Abstract
Objectives
The purpose of this study was to describe the electrophoretic patterns of proteinuria in cats at risk of and cats with chronic kidney disease (CKD), and to investigate whether the presence of high-molecular-weight (HMW) and low-molecular-weight (LMW) proteins were associated with CKD, proteinuria and/or disease progression.
Methods
Healthy cats at risk of developing renal disease (n = 17) and cats affected with CKD at different stages (n = 22) were prospectively enrolled and sampled over time. Seventy urine samples were included and assayed with a commercially available sodium dodecyl sulfate–agarose gel electrophoresis (SDS-AGE) method. Each sample (gel lane) was inspected to identify albumin, HMW and LMW proteins, and an electrophoretic pattern (albuminuria, glomerular, tubular, mixed or negative) was assigned accordingly. Fisher’s exact test was used to assess the distribution of HMW and LMW proteins in cats grouped according to International Renal Interest Society stage and to the magnitude of proteinuria, and to assess if HMW and LMW proteins at the time of inclusion were associated with the development and progression of CKD.
Results
In samples of cats at risk, the most common pattern was glomerular (84.6%); glomerular pattern was also common in cats with CKD (54.2%), although mixed proteinuria and tubular proteinuria were also present (29.5% and 11.4%, respectively). The presence of LMW proteins was associated with CKD (P <0.0001) and to a urine protein:creatinine ratio >0.2 (P = 0.025). Both HMW and LMW proteins were not associated with progression of CKD within 6 months (n = 14).
Conclusions and relevance
Our results showed that HMW proteinuria is common in healthy cats at risk of developing CKD, although the pathological significance needs to be confirmed. The detection of LMW proteins in urine of cats suspected to be affected by CKD, especially in non-azotaemic, non-proteinuric or borderline proteinuric cats, suggests the presence of kidney damage.
Keywords: Chronic kidney disease, proteinuria, sodium dodecyl sulfate–agarose gel electrophoresis, urine protein:creatinine ratio
Introduction
In the diagnostic work-up of feline chronic kidney disease (CKD), the accurate evaluation of proteinuria is routinely performed using the urinary protein:creatinine (UPC) ratio on spot urine samples. This quantitative method provides information about the severity of proteinuria and gives prognostic indications. 1 Despite these unquestionable advantages, the UPC ratio does not provide information about the source of proteinuria and which part of the nephron is mainly affected.
Qualitative evaluation of proteinuria with one-dimensional (1D) electrophoresis allows separation and identification of proteins on the gel as bands and, depending on the types of bands identified, renal proteinuria can be classically subdivided into glomerular, tubular or mixed. 2 Glomerular proteinuria occurs when the selective permeability of the glomerulus is altered, leading to overfiltration of albumin and of proteins with a molecular weight (MW) higher than that of albumin. Tubular proteinuria is characterised by the presence of protein with an MW lower than that of albumin: normally these proteins freely pass the glomerulus and are reabsorbed in the proximal tubule and, therefore, their presence is attributed to tubular damage. 3 When both glomerular filtration and tubular reabsorption are disturbed, mixed proteinuria can occur. 4
Of the mono-dimensional electrophoretic methods, sodium dodecyl sulfate (SDS)-agarose gel electrophoresis (SDS-AGE) is easier, more rapid and less expensive to perform than sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), which is considered to be the gold standard for qualitative analysis of urinary proteins. Moreover, in clinical practice it is preferred for the lower toxicity for laboratory personnel. In dogs affected with CKD, SDS-AGE was shown to have good sensitivity for the identification of renal damage.5,6 Sensitivity for both glomerular and tubular damage was 100% and 92%, respectively, when compared with histopathology. 5 In addition, the presence of proteins with a very low MW (12–15 kDa) was associated with higher severity of tubular damage on histopathology. 6 In another recent study, the sensitivity of SDS-AGE to predict glomerular damage in Dogue de Bordeaux dogs, suspected to be affected with glomerulonephropathy on the basis of persistent proteinuria, was about 90%. 7
Little information is available about the electrophoretic patterns and their clinical relevance in feline CKD. Recently, a study evaluated SDS-AGE in cats affected with familiar renal amyloidosis revealed a mixed pattern, consistent with the pattern of amyloid deposition histologically. 8 To date, no information is available about the electrophoretic protein patterns in healthy cats at risk of developing CKD and in cats affected with naturally occurring CKD.
The aims of this study were: (1) to describe the electrophoretic patterns of proteinuria in healthy cats at risk of developing CKD and in cats affected with CKD at different stages, using a commercially available SDS-AGE method; (2) to investigate the possible association between glomerular or tubular proteinuria and CKD; (3) to assess whether the type of proteinuria may predict the development of CKD in healthy cats at risk and the progression of disease in cats with CKD over 6 months of age.
Materials and methods
Patients
Urine samples were collected from 39 client-owned cats presented for routine health screening at the Veterinary Teaching Hospital of University of Milan from September 2014 to April 2017. Informed consent was signed by the owners and, according to the ethical committee statements of the University of Milan (decision number 2/2016), biological samples collected in this setting could be used also for research purposes.
Samples included in this study were part of a prospective study in which healthy cats at risk of developing CKD and cats affected by CKD at any stage of disease were enrolled and monitored over a period of 18 months.
