Abstract
Objectives
Phage–gonadotropin-releasing hormone (GnRH) constructs with potential contraceptive properties were generated in our previous study via selection from a phage display library using neutralizing GnRH antibodies as selection targets. In mice, these constructs invoked the production of antibodies against GnRH and suppressed serum testosterone. The goal of this study was to evaluate this vaccine against GnRH for its potential to suppress reproductive characteristics in cats.
Methods
Sexually mature male cats were injected with a phage–GnRH vaccine using the following treatment groups: (1) single phage–GnRH vaccine with adjuvant; (2) phage–GnRH vaccine without adjuvant and half-dose booster 1 month later; or (3) phage–GnRH vaccine with adjuvant and two half-dose boosters with adjuvant 3 and 6 months later. Anti-GnRH antibodies and serum testosterone, testicular volume and sperm characteristics were evaluated monthly for 7–9 months.
Results
All cats developed anti-GnRH antibodies following immunization. Serum antibody titers increased significantly after booster immunizations. In group 3, serum testosterone was suppressed 8 months after primary immunization. Total testicular volume decreased in group 1 by 24–42% and in group 3 by 15–36% at 7 months after immunization, indicating potential gonadal atrophy. Vacuolation of epididymides was observed histologically. Although all cats produced sperm at the conclusion of the study, normal morphology was decreased as much as 38%. Phage alone produced no local or systemic reactions. Immunization of phage with AdjuVac produced unacceptable injection site reactions.
Conclusions and relevance
Our phage-based vaccine against GnRH demonstrated a potential for fertility impairment in cats. Future research is required to optimize vaccine regimens and identify animal age groups most responsive to the vaccine. If permanent contraception (highly desirable in feral and shelter cats) cannot be achieved, the vaccine has a potential use in zoo animals or pets where multiple administrations are more practical and/or reversible infertility is desirable.
Keywords: Fertility control, filamentous phage, gonadotropin-releasing hormone, GnRH
Introduction
The domestic cat is an important target species for population control as millions of free-roaming cats inhabit rural and urban areas worldwide. This is a serious animal welfare problem and a growing concern for public health as cats can serve as a reservoir for viruses and parasites that cause zoonotic diseases. While surgical sterilization remains the ‘gold standard’ for permanent sterilization, contraception of cats with anti-fertility vaccines is recognized as a promising tool for managing their numbers.1–4
Gonadotropin-releasing hormone (GnRH) is an attractive target for animal contraception because the same contraceptive product targeting GnRH might be effective in multiple mammalian species, as well as in both sexes. It is a hypothalamic peptide that specifically binds to the GnRH receptor on gonadotrope cells in the pituitary gland. Through binding to this receptor, GnRH regulates synthesis and secretion of pituitary gonadotropins that are essential for gametogenesis and synthesis of gonadal steroids crucial for animal reproduction. GnRH vaccines stimulate production of antibodies that inactivate endogenous GnRH causing reduced release of gonadotropic hormones followed by gonadal atrophy. GnRH is a well-studied target in animal immunocontraception with continuing interest.5–7 Given that GnRH is a small (10 amino acids) ‘self’ protein, it requires conjugation to a larger carrier protein for increased antigenicity. Most of the GnRH-based vaccines are chemical conjugates of multiple GnRH copies with carrier proteins such as tetanus toxoid, 8 diphtheria toxoid, 9 keyhole limpet hemocyanin, 10 Concholepas concholepas hemocyanin (blue protein) 4 or ovalbumin. 11
The vaccine used in this study utilizes filamentous phage virions as a carrier. Phage virions are genetically engineered to display GnRH-like peptides as fusions to the phage coat proteins. 12 Phages are highly immunogenic due to their particulate nature and nanoscale size. Methods for engineering phage–peptide constructs are well established and easy to reproduce. Distinct from chemical conjugates, phage-based preparations possess excellent batch-to-batch consistency. As phages are bacterial viruses, they are not pathogenic for animals. Filamentous phages have been used for immunization of mice, dogs, goats, sheep and pigs, with no local or systemic adverse reactions.13–15 In addition to these characteristics, low cost and phage stability in various environmental conditions make phages an attractive antigen delivery system for vaccine development, including vaccines for suppression of fertility.16,17
Recently, we have developed several phage-based vaccines against GnRH that were tested in male mice and demonstrated promising contraceptive properties. 12 These proof of principle results in mice warranted the next logical step in evaluating the vaccines in the target species, cats. Consequently, the objectives of the study were focused on evaluation of the vaccines for immune responses and effects on reproductive parameters (testosterone, semen production and quality, testicle size and histology) in cats.
Materials and methods
Animal inclusion criteria and description
The cats used in this study were obtained from and maintained at a breeding colony at the Scott-Ritchey Research Center, Auburn University College of Veterinary Medicine. Fifteen intact male domestic cats made up three experimental groups (five cats/group): (1) single phage–GnRH vaccine with AdjuVac adjuvant; (2) phage–GnRH vaccine without adjuvant and half-dose booster 1 month later; or (3) phage–GnRH vaccine with AdjuVac adjuvant and two half-dose boosters with AdjuVac and Thermogel adjuvants 3 and 6 months later, respectively. All cats were post-pubertal prior to the start of the study. In group 1, four cats were 9–10 months old and one cat was 18 months old. In group 2, cats were 12–13 months and in group 3 cats were 11–12 months old. Cats in all groups served as self-controls. Post-immunization parameters were compared with pre-immunization pa-rameters collected from the same cats. For histological evaluation, testes of treated cats were compared with two control cats (12 months old) from the same colony.Cats were housed in small groups of males only in indoor runs under artificial lights. All animal procedures were approved by the Auburn University Institutional Animal Care and Use Committee (IACUC). Prior to vaccination, cats were assessed for general health by a complete physical examination, complete blood count and serum chemistry. A second complete blood count and serum chemistry was performed at the conclusion of the study, at which time cats were neutered surgically and adopted into private homes per IACUC guidelines.
