Abstract
Practical relevance:
Abdominal ultrasound plays a vital role in the diagnostic work-up of many cats presenting to general and specialist practitioners. The biliary tree encompasses the liver, gall bladder and bile ducts, although only diseases affecting the latter two are discussed here. Diseases of the bile ducts and gall bladder are more common than those of the liver parenchyma and ultrasound plays an important role in their diagnosis.
Clinical challenges:
Despite ultrasonography being a commonly used modality, many practitioners are not comfortable performing an ultrasound examination or interpreting the resulting images. Even differentiating between normal variation and pathological changes can be challenging for all but the most experienced. In addition, a lack of pathological change does not necessarily rule out disease; for example, absence of gall bladder and/or extrahepatic biliary distension is not sufficient to exclude the possibility of biliary obstruction, and in many cases of cholangitis the liver and biliary tree are unremarkable on ultrasound examination.
Equipment:
Ultrasound facilities are readily available to most practitioners, although use of ultrasonography as a diagnostic tool is highly dependent on operator experience.
Aim:
This review, part of an occasional series on feline abdominal ultrasonography, discusses the appearance of the normal and diseased biliary system. It is aimed at general practitioners who wish to improve their knowledge and confidence in feline abdominal ultrasound and is accompanied by high-resolution images. Percutaneous ultrasound-guided cholecystocentesis is also covered. Ultrasound examination of the liver was discussed in an article published in January 2019 and an upcoming article will cover hepatic vascular anomalies.
Evidence base:
Information provided in this article is drawn from the published literature and the author’s own clinical experience.
Keywords: Ultrasound, biliary tree, gall bladder, cystadenoma, mucocele, extrahepatic obstruction, cholecystocentesis
Normal appearance of the biliary tree
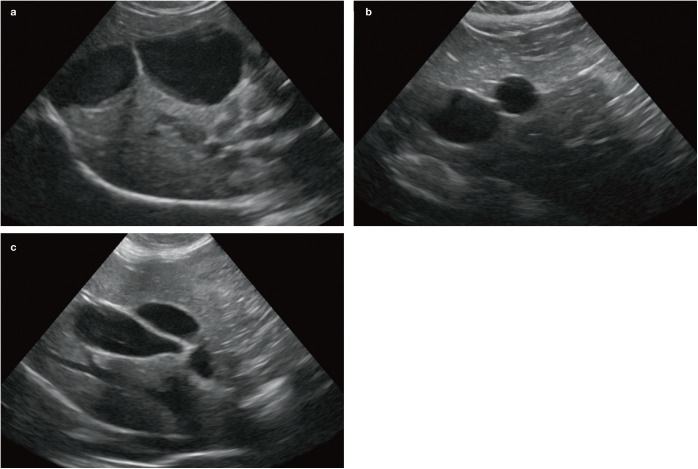
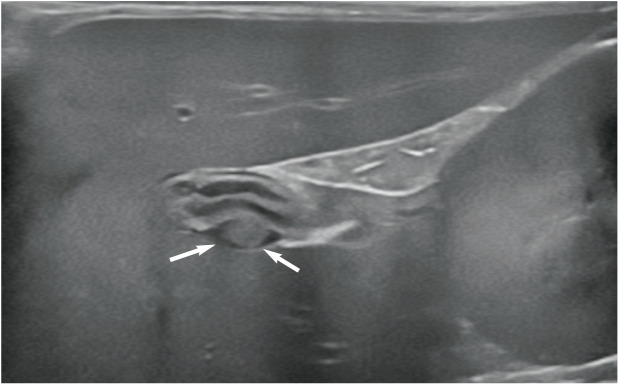
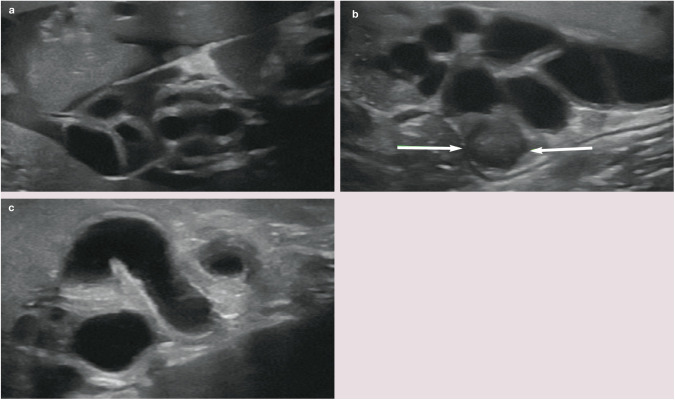
The gall bladder is located to the right of the midline between the two parts of the right medial liver lobe. 1 On average, 1 in 8 cats have some form of accessory gall bladder. 2 In some individuals, the gall bladder is bilobed (Figure 1). This represents a congenital anomaly that arises during embryonic development and is considered an incidental finding. Two different types of bilobed gall bladder have been described.3,4 In the first type, the gall bladder lumen is partially divided by an internal longitudinal septum. The two resulting chambers communicate at their proximal extent by a single shared cystic duct and the external appearance of the gall bladder is normal. 5 In the second type, the fundus of the gall bladder is completely divided and the two cavities fuse only at the neck, resulting in a ‘V’ or valentine heart shape when viewed externally. Duplex gall bladders, which have rarely been reported in the cat, involve two entirely separate cavities, each supplied by their own cystic duct.6–8
Figure 1.
(a–c) Typical appearance of a bilobed gall bladder in three cats
The gall bladder wall may or may not be visible with ultrasound (Figure 2). When seen, it forms an echogenic line <1 mm in thickness. 9 Gall bladder volume (ml) may be estimated during ultrasound examination using the ellipsoid formula (0.52 × length [mm] × height [mm] × width [mm]).10,11 Using this formula, a study conducted on 30 healthy, fasted adult cats, recorded a relatively wide range in gall bladder volume of 0.84–.5 ml, with a mean volume of 2.41 ml. 11 The authors of the study concluded that there does not appear to be any relationship between gall bladder volume and body weight. Gall bladder contractility can also be assessed ultrasonographically via a subcostal or right intercostal acoustic window, by using the ellipsoid formula to calculate the pre- and postprandial gall bladder volume. Using a subcostal window, mean pre- and postprandial (2 h after food) volumes of 2.47 ± 1.16 ml and 0.88 ± 0.13 ml, respectively, have been reported in the normal cat. 12
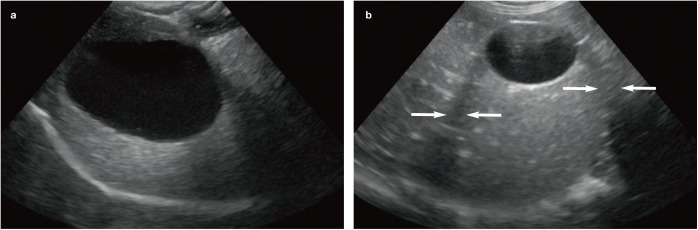
Figure 2.
