Abstract
Objectives
This study sought to determine if bupivacaine targeted at specific, potentially painful sites could enhance postoperative analgesia in routine feline ovariohysterectomies. A secondary objective was to assess the utility of multiple acute pain scales for cats in a high-volume surgery setting.
Methods
Two hundred and twelve cats were included in a prospective, randomized, double-blinded, placebo-controlled clinical trial. Anesthesia included buprenorphine, ketamine, dexmedetomidine and isoflurane. A ventral midline ovariohysterectomy was performed and cats were administered bupivacaine (2 mg/kg), placebo control (0.9% saline) or sham control (observation only) intraoperatively at the ovarian suspensory ligaments and vessels, uterine body and incisional subcutaneous tissues. Two pain scales were used to assess cats postoperatively. Initially, a multidimensional composite pain scale (MCPS) and a 0–10 numeric pain rating scale (NRS) were used. Subsequently, the MCPS was replaced with a modified Colorado State University Feline Acute Pain Scale (mCSU). Pain scores for the test groups were compared using a one-way ANOVA and a Holm–Bonferroni post hoc analysis when a difference was found (P <0.05).
Results
Pain for the bupivacaine group was lower than the control groups at 1 h post-recovery and discharge, attaining significance with higher body weights. The P values were 0.008 and 0.004 for 1 h post-recovery and discharge, respectively. Pain scores between evaluators for the MCPS and NRS correlated poorly with r values for 1 h post-recovery and discharge of −0.08 and 0.22, respectively. Additionally, the MCPS proved difficult to use and time consuming, especially for feral and fractious patients, and was replaced with the mCSU.
Conclusions and relevance
Targeted bupivacaine reduced early postoperative pain scores following routine feline ovariohysterectomies. The technique used was simple, requiring just over a minute to perform at minimal additional cost. The MCPS was not ideal for use in a high-volume spay setting.
Keywords: Bupivacaine; analgesia; pain, postoperative; hysterectomy, veterinary; ovariectomy, veterinary; anesthetics, local; anesthesia
Introduction
Although feline ovariohysterectomy is commonly performed by most veterinarians, high-volume spay–neuter practices have emerged as a rapidly growing area of clinical practice. An important component of the success of these spay–neuter programs is use of safe, efficient protocols for anesthesia carefully designed to facilitate neutering large numbers of animals in a short period. Balanced anesthesia is essential and involves administering combinations of drugs to safely provide effective analgesia, loss of consciousness, muscle relaxation and immobility without patient compromise. 1
Effective analgesia is key for patients undergoing surgical neutering. Multimodal analgesia involves the use of multiple analgesic agents with varying mechanisms of action to minimize pain. Using multiple analgesic drugs can potentially improve the overall analgesia achieved. Additionally, the doses of the drugs used can be reduced with the goal of lessening the incidence and severity of potentially adverse effects compared with a single agent. 1
Postoperative analgesia contributes to successful case outcomes and helps reduce pain-induced deleterious effects that can hinder patient recovery.2–4 In humans, poorly controlled acute postoperative pain is associated with increased morbidity, functional and quality-of-life impairment, delayed recovery time, prolonged duration of opioid use and higher healthcare costs. Additionally, the presence and intensity of acute pain during or after surgery is predictive of the development of chronic or maladaptive pain. 4 Postoperative analgesia requirements vary among patients with differences in surgical complexity, technique, patient age, and individual responses to pain and analgesic agents. 1
Industry guidelines state that local anesthetics should be used, insofar as possible, with every surgical procedure as part of multimodal analgesia. 3 They prevent pain by blocking and interrupting nerve conduction through sodium channel blockade.5,6 Bupivacaine is a commonly used local agent for regional and infiltration anesthesia. The time to maximum effect is typically within 15–30 mins, and it has a longer duration of anesthesia when compared with other commonly used local anesthetics such as lidocaine. 7 The duration of action, commonly reported in veterinary medicine as 4–6 h, is dependent on several factors, including site of injection, route of administration, concentration and volume administered. One key factor determining duration of action is the extent to which the bupivacaine remains near the targeted nerves. The longer the drug remains in the proximity, the more likely the drug will affect the nerve membrane. Additionally, in humans, it has been noted that there is a period of analgesia that persists after the return of sensation, during which time analgesic needs are reduced. 8
The objective of this study was to determine if bupivacaine targeting specific, potentially painful sites could enhance postoperative analgesia in routine feline ovariohysterectomies. A secondary objective was to assess the utility of multiple acute pain scales in postoperative cats in a high-volume setting. It was hypothesized that targeted application of discrete volumes of bupivacaine intraoperatively would result in reduced postoperative pain scores in cats during the period from anesthesia recovery to same-day hospital discharge.
