and DIP3 cats (P = 0.0108) by VAS 3 h postoperatively. Rescue analgesia was required by two animals in the control group and one each in the DIP1 and DIP2 groups. There was no difference in SOD or CAT among groups. On day 5, MPO was more active in DIP2 than in DIP3 cats (P = 0.0274). No difference in lipoperoxidation among treatment and control cats was found. CBC remained constant and without statistical difference among groups. Control, DIP2 and DIP3 cats presented a similar percentage of HBs on day 10. Biochemical variables were similar among groups and times.
Conclusions and relevance
The administration of dipyrone in cats, when used in combination with tramadol, did not ensure better analgesia than tramadol alone. Dipyrone did not significantly affect biochemical variables and oxidative markers, despite minimal, clinically irrelevant, hematological differences between groups.
Keywords: Metamizole, tramadol, hematological effects, erythrocyte oxidation
Introduction
Dipyrone or metamizole is a pyrazolone derivative drug devoid of deleterious gastrointestinal and renal effects and is used for treating postoperative pain. It has a low anti-inflammatory activity and generates analgesia through additional pathways, such as activating the opioid and cannabinoid systems while inhibiting prostaglandin synthesis. 1 The analgesic, hematological and biochemical effects of this drug have been studied in humans, mice and, most recently, dogs.2–7 However, studies about the effects of dipyrone on cats are scarce. Although this drug is unavailable in some countries, the results of a survey conducted via an online questionnaire addressed to veterinarians in Brazil and other South American countries showed that it is consistently used in feline medicine. However, the recommended dose for cats is widely variable.8,9 It is believed that adverse effects in cats are related to hepatic metabolism and biotransformation to active metabolites.
Although the satisfactory analgesic effect of dipyrone for surgical procedures such as ovariohysterectomy (OVH) has already been described in dogs,5,10 based on the low amount of analgesic rescues performed in animals receiving this drug postoperatively, in cats its analgesic properties are unknown. Tramadol, an opioid-derived analgesic, is routinely used in feline practice, combined with non-steroidal anti-inflammatory drugs, to ensure an adequate analgesic comfort postoperatively.
Cats have some particularities regarding hepatic drug metabolism. The activity of cytochrome P450 complex isozymes is lower in cats, suggesting they may require reduced drug doses or longer dose intervals to avoid hepatotoxicity. 11 Also, cats have only two isoforms of the gamma-glutamyl transpeptidase enzyme, the main glucuronidation catalyst, which is a necessary step in the biotransformation of drugs, toxins and endogenous compounds, 12 contributing to the slow metabolism and toxicity. In addition, feline erythrocytes have eight oxidizable sulfhydryl groups (six more than other mammals), which make their cells more unstable and susceptible to oxidation, leading to protein denaturation and to Heinz body (HB) formation.
Impairment of the intracellular antioxidants superoxide dismutase (SOD) and catalase (CAT) have been used as markers of erythrocyte oxidation in studies involving acetaminophen toxicity.13,14 Additionally, extravascular hemolysis can be caused by both cell oxidation and direct cellular damage. Also, antioxidant activity impairment leads to an increase in the mechanisms of inflammation and lipid peroxidation, as indicated by increases in myeloperoxidase (MPO) activity and lipoperoxidation markers. 15
The aim of this study was to evaluate the effects of the use of dipyrone and tramadol administered for five consecutive days on postoperative pain, hematological and biochemical parameters, and oxidative stress status on the erythrocytes.
Materials and methods
The study was approved by the Ethics Committee on Animal Use of the Federal University of Santa Maria (number 8229080517). Written informed consent was obtained from clients. Thirty-two female cats (mean ± SD weight 2.5 ± 1.12 kg; mean ± SD age 2.8 ± 0.51 years) of mixed breed and considered healthy by physical, hematological and biochemical evaluation were selected to participate in this study. Inclusion criteria comprised tolerance to manipulation, evidenced by the absence of fear-based aggression to blood collection, limb trichotomy and measurement of systolic blood pressure with Doppler sphygmomanometry.
Cats were kept in individual cages in a calm and quiet room with food and water provided ad libitum for 8 days. This period was divided into 3 days of acclimatization to the new environment and 5 days of the experimental phase. Animals returned 10 days after surgery for blood collection and re-evaluation of the surgical wound.
Animals were randomly assigned to four groups by drawing pieces of paper with group identifications from a bag: control (1 ml NaCl 0.9% IV q8h; n = 8), DIP1 (25 mg/kg dipyrone IV q24h; n = 8), DIP2 (25 mg/kg dipyrone IV q12h; n = 8) and DIP3 (25 mg/kg dipyrone IV q8h; n = 8). Dipyrone was diluted in NaCl 0.9% to reach a final volume of 1 ml. All treatments were administered for 5 days.
