Abstract
Objectives
Despite the high prevalence and increasing awareness of chronic musculoskeletal pain in cats, approved treatment options are completely lacking in the USA, and few other options have sufficient safety and efficacy data. Knowledge of current prescribing practices should inform future research of putative therapies. We aimed to determine which drug and non-drug therapies were being used by general practitioners for the treatment of musculoskeletal pain in cats and to understand demographic influences on prescribing practices.
Methods
We distributed a survey to 36,676 veterinarians who were members of the Veterinary Information Network in January 2017. Within 3 weeks, 1056 practitioners completed the survey. The survey included demographic and background information, questions on prescribing frequency and dosing regimen of 13 drug and non-drug therapies and questions on preferred medication formulations and dosing frequencies. Descriptive statistics were used, as well as χ2 testing to evaluate relationships between demographic variables and prescription practices.
Results
Gabapentin was prescribed most frequently (71% of respondents), followed by joint supplements (67.8%), meloxicam (64.0%), opioids (62.6%), fish oil (62.1%) and polysulfated glycosaminoglycans (61.9%). Years in practice appeared to influence prescribing habits, with practitioners graduated for >20 years prescribing glucocorticoids more frequently than other age groups (P = 0.0002), whereas recent graduates (<1 year) reported prescribing therapies less frequently across all categories.
Conclusions and relevance
These results show a contrast between therapies prescribed by practitioners and what is supported by evidenced-based literature. Future research evaluating the safety and efficacy of gabapentin should be prioritised.
Keywords: Musculoskeletal pain, analgesics, surveys, questionnaires
Introduction
Estimates of the population of pet cats in the USA exceed 94 million, compared with approximately 90 million dogs. 1 Despite this, the ability of veterinarians to assess and treat pain in cats has lagged behind that of dogs. Despite increasing interest and research to bridge this information gap, most information on pain control in cats focuses on perioperative pain and analgesia.2–5 Less information exists about conditions resulting in chronic pain; consequently, more cats with these conditions remain undiagnosed and under-treated.6–8 The gradual onset and subtle behavioural changes seen in chronic pain situations, coupled with cats’ abilities to mask clinical signs, likely contribute to this disparity, making identification and measurement of chronic pain problematic.
Degenerative joint disease (DJD), which encompasses osteoarthritis (OA), commonly produces chronic musculoskeletal pain in cats, with an estimated ~60–90% of all cats having radiographic changes consistent with the disease.9–12 Of cats with radiographic evidence of DJD, an estimated 40% have DJD-associated pain (unpublished author observations from patient examinations). 9
Whereas OA includes degenerative changes of synovial joints, DJD is inclusive of degenerative changes in synovial, cartilaginous or fibrous joints. 13 When degenerative changes of both the appendicular and axial skeleton are being considered, DJD is the more correct term. For ease of understanding, the term chronic musculoskeletal pain/arthritis pain was used in the survey.
In order to guide future research of new analgesics for feline DJD, investigators should have a better understanding of what agents are currently being used and how they are being utilised. Although consensus statements and guidelines on treatment and management of chronic pain in cats exist,4,14 investigators have only evaluated the efficacy of a few suggested therapies appropriated. None of these are approved for the treatment of DJD in the USA. Only the non-steroidal anti-inflammatory drug (NSAID) meloxicam, 15 one ‘joint support diet’ 16 and a novel therapy (anti-nerve growth factor antibody) have undergone placebo-controlled trials and shown evidence of efficacy. 17 Furthermore, data on current prescribing practices can help inform future research and ensure clinical relevancy, but are currently lacking.
In order to determine which drug and non-drug therapies were being used by veterinarians for the treatment of DJD-associated pain in cats, we performed a survey through the Veterinary Information Network (VIN).
Materials and methods
Survey development involved a collaboration between experts in the fields of veterinary pharmacology, veterinary orthopedics, feline medicine and survey development and distribution. The survey focused primarily on pharmacological therapies and orally administered supplements, and excluded non-drug therapies such as acupuncture, stem cell- or platelet-rich plasma therapy, surgery, rehabilitation and laser therapy. The survey underwent several revisions with the final version comprising 107 questions, including questions regarding demographics/background, questions on 13 specific therapies and several options that allowed for other therapies to be entered as free text. Demographic and background information included years in practice, type of practice/species seen, frequency of presentation of suspected feline chronic musculoskeletal pain/arthritis cases, frequency that treatment was recommended for these and the frequency with which owners elected to treat the suspected chronic musculoskeletal pain/arthritis. Data were also collected as to whether the practitioner routinely used lean weight or true body weight (or ‘depends on therapy’) to calculate dosages.
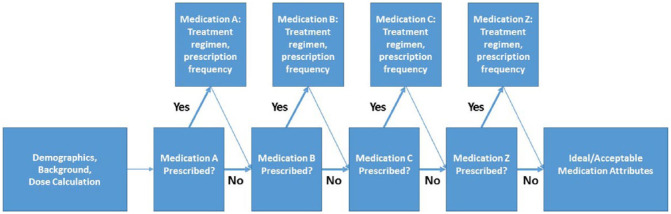
For each of the therapies included in the survey, practitioners were instructed to indicate their typical maintenance dose (clinically relevant ranges on a mg/kg or mg/lb basis, where applicable), dosing frequency, treatment duration, preferred formulation and the percentage of cats with DJD-associated pain prescribed the therapy. In order to facilitate completion, questions were blocked by therapeutic agent, which allowed respondents to bypass questions pertaining to therapies they did not use. Figure 1 shows the general survey flow and logic.
Figure 1.
Flow diagram of survey logic. Survey logic was used to allow respondents to bypass questions regarding medications the respondent indicated they did not prescribe
At the end of the survey, respondents were instructed to indicate their ideal therapeutic formulation and frequency of administration, followed by formulations and dosing frequencies that were considered acceptable. Final comments were also permitted at the end of the survey. Survey logic requiring participants to select an answer before moving to the next question was not implemented. The full survey is available as supplementary material.
The survey was distributed electronically via email to 36,676 clinicians, all belonging to an online community and information service for veterinarians (VIN). Individual sex and nationality demographic information was not collected during the survey but was available through the VIN database. The survey remained open from 17 January 2017 to 9 February 2017. Results were automatically recorded into an online database and transferred to a spreadsheet program for easier data management after the survey was closed (Microsoft Excel).
