Abstract
Practical relevance:
Prior to 1990 papillomaviruses (PVs) were not recognised to infect or cause disease in domestic cats. Since this time, the use of histology, immunohistochemistry and, more recently, molecular techniques has revealed that PVs almost certainly cause feline viral plaques and Bowenoid in situ carcinomas, oral papillomas and feline sarcoids. In addition, there is increasing evidence that PVs play a significant role in the development of feline cutaneous squamous cell carcinomas, one of the most common skin cancers of cats. Recent studies have also revealed that most cats are asymptomatically infected with PVs. This raises a critical question that is currently unanswered: why do only a small proportion of infected cats develop disease? In the future it may be possible to prevent PV-induced diseases by using a vaccine to prevent PV infection. Alternatively, novel therapies may be developed that prevent PVs from causing clinical disease by stimulating the host immune response.
Clinical challenges:
A recognition of the skin diseases caused by PVs is important to more accurately predict disease progression. Unfortunately, there are currently no non-surgical treatments that have been proven to be beneficial in cats and clinical management of PV-induced skin disease in cats can be challenging.
Global importance:
PVs have a worldwide distribution and negatively impact feline health and welfare globally.
Audience:
This review is aimed at clinicians, especially those who regularly treat cats with skin disease. The review will also be useful to oncologists and researchers who have an interest in how cancer develops in cats.
Evidence base:
In producing this update the authors have drawn on recently published peer-reviewed literature.
Keywords: Virus, papillomavirus, viral plaque, Bowenoid in situ carcinoma, skin cancer
Papillomaviruses
Papillomaviruses (PVs) are small, non-enveloped, icosahedral, double-stranded DNA viruses. Most animals are infected by PVs and hundreds of different PV types are recognised. 1 Five different Felis catus papillomavirus (FcaPV) types are currently known to infect domestic cats including: FcaPV-1, a lambdapapillomavirus; FcaPV-2, a dyothetapapillomavirus; and FcaPV-3, -4 and -5, which probably form their own separate PV genus. 2 Cats are also dead-end hosts for Bos taurus papillomavirus (BPV)-14, a deltapapillomavirus. 3
Virus discovery
PVs were first reported as having a role in feline skin disease in 1990 when PV virions were observed in a cutaneous plaque. 4 The PV present within the plaque was fully sequenced and classified as FcaPV-1 in 2002. 5
PVs were first associated with Bowenoid in situ carcinomas (BISCs) in 1997 after PV antigens were detected in around half of BISCs by immunohistochemistry. 6 In 2007, PCR was used to amplify PV DNA from 11/18 BISCs, and revealed that the same PV type was present in most BISCs. 7 This PV type was fully sequenced and classified as FcaPV-2 in 2009. 8
FcaPV-3 was amplified from a feline BISC in 2013. 9
FcaPV-4 was amplified in 2014 from an oral swab of a cat that showed no clinical lesions attributed to the PV infection. 10
FcaPV-5 was amplified from a viral plaque in 2017. 2 This viral plaque was unusual because PV infection had resulted in marked hyperplasia of the sebaceous glands and evidence of PV infection was visible close to the base of the hair follicles. 11
A role for PVs in feline sarcoids was first proposed in 2001. 12 Subsequent studies revealed that all tested feline sarcoids contained the same ‘FeSarPV viral sequence,12–14 which was determined to be of bovine origin and classified as BPV-14 in 2015. 3
While six PV types are currently recognised to infect cats, evidence from other species suggests that cats are likely to be infected with many additional PV types that have yet to be recognised.
Epidemiology
PVs have co-evolved with their hosts over a long time. As the host and virus are in such good balance the vast majority of PV infections in all species are asymptomatic.
In cats, the majority of the research into the epidemiology of PV infections has focused on FcaPV-2. Such studies have revealed that almost all cats are infected with FcaPV-2. 15 Additionally, FcaPV-2 is detectable in very young kittens, suggesting that, like some cutaneous human papillomavirus (HPV) types, infection occurs around the time of birth. 16 Transplacental infection by FcaPV-2 may also be possible. 17 While almost all cats are infected by FcaPV-2, most infections do not induce a humoral response and serum antibodies against this PV type are only detectable in around a quarter of cats. 18
With the important exception of the delta-papillomaviruses, such as BPV-14, PVs are strictly host-specific. 19 Therefore, while HPV DNA has been amplified from feline samples, 20 sample contamination appears the most likely explanation, and current evidence suggests that HPVs are unlikely to infect cats and feline PVs are unlikely to infect people.
Feline sarcoids are thought to be caused by cross-species infection by BPV-14 and this PV has never been detected in any non-sarcoid feline sample.13,21 Unsurprisingly, feline sarcoids are restricted to cats that have contact with cattle.
