Abstract
Objectives
Feline asthma (FA) and feline chronic bronchitis (CB) are common respiratory conditions in cats, frequently referred to as ‘feline lower airway disease’. However, the aetiologies of both inflammatory airway diseases are probably different. Little is known about the differences in signalment, clinical signs, laboratory abnormalities and radiographic features between cats with these two airway diseases. The aim of the study was to investigate whether certain parameters can help in differentiating between both diseases, as distinguished by airway cytology.
Methods
Seventy-three cats with FA and 24 cats with CB were included in the retrospective study. Inclusion criteria were compatible clinical signs and a cytological evaluation of bronchoalveolar lavage fluid indicating either FA (eosinophilic inflammation) or CB (neutrophilic inflammation) without cytological or microbiological evidence of bacterial infection. Parameters of signalment, physical examination, haematology and thoracic radiographs of both disease groups were compared statistically (P <0.05).
Results
The median age of cats with FA was 6 years, and was 7.5 years in cats with CB (P = 0.640). The most commonly reported clinical signs in both groups were a cough (95% FA/96% CB; P = 1.000), pathological pulmonary auscultatory sounds (82% FA/79% CB; P = 0.766) and dyspnoea (73% FA/79% CB; P = 0.601). Abnormal radiographic lung patterns were detected in 94% of cats with FA and 91% with CB (P = 0.629), respectively. Blood eosinophilia was significantly more common in cats with FA (40%) compared with CB (27%) (P = 0.026).
Conclusions and relevance
The study indicates that a differentiation of FA and CB by means of signalment, a single clinical sign, and haematological and radiographic findings is not possible.
Keywords: Feline lung disease, eosinophils, respiratory distress, lower airway disease, dyspnoea, cough
Introduction
Feline asthma (FA) and feline chronic bronchitis (CB) represent the main chronic lower respiratory diseases in cats. 1 About 1% of the feline population is affected. 1 The exact definition of FA and CB is still controversially discussed.2–5 FA is thought to result from a T-cell-based type 1 hypersensitivity reaction.6,7 CB has been defined as an inflammatory respiratory condition with increased neutrophilic content (>7%) in the bronchoalveolar lavage fluid (BALF) in the absence of increased eosinophils. 8 The condition is characterised by neutrophilic inflammation, mucosal oedema, increased mucus production and mucosal hypertrophy.9,10 Primary insults, such as trauma, toxins or previous infections are thought to play a role in the pathogenesis of CB.10–12 In human medicine, inhaled noxious agents, such as cigarette smoke, are a major factor in the development of CB, 13 which have been discussed as potential triggers for airway disease in veterinary medicine as well.12,14
To date, still little is known about the population characteristics of affected cats with both diseases. Cats with FA were described as young at onset of the disease. 3 Siamese cats were over-represented in some studies about feline chronic bronchial diseases.5,15,16 Cats with both conditions can be presented with a cough, abnormal respiratory sounds and dyspnoea; however, some authors mention severe dyspnoea as a clinical sign more common in cats with FA.10,17
In cats with chronic inflammatory bronchial diseases, thoracic radiographs typically reveal bronchial or bronchointerstitial lung patterns but have been reported as normal in up to 23% of patients. 16 Peripheral eosinophilia has been described in about 20% of patients with FA,7,18 while erythrocytosis can be seen as a consequence of many chronic respiratory diseases resulting from hypoxaemia. 19
To date, cats with CB have not been well described regarding signalment, clinical, laboratory and radiographic abnormalities. It is unknown whether typical diagnostic features described in cats with FA are equally common in cats with CB.
Therefore, the aim of the study was to investigate diagnostic parameters in cats with FA and CB and to compare these between both groups to identify potential markers for non-invasive clinical differentiation between both disease complexes.
Materials and methods
The retrospective study included cats that were presented to the Clinic of Small Animal Medicine of LMU University of Munich, Germany, from 2003 to 2016.
Ethical approval
Owing to the retrospective study design, no approval from the ethical review committee was needed. All diagnostic tests were performed for clinical use only and with pet owner agreement.