Preparation of injections and immunization of cats
To obtain suitable quantities of phage for experiments in cats, phage was amplified in bacteria and purified using standard techniques. 18 Phage concentration in virions per milliliter was assessed by reading the absorbance at 269 nm using a NanoDrop 2000 spectrophotometer (ThermoFisher Scientific). Phage used in the study was generated in our previous work 12 to display EHPSYGLA fusion peptide. (Note: the peptide sequence is presented in single-letter amino acid code. Amino acid residues that are identical to the GnRH sequence, EHWSYGLRPG, are underlined.) This phage–GnRH construct was obtained via isolation from an 8-mer landscape phage display library using neutralizing anti-GnRH antibody as a selection target.
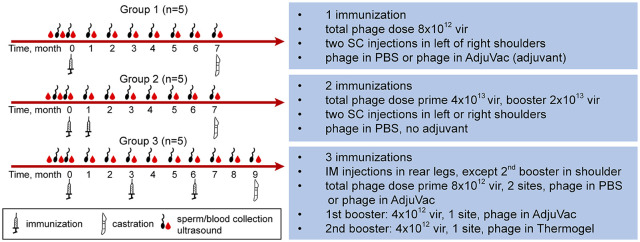
Cats were immunized with phage–adjuvant mixtures (groups 1 and 3) or with phage alone (group 2). Injections containing phage alone were prepared in phosphate buffered saline (PBS). Injections containing AdjuVac United States Department of Agriculture (USDA) were prepared by mixing phage in PBS with AdjuVac at a 1:1 ratio. To prepare injections containing Thermogel (AK97; Polyscitech), the polymer was first solubilized in PBS at 1:5 w/v ratio and then mixed with phage in PBS. Group 1 cats received phage with AdjuVac subcutaneously (SC) in the left scapular region and phage with PBS SC in the right scapular region. Group 2 cats received both injections (phage in PBS only) SC in the left (initial vaccine) and right (booster) scapular region. Group 3 cats received three intramuscular injections. The initial vaccine was administered into the semi-membranosus muscle of the left hindlimb, the second vaccine was administered into the triceps muscle and the third injection was administered into the right semi-membranosus muscle. All injections were delivered in a volume of 0.5 ml. The study design (phage doses, adjuvants, route/location of immunizations, time of boosters, duration of experiments, etc) is detailed in Figure 1.
Figure 1.
Study design. Group 1 received one-time immunization. A total phage dose of 8 × 1012 vir was split into two equal injections. The injections were given subcutaneously (SC) in the left and right shoulders. One of the injections contained phage–gonadotropin-releasing hormone (GnRH) in phosphate buffered saline (PBS) and the other had phage mixed with AdjuVac. Group 2 received two immunizations, a prime immunization followed by a booster immunization 1 month later. The prime immunization was a total phage dose of 4 × 1013 vir split into two equal injections given SC in the left and right shoulders. The booster immunization was 2 × 1013 vir split between two shoulders. All injections contained phage in PBS (no adjuvant). Group 3 received three immunizations, a prime immunization followed by two boosters 3 months apart. The prime immunization was a total phage dose of 8 × 1012 vir split between two injections. The injections were given intramuscularly (IM) in the left and right rear legs (semitendinosus muscle). The first booster was 4 × 1012 vir combined with AdjuVac given IM on the site of ‘phage in PBS’ primary immunization. The second booster was 4 × 1012 vir combined with Thermogel (adjuvant) given IM in the left shoulder. Blood was collected from each cat for three times prior to immunization to obtain negative control samples for GnRH antibody and to establish baseline testosterone values. This was followed by monthly post-immunization blood collections for the rest of the study. Testicular measurements and sperm collections were performed prior to immunization and then monthly post-immunization
Detection of GnRH antibody and testosterone in sera
Blood was collected weekly for 3 weeks before primary immunization to allow determination of pre-vaccination values, and then monthly post-immunization for the remainder of the experimental period. To avoid testosterone fluctuations owing to circadian rhythm, blood was collected at the same time of the day throughout the study. GnRH antibodies were detected by ELISA using a synthetic GnRH peptide (New England Peptide) bi 879814 otinylated at C terminus (NH2-EHWSYGLRPG-Lys-biotin-CONH2) to serve as the detector molecule. 12 Cat serum was diluted 1:100 and then tested at two-fold dilutions to endpoint titers. The endpoint titer was defined as the highest dilution providing an optical density twice that of the pre-immune sera. To measure serum testosterone, a Mouse and Rat Testosterone ELISA kit (ALPCO) was used as directed by the manufacturer. Two controls of known testosterone concentrations in rat serum (Rat Control Set; ALPCO) were run with each assay and cat serum obtained from neutered cats originating in the same colony was used as a negative control.
Semen collection and evaluation
Semen was collected at least twice prior to vaccination to obtain a baseline and then monthly for the duration of the study by electroejaculation under general anesthesia as per the method of Johnson. 19 Total sperm number was determined using SP-100 Nucleocounter (ChemoMetec). Motility characteristics of the spermatozoa were evaluated using computer-assisted sperm analysis software (Canine SpermVision SAR; MOFA). Morphologic analysis was performed using eosin–nigrin stain and counting 100 cells on × 100 oil immersion.
