Abstract
Objectives
The aims of this study were to describe the treatment outcomes following oral administration of a fixed dose (138 MBq; 3.7 mCi) of radioiodine in hyperthyroid cats and to examine the correlation between total thyroxine (TT4) concentrations before and after treatment.
Methods
This was a retrospective cohort study that documented the TT4 concentration and clinicopathological parameters at the time of diagnosis and after treatment. Logistic regression was used to assess the relationship between TT4 concentrations before and after treatment. The difference in pre- and post-treatment variables between cats that had TT4 concentrations below or within the reference interval (RI) was compared by the Mann–Whitney U-test.
Results
Of 161 cats, 133 (82.6%) cats had TT4 concentrations within the RI, four (2.5%) cats had TT4 concentrations above the RI and 24 (14.9%) cats had TT4 concentrations below the RI after treatment. The severity of hyperthyroidism at diagnosis, as measured by the percentage of TT4 elevation above the upper limit of the RI, had no impact on the odds of cats having low TT4 concentrations after treatment (odds ratio 1.00; 95% confidence interval 0.96–1.05; P = 0.828).
Conclusions and relevance
When using an orally administered fixed dose of radioiodine for the treatment of feline hyperthyroidism, TT4 concentrations at diagnosis cannot be used to predict TT4 concentrations after treatment. The proportion of cats with TT4 concentrations below the lower limit of the RI after treatment was 14.9%. Further work is required to optimise oral radioiodine dosing to achieve maximal euthyroid outcomes.
Keywords: Hyperthyroidism, iodine, administration, oral, treatment outcomes, azotaemia
Introduction
Untreated hyperthyroidism can induce protein malnutrition and haemodynamic changes that have an adverse impact on the kidneys and cardiovascular system. 1 Treatment options include antithyroid medication (thioureylenes), iodine-restricted diet, thyroidectomy and radioiodine therapy. Radioiodine is the curative treatment of choice based on its ability to target all abnormal thyroid tissue, regardless of location.1,2 Parenteral administration is most widely reported.3–9 Oral (PO) radioiodine administration is also effective,10–12 but has not been widely adopted, possibly owing to the risk of vomiting following administration, which may lead to treatment failure and increased personnel health and safety risks. However, oral administration is used commonly in Australia, largely because injectable radioiodine is costly and difficult to obtain.11,13
It has been recognised that the development of iatrogenic hypothyroidism after the treatment of hyperthyroid cats is detrimental to renal function and may have a negative impact on long-term survival.14,15 As a result, the goal of radioiodine treatment is to maximise the number of cats that achieve euthyroidism.
The method of determining the dose of radioiodine to be given to hyperthyroid cats varies between institutions. Several methods of calculating individualised doses have been reported.5,9,16 A fixed dose, typically between 148 and 185 megabecquerels (MBq; 4–5 millicuries [mCi]), has also been commonly used, irrespective of delivery route and severity of hyperthyroidism, owing to outcomes comparable with those of calculated dose protocols.4,6,10,17,18 A lower fixed radioiodine dose of 75 MBq (2 mCi) subcutaneously has been more recently suggested for mild to moderate hyperthyroidism (total thyroxine [TT4] >51 to <167 nmol/l) in order to lower the prevalence of iatrogenic hypothyroidism in cats following treatment. 19
It is uncertain whether data from cats treated by the parenteral route can be extrapolated to cats treated orally. Furthermore, there is limited recent literature regarding the outcome of oral fixed dose radioiodine treatment of hyperthyroidism in cats.
Thus, the first aim of this retrospective study was to describe the percentage of cats treated for hyperthyroidism with a fixed dose of approximately 138 MBq (3.7 mCi) of radioiodine orally that have TT4 concentrations within the reference interval (RI), below the lower limit of the RI or above the upper limit of the RI after treatment. Secondly, the study aimed to assess whether the severity of hyperthyroidism (as defined by severity of increase in TT4 concentration above the upper limit of the RI at the time of diagnosis) had an impact on the TT4 concentration after treatment. It was hypothesised that cats with TT4 concentrations only mildly elevated above the RI at the time of diagnosis would be more likely to have a TT4 concentration below the RI after radioiodine treatment when compared with cats that had severe elevations in TT4 concentrations at diagnosis.
Materials and methods
Study design and data collection
This was a retrospective cohort study of client-owned hyperthyroid cats that had been treated with a fixed dose of radioiodine orally at the U-Vet Animal Hospital of the University of Melbourne, VIC, Australia. Ethical approval was granted by the University of Melbourne Animal Ethics Committee (AEC application ID 1613858). Any cat treated between February 2011 and January 2018 was eligible for inclusion in the study.Cats included in this study had been treated using an established standard radioiodine treatment protocol. As part of the protocol, cats were eligible for treatment with fixed dose radioiodine after a definitive diagnosis of hyperthyroidism was made based on the presence of clinical signs consistent with hyperthyroidism (eg, weight loss despite polyphagia, polydipsia and polyuria, poor coat) 1 and one of the following diagnostic test results: (1) a serum TT4 concentration above the upper limit of the RI; and (2) a serum TT4 concentration within the high–normal RI and an elevated free thyroxine concentration. Furthermore, at a minimum, serum creatinine concentration and urine specific gravity (USG), performed within 1 month of proposed radioiodine therapy, were assessed to evaluate renal function in candidate cats. All cats had to be either ⩽International Renal Interest Society (IRIS) stage 1 chronic kidney disease (CKD) before any thioureylene medications were given or ⩽IRIS stage 2 CKD once hyperthyroidism had been controlled medically with thioureylenes for a minimum of 4 weeks. 20
Cats with a significant concurrent disease or suspicion of thyroid carcinoma based on clinical history, physical examination and diagnostic evaluation were excluded from fixed dosing. In particular, cats that had severe clinical signs attributable to hyperthyroidism and TT4 concentrations >200 nmol/l at diagnosis, those with a poor clinical and TT4 response to increasing and appropriate doses of thioureylene therapy for hyperthyroidism, 21 or those with fixed or large (>2 cm in longest dimension) thyroid masses had thyroid scintigraphy performed and calculated dose therapy rather than fixed dosing.22–25 Cats that had undergone prior surgical thyroidectomy for hyperthyroidism were also excluded from the study on the basis that reduced thyroid tissue might increase the potential for hypothyroidism following radioiodine treatment.