Statistical analysis involved the use of statistical software (JMP Pro 13; SAS). Summary descriptive statistics are presented when most appropriate. χ2 testing for independence was used to evaluate relationships between number of years in practice or proportion of feline caseload and therapies prescribed, or between ideal medication formulation and ideal frequency of administration. Fisher’s exact test was used to evaluate for any relationships between prescription of one therapeutic agent and the concurrent prescription of gabapentin. Sided tests were used, based on individual hypotheses: a right-sided test was used when a positive correlation between the therapy and gabapentin was suspected, whereas a left-sided test indicates a suspected negative correlation. Significance was set at a P value of ⩽0.05, before any Bonferroni corrections.
Results
Of the 36,676 veterinarians receiving the survey via email, 1056 completed the survey (defined as the respondent navigating through the final question or the survey logic ending the survey), for a completed response rate of 2.9%. Two respondents indicated that they either never recommended treatment for chronic musculoskeletal pain in cats, or clients never elected to administer the selected treatments, resulting in no further collected information. Therefore, the maximum total number of responses per question was 1054.
Most respondents were female (77.3%). Respondents were also primarily from the USA and Canada (77.0% and 13.6%, respectively), with some respondents residing in Australia (2.9%), the UK (1.3%) and New Zealand (1.1%). The level of clinical experience represented by the survey respondents ranged from <1 to >20 years in practice. All experience groups, excepting the ‘<1 year’ group, were well represented (>150 respondents); those with the most experience (‘20+ years of practice’) represented >40% of all respondents (number of responses = 440). More than 70% of respondents indicated that cats represented 25–50% of their caseload. A small subset of respondents (6.2%) indicated that they practised in feline-only clinics. Demographics of respondents completing the survey are summarised in Table 1.
Table 1.
Respondent demographic information, including sex, nationality, years of experience and feline portion of caseload
| Responses (%) | ||
|---|---|---|
| Respondent sex | Respondents (n = 1037) | |
| Male | 235 | 22.7 |
| Female | 802 | 77.3 |
| Respondent nationality | Respondents (n = 1042) | |
| USA | 802 | 77.0 |
| Canada | 142 | 13.6 |
| Australia | 30 | 2.9 |
| UK | 14 | 1.3 |
| New Zealand | 11 | 1.1 |
| Other | 43 | 4.1 |
| How long have you been in clinical practice? | Responses (n = 1054) | |
| <1 year | 32 | 3.0 |
| 1–5 years | 183 | 17.4 |
| 6–10 years | 160 | 15.2 |
| 10–20 years | 239 | 22.7 |
| >20 years | 440 | 41.7 |
| Approximately what percentage of your patients are cats? | Responses (n = 1039) | |
| <25% | 121 | 11.6 |
| 25–50% | 751 | 72.3 |
| 51–75% | 97 | 9.3 |
| 76–99% | 6 | 0.6 |
| 100% (feline only) | 64 | 6.2 |
Questions are presented as they appeared in the survey
Additional background questions collected data on the frequency of cases suspected to have chronic musculoskeletal pain/arthritis, how often respondents recommended treatment of this pain and, finally, how often respondents believed that owners followed these recommendations. Approximately 90% of respondents indicated they saw suspected cases of chronic musculoskeletal/arthritis pain in cats at least monthly, with 46.8% indicating that they saw suspected cases weekly and 15.8% indicating they saw cases daily. While only 26% of respondents indicated that they recommended treatment for pain in all of these suspected cases, 33.6% indicated they recommended treatment in >75% of cases, and 80% of respondents indicated they recommended treatment in at least 50% of these cases. However, respondents mostly believed that only 25–50% of owners followed these treatment recommendations. Full background information on frequency of cases seen, frequency of practitioner treatment recommendations and frequency of owners believed to be following treatment recommendations can be seen in Table 2.
Table 2.
Additional respondent demographic information, including the frequency at which suspected cases are seen, how often the veterinarian recommended treatment for these cases and how often the respondent estimates the owners elect to treat these cases
|
Responses (%) |
||
|---|---|---|
| Approximately how often do you see cats with suspected chronic musculoskeletal pain/arthritis, whether based on patient history, exam or radiographic findings? | Responses (n = 1056) | |
| Daily | 167 | 15.8 |
| Weekly | 494 | 46.8 |
| Monthly | 285 | 27.0 |
| Quarterly | 95 | 9.0 |
| Yearly | 15 | 1.4 |
| Never | 0 | 0 |
| How often do you recommend therapy for these cats? | Responses (n = 1055) | |
| Never or 0% | 1 | 0.1 |
| <25% of cases | 65 | 6.2 |
| 25–50% of cases | 136 | 12.9 |
| 50–75% of cases | 225 | 21.3 |
| 75–100% of cases | 354 | 33.6 |
| 100% of cases | 274 | 26.0 |
| How frequently do clients elect to administer the recommended treatments? | Responses (n = 1055) | |
| Never or 0% | 1 | 0.1 |
| <25% of cases | 277 | 26.3 |
| 25–50% of cases | 359 | 34.0 |
| 50–75% of cases | 278 | 26.4 |
| 75–100% of cases | 120 | 11.4 |
| 100% of cases | 20 | 1.9 |
Questions are presented as they appeared in the survey
Seventy-one percent of respondents reported prescribing gabapentin (Neurontin; Pfizer) for treating DJD-associated pain in cats. Slightly fewer (67.8%) prescribed joint supplements, meloxicam (64.0% [Metacam; Boehringer Ingelheim]), opioids (62.6%), fish oil (62.1%) and polysulfated glycosaminoglycans (PSGAGs; 61.9% [Adequan; Elanco]). Very few respondents reported prescribing other NSAIDs (eg, aspirin and carprofen, 5.6%) or triamcinolone (1.4%). Full data on the percentage of respondents prescribing individual therapies are presented in Table 3.
Table 3.