Normal appearance of the gall bladder. (a) The wall of the gall bladder is not visible. The gall bladder appears large relative to the liver. This was a normal finding in this cat, which was fasted prior to ultrasound examination. Note the acoustic enhancement distal to the gall bladder due to reduced attenuation of the ultrasound beam as it passes through the bile. (b) The wall of the gall bladder is visible as a thin echogenic line. The hypoechoic regions either side of the gall bladder (arrows) are the result of edge shadowing due to refraction

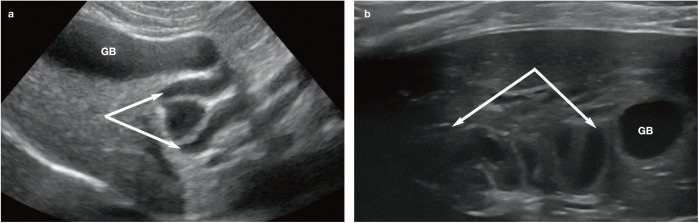
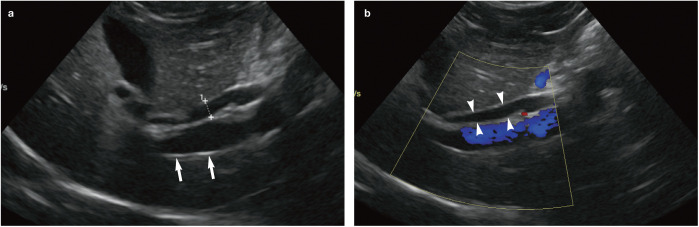
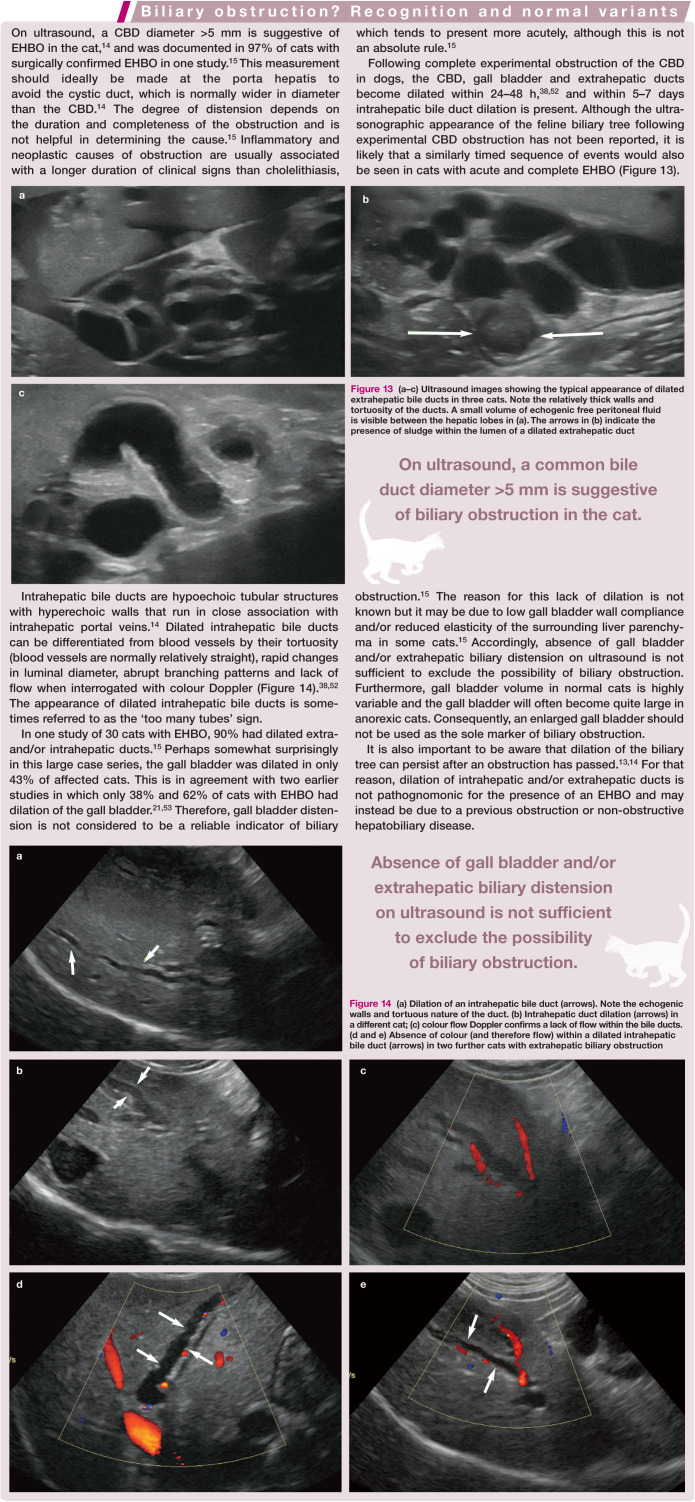
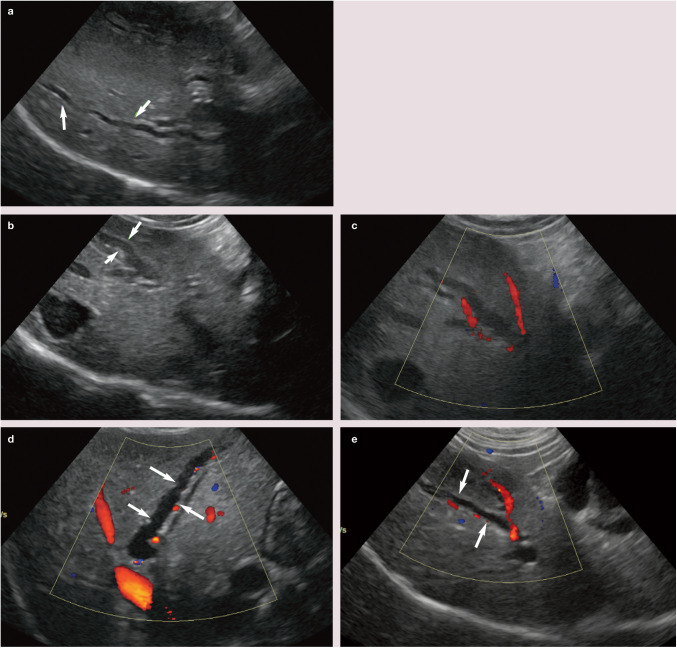
The feline common bile duct (CBD) is long and tortuous compared with its canine counterpart (Figure 3). 13 It can be routinely identified on ultrasound as a tubular anechoic structure with echogenic walls located ventral to the portal vein at the porta hepatis, using either a subcostal approach (Figure 4a) or right cranial intercostal window. 14 The normal CBD may be differentiated from blood vessels by its thicker walls, tortuosity and absence of flow signal when interrogated with colour Doppler (Figure 4b). 14 It can usually be followed caudally without too much difficulty to the junction where it merges with the major pancreatic duct prior to its entry into the duodenum via the major duodenal papilla (Figure 5). 14 The normal CBD should measure no greater than 4 mm in diameter and the wall of the duct should be <1 mm in thickness. 14 Intra- and extrahepatic ducts are not normally visible unless pathologically dilated. 14 ’ 15
Figure 3.
(a,b) Ultrasound images showing the gall bladder (GB) and normal tortuosity of the cystic and common bile ducts (both indicated by arrows)
Figure 4.
(a) The common bile duct (indicated by measuring calipers) can be found ventral to the portal vein (arrows) at the porta hepatis. (b) Colour flow Doppler confirms the presence of hepatopetal flow within the portal vein (ie, flow travelling from right to left in the image) and an absence of flow within the common bile duct (arrowheads)
Figure 5.
Ultrasound image showing the normal appearance of the major duodenal papilla (arrows). The cranial pole of the right kidney is just visible on the right side of the image
Abnormalities of the biliary tree
Gall bladder wall thickening
Diseases of the gall bladder and bile ducts are more common than diseases of the liver parenchyma in cats. 16 A gall bladder wall thickness >1 mm is reported to be an accurate predictor of gall bladder disease in cats, although a thickness <1 mm does not rule out mild or chronic inflammation (Figure 6). 9 Diffuse gall bladder wall thickening is considered to be a non-specific change, and in humans and dogs has been reported in association with both primary hepatobiliary and systemic disease.17–20 In cats, wall thickening can occur as a result of inflammation (cholecystitis, cholangitis, cholangiohepatitis), oedema (especially due to biliary obstruction) and mucosal gland hyperplasia.9,21,22 Diffuse gall bladder wall thickening was identified in 55% of cats with hepatobiliary disease in one study in which the gall bladder wall appeared either diffusely hyperechoic or had a double-rim appearance resulting from a hypoechoic layer sandwiched between two echogenic layers. 9
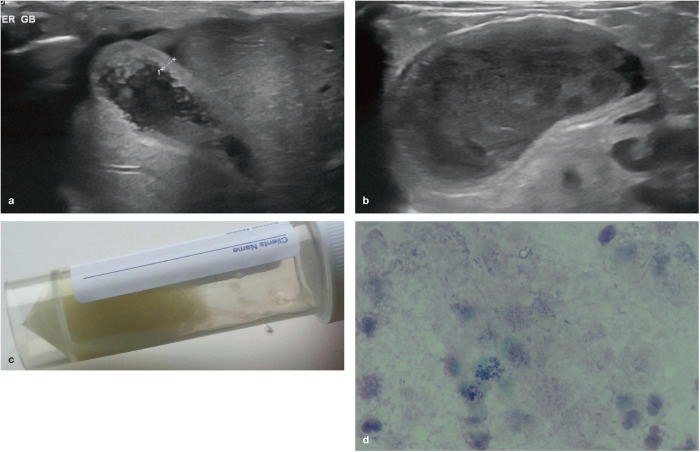
Figure 6.
