Abstract
Objectives
The aim of the study was to evaluate the appetite-stimulating effect of gabapentin by comparing it with mirtazapine in healthy cats in the first 8 h after ovariectomy surgery.
Methods
This double-masked, placebo-controlled, prospective clinical trial included 60 healthy cats presented to the hospital for ovariectomy: 20 received gabapentin, 21 received mirtazapine and 19 received a placebo immediately before and 6 h after surgery. Food was offered at 2, 4, 6 and 8 h post-ovariectomy. After each meal, food intake was measured. Data were analysed using repeated-measure ANOVA and a linear mixed-model analysis. Post-hoc Tukey’s honest significant difference test was performed for multiple comparisons.
Results
Food intake increased in both treatment groups vs placebo. No statistically significant difference was found between cats treated with gabapentin or mirtazapine.
Conclusions and relevance
Cats receiving gabapentin ate more than cats in the placebo group. Thirty percent of cats in the gabapentin group covered their resting energy requirements, while none of the cats in the placebo group did. Gabapentin and mirtazapine produced similar effects on food intake.
Keywords: Appetite, appetite-stimulating drug, nutrition, food intake, gabapentin, mirtazapine, orexigenic effect
Introduction
Inadequate food intake in hospitalised animals is a very common but often overlooked problem in small animal clinics. 1 Insufficient calorie intake coupled with possible nutrient deficiencies translates into progressive weight loss and muscle wasting, a pattern that eventually results in poorer outcomes. 2
Mechanisms behind appetite regulation are extremely complex and not entirely understood. In the ideal situation, the interaction between satiation and adiposity signals dictates and controls energy intake to maintain the ideal body weight (BW): a healthy animal, which lost weight owing to insufficient calorie intake not meeting its energy requirement, produces less anorexigenic hormones and peptides, while the orexigenic stimuli are boosted, resulting in increased food intake and weight gain.3,4 However, the fine equilibrium in the gut–brain axis can frequently be altered in hospitalised animals leading to a reduced or completely lost appetite.
Inflammation, either caused by illness, injury or surgery, can easily alter the neural regulation of appetite on several levels: interleukin-1 and tumour necrosis factor alpha can exert anorexigenic effects by modulation of leptin, neuropeptide Y and corticotropin-releasing hormone, while other inflammatory cytokines can suppress ghrelin’s orexigenic signal.5–7 Moreover, non-homeostatic factors not linked to energy need (eg, stress related to the unfriendly novel environment) or the adverse effects of certain medications such as nausea (eg, metronidazole and doxycycline), altered gastrointestinal motility (eg, opioids) or changed taste perception (eg, cardiac medications in human medicine), could also interfere with the patient’s food intake. 1
An important starting point for clinicians is to have a protocol for the management of nutritional support in hospitalised patients as it has been demonstrated, both in human and veterinary medicine, that early nutrition improves outcome and shortens the hospitalisation time.8–10
The first step is to recognise and describe if food intake is adequate, meaning that appetite is normal and the patient is spontaneously consuming enough calories to maintain its ideal BW (or at least enough to avoid unintentional weight loss). 1 If this is not the case, we can identify two other scenarios in which appetite is not normal but partially (hyporexia) or completely lost (anorexia): the first is when food intake is inadequate, meaning that the patient’s ideal BW could not be maintained or reached (if underweight); the second is when food intake is totally absent.1,11 In these two situations, food intake must be encouraged and it is important to supply nutrients through the most natural feeding route.