Rank-order percentages of respondents indicating that they prescribed a medication for the treatment of chronic musculoskeletal pain/arthritis in cats (median number of prescribed therapeutics is also included)
| Therapeutic | Respondents prescribing (%) |
|---|---|
| Gabapentin | 71.0 |
| Joint supplement | 67.8 |
| Meloxicam | 64.0 |
| Opioids | 62.6 |
| Fish oil | 62.1 |
| PSGAGs | 61.9 |
| Robenacoxib | 50.0 |
| Joint diets | 41.6 |
| Prednisolone | 34.4 |
| Tramadol | 34.0 |
| Methylprednisolone | 8.6 |
| Amitriptyline | 7.5 |
| Amantadine | 6.9 |
| Other NSAID | 5.6 |
| Triamcinolone | 1.4 |
| Median number prescribed per practitioner | 4 |
PSGAGs = polysulfated glycosaminoglycans; NSAID = non-steroidal anti-inflammatory drug
Years in practice and patterns of prescribing (Table 4) were compared. Opioids, PSGAGs and prednisolone, several ‘other’ options and some groupings of therapies by class or mechanism of action (eg, ‘any opioids’ or ‘any steroids’) showed an association. In general, practitioners with between 6 and 20 years of experience were most likely to prescribe some sort of therapy, and those with <1 year of experience were least likely to prescribe any given therapy. Practitioners with >20 years of experience were most likely to prescribe glucocorticoids (Table 5).
Table 4.
χ2 test for independence, showing where prescription of therapeutics varied significantly with years in practice
| Therapeutic | χ2 P value |
|---|---|
| Meloxicam | 0.003* |
| Robenacoxib | 0.005* |
| NSAID other | 0.001 † |
| Any NSAID | 0.838 |
| Gabapentin | 0.086 |
| PSGAGs | <0.001 † |
| Fish oil | 0.195 |
| Joint support diet | 0.036* |
| Joint supplement, other | 0.001 † |
| Any oral joint supplement | 0.010 † |
| Tramadol | 0.191 |
| Opioids | <0.001 † |
| Any opioid | <0.001 † |
| Amantadine | 0.237 |
| Amitriptyline | 0.003* |
| Amantadine or amitriptyline | 0.010* |
| Prednisolone | <0.001 † |
| Methylprednisolone | 0.004* |
| Triamcinolone | 0.111 |
| Any steroid | <0.001 † |
Denotes significance at P = 0.05
Denotes significance at P = 0.05 with Bonferroni correction (n = 20)
NSAID = non-steroidal anti-inflammatory drug; PSGAGs = polysulfated glycosaminoglycans
Table 5.
Prescribing rates of therapeutics by years in practice
| Therapeutic | Years in practice | ||||
|---|---|---|---|---|---|
| <1 year | 1–5 years | 6–10 years | 10–20 years | >20 years | |
| Meloxicam* | 46.9 | 56.3 | 59.4 | 70.7 | 66.4 |
| Robenacoxib* | 53.1 | 59.6 | 56.9 | 45.2 | 45.9 |
| Other NSAIDs † | 6.3 | 1.1 | 3.8 | 4.2 | 8.9 |
| Any NSAID | 84.4 | 85.2 | 85.6 | 83.3 | 82.3 |
| Gabapentin | 54.8 | 71.0 | 76.9 | 73.2 | 68.9 |
| PSGAGs † | 34.4 | 50.3 | 58.8 | 67.4 | 66.8 |
| Fish oil | 50.0 | 62.5 | 67.8 | 64.8 | 59.3 |
| Joint support diet* | 25.0 | 45.2 | 50.0 | 41.4 | 38.6 |
| Other joint supplement † | 41.9 | 65.5 | 75.8 | 72.5 | 65.3 |
| Any oral joint supplement † | 75.0 | 90.7 | 92.5 | 90.4 | 85.7 |
| Tramadol | 31.3 | 30.6 | 41.9 | 34.7 | 32.3 |
| Other opioids † | 46.9 | 72.1 | 73.1 | 64.9 | 54.8 |
| Any opioid † | 59.4 | 79.2 | 84.4 | 76.6 | 69.1 |
| Amantadine | 3.1 | 4.4 | 5.0 | 8.8 | 8.0 |
| Amitriptyline* | 3.1 | 2.2 | 6.9 | 6.3 | 10.9 |
| Amantadine or amitriptyline* | 6.3 | 5.5 | 11.3 | 12.6 | 15.5 |
| Prednisolone † | 9.4 | 15.3 | 19.4 | 37.7 | 48.0 |
| Methylprednisolone* | 6.3 | 3.3 | 6.3 | 7.9 | 12.3 |
| Triamcinolone | 0.0 | 0.0 | 0.6 | 1.3 | 2.5 |
| Any steroid † | 59.4 | 79.2 | 84.4 | 76.6 | 69.1 |
Denotes significance at P = 0.05
Denotes significance at P = 0.05 with Bonferroni correction (n = 20)
NSAID = non-steroidal anti-inflammatory drug; PSGAGs = polysulfated glycosaminoglycans
Subsequently, relationships between the percentage of cats seen in the practice and prescribed therapies (Table 6) were explored. There appeared to be little relationship between the percentage of cats seen in the practice and prescription of individual therapies. In some instances, clinicians with high feline caseloads (76–99%) prescribed certain therapies at the highest frequency. However, this should be interpreted cautiously given the sample size for this group (number of responses = 6). That said, a positive relationship existed between the proportion of cats seen in the practice and the prescribing practices for PSGAGs. Similarly, practices seeing a higher proportion of cats reported higher prescribing frequencies of gabapentin, joint supplements, opioids and steroids (Table 7). A comparison of feline-only practitioners against all other respondents revealed similar results (Table 8).
Table 6.