(a) Thickening of the gall bladder wall of unknown aetiology in a 14-year-old female neutered Russian Blue cat. (b) An enlarged gall bladder with a diffusely thickened wall and echogenic sludge in a 14-year-old female neutered domestic shorthair cat with neutrophilic cholangiohepatitis; (c) cholecystocentesis revealed pale yellow bile and (d) bile culture confirmed a heavy growth of Escherichia coli. Courtesy of Andrew Kent, Willows Veterinary Centre and Referral Service
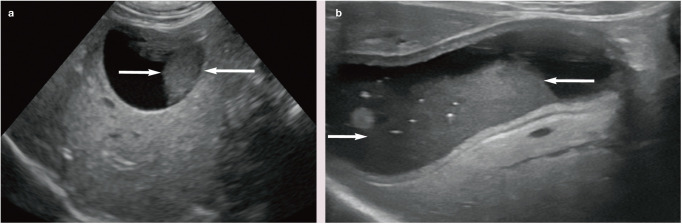
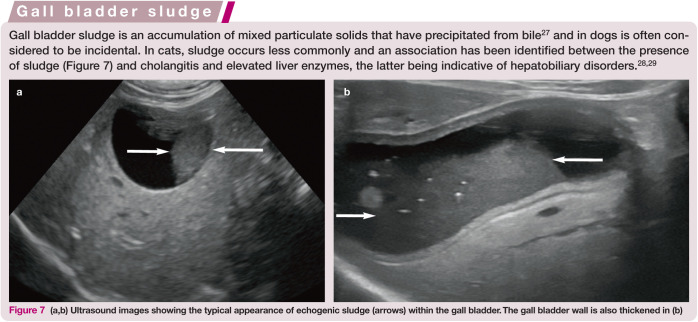
Figure 7.
(a,b) Ultrasound images showing the typical appearance of echogenic sludge (arrows) within the gall bladder. The gall bladder wall is also thickened in (b)
Neoplasia arising from the gall bladder epithelium is very rare in the cat. Adenocarcinomas have occasionally been reported, although the ultrasonographic appearance has not been described.23,24 Primary gall bladder lymphoma has been reported in the cat and described ultrasonographically as severe generalised mural thickening (up to 14 mm) almost obliterating the gall bladder lumen. 25 In a second case of gall bladder lymphoma, the appearance was that of multiple sessile hyperechoic nodules protruding from the wall into the lumen. 26
Pseudothickening of the gall bladder wall due to peritoneal effusion has also been described and is believed to be the result of the acoustic interface between the peritoneal fluid and the wall of the gall bladder. 13
Inflammation of the biliary tree
Cholangitis is a common inflammatory disorder of the biliary system in cats; two distinct forms, neutrophilic and lymphocytic, have been recognised depending on the predominant inflammatory cell type. 22 Since concurrent involvement of the liver parenchyma is common, the term cholangitis/cholangiohepatitis complex (CCHC) is sometimes used. 22
In many cases, the liver and biliary tree are unremarkable on ultrasound examination.22,30 When abnormalities are present they include: gall bladder and/or CBD wall thickening; hyperechoic gall bladder contents, possibly due to either gall bladder dysfunction or biliary stasis; choleliths and/or choledocholiths; and dilation of the CBD (Figure 8).22,31–34 Changes to the appearance of the gall bladder in cats with neutrophilic cholangitis may be the result of concurrent bacterial cholecystitis (infectious inflammation of the gall bladder).22,35 Dilation of the CBD in cats with cholangitis can be the result of inflammation-induced biliary stasis or obstruction of the lumen by biliary sludge or a calculus (choledocholith). 34 Choledocholiths may be mineralised or non-mineralised and can be both a cause and consequence of biliary obstruction; if obstruction is complete, regardless of the underlying cause, rupture of the gall bladder or CBD can occur.6,21,36
Figure 8.
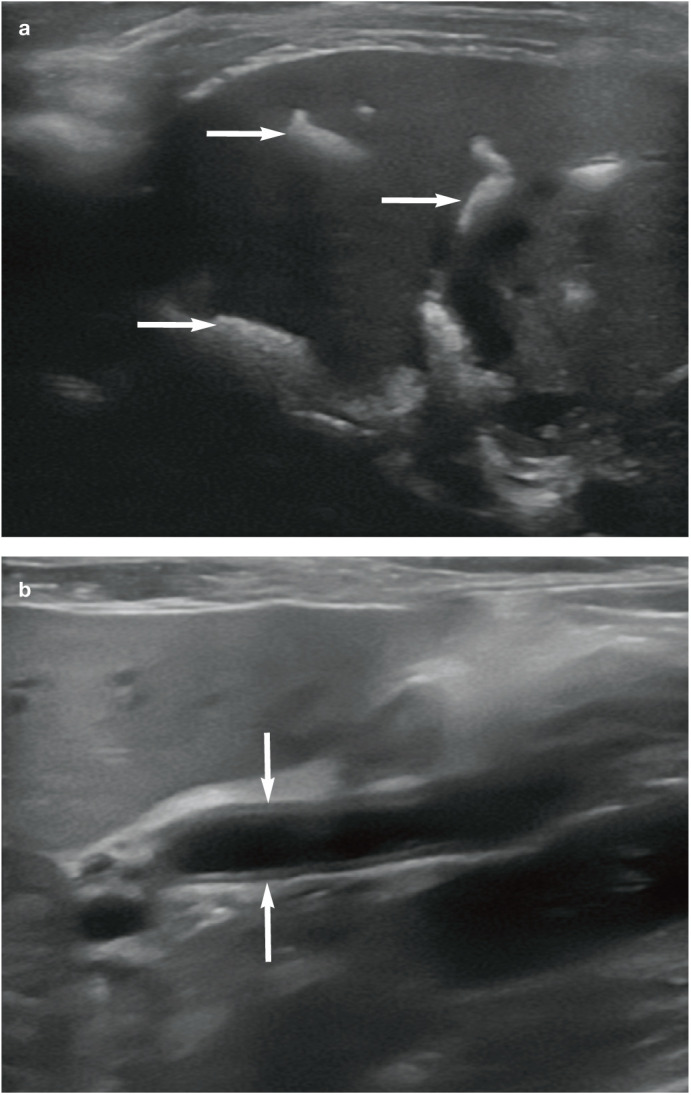
(a) Multiple tortuous hyperechoic tracts (arrows) throughout the liver, which are associated with distal acoustic shadowing and represent extensive choledocholithiasis, in a 16-year-old male neutered domestic shorthair cat with advanced renal failure. (b) Dilation of the common bile duct (arrows) in a 14-year-old female neutered domestic shorthair cat with neutrophilic cholangiohepatitis
Mineralised choleliths and choledocholiths are easily recognisable on ultrasound examination due to their hyperechoic interface and associated distal acoustic shadowing (Figure 9). The latter can be accentuated by placing the cholelith within the focal zone of the transducer and by using a high frequency.37,38 Gall bladder choleliths are often incidental although, as mentioned above, they can cause biliary obstruction if they migrate from the gall bladder into the CBD.
Figure 9.