Testes size and histology
Testicular measurements were recorded using an ultrasound prior to immunization, then once monthly for the duration of the study period. The length (L), width (W) and height (H) of each testicle were obtained independently by a single investigator. Testicular volume (TV) for each testicle was calculated using the formula: TV = L × W × H × 0.5233. 20 The TV of the left and right testicles was added together to obtain the total testicular volume (TTV).
At the time of neuter, cat testes with attached epididymis were surgically removed, fixed in modified Davidson’s fluid, 21 and processed for preparation of hematoxylin and eosin-stained sections for histologic evaluation. Testis and epididymis from two age-matched untreated cats were also collected and evaluated. Soft tissue nodules that appeared at immunization sites with AdjuVac were biopsied at the time of neuter and evaluated for neoplasia.
Statistical analysis
Data were analyzed using the general linear model for repeated-measures ANOVA. For each group of cats each time point post-immunization was compared with pre-immunization month 0 using contrasts.
Results
GnRH antibody responses
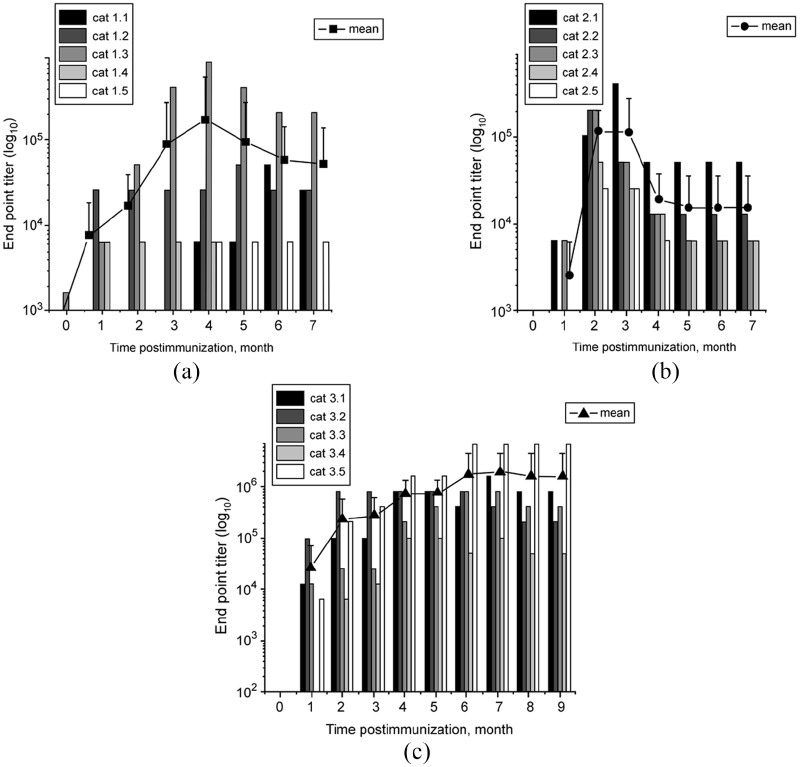
Although the vaccine stimulated production of GnRH antibodies in all immunized cats, their titer and duration depended on the type of treatment, and also varied between individual cats within the same treatment group. All cats in group 1 developed antibodies against GnRH; however, the antibody profiles were not uniform across the immunized animals (Figure 2a). The differences included the antibody initial rise, duration and strength. One cat (1.3) developed detectable GnRH antibody as early as 2 weeks after immunization. This cat also developed the highest level of antibodies for this group, with an endpoint titer of 819,200 at month 4. Two cats (1.1 and 1.5) developed detectable antibodies relatively late, 4 months after immunization. One cat was considered a short-term responder with an antibody duration of 4 months. As a group, the antibodies generally peaked around months 3–5 (Figure 2a). Four of five cats maintained the antibodies until the end of the study.
Figure 2.
Gonadotropin-releasing hormone (GnRH) antibodies in cat sera following immunization with phage–GnRH. (a) Group 1; (b) group 2; (c) group 3. The immunization schedule is given in Figure 1. Samples were collected prior to immunization followed by monthly post-immunization collections for 7 (groups 1 and 2) or 9 (group 3) months. The antibodies were detected in sera by ELISA. All measurements were performed in duplicate. The data are presented as endpoint titers (defined as the highest dilution providing an optical density twice that of the pre-immune sera). Each bar represents an individual cat. Lines connecting monthly data points with symbols represent means of antibody titers ± SD for each group
While the antibody titers varied significantly between cats in group 2, reactions to immunization in all cats followed the same pattern. The antibodies peaked around 1.5–2 months after primary immunization, then de-creased and plateaued around 3–4 months, maintaining approximately the same level until the end of the study (Figure 2b). Cat 2.1 developed and maintained the highest antibody titer for this group, with a peak value of 409,600. Cat 2.5 developed the lowest antibody level, with a peak value of 25,600. It was also considered a short-term responder, with no detectable GnRH antibodies after 4 months following primary immunization.
Similarly to groups 1 and 2, all cats in group 3 developed GnRH antibodies. The antibody response varied significantly between the cats (Figure 2c). Each cat, except cat 3.2, had a large increase in antibody titer after the first booster immunization (month 3). Cats 3.1 and 3.4 also showed an increase in antibody titer after the second booster immunization (month 6). Cat 3.5 had the highest antibody titer of 6,553,600, which was reached at month 6 and persisted through to the end of the study. Cat 3.4 was the slowest to develop antibodies with no detectable antibodies until 2 months after primary immunization and had the lowest titer for group 3. The mean GnRH titers from cats in group 3 were greater than groups 1 and 2 (P <0.05) at most time points.