Cats were included in the ‘at-risk’ group based on the following criteria: (1) age >8 years; or (2) cats of any age belonging to breeds predisposed to CKD. Included breeds were Persian, Abyssinian and Maine Coon, which have a predisposition to renal amyloidosis, 9 a predisposition to polycystic kidney disease 10 and an increased prevalence of CKD, 11 respectively. All cats included in the at-risk group were clinically healthy and did not have laboratory abnormalities consistent with CKD or any other systemic disease.
Cats of any age and breed with a diagnosis of CKD were included in ‘CKD’ groups. Diagnosis of CKD was made on the basis of clinical and laboratory results. Specifically, azotaemic CKD was diagnosed in the case of increased serum creatinine (laboratory reference interval [RI] <1.6 mg/dl) and concurrent inadequate urinary concentration ability. Non-azotaemic CKD was diagnosed in cases of serum creatinine <1.6 mg/dl and one of the following abnormalities: (1) inadequate urinary concentration ability (persistent urine specific gravity [USG] <1.035); (2) abnormal renal imaging findings; or (3) increasing serum creatinine concentration (>0.3 mg/dl) 12 in samples collected serially, not exceeding the RI. 13 These cats were staged in accordance with the International Renal Interest Society (IRIS) staging guidelines. 14 All the cats diagnosed with non-azotaemic CKD were IRIS stage 1. 14
Patients were excluded from both groups in the case of systemic infectious, endocrine or cardiovascular diseases, or malignant tumours. Additional exclusion criteria were the administration of drugs affecting systolic blood pressure (SBP; eg, corticosteroids, calcium-channel blockers, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, aldosterone inhibitors, opioids), diuretics or anti-inflammatory drugs in the 4 weeks before inclusion. Moreover, cats with urinary tract infections, haematuria, active sediment or acute kidney injury were excluded.
Cats at risk and cats affected by CKD at IRIS stage 1 were checked every 6 months, whereas cats with CKD at IRIS stages 2–4 were checked every 3 months.
Progressive CKD was identified in case of progression to a higher IRIS stage (eg, from at risk to IRIS 2 or from IRIS stage 3 to IRIS stage 4) within 6 months.
Sample collection
SBP measurement, blood and urine collection were performed at the time of inclusion (T0) and at each checkpoint clinical visit.
Urine samples (8–10 ml) were collected from each cat by ultrasonographically-guided cystocentesis and were sent within the syringe to the internal clinical pathology laboratory. Complete urinalysis (including USG, dipstick analysis and sediment evaluation) was carried out on each sample as previously reported, 15 whereas urine culture was performed whenever infection was suspected. Urinary tract infection was suspected whenever compatible clinical signs and/or detection of pyuria at the microscopic sediment evaluation (ie, >5 urinary white blood cells per high power field, × 400) were detected. Urinary protein (UP) and urinary creatinine (UC) were measured on urine supernatant. The UP was measured with pyrogallol red molybdate method on undiluted supernatant and the UC with modified Jaffe method diluting supernatants 1:20 with distilled water. Both these tests were run with an automated biochemical analyser (Cobas Mira; Roche Diagnostics). The remaining supernatant was aliquoted (400 μl) and stored at –20°C within 4 h of collection.
Urinalysis
All available urine samples collected from cats (irrespective of time of collection) were selected and used for analysis. Samples were analysed after a maximum of 12 months of storage. The day before analysis, aliquots were gently thawed overnight at +4°C and were warmed at room temperature 1 h before the run.
SDS-AGE was performed using commercially available electrophoretic gels (Hydragel 5 Proteinuria; Sebia Italia) applied to a semi-automated analyser (Hydrasis; Sebia Italia). Electrophoretic gels are made by agarose buffered at pH 7 ± 0.5 and held five wells. Sample preparation and migration procedure were performed according to the manufacturer’s instruction. Specifically, after a brief vortexing of the supernatant, 80 μl of each sample were mixed with 20 μl of the supplied diluents (containing SDS to denature and negatively charge all the proteins, and bromophenol blue to mark migration). Control material (Molecular Mass Control; Sebia Italia) containing proteins with known molecular weight (ie, lysoxyme, 14.3 kDa; triosephosphate isomerase, 26.6 kDa; bovine albumin, 66 kDa; human IgG, 150 kDa) was included in each run, after treatment with SDS as described above for samples. Five microlitres of treated supernatants and control material were applied in the wells and gel was loaded in the analyser. Migration was carried out under a condition of constant power (10 W) at 20°C controlled by Peltier effect to accumulate 60 Volts per hour (V/h) for about 15 mins. After migration, electrophoretic gels were manually transferred to the staining module of the instrument and automatically stained with acid violet. Gels were then fixed with glycerin solution (1:8 in distilled water) and then dried.
