Abstract
The gonococcal pilus is a primary virulence factor, providing the initial attachment of the bacterial cell to human mucosal tissues. Pilin, the major subunit of the pilus, can carry a wide spectrum of primary amino acid sequences which are generated by the action of a complex antigenic variation system. Changes in the pilin amino acid sequence can produce different pilus-dependent colony morphotypes, which have been previously shown to reflect phase variation of pili on the bacterial cell surface. In this study, we further examined the relationships between changes in pilus-dependent colony morphology, pilin sequence, pilus expression, and pilus function in Neisseria gonorrhoeae FA1090. A group of FA1090 colony variants expressed different pilin sequences and demonstrated different levels of pilin, S-pilin, and pilus expression. The analysis of these colony variants shows that they do not represent two distinct phases of pilus expression, but that changes in pilin protein sequence produce a spectrum of S-pilin production, pilus expression, and pilus aggregation levels. These different levels of pilus expression and aggregation influence not only colony morphology but also DNA transformation efficiency and epithelial cell adherence.
The gram-negative diplococcus Neisseria gonorrhoeae (the gonococcus) is an obligate human pathogen which has been documented as infecting humans as far back as the 5th century B.C. (30). Today, gonorrhea remains a worldwide health problem, with an estimated annual incidence of 62 million cases (69). Attempts at vaccine development have been unsuccessful (1, 65), due in part to the ability of N. gonorrhoeae to antigenically and phase vary a number of surface components. Pili, long filamentous appendages composed of numerous copies of the protein subunit pilin (61), play a crucial role in the initiation of disease by providing for the primary attachment of the bacterial cell to human mucosal tissues (56). Pilus-mediated adherence has been studied both in vitro and in vivo. Piliation of gonococci enhances adherence to epithelial cells in tissue culture (4, 40, 66) and in organ culture (10). Studies with human volunteers have confirmed that pili are important for establishing infection (23, 24, 62).
In addition to adherence, pili are required for full natural DNA transformation efficiency of N. gonorrhoeae. Nonpiliated cells are greatly reduced in competence (48, 53), and some pilus phase variants demonstrate intermediate levels of transformation competence (13). Gonococcal transformation is linked to the presence of the 10-bp gonococcal DNA transformation uptake sequence (14). Pilus assembly in the presence of the PilC protein has also been shown to be required for efficient DNA transformation (42).
Pilus antigenic variation, which occurs by changes in the pilin primary amino acid sequence, is an important defense mechanism of gonococci and may also influence tissue tropism (20, 26, 43). Pilin sequence changes are mediated predominantly by nonreciprocal recombination events (17). Variant sequences from silent copies of partial pilin information (pilS) transfer to the pilin expression gene (pilE) (15, 46) in a RecA-dependent fashion (25). A conserved DNA sequence, the Sma/Cla repeat, is found at the 3′ end of all pilS and pilE loci (32) and is required for efficient antigenic variation to occur (68). While the precise molecular mechanisms used to allow transfer of pilS sequences to pilE are largely unknown, there are molecular models that predict the types of recombination reactions that lead to antigenic variation (15, 18). Additionally, several transposon-induced mutations which interfere with pilin variation and repair of UV damage have been isolated (31).
The N. gonorrhoeae pilus also undergoes phase variation, the reversible switch between piliated and nonpiliated states. A number of mechanisms, some of which overlap with antigenic variation, have been proposed to contribute to pilus phase variation. Pilus-dependent colony morphology has been used extensively as a sensitive indicator of pilus expression (57–59) and is the main phenotypic screen used for phase variation. A tight-edged, domed (P+) colony correlates directly with piliation, whereas a flat colony without a distinct edge (P−) correlates with a lack of pilus expression (61). The very flat colonies seen in studies with gonococcal strain MS11 often harbor either a deletion of pilE (3) or an extended pilE with a duplication of its 3′ portion, called an L-pilin. L-pilin variants synthesize an overlong pilin protein which is not polymerized into pili (16). Gonococci also form colony morphotypes which are intermediates between P+ and P−. These intermediate colony variants often correlate with a truncated, secreted form of the pilin protein, S-pilin (16, 60). S-pilin lacks the conserved N-terminal 39 amino acids of the mature pilin protein (16), a region which is implicated in pilin polymerization (39). The role of these alternate pilins in N. gonorrhoeae pathogenesis, transformation, and adherence has not been determined, although S-pilin formation has been proposed to allow for the release of otherwise toxic pilin monomers that cannot be efficiently assembled into pili (16, 22, 25).
The majority of studies on gonococcal pilus phase variation (3, 34, 45, 58–60, 63) and S-pilin production (16) have utilized N. gonorrhoeae MS11. However, strain FA1090 has been extensively used to study the Opa protein gene family (7, 8, 36) and to examine the dynamics of Opa and pilin variation in human volunteers (19, 49). Additionally, because FA1090 is the first N. gonorrhoeae strain for which the entire genome sequence will be determined (41), it is evident that FA1090 will be used extensively in future studies of gonococcal pathogenesis. Therefore, to investigate the process of pilus phase variation in strain FA1090, we isolated a series of in vitro-generated pilus colony morphology variants and characterized the changes in pilin expression, pilus expression, and pilus function when variant pilin proteins were expressed.
MATERIALS AND METHODS
Bacterial strains and media.
Gonococci were grown on GC Medium Base (GCB; Difco) plus Kellogg supplements (24) at 37°C in 5% CO2. Variants were derived from N. gonorrhoeae FA1090. Prior to phenotypic analyses, all variants were transformed with plasmid DNA carrying an isopropyl-β-d-thiogalactopyranoside (IPTG)-regulatable gonococcal recA allele, recA6, which has been described previously (47). Briefly, the recA6 allele is composed of the gonococcal recA coding sequence with a promoter region consisting of two tandem lac promoter/operator sequences (tac-UV5) and an associated lacIq gene and tetracycline resistance marker. Transformation with this cloned DNA results in the introduction of this cassette into the corresponding location relative to the chromosomal recA gene. In the absence of IPTG induction, recA6 strains are phenotypically Rec− (47) and do not undergo antigenic variation (31), thus allowing for the creation of a population with a stable pilE sequence. An IPTG concentration of 1 mM in the media allows for maximal induction of recA transcription and restoration of transformation competence to near-wild-type levels (47).
PCR amplification and DNA sequencing.
The expressed pilin genes were amplified by using oligonucleotide primers PILRBS and SP3A (70) in a PTC-100 thermocycler (MJ Research). Absence of an approximately 720-bp product indicated a deleted pilE gene. The PCR products were purified over CL6B-Sepharose (Sigma) spin columns to remove excess primers and deoxynucleoside triphosphates. PCR-generated pilin template (10 to 50 ng) was used in fmol sequencing (Promega) as recommended by the manufacturer, using [γ-32P]ATP (Amersham) end-labeled primers CONSTF2, CYS1F, CYS1R, and PILEND (70).
Analysis of full-length pilin and S-pilin production.
Gonococci were grown in 5 ml of GC Liquid medium (GCL; 1.5% proteose peptone no. 3 [Difco], 0.4% K2HPO4, 0.1% KH2PO4, 0.1% NaCl) with Kellogg supplements and 0.042% sodium bicarbonate (35) at 37°C for 18 to 20 h in a rotator. Cells and pili were sedimented in a SW50.1 rotor at approximately 200,000 × g for 1 h. S-pilin in the supernatant was precipitated with trichloroacetic acid as described by Haas et al. (16). Both the cell pellet and the supernatant fractions were separated on sodium dodecyl sulfate (SDS)–17 to 21% polyacrylamide gradient gels and either stained with Coomassie brilliant blue R-250 (Sigma) or transferred to an Immobilon-P membrane (Millipore), using a Trans-Blot Cell (Bio-Rad). The membranes were blocked with MegaBlocI (CEL Associates) as recommended by the manufacturer and probed with pilin monoclonal antibody (MAb) 1E8/G8 (gift from M. Koomey and M. Blake) (11) at a dilution of 1:1,000 or MAb SM1 (gift from M. Virji) (67) at a dilution of 1:2,000. Horseradish peroxidase-linked anti-mouse secondary antibody (Amersham) was used at a dilution of 1:10,000, and the immunoblots were developed by using enhanced chemiluminescence reagents (Amersham).