Testosterone, testicular and semen parameters
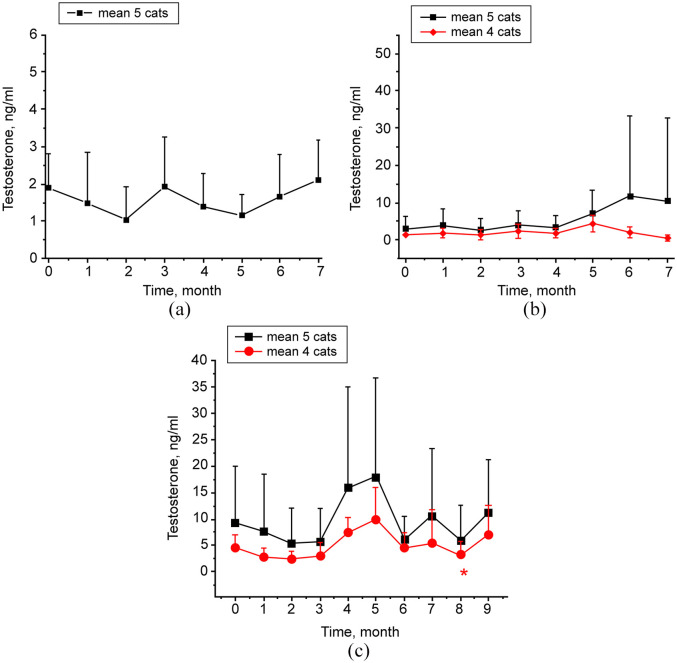
Throughout the study period post-immunization, there was no significant change in mean testosterone concentrations in group 1 cats compared with baseline values (Figure 3a). Baseline values in serum testosterone in group 2 cats varied broadly. Four of the cats (cats 2.2–2.5) ranged from 0.54 to 2.12 ng/ml, while cat 2.1 measured 9.08 ng/ml. Also, cat 2.1 had much higher serum testosterone levels post-immunization than other cats in group 2, reaching 50 ng/ml at months 6 and 7. Therefore, testosterone for group 2 cats was analyzed both with and without cat 2.1 (Figure 3b). Using either approach, there was no statistically significant difference in serum testosterone for group 2 cats throughout the study period. Similarly to group 2, baseline values in serum testosterone in group 3 cats ranged broadly. Four of the cats (cats 3.2–3.5) ranged from 2.43 to 7.55 ng/ml, while cat 3.1 measured significantly higher at 27.95 ng/ml. Also, cat 3.1 had much higher post-immunization testosterone levels (>50 ng/ml) than any of the other cats in group 3. Therefore, testosterone for group 3 cats was evaluated with and without cat 3.1 (Figure 3c). When analyzed as a group of four cats, the mean testosterone was significantly reduced at month 8 after primary immunization compared with pre-immunization value (P <0.05).
Figure 3.
Testosterone in cat sera following immunization with phage–gonadotropin-releasing hormone. Serum samples were collected prior to immunization followed by post-immunization collections performed monthly. All samples were evaluated in duplicate using ELISA. Data are presented as group means ± SD. (a) Group 1. Data analysis was performed for five cats. (b) Group 2. Black line is data analysis for five cats. Red line is data analysis for four cats, excluding cat 2.1 with abnormally high testosterone. (c) Group 3. Black line is data analysis for five cats. Red line is data analysis for four cats, excluding cat 3.1 with abnormally high testosterone. P <0.05 is noted with an asterisk. P value refers to post-treatment vs pretreatment
The TTV was decreased in 4/5 cats in group 1 from the start to the end of the study. At the time of neuter, the maximum TTV decrease of 42.4% was observed in cat 1.1 and the minimum decrease of 24.5% in cat 1.4. One cat in this group showed an increased TTV of 19.4%. Thus, when evaluated as a group, the differences between post- and pre-immunization TTV values were not statistically significant (Figure 4). In group 2 cats, there were no significant changes of mean TTV values, except at two separate post-immunization time points (months 3 and 6), when the TV increased. These changes were sporadic and did not constitute a trend. TTV declined in all five cats in group 3. These TTV changes were significant at multiple time points, including months 2, 3, 4, 5, 6 and 8 (Figure 4). The overall trend in testicular size in this group was downward.
Figure 4.
