Abstract
Objectives
Leishmaniosis is a vector-borne disease and in European countries is caused by Leishmania infantum. Cats are considered secondary reservoirs of the infection in endemic areas. The objective of this retrospective study is to describe the clinical findings, diagnosis, treatment and outcome of feline leishmaniosis (FeL) in 16 cats in Spain.
Methods
Medical records of cats diagnosed with leishmaniosis were retrospectively reviewed for cases that met the following inclusion criteria: identification of Leishmania organisms and/or DNA on cytological and/or histological specimens and/or a high anti-Leishmania antibody titre, compatible clinical findings and pathological abnormalities.
Results
Sixteen cats met the inclusion criteria, all of which were living in areas endemic for canine leishmaniosis. Systemic signs were present in 11 cases (68.8%). The most common clinical signs on presentation included cutaneous lesions in 12 cats (75%), ocular disease in six cats (37.5%) and anorexia in six cats (37.5%). A polyclonal gammopathy was noted in 12 cats (85.7%). Non-regenerative anaemia and renal abnormalities were present in six (37.5%) and five patients (31.3%), respectively. In nine cats (56.3%), immunosuppressive conditions/comorbidities were identified. The diagnosis was made in eight of the cats (50%) by cytology, but a combination of diagnostic tests was needed for definitive diagnosis in the remaining patients. Twelve cats (75%) were treated specifically for leishmaniosis. Five of the 12 cats (41.7%) did not improve with treatment. The median survival time in the group of patients treated specifically for leishmaniosis was 17 months. Median survival of patients treated with concomitant diseases was 13 months vs 41 months in those without, although this was not statistically significant (P = 0.557).
Conclusions and relevance
Presentation of FeL appears to be similar to canine leishmaniosis but with some specific features: ulcerative and nodular skin lesions are the predominant cutaneous signs; cats with immunosuppressive conditions or coexisting diseases were more commonly present than typically seen in dogs (mainly feline immunodeficiency virus). A combination of diagnostic tests may be needed for definitive diagnosis.
Keywords: Leishmaniosis, retrospective study, leishmania, case series
Introduction
Leishmaniosis is a zoonotic parasitic disease caused by Leishmania species worldwide. Leishmania infantum is the protozoan responsible for this disease in European countries and it is transmitted by a vector of the genus Phlebotomus.1–3 Dogs are considered the main reservoir host and cats are considered secondary reservoirs of this disease.1,4–8
Since 1977, 53 natural cases of feline leishmaniosis (FeL) have been described worldwide.9–16 Most cases of FeL have been reported in countries in the Mediterranean basin, although it has also been reported in southern USA, Central and South America, Brazil and Iran.4,17–22 Different studies have identified that the prevalence of Leishmania infection in cats in endemic areas varies from 0% to 68.5%.4,23–26
Most infected cats are asymptomatic. Common clinical manifestations of the disease previously reported involve cutaneous and mucocutaneous lesions, with or without visceral signs.4,9–13,24,27–35 Nodules and ulcerations are the most common cutaneous and mucocutaneous findings. 4 Ocular lesions have been described in a third of cases.4,10,12,14,36–40
To the authors’ knowledge, the published literature on FeL includes case reports and a few retrospective case series on clinical signs and skin lesions. The objective of this study was to describe the clinical findings, diagnosis, treatment and outcome in 16 cats with leishmaniosis diagnosed in Spain.
Materials and methods
Cats with a diagnosis of leishmaniosis were reviewed at the Universitat Autònoma de Barcelona Hospital (UAB) between 2000 and 2015. More cases were recruited from private practices over Spain via an electronic survey through the Small Animal Spanish Veterinary Association (AVEPA) feline medicine working group and online forums (dermatology, feline medicine and internal medicine). Sixteen cats were enrolled: six cats were diagnosed at UAB, four cats at Ars Veterinaria Hospital (Barcelona) and the other six cats at different practices in the Barcelona area, Mallorca and Valencia. Inclusion criteria for this study were as follows: identification of Leishmania organisms and/or DNA on cytological and/or histological specimens and/or a high anti-Leishmania species antibody titre, along with compatible clinical findings and pathological abnormalities. Cats with only positive antibody titres and lacking additional clinical details were excluded. Sixteen cases met the criteria for case selection. Data collected included signalment, lifestyle, clinical signs, physical examination findings, clinicopathological abnormalities, diagnostic tests, retroviral status, concurrent diseases and/or immunosuppressive conditions, specific treatment, treatment response, outcome and survival.
Survival data and curves were generated by the Kaplan–Meier method, and survival plots were compared by use of the log-rank test. Kaplan–Meier survival curve construction comparing cats treated with concomitant diseases and those without concomitant disease was performed. For this analysis, any cat that died or was euthanased was classified as dead, and any cat still alive at the time it was lost to follow-up was censored.
Statistical analyses were performed with SPSS Statistics for Macintosh version 25.0 (IBM) and descriptive statistics were used to report baseline data. P values <0.05 were considered to be significant.
Three cases included in this study had been previously published as case reports, one in Veterinary Opthalmology, 37 one in Clínica Veterinaria de Pequeños Animales (AVEPA journal) 9 and the final as a poster at the Southern European Veterinary Conference in 2016. 41
Results
Seven cats were male (all neutered), eight were female (seven neutered and one intact) and in one case sex was not recorded. Fourteen cats were domestic shorthairs and two were Siamese. Age at diagnosis was known for 13 cats; mean age was 7 years (range 3–21 years). Seven cats were outdoor, one was indoor and the lifestyle was not known in the remaining cases.
Systemic signs were present in 11/16 cats (68.8%) (Table 1). Seven of these 11 cats also had cutaneous signs (63.6%).
Table 1.
Clinical abnormalities reported for 16 cats at the time of diagnosis
| Clinical signs and physical examination findings | n (%) |
|---|---|
| Cutaneous lesions | 11 (68.8) |
| Ocular signs | 6 (37.5) |
| Anorexia | 6 (37.5) |
| Weight loss | 5 (31.3) |
| Lethargy | 5 (31.3) |
| Generalised lymphadenopathy | 4 (25.0) |
| Stomatitis | 3 (18.8) |
| Glossitis | 2 (12.5) |
| Fever | 2 (12.5) |
| Icterus | 1 (6.3) |
| Vomiting and diarrhoea | 1 (6.3) |
Cutaneous lesions were present in 12/16 cats (75%) (Figures 1 and 2). Skin disease without systemic signs was seen in 5/16 cases (31.3%). The skin lesions observed included the following: nodules in the facial area and extremities (one cat); nodules solely in the facial area (three cats); ulcerated nodules in the extremities (one cat); ulcerative lesions affecting the paws (two cats); nodule on one footpad (one cat); ulcers in peri-ocular area and pressure points (one cat); single ulcer on the bridge of the nose (one cat); multifocal ulcers over trunk, face and extremities (one cat); exfoliative dermatitis (three cats); and focal ventral alopecia (three cats).
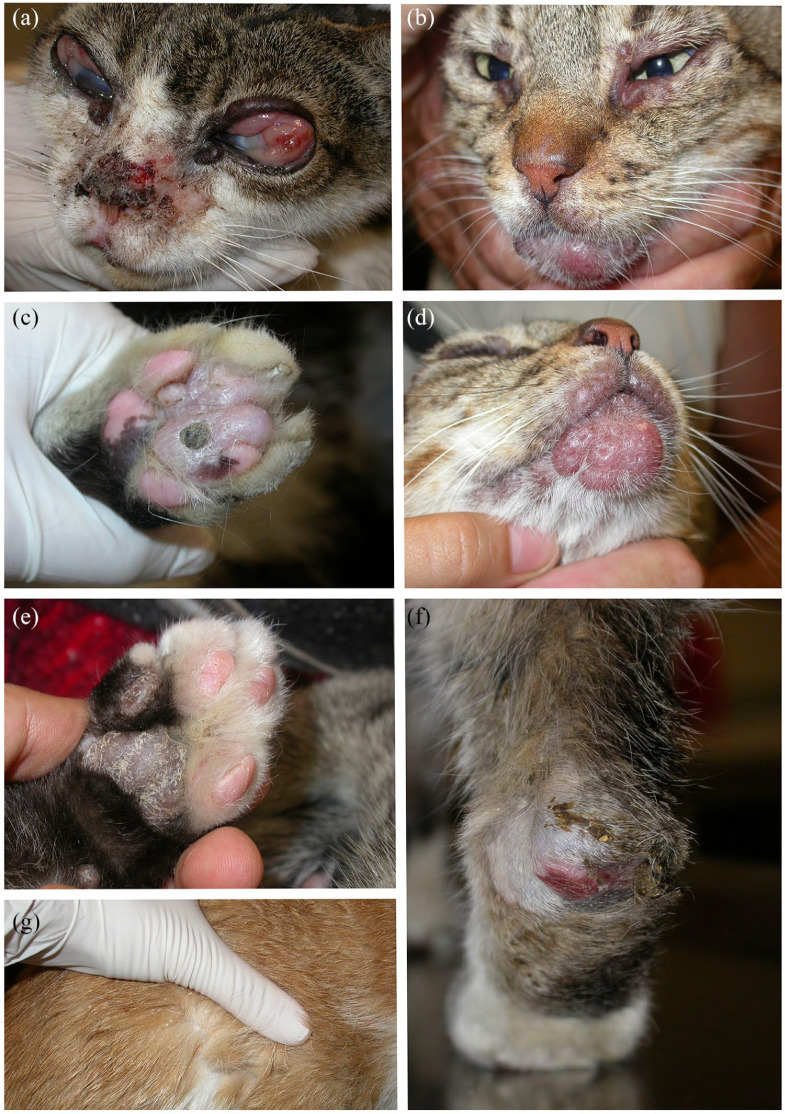
Figure 1.