On the third day of acclimatization, animals were fasted for 8 h prior to anesthesia. Cats were premedicated with acepromazine (0.05 mg/kg), midazolam (0.3 mg/kg) and pethidine (4 mg/kg) intramuscularly. After 15 mins, a 22 G catheter was placed in the left cephalic vein to administer medication and fluid therapy with NaCl 0.9% (3 ml/kg/h) throughout the procedure. General anesthesia was induced with propofol (5 ± 1.3 mg/kg IV) and maintained with isoflurane carried in oxygen. After stabilization of the anesthetic plane, OVH by celiotomy was performed by a proficient surgeon. Throughout the anesthetic period, heart rate, respiratory rate, esophagal temperature, systolic arterial pressure, peripheral capillary oxygen saturation and end-tidal carbon dioxide partial pressure were monitored continuously using a multi-parameter monitor (Mindray PM 7000) and vascular Doppler (Parks), and recorded every 5 mins. At the end of the surgery, tramadol (2 mg/kg) was administered subcutaneously to all cats (repeated every 8 h for 5 days) and saline or dipyrone intravenously (IV) according to the group.
Postoperative pain was assessed by two evaluators blinded to the treatment, using a visual analog scale (VAS), the UNESP-Botucatu Multidimensional Composite Pain Scale (UNESP) and the Glasgow Feline Composite Measure Pain Scale (GFCMPS) at the third day of adaptation (preoperatively) and 3, 6, 12, 24, 36 and 48 h after extubation. Rescue analgesia was performed with methadone (0.1 mg/kg) subcutaneously if scores exceeded 8 points in UNESP, 5 in GFCMPS or 30 mm on VAS. Pain scores were re-evaluated 30 mins after methadone administration. Pain scores used to perform statistical tests were those obtained prior to the analgesic rescue.
For blood collection, baseline values were divided into preoperative (collection prior to surgery) and extubation values; extubation values were considered to be the blood collected prior to the administration of dipyrone, at the end of the surgery. Venous blood was collected: preoperatively, at extubation and then daily for five consecutive days and on day 10 after surgery to determine the HB percentage and a complete blood count (CBC); at extubation and at days 3, 5 and 10 after the procedure to evaluate the oxidative status by measuring the activity of SOD, CAT and MPO enzymes and lipid peroxidation; and preoperatively and at days 5 and 10 for biochemical measurements (aspartate aminotransferase [AST], creatinine and urea). Venous blood samples were collected from a cephalic vein catheter, which was replaced every 48 h during the 5 days of the experiment. Prior to each blood collection, the dead space of the catheter was washed with 0.5 ml of saline. Half a millilitre of blood was collected and discarded, then, immediately, blood was collected by dripping directly into the collection tubes containing no anticoagulant, citrate or EDTA, totalling 0.5–2.5 ml of blood.
Glucose levels were measured using a glucometer (Accu-Check Active; Roche) preoperatively and 1 and 24 h after extubation.
Lipid peroxidation was assessed by using the thiobarbituric acid method, which measures malondialdehyde (MDA)-reactive products. 16 CAT and SOD activities were determined as described by Nelson and Kiesow 17 and McCord and Fridovich, 18 respectively.
The MPO activity was estimated in serum, according to the method of Metcalf et al. 19
According to a power analysis calculation, a study population of 14 cats per group would be needed to detect a 70% difference between groups, considering standard alpha and a power of 90%. All results are presented as mean ± SD. The data were analyzed using GraphPad Prism 6.00 for Windows. The Shapiro–Wilk and Kolmogorov–Smirnov tests were used to assess normality. The data regarding pain scores on each pain scale, HBs, hematocrit, leukocytes, platelets, AST, creatinine, urea, CAT, SOD, MPO and MDA were analyzed by means of a repeated measures ANOVA with one grouping factor (control, DIP1, DIP2 and DIP3) and one repeat factor (time 0–48 h), followed by multi-comparison Tukey’s post-hoc test. The Kruskal–Wallis test at 5% significance was used to compare groups for non-parametric data, number of analgesic rescues and glycemia. Cats that received rescue analgesia were not excluded from statistical analysis. The significance level was defined as P <0.05.
Results
No adverse effects such as vomiting, diarrhea, salivation and anorexia were observed at any of the administration times. Twenty-eight cats participated in the experiment until its end. Four animals, one from each group, were removed because they demonstrated aggressive behavior despite being docile in the selection, making it impossible to handle them without stress. Age, weight and duration of anesthesia and surgery were similar between groups (Table 1).
Table 1.