Inclusion criteria
Inclusion criteria for cats with FA and CB were clinical signs typical for inflammatory bronchial disease, including a cough, abnormal pulmonary auscultatory sounds or dyspnoea. Dyspnoea was defined as difficult or laboured breathing, 20 characterised by changes in type of respiration (eg, open-mouth breathing) with or without increased respiratory rate. Cats with CB had to show clinical signs for 3 months or longer, based upon the definition of CB in human medicine.21,22 Pretreatment with antibiotics or glucocorticoids within the 3 weeks before presentation was an exclusion criterion. Coexisting diseases, for example lungworm infection in outdoor cats or cardiac diseases in cats with a heart murmur, were investigated with non-invasive tests. Lack of a full diagnostic work-up or non-diagnostic BALF cytology also led to exclusion. Diagnostic work-up included history, physical examination, thoracic radiographs, haematology (erythrocytes, differential blood count), Baermann faecal examination in outdoor cats, bronchoscopy and bronchoalveolar lavage (BAL).
BALF cytology in cats with FA had to show an increased proportion of eosinophils (>20%) or a mixed eosinophilic–neutrophilic inflammatory pattern, with eosinophils constituting >20%. BALF cytology in patients with CB had to show an increased proportion of mature neutrophils (>10%) without cytological evidence of pathogens and a negative bacterial culture. Cell counts were estimated based on the evaluation of a non-blinded board-certified clinical pathologist. Cats with a positive PCR result for Mycoplasma species were not excluded, because the significance of Mycoplasma species as a primary respiratory pathogen has not been fully clarified in cats. 23
Study population
Patients were selected from the records of 640 cats with suspected inflammatory bronchial disease.
Seventy-three cats diagnosed with FA and 24 cats with CB met the inclusion criteria.
Diagnostic procedure
To investigate the predominance of specific breeds regarding regional bias, the patient groups were retrospectively compared with the clinic population for this parameter.
Thoracic radiographs were performed and interpreted by the veterinarian in charge. Haematology and differential white blood cell counts were performed using an automated cell counter and were controlled manually by a laboratory technician, if values were outwith the reference interval (RI).
Bacterial cultures were performed at the Institute for Infectious Diseases and Zoonoses, LMU University of Munich (Germany). Mycoplasma species were detected by cultivation on modified Hayflick-Agar or by real-time PCR (IDEXX Laboratories).
Bronchoscopy and BAL were not performed in a standardised fashion: different types of bronchoscopes were used and cats were anaesthetised with different anaesthetic protocols. BAL was performed endoscopically over the working channel in 38 cats, and with a blind approach using a sterile catheter inserted into a sterile endotracheal tube in 35 cats. In 24 cats the type of BAL procedure was not reported. Normally 2–3 aliquots of about 3 ml of sterile NaCl were used for the BAL procedure.
BALF was immediately centrifuged with a Cytospin at 1000 rounds per min for 5 mins. The sediment was spread on a slide and stained with a modified Wright stain. For cytology, at least 100 cells were evaluated at five different sites on each smear.
Statistical analysis
Statistical evaluation was performed with the software RStudio version 1.0.153.
A χ2 test was used for dichotomous data with sample sizes >5. Discrete numerical data were evaluated for normal distribution by the Shapiro–Wilk test and calculated by the Wilcoxon rank-sum test with correction for continuity. Fisher’s exact test was used for nominal data with sample sizes ⩽5. McNemar’s test was used on paired nominal data, and unpaired t-test for continuous data. The level of significance was set at P <0.05 for all comparisons.
Results
Study population
The median age at time of diagnosis was 6.2 years for cats with FA (range 1–15 years) and 7.5 years for CB patients (range 1–17 years) (P = 0.640).
The group with FA consisted of 22 female cats (20 spayed), and 51 male cats (37 castrated). The CB group included 10 females (five spayed) and 14 male cats (12 castrated) (P = 0.324).