Materials and methods
This trial was a prospective, randomized, double-blind, placebo-controlled clinical study. The study was reviewed and approved by the Board of Directors of the Hill Country Animal League (HCAL). Owner consent was required before entering patients into the trial.
Animals
Two hundred and sixty-seven healthy female cats presented for elective ovariohysterectomy to HCAL, a non-for-profit high-volume spay-and-neuter practice, were entered into the study. Cats were weighed and confirmed to be ⩾0.9 kg body weight and ⩾2 months of age, as reported by owner/agent and confirmed by dentition. Cats determined intraoperatively to be pregnant cats, in estrus or experiencing an intraoperative complication were excluded. Additionally, cats were excluded from the study in the case of having only partial data available.
Group assignment
Cats were block-randomized sequentially by day. A random number generator (www.random.org) was used to assign days of surgery to one of three groups: treatment (bupivacaine), placebo control (saline) and sham control (sham). Cats having surgery on the same day were assigned to the same group and had the same intervention. Treatment days were blinded to all participants. Surgeons and operating room staff were blinded to treatment drugs. Surgical anesthesia recovery personnel and pain evaluators were not apprised of groupings or intraoperative interventions. Additionally, owners were not informed of treatment or control status of their cat.
Anesthesia, analgesia and ovariohysterectomy
Owners withheld food from cats, beginning the night before surgery. Buprenorphine (0.01 mg/kg IM) was administered preoperatively (Buprenorphine Injection PF; Roadrunner Pharmacy MFG). Equal volumes of ketamine (100 mg/ml [KetaVed; VEDCO]) and dexmedetomidine (0.5 mg/ml [Dexmedetomidine HCl; Putney]) were mixed in a single sterile vial. Anesthesia was induced by intramuscular injection of 0.3 ml for cats weighing 0.9–1.8 kg. Cats weighing >1.8 kg were administered 0.4 ml. Total dosage of induction drugs ranged from 3.75 mg/kg to 16.5 mg/kg for ketamine and from 0.02 mg/kg to 0.04 mg/kg for dexmedetomidine. Each animal was intubated, and isoflurane was administered to maintain a surgical plane of anesthesia with a vaporizer setting range of 0.5–1% (Fluriso; MWI). Oxygen flow was 2 l/min using a non-rebreathing circuit. In the event of any difficulty with intubation, cats were administered isoflurane by mask to facilitate the procedure.
Anesthesia was monitored by operating room technicians with oversight from the surgeon. Owing to the short duration of the surgical procedure, patients were typically monitored without the use of specialized equipment. Circulatory function was monitored using a gross assessment of peripheral perfusion (pulse quality, mucous membrane color and capillary refill time). Ventilation was qualitatively assessed via observation of the thoracic wall movement and observation of breathing bag movement. Auscultation of heart beat and breath sounds with an external stethoscope was performed by exception, when there was a concern noted. Similarly, pulse oximetry was used by exception to assess oxygenation and pulse rate.