Thioureylene drugs were discontinued at least 1 week prior to treatment, and an iodine-restricted diet was discontinued at least 4 weeks prior to treatment. The fixed dose used is within the fixed dose range (55–227 MBq) used orally and parenterally at other institutions.4,6,10,18 It was selected based on this and the availability of commercial radioiodine capsules of 150 MBq (4.0 mCi) at the time of dispatch, which decayed predictably during overnight transport to give a final activity of approximately 138 MBq (3.7 mCi) at the time of oral administration the following morning. The dose was also influenced by a previous retrospective assessment of treatment outcomes at the same institution using a higher administered fixed oral dose of 154 MBq (4.2 mCi). This previous study recommended a dose reduction owing to a 7% incidence of clinical hypothyroidism in the study population at 2–11 months post-radioiodine treatment. 17
The actual dose of radioiodine in individual capsules was measured within 1 h prior to administration using a radionuclide dose calibrator (Atomlab 100 Plus Dose Calibrator; Biodex Medical Systems) and was recorded for each cat.Cats eligible for radioiodine therapy were admitted the day before the actual administration of radioiodine. Maropitant citrate (Cerenia; Zoetis) was administered at 1 mg/kg subcutaneously before the evening feed to minimise the risk of vomiting over the next 24 h, as previous incidences of vomiting following radioiodine capsule administration had been recorded at the institution in unmedicated cats. Cats were fasted for approximately 12 h before radioiodine therapy as an additional precaution to reduce the incidence of vomiting. 11 Sedation of the cats is a requirement for staff occupational health and safety, and sedatives were given to effect to reduce anxiety, movement and risk of biting or rejecting the oral capsule, while maintaining the swallowing reflex. Various sedation protocols were used, but the predominant sedative used was a 50:50 v/v solution of ketamine 100 mg/ml (Ceva Animal Health) and diazepam 5 mg/ml (Troy Laboratories) administered intravenously. The maximum dose administered was 25 mg for ketamine and 1.25 mg for diazepam. If the cat was fractious and an intravenous injection was unable to be administered, or if there were contraindications for the use of ketamine or diazepam, intramuscular administration of a combination of midazolam 0.2 mg/kg (Hypnovel; Roche Products), butorphanol 0.2 mg/kg (Butorphanol; Ausrichter) and alfaxalone 2 mg/kg (Alfaxan; Jurox), or a combination of medetomidine 5 μg/kg (Medetomidine; Troy Laboratories) and butorphanol 0.2 mg/kg, was used. After oral administration of a radioiodine capsule, 5 ml water was given via syringe into the cat’s mouth to encourage swallowing and orogastric transport of the capsule.The approximate location of the capsule in the stomach was confirmed using a Geiger counter (RAM GENE-1F radiation contamination meter; Rotem Radiation). Treated cats were monitored continuously until recovery from sedation and then offered a meal of their usual diet at 50% of their daily energy requirements. In addition to monitoring appetite, demeanour, urination and defaecation at least three times daily while hospitalised, cats were particularly observed every 3 h during the 9 h following treatment for vomiting; if any was observed, this was recorded.
Clinical and diagnostic data of cats were collected from U-Vet and/or referring veterinary practice databases at two separate time points: (1) baseline (referring to time of diagnosis of hyperthyroidism; however, in cases where hyperthyroidism had been previously managed with thioureylene medications, renal parameters within 4 weeks of schedule radioiodine therapy were used); (2) recheck, referring to at least 1 month after radioiodine treatment. If cats had rechecks performed at more than one time point after radioiodine treatment, only the first recheck that was performed at least 1 month after treatment was included for data analysis. For each cat, radioiodine treatment information and the TT4 concentrations, renal parameters and clinical signs attributable to hyperthyroidism at each of the two time points were collated using REDCap electronic data capture tools hosted by the University of Melbourne. 26
The primary clinicopathological parameter of interest was the TT4 concentration; as multiple reference laboratories were used, TT4 results were expressed as a percentage (TT4%) above or below the upper or lower reference limit, respectively, for each laboratory. Renal parameters (serum creatinine concentration and urinalysis) were assessed where available. Renal azotaemia was suspected if serum creatinine was ⩾140 µmol/l (⩾1.6 mg/dl) in conjunction with inadequate urine concentrating ability as demonstrated by a USG of <1.035. Staging of CKD was based on the IRIS guidelines. 20
Clinical records of cats with missing TT4 concentrations at one or both time points were excluded from the study.
Statistical analysis
Logistic regression analysis was used to estimate the odds ratio (OR) for the outcome of a TT4 concentration below the RI, using TT4 at diagnosis expressed as a percentage (TT4%) above the upper limit of the RI, as the explanatory variable. Analysis was performed in 10% increments (eg, cats with TT4% 0–10% above the RI, 10–20% above the RI and so on up to 330%), to allow meaningful interpretation of the ORs given the highly variable TT4% results between individual cats. The Pearson correlation coefficient was used to assess the relationship between TT4% baseline and recheck.