Gels were visually inspected to identify the presence and types of electrophoretic bands. Bands of the samples were compared with the bands of the control lane and three possible types of bands were assigned to each sample: albumin, glomerular (high-molecular-weight [HMW] proteins) and tubular (low-molecular-weight [LMW] proteins) bands. According to the types of bands identified, one of the following patterns was assigned for each sample: negative (lack of bands); albuminuria; glomerular; tubular; or mixed (Figure 1). To the study aims, two different classifications of samples were applied: one on the basis of the presence or absence of glomerular proteins, on which samples with glomerular bands (ie, samples with glomerular or mixed pattern) were compared with samples without glomerular bands (ie, negative samples and samples with albuminuria or ‘pure’ tubular pattern); and one on the basis of the presence or absence of tubular proteins, on which samples with tubular bands (ie, samples with tubular or mixed pattern) were compared with samples without tubular bands (ie, negative samples and samples with albuminuria or ‘pure’ glomerular patterns).
Figure 1.
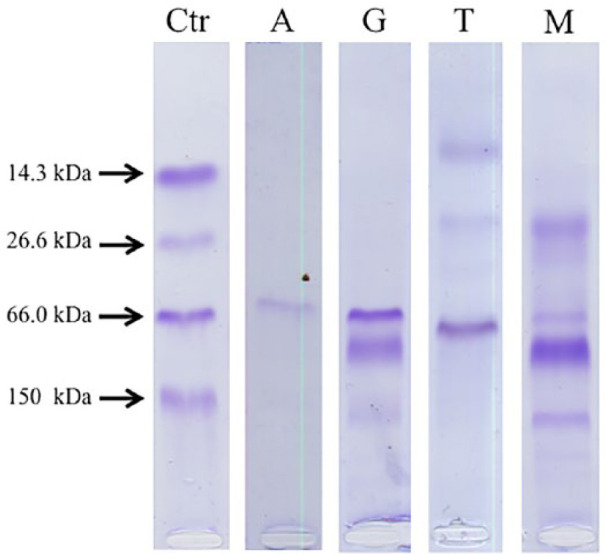
Sodium dodecyl sulfate–agarose gel electrophoresis patterns. The first lane shows the control (Ctr) sample having a marker with known molecular weight (MW), corresponding to lysozyme (14.3 kDa), triose phosphate isomerase (26.6 kDa), albumin (66 kDa) and IgG (150 kDa). Samples with bands of MW equal to that of albumin were considered albumininuric (A). Glomerular pattern (G) was identified when bands corresponding to proteins with MW higher than albumin were present. Tubular pattern (T) was identified when bands corresponding to proteins with MW lower than albumin were present. Mixed pattern (G) was identified when bands corresponding to proteins with MW higher and lower than albumin were present
Statistical analysis
Statistical analysis and descriptive statistics of continuous variables were performed with a commercially available software (GraphPad Prism 5.0; GraphPad Software). A P value <0.05 was considered statistically significant. Normality distribution of continuous variables was assessed by the Kolmogorov–Smirnov test.
Fisher’s exact test was used to verify the null hypothesis that the glomerular bands and tubular bands were equally distributed in samples grouped according to the health status or grouped according to the magnitude of proteinuria. Specifically, cats at risk were compared with cats with CKD and samples with UPC <0.2, corresponding to IRIS sub-stage ‘non-proteinuric’ (NP), were compared with samples having a UPC ⩾0.2, corresponding to IRIS substage ‘borderline proteinuric’ (BP) and ‘proteinuric’.
The non-parametric Mann–Whitney U-test was used to compare serum creatinine concentration, UP, UC and UPC between cats with and without glomerular proteinuria and between cats with and without tubular proteinuria.
Fisher’s exact test was also used to verify whether the presence of glomerular or tubular bands at the time of inclusion (T0) were associated with progression of CKD. To this aim, the number of samples with glomerular bands and tubular bands were compared between the group of cats that remained at the same IRIS stage (including cats at risk) within 6 months (‘stable’ group) and the group of cats with progressive CKD (‘progressive’ group).
Results
Samples
Thirty-nine cats fulfilled the inclusion criteria, 17 of which were classified as at risk and 22 were affected by CKD. Of the cats at risk, eight were male (all neutered except one) and nine were female (all neutered); 15 were domestic shorthairs; there was one Persian and one Maine Coon cat. Mean ± SD age was 132 ± 33.8 months (11 ± 2.8 years) and ranged from 67 to 169 months (5.6–14.1 years). Of the cats affected by CKD (four IRIS stage 1, 13 IRIS stage 2, three IRIS stage 3 and two IRIS stage 4 at the time of inclusion), nine were male (all neutered) and 13 were female (all neutered); 12 were domestic shorthairs and there were two each of Siamese, Exotic Shorthair, Siberian and Norwegian Forest Cats; there was one Persian and one Chartreux. Mean ± SD age was 140 ± 49.7 months (11.6 ± 4.1 years) and ranged from 72 to 226 months (6.0–18.8 years).
Seventy urine samples, collected either at the time of inclusion or during follow-up, were available for analysis. The median and range of UP, UC and UPC in the different IRIS stages and the frequency of cats grouped according to the IRIS substage of proteinuria in each IRIS stage are shown in Table 1.
Table 1.