An ovariohysterectomy via midline celiotomy was performed by one of three surgeons well experienced in high-volume surgery. Procedures were performed aseptically following published guidelines for elective surgery on shelter animals. 1 Overall times required for surgery were measured in a subset of cats enrolled. Surgical anesthesia recovery was performed by solely dedicated, experienced technicians who remained with patients continuously until the end of the anesthetic period.
Interventions
Bupivacaine group cats were administered targeted injections of 0.5% bupivacaine (2 mg/kg total dose [Marcaine, 0.5%; Hospira]). Bupivacaine was equally divided by volume and injected into four sites intraoperatively: the (1) right and (2) left suspensory ligaments of the ovary, mesovarium, and the pedicles of ovarian vessels; (3) the uterine body just caudal to the bifurcation; and (4) the subcutaneous tissue between the closed abdominal muscle fascia and subcuticular layers. The saline group was administered an equal volume of 0.9% saline at the same locations. The sham group was not administered any drug; rather, each injection site was carefully identified intraoperatively by the surgeon. Times required for administering interventions were measured in a subset of cats enrolled.
Interventional technique
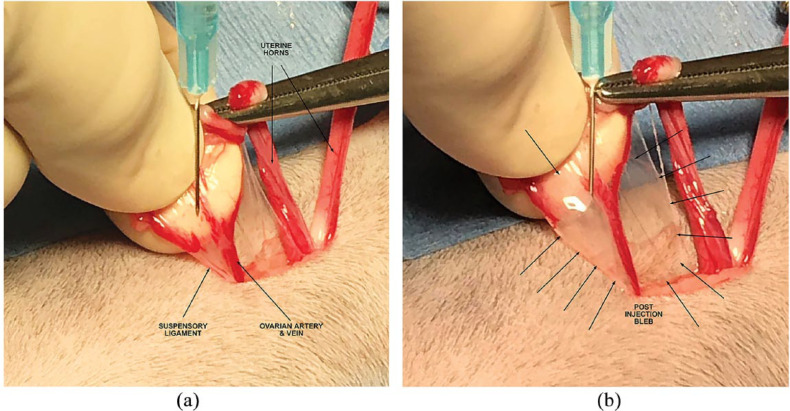
After entry into the abdomen via midline incision, one horn of the uterus was located and gently elevated from the abdomen. The uterine horn was followed cranially to its corresponding ovary. The ovary was exteriorized, and a clamp was placed across its proper ligament. With the assistance of the operating room technician, a sterile syringe with a 25 G needle was used to draw up the appropriate volume of the drug to be administered. The ovarian vessels and suspensory ligament were separated using a fanning motion with thumb and index or middle finger. The needle was inserted near the suspensory ligament with care taken not to enter any blood vessel. The drug was infiltrated until a bleb was formed incorporating the ligaments of the ovary, mesovarium, and the tissues surrounding the ovarian vessels (Figure 1a,b). The vessels were then ligated and transected. The uterus was traced caudally to the uterine body and then cranially to the contralateral ovary. The procedure was then repeated for this ovary. The uterus was again followed caudally to the uterine body. The uterine body was clamped caudal to the bifurcation. The uterine body was infiltrated entering on the dorsal aspect of the uterus caudal to the clamp (Figure 2). The uterine tissues were observed to be well infiltrated circumferentially. The uterus was then ligated and transected. After closure of the abdominal wall, the last portion of the drug administered was infiltrated into the subcutaneous tissue between the closed abdominal muscle fascia and subcuticular layers prior to closure of the skin.
Figure 1.
Photographs of the area targeted for local anesthetic application near the ovary (a) before injection and (b) after injection showing a bleb, approximately 1 cm in diameter encompassing suspensory ligaments of the ovary, mesovarium and the pedicles of ovarian vessels. An additional few drops of local anesthetic are applied topically to the cut ends of the tissue after ligation and transection
Figure 2.