Descriptive statistics were used to summarise historical, clinical and clinicopathological parameters. Based on visual inspection of scatterplots, normality was not assumed, and results are reported as the median and interquartile range (IQR). The Mann–Whitney U-test (MWU) was used to compare the TT4% at the time of diagnosis for cats that had thioureylene medications and those that did not. The MWU was also used to compare the TT4% above the upper limit of the RI at diagnosis, duration from diagnosis to radioiodine treatment, duration of thioureylene treatment prior to radioiodine treatment, duration from radioiodine treatment to recheck, body weight at time of treatment, age, and creatinine concentration and USG at diagnosis and at recheck for cats with a TT4 concentration below the RI vs within the RI at recheck. Similarly, categorical variables were compared between groups using the χ2 test. There were too few cats with a TT4 above the RI at recheck (ie, remaining hyperthyroid) to perform a comparison between this group and the groups of cats with TT4 within or below the RI, respectively.
Finally, logistic regression was employed to estimate the odds of azotaemia at recheck, comparing cats with TT4 concentrations below the lower limit of the RI with those that had TT4 concentrations within the RI at recheck. For all analyses, statistical significance was defined as P ⩽0.05.
Results
Between February 2011 and January 2018, 277 cats received a single oral fixed dose of radioiodine. Of these, 162 cats had the TT4 concentration at both diagnosis and recheck available and 161 cats were included in the study. One cat with TT4 values available was excluded owing to history of a thyroidectomy performed before radioiodine treatment. The remaining 115 cats were excluded because TT4 concentrations after treatment were not available. The mean actual dose of oral radioiodine that included cats received was 132.6 MBq (3.6 mCi) (SD 4.7 MBq [0.1 mCi]). No complications regarding radioiodine capsule administration, such as hypersalivation or vomiting, were documented.
Signalment of the study population
The included study population consisted of 141 domestic shorthair, seven domestic mediumhair and five domestic longhair cats; two each of Ragdoll, Tonkinese and British Shorthair; and one each of Russian Blue and Scottish Fold. There were 75 (47%) male and 86 (53%) female cats; all cats were neutered. Median age was 12.8 years (IQR 11.0–14.2).
Clinical data at diagnosis of hyperthyroidism
The most frequently documented clinical signs attributable to hyperthyroidism at the time of diagnosis are outlined in Table 1. Additionally, over-grooming and vocalisation was noted in five cats per category. Altered behaviour such as urine marking, increased irritability or aggression were each documented once in individual cats.
Table 1.
Common clinical signs attributable to hyperthyroidism at the time of diagnosis
| Clinical sign | n | Percentage of the sample population |
|---|---|---|
| Weight loss | 131 | 81 |
| Polyphagia | 96 | 60 |
| Cardiac murmur and/or gallop rhythm | 85 | 53 |
| Vomiting | 60 | 37 |
| Polyuria and polydipsia | 49 | 30 |
| Hyperactivity | 33 | 20 |
| Tachypnoea or open-mouth breathing | 30 | 19 |
| Diarrhoea | 16 | 10 |
| Hyporexia | 15 | 9 |
| Generalised weakness | 8 | 5 |
Of the 161 cats, 114 (71%) had received thioureylene medications prior to radioiodine treatment for a median of 104.0 days (IQR 62.0–217.0). The median TT4% above the upper limit of the RI at the time of diagnosis for medicated and unmedicated cats were 74.2% (IQR 34.7–150.0%) and 100.0% (IQR 33.3–188.3%), respectively; there was no statistically significant difference between these groups (MWU; P = 0.378). One cat had been fed an iodine restricted diet exclusively (Y/D Feline Prescription Diet; Hill’s Pet Nutrition) for 24 days without noticeable clinical improvement or reduction in TT4.
Evaluation of TT4 before and after radioiodine treatment
At the time of diagnosis, the median TT4% above the upper limit of the RI was 80.0% (IQR 34.4–150.0%). The median duration from diagnosis of hyperthyroidism to radioiodine treatment was 79.5 days (IQR 39.8–174.8) overall. This duration did not differ significantly between cats that had TT4 concentrations within (78.0 days; IQR 39.0–167.0) and below (73.5 days; IQR 42.5–302.0) the RI at recheck (MWU; P = 0.595).
At the time of recheck, 133 (82.6%) cats had a TT4 concentration within the RI, four (2.5%) cats had a TT4 concentration above the RI and 24 (14.9%) cats had a TT4 concentration below the RI. The median duration from treatment to recheck was 135.0 days (IQR 108.0–184.5 days). There were 41 cats with a follow-up duration of 49–108 days, 80 cats with a follow-up duration of 109–183 days and 40 cats with a follow up duration of 184–1261 days. Of the 41 cats reassessed at 49–108 days, seven had a TT4 concentration below the RI, 33 had a TT4 concentration within the RI and one cat remained hyperthyroid. Of the 80 cats reassessed at 109–183 days, 68 and 11 cats had TT4 concentrations within and below the RI, respectively and one cat remained hyperthyroid. Of the 40 cats reassessed at >184 days, 32 and six cats had TT4 concentrations within and below the RI, respectively, and two cats remained hyperthyroid.