Analysis of PilC production.
Variants RM0, RM5, RM11, and RM21 were cultured on GCB plates for approximately 19 h. Gonococci were collected with Dacron swabs (Puritan), suspended in GCL, pelleted, and resuspended in 5× sample buffer (5). Cell lysates were separated on SDS–7.5% polyacrylamide gels and transferred to a nitrocellulose membrane (Micron Separations, Inc.), using a Trans-Blot Cell. Membranes were blocked as described above and probed with preabsorbed polyclonal PilC antisera (gift from J. Pfeifer and S. Normark) (37). The immunoblots were then probed with alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G secondary antibody (Promega) at a dilution of 1:7,500 and developed colorimetrically, using nitroblue tetrazolium (330 μg ml−1) and 5-bromo-4-chloro-3-indolylphosphate (165 μg ml−1) (Sigma) in buffer containing 100 mM Tris, 100 mM NaCl, and 5 mM MgCl2 (pH 9.5).
Electron microscopy.
Gonococci were grown for 18 to 20 h on plates, and poly-l-lysine (1 μg ml−1)-treated, Formvar-coated grids (Ladd Research Industries, Inc.) were used to lift cells from colonies. Alternatively, to examine variants which expressed only a few pili per cell, 200 μl of 16- to 18-h liquid cultures of N. gonorrhoeae were centrifuged at approximately 2,600 × g onto poly-l-lysine (1 μg ml−1)-treated, Formvar-coated grids placed in microcentrifuge tubes. The grids were then fixed and negatively stained essentially as described by McGee et al. (29). Grids were incubated in drops of the following solutions: 1% glutaraldehyde in 0.1 M cacodylate buffer for 2 min, twice in sterile water for 3 s, and 1% phosphotungstic acid (pH 6.0) for 1 min. Grids were viewed in a Jeol JEM-100 CX II transmission electron microscope at 60 kV.
Gonococcal transformation.
Gonococci were grown on plates for 16 to 18 h, collected with Dacron swabs, and suspended to a density of 108 CFU per ml in GCL. Twenty microliters of cells was added to 200 μl of GCL that contained 5 mM MgSO4 (48), 1 mM IPTG, and ∼2 μg of cloned gonococcal DNA carrying an erythromycin resistance (Ermr) gene insertion (31). After 15 min at 37°C, the transformation mixes were diluted into 2 ml of GCL plus Kellogg supplements and 1 mM IPTG and then incubated at 37°C in 5% CO2 for 5 h. The transformation mixes were diluted and plated on GCB with 2 μg of erythromycin ml−1 to select transformants and on GCB to determine CFU.
Adherence assays.
The Chang conjunctival cell line (ATCC CCL 20.2) was maintained in RPMI 1640 supplemented with 5% fetal bovine serum (Gibco BRL) (RPMI-FBS), penicillin (100 U ml−1), streptomycin (100 μg ml−1; Gibco BRL), and amphotericin B (2.5 μg ml−1; Biologos, Inc.) at 37°C in 5% CO2. Cells were grown in 75-cm2 culture flasks (Corning), split 1:15, and passaged every 4 days.
Opa− gonococci were used for all adherence assays. The Opa status of each variant was determined by Western analysis. Variants were cultured and collected for analysis as described for analysis of PilC production. Cell lysates were separated on SDS–12.5% polyacrylamide gels, transferred to nitrocellulose, and blocked as described above. Membranes were probed with a panel of five MAbs which are specific for FA1090 Opa proteins (gift from J. Cannon) (2). The immunoblots were then probed with alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G secondary antibody (Promega) used at a dilution of 1:7,500 and developed as described for analysis of PilC production.
Twenty-four hours prior to the assay, Chang cells were plated in 24-well culture dishes (Corning) at a concentration of 2 × 105 cells/well in RPMI-FBS and incubated at 37°C in 5% CO2. Eighteen hours prior to the assay, Opa− variants were passaged on GCB and grown overnight at 37°C in 5% CO2. Gonococci were collected from plates with Dacron swabs, suspended in RPMI-FBS, and diluted to approximately 6 × 107 CFU/ml. In the 24-well culture dishes, 1 ml of gonococcal suspension/well was incubated with the Chang cell monolayers (approximately 80% confluent) for 2.5 h at 37°C in 5% CO2. Monolayers were then washed five times with incomplete phosphate-buffered saline (Gibco BRL) to remove any nonadherent gonococci and then incubated with a 1% saponin (Sigma)–phosphate-buffered saline solution for 10 min to disrupt the monolayers. The cell suspensions were diluted in RPMI-FBS and plated on GCB to allow counting of adherent CFU.
Colony variation assay.
Variant RM11.2 recA6 was grown on GCB plates with IPTG induction (1 mM) for either 18 or 24 h. Gonococci were then collected from plates with a Dacron swab, suspended in GCL, diluted, plated to allow for 100 to 500 CFU per plate, and grown for approximately 24 h. Colonies were observed in a stereomicroscope. Colonies that had a morphology which lacked a defined edge and/or were less domed than RM11.2 recA6 colonies were picked with a sterile loop, patched onto GCB plates, and then suspended in 5 μl of colony lysate solution (31). Each colony was categorized as either a flat colony without a distinct edge (P−) or an intermediate colony lacking the dark ring found at the edge of most P+ colonies but more domed than P− colonies (P+/−). PCR analysis was used as described above to determine whether a pilE gene of the appropriate size was present in each colony. A subset of colonies which had an intact pilE were passaged to allow for Western analysis of pilin protein production. Whole-cell lysates were separated on SDS–15% polyacrylamide gels, transferred to nitrocellulose membranes, blocked, and probed with MAb 1E8/G8 as described above for analysis of pilin production. The immunoblots were then probed with secondary antibody and developed colorimetrically as described above for detection of Opa proteins.
Nucleotide sequence accession numbers.
The DNA sequences of the coding regions of variants RM0.1, RM11.1, RM11.2, RM11.6, RM11.9, RM5, and RM21 have been submitted to the GenBank database under accession no. AF043646, AF043650, AF043651, AF043652, AF043653, AF043647, and AF043649, respectively. The variable DNA sequence of variants RM0 and RM11 can be found under GenBank accession no. U58840.
RESULTS
Isolation of FA1090 colony morphology variants.
Most studies investigating the molecular mechanisms of gonococcal pilin antigenic variation have used N. gonorrhoeae MS11 (3, 15, 17, 34, 46, 60). Because N. gonorrhoeae FA1090 has been used to study pilin variation in human volunteers (49) and is currently being used to determine the frequency of pilin antigenic variation (50), we chose strain FA1090 to study the relationships between pilus-based colony morphology, pilin and S-pilin production, piliation, and pilus function. Numerous studies have examined the phenotypes associated with a P− colony morphology (13, 16, 23, 40, 42, 45, 53, 60), but there has been little investigation into pilus expression, aggregation, and function of variants which express only a few pili. To enrich for colonies which expressed a small number of pili and produced a substantial amount of S-pilin, we collected several independently isolated colony morphology variants of FA1090 that had defined edges characteristic of P+ colonies, lacked the dark ring found at the edge of most P+ colonies, and were more domed than variants with a deleted pilE (ΔpilE). To test whether any of these intermediate, or P+/−, colony variants were ΔpilE or L-pilin variants, we screened all variants by PCR amplification to determine the size of pilE. In most of these P+/− colony variants, a pilE of the appropriate size was detected (data not shown). From this screen, four independently derived P+/− colony variants with an intact pilE were chosen: RM0, RM5, RM11, and RM21. Since P− colony morphology has been associated with mutations in PilC, a protein essential for pilus biogenesis (21), we screened these variants for the expression of PilC. All of these variants were PilC+ by immunoblot analysis using an anti-PilC antiserum which recognizes both PilC1 and PilC2 (37) (data not shown).
Many P− colony variants revert to a P+ colony morphology at high frequencies (34, 45, 61). To study phenotypic differences between P+/− and P+ variants, a single P+ colony morphology variant was isolated from variant RM0 (RM0.1) and four P+ variants were isolated from variant RM11 (RM11.1, RM11.2, RM11.6, and RM11.9). These five P+ variants exhibited a subtle range of P+ colony morphotypes by blinded comparisons (data not shown). However, all of these variants expressed a colony morphology which was more P+ than the parental P+/− variants. Each P+/− and P+ variant was then transformed with cloned DNA carrying the recA6 allele, an IPTG-regulated recA gene (47), to create a population with a stable pilin sequence due to the lack of antigenic variation (31). In each case, a recA6 transformant that retained the colony morphology and pilE sequence of the original Rec+ variant was identified.