Demographic data, operation and anesthesia times
| Groups |
||||
|---|---|---|---|---|
| Control (n = 7) | DIP1 (n = 7) | DIP2 (n = 7) | DIP3 (n = 7) | |
| Age (months) | 26 ± 8.0 | 24 ± 0.0 | 22 ± 3.4 | 24 ± 16.3 |
| Weight (kg) | 3.3 ± 0.5 | 2.9 ± 0.6 | 2.6 ± 0.4 | 2.8 ± 0.3 |
| Anesthesia time (mins) | 37 ± 5.2 | 38 ± 9.7 | 40 ± 7.0 | 38 ± 10 |
| Surgical time (mins) | 21 ± 4.2 | 20 ± 6.1 | 24 ± 5.9 | 22 ± 6.5 |
Data are presented as mean ± SD
Control = saline; DIP1 = dipyrone 25 mg/kg q24h; DIP2 = dipyrone 25 mg/kg q12h; DIP3 = dipyrone 25 mg/kg q8h
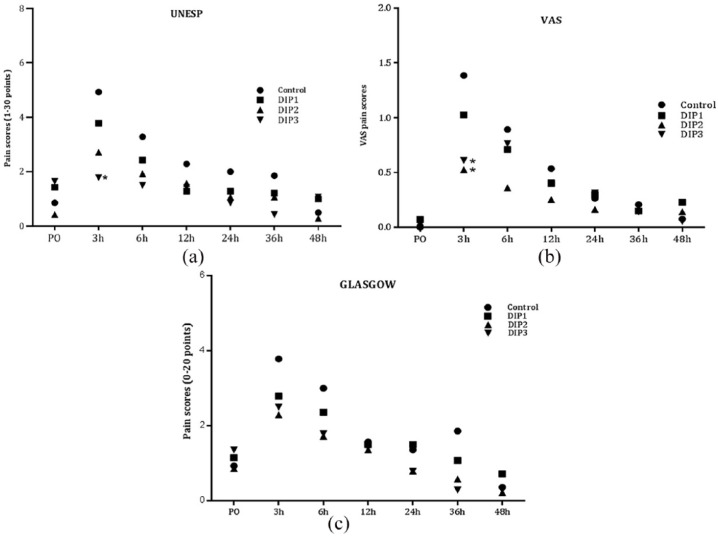
The analgesia results revealed that the pain scores in the control group were significantly higher than those obtained in the DIP3 group (P = 0.0065) on UNESP, and DIP2 (P = 0.0035) and DIP3 groups (P = 0.0108) on VAS at 3 h postoperatively (Figure 1). At that time, two animals from the control group and one from the DIP1 group received rescue analgesia. At 6 h, one animal in the DIP2 group also received rescue analgesia. Rescue analgesia was performed only according to UNESP once the necessary score for rescue analgesia was not reached in the other scales. There was no significant difference between groups (P >0.999) regarding the number of analgesic rescues performed. No difference was observed between groups at any time point of evaluation by the GFCMPS, although the scores obtained by cats in the control group were higher in 4/6 evaluation time points. Glucose concentration was similar between groups and between evaluated times (P = 0.4998).
Figure 1.
Mean pain scores on the (a) UNESP-Botucatu Multidimensional Composite Pain Scale (UNESP) (b) visual analog scale (VAS) or (c) Glasgow Feline Composite Measure Pain Scale for cats (Glasgow) over time for groups receiving saline or dipyrone (n = 7). *Denotes significant difference (P <0.05) in comparison with control. PO = preoperatively; control = saline; DIP1 = dipyrone 25 mg/kg q24h; DIP2 = dipyrone 25 mg/kg q12h; DIP3 = dipyrone 25 mg/kg q8h
Values obtained for SOD, CAT and MPO are presented in Tables 2 and 3. There was no significant difference between groups for SOD and CAT. MPO activity remained constant over time within each treatment. However, MPO was higher in DIP2 cats than in DIP3 cats (P = 0.0274) on day 5 of treatment. No significant difference was found between any other group on the other evaluation periods.
Table 2.
Superoxide dismutase (SOD) and catalase (CAT)
| Time | SOD (pM/mg protein/mins) |
CAT (pM/mg protein/mins) |
||||||
|---|---|---|---|---|---|---|---|---|
| Control | DIP1 | DIP2 | DIP3 | Control | DIP1 | DIP2 | DIP3 | |
| AE | 3.9 ± 3.1 | 3.3 ± 0.9 | 3.5 ± 1.5 | 2.5 ± 1.18 | 15.0 ± 7.8 | 22.1 ± 24.7 | 17.2 ± 7.5 | 17.6 ± 8.6 |
| Day 3 | 2.8 ± 1.4 | 2.4 ± 0.6 | 3.5 ± 1.4 | 3.2± 1.24 | 23.6 ± 9.7 | 17.3 ± 6.7 | 18.6 ± 4.0 | 23.3 ± 14 |
| Day 5 | 2.8 ± 1.1 | 2.9 ± 0.8 | 3.1 ± 0.5 | 2.7 ± 0.95 | 17.5 ± 8.7 | 24.9 ± 15.5 | 23.2 ± 9.3 | 17.3 ± 13.4 |
| Day 10 | 2.9 ± 0.3 | 2.6 ± 0.7 | 2.6 ± 1.3 | 2.86 ± 0.99 | 24.7 ± 10.0 | 19.2 ± 8.5 | 28.7 ± 10.5 | 19.8 ± 8.6 |
Data are mean ± SD
Control = saline; DIP1 = dipyrone 25 mg/kg q24h; DIP2 = dipyrone 25 mg/kg q12h; DIP3 = dipyrone 25 mg/kg q8h; AE = at extubation
Table 3.