Overall, the median duration from treatment to recheck did not differ significantly between cats that had TT4 concentrations within, and cats that had TT4 concentrations below, the RI at recheck (Table 2). Furthermore, age, sex, body weight, breed, TT4% above the upper reference limit at diagnosis and duration of thioureylene medications prior to radioiodine treatment did not differ between these two groups of cats (Table 2). When all cats were included, there was a statistically significant, but weak, correlation between the TT4% at diagnosis and at recheck (r = 0.20, P = 0.012). However, with exclusion of the cats that remained hyperthyroid after radioiodine treatment, the TT4% at diagnosis and after treatment were poorly correlated (r = 0.08, P = 0.320).
Table 2.
Selected clinicopathological data from cats with total thyroxine (TT4) concentrations within or below the reference interval (RI) after radioiodine treatment
| TT4 concentration within RI (n = 133) |
TT4 concentration below the RI (n = 24) |
P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Median | IQR | n | Median | IQR | ||||
| TT4% above RI at baseline | 133 | 86.0 | 32.5–150.0 | 24 | 69.2 | 45.5–135.0 | 0.805 † | ||
| Duration from diagnosis to I131 treatment (days) | 133 | 78.0 | 39.0–167.0 | 24 | 73.5 | 42.5–302.0 | 0.595 † | ||
| Duration of antithyroid treatment prior to I131 (days) | 90 | 104.0 | 63.8–207.3 | 22 | 84.0 | 54.5–328.8 | 0.608 † | ||
| Duration from I131 treatment to recheck (days) | 133 | 132.0 | 109.0–182.5 | 24 | 145.5 | 107.3–185.0 | 0.614 † | ||
| Body weight at time of I131 treatment (kg) | 133 | 4.3 | 3.7–5.1 | 24 | 4.7 | 3.6–5.4 | 0.537 † | ||
| Age (years) | 133 | 12.8 | 11.0–14.2 | 24 | 13 | 11.4–14.1 | 0.643 † | ||
| Creatinine at diagnosis (µmol/l) | 126 | 100.0 | 80.0–122.3 | 23 | 121.0 | 103.0–150.0 | 0.034 † | ||
| USG at diagnosis | 120 | 1.045 | 1.038–1.050 | 22 | 1.041 | 1.026–1.050 | 0.112 † | ||
| Post-treatment creatinine (µmol/l) | 123 | 139.0 | 120.0–170.0 | 24 | 172.5 | 134.0–207.5 | 0.006 † | ||
| Post-treatment USG | 82 | 1.039 | 1.024–1.050 | 17 | 1.026 | 1.016–1.043 | 0.039 † | ||
| Sex | 63 MN | 70 FN | 9 MN | 15 FN | 0.372* | ||||
| Breed | 125 domestic | 8 purebred | 24 domestic | 0 purebred | 0.217* | ||||
Domestic breed refers to all non-purebred cats (domestic shorthair, domestic mediumhair and domestic longhair)
χ2test
Mann–Whitney U-test
MN = male neutered; FN = female neutered; IQR = interquartile range; USG = urine specific gravity; I131 = radioiodine
The TT4 concentration at diagnosis (expressed as TT4% above the upper limit of the RI) did not predict whether cats would have a TT4 concentration below the lower limit of the RI at recheck (OR 1.00, 95% CI 0.96–1.05; P = 0.828) (Figure 1). The distribution of TT4% at the time of diagnosis in cats with and without low TT4 concentrations at recheck is shown in Figure 2.
Figure 1.
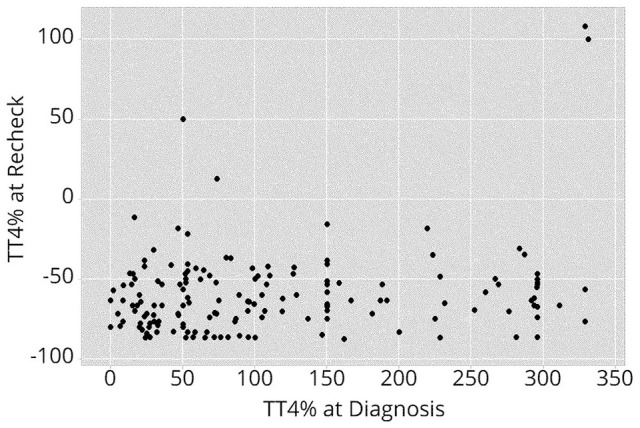
Scatterplot depicting the relationship between total thyroxine (TT4) concentration expressed as a percentage above or below the upper limit of the reference interval (TT4%) at diagnosis and at recheck.
TT4% of 0 (at diagnosis or recheck) indicates the upper reference limit of the respective laboratory. Each dot represents an individual cat
Figure 2.
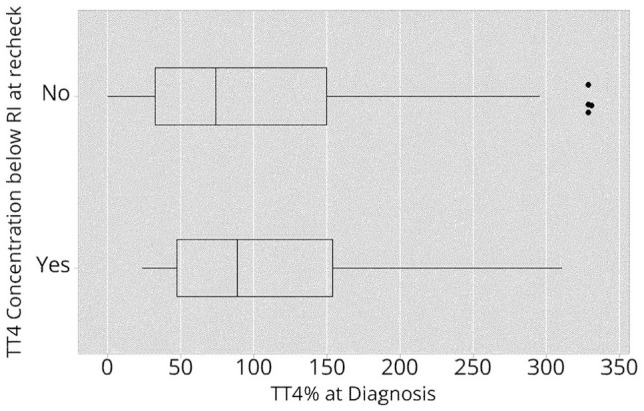
Distribution of total thyroxine (TT4) concentrations at diagnosis expressed as a percentage above the upper limit of the reference interval (TT4%) in cats with or without a TT4 concentration below the lower limit of the reference interval (RI) at recheck. Data are shown as a box plot. The horizontal line depicts the range. The box and vertical line show interquartile range and median, respectively. The asterisks represent outliers
Renal parameters before and after radioiodine treatment
At baseline, 139/161 (86.3%) cats had clinicopathological data regarding their renal function available, including creatinine concentration and USG. Eight of 139 (5.7%) cats had concurrent USG <1.035 and azotaemia consistent with IRIS stage 2 CKD. At the time of recheck, concurrent creatinine and USG measurements were available for 98 cats, of which 31/98 (31.6%) were concurrently azotaemic with a USG <1.035. The creatinine concentration in 25 cats was consistent with IRIS stage 2 CKD, in five cats with IRIS stage 3 CKD and in one cat with IRIS stage 4 CKD.