Therapeutic prescription frequency by percentage of feline patients
| Therapeutic | Percentage feline patients | ||||
|---|---|---|---|---|---|
| <25% | 25–50% | 51–75% | 76–99% | 100% | |
| Meloxicam | 58.7 | 62.5 | 73.2 | 66.7 | 75.0 |
| Robenacoxib | 45.5 | 52.1 | 41.2 | 50.0 | 48.4 |
| NSAID other | 4.1 | 5.7 | 8.2 | 16.7 | 3.1 |
| Any NSAID | 78.5 | 84.0 | 84.5 | 83.3 | 85.9 |
| Gabapentin* | 62.8 | 70.9 | 69.1 | 100.0 | 87.5 |
| PSGAGs † | 66.9 | 58.9 | 63.9 | 100.0 | 81.3 |
| Fish oil | 58.3 | 62.9 | 54.8 | 66.7 | 67.7 |
| Joint support diet | 43.5 | 42.0 | 32.3 | 16.7 | 46.8 |
| Other joint supplement* | 57.9 | 68.5 | 61.9 | 100.0 | 82.1 |
| Any oral joint supplement | 86.0 | 89.5 | 81.4 | 83.3 | 89.1 |
| Tramadol | 33.1 | 35.2 | 30.9 | 0.0 | 25.0 |
| Other opioids* | 47.1 | 63.5 | 67.0 | 66.7 | 68.8 |
| Any opioid* | 62.8 | 76.0 | 75.3 | 66.7 | 75.0 |
| Amantadine | 5.8 | 6.7 | 7.2 | 0.0 | 9.4 |
| Amitriptyline | 5.8 | 6.9 | 9.3 | 0.0 | 14.1 |
| Amantadine and amitriptyline | 10.7 | 11.5 | 12.4 | 0.0 | 20.3 |
| Prednisolone | 33.9 | 34.0 | 32.0 | 50.0 | 40.6 |
| Methylprednisolone | 10.7 | 8.3 | 10.3 | 16.7 | 6.3 |
| Triamcinolone | 2.5 | 1.6 | 0.0 | 0.0 | 0.0 |
| Any steroid* | 62.8 | 76.0 | 75.3 | 66.7 | 75.0 |
Denotes significance at P = 0.05
Denotes significance at P = 0.05 with Bonferroni correction (n = 20)
NSAID = non-steroidal anti-inflammatory drug; PSGAGs = polysulfated glycosaminoglycans
Table 7.
χ2 test for independence showing where prescription of therapeutics varied significantly with percentage of feline patients
| Therapeutic | χ2 P value |
|---|---|
| Meloxicam | 0.057 |
| Robenacoxib | 0.256 |
| NSAID other | 0.424 |
| Any NSAID | 0.637 |
| Gabapentin | 0.005* |
| PSGAGs | 0.001 † |
| Fish oil | 0.427 |
| Joint support diet | 0.219 |
| Joint supplement, other | 0.008* |
| Any oral joint supplement | 0.183 |
| Tramadol | 0.179 |
| Opioids | 0.006* |
| Any opioid | 0.044* |
| Amantadine | 0.851 |
| Amitriptyline | 0.213 |
| Amantadine or amitriptyline | 0.246 |
| Prednisolone | 0.721 |
| Methylprednisolone | 0.716 |
| Triamcinolone | 0.480 |
| Any steroid | 0.044* |
Denotes significance at P = 0.05
Denotes significance at P = 0.05 with Bonferroni correction (n = 20)
NSAID = non-steroidal anti-inflammatory drug; PSGAGs = polysulfated glycosaminoglycans
Table 8.
χ2 test for independence showing where prescription of therapeutics varied significantly between feline-only and all other practitioners
| Therapeutic | χ2 P value |
|---|---|
| Meloxicam | 0.057 |
| Robenacoxib | 0.796 |
| NSAID other | 0.375 |
| Any NSAID | 0.600 |
| Gabapentin | 0.003* |
| PSGAGs | 0.001 † |
| Fish oil | 0.342 |
| Joint support diet | 0.397 |
| Joint supplement other | 0.018* |
| Any oral joint supplement | 0.851 |
| Tramadol | 0.118 |
| Opioids | 0.296 |
| Any opioid | 0.936 |
| Amantadine | 0.427 |
| Amitriptyline | 0.040* |
| Amantadine or amitriptyline | 0.039* |
| Prednisolone | 0.283 |
| Methylprednisolone | 0.484 |
| Triamcinolone | 0.321 |
| Any steroid | 0.936 |
Denotes significance at P = 0.05
Denotes significance at P = 0.05 with Bonferroni correction (n = 20)
NSAID = non-steroidal anti-inflammatory drug; PSGAGs = polysulfated glycosaminoglycans
Next, associations between the prescription of one therapy and the concurrent frequency of gabapentin prescription were examined. Prescription of almost every therapy correlated positively with prescription of gabapentin, excepting meloxicam, (no relationship) and certain steroids (Table 9); in other words, respondents who prescribed gabapentin more frequently also prescribed other therapies more frequently.
Table 9.
Fisher’s exact test of effect of a prescription of a therapeutic on gabapentin prescription frequency
| Therapeutic | Fisher’s exact P value |
|---|---|
| Meloxicam | 0.813 R |
| Robenacoxib | <0.001 R † |
| NSAID other | 0.016 L* |
| Any NSAID | 0.044 R* |
| Gabapentin | NA |
| PSGAGs | <0.001 R † |
| Fish oil | <0.001 R † |
| Joint support diet | 0.028 R* |
| Joint supplement other | 0.009 R* |
| Any oral joint supplement | <0.001 R † |
| Tramadol | <0.001 R † |
| Opioids | <0.001 R † |
| Any opioid | <0.001 R † |
| Amantadine | <0.001 R † |
| 0.001 R † | |
| Amantadine or amitriptyline | <0.001 R † |
| Prednisolone | 0.029 L* |
| Methylprednisolone | 0.002 L † |
| Triamcinolone | 0.674 L |
| Any steroid | 1.000 L |
‘L’ denotes the hypothesis that gabapentin is prescribed more when drug or non-drug oral therapeutic is not prescribed; ‘R’ denotes the hypothesis that gabapentin is prescribed more when drug or non-drug oral therapeutic is prescribed
Denotes significance at P = 0.05
Denotes significance at P = 0.05 with Bonferroni correction (n = 19)
NSAID = non-steroidal anti-inflammatory drug; PSGAGs = polysulfated glycosaminoglycans; NA = not applicable
Respondents were asked whether they calculated therapeutic doses based on true or lean body weight. Approximately half indicated that their calculations depended on the drug, whereas the remainder skewed towards total body weight (Table 10).
Table 10.