Breeds included domestic shorthair (DSH; FA 63%/CB 46%), Siamese (FA 4%/CB 4%), Burmese (FA 3%/CB 8%), Persian (FA 4%/CB 4%) and other breeds (FA 23%/CB 34%). In some cats (FA 3%/CB 4%), the breed was unknown. During the time of the study, 21,204 cats were presented to the Clinic of Small Animal Medicine for other reasons. There was neither a significant difference in breed distribution between the clinic population (67% DSH/33% other breeds) and the patient groups with FA (P = 0.832) or CB (P = 0.089) during the observed time, nor a difference in breed distribution between the groups of FA and CB (P = 0.213).
Most patients were excluded because of pretreatment with antibiotics or glucocorticoids (n = 66), bacterial infection in neutrophilic BALF (n = 8), lack of a full diagnostic work-up, including BAL (n = 514), or concurrent conditions that could have been responsible for clinical signs (n = 78). Twenty cats were excluded as BALF cytology was non-diagnostic (eg, low cellularity, destroyed cells, poor quality of the smear).
Clinical signs
The most frequently observed clinical signs in both groups were a cough (FA 95%/CB 96%, P = 1), pathological respiratory auscultatory sounds (FA 82%/CB 79%, P = 0.766) and dyspnoea (FA 73%/CB 79%, P = 0.601). Open-mouth breathing (FA 40%/CB 25%, P = 0.228) and decreased activity level (FA 21%/CB 18%, P = 0.766) were reported in some cats. Seventeen percent of CB and 38% of FA patients were presented as emergencies and had to be hospitalised and treated at the intensive care unit. Nasal discharge was observed significantly more often in patients with CB (17%) than in cats with FA (3%) (P = 0.031). Other clinical signs that were described included sneezing (FA 5%/CB 13%, P = 0.359), restlessness (FA 12%/CB 25%, P = 0.191) and vomiting/regurgitation (FA 15%/CB 29%, P = 0.139).
Thoracic radiographs
Thoracic radiographs were not available for review in most cases, but written reports were available for most cats (FA 69/73, CB 22/24). Radiographic changes were documented in 94% of cats with FA and 91% of cats with CB (P = 0.629). The predominant lung pattern described in most cases was bronchial (FA 62%/CB 41%, P = 0.112) or bronchointerstitial (FA 26%/CB 23%, P = 0.916). There was no significant difference regarding the presence of pathological radiographic findings when both groups were compared (P = 0.128).
Laboratory parameters
Increased numbers of blood eosinophilic granulocytes above the RI were present in 39.6% of patients with FA (n = 21/53) and 27.3% (n = 6/22) of cats with CB (P = 0.026) (Figure 1). For the parameters erythrocytes, leukocytes, neutrophilic/basophilic granulocytes and monocytes no significant differences were detected between groups (Table 1). Erythrocytosis was observed in 37.0% (n = 27/73) of cats with FA and 29.2% with CB (n = 7/24) (P = 0.504).
Figure 1.
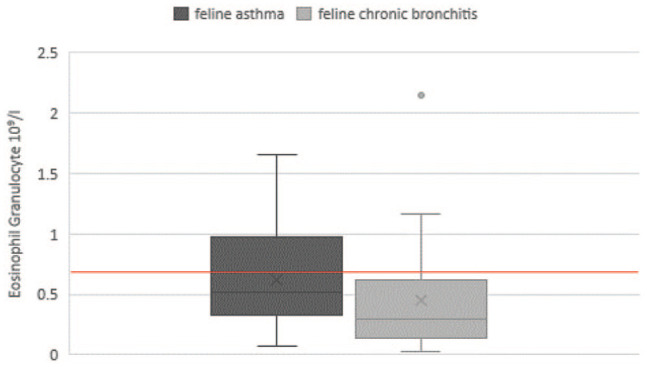
Haematological eosinophil cell count of cats with feline asthma and chronic bronchitis. The reference interval of eosinophil granulocytes is <0.6 × 109/l
Table 1.