While in some specific cases parenteral nutrition could be the only possible alternative, enteral nutrition is always the first choice because it preserves the luminal enzymatic activity and the integrity of the intestinal mucosa, avoiding bacterial translocation and subsequent development of sepsis.12–16 Intuitively, oral food intake must always be attempted before thinking of any other assisted enteral route via feeding tubes, because, while some may have the same positive results on the intestinal barrier’s integrity, they are not devoid of complications. 17
While there is no unanimous consensus, it is generally accepted that dogs and cats consuming less than their resting energy requirement (RER = 70 × BW[kg]0.75) for more than 3–5 days must begin an assisted refeeding plan.1,11,18 This time frame highly depends on body condition score (BCS), the presence of clinical signs of malnutrition and underlying diseases that in certain specific cases could require immediate intervention (eg, feline hepatic lipidosis). 11
Syringe oral nutrition, apart from being time consuming and not practical in terms of food volumes, is generally not advised because of the risk of aspiration pneumonia, and also for the strong stress component that could lead to the development of ‘food aversions’. 19
To enhance adequate food intake in the hyporectic or anorectic patient, pharmacological stimulation of appetite can be considered. A variety of drugs, such as anabolic steroids, corticosteroids, benzodiazepines and megestrol acetate, exhibit to a certain extent some short-term effects on appetite enhancement. Unfortunately, these drugs can also have some important and unpredictable adverse effects, which have led to veterinarians often being advised against their use. 20
In cats, only two molecules are currently recommended as appetite-stimulating drugs. 20 The first is cyproheptadine hydrochloride, a serotonin antagonist antihistamine that has not yet received Food and Drug Administration (FDA) approval for appetite stimulation in cats. 21 The second is mirtazapine, a tetracyclic antidepressant and antagonist of several serotonin receptor subtypes. A transdermal formulation of the latter has recently been approved by the FDA for appetite stimulation in cats and can currently be considered the gold standard in this species, especially in patients with chronic kidney disease (CKD).22,23
The present study focuses on alternative pharmacological solutions in the management of hyporexia or anorexia in cats. In particular, it aims to evaluate the possibility of an appetite-stimulating effect of gabapentin, an anticonvulsant and neuropathic pain analgesic, by comparing it with mirtazapine, the orexigenic effect of which has already been proven. 23 For the purpose of keeping unknown stimuli affecting appetite and food intake to a minimum, the effect being investigated was evaluated in healthy cats hospitalised for ovariectomy surgery.
Materials and methods
Animals
Client-owned cats were admitted to the veterinary teaching hospital of the University of Toulouse (École Nationale Vétérinaire de Toulouse, France) for ovariectomy surgery via linea alba incision. Only cats which, based on history and complete physical examination, were assessed as ‘healthy’ (American Society of Anesthesiologists’ class 1 of the physical status scale adapted from human medicine) were included in the study. Patients with aggressive behaviour and patients that received other drugs with an appetite-stimulating effect (eg, benzodiazepines) were excluded. Sixty female domestic shorthair cats met the study inclusion criteria. Cats were aged <12 months (range 6–12 months) and mean BW was 2.85 kg (range 2–3.9 kg).
Using an online random number generator, cats were split into three homogeneous groups. Homogeneity for BW and age was tested via Barlett’s test for the assumption of equal variances, followed by one-way ANOVA. One group received gabapentin (n = 20), the second group received mirtazapine (n = 21), and the third group, which acted as a control group, received a placebo (n = 19) (Table 1).
Table 1.
Details of study population according to group
| Parameter | Gabapentin group (n = 20) |
Mirtazapine group (n = 21) |
Placebo group (n = 19) |
|---|---|---|---|
| Age (months) | 7.70 ± 1.91 | 7.88 ± 1.86 | 7.74 ± 1.80 |
| Body weight (kg) | 2.84 ± 0.38 | 2.85 ± 0.51 | 2.87 ± 0.46 |
All cats were female domestic shorthairs. Data are mean ± SD
Drug administration
Drug doses were always prepared during patients’ premedication, to ensure fast administration and to avoid any possible degradation of gabapentin in aqueous solution.24,25 This preparation was always carried out by the same operator who was the only person in the study to know what the cats were given. Once the anonymised syringe was ready, it was given to the operator in charge of drug administration. To avoid drug recognition by the operator due to macroscopic or volume differences, syringes were made non-transparent with adhesive opaque tape.
To prepare the drug doses, a gabapentin 100 mg capsule was dissolved in 5 ml of water or a mirtazapine 15 mg tablet was dissolved in 2 ml/kg of water; a volume of the solution that resulted in either 5 mg/kg of gabapentin and 1.88 mg/cat of mirtazapine was then drawn into the anonymised syringes. Placebo syringes were filled with a volume of water for injections (not containing any active molecule) similar to the amount drawn for mirtazapine or gabapentin for a cat of the same BW. Details of the calculations behind the amount of diluted drug or water for injections drawn into the syringes are shown in Table 2.
Table 2.