Diseases associated with papillomaviruses in cats
PVs infect stratified epithelium and the life cycle of the virus is intimately associated with the progression of cells from the basal to the superficial cell layers. 22 A fundamental property of PVs is that viral replication is dependent on stimulating cell division within normally post-mitotic keratinocytes. Additionally, the greater the increase in keratinocyte cell division induced by the virus, the greater the number of viral particles that will be produced as a result of the infection. 22 All PVs produce proteins that alter normal cell regulation, and the diseases caused by PVs in cats (Table 1) are all due to increased cell replication and growth.
Table 1.
Summary of diseases that have been evaluated for a potential papillomavirus (PV) aetiology in cats
| Disease | Associated PV types | Strength of association | Histological evidence of PV infection † | p16CDKN2a protein immunostaining expected † |
|---|---|---|---|---|
| Viral plaque/Bowenoid in situ carcinoma | FcaPV-2, -3, -5 | High | Yes | Yes |
| Cutaneous squamous cell carcinoma | FcaPV-2 | Moderate | No | Yes |
| Basal cell carcinoma | FcaPV-3 | Unknown * | Yes | Yes |
| Oral papilloma | FcaPV-1 | High | Yes | Yes |
| Oral squamous cell carcinoma | None | Minimal | No | No |
| Feline sarcoid | BPV-14 | High | No | No |
FcaPV = Felis catus papillomavirus; BPV = Bos taurus papillomavirus
Although some basal cell carcinomas (BCCs) are strongly associated with PV infection in cats, the proportion of BCCs that are associated is unknown
Immunostaining is expected to be present in lesions that are thought to be caused by PVs. For example, not all squamous cell carcinomas contain evidence of a PV aetiology, but those putatively caused by PVs will consistently contain pi6CDKN2a protein immunostaining
Occasionally in cats, PVs are able to induce a rapid, albeit transient, increase in cell replication that results in a self-resolving hyperplastic papilloma (wart); 20 however, more frequently, PVs cause more modest cell replication that leads to the development of a raised plaque. The increased replication of cells within the plaque results in an increased rate of DNA mutations in these cells, predisposing to neoplastic transformation. Whether PVs directly cause DNA mutations in infected cells of cats is currently unknown. Unlike the human high-risk PVs that are well recognised to cause neoplasia by accidental integration into the cellular DNA, 23 there is currently no evidence that FcaPV DNA becomes integrated. Therefore, it may simply be that the chronically increased cell replication and altered cell regulation caused by the PVs predispose to the accumulation of spontaneous mutations and subsequent neoplastic transformation. Additionally, PV infection could act as a co-factor, potentiating the effect of other carcinogens such as ultraviolet (UV) light.24,25
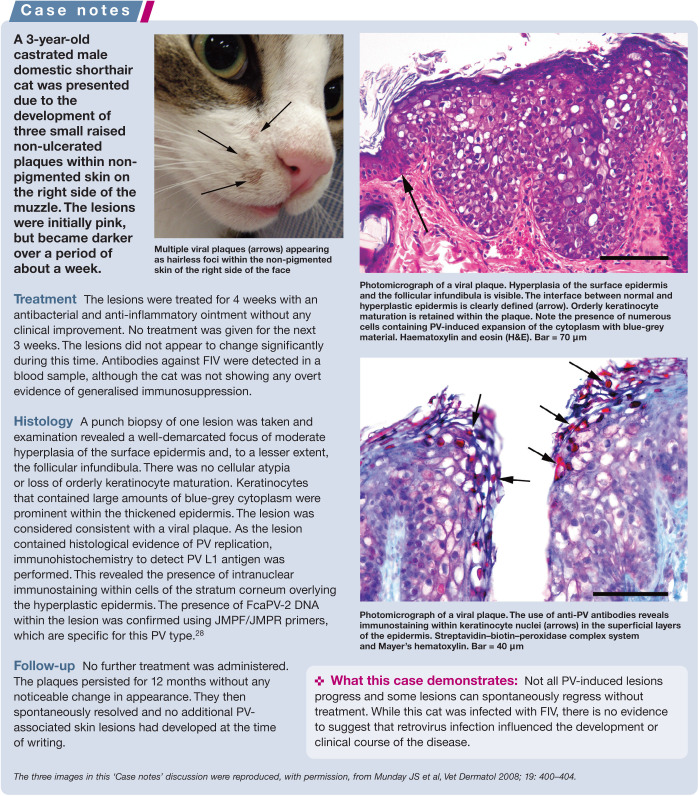
Most cats are infected by PVs, but PV-induced disease is rare. 15 This suggests that a key process in the pathogenesis of these diseases is the inability of the host defences to inhibit PV replication. Without inhibition of replication by the host, the PV is able to induce marked epithelial proliferation. If this epithelial proliferation results in the development of a clinically visible lesion, the infection will progress from an asymptomatic to a clinical infection. 26 Currently, the mechanisms that might lead to reduced defences are poorly understood and cats with seemingly normal immune systems can develop PV-induced disease. While coinfection by feline immunodeficiency virus (FIV) has been reported in some cases of PV-induced skin disease in cats, 27 there is currently little evidence for a significant role of either FIV or feline leukaemia virus in disease development (see ‘Case notes’ on page 3).