Haematological parameters of cats with feline asthma (FA) and chronic bronchitis (CB)
| Parameter | Number of patients (FA/CB) |
FA | CB | RI | P value |
|---|---|---|---|---|---|
| WBCs | 73/24 | 9.97 ± 4.00 | 11.34 ± 4.90 | 1–11/ (–18 stress) ×109/l | 0.111 |
| Lymphocytes | 58/24 | 1.96 ± 1.92 | 1.84 ± 1.65 | 1–6 ×109/l | 0.541 |
| Eosinophilic granulocytes | 53/22 | 0.52 ± 0.42 | 0.29 ± 0.49 | <0.6 ×109/l | 0.026 |
| Neutrophilic granulocytes | 58/22 | 5.95 ± 3.48 | 6.29 ± 4.77 | 3–12 ×109/l | 0.395 |
| Basophilic granulocytes | 23/12 | 0.01 ± 0.06 | 0.02 ± 0.02 | <0.04 ×109/l | NA |
| Monocytes | 55/21 | 0.29 ± 0.24 | 0.46 ± 0.40 | <0.5 ×109/l | 0.128 |
| RBCs | 73/24 | 9.74 ± 1.25 | 9.21 ± 1.68 | 5–10 ×1012/l | 0.504 |
Data are mean ± SD unless otherwise indicated
RI = reference interval; WBCs = white blood cells; RBCs = red blood cells; NA = not available
BALF
Alveolar macrophages could be detected in 48 samples, in four FA cats BALF cytology was of poor cellularity, but a clear cytological diagnosis was possible. In 29 FA cats BALF cytology was mixed dominated eosinophilic and partial neutrophilic, 44 were only eosinophilic.
Bacterial growth was present in 21.9% (n = 16/73) of BALF samples in cats with FA. Bacterial species included Pasteurella multocida (n = 9), Staphylococcus species (n = 4), Streptococcus species (n = 3), Chryseobacterium indologenes (n = 1), Bordetella bronchiseptica (n = 1) and Pseudomonas species (n = 1). Bacterial culture was negative in all samples from cats with CB (exclusion criterion). Mycoplasma species were detected in BALF of 5/73 (three PCR/three culture, one cat positive in both) cats with FA and in 6/24 (four PCR/two culture) cats with CB (P = 0.022).
Discussion
Although feline inflammatory bronchial disease is a common condition in cats, research so far has focused mainly on the diagnostic investigation and treatment of FA. The purpose of the present study was to compare diagnostic features of cats with a cytological diagnosis of CB and FA. The current study did not reveal any specific diagnostic parameters that proved to be more helpful in discriminating between both diseases, as distinguished by airway cytology.
In the present study, most cats were middle-aged when presented for a work-up of respiratory signs, which matches findings in other studies investigating feline bronchial disease.16,24 There was no significant difference between both groups for the parameter age, and cats were within a wide age range, although the median age of patients with CB was 1.5 years older than in patients with FA. Age cannot be used to differentiate between the conditions, despite it being suggested that cats with FA are presented at a younger age,1,10 but small patient groups can lead to variations. Furthermore, the course of disease in patients with FA is reported to be more acute and severe, 10 and thus owners might consult a veterinarian earlier than owners of cats with CB.
In contrast to other reports, no significant over-representation of Siamese or other purebred cats could be detected in either disease group compared with the clinic population.5,16 It should be considered that regional preferences for cat breeds can make it difficult with compare these results with other studies. As in other investigations about feline chronic bronchial disease, no clear sex predisposition could be detected.7,15,24,16
The main reason for exclusion was the lack of a full diagnostic work-up, most likely linked to the financial or anaesthetic concerns of the owners.
In both disease groups, a cough represented the predominant clinical sign, as described in multiple previous studies.5,25 In the present study, only one cat with CB showed dyspnoea without a history of cough. However, owing to the inclusion criterion of a disease history of at least 3 months, there might have been an exclusion of other cats with CB showing predominantly dyspnoea without cough as a clinical feature. Besides a cough, abnormal respiratory auscultatory sounds and dyspnoea were present in most cases. However, when compared statistically, all three common clinical signs were equally represented in both disease groups and therefore not helpful in differentiating between these inflammatory conditions. These results are comparable to other studies.1,8
Bronchoconstriction, leading to dyspnoea, is thought to represent one of the hallmarks in the pathogenicity of FA,12,26 and reversible bronchoconstriction has been proposed to discriminate between both conditions by other authors.8,12,27 Nonetheless, airway hyperreactivity can be a feature of other lower airway disorders and, by itself, is not specific for asthma. 12 There are probably additional factors contributing to dyspnoea in cats with CB. Chronic remodelling and thickening of lower airway mucosa, airway muscles and epithelium because of local inflammation and obstruction of airways with mucus has been described in both disorders.9,27 It is well known that small changes in bronchial diameter can lead to massive airflow limitation, and inflammation leads to inappropriate airway smooth muscle contraction. 27 Therefore, promotive triggers, such as irritants or secondary infections, might be able to provoke respiratory distress in cats with CB as well. Respiratory distress in CB is thought to be less common and a rather chronic condition.8,12 Although open-mouth breathing, indicating severe respiratory distress, was seen more commonly in cats with FA than with CB in the present study, the difference between groups for this parameter was not significant. These findings agree well with the findings of Allerton et al. 1 In their study, cats with FA were not more likely to suffer from dyspnoea but were given a higher dyspnoea score.