Skin and ocular lesions in patients with feline leishmaniosis. (a) Patient 7. Papules on the eyelids. Corneal oedema and severe chemosis and proliferative conjunctivitis. Ulcers and crusts on the bridge of the nose and dorsal planum nasale. Focal alopecia. (b) Patient 6. Papules on the eyelids and chin. (c) Patient 8. Nodule with a central crust with an underlying ulcer on a digital footpad. (d) Patient 6. Papules on the dorsal lips and a plaque on the chin. (e) Patient 6. Mild footpad hyperkeratosis. (f) Patient 7. Pressure point ulcer, alopecia and crusts on the hock. (g) Patient 4. Generalised scaling
Figure 2.
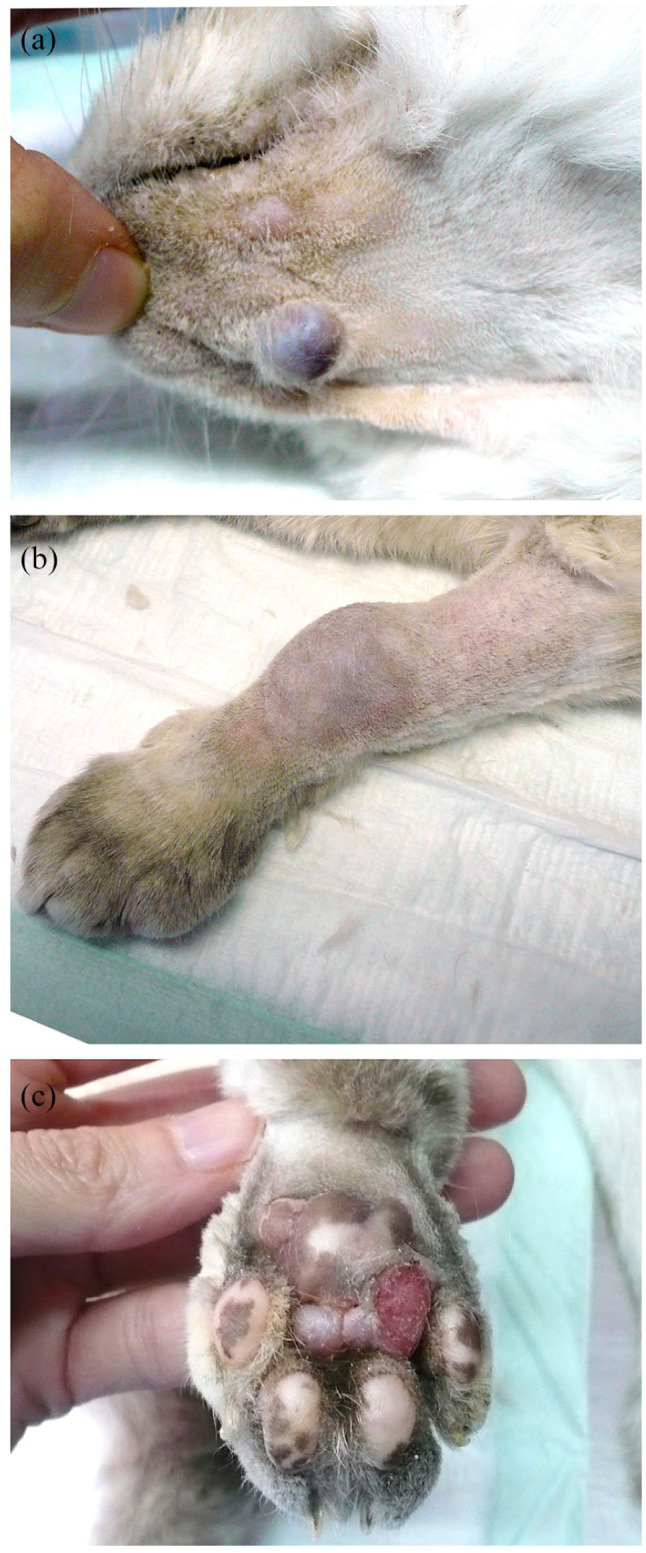
Skin lesions in patient 1: (a) exophytic nodule on the chin; (b) nodule on the left carpal area; and (c) ulcer on the left metatarsal footpad
Ocular disease was present in 6/16 (37.5%) cats. Systemic signs were seen in 4/6 cats with concurrent ocular signs. Corneal oedema and panuveitis were present in three cats, each presenting additional problems, including: melting keratitis and corneal perforation (one cat); chorioretinitis alongside exophthalmus (one cat); and chemosis with proliferative conjunctivitis (one cat). Chemosis, proliferative conjunctivitis and palpebral nodules with no other lesions were present in one cat. Conjunctival and palpebral nodules were seen in another cat, which also had multiple oral nodules and glossitis. Conjunctivitis and uveitis were present in one cat.
Stomatitis was present in 3/16 cats (18.8%), one of them with oral dysphagia. Glossitis was identified in 2/16 cats (12.5%), one cat had both stomatitis and glossitis, and the other cat had multiple oral nodules. These two cats presented oral dysphagia. Two cats (one with stomatitis and one with both glossitis and stomatitis) had been previously treated with long-term glucocorticoids, but the remaining three cats had not received any recent glucocorticoid treatment. Hepatomegaly was present in 2/16 cats (12.5%), splenomegaly in 1/16 cats (6.3%) and renomegaly in 1/16 cats (6.3%). One cat presented with neurological signs suspected to be secondary to diffuse central nervous system disease.
Complete blood count and serum biochemistry were available for all cats (Table 2). No abnormalities were found in 4/16 (25%) cats. Platelet counts were normal in all cases. Polyclonal gammopathy was present in 12/14 cats.
Table 2.
Clinicopathological abnormalities in the 16 cats infected with Leishmania species
| Laboratory abnormalities | n (%) |
|---|---|
| Polyclonal gammopathy* | 12/14 (85.7) |
| Non-regenerative anaemia (normocytic normochromic) | 6/16 (37.5) |
| Proteinuria † | 4/16 (25.0) |
| Azotaemia (increased creatinine) | 3/16 (18.8) |
| Alpha-2 globulin elevations | 3/16 (18.8) |
| Hyperproteinaemia | 2/16 (12.5) |
| Bilirubinaemia | 2/16 (12.5) |
| Neutrophilic leukocytosis | 2/16 (12.5) |
| Hypophosphataemia | 1/16 (6.3) |
| Hyperphosphataemia | 1/16 (6.3) |
| Hypoalbuminaemia | 1/16 (6.3) |
| Hyperglycaemia | 1/16 (6.3) |
| Neutropenia | 1/16 (6.3) |
| Increased alanine aminotransferase | 1/16 (6.3) |
| Creatine kinase elevation | 1/16 (6.3) |
Serum electrophoresis was performed on the serum of 14 cats
Range of proteinuria (urine protein:creatinine ratio 1.8–6)
In 9/16 (56.3%) cats, immunosuppressive conditions or coexisting diseases were identified (Table 3). Feline leukaemia virus (FeLV) and feline immunodeficiency virus (FIV) testing (in-house ELISA to detect antibodies against FIV or FeLV antigen in blood) was conducted in all but two cats. Five of 14 (35.7%) cats were FIV positive; two of them were suspected to be in an advanced state of immunosuppression due to the presence of infectious or opportunistic diseases (Table 3). All cats tested negative for FeLV. Four of 16 cats were receiving high doses and/or long-term glucocorticoids; one for chronic bronchial disease (also FIV positive), which received oral and inhaled glucocorticoids; and three for chronic gingivostomatitis, which were receiving a combination of subcutaneous and oral glucocorticoids (see specific doses in Table 3). One of these three was also FIV positive. One of these cats developed type 2 diabetes mellitus secondary to oral and subcutaneous glucocorticoids. One cat was pregnant at the time of diagnosis and one cat was 21 years old at the time of diagnosis; both conditions may be associated with immunosuppression.