Myeloperoxidase (MPO) and malondialdehyde (MDA)
| Time points | MPO (µM quinone imine produced in 30 mins) |
MDA (nmol/ mg protein) |
||||||
|---|---|---|---|---|---|---|---|---|
| Control | DIP1 | DIP2 | DIP3 | Control | DIP1 | DIP2 | DIP3 | |
| AE | 3.4 ± 1.5 | 2.3 ± 0.7 | 2.8 ± 0.7 | 2.0 ± 0.6 | 1.29 ± 1.18 | 0.51 ± 0.23 | 1.18 ± 0.32 | 0.83 ± 0.38 |
| Day 3 | 3.0 ± 0.8 | 2.4 ± 0.8 | 2.6 ± 0.8 | 2.4 ± 1.2 | 1.18 ± 0.47 | 0.80 ± 0.36 | 0.95 ± 0.52 | 0.68 ± 0.46 |
| Day 5 | 3.3 ± 0.9 | 2.4 ± 0.6 | 3.3 ± 2.3 | 1.6 ± 0.6* | 1.10 ± 0.90 | 0.51 ± 0.23 | 1.17 ± 0.54 | 0.71 ± 0.40 |
| Day 10 | 3.1 ± 1.2 | 2.4 ± 0.7 | 2.1 ± 0.6 | 2.7 ± 0.9 | 0.93 ± 0.39 | 0.51 ± 0.49 | 0.97 ± 0.45 | 0.96 ± 0.34 |
Significantly different from DIP2 (P <0.05)
Control = saline; DIP1 = dipyrone 25 mg/kg q24h; DIP2 = dipyrone 25 mg/kg q12h; DIP3 = dipyrone 25 mg/kg q8h; AE = at extubation
Regarding MDA levels, no significant difference on lipoperoxidation between treatments and control group was found at any of the times assessed, and it was not altered over time within each group (P = 0.2323) (Table 3).
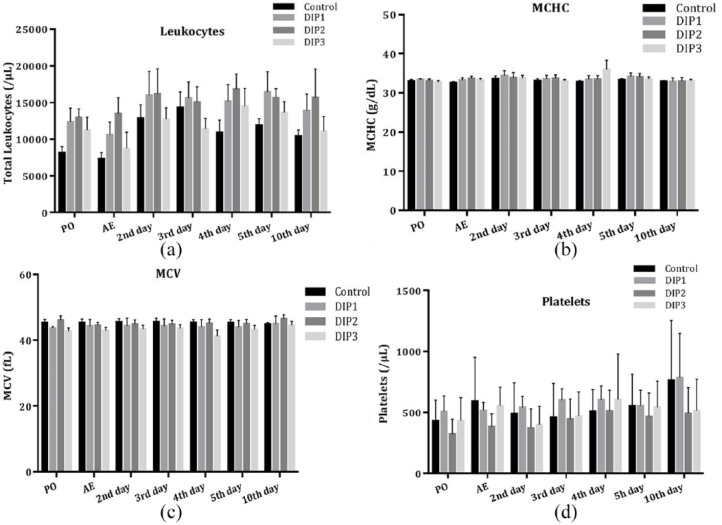
The concentration of platelets, leukocytes, mean cell volume and mean cell hemoglobin concentration (Figure 2) remained constant throughout the study period and without statistical difference among groups at any of the evaluated time points. No clinical signs of coagulopathy, such as petechiae, bruising or delayed healing, were detected. The percentage of HBs did not change over time in each group. However, on day 10, all treatment groups had a significantly higher percentage of HBs than the control group. The percentage of HBs in cats in groups DIP2 and DIP3 were significantly higher than in cats in the DIP1 group (P = 0.041 and P = 0.0423, respectively) (Table 4).
Figure 2.
(a) Mean leukocytes, (b) mean cell hemoglobin concentration (MCHC), (c) mean cell volume (MCV) and (d) platelets over time for groups receiving saline or dipyrone (n = 7). No difference between groups or time points assessed was observed for these variables. Error bars indicate SD. PO = preoperatively; AE = at extubation; control = saline; DIP1 = dipyrone 25 mg/kg q24h; DIP2 = dipyrone 25 mg/kg q12h; DIP3 = dipyrone 25 mg/kg q8h
Table 4.
Heinz bodies
| Time points | Heinz bodies (%) |
|||
|---|---|---|---|---|
| Control | DIP1 | DIP2 | DIP3 | |
| PO | 0.71 ± 0.90 | |||
| AE | 0.75 ± 0.99 | 0.46 ± 0.76 | 1.07 ± 1.14 | 0.57 ± 0.76 |
| Day 2 | 0.71 ± 0.92 | 0.59 ± 0.86 | 1.01 ± 1.02 | 0.54 ± 0.73 |
| Day 3 | 0.53 ± 0.59 | 0.61 ± 0.70 | 1.11 ± 1.13 | 0.54 ± 0.62 |
| Day 4 | 0.65 ± 0.88 | 0.52 ± 0.80 | 1.15 ± 1.09 | 0.50 ± 0.50 |
| Day 5 | 0.41 ± 0.44 | 0.27 ± 0.23 | 0.95 ± 1.21 | 0.58 ± 0.46 |
| Day 10 | 0.32 ± 0.32* | 0.53 ± 0.55 † | 1.00 ± 1.09 | 0.72 ± 0.44 |
Data are mean ± SD
Significantly different from DIP1, DIP2 and DIP3
Significantly different from control, DIP2 and DIP3 (P <0.05)
Control = saline; DIP1 = dipyrone 25 mg/kg q24h; DIP2 = dipyrone 25 mg/kg q12h; DIP3 = dipyrone 25 mg/kg q8h; PO = preoperatively; AE = at extubation
Hematocrit values were lower in the DIP2 and DIP3 groups than in the control group (P = 0.0070 and 0.0293, respectively) on day 2 of treatment, although within the reference interval (RI) for cats. The difference between DIP2 and control cats persisted up to day 3 (P = 0.0140), from which all groups showed similar hematocrit values. There was no difference within each group for this parameter at any time point (Table 5).