Feline viral plaques/Bowenoid in situ carcinomas
While initially thought to be separate disease entities, viral plaques and BISCs are probably best considered mild and severe forms of the same disease process.26,29 These rare lesions are pre-cancerous and can progress to invasive squamous cell carcinomas (SCCs), although the proportion of lesions that remain static vs the proportion that progress is currently unknown.
Viral plaques/BISCs typically develop on cats between the ages of 8 and 14 years. 29 They most frequently appear around the face, head and neck, although they can be found anywhere on the body. Lesions are often multiple and pigmented, and can develop within pigmented or non-pigmented, haired or non-haired skin. 29 Viral plaques are generally less than 1 cm in diameter, slightly raised, hairless lesions covered in a thin crust. BISCs are larger and are typically markedly raised ulcerated lesions covered by a significant serocellular crust or a thick layer of keratin.
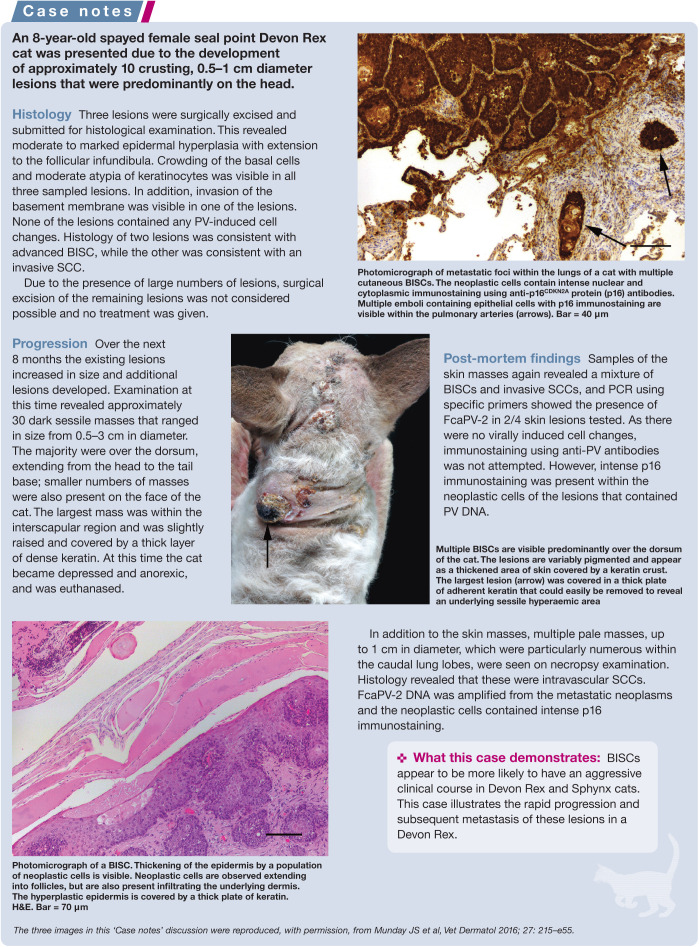
FcaPV-2 is the predominant cause of feline viral plaques/BISCs, although FcaPV-3 and FcaPV-5 have also been associated with lesion development (Table 1).2,30,31 Devon Rex and the closely related Sphynx breeds of cat appear to develop BISCs at an earlier age than other breeds, and the BISCs that develop in these cats tend to be highly aggressive and can rapidly metastasise (see ‘Case notes’ on page 5).32,33
Cutaneous squamous cell carcinomas
SCCs are one of the most common skin cancers of cats and, while the role of UV light in cancer development cannot be disputed, there is increasing evidence that FcaPV-2 may also cause SCC development. This evidence includes the detection of PV DNA more frequently in SCCs than in non-SCC skin samples,28,34 the detection and localisation of FcaPV-2 RNA within a proportion of SCCs but not samples of normal skin, 35 and the demonstration that FcaPV-2 proteins can influence neoplastic transformation of a cell.24,36 In addition, p16CDKN2a protein (p16) immunostaining (see later) is visible in a proportion of SCCs.37,38
The use of PCR and immunohistochemistry allows feline cutaneous SCCs to be subdivided into PV-positive and PV-negative lesions. Current evidence suggests that around 80% of SCCs from areas of the body protected from UV exposure are caused by PV infection. 34 UV light is a well recognised cofactor in PV-induced cutaneous cancers in people. 39 It appears probable that the same is true for cats and current evidence suggests that PVs could be the primary cause, or a cofactor in the development, of between a quarter and a third of all feline cutaneous SCCs.35,36
Basal cell carcinomas
A potential role of PVs in the development of basal cell carcinomas (BCCs), which are rare feline skin neoplasms, was first proposed after PV-induced cell changes were noted in the epidermis overlying a proportion of neoplasms. 40 More recently, feline BCCs that contain PV-induced cell changes within the neoplastic cells have been reported and both FcaPV-3 and a novel feline PV type have been amplified from these tumours.41,42 Interestingly, FcaPV-2 has not been detected within a BCC.