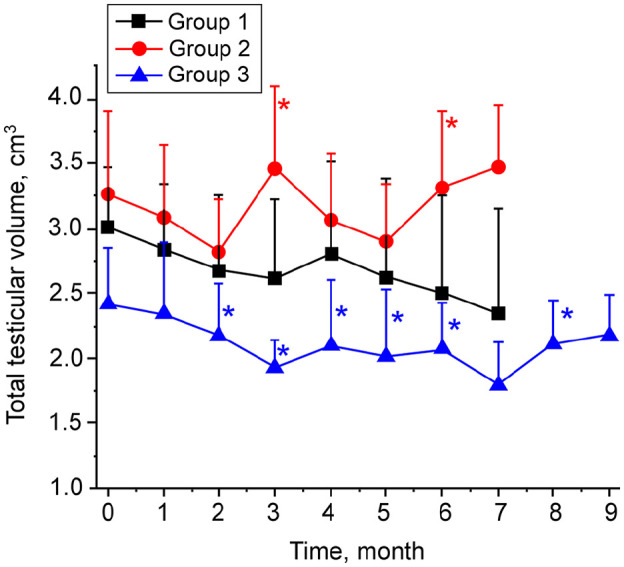
Total testicular volume in cats immunized with phage–gonadotropin-releasing hormone. Testicular size was measured via scrotal ultrasound prior to immunization and then monthly post-immunization for the rest of the experiment. The procedure was carried out by placing an ultrasound probe on the external surface of scrotum and measuring length (L), width (W) and height (H) of each testicle. Testicular volume was calculated by the formula L × W × H × 0.5233, and the total testicular volume for each cat was found by adding the volume of both testicles. All measurements of L, W and H were performed in duplicate. Data are presented as group means ± SD. P <0.05 is noted with an asterisk. P value refers to post-treatment vs pretreatment
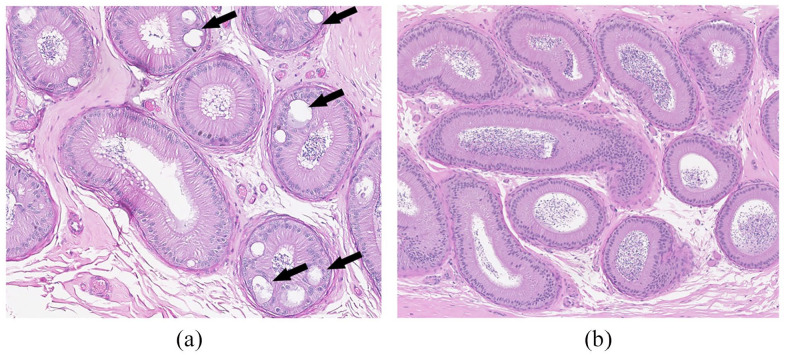
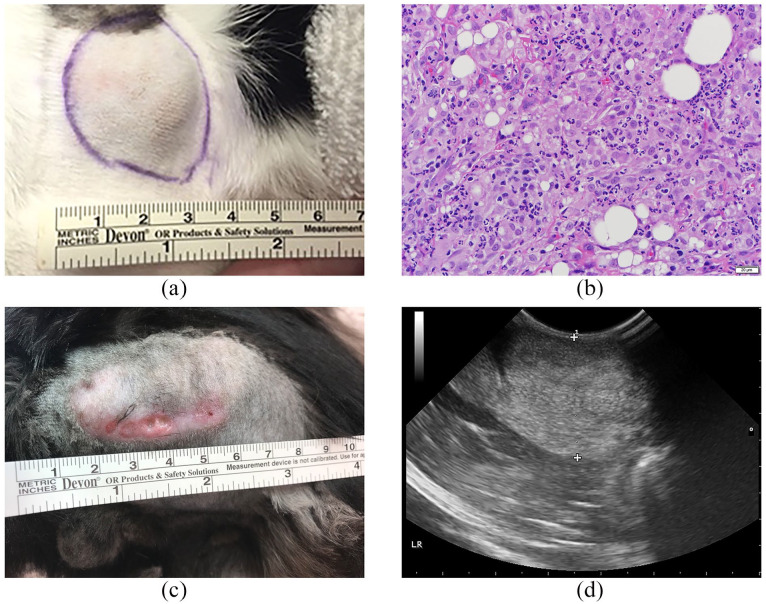
In addition, testes and epididymides of all five cats in group 3 were evaluated histologically. There were no vaccine-related changes detectable by light microscopy in the testes. All immunized cats had mild vacuolation in the epididymis of the corpus (Figure 5), which was not present in samples from untreated cats. Vacuolation was characterized by macrovesicles with proteinaceous material within the epithelial lining. Occasionally, these vacuoles had a rim of epithelial cells, compatible with the pseudoglandular appearance of cribriform change. Many vacuoles had no epithelial lining and were interpreted to be intracytoplasmic vacuoles.
Figure 5.
Histologic evaluation of cat epididymis (representative images). (a) Corpus of epididymis of a treated cat in group 3. Note the vacuolation shown by the arrows. (b) Corpus of epididymis of an age-matched cat from the same cat colony housed under identical conditions (not treated). Hematoxylin and eosin staining, magnification × 10
The total sperm number for each cat in all cat groups varied throughout the study. No consistent changes in sperm production were observed. All cats continued to produce sperm at the end of the experiments. Progressive sperm motility did not change significantly in all groups (not shown).
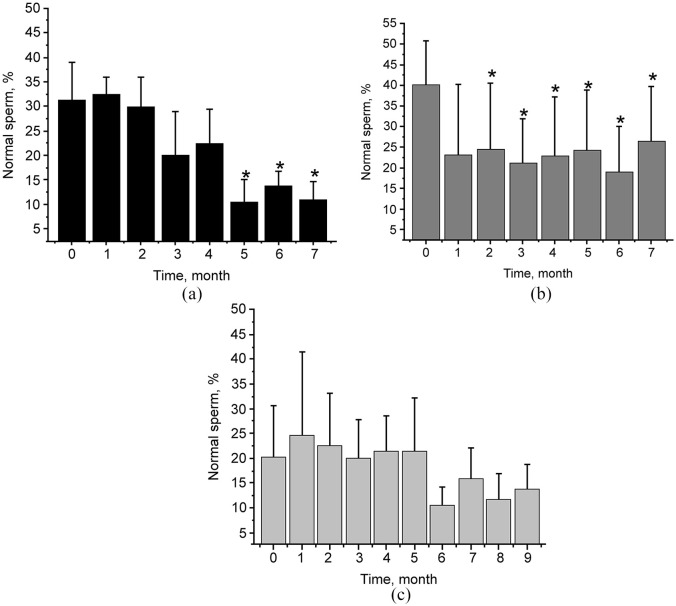
There was a statistically significant decrease in the relative number of morphologically normal sperm in groups 1 (at 5, 6 and 7 months post-immunization) and 2 (at post-immunization months 2–7), but there was no such effect in group 3. The mean sperm morphology data for each group at each collection are shown in Figure 6. The sperm abnormalities most commonly observed were abnormal heads, abnormal midpieces and tightly coiled tails, all indicating testicular insult.