Analysis of pilin production by the FA1090 colony variants.
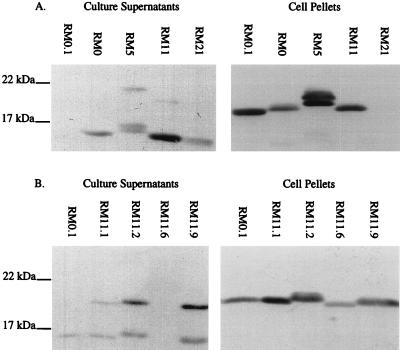
In previous studies, P− colony variants that carried an intact pilE gene were originally characterized as missense or nonsense mutants and could be differentiated by determining whether a full-length pilin protein was expressed (3, 16, 59). In addition, many P− colony variants have been shown to produce the truncated S-pilin form of the pilin protein (9, 12, 16, 21, 25). We used the broadly cross-reactive MAbs 1E8/G8 (11) (Fig. 1) and SM1 (67) (data not shown) for immunoblotting to detect both full-length pilin in cell pellets and S-pilin forms in supernatants of the FA1090 colony variants. As expected, the P+/− colony morphology variants RM0 recA6, RM5 recA6, RM11 recA6, and RM21 recA6 all produced significant amounts of S-pilin (Fig. 1A). Surprisingly, four of the five P+ colony variants derived from RM0 recA6 and RM11 recA6 also produced detectable amounts of S-pilin (Fig. 1B) even though they are phenotypically RecA− and therefore do not contain subpopulations with variant pilE sequences. In fact, RM11.2 recA6, which expressed one of the strongest piliated colony morphologies, produced a relatively large amount of S-pilin (Fig. 1B).
FIG. 1.
Pilin immunoblots of N. gonorrhoeae FA1090 colony variants. Soluble supernatants and cell pellets were separated on SDS-gradient polyacrylamide gels and probed with the broadly cross-reactive MAb 1E8/G8. (A) P+/− variants and P+ variant RM0.1 for comparison; (B) P+ variants. All variants contained the recA6 allele. RM0.1 S-pilin reacted poorly with MAb 1E8/G8 (A) but is detectable after a more extended development of the immunoblot (B).
Full-length pilin was detected in the cell pellets of all variants except RM21 recA6 (Fig. 1). Differences in pilin protein mobility were observed in these variants, indicating that they contained different pilin primary protein sequences (58, 63). These sizes were consistent with the predicted amino acid sequence of each variant (see Fig. 4). In variant RM5 recA6, a doublet band was detected in both full-length pilin and S-pilin forms (Fig. 1A); a doublet was also present in variant RM11.2 recA6 but was harder to resolve (Fig. 1B). We do not know the molecular basis for these doublets, but they are likely to be two differentially glycosylated forms of pilin since only these two variants carry the glycosylation site, serine 63 (39, 55) (see Fig. 4). These analyses showed that in almost all of these colony variants, both full-length pilin and S-pilin were present, but in apparently different amounts.
FIG. 4.
Predicted variable pilin amino acid sequences of FA1090 pilE variants. Variant names and lineages are shown on the left. DNA sequences were determined from PCR-amplified pilE DNA, but only the variable amino acid sequences are shown. The SV, cys1, HVL, cys2, and hypervariable tail (HVT) regions of pilE are indicated on the top. −, no changes relative to variant RM0; ∗, deletions relative to variant RM0; #, silent changes in DNA sequence not reflected in the amino acid sequence. Amino acid residues that have never varied in an expressed gene on any pilin variant from any reported N. gonorrhoeae strain are indicated in bold on the variant RM0 sequence. Patterned boxes represent nucleotide stretches of the pilE DNA sequences that can be mapped to a particular silent copy. The legend shows the silent locus and silent copy sources of each variable DNA sequence; for example, S1C1 indicates pilS1 copy 1 DNA sequences. Sequences at the junction of two different adjacent silent copy sequences that are identical in those silent copies are indicated as shared sequences; for example, RM0.1 has pilS6 copy 1-specific sequences and pilS2 copy 1-specific sequences, and the open box shows the sequence where these two silent copies are identical.
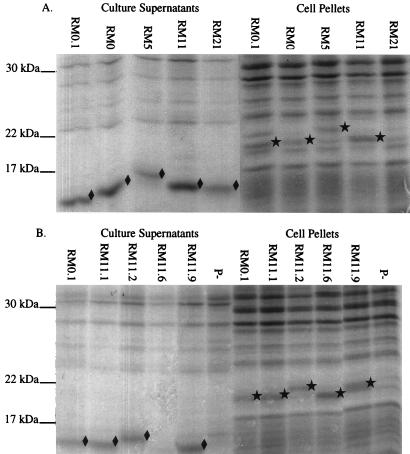
The variance in mobility of the pilins and S-pilins suggested that each of these FA1090 variants expressed different pilin gene sequences. MAb 1E8/G8 has been used to detect a wide variety of pilin variants (11), but we did not know whether it reacted with similar affinity to each variant pilin protein and could be used to quantitate relative amounts of pilin and S-pilin production. We therefore visualized the pilin proteins on Coomassie brilliant blue-stained SDS-polyacrylamide gradient gels (Fig. 2). Since there were few proteins of similar mobility in the culture supernatant samples, the S-pilin proteins were easily detectable. In contrast, the full-length pilin bands were difficult to identify due to the presence of comigrating proteins. However, by aligning an immunoblot with the stained gel, we could identify the full-length pilin bands in the cell pellets. Comparison of band intensities between the stained gel and immunoblot clearly showed differential reactivities of MAb 1E8/G8 to the different variants, and also differential reactivities between the two forms of pilin from the same variant. Differential reactivities of MAb SM1, which reacts with conserved amino acids 48 to 53 of pilin (38), were also observed (data not shown). Because both 1E8/G8 and SM1 react with conserved epitopes, our data demonstrate that the binding of these MAbs is influenced by the conformation and/or differential modifications of the pilin protein, and neither can be used to accurately quantify pilin and S-pilin protein levels between different variants or forms.
FIG. 2.
Stained SDS-polyacrylamide gels of FA1090 colony variants. Soluble supernatants and cell pellets were separated on SDS-gradient polyacrylamide gels and stained with Coomassie brilliant blue. (A) P+/− variants and P+ variant RM0.1 for comparison; (B) P+ variants and a nonpiliated (ΔpilE) control (P−). All P+ and P+/− variants contained the recA6 allele. S-pilin bands (⧫) and full-length pilin bands (★) as identified by parallel immunoblotting are marked.
A wide range of pilin phenotypes were exhibited by the variants (Table 1). For example, P+/− variant RM21 recA6 did not produce detectable full-length pilin and produced a moderate amount of S-pilin which reacted well with MAb 1E8/G8. P+ variant RM0.1 recA6 produced S-pilin and full-length proteins in roughly equal amounts (Fig. 2B), but MAb 1E8/G8 reacted well only with the full-length form (Fig. 1B). Finally, P+ variant RM11.6 recA6 did not produce detectable S-pilin and expressed full-length pilin protein, which was easily detected in stained gels (Fig. 2B), but reacted poorly with MAb 1E8/G8 (Fig. 1B). The remainder of the variants expressed both full-length and S-pilin forms which reacted equally well with the antibody. As a group, the P+ colony variants all expressed full-length pilin, while all of the P+/− colony variants expressed S-pilin. However, the levels of S-pilin and full-length pilin produced by each variant did not directly correlate with colony morphology.
TABLE 1.