Although typically these are single ulcerated raised lesions, a cat with multiple cutaneous PV-associated BCCs has been reported. 42 Currently too few feline cutaneous BCCs have been evaluated to determine what proportion are associated with PV infection and whether, like SCCs, they can be subdivided into PV-positive and PV-negative lesions.
Oral papillomas
There are few reports of oral papillomas in cats, although the true incidence is unknown as these are self-resolving lesions and do not cause dysphagia. They typically appear as multiple exophytic filiform masses on the ventral surface of the tongue. Histologically they contain prominent virally induced changes and are thought to be caused by FcaPV-1. 43
Oral squamous cell carcinomas
Oral SCCs are the fourth most common neoplasm of cats and an important cause of mortality. While bacterial toxins released into the mouth due to poor dental hygiene are hypothesised to promote cancer development, the cause of the high rate of oral SCCs in cats has not been definitively determined. 44 Around a quarter of human oral SCCs are thought to be caused by PV infection. 45 However, while PV DNA sequences have been detected in a small proportion of feline oral SCCs,31,46 there is currently no evidence that PVs cause feline oral SCCs.
Feline sarcoids
As the causative PV has a bovine definitive host, feline sarcoids are restricted to cats with outside access in rural areas and appear to be most common in cats that live in dairy barns. 12 Fighting is hypothesised to be important in allowing the PV entry into the dermis and sarcoids are more common in male cats. 12
Feline sarcoids typically develop around the face, especially the nasal philtrum, although they can also develop on the extremities and tail. Like equine sarcoids, these are mesenchymal neoplasms and histology reveals a proliferation of dermal mesenchymal cells covered by hyperplastic epithelium. PV DNA is present within the proliferating mesenchymal cells, 14 but as feline sarcoids do not support viral replication, they do not contain histological evidence of PV infection. Their behaviour is similar to equine sarcoids, with local invasion and frequent recurrence following surgical excision (see ‘Case notes’ above), but no metastatic potential.
Investigation of a papillomavirus aetiology
Histology
Histology can reveal PV-induced cell changes (enlarged keratinocytes with smudged blue-grey cytoplasm or with a shrunken nucleus surrounded by a clear halo [koilocytes]) within some lesions and their presence can be a useful diagnostic feature. Such cell changes occur as a result of PV replication within a cell and are expected to be visible in recently developed viral plaques/BISCs, oral papillomas and a proportion of BCCs. Viral replication does not occur in feline sarcoids or in the less well-differentiated cells of an advanced BISC or SCC, and PV-induced cell changes are not expected to be present in these lesions.
If histological evidence of PV-induced cell changes is not present within a lesion, there are other methods available to investigate a potential PV aetiology – notably immuno-histochemistry and molecular techniques.
Immunohistochemistry
Immunohistochemistry has the advantage of being able to be performed at most veterinary or human diagnostic laboratories. Options available for immunohistochemistry include using antibodies to detect either the presence of PV L1 protein or increased p16 within the cell. The PV L1 protein forms the capsid that surrounds the viral particle. This protein is targeted because large amounts are produced in the process of viral replication. The disadvantage of using antibodies against the L1 protein is that this protein is only produced late in the process of viral replication. Therefore, if no viral replication is present, no immunostaining will be visible. Viral replication appears to be rarely present in advanced BISCs and in PV-positive SCCs and, in the authors’ experience, PV L1 protein immuno-staining is rarely present in lesions that do not contain histologically visible PV-induced cell changes. An additional disadvantage of using antibodies against the PV L1 protein is that it is unknown if the commercially available anti-bodies, which have generally been created using human or bovine PV antigens, are able to cross-react with the L1 protein of the different FcaPV types.
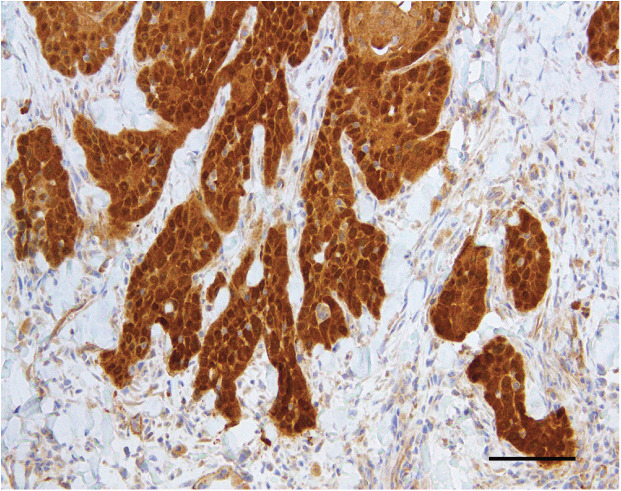
Human diagnostic laboratories rarely use anti-PV antibodies to determine a PV aetiology of a neoplasm. Instead, to differentiate between a PV-positive oral SCC and a PVnegative oral SCC they will typically use immunohistochemistry to detect increased cell p16. 48 Cancers that are caused by PVs consistently contain increased p16 because PVs consistently promote cell replication by altering pathways that also increase p16. 49 This differentiation is of critical clinical importance for human oral SCCs because PV-positive neoplasms are more likely to respond to treatment and have a more favorable prognosis than PV-negative cancers.50 In cats, studies have shown good correlation between the presence of increased p16 within the neoplastic cells and the presence of PV DNA or RNA within the neoplasm (Figure 1).36,37
Figure 1.