Therapeutic dose calculation methods
| How do you calculate dosing? | Responses (n = 1053) | Respondents (%) |
|---|---|---|
| Always on actual body weight | 267 | 25.4 |
| Always on lean body weight | 167 | 15.9 |
| It depends on the drug | 585 | 55.6 |
| Other | 34 | 3.2 |
For most therapies, doses and frequencies used long term appear to be similar to label dosages, regardless of label indications (eg, robenacoxib [Onsior; Elanco], which is only approved for perioperative pain in the USA, yet is used for chronic musculoskeletal pain), reports and reviews in the veterinary literature,15,18,19 and veterinary formularies (Table 11). 20 Duration of treatment varied by therapy, with the joint supplements generally being used for longer durations than NSAIDs or glucocorticoids. Respondents prescribed most therapies to <25% of cats with DJD. Respondents indicated that they prescribed a few therapies to 26–50% of cats with DJD (eg, gabapentin, fish oil, PSGAGs), whereas they prescribed joint supplements to 51–75% of cats with DJD.
Table 11.
Summary data of typical dosing regimen (including dose, frequency, duration and formulation), as well as percentage of patients prescribed the therapeutic
| Drug or non-drug therapeutic | Dose amount | Dosing frequency | Dosing duration | Most common formulation | Percentage of patients prescribed |
|---|---|---|---|---|---|
| Meloxicam | 0.02–0.04 mg/kg (<0.02 to >0.1 mg/kg) |
q24h (q12h–q72h; PRN or other) |
15–30 days (1 day to >120 days) |
Liquid | <25% (<25% to all cats) |
| Robenacoxib | 1–2.4 mg/kg (<1 to >2.4 mg/kg) |
q24h (q12h–q72h; PRN or other) |
4–7 days (1 day to >120 days) |
Tablet | <25% (<25% to all cats) |
| Other NSAID | NA | NA | 61–120 days (1 day to >120 days) |
Tablet | <25% (<25% to all cats) |
| Gabapentin | 5.1–10 mg/kg (<5 to >20 mg/kg) |
q12h (q12h–q48h; PRN or other) |
61–120 days (2–3 days to >120 days) |
Compounded | 26–50% (<25% to all cats) |
| PSGAGs | 5 mg/kg (<5 to >5 mg/kg) |
q14d (q24h–q30d; other) |
>120 days (1 day to >120 days) |
NA | 26–50% (<25% to all cats) |
| Fish oil | 50.1–100 mg/kg (<25 to >400 mg/kg) |
q48h (q12h–q48h; PRN or other) |
>120 days (11–14 days to >120 days) |
Liquid | 26–50% (<25% to all cats) |
| Joint diets | NA | NA | >120 days (1 day to >120 days) |
NA | <25% (<25% to all cats) |
| Joint supplements | NA | q24h (q24h–q30d; other) |
>120 days (1 day to >120 days) |
Capsule or tablet | 51–75% (<25% to all cats) |
| Tramadol | 2.0–4.0 mg/kg (<2 to >4 mg/kg) |
q12h (q8h–q24h; PRN or other) |
15–30 days (2–3 days to >120 days) |
Tablet | <25% (<25% to all cats) |
| Opioids | 0.01–0.02 mg/kg (0.005–0.5 mg/kg) |
q12h (q8h–q72h; PRN or other) |
8–10 day (1 days to >120 days) |
Liquid | <25% (<25% to all cats) |
| Amantadine | 3.0–5.0 mg/kg (<3 to >5 mg/kg) |
q24h (q12h–q24h) |
31–60 days (2–3 days to >120 days) |
Liquid | <25% (<25% to 51–75%) |
| Amitriptyline | 0.5–1.0 mg/kg (<0.5 to >2.5 mg/kg) |
q24h (q12h–q24h) |
61–120 days (4–7 days to >120 days) |
Tablet | <25% (<25% to 51–75%) |
| Prednisolone | 0.5–1.0 mg/kg (<0.5 to >2.0 mg/kg) |
q24h (q12h–q72h; PRN or other) |
15–30 days (2–3 days to >120 days) |
Tablet | <25% (<25% to 76–99%) |
| Methylprednisolone | 10.0–20.0 mg/kg (<10.0 to >20.0 mg/kg) |
q30d (q7d–q60d; PRN or other) |
61–120 days (1 day to >120 days) |
NA | <25% (<25% to 76–99%) |
| Triamcinolone | <0.5 mg/kg (<0.5 to 0.5–1.0 mg/kg) |
One response: q48h Remaining: PRN or other |
15–30 days (1 day to >120 days) |
Tablet | <25% (<25% to 51–75%) |
Data are presented as median (range)
PRN = pro re nata (as needed); NSAID = non-steroidal anti-inflammatory druge; NA = not applicable; PSGAG = polysufated glycosaminoglycan
Finally, practitioners were asked about their perceived ideal and acceptable therapeutic formulations and dosing frequencies. The results indicate that respondents considered liquid therapies dosed once daily most ideal. Almost all formulations were deemed acceptable, and >40% of respondents accepted any dosing frequency less than three times daily (Table 12). There was a relationship between ideal formulation and ideal frequency of administration (P <0.0001). The responses for parenteral (subcutaneous or intramuscular) formulations appeared to contribute the most to this relationship, with responses clustering around less frequent administration (eg, once monthly) when compared with oral routes of administration (eg, once daily). Additionally, clinicians with ⩽10 years of experience preferred liquid formulations, whereas clinicians with ⩾10 years of experience preferred parenteral formulations (P = 0.007). When asked about what other formulations would be considered acceptable, answers included ‘transdermal preparations’, ‘whatever the owner/cat would tolerate’, ‘compounded chews/treats’ and mentions of ‘alternative medicine’ techniques.
Table 12.