Table 3.
Signalment, concomitant diseases, diagnosis, specific treatment for leishmaniosis and outcomes of this group of patients
| Patient | Signalment (age, sex, neutered status, breed) | Clinical signs | Concomitant disease and/or immunosuppresive drugs | Diagnosis |
Treatment |
Individual survival since diagnosis | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|
| Diagnostic test to achieve diagnosis | Other diagnostic tests performed | Treatment for leishmaniosis | Duration of treatment | ||||||
| 1 | AU, FN, DSH | Nodules in the facial area (conjunctival and palpebral nodules) and extremities Ulcerative lesions in the pawGlossitis |
None | Cytology (skin) | Histopathology (skin) qPCR (blood) | Allopurinol (10 mg/kg PO q12h) | 2 months | 42 months | No response to treatment Euthanasia 42 months after diagnosis (worsening of nodules and glossitis) |
| 2 | 5y, FN, DSH | Lymphadenopathy Lethargy Ulcers in periocular area and pressure points |
None | Histopathology (skin) | qPCR (skin) | None | None | Euthanasia at the time of diagnosis | Euthanasia at the time of diagnosis |
| 3 | AU, SU, DSH | Lethargy Anorexia Single ulcer on the bridge of the nose |
FIV+ | Cytology (spleen) | Histopathology (spleen) PCR (spleen) |
Allopurinol (10 mg/kg PO q12h) Splenectomy |
2 months | 2 months | No response to treatment. Euthanasia 2 months after diagnosis |
| 4 | 15y, FN, Siamese | Conjunctivitis and uveitis Facial exfoliative dermatitis, generalised scaling Weight loss |
Chronic bronchial disease Chronic treatment with oral methylprednisolone (1–2 mg/kg/day) and inhaled fluticasone FIV+ |
Histopathology (skin) | Serology | Allopurinol (10 mg/kg PO q24h) Retreated with meglumine antimoniate (50 mg/kg SC q24h) |
13 months | 13 months | 12 months in remission, then retreated with meglumine antimoniate without response and development of nephrotic syndrome Euthanasia |
| 5 | 4y, MN, DSH | Chronic stomatitis Glossitis, generalised scaling |
Chronic stomatitis Chronic treatment with oral prednisolone (2 mg/kg/24 h) and IFNω FIV+ |
Serology | None | Allopurinol (10 mg/kg PO q24h) + meglumine antimoniate (50 mg/kg SC q24h) | 5 days | 5 days | Death due to kidney disease (development of AKI) |
| 6 | 3y, MN, DSH | Anorexia Nodules on the pinnae margins, periocular skin and chin and mild footpad hyperkeratosis |
None | Cytology (skin and lymph node) | Serology qPCR (blood) |
Allopurinol (10 mg/kg PO q12h) |
4 months | More than 24 months (lost to follow-up) | Clinically healthy after 24 months Resolution of dermatitis |
| 7 | 21y, FN, DSH | Anorexia, chemosis, corneal oedema, panuveitis, proliferative conjunctivitis Multifocal ulcerative dermatitis (trunk, face and extremities), gingival oral mucosa plaque, stomatitis, focal alopecia |
21y at the time of diagnosis | Cytology (palpebral conjunctiva) | Serology qPCR (blood) |
Allopurinol (10 mg/kg PO q12h) + meglumine antimoniate (50 mg/kg SC q24h) | 12 months | More than 12 months (lost to follow-up) | Good clinical response to treatment after 12 months (resolution of clinical signs), then lost to follow-up |
| 8 | AU, MN, DSH | Skin nodule on footpad, ulcer on digital footpad, generalised scaling and focal alopecia | FIV+ | Cytology (lymph node) | Histopathology (skin) serology | Allopurinol (50 mg/cat PO q12h) |
12 months | More than 12 months (lost to follow-up) |
Good clinical response to treatment after 12 months (resolution of clinical sings) Lost to follow-up (moved to Germany) |
| 9 | 8y, FN, DSH | Chronic stomatitis, corneal oedema, panuevitis, melting keratitis, corneal perforation Lethargy, fever |
Chronic stomatitis Chronic treatment with SC methylprednisolone acetate (10 mg/cat) or oral methylprednisolone (1–2 mg/kg/day) Type 2 DM |
Cytology (bone marrow) | Histopathology (eye) PCR (bone marrow) Serology |
Allopurinol (10 mg/kg PO q12h) Enucleation |
6 months | More than 9 months (lost to follow-up) | Good response to treatment Clinically healthy after 9 months |
| 10 | 12y, FN, DSH | Corneal oedema, panuveitis, chorioretinitis, exophthalmus, nodules in facial area, lethargy, weight loss | FIV+ | Serology | qPCR (blood) | Allopurinol (10 mg/kg PO q12h) + miltefosine (2 mg/kg PO q24h) | 1.5 months | 1.5 months | No response to treatment. Euthanasia |
| 11 | 7y, FE, DSH | Chemosis, proliferative conjunctivitis, nodules in facial area |
Pregnant at the time of diagnosis FeLV/FIV status not known* |
Histopathology (skin) | Serology PCR (skin) |
None | None | 24 months | Euthanasia 24 months after diagnosis (worsening of mucocutaneous nodules in mouth, eyelids) |
| 12 | 7y, MN, DSH | Weight loss, anorexia, lethargy, lymphadenopathy | None FeLV/FIV status not known* |
Cytology (liver) | None | None | None | Euthanasia at the time of diagnosis | Euthanasia at the time of diagnosis |
| 13 | 3y, MN, DSH | Ulcerated nodules in extremities | None | Histopathology (skin) | Immunohistochemistry (skin) | Allopurinol (10 mg/kg PO q12h) Nodulectomy |
1.5 months | 18 months | Euthanasia 18 months after diagnosis for recurrence of clinical signs (ulcerated nodules) |
| 14 | 4y, MN, Siamese | Neurological signs (ataxia, circling, head tilt), weakness, weight loss, cachexia, lymphadenopathy Ventral alopecia |
Chronic stomatitis Chronic treatment with SC methylprednisolone acetate (10 mg/cat) or oral methylprednisolone (1–2 mg/kg/day) |
Cytology (lymph node) | Serology | Meglumine antimoniate (300 mg/cat SC q24h) | 4 months | 90 months | Good response to treatment after 4 months (resolution of clinical signs) Developed kidney disease 90 months after treatment |
| 15 | 7y, MN, DSH | Anorexia, vomiting, diarrhoea, fever, icterus, lymphadenopathy, chronic stomatitis | None | qPCR (blood) | Serology | Allopurinol (10 mg/kg PO q12h) + meglumine antimoniate (50 mg/kg SC q24h) | Not known | 47 months | Good response to treatment (resolution of clinical signs) Developed kidney disease 30 months after treatment |
| 16 | 3y, FN, DSH | Weight loss Anorexia |
None | Serology | qPCR (spleen and skin) Histopathology (spleen) |
None | None | Euthanasia at the time of diagnosis | Euthanasia at the time of diagnosis due to AKI and severe clinical signs |
May be clinically relevant
AU = age unknown; FN = female neutered; DSH = domestic shorthair; qPCR = real-time PCR; y = years; SU = sex unknown; FIV = feline immunodeficiency virus; MN = male neutered; IFNω = interferon-omega; AKI = acute kidney injury; SC = subcutaneous; DM = diabetes mellitus; FE = female entire; FeLV = feline leukaemia virus
Diagnosis was obtained in 8/16 (50%) cats by cytology of the following: skin lesions (n = 1), lymph nodes (n = 2), skin lesion and lymph node (n = 1), palpebral conjunctiva (n = 1), bone marrow (n = 1), spleen (n = 1) and liver (n = 1). Histopathology was used to obtain a diagnosis in four cats (skin) and to confirm cytology results in five cats (two skin, two spleen and one eye). Leishmania species antibody titres were performed in 11/16 cats (immunofluorescence or ELISA), with high positive antibody titres in six cats, medium or low positive titres in four cats and negative titres in one cat. Antibody titres were the principal test to obtain a diagnosis in 3/11 cats and were used to confirm a diagnosis in 8/11 cats that had previous histological or cytological detection of Leishmania species. Immunohistochemistry was used in one cat to detect Leishmania species in a skin sample. PCR was performed in 10/16 cats in different tissues (Table 3). In three cases, qualitative PCR was performed and in seven cases a quantitative real-time PCR (TaqMan assay) was performed. PCR results were positive in all cases. Only in one case was blood PCR the test used to obtain the diagnosis (Table 3).