Calculation for the dilution of drugs and amount of solution drawn into the anonymised syringes
| BW (kg) | Gabapentin (100 mg in 5 ml; dose 5 mg/kg) | Mirtazapine (15 mg in 2 ml/kg; dose 1.88 mg/cat) | Placebo (ml) | ||
|---|---|---|---|---|---|
| 2.0 | 100 : 5 = (5 × 2.0) : x | x* = 0.500 ml | 15 : (2 × 2.0) = 1.88 : x | x* = 0.501 ml | 0.50 |
| 2.5 | 100 : 5 = (5 × 2.5) : x | x* = 0.625 ml | 15 : (2 × 2.5) = 1.88 : x | x* = 0.627 ml | 0.65 |
| 3.0 | 100 : 5 = (5 × 3.0) : x | x* = 0.750 ml | 15 : (2 × 3.0) = 1.88 : x | x* = 0.752 ml | 0.75 |
| 3.5 | 100 : 5 = (5 × 3.5) : x | x* = 0.875 ml | 15 : (2 × 3.5) = 1.88 : x | x* = 0.877 ml | 0.90 |
| 4.0 | 100 : 5 = (5 × 4.0) : x | x* = 1.000 ml | 15 : (2 × 4.0) = 1.88 : x | x* = 1.003 ml | 1.00 |
x* represents the amount of solution drawn into the syringe in order to contain the desired drug doseBW = body weight
Frequency of administration was calculated based on the pre-established aim to evaluate the appetite-stimulating effect in an 8 h window. Thus, mirtazapine’s half-life (t½ = 16 h) allowed only one administration vs the two needed for gabapentin (t½ = 3 h).23,26,27 In respect of the double-blind protocol, at the time of the second gabapentin administration, a placebo syringe was prepared for patients that already received mirtazapine and also for the control group.
Study design
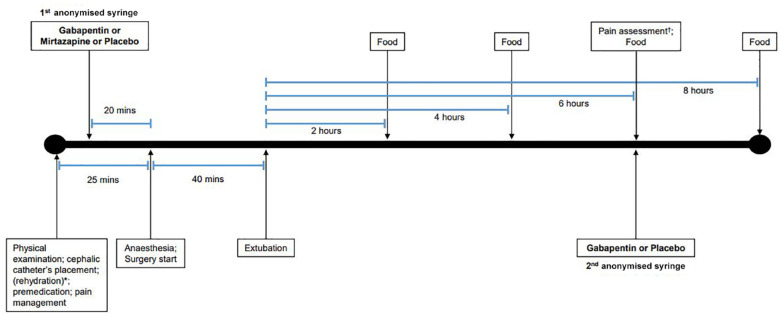
The study was conducted as a double-blinded, placebo-controlled, prospective trial. Figure 1 shows the study timeline.
Figure 1.
Graphical representation of the study timeline.
*When dehydration was present, premedication, pain management and timeline progression were delayed until correction of calculated fluid deficit.
†Pain was assessed via the Colorado State University Feline Acute Pain Scale
All procedures began with physical evaluation and body condition scoring. 28 Dehydration was assessed on skin, mucous membranes, eyes and by measuring pulse rate. 29 When needed, cats were rehydrated with 0.9% saline solution. Premedication was achieved with 50 µg/kg intramuscular (IM) acepromazine maleate coupled with 0.2 mg/kg IM methadone and followed by oral (PO) administration of the first anonymised syringe. After placement of a cephalic catheter, induction of anaesthesia was obtained with intravenous (IV) alfaxalone at 4 mg/kg. Endotracheal intubation was eased by local spraying with lidocaine. Anaesthesia was maintained with oxygen and 1.5% isoflurane gas. Depth of anaesthesia and physiological parameters were monitored during the entire procedure. Cats were positioned in dorsal recumbency for surgery. Mean operative time was 40 mins (range 37–51).
Extubation was performed when effective ventilator movements were present and oxygen saturation was >95% while inhaling room air. Postoperative assessment of physiological parameters was monitored until full recovery; pain was managed with 20 µg/kg IV of buprenorphine. Pain was assessed via the Colorado State University Feline Acute Paine Scale before giving the second anonymised syringe PO 6 h after extubation. 30 Details per group on procedure times and pain scoring are shown in Table 3.
Table 3.
Procedure timing and pain score according to group
| Parameters | Gabapentin group (n = 20) |
Mirtazapine group (n = 21) |
Placebo group (n = 19) |
|---|---|---|---|
| Premedication to anaesthesia (mins) | 21.10 ± 3.35 | 24.14 ± 2.31 | 24.11 ± 2.16 |
| Operative time (mins) | 41.25 ± 3.84 | 40.76 ± 3.53 | 41.16 ± 3.80 |
| Premedication to first food offer (mins) | 185.35 ± 5.16 | 184.90 ± 4.00 | 185.26 ± 3.87 |
| Colorado State University Feline Acute Pain Scale | 0.38 ± 0.44 | 0.14 ± 0.28 | 0.36 ± 0.38 |
Data are mean ± SD
Diet
Cats were fed a convalescence canned diet (metabolisable energy of the diet supplied by 30% protein, 60% fat and 10% nitrogen-free extract). Food was offered at 2, 4, 6 and 8 h after extubation. Environmental stressors were kept to a minimum to avoid non-homeostatic anorectic stimuli. Cats that were asleep were gently stimulated just before food presentation.