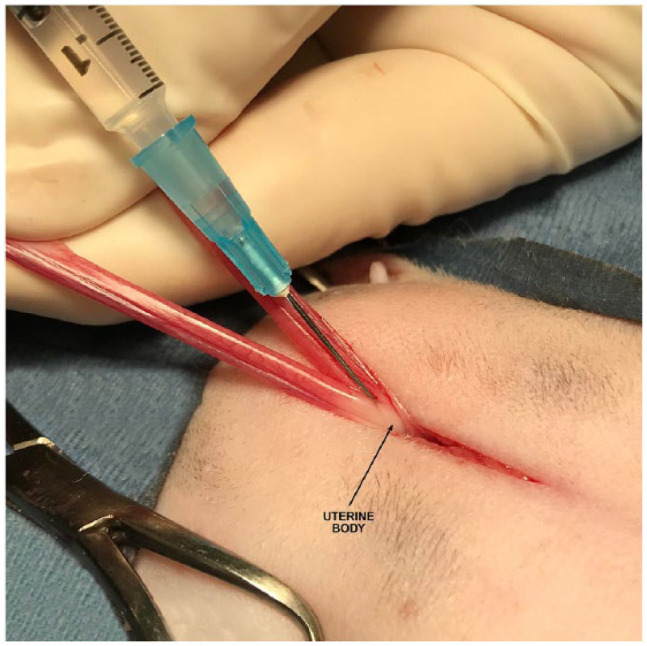
Photograph of the location targeted for local anesthetic infusion into the uterine body. An additional few drops of local anesthetic are applied topically to the cut ends of the tissue after ligation and transection
Pain assessments
Two technicians, each previously trained on a specific pain scale, assessed pain throughout the study. Assessments were made 1 h post-anesthesia recovery and immediately prior to same-day discharge from the hospital (⩽7 h post-anesthesia recovery) by the same technicians using their respective pain scale. There was no inter-observer communication related to pain scoring for enrolled cats.
Two pain scales were used to assess cats postoperatively. In the first phase of the study, a modified UNESP-Botucatu multidimensional composite pain scale (MCPS)9,10 and a 0–10 numeric pain rating scale (NRS) 11 were used. Physiological variables (subscale 3) of the MCPS, including arterial blood pressure and appetite, were not measured. In the final phase of the study, the MCPS was replaced with a modified Colorado State University Feline Acute Pain Scale (mCSU). 12 The CSU was modified to not include palpation of the surgical site owing to commonly seen aggression in fractious and feral cats.
Rescue analgesia was provided to any cat assessed a pain score considered moderate to severe. For the MCPS, a cut-off point above which interventional analgesics were used was >2 for subscale 1 ‘pain expression’ (scale range 0–12 points), and >3 for subscale 2 ‘psychomotor change’ (scale range 0–12 points). 9 NRS moderate pain scores were 4–6, and severe pain scores were 7–10. 11 The threshold for rescue analgesia for the mCSU was a pain score ⩾2.
Statistical analysis
Baseline data for treatment and control groups were analyzed for comparability of groups. A one-way ANOVA was used to compare age, weight and breed.
Postoperative pain scores among the three groups (bupivacaine, saline and sham) were compared. Scores from different pain scales were normalized to a maximum value of 1 by dividing the assigned score by its respective scale’s maximum number. The normalized scores from the two evaluators were averaged for each observation of each cat. Averaged, normalized scores from the three groups were compared using a one-way ANOVA to determine potential differences among pain score averages. A Holm–Bonferroni post-hoc analysis was performed to compare between groups when a difference was found via ANOVA.
Correlation analysis was used to measure the strength of the relationship between evaluators’ scores, and Bland–Altman agreement analysis was used to assess scoring agreement.
A P value of <0.05 was considered significant. Statistical analysis was performed using commercially available software (Microsoft Excel).