Four of 16 cats died without attempting any specific treatment (three were euthanased at the time of diagnosis and one cat lived for 2 years and was euthanased owing to worsening of cutaneous nodules). The remaining 12 cats were treated specifically for leishmaniosis and three of them had surgical interventions as part of their treatment (Table 3, Figure 3).
Figure 3.
Treatment and outcome of the 16 cats in the study
Five of 12 cats did not improve with the treatment and three of them died or were euthanased during the initial treatment period (Table 3 and Figure 3).
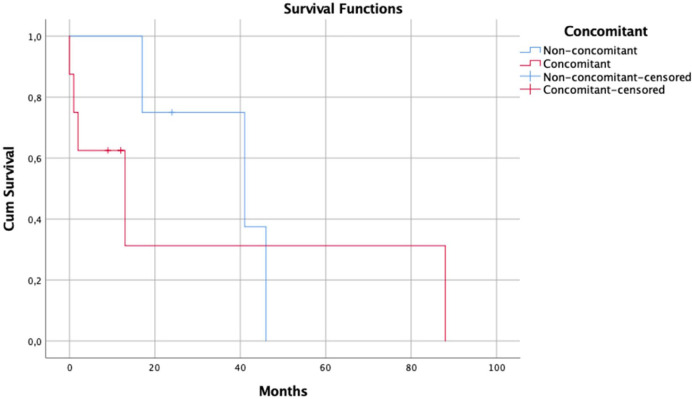
The median survival time in the group of patients treated specifically for leishmaniosis was 17 months. In the treatment group, a comparison of median survival time was made between cats with concomitant diseases or known immunosuppression and those cats without any concomitant diseases or immunosuppression (Figure 4). Median survival of the patients treated with concomitant diseases was 13 months vs 41 months for the treated patients without concomitant diseases, although this was not statistically significant (P = 0.557).
Figure 4.
Kaplan–Meier survival curve for cats treated specifically for leptospirosis, comparing those with concomitant diseases or immunosuppression (red line) and those with no concomitant diseases (blue)
In the group of patients without specific treatment (four cats), only one cat was not euthanased at the time of diagnosis, so median survival time could not be calculated for these patients.
Discussion
The most common clinical signs reported in this group included skin or mucocutaneous lesions in 75% of patients. Of these, 50% of cats also presented with systemic signs (including anorexia, weight loss and lethargy). It is also noteworthy that most of the ocular signs in this study appeared with systemic signs (66.7%). Our results are similar to previously reported cases, in which some cats showed only dermatological lesions,4,26–30 while others demonstrated a combination of skin or ocular lesions with systemic signs.9–13,24,31–35
Based on the combined findings of this study, in conjunction with previously published cases, FeL seems to be characterised predominantly by cutaneous lesions, including cases of nodular, alopecic, scaling and ulcerative dermatitis.24,29,31,33,39,42–47 This study identified that the most common presenting sign was ulcerative dermatitis, followed by nodular dermatitis, exfoliative dermatitis and alopecia. The presentation of leishmaniosis in cats may differ from the canine presentation based on our data and also based on previous literature.24,29,31,33,39,42–49 In canine leishmaniosis, exfoliative dermatitis is the most common presentation followed by ulcerative dermatitis and nodular dermatitis.48,50–73
Each presentation may reflect a different host–parasite relationship.48,56,74 In dogs, susceptibility to infection and disease progression is mediated predominantly by a non-protective T helper 2- and a T helper 1-oriented immune response, which stimulates phagocytosis by macrophages and consequent phagocyte-based parasite intracellular elimination. The association between clinical presentation and immune response has not been fully investigated in feline patients, but species-specific differences in the feline innate and adaptive immune responses might account for the observed lower prevalence of L infantum infection, as well as clinical leishmaniosis, in cats vs dogs. Recently, it has been described that cats from endemic areas are able to activate a cell-mediated adaptive immune response. 75 However, other authors have suggested that the humoral immune response is protective in FeL,1,76 highlighting the potential differences in the immune response between these two species. In some dogs, the simultaneous presence of more than one presentation could be due to other factors, such as skin vulnerability to mechanical trauma and/or to vascular compromise,48,77 and that might be the case in some cats.
In this group of patients, ocular signs were the second most common presentation described, observed in 37.5% of cases. Corneal oedema and panuveitis were the most common reported findings, although chorioretinitis, chemosis, conjunctivitis and melting keratitis were also seen in this population of cats. Ocular manifestations are frequently found in dogs and cats affected by leishmaniosis. Ocular signs occur in 16% to 80% of affected dogs.78,79 Blepharitis, keratoconjunctivitis and anterior uveitis were described as the most frequent signs in canine patients.78,79 Ocular lesions have been reported in approximately one-third of affected cats. 4 In cats, the most common ocular signs observed based on previous case reports were unilateral or bilateral uveitis, with occasionally a pseudotumoral granulomatous pattern and panopthalmitis.12,14,36–38 Blepharitis and conjunctivitis have also been observed in many reports of feline cases.10,39,40 In our case series, results were similar to those published in the literature, with a wide range of different clinical presentations. These results may reflect a considerable variability in the prevalence and type of eye lesions observed in our study, as happens in the canine population. 78
Most of our patients (n = 14/16) presented with cutaneous/mucocutaneous and/or ocular involvement, but a minority (n = 2/16) presented with non-specific clinical signs such as weight loss, anorexia, lethargy and lymphadenopathy. It is also interesting that FeL presentation may range from mild to severe and from acute to chronic.
Information regarding clinicopathological abnormalities seen in FeL is scarce and mainly based on case reports.4,14,49,80 In our case series, a normocytic normochromic non-regenerative anaemia was the most frequent haematological abnormality. Mild-to-severe normocytic normochromic non-regenerative anaemia is also the most frequent haematological abnormality reported in clinical cases.4,49 Hypergammaglobulinaemia was present in 87.5% of the cases in this study; however, it is remarkable that hyperproteinaemia was only present in 12.5% of the cases. Hyperproteinaemia with hypergammaglobulinaemia has also been described in many FeL cases, as also found in canine leishmaniosis.14,49,80 Polyclonal gammopathy occurs in many infectious and inflammatory diseases and is not specific for FeL. Despite this, it may be useful to evaluate the response to treatment or disease status in FeL as in dogs, but this is speculative and has not been evaluated to date. In this case series, the presence of renal disease appears to be similar to dogs. This presentation may be acute or chronic, or it may even appear with the course of the disease.
Proliferative and ulcerative chronic inflammation of the oral mucosa associated with FeL can be included in the list of possible causes of the feline chronic gingivostomatitis syndrome (FCGS). 4 This immune-mediated disease is considered multifactorial and has been associated with infectious and non-infectious agents.81–84 Infectious agents such as FeLV, FIV, feline calicivirus and feline herpesvirus-1, along with a wide variety of bacteria, have been isolated in cats with FCGS. These suspected pathogens can also be present in healthy animals, making it less consistent with a clear causal relationship.81,85–94 It is remarkable that stomatitis has been reported in around a quarter of FeL cases.4,19,48,78,80 In this case series, the prevalence of this syndrome is similar to previous reports. However, owing to the retrospective and multicentric nature of this study, there was no consistent information on the severity of the stomatitis and precise location of the lesions. Leishmaniosis may have been the cause of disease in this group of patients; however, other concomitant diseases could not be ruled out based on our data. Only by histopathological identification of the parasite in oral lesions would it be possible to differentiate between both diseases. Owing to the retrospective nature of this study, this could not be performed.
In previous studies, the clinical disease of FeL has been associated with an impaired immunocompetence due to several factors, including retroviral infections (FIV and FeLV), immunosuppressive treatment and concomitant debilitating diseases such as malignant neoplasia or diabetes mellitus.6,21,25,26,95–101 In our group of patients, possible immunosuppressive conditions (eg, pregnancy, age) or concurrent diseases were identified in 9/16 (56%) cases. In this case series, one-third of the cats were FIV positive, making it the most frequent concomitant disease found. Prevalence rates of FIV and FeLV in the region where these cats live have been previously reported to be 2.6–7.4% and 6.0–8.5%, respectively.102,103 Both FIV and/or FeLV infections have been referred as FeL predisposing factors explained by the ensuing immunosuppression.1,104–106 Supporting studies found a high positivity (~70%) of cats to both leishmaniosis and FIV, 104 and even a statistically significant correlation with FeL and both FIV 99 and FeLV.1,98 However, other studies failed to corroborate this finding.1,6,31,44,101,107–112 The cause–effect relationship between various aetiological and pathogenic factors is not always easy to establish.4,13 Full screening for other pathogens was not performed in all cases, but no other diseases were identified in the study population.