Food intake was measured every meal and expressed as grams of food per metabolic weight (g/kg BW0.67): 50 g food were weighed, put into a bowl and left in the cage, far from the urination/defaecation area, for a 15 min window during which the cat’s Elizabethan collar (E-collar) was momentarily removed. If any food was left, it was weighed again and the difference was noted. The same weighing scale was used for the duration of the study.
Data processing and statistical analysis
Data were analysed with R version 3.5.2 and RStudio 1.2.5001 using repeated-measure ANOVA and a linear mixed-model analysis. In the model, food intake was the response variable, with treatment, time and their interaction as explanatory variables. Cats were nested in treatment as random effects. Post-hoc Tukey’s honest significant difference test was performed for multiple comparison. The same combination of statistical tests was used to assess the interaction between food intake and pain score at each time food was offered and also considering total amount eaten during the whole study. A P value <0.05 was determined significant.
Results
All cats were in good condition according to BCS (4–5/9). Correction of mild dehydration (⁓5%) was necessary only in one cat in the placebo group. All calculations of energy requirements were based on ideal BW after rehydration.
Food intake (g/kg BW0.67) was greater in both gabapentin (P <0.01) and mirtazapine (P <0.01) groups vs placebo. No statistically significant differences were found between the two treatment groups (P = 0.48) (Figure 2).
Figure 2.
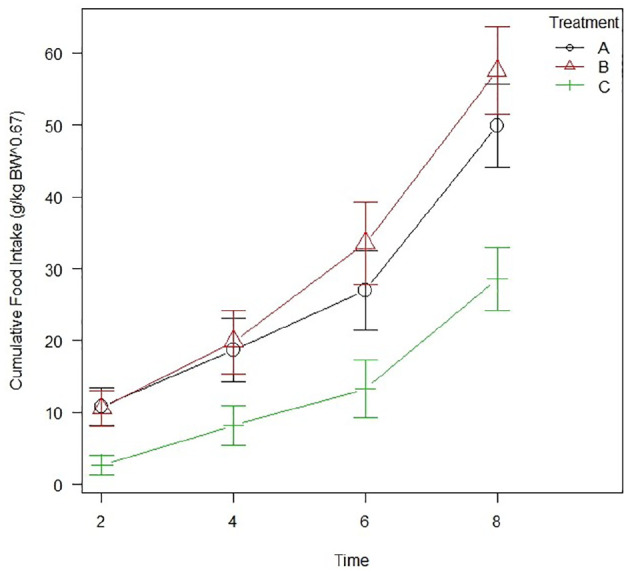
Line graph showing the cumulative food intake (g/kg body weight [BW]0.67) across the whole study. Markers at each time interval indicate group average, with error bars denoting SEM. Group A = gabapentin; group B = mirtazapine; group C = placebo
When appetite-stimulating effect was analysed as the percentage of cats eating the total amount of food offered at each interval, at 2 h post-extubation none (0%) of the cats in the placebo group ate all the 50 g offered, in contrast to the 35% and 24% of cats in the gabapentin and mirtazapine groups, respectively. None of these cats showed any problem related to early feeding after anaesthesia. At 4 h and 6 h no big differences were present between the three groups. At 8 h, 85% of gabapentin cats and 86% of mirtazapine cats ate the total amount of food provided compared with 42% of the cats in the placebo group (Figure 3).
Figure 3.