Descriptive statistic of serum creatinine and urea, urine specific gravity (USG), urinary protein (UP), urinary creatinine (UC) and urinary protein:creatinine (UPC) ratio at the different International Renal Interest Society (IRIS) stages
| At risk (n = 26) |
IRIS stage 1 (n = 12) |
IRIS stage 2 (n = 23) |
IRIS stage 3 (n = 7) |
IRIS stage 4 (n = 2) |
|
|---|---|---|---|---|---|
| Serum creatinine (mg/dl) |
1.29 (0.79–1.54) |
1.26 (0.89–1.58) |
1.97 (1.63–2.56) |
3.56 (3.18–4.85) |
6.19 (5.31–7.07) |
| Urea (mg/dl) |
60.0 (33–107) |
52.5 (31–151) |
70.5 (46–171) |
137.0 (106–221) |
157.5 (154–161) |
| USG | 1050 (1022–1086) |
1040 (1020–1050) |
1034 (1016–1046) |
1013 (1010–1025) |
1011 (1009–1013) |
| UP (mg/dl) |
24.7 (1.0–97.6) |
35.7 (5.2–61.4) |
28.0 (6.1–171.3) |
18.5 (2.8–39.1) |
7.7 (4.4–11.0) |
| UC (mg/dl) |
189.5 (50.4–401.4) |
229.1 (132.6–510.8) |
174.2 (61.4–564.4) |
174.2 (37–163.2) |
36.6 (45.7–47.6) |
| UPC | 0.15 (0.01–0.50) |
0.13 (0.04–0.34) |
0.17 (0.03–0.78) |
0.17 (0.02–0.71) |
0.20 (0.16–0.24) |
| NP | 17 | 8 | 15 | 4 | 1 |
| BP | 9 | 4 | 3 | 1 | 1 |
| P | – | – | 5 | 3 | – |
Data are median (range). The number of cats grouped according to the IRIS substage of proteinuria in each IRIS stage is also reported
NP = non-proteinuric; BP = borderline proteinuric; P = proteinuric
Electrophoretic patterns, glomerular bands and tubular bands
The electrophoretic patterns in samples grouped according to health status (at risk vs CKD) and according to IRIS substage of proteinuria are shown in Table 2. All the scanned lanes are shown in Figures 1–3 in the supplementary material.
Table 2.
Number (%) of the electrophoretic patterns in samples grouped according to the health status (at risk vs chronic kidney disease) and according to International Renal Interest Society substage of proteinuria
| At risk (n = 26) | CKD (n = 44) | NP (n = 45) | BP (n = 17) | P (n = 8) | |
|---|---|---|---|---|---|
| Glomerular | 22 (84.6) | 24 (54.5) | 34 (75.6) | 10 (58.9) | 2 (25.0) |
| Tubular | 1 (3.8) | 5 (11.4) | 2 (4.4) | 2 (11.8) | 2 (25.0) |
| Mixed | – | 13 (29.5) | 6 (13.3) | 4 (23.5) | 3 (37.5) |
| Negative | 3 (11.6) | 2 (4.5) | 3 (6.7) | 1 (5.9) | 1 (12.5) |
CKD = chronic kidney disease; NP = non-proteinuric; BP = borderline proteinuric; P = proteinuric
HMW proteins (glomerular and mixed pattern) were present in 49/70 samples, whereas LMW (tubular and mixed pattern) were present in 19/70. The presence of glomerular bands was not significantly different (P = 0.723) between the at-risk and CKD groups (Figure 2a). Conversely, tubular bands were significantly more frequent (P = 0.0006) in cats with CKD than in cats at risk (Figure 2b). The number of samples with glomerular and tubular bands in the different groups divided according to IRIS staging (including only cats with CKD) is displayed in Figure 3.
Figure 2.
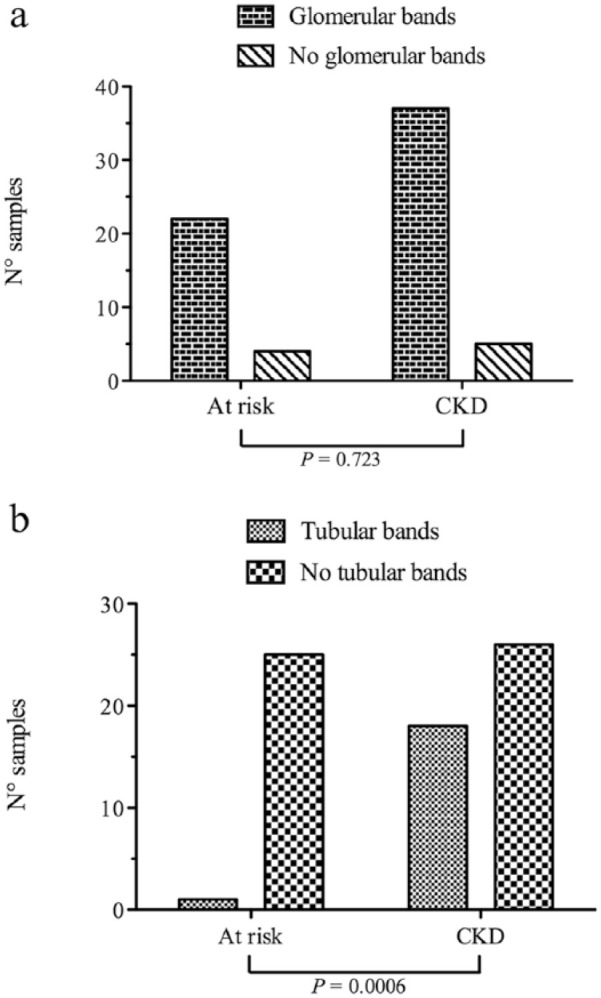
Distribution of (a) samples with or without glomerular bands and (b) samples with or without tubular bands in the group of cats at risk and in the group of cats affected by chronic kidney disease (CKD)
Figure 3.