Most diagnostic techniques for Leishmania species infection currently used in cats are the same available for dogs. Diagnosis of FeL is based on serological, cytological, histological or PCR methods. 4 It is remarkable that diagnosis was obtained in 50% of our cases by cytology. This technique represents a rapid, inexpensive and simple procedure to achieve diagnosis in many cases; additionally, it is a highly specific and non-invasive technique. However, histopathology was the diagnostic method in 4/16 cases. In 2/4 of these cases, serology was also performed in order to support histopathology results. This may indicate the importance of serology as a screening test when leishmaniosis is suspected as a differential diagnosis, avoiding more invasive tests in many cases. However, serology may not be enough to reach a diagnosis in negative or low positive cases, 4 and so a combination of diagnostic tests may be needed for definitive diagnosis. Discrepancies can be seen in cats, as occurs in dogs, when serological and molecular tests are used at the same time.4,80,113 The sensitivity and specificity of serological and molecular tests may be influenced by many factors, and this may result in a lack of consistency between tests results.
PCR was performed in 10/16 cases in different tissues and was positive in all of them. PCR may be more sensitive than cytology and histology, but some investigations have shown that animals with increased titres of anti-Leishmania antibodies presented decreased positivity in PCR, whereas the greatest identification of genetic material through PCR occurred more frequently in cats with reduced antibody titres.1,76,105 This suggests that the immune response in cats differs from that observed in dogs, which might explain the high number of asymptomatic infected cats as well as the variable clinical manifestation of the disease. 1
In this case series, median survival time was greater than a year (17 months) in the group that received treatment. However, median survival time could not be calculated in the non-treated patients owing to the small sample size of the group, since all but one of the cats were euthanased at diagnosis. In the five patients that showed no improvement with leishmaniosis treatment, survival time ranged from 5 days to 3.5 years (median 60 days). In the group of patients that showed a clinical response to treatment, survival times ranged from 9 months to 7.5 years (median 407 days). Cats positive for FIV and cats on chronic corticosteroids treatment (considered concomitant immunosuppressive conditions) were present in both groups.
According to the retrospective nature of this study and variability in the treatment of each case, it is difficult to establish the best treatment and an accurate prognosis. In a recent study, 114 prognosis was not influenced by therapy or the retroviral status of the patients.
Treatment of cats with clinical FeL is still not based on scientific evidence, but on clinical experience from published case reports and on the off-label use of the most common drugs prescribed to dogs.4,114–120 This means that the efficacy and safety of these protocols have never been evaluated in controlled studies. Interestingly, median survival time in the group of animals treated specifically for leishmaniosis without concomitant diseases was longer than in the group with concomitant diseases (Figure 4); however, no statistical differences were seen between groups. Owing to the relatively small number of cases in each group a definitive conclusion could not be made with the information available.
The main limitations of this study, as with all retrospective studies, are its variability the management and diagnosis of each case. Extensive clinical information was not available for all cases and there was inconsistency in the follow-up periods and evaluations of the cases, making it more difficult to draw solid conclusions.
Conclusions
The most common clinical signs reported in this study were cutaneous lesions followed by ocular abnormalities, in which some cats showed a combination of skin or ocular lesions with systemic signs. Immunosuppressive conditions or coexisting diseases were identified in more than half of the cases, with FIV coinfection having the greatest prevalence. It should be taken into consideration that FeL clinicopathological abnormalities may be non-specific. Diagnosis in FeL was made by serological, cytological, histological or PCR methods, or a combination of these, but the diagnosis was obtained in 50% of the cases by cytology. Owing to the retrospective nature of this study and variability in the treatment of each case, it is difficult to establish the best treatment and provide an accurate prognosis, although median survival time in the group of animals treated specifically for leishmania without concomitant disease was longer than in the group with concomitant diseases, but not significantly so. The combination of cutaneous lesions and/or ocular lesions with other clinical signs in an endemic area should increase the suspicion of leishmaniosis.
Acknowledgments
We would like to thank Dr Jordi Puig for his help with the statistical analysis.
Footnotes
Accepted: 6 January 2020
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work involved the use of non-experimental animal(s) only (owned or unowned), and followed established internationally recognised high standards (‘best practice’) of individual veterinary clinical patient care. Ethical approval from a committee was therefore not necessarily required.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work for the procedure(s) undertaken. For any animals or humans individually identifiable within this publication, informed consent (either verbal or written) for their use in the publication was obtained from the people involved.
ORCID iD: Ana Fernandez-Gallego  https://orcid.org/0000-0002-8290-7559
https://orcid.org/0000-0002-8290-7559
Mar Bardagí  https://orcid.org/0000-0001-6987-5356
https://orcid.org/0000-0001-6987-5356
References
- 1. Alves Soares CS, Cancela Duarte S, Ramalho Sousa S. What do we know about feline leishmaniosis? J Feline Med Surg 2016; 18: 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Afonso MO, Alves-Pires C. Bioecologia dos vectores. In: Santos-Gomes G, Fonseca IP. (eds). Leishmaniose canina. Lisbon: Merial, 2008, pp 27–39. [Google Scholar]
- 3. Simões-Mattos L, Bevilaqua C, Mattos M, et al. Feline leishmaniosis: uncommon or unknown? Rev Port Ciências Vet 2004; 550: 79–87. [Google Scholar]
- 4. Pennisi MG, Cardoso L, Baneth G, et al. LeishVet update and recommendations on feline leishmaniosis. Parasit Vectors 2015; 8. DOI: 10.1186/s13071-015-0909-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gramiccia M. Recent advances in leishmaniosis in pet animals: epidemiology, diagnostics and anti-vectorial prophylaxis. Vet Parasitol 2011; 181: 23–30. [DOI] [PubMed] [Google Scholar]
- 6. Solano-Gallego L, Rodriguez-Cortes A, Iniesta L, et al. Cross-sectional serosurvey of feline leishmaniosis in ecoregions around the Northwestern Mediterranean. Am J Trop Med Hyg 2007; 76: 676–680. [PubMed] [Google Scholar]
- 7. da Silva AV, de Souza Cândido CD, de Pita Pereira D, et al. The first record of American visceral leishmaniosis in domestic cats from Rio de Janeiro, Brazil. Acta Trop 2008; 105: 92–94. [DOI] [PubMed] [Google Scholar]
- 8. Maroli M, Pennisi MG, Di Muccio T, et al. Infection of sandflies by a cat naturally infected with Leishmania infantum. Vet Parasitol 2007; 145: 357–360. [DOI] [PubMed] [Google Scholar]
- 9. Dalmau A, Ossò M, Oliva A, et al. Leishmaniosis felina a propósito de un caso clínico. ¿Nos olvidamos de que existe? Clin Vet Peq Anim 2008; 28: 233–237. [Google Scholar]
- 10. Navarro JA, Sánchez J, Peñafiel-Verdú C, et al. Histopathological lesions in 15 cats with leishmaniosis. J Comp Pathol 2010; 143: 297–302. [DOI] [PubMed] [Google Scholar]