Feline cutaneous SCC. This neoplasm contained PV DNA and was from an area of the body that was protected from solar exposure. Note the consistent intense nuclear and cytoplasmic immunostaining against p16CDKN2A protein (p16). Bar = 50 μm
Advantages of using p16 immunostaining to determine a PV aetiology in a feline lesion include a consistent increase in the protein regardless of whether or not the PV is replicating and the known cross-reactivity of the antihuman G175-405 p16 clone antibodies to the feline p16 protein. However, the specificity of this test for a PV aetiology in cats has not been definitively shown and it remains possible that spontaneous mutations within a cell’s DNA could also result in increases in p16 that are independent of a PV infection.
Molecular techniques
Molecular tests to detect viral nucleic acids such as PCR and in situ hybridisation can also be used to investigate a PV aetiology for a lesion. The advantage of these techniques is that very small quantities of PV DNA or RNA can be detected. However, as asymptomatic PV infections are so common, simply detecting the presence of a PV within a lesion does not prove that the lesion was caused by the PV. In addition, these tests are not routinely available and are currently restricted to use in a research setting.
Treatment approaches
Surgical excision
There have been few studies evaluating treatment options for PV-induced lesions in cats. However, complete surgical excision is suggested for single or small numbers of viral plaques/BISCs, SCCs and BCCs. While surgical excision of a viral plaque/BISC is expected to be curative, clients should be advised that affected cats are more likely to develop additional lesions of this type. More aggressive treatment is suggested for Devon Rex and Sphynx cats due to the observation that BISCs can rapidly progress to metastatic SCCs in these breeds.32,33
Non-surgical therapies
Evidence supporting non-surgical therapies to treat viral plaques/BISCs is limited. However, as these lesions are confined to the epidermis, treatment using cryotherapy is expected to be successful. In humans, CO2 laser is considered one of the best treatment modalities for genital warts. 51 As this modality was recently used to successfully treat numerous large viral plaques on a dog, 52 CO2 laser could be a useful treatment for larger lesions in cats.
Alternatively, imiquimod cream stimulates toll-like receptors and locally increases inter-feron alpha (a) and tumor necrosis factor-a. 53 While initially marketed as a treatment for human genital warts, imiquimod does not specifically target PV-induced lesions. In humans, this treatment has also been used for BCCs and actinic lesions, although imiquimod is only recommended as a primary treatment for pre-neoplastic or neoplastic skin lesions if better established therapies are not available. 54 In an uncontrolled study of 12 cats with BISCs, imiquimod resulted in partial resolution of at least one BISC in all 12 cats and complete resolution of at least one BISC in five cats. 55 However, significant side effects were reported including local erythema in five cats and systemic toxicity in two cats. While there is anecdotal evidence supporting the use of imiquimod cream, additional controlled studies are required to determine the efficacy and safety of this treatment. Evidence from human studies suggests that imiquimod may be equally effective in PV-induced and non-PV-induced pre-neoplastic feline lesions, although its use should probably be restricted to situations where other treatments are impractical.
Photodynamic therapy has also been reported to be effective for feline cutaneous in situ carcinomas, although whether any of the treated lesions were PV induced is uncertain. 56
There is no evidence that autologous vaccines influence the resolution of an established PV-induced lesion in any species and it seems unlikely that such vaccines would be useful to treat PV-induced lesions in cats.
Complete excision of a feline sarcoid is expected to be curative; however, given the common location of these neoplasms, this can be difficult and they are prone to local recurrence.
Conclusions
The role of PVs in the development of disease in cats is increasingly being recognised. This increased recognition will, in turn, allow further subclassification of feline skin diseases (eg, into PV-positive or PV-negative SCC), which may allow for a more accurate prognosis and better targeted therapies. Additionally, recognition of the role that PVs play in skin disease in cats raises the possibility that the PV-induced diseases can be prevented – either by protective vaccination or by enabling the host immune system to control virus replication and prevent the virus from causing clinical disease.

Key Points
Most cats harbour asymptomatic PV infections.
It is unknown why a minority of infections result in clinical disease, but the development of disease is likely to be due to an inability of the host to maintain low levels of PV replication.
PVs cause viral plaques/BISCs, which can become large and multiple, and can progress to SCCs. Surgical excision should be curative, but additional lesions are likely. Other unproven treatment options include cryotherapy, CO2 laser and imiquimod
PVs may cause a proportion of cutaneous SCCs and BCCs, although the role of the virus in neoplastic transformation is currently unclear.