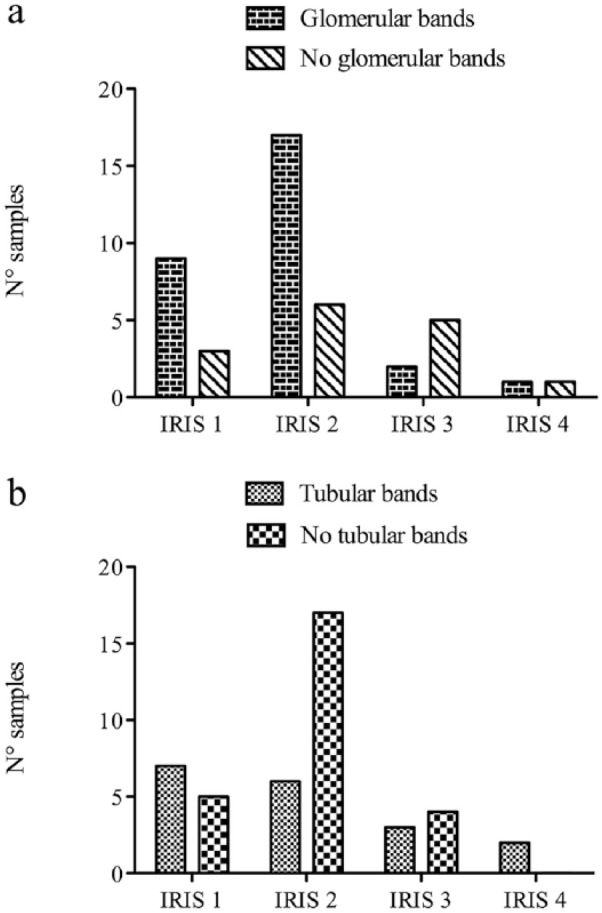
Distribution of (a) samples with or without glomerular bands and (b) samples with or without tubular bands at the different International Renal Interest Society (IRIS) stages (ie, stage 1, stage 2, stage 3 and stage 4)
Cats with proteinuria (UPC ⩾0.2) were more likely to have tubular bands than cats without proteinuria. However, there was no difference in the prevalence of glomerular bands between cats with and without proteinuria (P = 0.183; Figure 4a). The association between proteinuria and the presence of tubular bands was found in all cats (P = 0.025; Figure 4b) and among CKD-only cats (P = 0.0003). The number of samples with glomerular and tubular bands in the different groups divided according to IRIS substaging of proteinuria (including only cats with CKD) is displayed in Figure 5.
Figure 4.
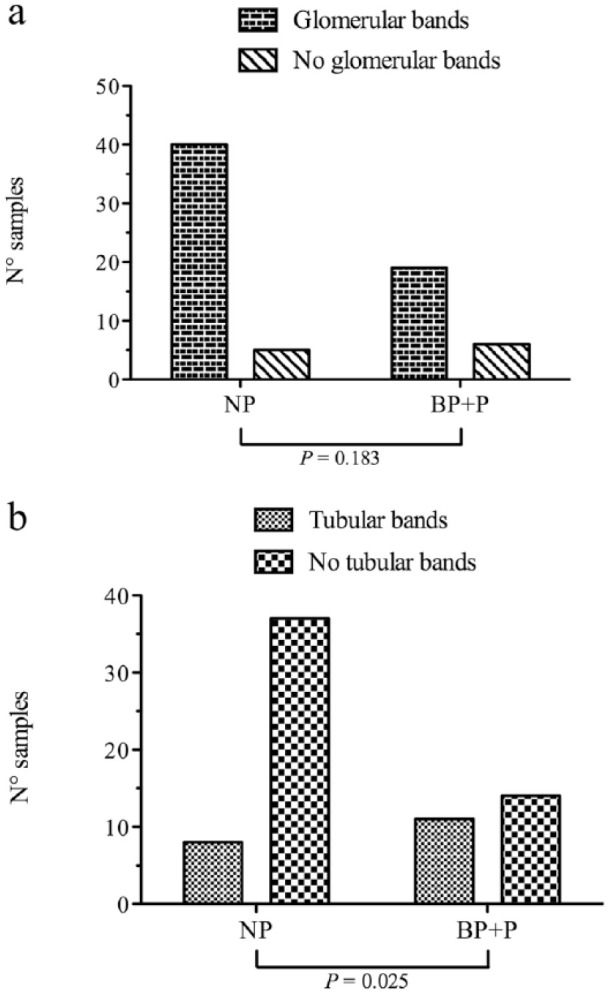
Distribution of (a) samples with or without glomerular bands and (b) samples with or without tubular bands in non-proteinuric (NP) cats (urinary protein:creatinine [UPC] ratio <0.2) and cats with UPC ⩾0.2 (BP + P) (borderline proteinuric [BP] + proteinuric [P])
Figure 5.