Nasal discharge occurred more commonly in cats with CB than in patients with FA. A coexisting disease complex of FA and allergic rhinitis has been postulated for cats with experimentally induced FA, 28 but rhinitis has not been described in cats with naturally occurring CB. Nasal discharge has been observed in cats with FA and CB in this study but could have been non-related or caused by a secondary bacterial or viral infection. As infections, postnasal drip and irritations are discussed as predisposing conditions for CB,8,12,29 upper airway infections are noteworthy.
Cats with FA and CB did not show significant differences regarding their radiographic abnormalities. Bronchial and bronchointerstitial patterns dominated in both groups as expected. Radiographs of some patients were judged as physiological (FA 6%/CB 9%). In patients with feline lower airway diseases it has been described that up to 23% of cats present with physiological radiographic images. 16 The current study implies that this applies to patients with CB as well. Therefore, thoracic imaging can neither be used to differentiate between both diseases nor to rule out inflammatory bronchial disease. A study investigating radiographic abnormalities in cats with feline bronchial disease demonstrated only poor agreement between examiners for interpretation of radiographs. 30 In the present study, radiographs had been judged by the attending clinician at the time of presentation and not by a board-certified radiologist. The clinician was not blinded, which might have led to an overdiagnosis of typical lung pattern and a low number of physiological results.
CT scans in cats with respiratory diseases have been associated with a greater sensitivity regarding typical changes than conventional radiography and might therefore provide more detailed classification of bronchial diseases.4,29 Significantly more cats with FA showed increased numbers of eosinophils on haematology compared with cats with CB (Figure 1). Interleukin-5, which is released during mast cell degranulation in allergic asthma, stimulates eosinopoiesis.31–33 However, most cats (60%) with FA were presented with eosinophils within the RI in the present study. Previous studies also showed no consistent presence of eosinophilia in asthmatic cats.1,7,18 Interestingly, a certain proportion of cats with CB also showed eosinophilia in the present investigation. Eosinophilia can also be present in the course of other disease processes, such as lungworm infections, other parasites, or allergic skin or intestinal diseases. Therefore, peripheral eosinophilia can only provide an indication for the presence of FA but cannot be used for differentiation from CB.
Other haematological findings in both groups were elevated numbers of erythrocytes, probably a result of chronic respiratory distress and hypoxaemia. 19 As severe or long-term hypoxaemia is necessary to stimulate erythropoietin production, 34 this finding also underlines the fact that not only FA, but also CB, can lead to significant airflow reduction and oxygen deficit, as already indicated by the high number of cats with a history of dyspnoea.
In the present study, FA was defined as airway disease with an increased percentage of eosinophils in BALF cytology, and CB as a condition with an increased percentage of mature neutrophils without evidence of bacterial infection (excluding Mycoplasma species). Unfortunately, cytology results are not without flaws for the diagnosis of FA or CB. Cut-off values for eosinophils in BALF vary widely between studies,8,12,35 and even healthy cats can have eosinophils up to 28%. 36 A previous study has shown that the predominating cell type and the resulting cytological diagnosis can differ significantly between different lung segments in cats with inflammatory airway disease. 37 In the study by Ybarra et al, 37 almost 50% of cats with predominantly eosinophilic inflammation in one lung segment had a cytological diagnosis of predominantly neutrophilic inflammation in a sample from another lung segment. The number of lavages performed on each location can also lead to variant cytology results. 36 This raises the general question of how to differentiate between the chronic bronchial diseases at all, as cytology is not always reliable and clinical signs do not vary significantly.