Results
Animals
Phase 1
Fifty-nine cats entered the first phase of the study. Thirty-six cats met the inclusion criteria; 12 in the bupivacaine group, 17 in the saline group and seven in the sham group. There were no significant differences among groups regarding breed or body weight. Saline group ages were significantly older than the other groups, with an average age of 9.6 ± 3.5 months vs 5.7 ± 3.2 months and 5.1 ± 2.1 months in the bupivacaine and sham groups, respectively (Table 1). Of note, of the 23 cats excluded, 17 (74%) were eliminated owing to the inability of the evaluator to determine a MCPS score.
Table 1.
Demographics for cats enrolled in phase 1 of the clinical trial
| Group | n | Breed (%) | Mean ± SD body weight (kg) | Mean ± SD age (months) |
|---|---|---|---|---|
| Bupivacaine | 12 | DSH (83.3) Other (16.7) |
2.3 ± 0.8 | 5.7 ± 3.2 |
| Saline | 17 | DSH (82.4) Other (17.6) |
2.7 ± 0.5 | 9.6 ± 3.5 * |
| Sham | 7 | DSH (85.7) Other (14.3) |
2.3 ± 1.0 | 5.1 ± 2.1 |
| Overall | 36 | DSH (83.3) Other (16.7) |
2.6 ± 0.7 | 8.4 ± 3.9 |
Significantly (P <0.05) higher average age
DSH = domestic shorthair
Phase 2
Two hundred and eight cats entered the second phase of study. Thirty-two cats were excluded because of partial data. A similar percentage of each groups’ sample was excluded: 18%, 16% and 13% for the bupivacaine, saline and sham groups, respectively. One hundred and seventy-six cats met the inclusion criteria: 59 in the bupivacaine group, 41 in the saline group and 76 in the sham group. There were no significant differences among groups regarding percentages of breeds of cats included. Saline group ages were older than the other groups with an average age of 8.5 ± 4.8 months vs 5.0 ± 3.1 months and 4.2 ± 3.3 months in the bupivacaine and sham groups, respectively. Body weights differed among groups. Mean body weights (2.8 ± 0.8 kg) of cats in the saline group were greater than those cats in the bupivacaine (2.0 ± 0.8 kg) and sham (1.7 ± 0.7 kg) groups (Table 2).
Table 2.
Demographics of cats enrolled in phase 2 of the clinical trial
| Group | n | Breed (%) | Mean ± SD body weight (kg) | Mean ± SD age (months) |
|---|---|---|---|---|
| Bupivacaine | 59 | DSH (78.0) Siamese (5.1) DMH (10.2) Other (6.8) |
2.0 ± 0.8 | 5.0 ± 3.1 |
| Saline | 41 | DSH (78.0) Siamese (2.4) DMH (12.2) Other (7.3) |
2.8 ± 0.8 * | 8.5 ± 4.8 * |
| Sham | 76 | DSH (80.3) Siamese (6.6) DMH (10.5) Other (2.6) |
1.7 ± 0.7 | 4.2 ± 3.3 |
| Overall | 176 | DSH (79.0) Siamese (5.1) DMH (10.8) Other (5.1) |
2.0 ± 0.8 | 5.5 ± 4.0 |
Significantly (P <0.05) higher mean body weight and average age
DSH = domestic shorthair; DMH = domestic mediumhair
Surgical/treatment times
Surgical times were measured in 38 animals. Median surgical time, as measured from initial incision to closure of the surgical site, was 5.5 mins (range 4.5–8.8 mins). Of note, one surgeon used pedicle ties, whereas the other two surgeons used sutures for ovarian pedicles. Times required for administering interventions were measured in 29 cats. Times for calculating and drawing up the dosage into a syringe, and administering the intervention at each of the four sites were totaled. The median total time required for administering an intervention was 69 s (range 50 s–2.7 mins). The longest segment of time, median 30 s, was the dosage calculation and drawing up the calculated volume (range 11–89 s).