Cross-species infection by a bovine PV causes feline sarcoids. These neoplasms can be locally recurrent after excision but do not metastasise.
PVs cause oral papillomas in cats, but there is no evidence that PVs cause oral SCCs.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Contributor Information
John S Munday, School of Veterinary Science, Massey University, Palmerston North, 4410 New Zealand.
Claire R Sharp, College of Veterinary Medicine, School of Veterinary and Life Sciences, Murdoch University, Australia.
Julia A Beatty, University of Sydney, Faculty of Science, Sydney School of Veterinary Science, NSW 2006, Australia.
References
- 1. Rector A, Van Ranst M. Animal papillomaviruses. Virology 2013; 445: 213–223. [DOI] [PubMed] [Google Scholar]
- 2. Munday JS, Dittmer KE, Thomson NA, et al. Genomic characterisation of Felis catus papillomavirus type 5 with proposed classification within a new papillomavirus genus. Vet Microbiol 2017; 207: 50–55. [DOI] [PubMed] [Google Scholar]
- 3. Munday JS, Thomson N, Dunowska M, et al. Genomic characterisation of the feline sarcoid-associated papillomavirus and proposed classification as Bos taurus papillomavirus type 14. Vet Microbiol 2015; 177: 289–295. [DOI] [PubMed] [Google Scholar]
- 4. Carney HC, England JJ, Hodgin EC, et al. Papillomavirus infection of aged Persian cats. J Vet Diagn Invest 1990; 2: 294–299. [DOI] [PubMed] [Google Scholar]
- 5. Tachezy R, Duson G, Rector A, et al. Cloning and genomic characterization of Felis domesticus papillomavirus type 1. Virology 2002; 301: 313–321. [DOI] [PubMed] [Google Scholar]
- 6. LeClerc SMC, Haines EG. Papillomavirus infection in association with feline cutaneous squamous cell carcinoma in situ. Proceedings of the AAVD/ACVD meeting. 1997 April 20; Nashville, TN, USA, pp 125–126. [Google Scholar]
- 7. Munday JS, Kiupel M, French AF, et al. Detection of papillo-maviral sequences in feline Bowenoid in situ carcinoma using consensus primers. Vet Dermatol 2007; 18: 241–245. [DOI] [PubMed] [Google Scholar]
- 8. Lange CE, Tobler K, Markau T, et al. Sequence and classification of FdPV2, a papillomavirus isolated from feline Bowenoid in situ carcinomas. Vet Microbiol 2009; 137: 60–65. [DOI] [PubMed] [Google Scholar]
- 9. Munday JS, Dunowska M, Hills SF, et al. Genomic characterization of Felis catus papillomavirus-3: a novel papillomavirus detected in a feline Bowenoid in situ carcinoma. Vet Microbiol 2013; 165: 319–325. [DOI] [PubMed] [Google Scholar]
- 10. Dunowska M, Munday JS, Laurie RE, et al. Genomic characterisation of Felis catus papillomavirus 4, a novel papillomavirus detected in the oral cavity of a domestic cat. Virus Genes 2014; 48: 111–119. [DOI] [PubMed] [Google Scholar]
- 11. Munday JS, Marshall S, Thomson NA, et al. Multiple viral plaques with sebaceous differentiation associated with an unclassified papillomavirus type in a cat. NZ Vet J 2017: 1–17. [DOI] [PubMed] [Google Scholar]
- 12. Schulman FY, Krafft AE, Janczewski T. Feline cutaneous fibropapillomas: clinicopathologic findings and association with papillomavirus infection. Vet Pathol 2001; 38: 291–296. [DOI] [PubMed] [Google Scholar]
- 13. Munday JS, Knight CG, Howe L. The same papillo-mavirus is present in feline sarcoids from North America and New Zealand but not in any non-sarcoid feline samples. J Vet Diagn Invest 2010; 22: 97–100. [DOI] [PubMed] [Google Scholar]
- 14. Teifke JP, Kidney BA, Lohr CV, et al. Detection of papillo-mavirus-DNA in mesenchymal tumour cells and not in the hyperplastic epithelium of feline sarcoids. Vet Dermatol 2003; 14: 47–56. [DOI] [PubMed] [Google Scholar]
- 15. Thomson NA, Dunowska M, Munday JS. The use of quantitative PCR to detect Felis catus papillomavirus type 2 DNA from a high proportion of queens and their kittens. Vet Microbiol 2015; 175: 211–217. [DOI] [PubMed] [Google Scholar]
- 16. Thomson NA, Thomas DG, Weidgraaf K, et al. Felis catus papillomavirus type 2 DNA loads on kittens are transient and do not reflect their susceptibility to infection. J Feline Med Surg 2018; 20: 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Altamura G, Jebara G, Cardeti G, et al. Felis catus papillo-mavirus type-2 but not type-1 is detectable and transcrip-tionally active in the blood of healthy cats. Transbound Emerg Dis 2018; 65: 497–503. [DOI] [PubMed] [Google Scholar]