In the present study, a significant number of BALF samples were taken without visualisation, which is described as a method not inferior to bronchoscopic BAL regarding cytological value by a recent study. 38 The number of macrophages was not reported for all BALF samples and detailed information on the procedure (presence of surfactant, recovered fluid volumes) were not available owing to the retrospective nature of the study. Therefore, some deep airway washes instead of true alveolar washes might have been included in some cases. Samples were pooled for cytology and multi-segment BAL could not be ensured in all cases, which might have influenced cytology results. Mixed predominantly eosinophilic and partial neutrophilic cells were present in 29 cats with FA in the present study. This represents a common finding in FA, as chronic allergic airway inflammation may attract immigration of neutrophils, leading to ‘chronic asthmatic bronchitis’.8,12
Bacterial growth in BALF was defined as an exclusion criterion for cats with CB in the present study to ensure that no cats with bacterial bronchitis were included. However, detection of Mycoplasma species on culture or by PCR did not lead to exclusion, because the pathological role of this organism in feline bronchial disease is still under debate.23,39 Mycoplasma species were significantly more frequently detected in BALF of cats with CB than in cats with FA. In human medicine, Mycoplasma infections appear to play a role in exacerbation of asthma and chronic bronchitis.40,41 If Mycoplasma organisms potentially act as primary or secondary pathogens that can trigger an inflammatory response, or if they just represent mucosal commensals, is an interesting question for future research.
The high percentage of positive bacterial cultures in cats with FA highlights the importance of bacterial examinations as part of the work-up. These results could be explained by secondary bacterial infections, non-symptomatic bacterial colonisation or oropharyngeal contamination of BALF. 42
As a retrospective study, this study has limitations, such as small patient populations due to strict inclusion criteria and the fact that complete data were not available for all patients. Disease classification was only based on cytology results, which can vary strongly depending on method, cut-off values and observer. Radiographs were not interpreted by a single blinded radiologist; therefore, misinterpretation cannot be ruled out. Because of the clinical and retrospective design of the study, underlying conditions might not have been fully assessed and partial overlap in diseases cannot be excluded. Regardless of these aspects, this is the largest comparison of FA and CB in cats with naturally occurring inflammatory bronchial disease with a full clinical work-up.
Conclusions
Although FA and CB have been seen as distinct diseases with different pathogeneses, outcomes and responses to treatment, 12 no single clinical sign, radiographic finding or laboratory parameter can be used to distinguish between these conditions. In future studies, immunological investigations, allergy and lung function testing might lead to a more detailed understanding and offer new diagnostic options to characterise patients with feline lower airway disease.
Footnotes
Accepted: 23 July 2019
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work involved the use of non-experimental animal(s) only (owned or unowned), and followed established internationally recognised high standards (‘best practice’) of individual veterinary clinical patient care. Ethical approval from a committee was therefore not necessarily required.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work for the procedure(s) undertaken. No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Maike Grotheer  https://orcid.org/0000-0003-0058-8580
https://orcid.org/0000-0003-0058-8580