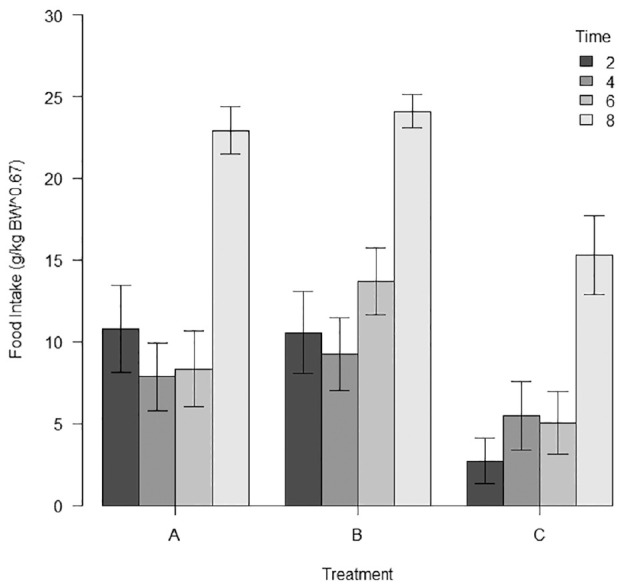
Bar graph showing the food intake per meal for each treatment. Error bars show SEM. Group A = gabapentin; group B = mirtazapine; group C = placebo; BW = body weight
When considering the whole duration of the study, the proportions of cats with a metabolisable energy intake sufficient to cover their resting energy requirement (RER = 70 × BW[kg]0.75) were none (0%) in the placebo group vs 30% and 38% in gabapentin and mirtazapine groups, respectively.
All cats scored <1.25 with the Colorado State University Feline Acute Pain Scale carried out 6 h after extubation. No statistically significant differences were noted between the groups’ pain scores. When evaluating the interaction between food intake and pain score, regardless of food intake at each time interval or the total amount eaten at the end of the study, no statistically significant differences were described.
Across the whole study, no mirtazapine or gabapentin side effects, and no complications during or after anaesthesia were observed.
Discussion
To the best of our knowledge, this is the first study to investigate a possible appetite-stimulating effect of gabapentin and to describe the immediate effects of mirtazapine and gabapentin on food intake. The results provide evidence of greater food intake (g/kg BW0.67) for cats receiving either gabapentin (P <0.006) or mirtazapine (P <0.00008) vs the control group. In terms of food intake, no statistically significant difference was found between the gabapentin and mirtazapine groups.
Gabapentin was compared with mirtazapine in this study because of the latter’s demonstrated appetite-stimulating effect in cats: the same dose of 1.88 mg/cat PO used in the present work showed positive effects on appetite, weight gain and reduction of vomiting in cats with CKD. 31 In the cited study, appetite was documented by the owners via a daily log sheet (decreased = −1; unchanged = 0; increased = 1) for a 3-week period and an appetite score was obtained by the sum of daily scores. Increased food intake was deduced via the owner scoring and by evidence of weight gain and increase of BCS, but no measurement of food intake or energy consumed were performed. 31
Our results show that at 2 h post-extubation, 35% and 24% of cats in the gabapentin and mirtazapine groups, respectively, ate the total amount of food offered vs 0% of control cats. A great difference was also seen at 8 h, with 42% of control cats eating the total amount of food offered vs 85% of gabapentin and 86% of mirtazapine cats. During the study, the amount of food divided into the four meals was unrelated to individual daily metabolic energy requirements (MER = 100 × BW[kg]0.67). 18 Fifty grams of moist pet food was systematically offered at every meal with the purpose of assessing the overall orexigenic effect of gabapentin and mirtazapine, considering the possibility of having some cats eating more than their MER. This did not happen in the control group, while it did for two cats given gabapentin and three given mirtazapine.
Interestingly, none of the cats in the control group consumed more than their RER, while 35% of gabapentin and 38% of mirtazapine cats did. According to guidelines for managing hyporexia in hospitalised pets, after 3–5 days of low voluntary food intake (below the individual RER), a different feeding route should be considered.1,11,17
Both drugs’ mechanisms of action are still not entirely understood and the appetite-stimulating effect has been explored and discussed only for mirtazapine.
Mirtazapine’s appetite-stimulating effect could be the result of several interactions. It has been speculated that its antagonism on H1 receptors could act by inactivating the brain satiety centre. 32 Its blockage of 5-HT2 receptors could also contribute, as it has been demonstrated that mice experimentally lacking these receptors develop obesity. 33 Furthermore, its antidepressant action mediated by antagonism of central presynaptic α2-receptors can overcome the negative feedback on noradrenaline (norepinephrine [NE]), resulting in the catecholamine’s increased availability. NE can then interact with different peripheral alpha receptors increasing appetite. 32
Comprehension and knowledge of gabapentin’s mechanism of action is still partly a conundrum. Although this drug was originally modelled as a structural analogue of the gamma-aminobutyric acid (GABA), a major inhibitory neurotransmitter in mammals, it has been shown that it does not work like a GABAergic drug, but instead it is completely inactive at GABA receptors. 34 It is currently used in human medicine as adjunctive treatment for refractory or complex partial seizures and for neuropathic pain, such as for diabetic neuropathy, cancer, post-herpetic and trigeminal neuralgia.35,36 In veterinary medicine, gabapentin finds an application in dogs’ refractory idiopathic epilepsy and it is also the most prescribed treatment for the alleviation of chronic musculoskeletal pain in cats.37–39 Actions leading to pain management have been ascribed to its binding to alpha 2 delta-1 subunits of voltage-gated calcium channels in the brain, inhibiting membrane and anterograde trafficking. 40
In the present study pain was assessed at 6 h after extubation via the Colorado State University Feline Acute Pain Scale, which was recently validated for inter-operator reliability for ovariohysterectomy in cats. 30 Despite the known effects of gabapentin in the management of chronic pain, no statistically significant differences were noted between the three groups. This could be related to the healthy study population, to the low postoperative pain level of ovariectomy or to the surgery analgesic protocol. 41 While no significant differences were found between pain scores in the present study, it is not possible to rule out the connection between gabapentin’s positive effect on appetite and its analgesic properties.