- 11. Ortuñez A, Gomez P, Verde MT, et al. Lesiones granulomatosas en la mucosa oral y lengua y muliplesnoduloscutaneos en un gato causado por Leishmaniainfantum. Proceedings of the Southern European Veterinary Conference; 2010 Sept 30–Oct 3; Barcelona, Spain. [Google Scholar]
- 12. Sanches A, Pereira AG, Carvalho JP. Um caso de leishmaniose felina. Vet Med 2011; 63: 29–30. [Google Scholar]
- 13. Pennisi MG, Hartmann K, Lloret A, et al. Leishmaniosis in cats: ABCD guidelines on prevention and management. J Feline Med Surg 2013; 15: 638–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richter M, Schaarschmidt-Kiener D, Krudewig C. Ocular signs, diagnosis and long-term treatment with allopurinol in a cat with leishmaniasis. Schweiz Arch Tierheilkd 2014; 156: 289–294. [DOI] [PubMed] [Google Scholar]
- 15. Altuzarra R, Movilla R, Roura X, et al. Computed tomographic features of destructive granulomatous rhinitis with intracranial extension secondary to leishmaniasis in a cat. Vet Radiol Ultrasound. Epub ahead of print 11 July 2018. DOI: 10.1111/vru.12666. [DOI] [PubMed] [Google Scholar]
- 16. Rivas AK, Alcover M, Martinez-Orellana P, et al. Clinical and diagnostic aspects of feline cutaneous leishmaniosis in Venezuela. Parasit Vectors 2018; 11. DOI: 10.1186/s13071-018-2747-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Campos Braga AR, Langoni H, Lucheis SM. Evaluation of canine and feline leishmaniasis by the association of blood culture, immunofluorescent antibody test and polymerase chain reaction. J Venom Anim Toxins Incl Trop Dis 2014; 20. DOI: 10.1186/1678-9199-20-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuhls K, Alam MZ, Cupolillo E, et al. Comparative microsatellite typing of new world Leishmania infantum reveals low heterogeneity among populations and its recent old world origin. PLoS Negl Trop Dis 2011; 5. DOI: 10.1371/journal.pntd.0001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pennisi MG, Persichetti MF. Feline leishmaniosis: is the cat a small dog? Vet Parasitol 2018; 251: 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Can H, Döşkaya M, Özdemir HG, et al. Seroprevalence of Leishmania infection and molecular detection of Leishmania tropica and Leishmania infantum in stray cats of İzmir, Turkey. Exp Parasitol 2016; 167: 109–114. [DOI] [PubMed] [Google Scholar]
- 21. Attipa C, Papasouliotis K, Solano-Gallego L, et al. Prevalence study and risk factor analysis of selected bacterial, protozoal and viral, including vector-borne, pathogens in cats from Cyprus. Parasit Vectors 2017; 10. DOI: 10.1186/s13071-017-2063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Metzdorf IP, da Costa Lima MS, de Fatima Cepa Matos M, et al. Molecular characterization of Leishmania infantum in domestic cats in a region of Brazil endemic for human and canine visceral leishmaniasis. Acta Trop 2017; 166: 121–125. [DOI] [PubMed] [Google Scholar]
- 23. Silaghi C, Knaus M, Rapti D, et al. Survey of Toxoplasma gondii and Neospora caninum, haemotropic mycoplasmas and other arthropod-borne pathogens in cats from Albania. Parasit Vectors 2014; 7. DOI: 10.1186/1756-3305-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poli A, Abramo F, Barsotti P, et al. Feline leishmaniosis due to Leishmania infantum in Italy. Vet Parasitol 2002; 106: 181–191. [DOI] [PubMed] [Google Scholar]
- 25. Pennisi MG, Masucci M, Catarsini O. Presenza di anticorpi anti-Leishmania in gatti FIV+ chevivono in zona endemica. Atti Soc Ital Sci Vet 1998; 52: 265–266. [Google Scholar]
- 26. Pennisi MG, Maxia L, Vitale F, et al. Studio dell’infezione da Leishmania mediante PCR in gatti che vivono in zona endemica. Atti Soc Ital Sci Vet 2000; 54: 215–216. [Google Scholar]
- 27. Grevot A, Jaussaud Hugues P, Marty P, et al. Leishmaniosis due to Leishmania infantum in a FIV and FeLV positive cat with a squamous cell carcinoma diagnosed with histological, serological and isoenzymatic methods. Parasite 2005; 12: 271–275. [DOI] [PubMed] [Google Scholar]
- 28. Laurelle-Magalon C, Toga I. Un cas de leishmaniose féline. Prat Med Chir Anim Comp 1996; 31: 255–261. [Google Scholar]
- 29. Rüfenacht S, Sager H, Müller N, et al. Two cases of feline leishmaniosis in Switzerland. Vet Rec 2005; 156: 542–545. [DOI] [PubMed] [Google Scholar]
- 30. Dunan N, Mary C, Garbe L, et al. A proposd’un cas de leishmaniosechez un chat de la régionmarseillaise. Bull Soc Fr Parasitol 1989; 7: 17–20. [Google Scholar]
- 31. Ozon C, Marty P, Pratlong F, et al. Disseminated feline leishmaniosis due to Leishmania infantum in southern France. Vet Parasitol 1998; 75: 273–277. [DOI] [PubMed] [Google Scholar]
- 32. Pocholle E, Reyes-Gomez E, Giacomo A, et al. Un cas de leishmaniose féline disseminé edans le sud de la France. Parasite 2012; 19: 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hervás J, Chacón-M De, Lara F, Sánchez-Isarria MA, et al. Two cases of feline visceral and cutaneous leishmaniosis in Spain. J Feline Med Surg 1999; 1: 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Costa Durão JFC, Rebelo E, Peleteiro MC, et al. Primeiro caso de leishmanioseem gato doméstico (Felis catus) detectado em Portugal (Concelho de Sesimbra). Nota Preliminar. Rev Port Cienc Vet 1994; 89: 140–144. [Google Scholar]
- 35. Ibba F. Un caso di rinitecronica in corso di leishmaniosi felina. Proceedings of the 62nd International SCIVAC Congress. Rimini: Società Culturale Italiana Veterinari per Animali da Compagnia; 2009 May 29–31; Rimini, Italy. [Google Scholar]
- 36. Hervás J, Chácon-Manrique de, Lara F, López J, et al. Granulomatous (pseudotumoral) iridociclitis associated with leishmaniasis in a cat. Vet Rec 2001; 149: 624–625. [DOI] [PubMed] [Google Scholar]
- 37. Leiva M, Lloret A, Pena T, et al. Therapy of ocular and visceral leishmaniasis in a cat. Vet Ophthalmol 2005; 8: 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Verneuil M. Leishmaniose oculaire féline: à proposd’un cas. J Fr Ophtalmol 2013; 36: e67–e72. [DOI] [PubMed] [Google Scholar]
- 39. Migliazzo A, Vitale F, Calderone S, et al. Feline leishmaniosis: a case with a high parasitic burden. Vet Dermatol 2015; 26: 69–70. [DOI] [PubMed] [Google Scholar]
- 40. Pennisi MG, Lupo T, Migliazzo A, et al. Feline leishmaniosis in Italy: retrospective evaluation of 24 clinical cases. Proceedings of the 5th World Congress on Leishmaniasis; 2013 May 13–17; Porto de Galinhas, Pernambuco, Brazil. [Google Scholar]
- 41. Fernandez-Gallego A, Pertegaz J, Feo L. Fallo renal agudo en un gato con leishmaniosis visceral. Poster presentation at the X SEVC – Southern European Veterinary Congress; 2016 Oct 20–22; Granada, Spain. [Google Scholar]
- 42. Di Mattia D, Fondevila D, Abramo F, et al. A retrospective histopathological, immunohistochemical and molecular study of the presence of Leishmania spp. in the skin of cats with head and neck ulcerative dermatitis. Vet Dermatol 2018; 29. DOI: 10.1111/vde.12535. [DOI] [PubMed] [Google Scholar]
- 43. Vides JP, Schwardt TF, Sobrinho LS, et al. Leishmania chagasi infection in cats with dermatologic lesions from an endemic area of visceral leishmaniosis in Brazil. Vet Parasitol 2011; 178: 22–28. [DOI] [PubMed] [Google Scholar]
- 44. Savani ES, de Oliveira Camargo MC, de Carvalho MR, et al. The first record in the Americas of an autochthonous case of Leishmania (Leishmania) infantum chagasi in a domestic cat (Felix catus) from Cotia County, São Paulo State, Brazil. Vet Parasitol 2004; 120: 229–233. [DOI] [PubMed] [Google Scholar]
- 45. de Souza AI, Silva Barros EM, Ishikawa E, et al. Feline leishmaniasis due to Leishmania (Leishmania) amazoniensis in Mato Grosso do Sul State, Brazil. Vet Parasitol 2005; 128: 41–45. [DOI] [PubMed] [Google Scholar]
- 46. Trainor KE, Porter BF, Logan KS, et al. Eight cases of feline cutaneous leishmaniasis in Texas. Vet Parasitol 2010; 47: 1076–1081. [DOI] [PubMed] [Google Scholar]