Phenotypes of FA1090 variants
| Varianta | Classb | % of cells expressing pilic | Pilin expressiond | S-pilin expressione | Transformation frequencyf | Adherenceh |
|---|---|---|---|---|---|---|
| RM11.2 | I | 80–95 | ++++ | ++++ | ++++ | +++++ |
| RM11.6 | I | 80–95 | ++++ | + | +++ | ++++ |
| RM11.9 | II | 80–95 | +++ | +++ | ++++ | ++ |
| RM11.1 | III | 80–95 | ++ | ++++ | ++g | +++ |
| RM0.1 | III | 80–95 | ++ | ++++ | ++ | +++ |
| RM0 | IV | 40–60 | + | ++++ | ++++ | ++++ |
| RM11 | IV | 40–60 | ++++ | +++ | +++ | +++ |
| RM5 | V | 5–10 | ++ | ++ | ++ | ++ |
| RM21 | V | 5–10 | +/− | +++ | ++ | ++ |
| ΔpilE | 0 | − | − | − | + |
Lineage of variants is depicted in Fig. 4.
Class of piliation phenotype as defined by TEM (Fig. 3).
Percentage of cells that expressed single or aggregated pili (Fig. 3).
Full-length pilin protein expression determined by densitometric analysis of Coomassie blue-stained gradient gels; density from comigrating bands was subtracted from final band density. ++++, high level of pilin produced; +, low level of pilin produced; +/−, no pilin detected on gels but a few pili detected by TEM; −, no pilin detected (Fig. 1 to 3).
S-pilin protein expression determined by densitometric analysis of Coomassie blue-stained gradient gels; no comigrating bands were detected in the ΔpilE control. ++++, high level of S-pilin produced; +, low level of S-pilin produced; −, no S-pilin detected (Fig. 1 and 2).
Transformation frequencies of each variant as determined by number of Ermr transformants per CFU. ++++, high level of transformation competence; +, low level of transformation competence; −, Ermr transformants were never detected. The transformation competence levels are not statistically different due to the spread of the data (Fig. 5A).
Could not be accurately determined due to consistently low CFU.
Adherence of gonococci to epithelial cells. ++++, high level of adherence; +, low level of adherence. The five adherence levels are significantly different (P < 0.05) from each other as calculated by Student’s t test (Fig. 5B).
Pilus expression of FA1090 colony variants.
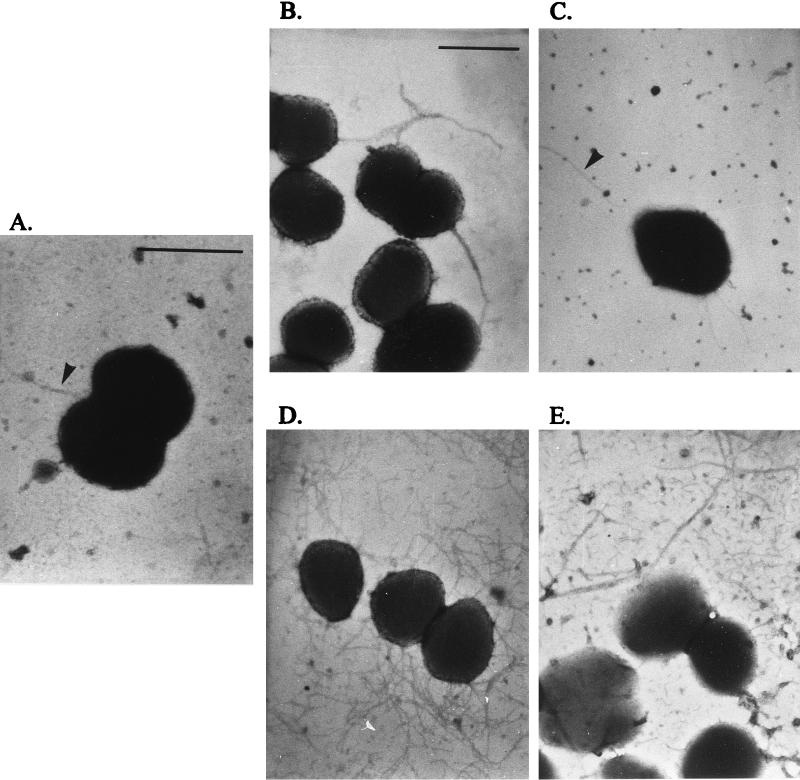
Pilus expression by individual diplococci or cocci (cells) of the FA1090 colony variants was ascertained using transmission electron microscopy (TEM). Because some variants had a pattern of pilus expression which closely resembled that of another variant, variants were divided into five piliation classes (Table 1). In classes I, II, and III, which encompass the five P+ variants, approximately 80 to 95% of individual cells expressed pili. Many pili which were minimally aggregated or singular were seen on variants RM11.2 recA6 and RM11.6 recA6 (class I) (Fig. 3D). RM11.9 recA6 (class II) expressed many pili on each cell, but all pili seen were aggregated into one or two large bundles per cell (Fig. 3E). RM0.1 recA6 and RM11.1 recA6 (class III) expressed fewer pili per cell than class I, with some pili in small bundles (Fig. 3C). In contrast to the first three classes, only 40 to 60% of the cells of class IV variants RM0 recA6 and RM11 recA6 expressed pili, and the individual fibers were usually aggregated into a single bundle of medium thickness (Fig. 3B). Finally, variants RM5 recA6 and RM21 recA6 (class V) rarely expressed detectable pili on their cell surface, with only 5 to 10% of individual cells expressing one or two detectable pilus fibers (Fig. 3A). The individual pili expressed by the class V variants were detected only when gonococci were directly sedimented onto grids from liquid culture, not from colony lifts (see Materials and Methods). Although RM21 recA6 did not produce enough full-length pilin for detection on immunoblots (Fig. 1A) or Coomassie blue-stained gels (Fig. 2A), this variant did express a small number of pili by TEM. This analysis showed that the pili expressed on these FA1090 colony variants were detectable on different proportions of the cells in each culture and also that pilin variation can cause changes in the extent of pilus aggregation.
FIG. 3.
Transmission electron micrographs of FA1090 colony variants. Representative examples of P+ cells are shown, but a combination of P+ and P− cells was observed (Table 1). (A) Class V variant RM5 recA6; (B) class IV variant RM0 recA6; (C) class III variant RM11.1 recA6; (D) class I variant RM11.2 recA6; (E) class II variant RM11.9 recA6. Arrowheads in panels A and C mark pili. The bar in panel A represents 1 μm in panels A and C; the bar in panel B represents 1 μm in panels B, D, and E.
Sequence analysis of the pilE gene of FA1090 colony variants.
To determine the predicted primary amino acid sequences of the colony variants and whether the primary sequence correlated with pilin, S-pilin, or pilus formation, we sequenced the pilE gene of each variant. The predicted amino acid sequences were then aligned and compared with all pilS sequences of FA1090 (52) (Fig. 4). Four of the five P+ colony variants (RM0.1 recA6, RM11.1 recA6, RM11.6 recA6, and RM11.9 recA6) contained hypervariable loop (HVL) (or mc2) sequences (18) from pilS2 copy 1. Three of these variants also expressed pilS2 copy 1 sequences in the semivariable (SV) region. The fifth P+ colony variant (RM11.2 recA6) retained the parental HVL sequences but instead acquired new sequences from pilS1 copy 5 in the 5′ portion of the SV region. The P+/− colony variants all contained pilS6 copy 1 HVL sequences except for RM21 recA6, which expressed pilS1 copy 1 HVL sequences. This analysis shows that a change in colony morphology usually correlates with sequence changes in the HVL region and occasionally with changes in the 5′ portion of the SV region of pilE. However, we could not correlate specific sequences or pilS copies with the levels of full-length pilin or S-pilin production.
Functional analysis of FA1090 colony variants.