Seventeen of 24 (70.8%) cats with a TT4 concentration below the lower limit of the RI at recheck had both serum creatinine concentration and USG documented. Eight of these 17 cats (47.0%) had concurrent azotaemia with a USG of <1.035; five consistent with IRIS stage 2 CKD, two consistent with IRIS stage 3 CKD and one consistent with IRIS stage 4 CKD.
Eighty of 133 (60.2%) cats with a TT4 concentration within the RI had complete data regarding renal function at recheck. Twenty-three cats (28.8%) had concurrent azotaemia with a USG <1.035, of which 20 cats had a creatinine concentration consistent with IRIS stage 2 CKD and three cats with IRIS stage 3 CKD.
Cats with a TT4 concentration below the lower limit of the RI after radioiodine treatment had a significantly higher median creatinine concentration at diagnosis and after treatment (MWU; P = 0.034 and P = 0.006, respectively) than cats with TT4 concentrations within the RI (Table 2). The median post-treatment USG concentration was not significantly different between these groups at diagnosis (MWU; P = 0.112). However, after treatment, USG was significantly lower in cats with TT4 concentrations below the lower limit of the RI than those with TT4 concentrations within the RI (MWU; P = 0.039). Cats with a TT4 concentration below the lower limit of the RI at recheck had significantly higher odds of concurrent renal azotaemia than cats that had a TT4 concentration within the RI at recheck (OR 3.62; 95% CI 1.26–10.39; P = 0.02).
Discussion
There is little recent information regarding treatment outcomes of hyperthyroid cats after oral radioiodine treatment. Hence, this study aimed to describe the proportion of cats that have TT4 concentrations within, above or below the upper and lower limits of the RI after receiving a fixed dose (approximately 138 MBq; 3.7 mCi) of oral radioiodine. Furthermore, we aimed to assess whether TT4 concentrations after treatment are dependent on the severity of hyperthyroidism (as assessed by TT4%) at diagnosis. The TT4% at diagnosis and after treatment correlated weakly, owing to the four cats that remained hyperthyroid at the time of recheck. When only the cats with a resolution of hyperthyroidism after radioiodine treatment were considered, that is, those with TT4 within or below the RI at recheck, no relationship between pre- and post-treatment TT4 concentration was detected. In clinical practice, this indicates that the odds for a below RI TT4 outcome does not depend on the pretreatment TT4 concentration.
The lack of relationship between pre- and post-treatment TT4 concentrations contrasts with existing studies using parenteral radioiodine therapy.18,19,27 Higher pretreatment TT4 concentrations have been found to correlate significantly with higher post-treatment TT4 concentrations. In addition, it has been shown that the prevalence of overt or subclinical iatrogenic hypothyroidism following treatment of mildly to moderately hyperthyroid cats reduces when lower radioiodine doses are used.19,27 Thus, at least among studies using parenteral radioiodine therapy, there appears to be a correlation between severity of hyperthyroidism and treatment outcome, which can be modulated by the radioiodine dose.
Veterinary studies that directly compare parenterally and orally administered radioiodine therapy with respect to doses required and outcome achieved are currently lacking. When humans received similar radioiodine doses either orally or intravenously in a randomised fashion, there was no difference in rates of resolution of hyperthyroidism. Unfortunately, the impact of the route of administration on the rate of iatrogenic hypothyroidism was not reported. 28 It has been suggested within the veterinary literature that oral doses of radioiodine should be higher than parenteral doses to account for reduced bioavailability associated with oral administration.10,11To date, there is a scarcity of published information regarding the bioavailability of radioiodine in cats. The absorption of iodine occurs primarily in the small intestine. In healthy humans, absorption is rapid and nearly complete in the fasted patient, with a baseline absorption fraction of 0.99. 29 Absorption is slowed when iodide is ingested with food, but is virtually complete after 3 h. 30 It remains unclear to what extent absorption of oral radioiodine is affected by gastrointestinal transit time, blood flow and absorptive capacity. These factors are likely altered in feline hyperthyroidism and variability in absorbed dose may explain the observed lack of relationship between pre- and post-treatment TT4 concentrations.18,19,27
Importantly, the results of this study do not support the concept that the radioiodine dose should be generally higher when it is administered orally. Indeed, the radioiodine dose used in this study is comparable to or lower than doses described in the literature for both parenteral and oral administration.9,12,17,19,31 The rate of resolution of hyperthyroidism was comparable between these studies, with persistent hyperthyroidism after radioiodine treatment reported in 1–4% of cases when assessed between 1 and 3 months post-treatment. This study assessed cats over a wider time frame but found a similar rate of persistent hyperthyroidism (2.5%).