- 47. Rougeron V, Catzeflis F, Hide M, et al. First clinical case of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis in a domestic cat from French Guiana. Vet Parasitol 2011; 181: 325–328. [DOI] [PubMed] [Google Scholar]
- 48. Saridomichelakis MN, Koutinas AF. Cutaneous involvement in canine leishmaniosis due to Leishmania infantum (syn. L. chagasi). Vet Dermatol 2014; 25. DOI: 10.1111.vde.1205. [DOI] [PubMed] [Google Scholar]
- 49. Pennisi MG, Venza M, Reale S, et al. Case report of feline leishmaniasis in four cats. Vet Res Comm 2004; 28 Suppl 1: 363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Koutinas AF, Polizopoulou ZS, Saridomichelakis MN, et al. Clinical considerations on canine visceral leishmaniasis (CVL) in Greece: a retrospective study of 158 spontaneous cases. J Am Anim Hosp Assoc 1999; 35: 376–383. [DOI] [PubMed] [Google Scholar]
- 51. Ciaramella P, Oliva G, DeLuna R, et al. A retrospective clinical study of canine leishmaniasis in 150 dogs naturally infected by Leishmania infantum. Vet Rec 1997; 141: 539–543. [DOI] [PubMed] [Google Scholar]
- 52. Slappendel RJ. Canine leishmaniasis. A review based on 95 cases in The Netherlands. Vet Q 1988; 10: 1–16. [DOI] [PubMed] [Google Scholar]
- 53. Kontos VJ, Koutinas AF. Old World canine leishmaniasis. Comp Cont Educ Pract Vet 1993; 15: 949–960. [Google Scholar]
- 54. Koutinas AF, Scott DW, Kontos V, et al. Skin lesions in canine leishmaniasis (Kala-Azar): a clinical and histopathological study on 22 spontaneous cases in Greece. Vet Dermatol 1992; 3: 121–130. [Google Scholar]
- 55. Papadogiannakis EI, Koutinas AF, Saridomichelakis MN, et al. Cellular immunophenotyping of exfoliative dermatitis in canine leishmaniosis (Leishmania infantum). Vet Immunol Immunopathol 2005; 104: 227–237. [DOI] [PubMed] [Google Scholar]
- 56. Ferrer L, Rabanal R, Fondevila D, et al. Skin lesions in canine leishmaniasis. J Small Anim Pract 1988; 29: 381–388. [Google Scholar]
- 57. Denerolle P. Leishmaniose canine: difficultes du diagnostic et du traitement (125 cas). Prat Med Chirurg Anim Comp 1996; 31: 137–145. [Google Scholar]
- 58. Koutinas AF, Saridomichelakis MN, Mylonakis ME, et al. A randomised, blinded, placebo-controlled clinical trial with allopurinol in canine leishmaniosis. Vet Parasitol 2001; 98: 247–261. [DOI] [PubMed] [Google Scholar]
- 59. Koutinas AF, Carlotti DN, Koutinas C, et al. Claw histopathology and parasitic load in natural cases of canine leishmaniosis (Leishmania infantum). Vet Dermatol 2010; 21: 572–577. [DOI] [PubMed] [Google Scholar]
- 60. Saridomichelakis MN, Koutinas AF, Bourdeau P. Questionnaire-based survey of canine leishmaniosis (Leishmania infantum) in Greece. J Hellenic Vet Med Soc 2009; 60: 503–526. [Google Scholar]
- 61. Abranches P, Silva-Pereira MC, Conceicao-Silva FM, et al. Canine leishmaniasis: pathological and ecological factors influencing transmission of infection. J Parasitol 1991; 77: 557–561. [PubMed] [Google Scholar]
- 62. Rallis T, Day MJ, Saridomichelakis MN, et al. Chronic hepatitis associated with canine leishmaniosis (Leishmania infantum): a clinicopathological study of 26 cases. J Comp Pathol 2005; 132: 145–152. [DOI] [PubMed] [Google Scholar]
- 63. de Amorim IF, da Silva SM, Figueiredo MM, et al. Toll receptors type-2 and CR3 expression of canine monocytes and its correlation with immunohistochemistry and xenodiagnosis in visceral leishmaniasis. PLoS One 2011; 6. DOI: 10.1371/journal.pone.0027679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Woerly V, Maynard L, Sanquer A, et al. Clinical efficacy and tolerance of miltefosine in the treatment of canine leishmaniosis. Parasitol Res 2009; 105: 463–469. [DOI] [PubMed] [Google Scholar]
- 65. Koutinas CK. Efficacy and safety of amphotericin B (lipid emulsion) in canine leishmaniosis (Leishmania infantum) and its effect on renal function, along with or without enalapril. Thesis, Aristotles University of Thessaloniki, 2006. [Google Scholar]
- 66. Petanides TA, Koutinas AF, Mylonakis ME, et al. Factors associated with the occurrence of epistaxis in natural canine leishmaniasis (Leishmania infantum). J Vet Intern Med 2008; 22: 866–872. [DOI] [PubMed] [Google Scholar]
- 67. Plevraki K, Koutinas AF, Kaldrymidou H, et al. Effects of allopurinol treatment on the progression of chronic nephritis in canine leishmaniosis (Leishmania infantum). J Vet Intern Med 2006; 20: 228–233. [DOI] [PubMed] [Google Scholar]
- 68. Vamvakidis CD, Koutinas AF, Kanakoudis G, et al. Masticatory and skeletal muscle myositis in canine leishmaniasis (Leishmania infantum). Vet Rec 2000; 146: 698–703. [DOI] [PubMed] [Google Scholar]
- 69. Lima TB, Batista ZS, Chaves DP, et al. Canine visceral leishmaniosis in an endemic area in Sao Luis island: clinical and serological status. Proceedings of the 3rd World Congress on Leishmaniosis; 2005 April 10–15; Palermo-Terrasini, Italy, p 160. [Google Scholar]
- 70. De Freitas JC, Nunes-Pinheiro DC, Lopes Neto BE, et al. Clinical and laboratory alterations in dogs naturally infected by Leishmania chagasi. Rev Soc Bras Med Trop 2012; 45: 24–29. [DOI] [PubMed] [Google Scholar]
- 71. De Freitas JC, Lopes-Neto BE, de Abreu CR, et al. Profile of anti-Leishmania antibodies related to clinical picture in canine visceral leishmaniasis. Res Vet Sci 2012; 93: 705–709. [DOI] [PubMed] [Google Scholar]
- 72. Rougier S, Hasseine L, Delaunay P, et al. One-year clinical and parasitological follow-up of dogs treated with marbofloxacin for canine leishmaniosis. Vet Parasitol 2012; 186: 245–253. [DOI] [PubMed] [Google Scholar]
- 73. Cortada VM, Doval ME, Souza Lima MA, et al. Canine visceral leishmaniosis in Anastacio, Mato Grosso do Sul state, Brazil. Vet Res Commun 2004; 28: 365–374. [DOI] [PubMed] [Google Scholar]
- 74. Ordeix L, Solano-Gallego L, Fondevila D, et al. Papular dermatitis due to Leishmania spp. infection in dogs with parasite specific cellular immune responses. Vet Dermatol 2005; 16: 187–191. [DOI] [PubMed] [Google Scholar]
- 75. Priolo V, Martinez-Orellana P, Pennisi MG, et al. Leishmania infantum-specific IFN-γ production in stimulated blood from cats living in areas where canine leishmaniosis is endemic. Parasit Vectors 2019; 12: 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Martín-Sánchez J, Acedo C, Muñoz-Pérez M, et al. Infection by Leishmania infantum in cats: epidemiological study in Spain. Vet Parasitol 2007; 145: 267–273. [DOI] [PubMed] [Google Scholar]
- 77. Fondevila D, Vilafranca M, Ferrer L. Epidermal immunocompetence in canine leishmaniasis. Vet Immunol Immunopathol 1997; 56: 319–327. [DOI] [PubMed] [Google Scholar]
- 78. Di Pietro S, Bosco VRF, Crinò C, et al. Prevalence, type, and prognosis of ocular lesions in shelter and owned-client dogs naturally infected by Leishmania infantum. Vet World 2016; 9: 633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Peña MT, Roura X, Davidson MG. Ocular and periocular manifestations of leishmaniasis in dogs: 105 cases (1993–1998). Vet Ophthalmol 2000; 3: 35–41. [DOI] [PubMed] [Google Scholar]
- 80. Solano-Gallego L, Koutinas A, Miró G, et al. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet Parasitol 2009; 165: 1–18. [DOI] [PubMed] [Google Scholar]
- 81. Rolim VM, Pavarani SP, Campos FS, et al. Clinical, pathological, immunohistochemical and molecular characterization of feline chronic gingivostomatitis. J Feline Med Surg 2017; 19: 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Frost P, Williams CA. Feline dental disease. Vet Clin North Am Small Anim Pract 1986; 16: 851–873. [DOI] [PubMed] [Google Scholar]
- 83. Williams CC, Aller MS. Gingivitis/stomatitis in cats. Vet Clin North Am Small Anim Pract 1992; 22: 1361–1383. [DOI] [PubMed] [Google Scholar]
- 84. Lyon KF. Gingivostomatitis. Vet Clin North Am Small Anim Pract 2005; 35: 891–911. [DOI] [PubMed] [Google Scholar]
- 85. Knowles JO, Gaskell RM, Gaskell CJ, et al. Prevalence of feline calicivirus, feline leukaemia virus and antibodies to FIV in cats with chronic stomatitis. Vet Rec 1989; 124: 336–338. [DOI] [PubMed] [Google Scholar]