Bianka Schulz  https://orcid.org/0000-0002-1785-6206
https://orcid.org/0000-0002-1785-6206
References
- 1. Allerton FJW, Leemans J, Tual C, et al. Correlation of bronchoalveolar eosinophilic percentage with airway responsiveness in cats with chronic bronchial disease. J Small Anim Pract 2013; 54: 258–264. [DOI] [PubMed] [Google Scholar]
- 2. Foster S, Allan G, Martin P, et al. Twenty-five cases of feline bronchial disease (1995–2000). J Feline Med Surg 2004; 6: 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trzil JE, Reinero CR. Update on feline asthma. Vet Clin North Am Small Anim Pract 2014; 44: 91–105. [DOI] [PubMed] [Google Scholar]
- 4. Reinero CR, Masseau I, Grobman M, et al. Perspectives in veterinary medicine: description and classification of bronchiolar disorders in cats. J Vet Intern Med 2019; 33: 1201–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Padrid P. Kirk’s current veterinary therapy XV e-book. St Louis, MO: Elsevier Health Sciences, 2013. [Google Scholar]
- 6. Tizard IR. Veterinary immunology e-book. 9th ed. St Louis, MO: Elsevier Health Sciences, 2017. [Google Scholar]
- 7. Corcoran B, Foster D, Fuentes VL. Feline asthma syndrome: a retrospective study of the clinical presentation in 29 cats. J Small Anim Pract 1995; 36: 481–488. [DOI] [PubMed] [Google Scholar]
- 8. Nafe LA, DeClue AE, Lee-Fowler TM, et al. Evaluation of biomarkers in bronchoalveolar lavage fluid for discrimination between asthma and chronic bronchitis in cats. Am J Vet Res 2010; 71: 583–591. [DOI] [PubMed] [Google Scholar]
- 9. Moses BL, Spaulding GL. Chronic bronchial disease of the cat. Vet Clin North Am Small Anim Pract 1985; 15: 929–948. [DOI] [PubMed] [Google Scholar]
- 10. Reinero C, DeClue A. Feline tracheobronchial disease. In: Fuentes VL, Johnson L, Dennis S. (eds). BSAVA manual of canine and feline cardiorespiratory medicine. 2nd ed. Quedgeley: Wiley, 2010, pp 280–284. [Google Scholar]
- 11. Chung-Hui L, Pei-Ying L, Huey-Dong W, et al. Association between indoor air pollution and respiratory disease in companion dogs and cats. J Vet Intern Med 2018; 32: 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reinero CR. Advances in the understanding of pathogenesis, and diagnostics and therapeutics for feline allergic asthma. Vet J 2011; 190: 28–33. [DOI] [PubMed] [Google Scholar]
- 13. Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001; 163: 1256–1276. [DOI] [PubMed] [Google Scholar]
- 14. Johnson LR. Diseases of airways. In: Johnson LR. (ed). Clinical canine and feline respiratory medicine. Ames, IA: Wiley, 2010, pp 113–117. [Google Scholar]
- 15. Moise N, Wiedenkeller D, Yeager A, et al. Clinical, radiographic, and bronchial cytologic features of cats with bronchial disease: 65 cases (1980–1986). J Am Vet Med Assoc 1989; 194: 1467–1473. [PubMed] [Google Scholar]
- 16. Adamama-Moraitou KK, Patsikas MN, Koutinas AF. Feline lower airway disease: a retrospective study of 22 naturally occurring cases from Greece. J Feline Med Surg 2004; 6: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taylor S. Feline lower airway disease: asthma and beyond. Vet Nurse 2017; 8: 17–23. [Google Scholar]
- 18. Lilliehöök I, Tvedten H. Investigation of hypereosinophilia and potential treatments. Vet Clin North Am Small Anim Pract 2003; 33: 1359–1378. [DOI] [PubMed] [Google Scholar]
- 19. Weiss DJ, Wardrop KJ. Schalm’s veterinary hematology. Ames, IA: John Wiley & Sons, 2011, pp 162–165. [Google Scholar]
- 20. Dickson D, Little CJL, Harris J, et al. Rapid assessment with physical examination in dyspnoeic cats: the RAPID CAT study. J Small Anim Pract 2018; 59: 75–84. [DOI] [PubMed] [Google Scholar]
- 21. Guerra S. Overlap of asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med 2005; 11: 7–13. [DOI] [PubMed] [Google Scholar]
- 22. Cruz AA. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. https://www.who.int/gard/publications/GARD_Manual/en/ (2007, accessed August 13, 2019).