Iatrogenic overt hypothyroidism, defined as a TT4 concentration below the lower limit of the RI with concurrent thyroid-stimulating hormone (TSH) above the upper limit of the RI, 32 causes a decrease in glomerular filtration rate and may lead to renal azotaemia.33,34 Survival time may be reduced in cats that develop renal azotaemia concurrently with overt hypothyroidism, compared with cats that remain non-azotaemic after receiving treatment for hyperthyroidism. 35 Treatment of overt hypothyroidism may help to resolve azotaemia.36,37 However, medical management of hypothyroidism often requires twice-daily oral administration of levothyroxine, 36 which can be difficult in non-compliant cats. To avoid this, it is best to find the appropriate radioiodine dose that resolves hyperthyroidism, yet also prevents hypothyroidism.
In the current study, cats with TT4 concentrations below the RI at recheck had significantly higher creatinine concentrations at baseline than cats with TT4 concentrations within the RI at recheck. It is possible that cats with higher creatinine concentrations at baseline were more prone to becoming hypothyroid after treatment, owing to reduced renal excretion (and therefore prolonged activity) of radioiodine. This has been shown in humans, where reduced renal function before radioiodine treatment was associated with reduced renal excretion of radioiodine, thus increasing its effective half-life. 38 An alternative explanation for differences in baseline creatinine concentrations in the respective groups of cats with TT4 concentrations below or within the RI at recheck is that the proportion of cats with non-thyroidal illness may have been higher in the group of cats with TT4 concentration below the RI at recheck. Unfortunately, this study was unable to differentiate overt hypothyroidism from non-thyroidal illness because clinical signs, scintigraphic images of the thyroid or concurrent free T4 and TSH concentrations were inconsistently recorded. For these reasons, we were also unable to differentiate euthyroid cats from cats with subclinical hypothyroidism, defined by a TT4 concentration within the low–normal RI and TSH above the RI. 32 Further studies are needed to evaluate the impact of the oral radioiodine dose on the prevalence of overt or subclinical hypothyroidism and to assess the prevalence and severity of renal dysfunction in cats with confirmed overt or subclinical hypothyroidism after oral radioiodine treatment.
The overall prevalence of renal azotaemia after radioiodine treatment in this study (31.6%) is similar to that previously reported (16–41%).19,39,40 Forty-seven percent of cats with TT4 concentrations below the lower limit of the RI at recheck had renal azotaemia, and this group of cats had significantly increased odds of azotaemia compared with cats that had TT4 concentrations within the RI (28.8% prevalence of renal azotaemia). This generally supports the findings of previous studies.14,15,33 However, in addition to the limitation of being unable to differentiate hypothyroid cats from those with non-thyroidal illness, the cross-sectional nature of the study means that cats were not followed over time. Thyroid function is known to change over time; transient iatrogenic hypothyroidism may persist for 6–12 months, 19 and development of late-onset hypothyroidism is also possible.33,41 Therefore, the results presented here may not reflect the long-term outcome of these cats and the proportion of cats with potentially reversible renal azotaemia remains uncertain. Further prospective studies are needed to evaluate thyroid function over time and to determine the optimal oral radioiodine dose for the current population of hyperthyroid cats.
In the interest of obtaining a large study population, the study focused mainly on TT4 concentrations; other parameters that may be used to assess severity or outcome, such as goitre size or clinical signs, were not thoroughly assessed. Such information was difficult to collate given the retrospective nature of this study. Goitre measurements and clinical signs are also subjective and affected by the palpation technique of the clinician and, to a lesser degree, inter-observer variability. 42
Another limitation of this retrospective study was that the durations from diagnosis to radioiodine treatment and from treatment to subsequent recheck varied substantially between cats. The impact of these pre- and post-treatment durations on the relationship between pre- and post-treatment TT4 concentrations could not be thoroughly assessed, because sample populations were too small for the variability observed. Prospective studies using consistent time frames from diagnosis to treatment and treatment to recheck would be better placed to assess the strength of the relationship between pre- and post-treatment TT4 concentration.
Nearly half of the cats treated with radioiodine in the period of interest did not have TT4 concentrations available at recheck and were therefore excluded. Regular rechecks of clinical status, TT4 concentrations and renal parameters are recommended to owners and vets at discharge from our institution. Thus, it is likely that recheck TT4 concentrations were not performed because vets or owners felt it would not change management or because there were financial constraints. Inclusion of all available cats may have revealed a relationship between pre- and post-treatment TT4 concentrations, particularly if the exclusion of cats resulted in a selection bias.
Conclusions
This study finds no direct correlation between the TT4 concentration at the time of diagnosis and the TT4 concentration after treatment in cats receiving a mean fixed dose of 132.6 MBq (3.6 mCi) of radioiodine orally. These findings contrast with the outcomes of parenteral radioiodine treatment studies where a relationship between severity of hyperthyroidism, radioiodine dose and treatment outcome was inferred. The odds of having low TT4 concentrations after treatment did not increase with lower TT4 concentrations at diagnosis. Therefore, the study results do not support a dose revision for orally administered radioiodine treatment based on the severity of the increase in TT4 at the time of diagnosis of hyperthyroidism. We suggest that extrapolation from conclusions established by studying a feline population that received radioiodine parenterally to a population treated with radioiodine orally should be approached with caution.
TT4 concentrations below the lower limit of the RI at recheck were seen in 14.9% of the cats studied here and abnormalities in renal parameters were more common in these cats than in the cats that had TT4 concentrations within the RI. This highlights the fact that renal and thyroid parameters should be checked regularly after oral radioiodine therapy. It is possible that many of the cats with TT4 concentrations below the lower limit of the RI suffered from iatrogenic hypothyroidism. Hence, a reduction of the oral radioiodine dose may be indicated, although the optimal oral radioiodine dose is ideally determined during further pharmacological and prospective clinical studies.