Table 5.
Hematocrit
| Time points | Hematocrit (%) |
|||
|---|---|---|---|---|
| Control | DIP1 | DIP2 | DIP3 | |
| PO | 26.72 ± 8.77 | |||
| AE | 27.65 ± 5.71 | 29.1 ± 4.97 | 27.88 ± 5.40 | 31.6 ± 6.59 |
| Day 2 | 35.51 ± 8.03 | 32.2 ± 5.90 | 26.65 ± 4.26* | 31.7 ± 2.90* |
| Day 3 | 35.80 ± 4.97 | 32.9 ± 4.47 | 27.55 ± 4.95* | 33.7 ± 3.91 |
| Day 4 | 29.68 ± 7.28 | 29.3 ± 4.11 | 27.43 ± 5.80 | 32.6 ± 4.14 |
| Day 5 | 32.06 ± 3.62 | 31.8 ± 2.94 | 27.14 ± 3.83 | 30.1 ± 3.91 |
| Day 10 | 36.48 ± 6.42 | 36.1 ± 4.66 | 32.27 ± 3.41 | 34.7 ± 5.94 |
Data are mean ± SD
Significantly different from control (P <0.05)
Control = saline; DIP1 = dipyrone 25 mg/kg q24h; DIP2 = dipyrone 25 mg/kg q12h; DIP3 = dipyrone 25 mg/kg q8h; PO = preoperatively; AE = at extubation
Biochemical variables evaluated – creatinine and urea – were not significantly different between groups (P = 0.7605 and P = 0.4498, respectively), with no difference in the periods of evaluation and always remaining within the RI for cats. Except for cats in DIP3, cats in all the other groups showed a significant reduction in creatinine concentration from preoperatively to days 5 and 10 after treatment (Table 6). Aspartate transaminase (AST), as well as albumin, hepatocellular lesion and liver function parameters, were not significantly different between groups (AST P = 0.8283; albumin P = 0.4763) and between the evaluated times (Table 7).
Table 6.
Creatinine and urea
| Times | Creatinine (mg/dl) |
Urea (mg/dl) |
||||||
|---|---|---|---|---|---|---|---|---|
| Control | DIP1 | DIP2 | DIP3 | Control | DIP1 | DIP2 | DIP3 | |
| PO | 1.4 ± 0.3 | 1.1 ± 0.16 | 1.3 ± 0.3 | 0.9 ± 0.4 | 15.0 ± 7.8 | 22.1 ± 24.7 | 17.2 ± 7.5 | 17.6 ± 8.6 |
| Day 5 | 0.9 ± 0.1 | 0.8 ± 0.14 | 0.8 ± 0.1 | 0.8 ± 0.1 | 17.5 ± 8.7 | 24.9 ± 15.5 | 23.2 ± 9.3 | 17.3 ± 13.4 |
| Day 10 | 1.2 ± 0.08* | 0.9 ± 0.08* | 0.9 ± 0.1* | 1.0 ± 0.1 | 24.7 ± 10 | 19.2 ± 8.5 | 28.7 ± 10.5 | 19.8 ± 8.6 |
Data are mean ± SD
Significantly different from preoperatively (PO) (P <0.05)
Control = saline; DIP1 = dipyrone 25 mg/kg q24h; DIP2 = dipyrone 25 mg/kg q12h; DIP3 = dipyrone 25 mg/kg q8h
Table 7.
Aspartate transaminase (AST) and albumin
| Times | AST (U/l) |
Albumin |
||||||
|---|---|---|---|---|---|---|---|---|
| Control | DIP1 | DIP2 | DIP3 | Control | DIP1 | DIP2 | DIP3 | |
| PO | 16.7 ± 7.4 | 18.0 ± 9.3 | 29 ± 12 | 13.6 ± 8.0 | 2.8 ± 0.23 | 2.6 ± 0.43 | 2.5 ± 0.47 | 2.5 ± 0.25 |
| Day 5 | 35.3 ± 8.8 | 33.8 ± 4.6 | 34.5 ± 10 | 39.6 ± 17 | 2.9 ± 0.53 | 2.9 ± 0.38 | 2.5 ± 0.34 | 2.5 ± 0.50 |
| Day 10 | 36.4 ± 7.1 | 28.1 ± 5.2 | 36.3 ± 9.05 | 28.8 ± 6.3 | 3.1 ± 0.25 | 2.9 ± 0.36 | 2.9 ± 0.23 | 3.0 ± 0.35 |
Data are mean ± SD
Control = saline; DIP1 = dipyrone 25 mg/kg q24h; DIP2 = dipyrone 25 mg/kg q12h; DIP3 = dipyrone 25 mg/kg q8h; PO = preoperatively
Discussion
In this study, dipyrone slightly improved postoperative pain. Also, this drug had no significant effect on oxidative stress, or the hematological and biochemical variables of cats receiving it for five consecutive days and 5 days after the end of the treatment.