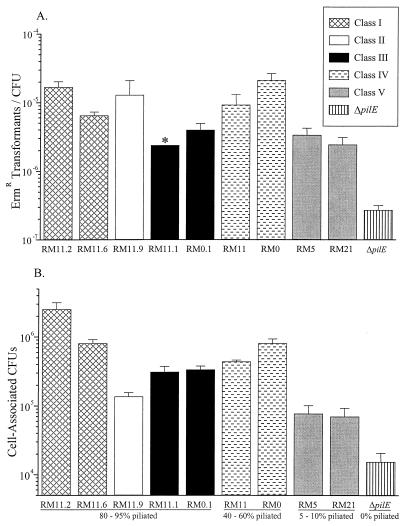
We determined whether pilus variation would affect the pilus-dependent functions of DNA transformation and epithelial cell adherence. Previous studies have shown that P+ colony variants are fully competent for DNA transformation, while P− colony variants, such as ΔpilE variants (48), are about 1,000-fold reduced in competence (53). In addition, some S-pilin-producing variants have been shown to be about 10-fold reduced in transformation competence (13). Therefore, the transformation competence of each variant was determined. Variants were transformed with cloned gonococcal DNA that confers Ermr (31). The transformation efficiencies ranged from <2 × 10−7 for the ΔpilE control to about 10−5 for RM11.2 recA6 (Fig. 5A). Although the variants exhibited different transformation efficiencies, the spread of the data did not allow for the variants to be classified into statistically different levels of competence. Both RM5 recA6 and RM21 recA6, which rarely expressed pili, were approximately 10-fold higher in efficiency than the ΔpilE control and about 10-fold lower than RM11.2 recA6. Interestingly, RM11 recA6 and RM0 recA6, which express detectable pili on only 40 to 60% of the cells, exhibited transformation frequencies similar to those of the class I and II variants, which express detectable pili on 80 to 95% of the cells. The transformation frequencies of class III variants RM11.1 recA6 and RM0.1 recA6 were lower than those of the class I and II variants, suggesting that the number of pili expressed per cell may influence competence. These data support the finding that pilus expression is important for full transformation competence (48, 53). However, these data also suggest that other factors such as the level of S-pilin production, pilin glycosylation, pilus expression, and pilus aggregation may influence transformation efficiency.
FIG. 5.
DNA transformation efficiency and epithelial cell adherence of FA1090 colony variants. All P+ and P+/− variants contained the recA6 allele. Values are mean ± standard error. Variants are grouped by piliation class as defined by TEM (Fig. 3). See also Table 1. (A) Average number of gonococci transformed to Ermr/total CFU. Three to nine experiments were performed per variant. ∗, the transformation frequency could not be accurately determined due to consistently low CFU, but is close to the value shown. (B) Average cell-associated N. gonorrhoeae CFU/well of subconfluent Chang conjunctival epithelial cell monolayers. Three to seven experiments were performed per variant.
In addition to DNA transformation, N. gonorrhoeae pili also enhance adherence to epithelial cells in tissue culture (4, 40, 66) and in organ culture (10). We therefore determined how well the different colony variants adhered to cultured Chang conjunctival epithelial cells. The P+/− colony variants RM5 recA6 and RM21 recA6 (class V) exhibited significantly greater adherence to Chang cells than did the ΔpilE control (Fig. 5B). Interestingly, the adherence levels of P+/− colony variants RM5 recA6 and RM21 recA6 and the P+ variant RM11.9 recA6 (class II) were not significantly different. P+/− variant RM11 recA6 (class IV) and P+ colony variants RM0.1 recA6 and RM11.1 recA6 (class III) all adhered to Chang cells at indistinguishable, intermediate levels, and RM0 recA6 (class IV) and RM11.6 recA6 (class I) adhered to Chang cells at a significantly greater level. Finally, RM11.2 recA6 (class I) adhered significantly better to the Chang cells than any of the other variants. These data show that there is no strict correlation between adherence to Chang cells and pilin protein sequence, full-length pilin or S-pilin expression levels, level of pilus expression or aggregation, or colony morphology. It is possible that several of these variables may in combination affect how gonococci adhere to epithelial cells, but further studies are necessary to determine the influence of each on pilus-mediated adherence.
Analysis of colony variation in RM11.2 recA6.
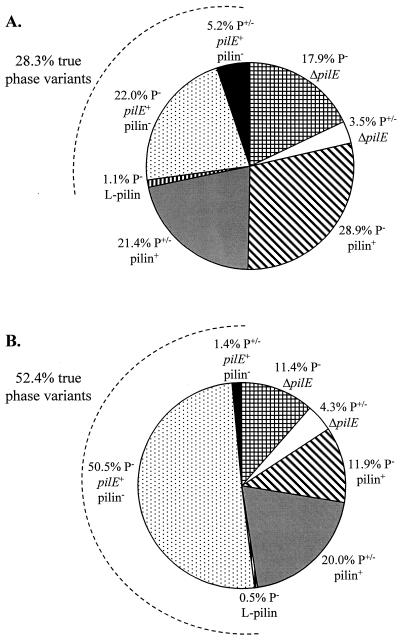
A change in gonococcal colony morphology from P+ to a P+/− or P− morphology can be caused by a variety of mechanisms, including deletion of pilE (34, 45), transfer of pilS sequences into pilE by antigenic variation (15, 60), and phase-variable expression of the pilus assembly protein PilC (21). Our previous analysis of FA1090 colony variants did not measure the relative contribution of each of these mechanisms to colony variation. Therefore, we used the defined P+ variant RM11.2 recA6 to analyze a large number of colonies with a changed colony morphology which were generated upon IPTG induction of a recA6 strain. Variant colonies were categorized as either P− or P+/−. The frequency at which these variant colonies arose, the size of the pilE gene (normal, deleted, or larger), and the absence or presence of pilin protein were determined. After 18 h of IPTG induction, 5.4% of total colonies exhibited a P− or P+/− morphology. The percentage of colony variants increased to 12.7% after 24 h of IPTG induction. Although the percentage of colony variants more than doubled between 18 and 24 h of IPTG induction, the percentage of P− (∼75%) versus P+/− (∼25%) colonies remained very similar (Fig. 6). As expected, pilE was deleted in some P− colonies, but a small percentage of P+/− colonies also harbored pilE deletions. However, the majority of P− and P+/− colonies had an intact pilE gene as determined by PCR analysis. Nearly all of the P+/− colonies also expressed pilin, which is not surprising since a P+/− colony morphology is thought to be conferred by pilus expression, albeit a reduced expression. A substantial number of P− colonies and a few P+/− colonies had an intact pilE but did not express pilin. These colony variants most likely harbored a pilE with a missense or nonsense mutation, resulting in a truncated pilin protein that could not be assembled into pili. Finally, a total of three L-pilin variants were detected among the P− colony variants, showing that this is not a phenomenon unique to strain MS11. This analysis demonstrates that colony morphology is not an accurate gauge of true pilus phase variation of gonococci. These data also indicate that colony morphology cannot be used as a reliable indicator of the absence or presence of pilin protein production or the pilE gene.
FIG. 6.
Phenotypes of RM11.2 recA6 colony variants. To generate colony variants, RM11.2 recA6 was induced with 1 mM IPTG on plates for either 18 (A) or 24 (B) h. Colonies with a morphology less P+ than RM11.2 recA6 were categorized as either flat and without a distinct edge (P−), or intermediate, lacking the dark ring found at the edge of most P+ colonies but more domed than P− colonies (P+/−). The presence (pilE+), absence (ΔpilE), or larger size (L-pilin) of the pilE gene was determined by PCR analysis of colony lysates from 173 colony variants at 18 h and 210 colony variants at 24 h. The presence (pilin+) or absence (pilin−) of pilin protein was determined by Western analysis of a subset of colonies from each time point (n = 32 at 18 h; n = 50 at 24 h). The percentage of colony variants representing true pilus phase variants is indicated by an arc (see Discussion).
DISCUSSION
The original aim of this study was to collect and characterize a variety of in vitro-generated pilus-dependent colony phase variants of strain FA1090 for comparison to variants obtained from human volunteer infections (49). Upon analysis of these apparent “phase” variants, we determined that most of them both expressed pili and released S-pilin and that they represented a spectrum of pilus expression levels. This spectrum of pilus expression levels is similar to the initial findings reported by Haas and others for strain MS11 (16). Phase variation is classically defined as a high frequency and reversible change between two phases of expression. However, a majority of N. gonorrhoeae colony phase variants do not represent one phase of pilus phase variation. Colony phase variants with a deleted pilE (32, 34, 45) are not reversible at high frequency (3, 59) and are therefore not authentic phase variants. Nor can we define S-pilin variants as pilus phase variants, since many highly piliated variants express S-pilin, and P+/− colony phase variants with reduced pilin expression do express a few pili. The few remaining pilus phase variant candidates include the L-pilin variants, which arise at a lower frequency than P+/− and other P− colony variants. L-pilin variants exhibit a P− colony morphology, do not express detectable pili (16), and switch to a P+ state at high frequency (13, 16). The only other true phase variants are those which have an intact pilE but express no pilin protein. The majority of these variants exhibit a P− colony morphology, and like the L-pilins, these variants revert to a P+ state at a high frequency (59). Therefore, we conclude that although gonococci undergo colony morphology changes that can indicate changes in pilus expression, true pilus phase variation occurs only in a subset of colony variants (Fig. 6). A change in colony morphology is not necessarily indicative of true pilus phase variation in the gonococcus.