Acknowledgments
The authors acknowledge the contribution of Dr Emma Roberts in the preparation of this manuscript, and thank all contributing referring veterinarians for their input and support.
Footnotes
Accepted: 27 September 2019
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work involved the use of experimental animals; or involved the use of client-owned animal(s) outside of established internationally recognised high standards (‘best practice’) of individual veterinary clinical patient care. The study therefore had ethical approval from an established committee as stated in the manuscript.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work for the procedure(s) undertaken. No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Lucia Yu  https://orcid.org/0000-0003-0911-6811
https://orcid.org/0000-0003-0911-6811
Lauren Lacorcia  https://orcid.org/0000-0002-1767-6248
https://orcid.org/0000-0002-1767-6248
Sue Finch  https://orcid.org/0000-0003-4261-0504
https://orcid.org/0000-0003-4261-0504
Thurid Johnstone  https://orcid.org/0000-0001-6643-9153
https://orcid.org/0000-0001-6643-9153
References
- 1. Scott-Moncrieff JC. Feline hyperthyroidism. In: Feldman EC, Nelson RW, Reusch C, et al. (eds). Canine and feline endocrinology. 4th ed. St Louis, MO: Elsevier Saunders, 2015, pp 137–187. [Google Scholar]
- 2. Peterson ME, Broome MR. Radioiodine for feline hyperthyroidism. In: Bonagura J, Twedt DC. (eds). Kirk’s current veterinary therapy. XV ed. St Louis, MO: Elsevier Saunders, 2014, pp e112–e122. [Google Scholar]
- 3. Turrel JM, Feldman EC, Hays M, et al. Radioactive iodine therapy in cats with hyperthyroidism. J Am Vet Med Assoc 1984; 184: 554–559. [PubMed] [Google Scholar]
- 4. Meric SM, Hawkins EC, Washabau RJ, et al. Serum thyroxine concentrations after radioactive iodine therapy in cats with hyperthyroidism. J Am Vet Med Assoc 1986; 188: 1038–1040. [PubMed] [Google Scholar]
- 5. Broome MR, Turrel JM, Hays MT. Predictive value of tracer studies for I-131 treatment in hyperthyroid cats. Am J Vet Res 1988; 49: 193–197. [PubMed] [Google Scholar]
- 6. Craig A, Zuber M, Allan GS. A prospective study of 66 cases of feline hyperthyroidism treated with a fixed dose of intravenous 131I. Aust Vet Pract 1993; 23: 2–6. [Google Scholar]
- 7. Mooney CT. Radioactive iodine therapy for feline hyperthyroidism: efficacy and administration routes. J Small Anim Pract 1994; 35: 289–294. [Google Scholar]
- 8. Theon AP, Van Vechten MK, Feldman E. Prospective randomized comparison of intravenous versus subcutaneous administration of radioiodine for treatment of hyperthyroidism in cats. Am J Vet Res 1994; 55: 1734–1738. [PubMed] [Google Scholar]
- 9. Peterson ME, Becker DV. Radioiodine treatment of 524 cats with hyperthyroidism. J Am Vet Med Assoc 1995; 207: 1422–1428. [PubMed] [Google Scholar]
- 10. Klausner JS, Johnston GR, Feeney DA, et al. Results of radioactive iodine therapy in 23 cats with hyperthyroidism. Minnesota J Vet Med 1987; 27: 28–32. [Google Scholar]
- 11. Malik R, Lamb WA, Church DB. Treatment of feline hyperthyroidism using orally administered radioiodine: a study of 40 consecutive cases. Aust Vet J 1993; 70: 218–219. [DOI] [PubMed] [Google Scholar]
- 12. Feeney DA, Jessen CR, Weichselbaum RC. Paired pre- and post-treatment serum biochemical parameters and thyroxine concentrations in a cohort of ninety-seven radioiodine treated hyperthyroid cats. Int J Appl Res Vet Med 2011; 9: 40–51. [Google Scholar]
- 13. Wong A, Teh H, Beatty J, et al. Risk factors for the development of chronic kidney disease and effect on survival after I131 treatment for feline hyperthyroidism. Research Communications from the Australian and New Zealand College of Veterinary Scientists, Science Week, 2011. http://oldwebsite.anzcvs.org.au/samedicine_assets/documents/2011%20SAM%20proceedings/Thompson%202011%20Wong%202011.pdf (2011, accessed May 9, 2018).
- 14. Williams TL, Elliott J, Syme HM. Association of iatrogenic hypothyroidism with azotemia and reduced survival time in cats treated for hyperthyroidism. J Vet Intern Med 2010; 24: 1086–1092. [DOI] [PubMed] [Google Scholar]
- 15. Peterson ME, Rishniw M. Hyperthyroid cats develop transient or persistent subclinical hypothyroidism after successful radioiodine treatment [abstract]. J Vet Intern Med 2017; 31: 222. [Google Scholar]
- 16. Morré WA, Panciera DL, Daniel GB, et al. Investigation of a novel variable dosing protocol for radioiodine treatment of feline hyperthyroidism. J Vet Intern Med 2018; 32: 1856–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dietze ME. Radioiodine treatment of hyperthyroidism in the cat. MS thesis, University of Melbourne, 1998. [Google Scholar]
- 18. Chun R, Garrett LD, Sargeant J, et al. Predictors of response to radioiodine therapy in hyperthyroid cats. Vet Radiol Ultrasound 2002; 43: 587–591. [DOI] [PubMed] [Google Scholar]
- 19. Lucy JM, Peterson ME, Randolph JF, et al. Efficacy of low-dose (2 millicurie) versus standard-dose (4 millicurie) radioiodine treatment for cats with mild-to-moderate hyperthyroidism. J Vet Intern Med 2017; 31: 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. International Renal Interest Society. IRIS staging of chronic kidney disease. http://www.iris-kidney.com/pdf/IRIS_2017_Staging_of_CKD_09May18.pdf (2017, accessed May 9, 2018).