- 18. Geisseler M, Lange CE, Favrot C, et al. Geno- and seroprevalence of Felis domesticus papillomavirus type 2 (FdPV2) in dermatologically healthy cats. BMC Vet Res 2016; 12: 147. DOI: 10.1186/s12917-016-0776-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sundberg JP, Van Ranst M, Montali R, et al. Feline papillomas and papillomaviruses. Vet Pathol 2000; 37: 1–10. [DOI] [PubMed] [Google Scholar]
- 20. Munday JS, Hanlon EM, Howe L, et al. Feline cutaneous viral papilloma associated with human papillomavirus type 9. Vet Pathol 2007; 44: 924–927. [DOI] [PubMed] [Google Scholar]
- 21. Munday JS, Knight CG. Amplification of feline sarcoid-associated papillomavirus DNA sequences from bovine skin. Vet Dermatol 2010; 21: 341–344. [DOI] [PubMed] [Google Scholar]
- 22. Doorbar J. The papillomavirus life cycle. J Clin Virol 2005; 32 Suppl 1: S7–15. [DOI] [PubMed] [Google Scholar]
- 23. Munday JS. Bovine and human papillomaviruses: a comparative review. Vet Pathol 2014; 51: 1063–1075. [DOI] [PubMed] [Google Scholar]
- 24. Altamura G, Corteggio A, Pacini L, et al. Transforming properties of Felis catus papillomavirus type 2 E6 and E7 putative oncogenes in vitro and their transcriptional activity in feline squamous cell carcinoma in vivo. Virology 2016; 496: 1–8. [DOI] [PubMed] [Google Scholar]
- 25. Munday JS, Kiupel M. Papillomavirus-associated cutaneous neoplasia in mammals. Vet Pathol 2010; 47: 254–264. [DOI] [PubMed] [Google Scholar]
- 26. Munday JS, Thomson NA, Luff JA. Papillomaviruses in dogs and cats. Vet J 2017; 225: 23–31. [DOI] [PubMed] [Google Scholar]
- 27. Egberink HF, Berrocal A, Bax HA, et al. Papillomavirus associated skin lesions in a cat seropositive for feline immunodeficiency virus. Vet Microbiol 1992; 31: 117–125. [DOI] [PubMed] [Google Scholar]
- 28. Munday JS, Kiupel M, French AF, et al. Amplification of papillomaviral DNA sequences from a high proportion of feline cutaneous in situ and invasive squamous cell carcinomas using a nested polymerase chain reaction. Vet Dermatol 2008; 19: 259–263. [DOI] [PubMed] [Google Scholar]
- 29. Wilhelm S, Degorce-Rubiales F, Godson D, et al. Clinical, histo-logical and immunohistochemical study of feline viral plaques and bowenoid in situ carcinomas. Vet Dermatol 2006; 17: 424–431. [DOI] [PubMed] [Google Scholar]
- 30. Munday JS, Fairley R, Atkinson K. The detection of Felis catus papillomavirus 3 DNA in a feline bowenoid in situ carcinoma with novel histologic features and benign clinical behavior. J Vet Diagn Invest 2016; 28: 612–625. [DOI] [PubMed] [Google Scholar]
- 31. O’Neill SH, Newkirk KM, Anis EA, et al. Detection of human papillomavirus DNA in feline premalignant and invasive squamous cell carcinoma. Vet Dermatol 2011; 22: 68–74. [DOI] [PubMed] [Google Scholar]
- 32. Munday JS, Benfell MW, French A, et al. Bowenoid in situ carcinomas in two Devon Rex cats: evidence of unusually aggressive neoplasm behaviour in this breed and detection of papillomaviral gene expression in primary and metastatic lesions. Vet Dermatol 2016; 27: 215–e55. [DOI] [PubMed] [Google Scholar]
- 33. Ravens PA, Vogelnest LJ, Tong LJ, et al. Papillomavirus-associated multicentric squamous cell carcinoma in situ in a cat: an unusually extensive and progressive case with subsequent metastasis. Vet Dermatol 2013; 24: 642–645, e161-162. [DOI] [PubMed] [Google Scholar]
- 34. Munday JS, Gibson I, French AF. Papillomaviral DNA and increased p16CDKN2A protein are frequently present within feline cutaneous squamous cell carcinomas in ultraviolet-protected skin. Vet Dermatol 2011; 22: 360–366. [DOI] [PubMed] [Google Scholar]
- 35. Hoggard N, Munday JS, Luff J. Localization of Felis catus papillomavirus type 2 E6 and E7 RNA in feline cutaneous squamous cell carcinoma. Vet Pathol 2018; 55: 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thomson NA, Munday JS, Dittmer KE. Frequent detection of transcriptionally active Felis catus papillomavirus 2 in feline cutaneous squamous cell carcinomas. J Gen Virol 2016; 97: 1189–1197. [DOI] [PubMed] [Google Scholar]