From this study we can also conclude that colony morphology is not a consistent indicator of pilus expression or pilus function and that many different molecular changes can result in colony morphology variation. Colony variants of FA1090 P+ variant RM11.2 recA6 demonstrated that neither the expression of pilin protein nor the absence or presence of an intact pilE gene correlated absolutely with colony morphology. Approximately 75% of the colony variants exhibited a flat or P− morphology. The remainder of the colony variants were P+/−. As expected, most P+/− colony variants had an intact pilE and expressed pilin and S-pilin (data not shown). However, the pilE gene was deleted in some of the P+/− colonies, and others had an intact pilE but did not express detectable pilin protein. A similar phenomenon was observed by Wainwright et al. (68), who found a P− colony variant which had the same pilE sequence and similar levels of pilin and PilC expression compared to its P+ parent. We do not know the molecular basis for these colony variants which do not accurately reflect pilus expression levels, but we assume that they are influenced by other factors.
A wide range of piliation phenotypes, defined by the level of pilus expression, the extent of pilus aggregation, and the percentage of cells expressing pili, were seen among the nine colony variants studied in detail. There were differences in the percentage of cells expressing pili between the P+ and the P+/− variants, and the number of pili per cell and the level of pilus aggregation differed among all the colony variants (Fig. 3; Table 1). A change in the pilin amino acid sequence was found when the colony piliation phenotype changed. However, we found no strict correlation between piliation class and a specific pilin amino acid sequence, its predicted isoelectric point (data not shown), or level of full-length pilin or S-pilin expression. Interestingly, sequence analysis showed that four of the five P+ colony variants isolated from two different P+/− colony variants had variable lengths of pilS2 copy 1. Also, three of four P+/− colony variants carried FA1090 pilS6 copy 1 sequences. This correlation of certain pilS copies with colony morphology is similar to findings for MS11 which have shown P+ colony variants carrying MS11 pilS1 copy 2 sequences (3, 32, 44, 63) and P− colony variants carrying MS11 pilS1 copy 5 sequences (3, 18, 60, 63). It is probable that a portion of these silent sequences confer a conformational change to pilin that influences pilus expression levels or pilus aggregation and therefore leads to either a P+, P+/−, or P− colony morphology. Our analysis suggests that changes in the HVL region or the amino-terminal portion of the SV region may be important in influencing pilin conformation. We do not know from this limited study whether the particular silent copies that affect pilus expression are influenced by the particular starting sequences present in pilE. Our speculation is that each combination of HVL and N-terminal SV sequences combines to influence pilin conformation and pilus expression. Alternatively, changes in posttranslational modification sites, such as the loss of serine 63 (39, 55) or serine 93 (54), may also play a role in this process.
TEM also demonstrated that individual cells within a population of a given variant had different levels of pilus expression. In the class IV variants, approximately 40 to 60% of the gonococci observed expressed pili, and in class V variants only 5 to 10% of the cells expressed detectable pili. Because these populations are phenotypically RecA−, this is not due to a subpopulation of different pilin variants in the population. We do not know the molecular basis for this partial expression but can offer several possible explanations. Studies with an IPTG-regulatable pilE have shown that when limited pilin is expressed, only a minor portion of the cells express one or two pili (27). This observation has led to the hypothesis that a threshold of full-length pilin must accumulate at a site of assembly prior to polymerization of pilin into a pilus. Both variants of class V, RM5 recA6 and RM21 recA6, also have relatively low levels of full-length pilin expression. Pili on these variants could be visualized when gonococci were sedimented onto the TEM grids, but pili could not be detected when the cells were directly lifted from plates. It is likely that the class V variants are expressing too little full-length pilin to assemble pili on all the cells. Alternatively, the pili on these two variants may be fragile, perhaps due to the pilin primary amino acid sequence or because most of the pili are in single unbundled fibers. A third explanation for differential pilus expression is that there are additional, perhaps epigenetic factors that are sensitive to the overall level of pilin protein available and influence whether or not pili are expressed.
Gonococcal pili are required for efficient DNA transformation (48, 53) and adherence to human mucosal tissues (4, 10, 40, 66). Because the variants in this study encompassed a wide range of full-length pilin and S-pilin expression, as well as many different piliation phenotypes, it was important to determine how these different phenotypes affected pilus-dependent functions. One striking result was that very few pili were needed to significantly increase DNA transformation efficiency and adherence to cultured epithelial cells over that of a nonpiliated (ΔpilE) control. The class V variants RM5 recA6 and RM21 recA6 had relatively low levels of pilin and pilus expression, and yet their transformation efficiencies were about 10 times higher than that of the ΔpilE control. Similar results have been obtained in studies using gonococcal derivatives with an IPTG-regulated pilE (27, 42). The class V variants RM5 recA6 and RM21 recA6 also exhibited adherence levels which were significantly higher than that of the ΔpilE control. This finding suggests that only a few pili are needed for primary attachment of N. gonorrhoeae to epithelial cells and DNA transformation. Conversely, the highly bundled class II variant RM11.9 recA6 adhered to Chang cells at levels similar to those for the class V variants, despite the fact that RM11.9 recA6 has many more pili per cell and a greater percentage of piliated cells. Because the pili of this variant are bundled into one or two large aggregates per cell, perhaps they can function only as one or two singular pili per cell. This observation supports the hypothesis that the aggregation of pili, rather than the pilin sequence, may be a significant factor in inhibiting the adherence of RM11.9 recA6. The idea that single, unbundled pili are necessary for maximum pilus function is supported by analysis of the class I P+ variant RM11.2 recA6, which exhibited a high level of adherence and DNA transformation competence. An alternate explanation of these data is that pilus assembly machinery is required for the expression of pilus-dependent functions (12, 42, 43, 64) and that this machinery is presented properly on the cell surface only when pili are expressed.
The role of these pilus variations in human infections remains uncertain. Essentially all clinical isolates express a strong piliation phenotype when cultured in vitro (23, 62). It is possible that the pilin variants which express few pili are at a selective disadvantage during infection and represent an inherent defect or a necessary intermediate in this complex antigenic variation system. Conversely, studies have shown that pili are not required for invasion of epithelial cells (28, 51). Underpiliated variants have also been suggested to function during transmission or dissemination of infection, or during chronic infection to avoid the immune response (33). The underpiliated variants may be present and/or function only in the subepithelial space or during disseminated infection and are therefore not found in clinical isolates from the genital tract. However, since variants expressing very few pili are capable of adherence and DNA transformation, other functions, such as the ability to form microcolonies (6), may be the reason that only highly piliated variants are isolated from the mucosal surface. We conclude that the frequent formation of under-piliated variants is likely to have a role in N. gonorrhoeae pathogenesis, but novel methods will be required to detect these variants in vivo.
ACKNOWLEDGMENTS
We thank M. Koomey and M. Blake for MAb 1E8/G8, M. Virji for MAb SM1, J. Pfeifer and S. Normark for the PilC antisera, and J. Cannon for the FA1090 ΔpilE strain and the anti-Opa MAbs. Finally, we thank Leslie Blount, Joe Dillard, Becky Howell-Adams, Ian Mehr, and Carla Serkin for critical reading of the manuscript.
This work was supported by PHS grant U01 AI31494. C.D.L. was supported by PHS grant T32 GM08061-14.