- 86. Tenorio AP, Franti CE, Madewell BR, et al. Chronic oral infections of cats and their relationship to persistent oral carriage of feline calici-, immunodeficiency, or leukemia viruses. Vet Immunol Immunopathol 1997; 29: 1–14. [DOI] [PubMed] [Google Scholar]
- 87. Reubel GH, George JW, Higgins J, et al. Effect of chronic feline immunodeficiency virus infection on experimental feline calicivirus-induced disease. Vet Microbiol 1994; 39: 335–351. [DOI] [PubMed] [Google Scholar]
- 88. Hargis AM, Ginn PE, Mansell JEKL, et al. Ulcerative facial and nasal dermatitis and stomatitis in cats associated with feline herpesvirus 1. Vet Dermatol 1999; 10: 267–274. [DOI] [PubMed] [Google Scholar]
- 89. Lommer MJ, Verstraete FJM. Concurrent oral shedding of feline calicivirus and feline herpesvirus 1 in cats with chronic gingivostomatitis. Oral Microbiol Immunol 2003; 18: 131–134. [DOI] [PubMed] [Google Scholar]
- 90. Quimby JM, Elston T, Hawley J, et al. Evaluation of the association of Bartonella species, feline herpesvirus 1, feline calicivirus, feline leukemia virus and feline immunodeficiency virus with chronic feline gingivostomatitis. J Feline Med Surg 2008; 10: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lee M, Bosward KL, Norris JM. Immunohistological evaluation of feline herpesvirus-1 infection in feline eosinophilic dermatoses or stomatitis. J Feline Med Surg 2010; 12: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sykes JE, Westropp JL, Kasten RW, et al. Association between Bartonella species infection and disease in pet cats as determined using serology and culture. J Feline Med Surg 2010; 12: 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dolieslager SMJ, Bennett D, Johnston N, et al. Novel bac- terial phylotypes associated with the healthy feline oral cavity and feline chronic gingivostomatitis. Res Vet Sci 2013; 94: 428–432. [DOI] [PubMed] [Google Scholar]
- 94. Henzel A, Brum MCS, Lautert C, et al. Isolation and identification of feline calicivirus and feline herpesvirus in southern brazil. Braz J Microbiol 2012; 43: 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ayllon T, Diniz PP, Breitschwerdt EB, et al. Vector-borne diseases in client-owned and stray cats from Madrid, Spain. Vector Borne Zoonotic Dis 2012; 12: 143–150. [DOI] [PubMed] [Google Scholar]
- 96. Pennisi MG, Lupo T, Malara D, et al. Serological and molecular prevalence of Leishmania infantum infection in cats from Southern Italy. J Feline Med Surg 2012; 14: 656–657. [Google Scholar]
- 97. Persichetti MF, Solano-Gallego L, Serrano L, et al. Detection of vector-borne pathogens in cats and their ectoparasites in southern Italy. Parasit Vectors 2016; 9. DOI: 10.1186/s13071-016-1534-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sherry K, Miró G, Trotta M, et al. A serological and molecular study of Leishmania infantum infection in cats from the Island of Ibiza (Spain). Vector Borne Zoonotic Dis 2011; 11: 239–245. [DOI] [PubMed] [Google Scholar]
- 99. Sobrinho LSV, Rossi CN, Vides JP, et al. Coinfection of Leishmania chagasi with Toxoplasma gondii, feline immunodeficiency virus (FIV) and feline leukemia virus (FeLV) in cats from an endemic area of zoonotic visceral leishmaniasis. Vet Parasitol 2012; 187: 302–306. [DOI] [PubMed] [Google Scholar]
- 100. Spada E, Canzi I, Baggiani L, et al. Prevalence of Leishmania infantum and co-infections in stray cats in northern Italy. Comp Immunol Microbiol Infect Dis 2016; 45: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Vita S, Santori D, Aguzzi I, et al. Feline leishmaniasis and ehrlichiosis: serological investigation in Abruzzo region. Vet Res Commun 2005; 29 Suppl 2: 319–321. [DOI] [PubMed] [Google Scholar]
- 102. Ravicini S, Pastor J, Hawley J, et al. Prevalence of selected infectious disease agents in stray cats in Catalonia, Spain. JFMS Open Rep 2016; 29. DOI: 10.1177/2055116916634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Solano-Gallego L, Hegarty B, Espada Y, et al. Serological and molecular evidence of exposure to arthropod-borne organisms in cats from northeastern Spain. Vet Microbiol 2006; 118: 274–277. [DOI] [PubMed] [Google Scholar]
- 104. Pennisi G. A high prevalence of feline leishmaniosis in southern Italy. Intervet Proceedings of the 2nd International Canine Leishmaniosis Forum; 2002 Feb 6–9; Seville, Spain. Boxmeer: Intervet, 2002, pp 39–48. [Google Scholar]
- 105. Costa T, Rossi C, Laurenti M, et al. Ocorrência de Leishmanioseem gatos de área endémica para leishmaniose visceral. Braz J Vet Res Animal Sci 2010; 3: 213–217. [Google Scholar]
- 106. Simões-Mattos L, Mattos MR, Teixeira MJ, et al. The susceptibility of domestic cats (Felis catus) to experimental infection with Leishmania braziliensis. Vet Parasitol 2005; 127: 199–208. [DOI] [PubMed] [Google Scholar]
- 107. Maia C, Gomes J, Cristóvão J, et al. Feline Leishmania infection in a canine leishmaniasis endemic region, Portugal. Vet Parasitol 2010; 174: 336–340. [DOI] [PubMed] [Google Scholar]
- 108. Maroli M, Pennisi MG, Di Muccio T, et al. Infection of sandflies by a cat naturally infected with Leishmania infantum. Vet Parasitol 2007; 145: 357–360. [DOI] [PubMed] [Google Scholar]
- 109. Marcos R, Santos M, Malhão F, et al. Pancytopenia in a cat with visceral leishmaniosis. Vet Clin Pathol 2009; 38: 201–205. [DOI] [PubMed] [Google Scholar]
- 110. Bourdoiseau G. Leishmanioseféline: actualités. Prat Med Chirurg Animal Comp 2011; 46: 23–26. [Google Scholar]
- 111. Coelho WM, do Amarante AF, Apolinario C, et al. Seroepidemiology of Toxoplasma gondii, Neospora caninum, and Leishmania spp. infections and risk factors for cats from Brazil. Parasitol Res 2011; 109: 1009–1013. [DOI] [PubMed] [Google Scholar]
- 112. da Silva SM, Rabelo PF, Gontijo NF, et al. First report of infection of Lutzomyia longipalpis by Leishmania (Leishmania) infantum from a naturally infected cat of Brazil. Vet Parasitol 2010; 174: 150–154. [DOI] [PubMed] [Google Scholar]
- 113. Foglia Manzillo V, Di Muccio T, Cappiello S, et al. Prospective study on the incidence and progression of clinical signs in naïve dogs naturally infected by Leishmania infantum. PLoS Negl Trop Dis 2013; 7. DOI: 10.1371/journal.pntd.0002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Pennisi MG, Persichetti MF, Migliazzo A, et al. Feline leishmaniosis: clinical signs and course in 14 followed up cases. Proceedings of the LXX Convegno SIS Vet. 2016 June 13–16; Palermo, Italy, pp 166–167. [Google Scholar]
- 115. Basso MA, Marques C, Santos M, et al. Successful treatment of feline leishmaniosis using a combination of allopurinol and N-methyl-glucamine antimoniate. JFMS Open Rep 2016; 2. DOI: 10.1177/2055116916630002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Brianti E, Celi N, Napoli E, et al. Parasitological, pathological and therapeutical findings in a case of feline leishmaniosis. In: Proceedings of the WAAVP Congress; 2015 Aug 16–20; Liverpool, UK, p 386. [Google Scholar]
- 117. Dedola C, Ibba F, Manca T, et al. Dermatitees foliativa associata a leishmaniosi in un gatto. In: Proceedings 2° Congresso Nazionale SIDEV; 2015 Jul 17–19. Aci Castello, Italy. [Google Scholar]
- 118. Maia C, Sousa C, Ramos C, et al. First case of feline leishmaniosis caused by Leishmania infantum genotype E in a cat with a concurrent nasal squamous cell carcinoma. JFMS Open Rep 2015; 1. DOI: 10.1177/2055116915593969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pimenta P, Alves-Pimenta S, Barros J, et al. Feline leishmaniosis in Portugal: 3 cases (year 2014). Vet Parasitol Reg Stud Reports 2015; 1–2: 65–69. [DOI] [PubMed] [Google Scholar]
- 120. Solano-Gallego L, Miró G, Koutinas A, et al. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors 2011; 4. DOI: 10.1186/1756-3305-4-86. [DOI] [PMC free article] [PubMed] [Google Scholar]