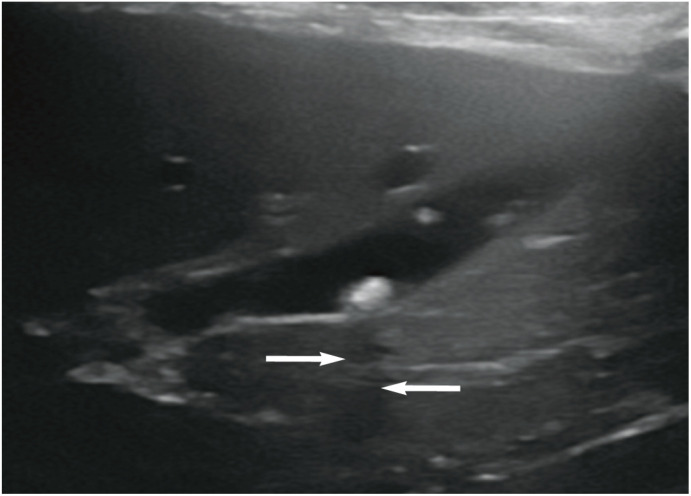
Ultrasound image showing the typical appearance of a cholelith. A well-defined hyperechoic structure is visible within the lumen of the gall bladder. Note the subtle acoustic shadow (arrows) distal to the stone as a result of mineralisation
Enlargement of the pancreas, most likely due to pancreatitis, has also been reported in cats with CCHC. 34 Both pancreatitis and inflammatory bowel disease are common concurrent findings in cats with CCHC due to the fusion of the CBD and pancreatic duct prior to their common entry into the duodenum. 39
Extrahepatic biliary obstruction
Obstruction of the CBD frequently occurs close to or at the level of the duodenal papilla 15 and can arise as a result of mural thickening, extraluminal compression or obstruction of the lumen. 14 As previously mentioned, specific causes of luminal obstruction in cats include choledocholithiasis, mucosal proliferation and biliary sludge (Figure 10).
Figure 10.
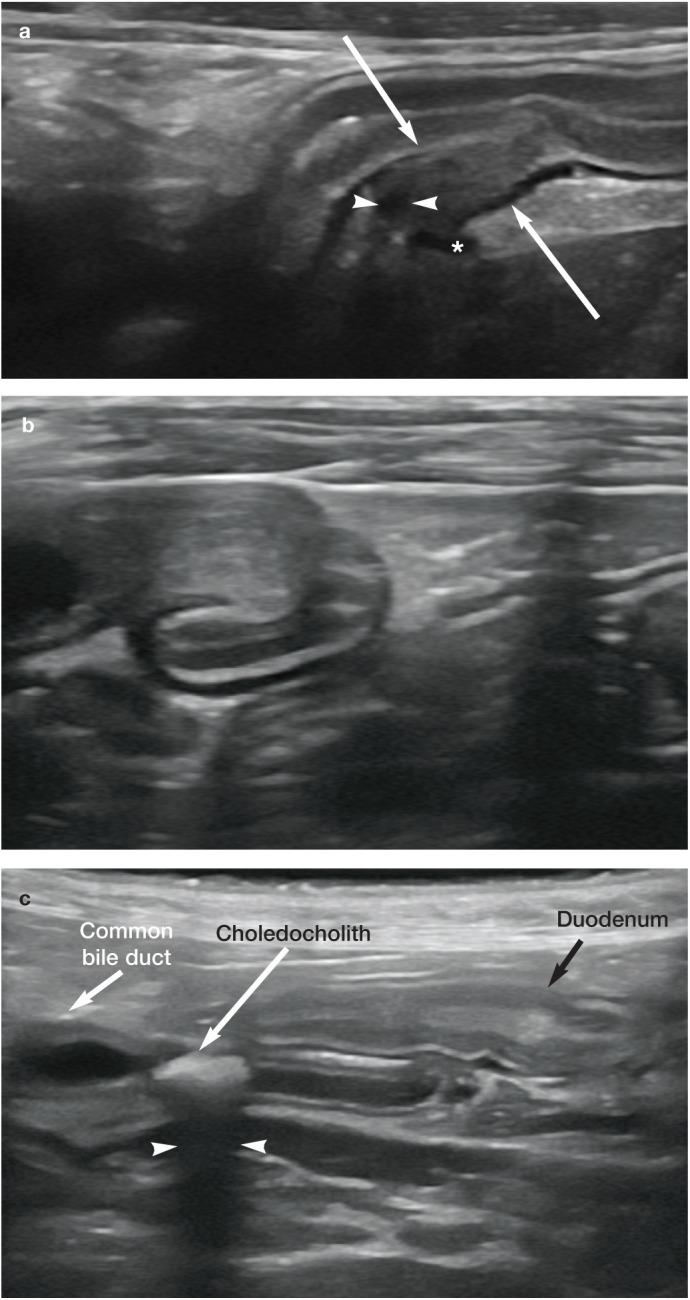
(a) Partial obstruction of the common bile duct, presumed to be due to biliary sludge or wall thickening, at the level of the duodenal papilla (arrows) in a 16-year-old cat with cholangiohepatitis and pancreatitis. The common bile duct (arrowheads) and pancreatic duct (asterisk) can be seen entering the duodenal papilla. (b) Obstruction of the common bile duct due to biliary sludge and/or thickening of the duct wall at the level of the duodenal papilla in a 3-year-old male neutered domestic shorthair cat with cholangiohepatitis. (c) Obstructive choledocholith in the common bile duct of a 15-year-old female neutered domestic shorthair cat. Note the strong clean distal acoustic shadowing (arrowheads) confirming the mineralised nature of the choledocholith
Pancreatitis is known to cause extraluminal obstruction of the CBD in some cats due to the close association of the right pancreatic limb with the distal portion of the duct.40,41 This may be recognised ultrasonographically as enlargement of the pancreas, reduced pancreatic echogenicity and/or an increase in echogenicity of the peripancreatic fat. Neoplasia arising from the CBD, pancreas or proximal duodenum is a less common cause of extrahepatic biliary obstruction (EHBO) (Figure 11).21,42–46 It is often not possible to definitively differentiate between an obstructive mass caused by inflammation and a neo-plastic mass since both conditions can share similar features on ultrasound. 15
Figure 11.
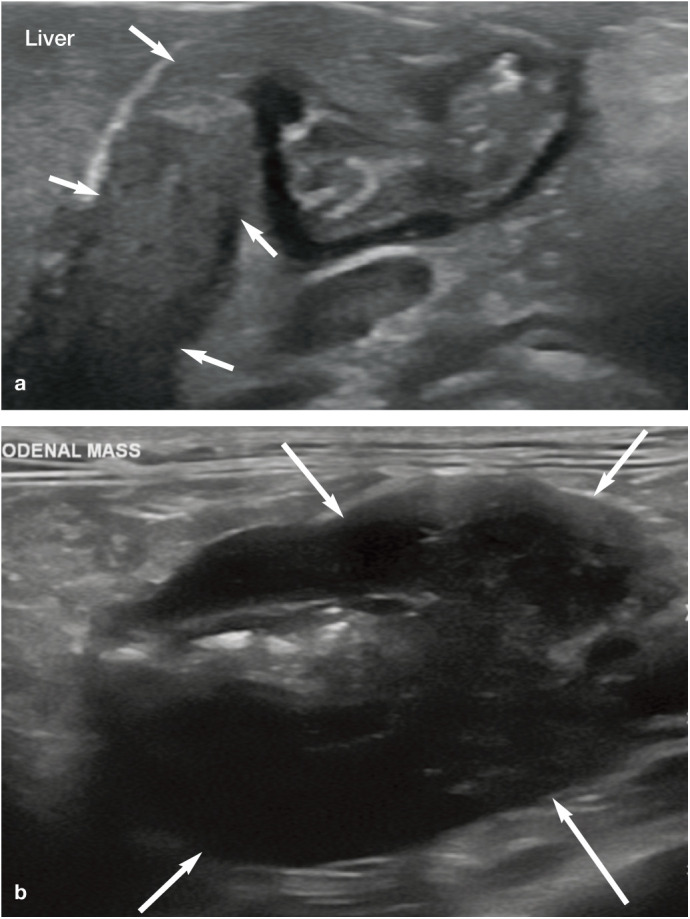
(a) Obstruction and effacement of the distal common bile duct by a cholangiocarcinoma (arrows) in a 15-year-old male neutered domestic longhair cat. (b) Large duodenal mass (arrows; most likely lymphoma, adenocarcinoma or mast cell tumour) at the level of the duodenal papilla that caused complete obstruction of the common bile duct in a 6-year-old male neutered domestic shorthair cat
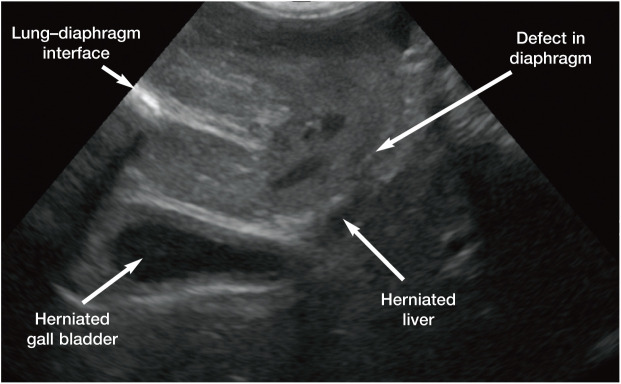
Unusual causes of EHBO reported in cats have included the presence of grass awns within the CBD, a foam earplug in the duodenum at the level of the major duodenal papilla, constriction of the CBD by cat hair as a result of involuntary transcavitary transplantation, diaphragmatic herniation (Figure 12) and severe liver fluke infestation.47–51 Ultrasound is helpful in such cases to confirm the need for surgical intervention.