REFERENCES
- 1.Arminjon F, Cadoz M, Morse S A, Rock J P, Sarafian S K. Abstracts of the 87th Annual Meeting of the American Society for Microbiology 1987. Washington, D.C: American Society for Microbiology; 1987. Bactericidal and opsonic activities of sera from individuals immunized with a gonococcal protein I vaccine, abstr. E-92; p. 118. [Google Scholar]
- 2.Barritt D S, Schwalbe R S, Klapper D G, Cannon J G. Antigenic and structural differences among six proteins II expressed by a single strain of Neisseria gonorrhoeae. Infect Immun. 1987;55:2026–2031. doi: 10.1128/iai.55.9.2026-2031.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergström S, Robbins K, Koomey J M, Swanson J. Piliation control mechanisms in Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1986;83:3890–3894. doi: 10.1073/pnas.83.11.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bessen D, Gotschlich E C. Interactions of gonococci with HeLa cells: attachment, detachment, replication, penetration, and the role of protein II. Infect Immun. 1986;54:154–160. doi: 10.1128/iai.54.1.154-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bollag D M, Edelstein S J. Protein methods. New York, N.Y: John Wiley & Sons, Inc.; 1991. [Google Scholar]
- 6.Chiang S L, Taylor R K, Koomey M, Mekalanos J J. Single amino acid substitutions in the N-terminus of Vibrio cholerae TcpA affect colonization, autoagglutination, and serum resistance. Mol Microbiol. 1995;17:1133–1142. doi: 10.1111/j.1365-2958.1995.mmi_17061133.x. [DOI] [PubMed] [Google Scholar]
- 7.Connell T D, Shaffer D, Cannon J G. Characterization of the repertoire of hypervariable regions in the Protein II (opa) gene family of Neisseria gonorrhoeae. Mol Microbiol. 1990;4:439–449. doi: 10.1111/j.1365-2958.1990.tb00610.x. [DOI] [PubMed] [Google Scholar]
- 8.Dempsey J A, Litaker W, Madhure A, Snodgrass T L, Cannon J G. Physical map of the chromosome of Neisseria gonorrhoeae FA1090 with locations of genetic markers, including opa and pil genes. J Bacteriol. 1991;173:5476–5486. doi: 10.1128/jb.173.17.5476-5486.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drake S L, Koomey M. The product of the pilQ gene is essential for the biogenesis of type IV pili in Neisseria gonorrhoeae. Mol Microbiol. 1995;18:975–986. doi: 10.1111/j.1365-2958.1995.18050975.x. [DOI] [PubMed] [Google Scholar]
- 10.Draper D L, Donegan E A, James J F, Sweet R L, Brooks G F. Scanning electron microscopy of attachment of Neisseria gonorrhoeae colony phenotypes to surfaces of human genital epithelia. Am J Obstet Gynecol. 1980;138:818–826. doi: 10.1016/s0002-9378(16)32743-0. [DOI] [PubMed] [Google Scholar]
- 11.Edwards M, McDade R L, Schoolnik G, Rothbard J B, Gotschlich E C. Antigenic analysis of gonococcal pili using monoclonal antibodies. J Exp Med. 1984;160:1782–1791. doi: 10.1084/jem.160.6.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freitag N E, Seifert H S, Koomey M. Characterization of the pilF-pilD pilus-assembly locus of Neisseria gonorrhoeae. Mol Microbiol. 1995;16:575–586. doi: 10.1111/j.1365-2958.1995.tb02420.x. [DOI] [PubMed] [Google Scholar]
- 13.Gibbs C P, Reimann B Y, Schultz E, Kaufmann A, Haas R, Meyer T F. Reassortment of pilin genes in Neisseria gonorrhoeae occurs by two distinct mechanisms. Nature. 1989;338:651–652. doi: 10.1038/338651a0. [DOI] [PubMed] [Google Scholar]
- 14.Goodman S D, Scocca J J. Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1988;85:6982–6986. doi: 10.1073/pnas.85.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haas R, Meyer T F. The repertoire of silent pilus genes in Neisseria gonorrhoeae: evidence for gene conversion. Cell. 1986;44:107–115. doi: 10.1016/0092-8674(86)90489-7. [DOI] [PubMed] [Google Scholar]
- 16.Haas R, Schwarz H, Meyer T F. Release of soluble pilin antigen coupled with gene conversion in Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1987;84:9079–9083. doi: 10.1073/pnas.84.24.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagblöm P, Segal E, Billyard E, So M. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature. 1985;315:156–158. doi: 10.1038/315156a0. [DOI] [PubMed] [Google Scholar]
- 18.Howell-Adams B, Wainwright L A, Seifert H S. The size and position of heterologous insertions in a silent locus differentially affect pilin recombination in Neisseria gonorrhoeae. Mol Microbiol. 1996;22:509–522. doi: 10.1046/j.1365-2958.1996.00128.x. [DOI] [PubMed] [Google Scholar]
- 19.Jerse A E, Cohen M S, Drown P M, Whicker L G, Isbey S F, Seifert H S, Cannon J G. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J Exp Med. 1994;179:911–920. doi: 10.1084/jem.179.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonsson A B, Ilver D, Falk P, Pepose J, Normark S. Sequence changes in the pilus subunit lead to tropism variation of Neisseria gonorrhoeae to human tissue. Mol Microbiol. 1994;13:403–416. doi: 10.1111/j.1365-2958.1994.tb00435.x. [DOI] [PubMed] [Google Scholar]
- 21.Jonsson A B, Nyberg G, Normark S. Phase variation of gonococcal pili by frameshift mutation in pilC, a novel gene for pilus assembly. EMBO J. 1991;10:477–488. doi: 10.1002/j.1460-2075.1991.tb07970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonsson A B, Pfeifer J, Normark S. Neisseria gonorrhoeae PilC expression provides a selective mechanism for structural diversity of pili. Proc Natl Acad Sci USA. 1992;89:3204–3208. doi: 10.1073/pnas.89.8.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellogg D S, Jr, Cohen I R, Norins L C, Schroeter A L, Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968;96:596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kellogg D S, Jr, Peacock W L, Deacon W E, Brown L, Pirkle C I. Neisseria gonorrhoeae. I. Virulence genetically linked to colonial variation. J Bacteriol. 1963;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koomey M, Gotschlich E C, Robbins K, Bergström S, Swanson J. Effects of recA mutations on pilus antigenic variation and phase transitions in Neisseria gonorrhoeae. Genetics. 1987;117:391–398. doi: 10.1093/genetics/117.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambden P R, Heckels J E, McBride H, Watt P J. The identification and isolation of novel pilus types produced by variants of N. gonorrhoeae P9 following selection in vivo. FEMS Microbiol Lett. 1981;10:339–341. [Google Scholar]
- 27.Long, C. D., H. A. Harvey, M. A. Apicella, and H. S. Seifert. Submitted for publication.