Ideal and acceptable therapeutic formulations and dosing frequencies, as indicated by respondents
| Responses (%) | ||
|---|---|---|
| What is your IDEAL treatment formulation for treating chronic musculoskeletal pain or arthritis in cats? | Responses (n = 1016) | |
| Tablet | 72 | 7.1 |
| Capsule | 27 | 2.7 |
| Liquid | 653 | 64.1 |
| Parenteral (SC or IM) | 131 | 12.9 |
| Other | 133 | 13.1 |
| What is your IDEAL dosing frequency for medications used to treat chronic musculoskeletal pain or arthritis in cats? | Responses (n = 1039) | |
| Thrice daily | 2 | 0.2 |
| Twice daily | 45 | 4.3 |
| Once daily | 545 | 52.4 |
| Twice weekly | 103 | 9.9 |
| Once weekly | 155 | 14.9 |
| Twice monthly | 22 | 2.1 |
| Once monthly | 122 | 11.7 |
| Once every other month | 45 | 4.3 |
| What formulations for treating chronic musculoskeletal pain or arthritis in cats would you consider acceptable? | Respondents (n = 1046; total responses = 3306) |
Practitioners considering acceptable (%) |
| Tablet | 793 | 75.8 |
| Capsule | 579 | 55.3 |
| Liquid | 1003 | 95.8 |
| Parenteral (SC or IM) | 732 | 69.9 |
| Other | 202 | 19.3 |
| What dosing frequencies for medications used to treat chronic musculoskeletal pain or arthritis in cats would you consider acceptable? |
Respondents (n = 1048;
total responses = 4749) |
|
| Thrice daily | 45 | 4.3 |
| Twice daily | 440 | 42.0 |
| Once daily | 934 | 89.1 |
| Twice weekly | 731 | 69.8 |
| Once weekly | 790 | 75.4 |
| Twice monthly | 609 | 58.1 |
| Once monthly | 698 | 66.6 |
| Once every other month | 502 | 47.9 |
Questions are presented as they appeared in the survey
SC = subcutaneous; IM = intramuscular
In total, 116 respondents specified prescribed therapies other than those populating the survey. Reponses included joint supplements and sources of omega 3 fatty acids (despite being included as choices earlier in the study; number of responses = 33), acupuncture (number of responses = 26), traditional Chinese medicine and/or herbs (number of responses = 18), laser therapy (number of responses = 18), homeopathic treatments (number of responses = 11), maropitant (number of responses = 7 [Cerenia; Zoetis]) and cannabidiol-containing products (number of responses = 4). Altogether, 225 respondents provided final comments at the end of the survey. These responses commonly included recommendations of other therapies, including acupuncture (number of responses = 54), laser therapy (number of responses = 49), traditional Chinese medicine (number of responses = 3), Assisi loops (number of responses = 2) and others. We did not include these responses in any statistical analysis.
Discussion
Our survey suggests that gabapentin is a very popular choice for treating chronic musculoskeletal pain in cats, both in terms of the proportion of respondents who prescribe gabapentin and the proportion of cats prescribed gabapentin. Joint supplements were also prescribed by a high proportion of respondents, and for a high proportion of cats. Similarly, despite the potential adverse events and the US Food and Drug Administration (FDA)-mandated ‘black box’ warning against using meloxicam beyond a single dose in the USA, many respondents reported prescribing NSAIDs. While the survey did not collect data on whether respondents were taking a multimodal approach in individual patients, half the respondents prescribed at least four therapies (although not necessarily to each cat). Therapies with lower risk of adverse effects (true or perceived) had a longer median duration of treatment and higher median proportion of cats being prescribed the therapy (eg, joint supplements, fish oil and PSGAGs vs NSAIDs and steroids).
We found differences in prescribing habits with years in practice. With few exceptions, respondents with <1 year of experience prescribed all therapies at the lowest rate. While speculative, reasons for this could be lack of comfort in treating the disease, concerns about off-label drug use, or simply due to fewer cases seen by that group. However, experience alone does not seem to correlate with an increased likelihood to prescribe therapies. While prescribing frequency increased as experience increased from 1 year up to 20 years, prescribing frequency decreased across all therapies seen in respondents with >20 years of experience. It is unclear whether this is related to the more recent emphasis on chronic pain in companion species, or other factors. One such factor might be practitioner sex – female physicians are perceived as more empathetic than their male colleagues are and are more likely to prescribe pharmacological agents as a first line of treatment for patients with lower back pain.21,22 Whether these relationships are present in veterinary medicine is unknown. Practitioner experience, age, sex and other factors all likely contribute to these differences in prescribing rates overall, and of specific drug classes like NSAIDs and steroids.
Major events and drug approvals can affect prescribing frequencies. The FDA released a ‘Boxed Warning’ on Metacam in late 2010. This might, in part, explain the reported lower prescription frequencies of meloxicam by less experienced practitioners. Similarly, robenacoxib gained approval (in the USA, for postoperative pain and inflammation for up to 3 days) in early 2011, which appears to be reflected by increased prescribing rates by less experienced veterinarians in our survey. Previous studies have shown that prescribing habits are closely associated with age of physician. 23 It is worth noting that since the development and distribution of the survey, the European Medicines Agency has adopted a positive opinion with regard to long-term administration of robenacoxib. The drug’s new label indication now awaits final approval by the European Commission. This would not have been reflected in the results at the time of the survey. Differences in drug approvals or formulation availability in countries outside of the USA could influence prescribing habits. It remains unknown whether these relationships are real or merely coincidental.
Our study highlights a disconnect between which therapies have clinical data to support their use and which therapies are used most often. For example, most respondents prescribed gabapentin to between 26% and 50% of cats with chronic DJD-associated pain. However, no clinical data exist supporting the use of gabapentin as an analgesic in cats, (or indeed in dogs) – the few studies examining analgesic effects of gabapentin found no effect.24–27 Similarly, many respondents reported prescribing joint supplements, with no clinical data supporting their use. 28 We suspect that an ‘appeal to authority’ fallacy might contribute to this, whether from numerous anecdotal accounts of use and efficacy, or from perceived experts in the field. 29 Additionally, the perceived high safety margin with joint supplements likely plays into ‘probably doesn’t hurt and might help’ thinking. Practitioners are also likely influenced by material they hear or read, and a search of Centre for Agriculture and Biosciences International abstracts indicates a steady increase in the number of review articles and conference proceedings discussing chronic pain in cats, despite only a handful of ‘source evidence’ studies – clinical studies evaluating efficacy of analgesics in cats with chronic pain.15–17,30–32 Indeed, with a paucity of data or evidence, review articles focused on dogma proliferate. 33
Overall, this suggests increasing pressure on practitioners to identify and address chronic musculoskeletal pain due to DJD, without the appropriate research or data to support fully informed and evidence-based treatment selections.