Figure 12.
Ultrasound image of a diaphragmatic hernia in a cat. The curvilinear hyperechoic line in the near field on the left side of the image represents the normal lung–diaphragm interface. This line should be continuous across the entire image; however, in this case the line ends abruptly in the centre of the image indicating the start of the defect in the diaphragm. Both the liver and gall bladder have herniated into the thorax. While there was no evidence of biliary obstruction in this cat, it is easy to see how the common bile duct could become compressed by adjacent structures

Figure 13.
(a–c) Ultrasound images showing the typical appearance of dilated extrahepatic bile ducts in three cats. Note the relatively thick walls and tortuosity of the ducts. A small volume of echogenic free peritoneal fluid is visible between the hepatic lobes in (a). The arrows in (b) indicate the presence of sludge within the lumen of a dilated extrahepatic duct
Figure 14.
(a) Dilation of an intrahepatic bile duct (arrows). Note the echogenic walls and tortuous nature of the duct. (b) Intrahepatic duct dilation (arrows) in a different cat; (c) colour flow Doppler confirms a lack of flow within the bile ducts. (d and e) Absence of colour (and therefore flow) within a dilated intrahepatic bile duct (arrows) in two further cats with extrahepatic biliary obstruction
Gall bladder mucoceles
Gall bladder sludge must be differentiated from a mucocele. Gall bladder mucoceles result from cystic mucinous hyperplasia, which causes an abnormal increase in mucin production and consequently gall bladder distension that can ultimately lead to wall necrosis and rupture. 1 Mucinous plugs associated with the mucocele can enter and travel along the CBD, resulting in EHBO. 54 Gall bladder mucoceles are extremely rare in cats and there are only a limited number of reports documenting the condition in this species.7,15,55 This may be due to the fact that cats have fewer mucous glands in the gall bladder wall than dogs. 56 Mucoceles may be associated with vomiting, anorexia, lethargy and elevated liver enzymes, although concurrent diseases such as hepatic lipidosis and EHBO may contribute to the clinical signs.7,55
In dogs, the typical ultrasonographic description of a gall bladder mucocele is that of a hyperechoic stellate or kiwi fruit-like pattern, the exact appearance varying with the age of the mucocele. 57 This differs somewhat from the appearance reported in cats. In one of the first reports of a mucocele in a cat, the gall bladder was described ultrasonographically as having a peripheral accumulation of immobile echogenic bile surrounding centrally located hypoechoic bile, with no evidence of a striated pattern. 55 It was postulated that this could represent an early stage of the disease. A second report described the presence of organised echogenic non-mobile gall bladder content in a cat with EHBO; a diagnosis of biliary mucocele was made at necropsy. 15 In the third and final report, with the exception of a duplex gall bladder, no other ultrasound changes were present to suggest a mucocele and the diagnosis was only made following histopathology. 7 On the basis of these reports, it would appear that the classic appearance of a mucocele in dogs cannot necessarily be extrapolated to cats.
Cysts
Hepatic cysts arise solely from the bile system in cats. 16
Solitary biliary cysts are occasionally identified in cats and may be congenital or acquired. They appear ultrasonographically as well-defined, thin-walled, fluid-filled structures with anechoic contents associated with strong distal acoustic enhancement (Figure 15).54,58 Biliary cysts are classified according to whether or not they communicate with the biliary tree. Cysts that communicate with the biliary tree are known as choledochal cysts and are defined as segmental cystic dilations of extrahepatic and/or intrahepatic biliary ducts. 59
Figure 15.
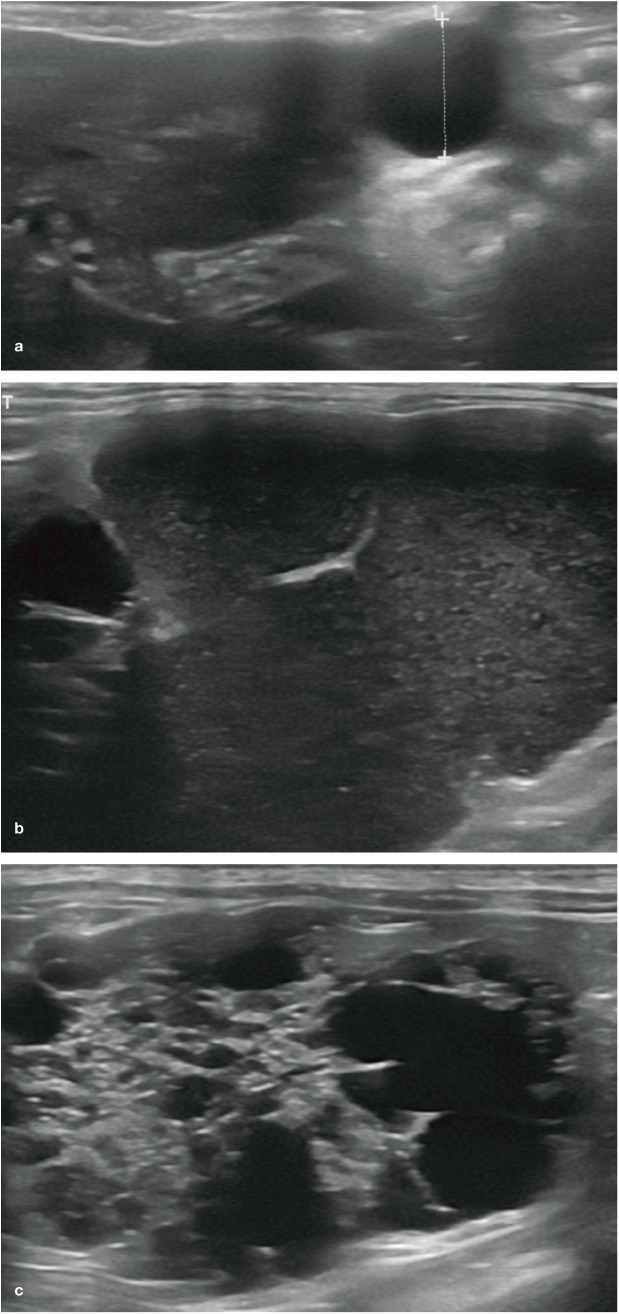
(a) Ultrasound image of a solitary, well-circumscribed anechoic spherical cyst (indicated by measuring calipers) at the caudal tip of a liver lobe. This represented an incidental finding of no clinical significance. (b) Large (>5 cm diameter) septated cystic structure containing echogenic fluid, associated with the liver of a 14-year-old male neutered Persian cat with polycystic kidney disease; (c) multiple variable-sized cysts were also present throughout the liver of the cat
Figure 16.