Evangelista et al 20 identified that tramadol used alone at a dose of 2 mg/kg provides less analgesia than 4 mg/kg in cats submitted to OVH. Zanuzzo et al 5 showed that 30% of bitches receiving only dipyrone in the postoperative period required analgesic rescue. The findings of the present investigation showed that cats receiving dipyrone in addition to tramadol were given less analgesic rescue than cats or dogs receiving tramadol or dipyrone alone, respectively. In this study, there was a 10% rate of analgesic rescue in the dipyrone groups vs 14% in cats receiving tramadol at 2 mg/kg, 20 and 30% in dogs receiving dipyrone alone. 5 Considering administration of tramadol alone, analgesic rescue in controls was worse than reported in Evangelista et al 20 in the 2 mg/kg group (29% vs 14%), which can probably be attributed to the different routes of administration, with intramuscular administration being superior to the subcutaneous route. Nevertheless, the use of dipyrone might have contributed to the effect of tramadol, which would explain the lesser requirement for supplemental analgesia in dipyrone groups, although administering dipyrone more frequently did not assure better analgesia.
Rescue analgesia was administered subcutaneously to promote less handling stress. As pain scores lowered to baseline values within 30 mins after methadone administration, rescue analgesia was obtained effectively.
Analgesic comfort, acclimatization period and blood collection by catheter possibly acted to reduce confinement and manipulation stress and its influence on the parameters measured. Surgical trauma, by itself, is capable of provoking a pro-oxidative state due to ischemia and reperfusion processes, as well as tissue trauma, hypothermia and postoperative pain, 21 which appears to be diminished with isoflurane or propofol anesthesia.22,23 The results of this work indicate that erythrocyte activity of SOD and CAT did not decrease owing to the procedures to which the animals were submitted, or as a consequence of the medications used. SOD and CAT are the main erythrocyte components of the defense system against reactive oxygen species and oxidative lesions are expected to occur with decreased activity of these enzymes. 24
MPO, an enzyme often used as an inflammatory marker, 15 showed similar levels of activity between control and treatment groups, indicating that the anti-inflammatory action of dipyrone was not effective in decreasing the inflammatory process. In addition, lipoperoxidation in dipyrone-treated groups, besides not surpassing cats in the control group, was unchanged throughout the administration period, without being altered even 5 days after the end of treatment.
MDA, formed from lipid peroxidation, is understood as a marker of oxidative stress and is related to MPO as this enzyme is the main catalyst for lipoperoxidation. Consequently, an increase in MPO activity may be accompanied by an increase in lipoperoxidation, as already demonstrated in the literature. 25 The results suggest that dipyrone is not significantly different from control regarding oxidative damage to erythrocytes. However, this finding should be interpreted in view of the small sample size, resulting in low statistical power. Oxidative stress triggered by the surgical procedure, such as OVH, is characterized by an increase in lipoperoxidation with a decrease in antioxidant enzymes in relation to preoperative values or to groups not submitted to surgery.26,27 In our study, all groups underwent the same procedures with a similar duration of time between them, so observed differences between groups and times for these parameters should be due to the medications administered.
Dipyrone metabolism involves hydrolysis to 4-methylamino antipyrine, which is metabolized to 4-amino antipyrine (AA) and 4-formylamino antipyrine in the liver, with cytochrome P450 oxygenase involvement. AA is also acetylated in 4-acetylamino antipyrine, and there is the subsequent formation of glucuronide metabolites. 28 Considering that cats have a relative deficiency in glucuronosyltransferase enzyme activity, 11 it is believed that dipyrone metabolism is slower in cats, making this drug contraindicated in this species. 29 Deficient dipyrone metabolism could expose erythrocytes to a higher formation of disulfide bridges and, consequently, excessive production of HBs. Hill et al 30 demonstrated that depletion of glutathione reductase is secondary to the formation of HBs, which is acutely produced and is related to a decrease in erythrocyte lifespan to 7–8 days. 31
In the present study, there was no increase in HB formation within each group over time, and the highest percentage of HBs in DIP2, in relation to the other groups, does not appear to have clinical significance as this group had already shown a higher percentage of HBs before the beginning of the proposed treatments. The results concerning HBs are supported by the absence of hemolysis, evidenced by the maintenance of hematocrit values above the feline RI throughout the evaluated period. It would be expected that with an increase in HBs, hematocrit should decrease. However, in our study the opposite happened: the increase in HBs was accompanied by a similar elevation in the magnitude of hematocrit in all dipyrone groups. Andress et al 32 induced feline erythrocytes to oxidative damage by administering propofol daily for 10 days, obtaining HB percentages above 15% from day 4 onwards; they also reported clinical signs such as anorexia and diarrhoea. Differently from that study, in the present investigation, there was no evidence of oxidative lesions on erythrocytes.
When used alone, dipyrone at 25 mg/kg decreases platelet aggregation in dogs without influencing blood coagulation or the viscoelastic properties of blood and platelet concentration. 6 This effect was also demonstrated in vitro, in which doses used for humans (1000 mg) caused platelet function suppression for a short period. 33 Although platelet function was not evaluated in the present study, our findings suggest that the combination of dipyrone and tramadol used up to three times daily does not decrease platelet concentration and does not influence blood coagulation, assessed by the absence of hematomas and petechiae.