There are, unfortunately, several issues that could result from this disconnect, relating to the risks of a lack of efficacy or unknown side effects. Perhaps most important is the risk of continued pain because of ineffective treatment, especially given that pain in cats can be difficult to detect, owing to both a lack of validated assessment tools and a robust caregiver placebo effect. 34 Conversely, there exists the possibility of a beneficial placebo-by-proxy effect. 34 This occurs when administration of a therapy (efficacious or not) causes a change in caregiver disposition/optimism, changed or increased interactions with the patient, and resulting improvement in patient quality of life and activity. Finally, without safety data from chronic administration, clinicians cannot predict infrequent or rare adverse effects, some of which could be serious.
Respondents preferred once-daily administration of a liquid by a wide margin (64.1% and 52.4% of responses, respectively). The preference for a liquid formulation is understandable, given the perceived difficulty of administering solid oral medications to many cats. Similarly, a liquid formulation may be preferable to parenteral routes of administration for owners that are uncomfortable using needles, or cats that resent injections. However, we could not understand the preference for once daily administration for oral routes of administration, especially given that we provided less frequent options including twice weekly, once weekly and once every other month. It might be that respondents were concerned about owner compliance without a set daily routine, or about therapeutic toxicity or efficacy with once-every-other-month administration. In contrast, the ideal dosing frequency for parenteral formulations clustered around less frequent administration.
Our study has several limitations. Perhaps foremost among these is the assumption that owners are following through with prescribed treatments. We cannot determine whether prescribed therapies were administered to the cats, and no data were collected on whether clients renewed prescriptions. There was also a limit in the number of questions that we could feasibly ask. While algorithms were used to attempt to reduce the number of questions any respondent answered (eg, bypassing questions that were not relevant to individual practitioners), several respondents commented on the survey being too long or arduous. The survey length might have reduced the response rate. However, our response rate did not differ from most survey response rates for surveys administered to VIN members. Therefore, our responses might simply reflect the ‘survey-responsive’ pool of veterinarians who are VIN members. As with any convenience sample, the results, including any perceived trends by practice type or years in practice, might not represent the actual population. The questions pertaining to dose of therapies were not open-ended, precluding the calculation of true averages for dosing regimens. These data would be useful for researchers designing clinical trials or efficacy studies. Also, owing to limitations in length, we were unable to collect data on typical treatment regimens for a patient, or to see whether respondents commonly prescribed multimodal therapy. These data would also help researchers evaluating potential synergy between medications, so that clinically relevant combinations could be evaluated. We were also unable to collect data on perceived efficacy, reasons for discontinuation of a therapeutic, or other similar considerations. These data would also be beneficial for maximising research impact.
The survey did not include non-pharmaceutical therapies, such as acupuncture, laser therapy or other alternative medicine practices. While many respondents included this information in comments, the data were not collected in a way to allow analysis. The survey respondents were primarily female and from the USA and Canada. This sex bias reflects VIN membership demographics (71% female) more so than the demographics of the veterinary profession.35,36 This means that important differences in prescribing habits, as well as licensed indications between sexes, countries or regions could not be been appreciated. Finally, survey logic requiring a respondent to select an answer before moving on was not used. This means that partial or incomplete data might have been collected even within a given therapeutic agent.
Conclusions
Our study highlights the wide knowledge gap that exists when it comes to treating chronic musculoskeletal pain in cats. Research is required into the efficacy of various therapies, and the pharmacokinetics and safety after chronic administration. Given our results, further research into gabapentin would appear most pertinent and pressing.
Supplemental Material
Full survey distributed to VIN members investigating prescribing practices for chronic musculoskeletal pain in cats.
Acknowledgments
The authors would like to thank Dr Mark G Papich for his assistance during survey development.
Footnotes
Accepted: 15 June 2018
Author note: This paper was presented, in part, at the 2017 Annual College of Veterinary Medicine Research Forum on 22 September at the College of Veterinary Medicine, North Carolina State University, Raleigh, NC, USA.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research was funded by the Comparative Pain Research Program of the College of Veterinary Medicine, North Carolina State University, Raleigh, USA. Dr Adrian was receiving a fellowship stipend from the Morris Animal Foundation (#D17FE-401) while the research was performed.
Supplementary material: Full survey distributed to VIN members investigating prescribing practices for chronic musculoskeletal pain in cats.
ORCID iD: Derek E Adrian  https://orcid.org/0000-0002-0226-6182
https://orcid.org/0000-0002-0226-6182
B Duncan X Lascelles  https://orcid.org/0000-0002-2950-9009
https://orcid.org/0000-0002-2950-9009
References
- 1. American Pet Products Association (APPA). 2017–2018 APPA national pet owners survey. Greenwich, CT: APPA, 2017. [Google Scholar]
- 2. Calvo G, Holden E, Reid J, et al. Development of a behaviour-based measurement tool with defined intervention level for assessing acute pain in cats. J Small Anim Pract 2014; 55: 622–629. [DOI] [PubMed] [Google Scholar]
- 3. Brondani JT, Luna SPL, Padovani CR. Refinement and initial validation of a multidimensional composite scale for use in assessing acute postoperative pain in cats. Am J Vet Res 2011; 72: 174–183. [DOI] [PubMed] [Google Scholar]