Both mirtazapine and gabapentin can have adverse effects in cats. The main side effects of mirtazapine include increased activity and vocalisation, while excitability and dysphoria are less frequent and dose dependent. 31 No behavioural changes were seen during the study with one oral administration at a low dose of 1.88 mg/cat. Gabapentin’s main side effects in cats are sedation and ataxia (anecdotal mentioning of diarrhoea, vomiting and hyper-salivation) with a dose ranging from 10 to 30.5 mg/kg.39,42,43 No side effects were registered during observations with the study’s lower dose of 5 mg/kg; however, activity and heart rate were not measured and a mild level of sedation cannot be ruled out.
Researchers have also shown that in vitro gabapentin causes a 10–15% decrease of NE, dopamine and serotonin either related to its action on calcium channels or to an overall change in monoamine metabolism.44–46 It can be speculated that this metabolic derangement could have consequences on the gut–brain axis management of appetite, but a lack of studies and knowledge on this drug’s mechanism of action do not currently allow for a clear explanation.
An open-label study by DeToledo et al is the only published paper evaluating weight gain in people treated for 12 months with high-dose gabapentin (range 1800–3000 mg/day). Overall, 57% of patients had a 5% increase of BW from baseline and 23% had a 10% increase. 47 To our knowledge, no studies have been published on food intake and appetite stimulation in veterinary patients.
In future studies, it would be interesting to measure the appetite-stimulating effect of gabapentin in diseased patients.
Conclusions
This is the first controlled study to evaluate the appetite-stimulating effect of gabapentin. The results have shown that cats receiving gabapentin at 5 mg/kg PO ate more than control cats. Furthermore, none of the cats in the placebo group ate enough to cover their RER across the study, while 35% of cats in the gabapentin group did. Administration of either gabapentin or mirtazapine appeared to produce similar effects on food intake.
Footnotes
Accepted: 5 March 2020
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work involved the use of nonexperimental animals only (owned or unowned), and followed established internationally recognised high standards (‘best practice’) of individual veterinary clinical patient care. Ethical approval from a committee was therefore not necessarily required.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work for the procedure(s) undertaken. No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Marco Fantinati  https://orcid.org/0000-0002-5297-1752
https://orcid.org/0000-0002-5297-1752
References
- 1. Johnson LN, Freeman LM. Recognizing, describing, and managing reduced food intake in dogs and cats. J Am Vet Med Assoc 2017; 251: 1260–1266. [DOI] [PubMed] [Google Scholar]
- 2. Brunetto MA, Gomes MOS, Andre MR, et al. Effects of nutritional support on hospital outcome in dogs and cats. J Vet Emerg Crit Care 2010; 20: 224–231. [DOI] [PubMed] [Google Scholar]
- 3. Woods SC, D’Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab 2008; 93: s37–s50. DOI: 10.1210/jc.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guyenet SJ, Schwartz MW. Regulation of food intake, energy balance, and body fat mass: implications for the pathogenesis and treatment of obesity. J Clin Endocrinol Metab 2012; 97: 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong S, Pinkney J. Role of cytokines in regulating feeding behaviour. Curr Drug Targets 2004; 5: 251–263. [DOI] [PubMed] [Google Scholar]
- 6. Plata-Salamán CR. Brain mechanisms in cytokine-induced anorexia. Psychoneuroendocrinology 1999; 24: 25–41. [DOI] [PubMed] [Google Scholar]
- 7. Iwakura H, Bando M, Ueda Y, et al. The effects of inflammatory cytokines on the expression of ghrelin. Endocr J 2017; 64: S25–S26. [DOI] [PubMed] [Google Scholar]