Figure 6.
Relative quantity of morphologically normal sperm cells in cats immunized with phage–gonadotropin-releasing hormone. Semen collection was accomplished by electroejaculation under general anesthesia. The morphologic analysis was performed using eosin–nigrin stain evaluating 100 cells/sample for defects. Relative numbers (%) of normal cells for individual cats prior to and post-immunization (monthly) are shown. Data are presented as group means ± SD. P <0.05 is noted with an asterisk. P value refers to post-treatment vs pretreatment. (a) Group 1; (b) Group 2; (c) Group 3
Injection site reaction
Group 1 cats developed variably sized firm nodules at the site of the adjuvant–phage injections approximately 2 weeks after immunization. There was no reaction using phage only. The nodules were firm, non-painful and located under the skin in the subcutaneous space (Figure 7a). The nodules increased in size initially, then slowly decreased in size over the later months of the study. By 8 months after initial immunization, only two cats still had palpable nodules. Nodules were biopsied at the time of neuter and confirmed to be inflammatory with no evidence of neoplasia (Figure 7b).
Figure 7.
Injection site reaction in cats immunized with phage–gonadotropin-releasing hormone combined with AdjuVac. (a) Physical appearance of an injection site in cat 1.3, representative of the nodules that developed in group 1 cats (subcutaneous immunization). (b) Representative histologic image of soft tissue nodules in cat 1.3 (group 1) biopsied from the injection site. (c) Physical appearance of an injection site in cat 3.1 (group 3, intramuscular immunization). The reaction appeared at the site of the initial vaccination 7 months after injection. The sterile abscess was observed. (d) Ultrasound image of the injection site nodule in cat 3.1. The reaction is characterized by hyperechogenicity (area between two crosses)
Group 2 cats received phage immunizations in saline. No reactions were palpable at the site of either immunization throughout the study.
Four cats in group 3 developed palpable nodules within 3 weeks of the initial injection and all five cats developed nodules following the 3-month booster. The nodules increased in size initially and ranged from 1 to 7 cm before gradually decreasing in size. They remained firm and non-painful. Three of five cats still had easily palpable nodules at the conclusion of the study. One cat (3.1) developed a sterile abscess at the initial injection site 7 months after immunization (Figure 7c,d). There were no reactions to phage or phage mixed with Thermogel.Future studies using phage vaccines will test alternative delivery methods that do not include AdjuVac owing to the observed reactions. Clinically, because of the risk of neoplasia associated with vaccines in the cat, the current recommendation is to place all vaccinations in a distal location to provide options for easier treatment (amputation) should a vaccine reaction become untreatable or neoplastic.
Discussion
In our recent study, 12 phages displaying GnRH-like peptides were identified via selection from a phage display library using anti-GnRH antibodies as a selection target. Such phages were shown to trigger the production of GnRH antibodies and to suppress testosterone when injected in mice. Here, phages displaying GnRH-like peptides were evaluated in one of the target species, the cat. Cats were immunized with either phage alone or in combination with one of the adjuvants: (1) AdjuVac shown to stimulate high GnRH antibody titers in cats with moderate injection site reactions;22,23 or (2) Thermogel, shown to elevate GnRH responses with no injection site reactions in mice. 12
The vaccine stimulated the production of GnRH antibodies in all immunized cats. The titer and duration of antibody production was variable with respect to the type of treatment, and also varied between individual cats within the same treatment group. These fluctuations in antibody responses might occur as a result of a varying rate and pattern of phage release from phage–adjuvant depots formed at injection sites in individual animals. A broad range of inter-individual responses in cats to another GnRH-based vaccine, GonaCon, was reported by others.22–24 GonaCon was also described to produce wide interspecies variability in GnRH antibody production.10,24–26
Phage–GnRH immunizations triggered significant changes in several reproductive parameters. In groups 1 and 2, the group mean of morphologically normal sperm declined at multiple post-immunization time points. Such decline might indicate the initiation of disruption of spermatogenesis and/or maturation of sperm in the epididymis. This decline in normal sperm is especially significant in the light of the fact that when young, sexually mature cats are collected regularly (as in the present study), their sperm characteristics generally improve.
Phage–GnRH also led to decreased TV. Any decrease in volume is biologically significant, as in young maturing male cats that are continuing to grow, the TV is expected to increase over time, not decrease. In this study, the TTV decreased as a trend (group 1) or demonstrated a statistically significant drop (group 3) in cats immunized with phage–AdjuVac, pointing to possible initiation of processes leading to gonadal atrophy. However, strong correlations between the testicular parameters and GnRH antibody titers were not established. Partially, as stated earlier, this could occur as a result of fluctuations in the release of the phage antigen from phage–AdjuVac depots formed at injection sites. Also, this might be a result of high variability of immunologic responses in individual cats common for this species in general, as well as owing to specific antibody responses to the GnRH antigen. Even though GnRH-like peptides displayed on phage are short, they might contain not one, but several, antigenic epitopes stimulating the production of polyclonal antibodies, some of which were neutralizing, causing testicular changes, while the others were not. The ratio of neutralizing to non-neutralizing antibodies could vary in individual cats, resulting in no strong correlations between the total GnRH antibody responses (as measured by ELISA) and quantitative testicular characteristics.