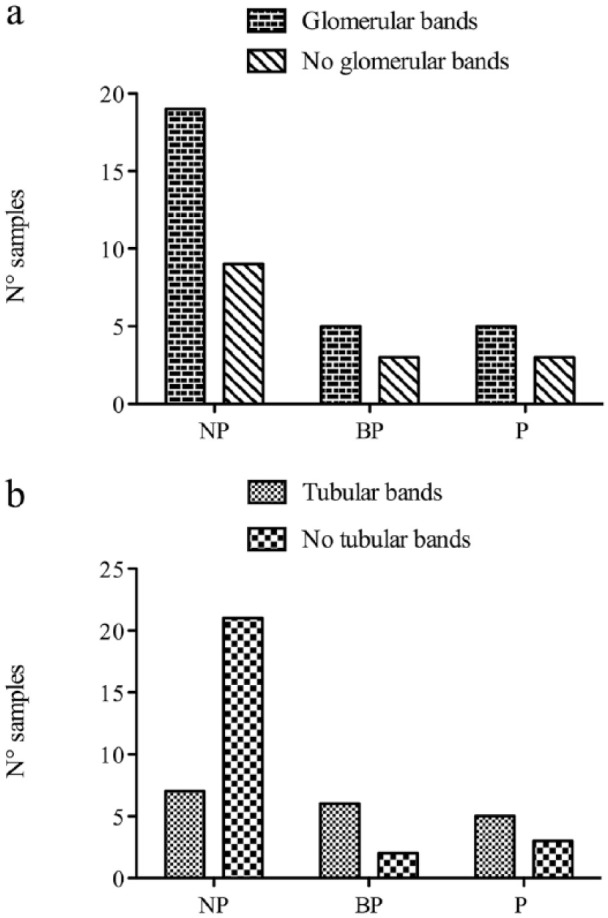
Distribution of (a) samples with or without glomerular bands and (b) samples with or without tubular bands in non-proteinuric (NP), borderline proteinuric (BP) and proteinuric (P) cats
Descriptive statistics of serum creatinine, UP, UC and UPC in samples with glomerular bands vs samples without and samples with tubular bands vs samples without, and the statistical comparison between groups, are shown in Table 3.
Table 3.
Descriptive statistics of serum creatinine, urinary protein (UP), urinary creatinine (UC) and urine protein:creatinine (UPC) ratio in samples with glomerular bands (n = 59), samples without glomerular bands (n = 11), samples with tubular bands (n = 19) and samples without tubular bands (n = 51)
| With glomerular bands | Without glomerular bands | P value | With tubular bands | Without tubular bands | P value | |
|---|---|---|---|---|---|---|
| Serum creatinine (mg/dl) | 1.54 (1.24–1.97) |
1.80 (1.22–3.86) |
0.309 | 1.99 (1.24–1.17) |
1.50 (1.17–1.87) |
0.055 |
| UP (mg/dl) |
28.00 (15.00–45.70) |
25.00 (12.60–38.80) |
0.383 | 39.10 (25.00–61.40) |
22.80 (12.40–38.80) |
0.024 |
| UC (mg/dl) |
186.4 (132.6–318.9) |
149.4 (50.4–305.2) |
0.110 | 174.2 (69.2–271.0) |
183.8 (132.6–313.4) |
0.439 |
| UPC | 0.15 (0.07–0.23) |
0.24 (0.05–0.50) |
0.309 | 0.22 (0.15–0.56) |
0.12 (0.06–0.20) |
0.003 |
Data are median (interquartile range). The P values of the comparison between groups for each parameter are also shown (significant differences are shown in bold)
Follow-up
Twenty-one cats had two urine samples available 6 months apart. The mean ± SD length of follow-up was 11.6 ± 5.0 months. Of the 21 cats included in this analysis, six patients completed the 18 month period of monitoring, three were excluded after 6 months owing to the onset of other diseases, and six, four and two were lost after 6, 12 and 15 months, respectively. Fourteen were cats at risk at T0, nine of which remained stable for 6 months, whereas five cats progressed to CKD (three to IRIS stage 1 and two to IRIS stage 2). Seven had a diagnosis of CKD at T0, six of which remained stable (one at stage 1 and five at stage 2), whereas one progressed (from stage 2 to stage 3). Neither the presence of glomerular nor tubular bands at T0 were associated with progression of CKD over 6 months (P = 0.1).
Discussion
This study evaluated the electrophoretic pattern of proteinuria in cats at risk of, and cats with, CKD, using a commercially available SDS-AGE method.
The presence of proteins with an MW equal to or higher than albumin and, in turn, the presence of a glomerular pattern, were a frequent finding in healthy cats at risk of developing CKD. It is possible that some healthy cats at risk of developing CKD have early slow progressive glomerular damage or diseases other than CKD that also affect older cats. However, glomerular damage was reported to be mild and uncommon in cats with CKD, especially in the early stages of CKD. 16 Therefore it is possible that the presence of HMW proteins in healthy non-proteinuric cats is not pathological. In line with this hypothesis, the majority of at-risk cats with this pattern remained healthy for months after urine collection (data not shown). The glomerular pattern was also common in samples without proteinuria (ie, UPC <0.2). The use of SDS-AGE to determine the nature of proteinuria in non-proteinuric cats at risk of developing CKD may identify glomerular proteinuria in many cats is of unknown, and potentially minimal, clinical significance. Similar results were found in a previous study in dogs, reporting that about 20% of samples with a UPC <0.2 yielded different patterns (ie, glomerular, tubular or mixed pattern) and showing that SDS-AGE in non-proteinuric samples had low specificity in detecting samples that actually are proteinuric. 17 The presence of HMW proteins has already been described in healthy non-proteinuric cats. 18 Specifically, using two-dimensional protein electrophoresis followed by mass spectrometry, it was demonstrated that, besides albumin, cauxin (70 kDa) was the most abundant protein, and uromodulin (95 kDa) and other HMW proteins were also present. 18 Therefore, it could be speculated that the glomerular bands found in our healthy population could represent these physiological proteins. However, further longitudinal studies with longer follow-up and additional assessment of proteinuria (quantitative and qualitative), combined with renal histopathological evaluation, are required to confirm this hypothesis.