- 8. Serón-Arbeloa C, Puzo-Foncillas J, Garcés-Gimenez T, et al. A retrospective study about the influence of early nutritional support on mortality and nosocomial infection in the critical care setting. Clin Nutr 2011; 30: 346–350. [DOI] [PubMed] [Google Scholar]
- 9. Liu DT, Brown DC, Silverstein DC. Early nutritional support is associated with decreased length of hospitalization in dogs with septic peritonitis: a retrospective study of 45 cases (2000–2009). J Vet Emerg Crit Care 2012; 22: 453–459. [DOI] [PubMed] [Google Scholar]
- 10. Harris JP, Parnell NK, Griffith EH, et al. Retrospective evaluation of the impact of early enteral nutrition on clinical outcomes in dogs with pancreatitis: 34 cases (2010–2013). J Vet Emerg Crit Care 2017; 27: 425–433. [DOI] [PubMed] [Google Scholar]
- 11. Delaney SJ. Management of anorexia in dogs and cats. Vet Clin North Am Small Anim Pract 2006; 36: 1243–1249. [DOI] [PubMed] [Google Scholar]
- 12. Buchman AL, Moukarzel AA, Bhuta S, et al. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. J Parenter Enter Nutr 1995; 19: 453–460. [DOI] [PubMed] [Google Scholar]
- 13. Alverdy J. The effect of nutrition on gastrointestinal barrier function. Semin Respir Infect 1994; 8: 563–567. [PubMed] [Google Scholar]
- 14. Gianotti L, Nelson JL, Alexander JW, et al. Post injury hypermetabolic response and magnitude of translocation: prevention by early enteral nutrition. Nutrition 1994; 10: 225–231. [PubMed] [Google Scholar]
- 15. Hadfield RJ, Sinclair DG, Houldsworth PE, et al. Effects of enteral and parenteral nutrition on gut mucosal permeability in the critically ill. Am J Respir Crit Care Med 1995; 152: 1545–1548. [DOI] [PubMed] [Google Scholar]
- 16. Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications the results of a meta-analysis. Ann Surg 1992; 216: 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perea S. Routes of nutritional support in small animals. In: Chan DL. (ed). Nutritional management of hospitalized small animals. Chichester: John Wiley & Sons, 2015, pp 14–20. [Google Scholar]
- 18. National Reaserch Council. Nutrient requirements of dogs and cats. Washington, DC: National Academies Press, 2006. [Google Scholar]
- 19. Holahan ML, Abood SK, McLoughlin MA, et al. Enteral nutrition. In: DiBartola SP. (ed). Fluid, electrolyte, and acid-base disorders in small animal practice. St Louis, MO: Elsevier, 2005, pp 623–646. [Google Scholar]
- 20. Agnew W, Korman R. Pharmacological appetite stimulation. J Feline Med Surg 2014; 16: 749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Norris CR, Boothe DM, Esparza T, et al. Disposition of cyproheptadine in cats after intravenous or oral administration of a single dose. Am J Vet Res 1998; 59: 79–81. [PubMed] [Google Scholar]
- 22. Quimby JM, Gustafson DL, Lunn KF. The pharmacokinetics of mirtazapine in cats with chronic kidney disease and in age-matched control cats. J Vet Intern Med 2011; 25: 985–989. [DOI] [PubMed] [Google Scholar]
- 23. Quimby JM, Gustafson DL, Samber BJ, et al. Studies on the pharmacokinetics and pharmacodynamics of mirtazapine in healthy young cats. J Vet Pharmacol Ther 2011; 34: 388–396. [DOI] [PubMed] [Google Scholar]
- 24. Zour E, Lodhi SA, Nesbitt RU, et al. Stability studies of gabapentin in aqueous solutions. Pharm Res 1992; 9: 595–600. [DOI] [PubMed] [Google Scholar]
- 25. McClain BC, Ennevor S. The use of gabapentin in pediatric patients with neuropathic pain. Semin Anesth 2000; 19: 83–87. [Google Scholar]
- 26. Adrian D, Papich MG, Baynes R, et al. The pharmacokinetics of gabapentin in cats. J Vet Intern Med 2018; 32: 1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Siao KT, Pypendop BH, Ilkiw JE. Pharmacokinetics of gabapentin in cats. Am J Vet Res 2010; 71: 817–821. [DOI] [PubMed] [Google Scholar]
- 28. Laflamme D. Development and validation of a body condition score system for cats: a clinical tool. Feline Pract 1997; 25: 13–17. [Google Scholar]