- 37. Munday JS, French AF, Peters-Kennedy J, et al. Increased p16CDKN2A protein within feline cutaneous viral plaques, bowenoid in situ carcinomas, and a subset of invasive squamous cell carcinomas. Vet Pathol 2011; 48: 460–465. [DOI] [PubMed] [Google Scholar]
- 38. Munday JS, French AF, Gibson IR, et al. The presence of p16 CDKN2A protein immunostaining within feline nasal planum squamous cell carcinomas is associated with an increased survival time and the presence of papillomaviral DNA. Vet Pathol 2013; 50: 269–273. [DOI] [PubMed] [Google Scholar]
- 39. Akgul B, Cooke JC, Storey A. HPV-associated skin disease. J Pathol 2006; 208: 165–175. [DOI] [PubMed] [Google Scholar]
- 40. Gross TL, Ihrke PJ, Walder EJ, et al. (eds). Epidermal tumors. In: Skin diseases of the dog and cat: clinical and histopatho-logic diagnosis. 2nd ed. Oxford, UK: Blackwell Science, 2005, pp 562–603. [Google Scholar]
- 41. Munday JS, French A, Thomson N. Detection of DNA sequences from a novel papillomavirus in a feline basal cell carcinoma. Vet Dermatol 2017; 28: 236–e60. [DOI] [PubMed] [Google Scholar]
- 42. Munday JS, Thomson NA, Henderson G, et al. Identification of Felis catus papillomavirus 3 in skin neoplasms from four cats. J Vet Diagn Invest 2018; 30: 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Munday JS, Fairley RA, Mills H, et al. Oral papillomas associated with Felis catus papillomavirus type 1 in 2 domestic cats. Vet Pathol 2015; 52: 1187–1890. [DOI] [PubMed] [Google Scholar]
- 44. Munday JS, Lohr CV, Kiupel M. Tumors of the alimentary tract. In: Meuton DJ. (ed). Tumors in domestic animals. 5th ed. Hoboken, NJ, John Wiley and Sons, 2017, pp 499–601. [Google Scholar]
- 45. Marur S, D’Souza G, Westra WH, et al. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 2010; 11: 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Munday JS, Knight CG, French AF. Evaluation of feline oral squamous cell carcinomas for p16CDKN2A protein immunoreactivity and the presence of papillomaviral DNA. Res Vet Sci 2011; 90: 280–283. [DOI] [PubMed] [Google Scholar]
- 47. Manos MM, Ting Y, Wright DK, et al. The use of polymerase chain reaction amplification for the detection of genital human papillomaviruses. Cancer Cells 1989; 7: 209–214. [Google Scholar]
- 48. Smeets SJ, Hesselink AT, Speel EJ, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer 2007; 121: 2465–2472. [DOI] [PubMed] [Google Scholar]
- 49. Parry D, Bates S, Mann DJ, et al. Lack of cyclin D-Cdk complexes in Rb-negative cells correlates with high levels of p16INK4/MTS1 tumour suppressor gene product. EMBO J 1995; 14: 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chung CH, Zhang Q, Kong CS, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol 2014; 32: 3930–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bertolotti A, Dupin N, Bouscarat F, et al. Cryotherapy to treat anogenital warts in nonimmunocompromised adults: systematic review and meta-analysis. J Am Acad Dermatol 2017; 77: 518–526. [DOI] [PubMed] [Google Scholar]
- 52. Knight EC, Munday JS, Stone BM, et al. Carbon dioxide laser treatment of extensive pigmented viral plaque lesions in a golden retriever dog. Vet Dermatol 2016; 27: 442–e117. [DOI] [PubMed] [Google Scholar]
- 53. Miller RL, Gerster JF, Owens ML, et al. Imiquimod applied topically: a novel immune response modifier and new class of drug. Int J Immunopharmacol 1999; 21: 1–14. [DOI] [PubMed] [Google Scholar]
- 54. Love W, Bernhard JD, Bordeaux JS. Topical imiquimod or fluorouracil therapy for basal and squamous cell carcinoma: a systematic review. Arch Dermatol 2009; 145: 1431–1438. [DOI] [PubMed] [Google Scholar]
- 55. Gill VL, Bergman PJ, Baer KE, et al. Use of imiquimod 5% cream (Aldara) in cats with multicentric squamous cell carcinoma in situ: 12 cases (2002-2005). Vet Comp Oncol 2008; 6: 55–64. [DOI] [PubMed] [Google Scholar]
- 56. Flickinger I, Gasymova E, Dietiker-Moretti S, et al. Evaluation of long-term outcome and prognostic factors of feline squamous cell carcinomas treated with photodynamic therapy using liposomal phosphorylated meta-tetra(hydrox-ylphenyl)chlorine. J Feline Med Surg. Epub ahead of print 23 January 2018. DOI: 1098612X17752196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Anderson LA. Prophylactic human papillomavirus vaccines: past, present and future. Pathology 2012; 44: 1–6. [DOI] [PubMed] [Google Scholar]