- 21. Daminet S, Kooistra HS, Fracassi F, et al. Best practice for the pharmacological management of hyperthyroid cats with antithyroid drugs. J Small Anim Pract 2014; 55: 4–13. [DOI] [PubMed] [Google Scholar]
- 22. Peterson ME, Broome MR, Rishniw M. Prevalence and degree of thyroid pathology in hyperthyroid cats increases with disease duration: a cross-sectional analysis of 2096 cats referred for radioiodine therapy. J Feline Med Surg 2016; 18: 92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Broome MR. Thyroid scintigraphy in hyperthyroidism. Clin Tech Small Anim Pract 2006; 21: 10–16. [DOI] [PubMed] [Google Scholar]
- 24. Guptill L, Scottmoncrieff JCR, Janovitz EB, et al. Response to high-dose radioactive iodine administration in cats with thyroid carcinoma that had previously undergone surgery. J Am Vet Med Assoc 1995; 207: 1055–1058. [PubMed] [Google Scholar]
- 25. Turrel JM, Feldman EC, Nelson RW, et al. Thyroid carcinoma causing hyperthyroidism in cats: 14 cases (1981–1986). J Am Vet Med Assoc 1988; 193: 359–365. [PubMed] [Google Scholar]
- 26. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peterson ME, Broome MR. Ultra-low doses of radioiodine are highly effective in restoring euthyroidism without inducing hypothyroidism in most cats with milder forms of hyperthyroidism: 131 cases [abstract]. J Vet Intern Med 2014; 28: 1031. [Google Scholar]
- 28. Schneider P, Biko J, Hanscheid H, et al. The route of administration (oral vs intravenous) does not influence dose or outcome in Graves’ disease and unifocal autonomy. Eur J Nucl Med Mol Imaging 2005; 32: 788–793. [DOI] [PubMed] [Google Scholar]
- 29. Leggett RW. A physiological systems model for iodine for use in radiation protection. Radiat Res 2010; 174: 496–516. [DOI] [PubMed] [Google Scholar]
- 30. Keating FR, Albert A. The metabolism of iodine in man as disclosed with the use of radioiodine. Recent Prog Hormone Res 1949; 4: 429–481. [Google Scholar]
- 31. Mooney CT. Radioactive iodine therapy for feline hyperthyroidism: efficacy and administration routes. J Small Anim Pract 1994; 35: 289–294. [Google Scholar]
- 32. Peterson ME. Feline focus: diagnostic testing for feline thyroid disease: hypothyroidism. Compend Contin Educ Vet 2013; 35: E4. [PubMed] [Google Scholar]
- 33. Finch NC, Stallwood J, Tasker S, et al. Thyroid and renal function in cats post low-dose radioiodine therapy [abstract]. J Vet Intern Med 2018; 32: 554. [Google Scholar]
- 34. Peterson ME, Nichols R, Rishniw M. Serum thyroxine and thyroid-stimulating hormone concentration in hyperthyroid cats that develop azotaemia after radioiodine therapy. J Small Anim Pract 2017; 58: 519–530. [DOI] [PubMed] [Google Scholar]
- 35. Williams TL, Peak KJ, Brodbelt D, et al. Survival and the development of azotemia after treatment of hyperthyroid cats. J Vet Intern Med 2010; 24: 863–869. [DOI] [PubMed] [Google Scholar]
- 36. Peterson ME, Guter JN. Iatrogenic feline hypothyroidism: challenges and complexities of thyroid hormone replacement in cats. J Vet Intern Med 2015; 29: 448. [Google Scholar]
- 37. Williams TL, Elliott J, Syme HM. Effect on renal function of restoration of euthyroidism in hyperthyroid cats with iatrogenic hypothyroidism. J Vet Intern Med 2014; 28: 1251–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vogel K, Opfermann T, Wiegand S, et al. Relationship between estimated glomerular filtration rate and biological half-life of I-131. Retrospective analysis in patients with differentiated thyroid carcinoma. Nuklearmedizin 2013; 52: 164–169. [DOI] [PubMed] [Google Scholar]
- 39. Boag AK, Neiger R, Slater L, et al. Changes in the glomerular filtration rate of 27 cats with hyperthyroidism after treatment with radioactive iodine. Vet Rec 2007; 161: 711–715. [DOI] [PubMed] [Google Scholar]
- 40. Peterson ME, Varela FV, Rishniw M, et al. Evaluation of serum symmetric dimethylarginine concentration as a marker for masked chronic kidney disease in cats with hyperthyroidism. J Vet Intern Med 2018; 32: 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nykamp SG, Dykes NL, Zarfoss MK, et al. Association of the risk of development of hypothyroidism after iodine 131 treatment with the pretreatment pattern of sodium pertechnetate Tc 99m uptake in the thyroid gland in cats with hyperthyroidism: 165 cases (1990–2002). J Am Vet Med Assoc 2005; 226: 1671–1675. [DOI] [PubMed] [Google Scholar]
- 42. Paepe D, Smets P, van Hoek I, et al. Within- and between-examiner agreement for two thyroid palpation techniques in healthy and hyperthyroid cats. J Feline Med Surg 2008; 10: 558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]