- 29. Davis H, Jensen T, Johnson A, et al. 2013 AAHA/AAFP fluid therapy guidelines for dogs and cats. J Am Anim Hosp Assoc 2013; 49: 149–159. [DOI] [PubMed] [Google Scholar]
- 30. Shipley H, Guedes A, Graham L, et al. Preliminary appraisal of the reliability and validity of the Colorado State University Feline Acute Pain Scale. J Feline Med Surg 2019; 21: 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Quimby JM, Lunn KF. Mirtazapine as an appetite stimulant and anti-emetic in cats with chronic kidney disease: a masked placebo-controlled crossover clinical trial. Vet J 2013; 197: 651–655. [DOI] [PubMed] [Google Scholar]
- 32. Nihalani N, Schwartz TL, Siddiqui UA, et al. Weight gain, obesity, and psychotropic prescribing. J Obes 2011; 2011. DOI: 10.1155/2011/893629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Curzon G, Gibson EL, Oluyomi AO. Appetite suppression by commonly used drugs depends on 5-HT receptors but not on 5-HT availability. Trends Pharmacol Sci 1997; 18: 21–25. [DOI] [PubMed] [Google Scholar]
- 34. Taylor CP, Gee NS, Su T-Z, et al. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res 1998; 29: 233–249. [DOI] [PubMed] [Google Scholar]
- 35. Honarmand A, Safavi M, Zare M. Gabapentin: an update of its pharmacological properties and therapeutic use in epilepsy. J Res Med Sci 2011; 16: 1062–1069. [PMC free article] [PubMed] [Google Scholar]
- 36. Vranken J. Mechanisms and treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem 2009; 9: 71–78. [DOI] [PubMed] [Google Scholar]
- 37. Govendir M, Perkins M, Malik R. Improving seizure control in dogs with refractory epilepsy using gabapentin as an adjunctive agent. Aust Vet J 2005; 83: 602–608. [DOI] [PubMed] [Google Scholar]
- 38. Platt SR, Adams V, Garosi LS, et al. Treatment with gabapentin of 11 dogs with refractory idiopathic epilepsy. Vet Rec 2006; 159: 881–884. [PubMed] [Google Scholar]
- 39. Guedes AGP, Meadows JM, Pypendop BH, et al. Assessment of the effects of gabapentin on activity levels and owner-perceived mobility impairment and quality of life in osteoarthritic geriatric cats. J Am Vet Med Assoc 2018; 253: 579–585. [DOI] [PubMed] [Google Scholar]
- 40. Field MJ, Cox PJ, Stott E, et al. Identification of the α2-δ-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci 2006; 103: 17537–17542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pereira MAA, Gonçalves LA, Evangelista MC, et al. Postoperative pain and short-term complications after two elective sterilization techniques: ovariohysterectomy or ovariectomy in cats. BMC Vet Res 2018; 14: 335. DOI: 10.1186/s12917-018-1657-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Haaften KA, Forsythe LRE, Stelow EA, et al. Effects of a single preappointment dose of gabapentin on signs of stress in cats during transportation and veterinary examination. J Am Vet Med Assoc 2017; 251: 1175–1181. [DOI] [PubMed] [Google Scholar]
- 43. Hudec CP, Griffin CE. Changes in the stress markers cortisol and glucose before and during intradermal testing in cats after single administration of pre-appointment gabapentin. J Feline Med Surg 2020; 22: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schlicker E, Reimann W, Göthert M. Gabapentin decreases monoamine release without affecting acetylcholine release in the brain. Arzneimittelforschung 1985; 35: 1347–1349. [PubMed] [Google Scholar]
- 45. Fink K, Meder W, Dooley DJ, et al. Inhibition of neuronal Ca 2+ influx by gabapentin and subsequent reduction of neurotransmitter release from rat neocortical slices. Br J Pharmacol 2000; 130: 900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reimann W. Inhibition by GABA, baclofen and gabapentin of dopamine release from rabbit caudate nucleus: are there common or different sites of action? Eur J Pharmacol 1983; 94: 341–344. [DOI] [PubMed] [Google Scholar]
- 47. DeToledo JC, Toledo C, DeCerce J, et al. Changes in body weight with chronic, high-dose gabapentin therapy. Ther Drug Monit 1997; 19: 394–396. [DOI] [PubMed] [Google Scholar]