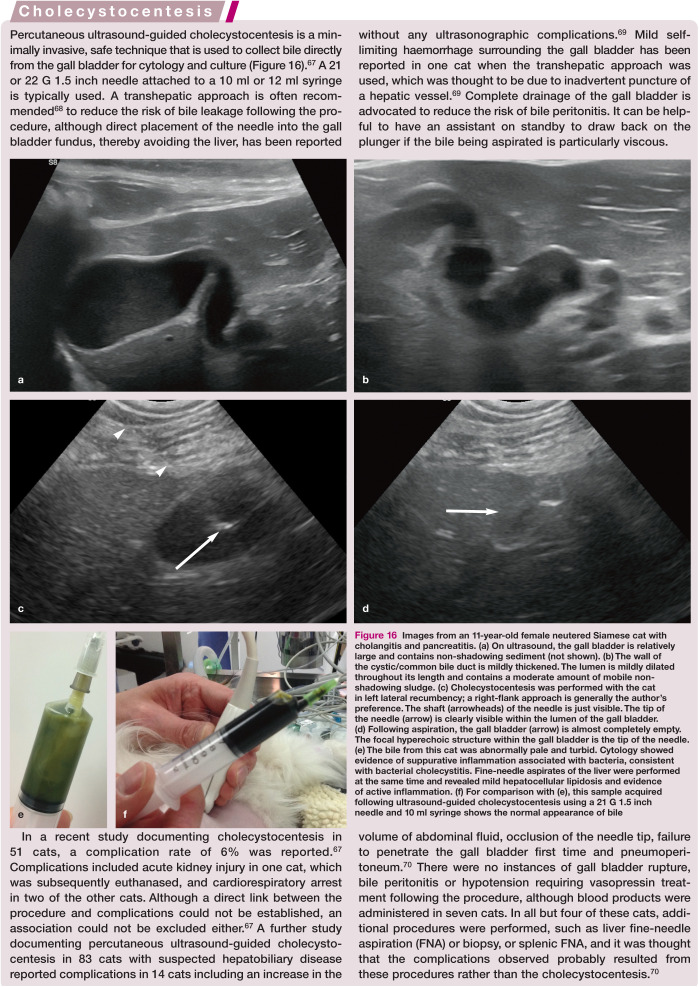
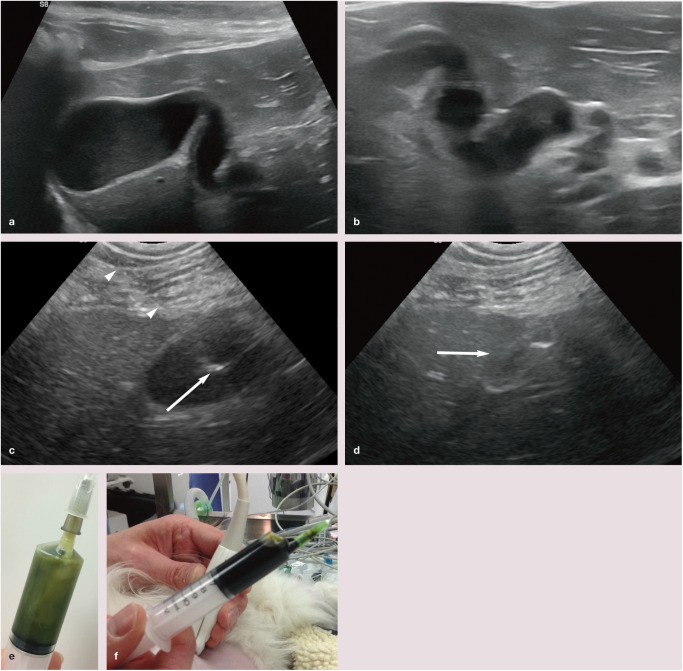
Images from an 11-year-old female neutered Siamese cat with cholangitis and pancreatitis. (a) On ultrasound, the gall bladder is relatively large and contains non-shadowing sediment (not shown). (b) The wall of the cystic/common bile duct is mildly thickened. The lumen is mildly dilated throughout its length and contains a moderate amount of mobile non-shadowing sludge. (c) Cholecystocentesis was performed with the cat in left lateral recumbency; a right-flank approach is generally the author’s preference. The shaft (arrowheads) of the needle is just visible. The tip of the needle (arrow) is clearly visible within the lumen of the gall bladder. (d) Following aspiration, the gall bladder (arrow) is almost completely empty. The focal hyperechoic structure within the gall bladder is the tip of the needle. (e) The bile from this cat was abnormally pale and turbid. Cytology showed evidence of suppurative inflammation associated with bacteria, consistent with bacterial cholecystitis. Fine-needle aspirates of the liver were performed at the same time and revealed mild hepatocellular lipidosis and evidence of active inflammation. (f) For comparison with (e), this sample acquired following ultrasound-guided cholecystocentesis using a 21 G 1.5 inch needle and 10 ml syringe shows the normal appearance of bile
Segmental dilation of the CBD consistent with choledochal cyst formation has been described in two cats.60,61 Both presented with non-specific clinical signs that included weight loss and both were icteric on physical examination. Ultrasonography revealed a 1.5 cm diameter cyst of the distal CBD in one cat and a 10 cm diameter cyst of the proximal CBD in the second.60,61 Concurrent dilation of the biliary tree was also noted at the time of the ultrasound examination.
More recently, the ultrasonographic findings in a case series of four cats with suspected choledochal cysts arising from the CBD have been reported. 59 All four were middle-aged to older neutered domestic shorthair cats with a history of chronic vomiting, reduced appetite and lethargy. Abdominal ultrasound in each case revealed segmental dilation of the CBD up to 5 cm in diameter, concurrent tubular to saccular intra- and extrahepatic bile duct dilation, accumulation of echogenic debris within the biliary tree and hepatomegaly. 59 No bile duct obstruction was identified in three of the cats described in this report (obstruction was considered equivocal in one case) and, despite marked dilation of the biliary tree on ultrasound, only one cat had (mild) hyper-bilirubinaemia. However, because the maximum diameter of the dilated CBD segment exceeded 5 mm in all cases, this could easily have led to a misdiagnosis of EHBO.
All six cats described above with choledochal cysts had evidence of a concurrent disease such as cholangitis, cholangio-hepatitis, pancreatitis or inflammatory bowel disease.
It is not clear whether choledochal cysts represent congenital or acquired biliary malformations. While some cats with choledochal cysts may exhibit only mild clinical signs despite marked dilation of the biliary tree, other choledochal cysts can result in biliary stasis and a predisposition to recurrent biliary tract infections. 59 It may be possible to manage some cats with medical treatment alone, although surgical intervention should be considered on a case-by-case basis.
Intra- or extrahepatic cysts containing bile that form separately from the biliary tree are known as bilomas or biliary pseudocysts and have been reported in a cat as a result of iatrogenic trauma caused during liver biopsy. 62 Biliary cysts can become so large that they occupy a significant portion of the abdominal cavity, causing mechanical compression and displacement of adjacent structures such as the stomach and liver.60,62,63 Multiple uni- or multilocular hepatic cysts have also been reported in association with pancreatic and renal cysts in a large proportion of cats with polycystic kidney disease (Figure 15b,c).64–66 Hepatic parenchyma surrounding uncomplicated cysts such as these is usually ultrasonographically unremarkable. 54

Key Points
Bilobed and duplex gall bladders are congenital, clinically insignificant anomalies that are present in around 1 in 8 cats.
The wall of the normal gall bladder and that of the CBD should be <1 mm diameter in the cat.
The normal CBD is readily visible in the cat. A duct diameter >5 mm is suggestive of EHBO in most cases.