The tubular proteins were significantly more frequent in our population of cats with CKD compared with healthy cats at risk. This result supports the fact that the main pathological feature of feline (idiopathic) CKD consists in chronic tubular interstitial nephritis. 19 In this setting, proteinuria is mainly secondary to tubular damage, increases as the CKD progresses 20 and correlates with interstitial fibrosis. 21 In our study, tubular proteins were identified at all stages of CKD, consistent with the occurrence of tubular damage occurring in the early stages of CKD. 21 Similar results were found in cats affected with renal amyloidosis, where LMW proteins (highlighted as mixed pattern) were frequently found at early stages of CKD.
The association between proteinuria and the presence of tubular bands was demonstrated in this study. Given that the severity of proteinuria increases as the CKD progress, 20 this finding could further support the theory that a higher UPC ratio corresponds to more severe tubulointerstitial damage. 21 Since UP was significantly higher in samples with tubular bands compared with those without, it could be possible that, given the higher urine dilution in cats with CKD, in some samples the protein concentration may not have been high enough to yield visible bands of tubular proteins on the gel using SDS-AGE. Therefore, using a more sensitive method such as SDS-PAGE, different stains or methods for concentration of proteins, the detection of tubular proteins could be enhanced. It is also worth noting that the frequency of tubular bands and therefore, presumably, of tubular damage, was similar in cats with borderline and overt proteinuria (Figure 5b). Currently, borderline proteinuria needs to be closely monitored and treatment is indicated only when it progresses to the proteinuric range (UPC >0.4). 22 Our results suggest that BP in cats with CKD could be considered pathological and that the diagnostic and therapeutic recommendation for cats with a UPC >0.4 should be transferred to cats with UPC >0.2. In accordance with this assumption, in cats with CKD the hazard ratio (HR) of death or euthanasia was 2.9 for borderline proteinuria (UPC 0.2–0.4) vs cats with a UPC <0.2, which is slightly lower than that found for a UPC >0.4 (HR 4.0). 20
Neither glomerular bands nor tubular bands were predictive of development or progression of CKD.Although few samples were included in this analysis, SDS-AGE appears more useful to qualitatively describe abnormal proteinuria (UPC >0.2) than to predict development and progression of azotaemia or proteinuria.
Conclusions
HMW proteins were frequently found in urine samples of cats at risk of developing CKD and were not associated with progression to CKD or proteinuria (UPC). Although the pathological significance of these findings needs to be confirmed, it is possible that these proteins could be physiologically present in urine and could be considered normal in this population of cats. Conversely, the presence of LMW proteins was associated with CKD and proteinuria, supporting the presence of tubulointerstitial damage in our population of affected cats. From a clinical point of view, the detection of LMW proteins in non-azotaemic cats or in cats with UPC <0.4 is potentially consistent with the presence of kidney damage and should be the subject of further diagnostic investigation.
Supplemental Material
Samples (n = 46) with glomerular pattern. The lane of the control material is indicated as ‘C’
Samples (n = 46) with glomerular pattern. The lane of the control material is indicated as ‘C’
Samples (n = 46) with glomerular pattern. The lane of the control material is indicated as ‘C’
Footnotes
Accepted: 8 January 2019
Author note: The preliminary results of this study were presented as abstract at the 18th ESVCP – ESVONC Congress, Nantes (France), 20–22 October 2016.
Supplementary material: The following files are available online:
Sodium dodecyl sulfate-agarose gel electrophoresis lanes of samples included in the study:
Figure 1: Samples (n = 46) with glomerular pattern. The lane of the control material is indicated as ‘C’.
Figure 2: Samples (n = 6) with tubular pattern. The lane of the control material is indicated as ‘C’.
Figure 3: Samples (n = 13) with mixed pattern. The lane of the control material is indicated as ‘C’.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Most of the samples included in this study were part of a study funded by the Winn Feline Foundation (Grant n° WZ14-009).