- 4. Epstein ME, Rodan I, Griffenhagen G, et al. 2015 AAHA/AAFP pain management guidelines for dogs and cats. J Feline Med Surg 2015; 17: 251–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson JA. Treatment of acute pain in cats. In: Egger CM, Love L, Doherty T. (eds). Pain management in veterinary practice. Ames, Iowa: John Wiley & Sons, 2013, pp 275–288. [Google Scholar]
- 6. Robertson SA. Osteoarthritis in cats: what we now know about recognition and treatment. Vet Med 2008; 103: 611–616. [Google Scholar]
- 7. Lorena SERS, Luna SPL, Lascelles BDX, et al. Current attitudes regarding the use of perioperative analgesics in dogs and cats by Brazilian veterinarians. Vet Anaesth Analg 2013; 41: 82–89. [DOI] [PubMed] [Google Scholar]
- 8. Lascelles BDX, Robertson SA. DJD-associated pain in cats: what can we do to promote patient comfort? J Feline Med Surg 2010; 12: 200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lascelles BD, Henry JB, Brown J, et al. Cross-sectional study of the prevalence of radiographic degenerative joint disease in domesticated cats. Vet Surg 2010; 39: 535–544. [DOI] [PubMed] [Google Scholar]
- 10. Clarke SP, Mellor D, Clements DN, et al. Prevalence of radiographic signs of degenerative joint disease in a hospital population of cats. Vet Rec 2005; 157: 793–799. [DOI] [PubMed] [Google Scholar]
- 11. Hardie EM, Roe SC, Martin FR. Radiographic evidence of degenerative joint disease in geriatric cats: 100 cases (1994–1997). J Am Vet Med Assoc 2002; 220: 628–632. [DOI] [PubMed] [Google Scholar]
- 12. Slingerland LI, Hazewinkel HA, Meij BP, et al. Cross-sectional study of the prevalence and clinical features of osteoarthritis in 100 cats. Vet J 2011; 187: 304–309. [DOI] [PubMed] [Google Scholar]
- 13. Resnick D. Degenerative disease of extraspinal locations. In: Resnick D. (ed). Bone and joint imaging. 2nd ed. Philadelphia, PA: WB Saunders, 1996, pp 321–354. [Google Scholar]
- 14. Mathews K, Kronen PW, Lascelles D, et al. Guidelines for recognition, assessment and treatment of pain: WSAVA Global Pain Council members and co-authors of this document. J Small Anim Pract 2014; 55: E10–E68. [DOI] [PubMed] [Google Scholar]
- 15. Gruen ME, Griffith E, Thomson A, et al. Detection of clinically relevant pain relief in cats with degenerative joint disease associated pain. J Vet Intern Med 2014; 28: 346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lascelles BDX, DePuy V, Thomson A, et al. Evaluation of a therapeutic diet for feline degenerative joint disease. J Vet Intern Med 2010; 24: 487–495. [DOI] [PubMed] [Google Scholar]
- 17. Gruen ME, Thomson AE, Griffith EH, et al. A feline-specific anti-nerve growth factor antibody improves mobility in cats with degenerative joint disease-associated pain: a pilot proof of concept study. J Vet Intern Med 2016; 30: 1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lascelles BDX, Gaynor JS, Smith ES, et al. Amantadine in a multimodal analgesic regimen for alleviation of refractory osteoarthritis pain in dogs. J Vet Intern Med 2008; 22: 53–59. [DOI] [PubMed] [Google Scholar]
- 19. Chew DJ, Buffington CA, Kendall MS, et al. Amitriptyline treatment for severe recurrent idiopathic cystitis in cats. J Am Vet Med Assoc 1998; 213: 1282–1286. [PubMed] [Google Scholar]
- 20. Papich MG. Saunders handbook of veterinary drugs: small and large animal. 4th ed. St Louis, MO: Elsevier, 2015. [Google Scholar]
- 21. Howick J, Steinkopf L, Ulyte A, et al. How empathic is your healthcare practitioner? A systematic review and meta-analysis of patient surveys. BMC Med Educ 2017; 17: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Veldhuijzen DS, Karhof S, Leenders ME, et al. Impact of physicians’ sex on treatment choices for low back pain. Pain Pract 2012; 13: 451–458. [DOI] [PubMed] [Google Scholar]
- 23. Holmes JS, Shevrin M, Goldman B, et al. Translating research into practice: are physicians following evidence-based guidelines in the treatment of hypertension? Med Care Res Rev 2004; 61: 453–473. [DOI] [PubMed] [Google Scholar]
- 24. Pypendop BH, Siao KT, Ilkiw JE. Thermal antinociceptive effect of orally administered gabapentin in healthy cats. Am J Vet Res 2010; 71: 1027–1032. [DOI] [PubMed] [Google Scholar]
- 25. Aghighi SA, Tipold A, Piechotta M, et al. Assessment of the effects of adjunctive gabapentin on postoperative pain after intervertebral disc surgery in dogs. Vet Anaesth Analg 2012; 39: 636–646. [DOI] [PubMed] [Google Scholar]
- 26. Crociolli GC, Cassu RN, Barbero RC, et al. Gabapentin as an adjuvant for postoperative pain management in dogs undergoing mastectomy. J Vet Med Sci 2015; 77: 1011–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Steagall PV, Benito J, Monteiro BP, et al. Analgesic effects of gabapentin and buprenorphine in cats undergoing ovariohysterectomy using two pain-scoring systems: a randomized clinical trial. J Feline Med Surg 2018; 20: 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vandeweerd JM, Coisnon C, Clegg P, et al. Systematic review of efficacy of nutraceuticals to alleviate clinical signs of osteoarthritis. J Vet Intern Med 2012; 26: 448–456. [DOI] [PubMed] [Google Scholar]
- 29. Fearnside WW, Holther WB. Fallacy; the counterfeit of argument. Englewood Cliffs, NJ: Prentice-Hall, 1959. [Google Scholar]
- 30. Gruen ME, Griffith EH, Thomson AE, et al. Criterion validation testing of clinical metrology instruments for measuring degenerative joint disease associated mobility impairment in cats. PLoS One 2015; 10: e0131839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Monteiro BP, Klinck MP, Moreau M, et al. Analgesic efficacy of tramadol in cats with naturally occurring osteoarthritis. PLoS One 2017; 12: e0175565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guillot M, Moreau M, Heit M, et al. Characterization of osteoarthritis in cats and meloxicam efficacy using objective chronic pain evaluation tools. Vet J 2013; 196: 360–367. [DOI] [PubMed] [Google Scholar]
- 33. Rishniw M, Pion PD. Is treatment of feline hypertrophic cardiomyopathy based in science or faith? A survey of cardiologists and a literature search. J Feline Med Surg 2011; 13: 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gruen ME, Dorman DC, Lascelles BDX. Caregiver placebo effect in analgesic clinical trials for cats with naturally occurring degenerative joint disease-associated pain. Vet Rec 2017; 180: 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. American Community Survey. 1-year public use microdata sample. https://factfinder.census.gov/faces/nav/jsf/pages/searchresults.xhtml?refresh=t: (2016, accessed May 15, 2018)
- 36. Mirza and Nacey Research. FVE survey of the veterinary profession in Europe. Arundel: Mirza and Nacey Research, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full survey distributed to VIN members investigating prescribing practices for chronic musculoskeletal pain in cats.