Dipyrone bioavailability in dogs, humans and rodents is close to 100% after oral, intramuscular or IV administration, being followed by renal excretion. 34 The literature demonstrates that high doses of dipyrone may be hepatotoxic. In dogs and rats, a single dose above 450 mg/kg caused increases in alkaline phosphatase, bilirubin and urea concentrations in addition to a decrease in hematocrit and an increase in HBs. 35 However, in humans, its use at clinical doses does not appear to compromise liver function, and the deterioration of renal function has rarely been observed. 28 In this study, serum creatinine and urea concentration were similar in all groups, regardless of whether or not they received dipyrone and of the frequency of administration. The high preoperative values were possibly due to fasting in this period. Likewise, AST activity, considered more sensitive for hepatic alterations than alanine aminotransferase in cats, 36 and albumin concentration demonstrate that the combination of drugs used does not appear to cause any gross alterations in renal or hepatic function.
To the best of our knowledge, there is a single study on the pharmacokinetics and pharmacodynamics of dipyrone in cats, 37 demonstrating a half-life of approximately 6 h, regardless of the route of administration. Also, evidence from studies in dogs and humans point to an effective duration of analgesia of – at most – 8 h, which justifies the dose tested in this study. 38
Limitations of this study include the lack of a group receiving only dipyrone as an analgesic. Also, this study was underpowered as, for logistical reasons and the exclusion of four cats, only seven animals per group could be included, even though allocating more cats to each group was planned. For these reasons, further investigation is warranted.
Conclusions
The findings of this study show that dipyrone administered to cats at 25 mg/kg once, twice or three times daily concomitantly to tramadol did not ensure better postoperative analgesia than tramadol alone. Dipyrone did not cause significant biochemical alterations or oxidative damage to erythrocytes, although there were minor, clinically irrelevant, hematological differences between the groups.
Footnotes
Accepted: 21 April 2019
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
ORCID iD: Luciana G Teixeira  https://orcid.org/0000-0002-4315-0303
https://orcid.org/0000-0002-4315-0303
References
- 1. Crunfli F, Vilela FC, Giusti-Paiva A. Cannabinoid CB1 receptors mediate the effects of dipyrone. Clin Exp Pharmacol Physiol 2015; 42: 246–255. [DOI] [PubMed] [Google Scholar]
- 2. Jasiecka A, Maślanka T, Jaroszewski JJ. Pharmacological characteristics of metamizole. Polish J Vet Sci 2014; 17: 207–214. [DOI] [PubMed] [Google Scholar]
- 3. Ince I, Aksoy M, Ahiskalioglu A, et al. A comparative investigation of the analgesic effects of metamizole and paracetamol in rats. J Invest Surg 2015; 28: 173–180. [DOI] [PubMed] [Google Scholar]
- 4. Kalchofner Guerrero KS, Schwarz A, Wuhrmann R, et al. Comparison of a new metamizole formulation and carprofen for extended post-operative analgesia in dogs undergoing ovariohysterectomy. Vet J 2015; 204: 99–104. [DOI] [PubMed] [Google Scholar]
- 5. Zanuzzo FS, Teixeira-Neto FJ, Teixeira LR, et al. Analgesic and antihyperalgesic effects of dipyrone, meloxicam or a dipyrone-meloxicam combination in bitches undergoing ovariohysterectomy. Vet J 2015; 205: 33–37. [DOI] [PubMed] [Google Scholar]
- 6. Zanuzzo FS, Teixeira-Neto FJ, Thomazini CM, et al. Effects of dipyrone, meloxicam, or the combination on hemostasis in conscious dogs. J Vet Emerg Crit Care 2015; 25: 512–520. [DOI] [PubMed] [Google Scholar]
- 7. Sarchahi AA, Vesal N, Khalighi F, et al. Effects of preanesthetic administration of metamizole on renal function, blood parameters and bone marrow cells in healthy dogs. Comp Clin Path 2017; 26: 657–662. [Google Scholar]
- 8. Hanson PD, Maddison JE. Nonsteroidal anti-inflammatory drugs and chondroprotective agents. In: Maddison JE, Page SW, Church DB. (eds). Small animal clinical pharmacology. St Louis, MO: Elsevier, 2008, pp 287–308. [Google Scholar]
- 9. Gaynor JS, Muir WW. Handbook of veterinary pain management. 3rd ed. St Louis, MO: Elsevier, 2014. [Google Scholar]
- 10. Imagawa VH, Fantoni DT, Tatarunas AC, et al. The use of different doses of metamizol for post-operative analgesia in dogs. Vet Anaesth Analg 2011; 38: 385–393. [DOI] [PubMed] [Google Scholar]
- 11. Van Beusekom CD, Schipper L, Fink-Gremmels J. Cytochrome P450-mediated hepatic metabolism of new fluorescent substrates in cats and dogs. J Vet Pharmacol Ther 2010; 33: 519–527. [DOI] [PubMed] [Google Scholar]