- 23. Schulz BS, Richter P, Weber K, et al. Detection of feline Mycoplasma species in cats with feline asthma and chronic bronchitis. J Feline Med Surg 2014; 16: 943–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dye JA, McKiernan BC, Rozanski EA, et al. Bronchopulmonary disease in the cat: historical, physical, radiographic, clinicopathologic, and pulmonary functional evaluation of 24 affected and 15 healthy cats. J Vet Intern Med 1996; 10: 385–400. [DOI] [PubMed] [Google Scholar]
- 25. Reinero CR, DeClue AE, Rabinowitz P. Asthma in humans and cats: is there a common sensitivity to aeroallegens in shared environments? Environ Res 2009; 109: 634–640. [DOI] [PubMed] [Google Scholar]
- 26. Byers CG, Nishi Dhupa B. Feline bronchial asthma: pathophysiology and diagnosis. Comp Cont Educ Pract 2005; 27: 418–425. [Google Scholar]
- 27. Padrid P. Chronic bronchitis and asthma in cats. In: Bonagura JD, Twedt DC. (ed). Kirk’s current veterinary therapy XIV. St Louis, MO: WB Saunders, 2009, pp 650–658. [Google Scholar]
- 28. Venema CM, Williams KJ, Gershwin LJ, et al. Histopathologic and morphometric evaluation of the nasal and pulmonary airways of cats with experimentally induced asthma. Int Arch Allergy Immunol 2013; 160: 365–376. [DOI] [PubMed] [Google Scholar]
- 29. Hahn H, Specchi S, Masseau I, et al. The computed tomographic “tree-in-bud” pattern: characterization and comparison with radiographic and clinical findings in 36 cats. Vet Radiol Ultrasound 2018; 59: 32–42. [DOI] [PubMed] [Google Scholar]
- 30. Gadbois J, d’Anjou M-A, Dunn M, et al. Radiographic abnormalities in cats with feline bronchial disease and intra- and interobserver variability in radiographic interpretation: 40 cases (1999–2006). J Am Vet Med Assoc 2009; 234: 367–375. [DOI] [PubMed] [Google Scholar]
- 31. Padrid PA, Qin Y, Wells TN, et al. Sequence and structural analysis of feline interleukin-5 cDNA. Am J Vet Res 1998; 59: 1263–1269. [PubMed] [Google Scholar]
- 32. Takatsu K, Nakajima H. IL-5 and eosinophilia. Curr Opin Immunol 2008; 20: 288–294. [DOI] [PubMed] [Google Scholar]
- 33. Busse WW. The relationship of airway hyperresponsiveness and airway inflammation: airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest 2010; 138: 4S–10S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stockmann C, Fandrey J. Hypoxia-induced erythropoietin production: a paradigm for oxygen-regulated gene expression. Clin Exp Pharmacol Physiol 2006; 33: 968–979. [DOI] [PubMed] [Google Scholar]
- 35. Padrid P, Feldman B, Funk K, et al. Cytologic, microbiologic, and biochemical analysis of bronchoalveolar lavage fluid obtained from 24 healthy cats. Am J Vet Res 1991; 52: 1300–1307. [PubMed] [Google Scholar]
- 36. Hawkins E, Kennedy-Stoskopf S, Levy J, et al. Cytologic characterization of bronchoalveolar lavage fluid collected through an endotracheal tube in cats. Am J Vet Res 1994; 55: 795–802. [PubMed] [Google Scholar]
- 37. Ybarra WL, Johnson LR, Drazenovich TL, et al. Interpretation of multisegment bronchoalveolar lavage in cats (1/2001–1/2011). J Vet Intern Med 2012; 26: 1281–1287. [DOI] [PubMed] [Google Scholar]
- 38. Hooi KS, Defarges AM, Sanchez AL, et al. Comparison of bronchoscopic and nonbronchoscopic bronchoalveolar lavage in healthy cats. Am J Vet Res 2018; 79: 1209–1216. [DOI] [PubMed] [Google Scholar]
- 39. Lee-Fowler T. Feline respiratory disease: what is the role of Mycoplasma species? J Feline Med Surg 2014; 16: 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hong S-J. The role of Mycoplasma pneumoniae infection in asthma. Allergy Asthma Immunol Res 2012; 4: 59–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cherry JD, Taylor-Robinson D, Willers H, et al. A search for mycoplasma infections in patients with chronic bronchitis. Thorax 1971; 26: 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baral RM. Lower respiratory tract disease. In: Little S. (ed). The cat-e-book: clinical medicine and management. 2nd ed. St Louis, MO: Elsevier Health Sciences, 2011. [Google Scholar]