Phage–GnRH was associated with vacuolation/cribriform changes of the corpus epithelium of the epididymides, indicating potential gonadal atrophy related to the treatment as well. The histologic changes demonstrated in the epididymis of immunized cats might disrupt sperm maturation. Similar types of changes in the epididymis were documented previously. 27 Intracytoplasmic vacuolation was reported as an effect of toxic compounds targeting the epididymis. 28
Usually, higher antigen doses lead to higher levels of antigen-specific antibodies and, as a consequence, to improved biologic effects. The present study was the first to inject a phage-based vaccine into cats. The lack of knowledge on the local or systemic reactions to phage inoculations in this species was a barrier for the use of high phage doses. To achieve the goal of contraception, much higher phage doses will be explored in cats in future studies. Increasing phage doses were shown to improve antibody responses in other studies.12,29 Importantly, high phage doses were shown to be safe in multiple species, including sheep, goats, pigs and rhesus macaques (the highest reported phage dose of 2 × 1014 virions per animal).13–15
The primary problem in developing vaccines for cats is identifying an adjuvant that renders the vaccine effective without generating unacceptable side effects, such as injection site reaction. The nodules observed following injections with AdjuVac adjuvant were inflammatory and not neoplastic, decreasing over time in the majority of cats. Similar reactions to an AdjuVac-containing vaccine GonaCon were reported by others. 1 As one cat in this study developed an abscess at the injection site, and several cats had large nodules (>7 cm), we concluded that combination of phage with AdjuVac is not acceptable for the use in cats. There are examples of other adjuvants that have been shown to be effective and safe by others. 30
Conclusions
This study demonstrated that phage–GnRH vaccination stimulates the production of GnRH antibodies that affect reproductive parameters in cats (decreased TV, vacuolation of the epididymis, increased percentage of sperm with abnormal morphology). The immunization regimen specific for each treatment group seems to be important. Ultimately, the regimen has to be optimized and the study group size increased to achieve a consistent and predictable reaction to phage–GnRH immunization in individual cats. It is also important to study the vaccine in cats of different ages as it might be more effective in young (pre-pubertal) animals. As GnRH peptide is conserved in all mammals, contraceptive vaccines against GnRH might be effective in multiple animal species and in both sexes. If permanent contraception cannot be achieved, the vaccine has a potential to be used in zoos, where the majority of animals require temporary, reversible contraception. Also, such contraceptive products could be used for pets where multiple administrations are practical and/or reversible infertility is desirable.
Acknowledgments
AdjuVac (lot number 03292017) was kindly supplied by Douglas Eckery at USDA-APHIS National Wildlife Research Center. The phage–GnRH clone used in this study was isolated previously from a landscape phage display library (a kind gift from Dr V Petrenko, Auburn University). Our special thanks for extensive technical help to JS Cannon, MS Korbely and CL Barstow at the Scott Ritchey Research Center cat colony.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was supported by Auburn University Intramural Grants Program, Merck-Merial Veterinary Scholars Program and the Scott-Ritchey Research Center, College of Veterinary Medicine, Auburn University. The GnRH phage used in the present study was developed previously on a project funded by the Found Animals Foundation, Michelson Grant in Reproductive Biology D0910-S10.
Ethical approval: This work involved the use of experimental animals; or involved the use of non-experimental animal(s) (owned or unowned) outside of established internationally recognized high standards (‘best practice’) of individual veterinary clinical patient care. The study therefore had ethical approval from an established committee as stated in the manuscript.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work for the procedure(s) undertaken. For any animals or humans individually identifiable within this publication, informed consent for their use in the publication (verbal or written) was obtained from the people involved.
ORCID iD: Aime Johnson  https://orcid.org/0000-0003-3895-7767
https://orcid.org/0000-0003-3895-7767
Accepted: 19 August 2019
References
- 1. Levy JK. Contraceptive vaccines for the humane control of community cat populations. Am J Reprod Immunol 2011; 66: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goericke-Pesch S, Wehrend A, Georgiev P. Suppression of fertility in adult cats. Reprod Domest Anim 2014; 49 Suppl 2: 33–40. [DOI] [PubMed] [Google Scholar]
- 3. Benka VA, Levy JK. Vaccines for feline contraception: GonaCon GnRH-hemocyanin conjugate immunocontraceptive. J Feline Med Surg 2015; 17: 758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vansandt LM, Kutzler MA, Fischer AE, et al. Safety and effectiveness of a single and repeat intramuscular injection of a GnRH vaccine (GonaCon) in adult female domestic cats. Reprod Domest Anim 2017; 52 Suppl 2: 348–353. [DOI] [PubMed] [Google Scholar]
- 5. Gupta SK, Minhas V. Wildlife population management: are contraceptive vaccines a feasible proposition? Front Biosci 2017; 9: 357–374. [DOI] [PubMed] [Google Scholar]