- 12. Court MH. Feline drug metabolism and disposition: pharmacokinetic evidence for species differences and molecular mechanisms. Vet Clin North Am Small Anim Pract 2013; 43: 1039–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Webb C, Twedt D, Fettman M, et al. S-adenosylmethionine (SAMe) in a feline acetaminophen model of oxidative injury. J Feline Med Surg 2003; 5: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pandey KB, Rizvi SI. Biomarkers of oxidative stress in red blood cells. Biomed Pap 2011; 155: 131–136. [DOI] [PubMed] [Google Scholar]
- 15. Faith M, Sukumaran A, Pulimood AB, et al. How reliable an indicator of inflammation is myeloperoxidase activity? Clin Chim Acta 2008; 396: 23–25. [DOI] [PubMed] [Google Scholar]
- 16. Jentzsch AM, Bachmann H, Fürst P, et al. Improved analysis of malondialdehyde in human body fluids. Free Radic Biol Med 1996; 20: 251–256. [DOI] [PubMed] [Google Scholar]
- 17. Nelson DK, Kiesow LA. Enthalpy of the composition of hydrogen peroxide by catalase at 25°C. Anal Biochem 1972; 49: 474–479. [DOI] [PubMed] [Google Scholar]
- 18. McCord JM, Fridovich I. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 1969; 244: 6049–6055. [PubMed] [Google Scholar]
- 19. Metcalf JÁ, Gallin JI, Nauseef WN, et al. Laboratory manual of neutrophil function. 5th ed. New York: Raven Press, 1986. [Google Scholar]
- 20. Evangelista MC, Silva RA, Cardozo LB, et al. Comparison of preoperative tramadol and pethidine on postoperative pain in cats undergoing ovariohysterectomy. BMC Vet Res 2014; 10: 252. DOI: 10.1186/s12917-014-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Braz MG, Braz LG, Braz JR, et al. Comparison of oxidative stress in ASA physical status I patients scheduled for minimally invasive surgery under balanced or intravenous anesthesia. Minerva Anestesiol 2013; 79: 1030–1038. [PubMed] [Google Scholar]
- 22. Lee JY, Kim MC. Effect of propofol on oxidative stress status in erythrocytes from dogs under general anaesthesia. Acta Vet Scand 2012; 54: 76. DOI: 10.1186/1751-0147-54-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee Y-M, Song BC, Yeum K-J. Impact of volatile anesthetics on oxidative stress and inflammation. Biomed Res Int 2015; 2015. DOI: 10.1155/2015/242709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem 2015; 97: 55–74. [DOI] [PubMed] [Google Scholar]
- 25. Begenik H, Soyoral YU, Erkoc R, et al. Serum malondialdehyde levels, myeloperoxidase and catalase activities in patients with nephrotic syndrome. Redox Rep 2013; 18: 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Serin G, Kiral F, Serin I. Acute effect of ovariohysterectomy on lipid peroxidation and some antioxidant levels in dogs. Bull Vet Inst Pulawy 2008; 52: 251–253. [Google Scholar]
- 27. Fatma G, Ondokuz Y, May O. Effect of ovariohysterectomy on some oxidative stress markers in the rat. Harran Üniv Vet Fak Derg 2016; 5: 124–128. [Google Scholar]
- 28. Nikolova I, Petkova V, Tencheva J, et al. Metamizole: a review profile of a well-known ‘forgotten’ drug. Part II: clinical profile. Biotechnol Biotechnol Equip 2013; 27: 3605–3619. [Google Scholar]
- 29. Maddison JE. Adverse drug reactions. In: Maddison JE, Page SW, Church DB. (eds). Small animal clinical pharmacology. St Louis, MO: Saunders Elsevier, 2008, pp 41–58. [Google Scholar]
- 30. Hill AS, O’Neill S, Rogers QR, et al. Antioxidant prevention of Heinz body formation and oxidative injury in cats. Am J Vet Res 2001; 62: 370–374. [DOI] [PubMed] [Google Scholar]
- 31. Harvey JW. The erythrocyte: physiology, metabolism, and biochemical disorders. In: Kaneko JJ. (ed). Clinical biochemistry of domestic animals. St Louis, MO: Elsevier, 2008, pp 173–240. [Google Scholar]
- 32. Andress JL, Day TK, Day DG. The effects of consecutive day propofol anesthesia on feline red blood cells. Vet Surg 1995; 24: 277–282. [DOI] [PubMed] [Google Scholar]
- 33. Hinz B, Cheremina O, Bachmakov J, et al. Dipyrone elicits substantial inhibition of peripheral cyclooxygenases in humans: new insights into the pharmacology of an old analgesic. FASEB J 2007; 21: 2343–2351. [DOI] [PubMed] [Google Scholar]
- 34. Nikolova I, Petkova V, Tencheva J, et al. Metamizole: a review profile of a well-known “forgotten” drug. Part I: pharmaceutical and nonclinical profile. Biotechnol Biotechnol Equip 2012; 26: 3329–3337. [Google Scholar]
- 35. Kramer M. Chronic toxicity of pyrazolones: the problem of nitrosation. Br J Clin Pharmacol 1980; 10: 313S–317S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Center SA. Interpretation of liver enzymes. Vet Clin North Am Small Anim Pract 2007; 37: 297–333. [DOI] [PubMed] [Google Scholar]
- 37. Lebkowska-Wieruszewska B, Kim TW, Chea B, et al. Pharmacokinetic profiles of the two major active metabolites of metamizole (dipyrone) in cats following three different routes of administration. J Vet Pharmacol Ther 2018; 41: 334–339. [DOI] [PubMed] [Google Scholar]
- 38. Volz M, Kellner H. Kinetics and metabolism of pyrazolones (propyphenazone, aminopyrine and dipyrone). Br J Clin Pharmacol 1980; 10: 299S–308S. [DOI] [PMC free article] [PubMed] [Google Scholar]