- 28.Makino S, van Putten J P, Meyer T F. Phase variation of the opacity outer membrane protein controls invasion by Neisseria gonorrhoeae into human epithelial cells. EMBO J. 1991;10:1307–1315. doi: 10.1002/j.1460-2075.1991.tb07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGee Z A, Gross J, Dourmashkin R R, Taylor-Robinson D. Nonpilar surface appendages of colony type 1 and colony type 4 gonococci. Infect Immun. 1976;14:266–270. doi: 10.1128/iai.14.1.266-270.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGrew R E. Encyclopedia of medical history. New York, N.Y: McGraw-Hill Book Co.; 1985. Gonorrhea; pp. 115–116. [Google Scholar]
- 31.Mehr I J, Seifert H S. Random shuttle mutagenesis: gonococcal mutants deficient in pilin antigenic variation. Mol Microbiol. 1997;23:1121–1131. doi: 10.1046/j.1365-2958.1997.2971660.x. [DOI] [PubMed] [Google Scholar]
- 32.Meyer T F, Billyard E, Haas R, Storzbach S, So M. Pilus genes of Neisseria gonorrhoeae: chromosomal organization and DNA sequence. Proc Natl Acad Sci USA. 1984;81:6110–6114. doi: 10.1073/pnas.81.19.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer T F, Gibbs C P, Haas R. Variation and control of protein expression in Neisseria. Annu Rev Microbiol. 1990;44:451–477. doi: 10.1146/annurev.mi.44.100190.002315. [DOI] [PubMed] [Google Scholar]
- 34.Meyer T F, Mlawer N, So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982;30:45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- 35.Morse S A, Bartenstein L. Factors affecting autolysis of Neisseria gonorrhoeae. Proc Soc Exp Biol Med. 1974;145:1418–1421. doi: 10.3181/00379727-145-38025. [DOI] [PubMed] [Google Scholar]
- 36.Murphy G L, Connell T D, Barritt D S, Koomey M, Cannon J G. Phase variation of gonococcal protein II: regulation of gene expression by slipped-strand mispairing of a repetitive DNA sequence. Cell. 1989;56:539–547. doi: 10.1016/0092-8674(89)90577-1. [DOI] [PubMed] [Google Scholar]
- 37.Nassif X, Beretti J L, Lowy J, Stenberg P, O’Gaora P, Pfeifer J, Normark S, So M. Roles of pilin and PilC in adhesion of Neisseria meningitidis to human epithelial and endothelial cells. Proc Natl Acad Sci USA. 1994;91:3769–3773. doi: 10.1073/pnas.91.9.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicolson I J, Perry A C, Virji M, Heckels J E, Saunders J R. Localization of antibody-binding sites by sequence analysis of cloned pilin genes from Neisseria gonorrhoeae. J Gen Microbiol. 1987;133:825–833. doi: 10.1099/00221287-133-4-825. [DOI] [PubMed] [Google Scholar]
- 39.Parge H E, Forest K T, Hickey M J, Christensen D A, Getzoff E D, Tainer J A. Structure of the fibre-forming protein pilin at 2.6 Å resolution. Nature. 1995;378:32–38. doi: 10.1038/378032a0. [DOI] [PubMed] [Google Scholar]
- 40.Punsalang A P, Jr, Sawyer W D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973;8:255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roe, B. A., S. P. Lin, L. Song, X. Yuan, S. Clifton, and D. W. Dyer. 1997. Gonococcal genome sequencing project. Web site URL: http://www .genome.ou.edu/gono.html
- 42.Rudel T, Facius D, Barten R, Scheuerpflug I, Nonnenmacher E, Meyer T F. Role of pili and the phase-variable PilC protein in natural competence for transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1995;92:7986–7990. doi: 10.1073/pnas.92.17.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rudel T, van Putten J P, Gibbs C P, Haas R, Meyer T F. Interaction of two variable proteins (PilE and PilC) required for pilus-mediated adherence of Neisseria gonorrhoeae to human epithelial cells. Mol Microbiol. 1992;6:3439–3450. doi: 10.1111/j.1365-2958.1992.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 44.Schoolnik G K, Fernandez R, Tai J Y, Rothbard J, Gotschlich E C. Gonococcal pili. Primary structure and receptor binding domain. J Exp Med. 1984;159:1351–1370. doi: 10.1084/jem.159.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Segal E, Billyard E, So M, Storzbach S, Meyer T F. Role of chromosomal rearrangement in N. gonorrhoeae pilus phase variation. Cell. 1985;40:293–300. doi: 10.1016/0092-8674(85)90143-6. [DOI] [PubMed] [Google Scholar]
- 46.Segal E, Hagblöm P, Seifert H S, So M. Antigenic variation of gonococcal pilus involves assembly of separated silent gene segments. Proc Natl Acad Sci USA. 1986;83:2177–2181. doi: 10.1073/pnas.83.7.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seifert H S. Insertionally inactivated and inducible recA alleles for use in Neisseria. Gene. 1997;188:215–220. doi: 10.1016/s0378-1119(96)00810-4. [DOI] [PubMed] [Google Scholar]
- 48.Seifert H S, Ajioka R S, Paruchuri D, Heffron F, So M. Shuttle mutagenesis of Neisseria gonorrhoeae: pilin null mutations lower DNA transformation competence. J Bacteriol. 1990;172:40–46. doi: 10.1128/jb.172.1.40-46.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seifert H S, Wright C J, Jerse A E, Cohen M S, Cannon J G. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J Clin Invest. 1994;93:2744–2749. doi: 10.1172/JCI117290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serkin C D, Seifert H S. Frequency of pilin antigenic variation in Neisseria gonorrhoeae. J Bacteriol. 1998;180:1955–1958. doi: 10.1128/jb.180.7.1955-1958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaw J H, Falkow S. Model for invasion of human tissue culture cells by Neisseria gonorrhoeae. Infect Immun. 1988;56:1625–1632. doi: 10.1128/iai.56.6.1625-1632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snodgrass, T. L., and J. G. Cannon. 1997. Personal communication.
- 53.Sparling P F. Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. J Bacteriol. 1966;92:1364–1371. doi: 10.1128/jb.92.5.1364-1371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stimson E, Virji M, Barker S, Panico M, Blench I, Saunders J, Payne G, Moxon E R, Dell A, Morris H R. Discovery of a novel protein modification: alpha-glycerophosphate is a substituent of meningococcal pilin. Biochem J. 1996;316:29–33. doi: 10.1042/bj3160029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stimson E, Virji M, Makepeace K, Dell A, Morris H R, Payne G, Saunders J R, Jennings M P, Barker S, Panico M, Blench I, Moxon E R. Meningococcal pilin: a glycoprotein substituted with digalactosyl 2,4-diacetamido-2,4,6-trideoxyhexose. Mol Microbiol. 1995;17:1201–1214. doi: 10.1111/j.1365-2958.1995.mmi_17061201.x. [DOI] [PubMed] [Google Scholar]
- 56.Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973;137:571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swanson J. Colony opacity and protein II compositions of gonococci. Infect Immun. 1982;37:359–368. doi: 10.1128/iai.37.1.359-368.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swanson J, Barrera O. Gonococcal pilus subunit size heterogeneity correlates with transitions in colony piliation phenotype, not with changes in colony opacity. J Exp Med. 1983;158:1459–1472. doi: 10.1084/jem.158.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swanson J, Bergström S, Barrera O, Robbins K, Corwin D. Pilus-gonococcal variants. Evidence for multiple forms of piliation control. J Exp Med. 1985;162:729–744. doi: 10.1084/jem.162.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swanson J, Bergström S, Robbins K, Barrera O, Corwin D, Koomey J M. Gene conversion involving the pilin structural gene correlates with pilus+ ⇔ pilus− changes in Neisseria gonorrhoeae. Cell. 1986;47:267–276. doi: 10.1016/0092-8674(86)90449-6. [DOI] [PubMed] [Google Scholar]
- 61.Swanson J, Kraus S J, Gotschlich E C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971;134:886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swanson J, Robbins K, Barrera O, Corwin D, Boslego J, Ciak J, Blake M, Koomey J M. Gonococcal pilin variants in experimental gonorrhea. J Exp Med. 1987;165:1344–1357. doi: 10.1084/jem.165.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Swanson J, Robbins K, Barrera O, Koomey J M. Gene conversion variations generate structurally distinct pilin polypeptides in Neisseria gonorrhoeae. J Exp Med. 1987;165:1016–1025. doi: 10.1084/jem.165.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tønjum T, Freitag N E, Namork E, Koomey M. Identification and characterization of pilG, a highly conserved pilus-assembly gene in pathogenic Neisseria. Mol Microbiol. 1995;16:451–464. doi: 10.1111/j.1365-2958.1995.tb02410.x. [DOI] [PubMed] [Google Scholar]
- 65.Tramont E C, Boslego J, Chung R, McChesney D C, Ciak J, Sadoff J, Piziak M, Brinton C, Wood S, Bryan J. Parenteral gonococcal pilus vaccine. In: Schoolnik G K, Brooks G F, Falkow S, Frasch C E, Knapp J S, McCutchan J A, Morse S A, editors. The pathogenic neisseriae. Washington, D.C: American Society for Microbiology; 1985. pp. 316–322. [Google Scholar]
- 66.Virji M, Everson J S. Comparative virulence of opacity variants of Neisseria gonorrhoeae strain P9. Infect Immun. 1981;31:965–970. doi: 10.1128/iai.31.3.965-970.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Virji M, Heckels J E. Antigenic cross-reactivity of Neisseria pili: investigations with type- and species-specific monoclonal antibodies. J Gen Microbiol. 1983;129:2761–2768. doi: 10.1099/00221287-129-9-2761. [DOI] [PubMed] [Google Scholar]
- 68.Wainwright L A, Pritchard K H, Seifert H S. A conserved DNA sequence is required for efficient gonococcal pilin antigenic variation. Mol Microbiol. 1994;13:75–87. doi: 10.1111/j.1365-2958.1994.tb00403.x. [DOI] [PubMed] [Google Scholar]
- 69.World Health Organization. Sexually transmitted diseases fact sheet. Geneva, Switzerland: World Health Organization; 1996. [Google Scholar]
- 70.Wright C J, Jerse A E, Cohen M S, Cannon J G, Seifert H S. Nonrepresentative PCR amplification of variable gene sequences in clinical specimens containing dilute, complex mixtures of microorganisms. J Clin Microbiol. 1994;32:464–468. doi: 10.1128/jcm.32.2.464-468.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]