ORCID iD: Marco Giraldi  https://orcid.org/0000-0003-3647-2539
https://orcid.org/0000-0003-3647-2539
Saverio Paltrinieri  https://orcid.org/0000-0001-7117-7987
https://orcid.org/0000-0001-7117-7987
References
- 1. Chakrabarti S, Syme HM, Elliott J. Clinicopathological variables predicting progression of azotemia in cats with chronic kidney disease. J Vet Intern Med 2012; 26: 275–281. [DOI] [PubMed] [Google Scholar]
- 2. Littman P. Protein-losing nephropathy in small animals. Vet Clin North Am Small Anim Pract 2011; 41: 31–62. [DOI] [PubMed] [Google Scholar]
- 3. Nabity MB, Lees GE, Dangott LJ, et al. Proteomic analysis of urine from male dogs during early stages of tubulointerstitial injury in a canine model of progressive glomerular disease. Vet Clin Pathol 2011; 40: 222–236. [DOI] [PubMed] [Google Scholar]
- 4. Bazzi C, Petrini C, Rizza V, et al. Characterization of proteinuria in primary glomerulonephritides. SDS-PAGE patterns: clinical significance and prognostic value of low molecular weight (‘tubular’) proteins. Am J Kidney Dis 1997; 29: 27–35. [DOI] [PubMed] [Google Scholar]
- 5. Zatelli A, Borgarelli M, Santilli R, et al. Glomerular lesions in dogs infected with Leishmania organisms. Am J Vet Res 2003; 64: 558–561. [DOI] [PubMed] [Google Scholar]
- 6. Zini E, Bonfanti U, Zatelli A. Diagnostic relevance of qualitative proteinuria evaluated by use of sodium dodecyl sulfate-agarose gel electrophoresis and comparison with renal histologic findings in dogs. Am J Vet Res 2004; 65: 964–971. [DOI] [PubMed] [Google Scholar]
- 7. Lavoué R, Trumel C, Smets PMY, et al. Characterization of proteinuria in Dogue de Bordeaux dogs, a breed predisposed to a familial glomerulonephropathy: a retrospective study. PLoS One 2015; 10. DOI: 10.1371/journal.pone.0133311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paltrinieri S, Sironi G, Giori L, et al. Changes in serum and urine SAA concentrations and qualitative and quantitative proteinuria in Abyssinian cats with familial amyloidosis: a five-year longitudinal study (2009–2014). J Vet Intern Med 2015; 29: 505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chew DJ, DiBartola SP, Boyce JT, et al. Renal amyloidosis in related Abyssinian cats. J Am Vet Med Assoc 1982; 181: 139–142. [PubMed] [Google Scholar]
- 10. Eaton KA, Biller DS, DiBartola SP, et al. Autosomal dominant polycystic kidney disease in Persian and Persian-cross cats. Vet Pathol 1997; 34: 117–126. [DOI] [PubMed] [Google Scholar]
- 11. Polzin DJ. Chronic kidney disease. In: Barges J, Polzin DJ. (eds). Nephrology and urology of small animals. Hoboken, NJ: Wiley-Blackwell, 2011, pp 433–471. [Google Scholar]
- 12. Braun JP, Lefebvre HP, Watson ADJ. Creatinine in the dog: a review. Vet Clin Pathol 2003; 32: 162–179. [DOI] [PubMed] [Google Scholar]
- 13. Syme H. CKD early diagnosis. http://www.iris-kidney.com/education/early_diagnosis.html (2016, accessed October 13, 2018).
- 14. Elliot J, Watson ADJ. IRIS staging system. http://www.iris-kidney.com/education/staging_system.html (2016, accessed August 18, 2018).
- 15. Rossi G, Bertazzolo W, Dondi F, et al. The effect of inter-laboratory variability on the protein:creatinine (UPC) ratio in canine urine. Vet J 2015; 204: 66–72. [DOI] [PubMed] [Google Scholar]
- 16. Brown CA, Elliott J, Schmiedt CW, et al. Chronic kidney disease in aged cats: clinical features, morphology, and proposed pathogeneses. Vet Pathol 2016; 53: 309–326. [DOI] [PubMed] [Google Scholar]
- 17. Giori L, Tricomi FM, Zatelli A, et al. High-resolution gel electrophoresis and sodium dodecyl sulphate-agarose gel electrophoresis on urine samples for qualitative analysis of proteinuria in dogs. J Vet Diagn Invest 2011; 23: 682–690. [DOI] [PubMed] [Google Scholar]
- 18. Ferlizza E, Campos A, Neagu A, et al. The effect of chronic kidney disease on the urine proteome in the domestic cat (Felis catus). Vet J 2015; 204: 73–81. [DOI] [PubMed] [Google Scholar]
- 19. McLeland SM, Cianciolo RE, Duncan CG, et al. A comparison of biochemical and histopathologic staging in cats with chronic kidney disease. Vet Pathol 2015; 52: 524–534. [DOI] [PubMed] [Google Scholar]
- 20. Syme HM, Markwell PJ, Pfeiffer D, et al. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria. J Vet Intern Med 2006; 20: 528–535. [DOI] [PubMed] [Google Scholar]
- 21. Chakrabarti S, Syme HM, Brown CA, et al. Histomorphometry of feline chronic kidney disease and correlation with markers of renal dysfunction. Vet Pathol 2013; 50: 147–155. [DOI] [PubMed] [Google Scholar]
- 22. Harley L, Langston C. Proteinuria in dogs and cats. Can Vet J 2012; 53: 631–638. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Samples (n = 46) with glomerular pattern. The lane of the control material is indicated as ‘C’
Samples (n = 46) with glomerular pattern. The lane of the control material is indicated as ‘C’
Samples (n = 46) with glomerular pattern. The lane of the control material is indicated as ‘C’