- 6. Aponte PM, Gutierrez-Reinoso MA, Sanchez-Cepeda EG, et al. Active immunization against GnRH in pre-pubertal domestic mammals: testicular morphometry, histopathology and endocrine responses in rabbits, guinea pigs and ram lambs. Animal 2018; 12: 784–793. [DOI] [PubMed] [Google Scholar]
- 7. Schaut RG, Brewer MT, Hostetter JM, et al. A single dose polyanhydride-based vaccine platform promotes and maintains anti-GnRH antibody titers. Vaccine 2018; 36: 1016–1023. [DOI] [PubMed] [Google Scholar]
- 8. Ladd A, Tsong YY, Walfield AM, et al. Development of an antifertility vaccine for pets based on active immunization against luteinizing hormone-releasing hormone. Biol Reprod 1994; 51: 1076–1083. [DOI] [PubMed] [Google Scholar]
- 9. Earl ER, Waterston MM, Aughey E, et al. Evaluation of two GnRH-I based vaccine formulations on the testes function of entire Suffolk cross ram lambs. Vaccine 2006; 24: 3172–3183. [DOI] [PubMed] [Google Scholar]
- 10. Miller LA, Johns BE, Killian GJ. Immunocontraception of white-tailed deer with GnRH vaccine. Am J Reprod Immunol 2000; 44: 266–274. [DOI] [PubMed] [Google Scholar]
- 11. Oonk HB, Turkstra JA, Schaaper WM, et al. New GnRH-like peptide construct to optimize efficient immunocastration of male pigs by immunoneutralization of GnRH. Vaccine 1998; 16: 1074–1082. [DOI] [PubMed] [Google Scholar]
- 12. Samoylov A, Cochran A, Schemera B, et al. Humoral immune responses against gonadotropin releasing hormone elicited by immunization with phage-peptide constructs obtained via phage display. J Biotechnol 2015; 216: 20–28. [DOI] [PubMed] [Google Scholar]
- 13. Chen X, Scala G, Quinto I, et al. Protection of rhesus macaques against disease progression from pathogenic SHIV-89.6PD by vaccination with phage-displayed HIV-1 epitopes. Nat Med 2001; 7: 1225–1231. [DOI] [PubMed] [Google Scholar]
- 14. Villa-Mancera A, Quiroz-Romero H, Correa D, et al. Induction of immunity in sheep to Fasciola hepatica with mimotopes of cathepsin L selected from a phage display library. Parasitology 2008; 135: 1437–1445. [DOI] [PubMed] [Google Scholar]
- 15. Segura-Velazquez R, Cervantes J, Acosta E, et al. Influenza vaccine: development of a novel intranasal and subcutaneous recombinant adjuvant. Vaccine 2013; 31: 4009–4016. [DOI] [PubMed] [Google Scholar]
- 16. Manoutcharian K. Phage vaccines and phage therapy. In: Petrenko V, Smith GP. (eds). Phage nanobiotechnology. London: The Royal Society of Chemistry, 2011, pp 245–258. [Google Scholar]
- 17. Samoylova TI, Braden TD, Spencer JA, et al. Immunocontraception: filamentous bacteriophage as a platform for vaccine development. Curr Med Chem 2017; 24: 3907–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brigati JR, Samoylova TI, Jayanna PK, et al. Phage display for generating peptide reagents. Curr Protoc Prot Sci 2008; 51: 18.9. [DOI] [PubMed] [Google Scholar]
- 19. Johnson AK. Evaluation of the tom: collection procedures, evaluation of sperm, and subsequent use. Clin Theriogenol 2014; 6: 219–223. [Google Scholar]
- 20. Gutiérrez-Reinoso M, Garciaa-Herreros M. Normozoospermic versus teratozoospermic domestic cats: differential testicular volume, sperm morphometry, and subpopulation structure during epididymal maturation. Asian J Androl 2016; 18: 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Latendresse JR, Warbrittion AR, Jonassen H, et al. Fixation of testes and eyes using a modified Davidson’s fluid: comparison with Bouin’s fluid and conventional Davidson’s fluid. Toxicol Pathol 2002; 30: 524–533. [DOI] [PubMed] [Google Scholar]
- 22. Levy JK, Miller LA, Cynda Crawford P, et al. GnRH immunocontraception of male cats. Theriogenology 2004; 62: 1116–1130. [DOI] [PubMed] [Google Scholar]
- 23. Levy JK, Friary JA, Miller LA, et al. Long-term fertility control in female cats with GonaCon, a GnRH immunocontraceptive. Theriogenology 2011; 76: 1517–1525. [DOI] [PubMed] [Google Scholar]
- 24. Fischer A, Benka VA, Briggs J, et al. Effectiveness of GonaCon as an immunocontraceptive in colony-housed cats. J Feline Med Surg 2018; 20: 786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baker DL, Powers JG, Ransom JI, et al. Reimmunization increases contraceptive effectiveness of gonadotropin-releasing hormone vaccine (GonaCon-Equine) in free-ranging horses (Equus caballus): limitations and side effects. PLoS One 2018; 13: e0201570. DOI: 10.1371/journal.pone.020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Massei G, Koon KK, Law SI, et al. Fertility control for managing free-roaming feral cattle in Hong Kong. Vaccine 2018; 36: 7393–7398. [DOI] [PubMed] [Google Scholar]
- 27. La Perle KM, Blomme EA, Sagartz JE, et al. Epididymal cribriform hyperplasia with nuclear atypia in p53 homozygous knockout mice on a mixed 129/Sv-FVB/N background. Comp Med 2002; 52: 568–571. [PubMed] [Google Scholar]
- 28. Vidal JD, Whitney KM. Morphologic manifestations of testicular and epididymal toxicity. Spermatogenesis 2014; 4: e979099. DOI: 10.4161/21565562.2014.979099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roehnisch T, Then C, Nagel W, et al. Chemically linked phage idiotype vaccination in the murine B cell lymphoma 1 model. J Transl Med 2013; 11: 267. DOI: 10.1186/1479-5876-11-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robbins SC, Jelinski MD, Stotish RL. Assessment of the immunological and biological efficacy of two different doses of a recombinant GnRH vaccine in domestic male and female cats (Felis catus). J Reprod Immunol 2004; 64: 107–119. [DOI] [PubMed] [Google Scholar]