Cholangitis/cholangiohepatitis may result in thickening of the wall of the gall bladder and/or CBD, biliary sludge, cholelithiasis and CBD dilation.
Gall bladder dilation is an unreliable indicator of biliary obstruction in the cat.
Hepatic cysts are biliary in origin in the cat and may be congenital or acquired, and incidental or clinically significant. They are typically thin-walled and associated with distal acoustic enhancement.
Percutaneous cholecystocentesis is a safe technique for obtaining a sample of bile for cytology, culture and sensitivity testing.
Footnotes
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Rademacher N. Liver. In: Barr F, Gaschen L. (eds). BSAVA manual of canine and feline ultrasonography. Gloucester, UK: BSAVA, 2011, pp 85–99. [Google Scholar]
- 2. Boyden EA. The accessory gall-bladder - an embryological and comparative study of aberrant biliary vesicles occurring in man and the domestic mammals. Am J Anat 1926; 38: 177–231. [Google Scholar]
- 3. Moentk J, Biller DS. Bilobed gallbladder in a cat: ultrasonographic appearance. Vet Radiol Ultrasound 1993; 34: 354–356. [Google Scholar]
- 4. Gross R. Congenital anomalies of the gallbladder. Arch Surg 1936; 32: 131–162. [Google Scholar]
- 5. Ergin I, Senel OO, Sen Y, et al. Bilobed gallbladder in a cat. Revue Med Vet 2013; 164: 453–456. [Google Scholar]
- 6. Moores AL, Gregory SP. Duplex gall bladder associated with choledocholithiasis, cholecystitis, gall bladder rupture and septic peritonitis in a cat. J Small Anim Pract 2007; 48: 404–409. [DOI] [PubMed] [Google Scholar]
- 7. Woods KS, Brisson BA, Defarges AM, et al. Congenital duplex gallbladder and biliary mucocele associated with partial hepatic cholestasis and cholelithiasis in a cat. Can Vet J 2012; 53: 269–273. [PMC free article] [PubMed] [Google Scholar]
- 8. Secrest SA, Bailey MQ. What is your diagnosis? Vesica fellea duplex. J Am Vet Med Assoc 2008; 233: 227–228. [DOI] [PubMed] [Google Scholar]
- 9. Hittmair KM, Vielgrader HD, Loupal G. Ultrasonographic evaluation of gallbladder wall thickness in cats. Vet Radiol Ultrasound 2001; 42: 149–155. [DOI] [PubMed] [Google Scholar]
- 10. Finn-Bodner ST, Park RD, Tyler JW, et al. Ultrasonographic determination, in vitro and in vivo, of canine gallbladder volume, using four volumetric formulas and stepwise-regression models. Am J Vet Res 1993; 54: 832–835. [PubMed] [Google Scholar]
- 11. Penninck DG, Brisson JO, Webster CR. Sonographic assessment of gallbladder volume in normal cats. Vet Radiol Ultrasound 2010; 51: 665–666. [DOI] [PubMed] [Google Scholar]
- 12. Diana A, Guglielmini C, Specchi S, et al. Ultrasonographic evaluation of preprandial and postprandial gallbladder volume in healthy cats. Am J Vet Res 2012; 73: 1583–1588. [DOI] [PubMed] [Google Scholar]
- 13. Center SA. Diseases of the gallbladder and biliary tree. Vet Clin North Am Small Anim Pract 2009; 39: 543–598. [DOI] [PubMed] [Google Scholar]
- 14. Leveille R, Biller DS, Shiroma JT. Sonographic evaluation of the common bile duct in cats. J Vet Intern Med 1996; 10: 296–299. [DOI] [PubMed] [Google Scholar]
- 15. Gaillot HA, Penninck DG, Webster CR, et al. Ultrasonographic features of extrahepatic biliary obstruction in 30 cats. Vet Radiol Ultrasound 2007; 48: 439–447. [DOI] [PubMed] [Google Scholar]
- 16. Otte CM, Penning LC, Rothuizen J. Feline biliary tree and gallbladder disease: aetiology, diagnosis and treatment. J Feline Med Surg 2017; 19: 514–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wegener M, Borsch G, Schneider J, et al. Gallbladder wall thickening: a frequent finding in various non-biliary disorders - a prospective ultrasonographic study. J Clin Ultrasound 1987; 15: 307–312. [DOI] [PubMed] [Google Scholar]
- 18. Colli A, Cocciolo M, Buccino G, et al. Thickening of the gallbladder wall in ascites. J Clin Ultrasound 1991; 19: 357–359. [DOI] [PubMed] [Google Scholar]
- 19. Shlaer WJ, Leopold GR, Scheible FW. Sonography of the thickened gallbladder wall: a nonspecific finding. AJR Am J Roentgenol 1981; 136: 337–339. [DOI] [PubMed] [Google Scholar]
- 20. Spaulding KA. Gallbladder wall thickness. Vet Radiol Ultrasound 1993; 34: 270–273. [Google Scholar]
- 21. Mayhew PD, Holt DE, McLear RC, et al. Pathogenesis and outcome of extrahepatic biliary obstruction in cats. J Small Anim Pract 2002; 43: 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marolf AJ, Leach L, Gibbons DS, et al. Ultrasonographic findings of feline cholangitis. J Am Anim Hosp Assoc 2012; 48: 36–42. [DOI] [PubMed] [Google Scholar]
- 23. Patnaik AK. A morphologic and immunocytochemical study of hepatic neoplasms in cats. Vet Pathol 1992; 29: 405–415. [DOI] [PubMed] [Google Scholar]
- 24. Foley P, Miller L, Graham K, et al. Cholecystadenocarcinoma in a cat. Can Vet J 1998; 39: 373–374. [PMC free article] [PubMed] [Google Scholar]
- 25. Geigy CA, Dandrieux J, Miclard J, et al. Extranodal B-cell lymphoma in the urinary bladder with cytological evidence of concurrent involvement of the gall bladder in a cat. J Small Anim Pract 2010; 51: 280–287. [DOI] [PubMed] [Google Scholar]
- 26. Baxter KJ, Hill RC, Parfitt SL, et al. Gastrointestinal small-cell lymphoma with gall bladder involvement in a cat. J Feline Med Surg 2012; 14: 267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shaffer EA. Gallbladder sludge: what is its clinical significance? Curr Gastroenterol Rep 2001; 3: 166–173. [DOI] [PubMed] [Google Scholar]
- 28. Zawie DA, Garvey MS. Feline hepatic disease. Vet Clin North Am Small Anim Pract 1984; 14: 1201–1230. [DOI] [PubMed] [Google Scholar]
- 29. Harran N, d’Anjou MA, Dunn M, et al. Gallbladder sludge on ultrasound is predictive of increased liver enzymes and total bilirubin in cats. Can Vet J 2011; 52: 999–1003. [PMC free article] [PubMed] [Google Scholar]
- 30. Sato AF, Solano M. Ultrasonographic findings in abdominal mast cell disease: a retrospective study of 19 patients. Vet Radiol Ultrasound 2004; 45: 51–57. [DOI] [PubMed] [Google Scholar]
- 31. Newell SM, Selcer BA, Girard E, et al. Correlations between ultrasonographic findings and specific hepatic diseases in cats: 72 cases (1985-1997). J Am Vet Med Assoc 1998; 213: 94–98. [PubMed] [Google Scholar]
- 32. Brain PH, Barrs VR, Martin P, et al. Feline cholecystitis and acute neutrophilic cholangitis: clinical findings, bacterial isolates and response to treatment in six cases. J Feline Med Surg 2006; 8: 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eich CS, Ludwig LL. The surgical treatment of cholelithiasis in cats: a study of nine cases. J Am Anim Hosp Assoc 2002; 38: 290–296. [DOI] [PubMed] [Google Scholar]
- 34. Callahan Clark JE, Haddad JL, Brown DC, et al. Feline cholangitis: a necropsy study of 44 cats (1986-2008). J Feline Med Surg 2011; 13: 570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cullen JM. Summary of the World Small Animal Veterinary Association standardisation committee guide to classification of liver disease in dogs and cats. Vet Clin North Am Small Anim Pract 2009; 39: 395–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baker SG, Mayhew P, Mehler SJ. Choledochotomy and primary repair of extrahepatic biliary duct rupture in seven dogs and two cats. J Small Anim Pract 2011; 52: 32–37. [DOI] [PubMed] [Google Scholar]
- 37. Larson MM. Ultrasound imaging of the hepatobiliary system and pancreas. Vet Clin North Am Small Anim Pract 2016; 46: 453–480. [DOI] [PubMed] [Google Scholar]
- 38. Nyland TG, Larson MM, Mattoon JS. Liver. In: Mattoon JS, Nyland TG. (eds). Small animal diagnostic ultrasound. 3rd ed. St Louis, MO: Elsevier Saunders, 2015, pp 332–399. [Google Scholar]
- 39. Simpson KW. Pancreatitis and triaditis in cats: causes and treatment. J Small Anim Pract 2015; 5: 40–49. [DOI] [PubMed] [Google Scholar]
- 40. Son TT, Thompson L, Serrano S, et al. Surgical intervention in the management of severe acute pancreatitis in cats: 8 cases (2003-2007). J Vet Emerg Crit Care (San Antonio) 2010; 20: 426–435. [DOI] [PubMed] [Google Scholar]
- 41. Mayhew PD, Weisse CW. Treatment of pancreatitis-associated extrahepatic biliary tract obstruction by choledochal stenting in seven cats. J Small Anim Pract 2008; 49: 133–138. [DOI] [PubMed] [Google Scholar]
- 42. Zawie DA, Shaker E. Diseases of the liver. In: Sherding RG. (ed). The cat: diseases and clinical management. New York: Churchill Livingstone, 1989, p 1015. [Google Scholar]
- 43. Pastor J, Majo N, Arbona C, et al. Sclerosing adenocarcinoma of the extrahepatic bile duct in a cat. Vet Rec 1997; 140: 367–368. [DOI] [PubMed] [Google Scholar]
- 44. Barsanti JA, Higgins RJ, Spano JS, et al. Adenocarcinoma of the extrahepatic bile duct in a cat. J Small Anim Pract 1976; 17: 599–605. [DOI] [PubMed] [Google Scholar]
- 45. Naus MJ, Jones BR. Cholelithiasis and choledocholithia-sis in a cat. N Z Vet J 1978; 26: 160–161. [DOI] [PubMed] [Google Scholar]
- 46. Wold AM. Obstructive jaundice in a cat resulting from choledocholithiasis. J Am Vet Med Assoc 1984; 185: 85–87. [PubMed] [Google Scholar]
- 47. Della Santa D, Schweighauser A, Forterre F, et al. Imaging diagnosis - extrahepatic biliary tract obstruction secondary to a duodenal foreign body in a cat. Vet Radiol Ultrasound 2007; 48: 448–450. [DOI] [PubMed] [Google Scholar]
- 48. Cornell KK, Jakovljevic S, Waters DJ, et al. Extra-hepatic biliary obstruction secondary to diaphragmatic hernia in two cats. J Am Anim Hosp Assoc 1993; 29: 502–507. [Google Scholar]
- 49. Linton M, Buffa E, Simon A, et al. Extrahepatic biliary duct obstruction as a result of involuntary transcavitary implantation of hair in a cat. JFMS Open Rep 2015; 1. DOI: 10.1177/2055116915610359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brioschi V, Rousset N, Ladlow JF. Imaging diagnosis -extrahepatic biliary tract obstruction secondary to a biliary foreign body in a cat. Vet Radiol Ultrasound 2014; 55: 628–631. [DOI] [PubMed] [Google Scholar]
- 51. Haney DR, Christiansen JS, Toll J. Severe cholestatic liver disease secondary to liver fluke (Platynosomum concinnum) infection in three cats. J Am Anim Hosp Assoc 2006; 42: 234–237. [DOI] [PubMed] [Google Scholar]
- 52. Nyland TG, Gillett NA. Sonographic evaluation of experimental bile duct ligation in the dog. Vet Radiol 1982; 23: 252–260. [Google Scholar]
- 53. Buote NJ, Mitchell SL, Penninck D, et al. Cholecysto-enterostomy for treatment of extrahepatic biliary tract obstruction in cats: 22 cases (1994-2003). J Am Vet Med Assoc 2006; 228: 1376–1382. [DOI] [PubMed] [Google Scholar]
- 54. d’Anjou MA Penninck D. Liver . In: Penninck D and d’Anjou MA (eds). Atlas of small animal ultrasonography Iowa: John Wiley & Sons, 2015, pp 183–238. [Google Scholar]
- 55. Bennett SL, Milne M, Slocombe RF, et al. Gallbladder mucocele and concurrent hepatic lipidosis in a cat. Aust Vet J 2007; 85: 397–400. [DOI] [PubMed] [Google Scholar]
- 56. Vielgrader HD. Sonographische und klinische Diagnostik bei Gallenblasen und Gallengangserkrankungen der Katze [dissertation]. Universitat Wien, 1998. [Google Scholar]
- 57. Choi J, Kim A, Keh S, et al. Comparison between ultrasonographic and clinical findings in 43 dogs with gallbladder mucoceles. Vet Radiol Ultrasound 2014; 55: 202–207. [DOI] [PubMed] [Google Scholar]
- 58. Scruggs SM, Bright JM. Chronic cardiac tamponade in a cat caused by an intrapericardial biliary cyst. J Feline Med Surg 2010; 12: 338–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Spain HN, Penninck DG, Webster CR, et al. Ultrasonographic and clinicopathologic features of segmental dilatations of the common bile duct in four cats. JFMS Open Rep 2017; 3. DOI: 10.1177/2055116917716881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Best EJ, Bush DJ, Dye C. Suspected choledochal cyst in a domestic shorthair cat. J Feline Med Surg 2010; 12: 814–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Grand JG, Doucet M, Albaric O, et al. Cyst of the common bile duct in a cat. Aust Vet J 2010; 88: 268–271. [DOI] [PubMed] [Google Scholar]
- 62. Berry CR, Ackerman N, Charach M, et al. Iatrogenic biloma (biliary pseudocyst) in a cat with hepatic lipidosis. Vet Radiol Ultrasound 1992; 33: 145–149. [Google Scholar]
- 63. Washizu M, Kobayashi K, Misaka K, et al. Surgery of hepatic cysts in a cat. J Vet Med Sci 1992; 54: 1051–1053. [DOI] [PubMed] [Google Scholar]
- 64. Stebbins KE. Polycystic disease of the kidney and liver in an adult Persian cat. J Comp Pathol 1989; 100: 327–330. [DOI] [PubMed] [Google Scholar]
- 65. Bosje JT, van den Ingh TS, van der Linde-Sipman JS. Polycystic kidney and liver disease in cats. Vet Q 1998; 20: 136–139. [DOI] [PubMed] [Google Scholar]
- 66. Crowell WA, Hubbell JJ, Riley JC. Polycystic renal disease in related cats. J Am Vet Med Assoc 1979; 175: 286–288. [PubMed] [Google Scholar]
- 67. Schiborra F, McConnell JF, Maddox TW. Percutaneous ultrasound-guided cholecystocentesis: complications and association of ultrasonographic findings with bile culture results. J Small Anim Pract 2017; 58: 389–394. [DOI] [PubMed] [Google Scholar]
- 68. McGahan JP, Walter JP. Diagnostic percutaneous aspiration of the gallbladder. Radiology 1985; 155: 619–622. [DOI] [PubMed] [Google Scholar]
- 69. Savary-Bataille KC, Bunch SE, Spaulding KA, et al. Percutaneous ultrasound-guided cholecystocentesis in healthy cats. J Vet Intern Med 2003; 17: 298–303. [DOI] [PubMed] [Google Scholar]
- 70. Byfield VL, Callahan Clark JE, Turek BJ, et al. Percutaneous cholecystocentesis in cats with suspected hepatobiliary disease. J Feline Med Surg 2017; 19: 1254–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]