Pain scores
An interim analysis was performed on cats in phase 1. A correlation analysis was used to measure the strength of the relationship of pain scores between evaluators. The r value for correlation of the MCPS and NRS 1 h post-recovery and at discharge were −0.08 and 0.22, respectively. A Bland–Altman analysis was used to further assess the agreement between evaluators’ normalized pain scores. A notable bias (–0.15) was observed, indicating NRS scores were higher than MCPS and suggesting the two pain scales were systematically producing difference results. These correlation and agreement analyses were determined to be poor, and this phase was discontinued.
Phase 2 mCSU and NRS pain scores were assessed 1 h post-anesthesia recovery and at discharge. Discharge observation times averaged 4.2 ± 0.05 h post-anesthesia recovery (range 1.7–7 h). A correlation analysis of mCSU and NRS pain scores were compared between the two evaluators. The r value for correlation between the evaluators’ scores 1 h post-recovery and at discharge were 0.40 and 0.47, respectively. When scores for both time periods were combined, the r value was 0.50. Bland–Altman agreement analysis revealed a much smaller bias (0.02) between evaluators using these two pain scales than with the MCPS and NRS. The strength of the relationship and agreement between evaluators’ pain scores was considered acceptable.
To account for age and weight differences among groups, data were stratified by weight into three strata: 0.9–1.5 kg, >1.5–2.7 kg and >2.7 kg. Anesthesia induction dosages varied among these strata. Ketamine induction dosages were: 10.0–16.5 mg/kg, 7.4–10.0 mg/kg and 3.75–7.4 mg/kg, respectively. Dexmedetomidine dosages were 0.05–0.08 mg/kg, 0.04–0.05 mg/kg and 0.02–0.04 mg/kg, respectively.
Averaged, normalized pain scores were compared within weight strata. There were no significant differences in pain scores among the three groups within the two lower body weight strata. Significant differences among pain scores were noted for both observation periods in the third stratum (>2.7 kg). Bupivacaine group pain scores were lower than the two control groups at both observation times. The P values were 0.008 and 0.004 for 1 h post-recovery and discharge, respectively (Tables 3–5).
Table 3.
Averaged, normalized pain scores for study cats weighing 0.9–1.5 kg (phase 2)
| Group | n | Observation time 1 | Observation time 2 |
|---|---|---|---|
| Bupivacaine | 24 | 0.16 ± 0.10 | 0.04 ± 0.07 |
| Saline | 3 | 0.24 ± 0.06 | 0.13 ± 0.12 |
| Sham | 42 | 0.19 ± 0.12 | 0.07 ± 0.09 |
| Total | 69 |
Data are mean ± SD
Table 4.
Averaged, normalized pain scores for study cats weighing >1.5–2.7 kg (phase 2)
| Group | n | Observation time 1 | Observation time 2 |
|---|---|---|---|
| Bupivacaine | 21 | 0.20 ± 0.12 | 0.13 ± 0.11 |
| Saline | 16 | 0.19 ± 0.10 | 0.11 ± 0.10 |
| Sham | 29 | 0.19 ± 0.12 | 0.10 ± 0.08 |
| Total | 66 |
Data are mean ± SD
Table 5.
Averaged, normalized pain scores for study cats weighing >2.7 kg (phase 2)
| Group | n | Observation time 1 | Observation time 2 |
|---|---|---|---|
| Bupivacaine | 14 | 0.15 ± 0.10* | 0.05 ± 0.07† |
| Saline | 22 | 0.26 ± 0.12 | 0.14 ± 0.09 |
| Sham | 5 | 0.30 ± 0.08 | 0.19 ± 0.09 |
| Total | 41 |
Data are mean ± SD
Significantly lower acute pain score averages 1 h post-anesthesia recovery (P = 0.008)
Significantly lower acute pain score averages at same day discharge (P = 0.004)
In all groups, pain scores 1 h post-recovery were significantly higher than scores at discharge. This finding held true throughout weight groupings.
No cat included in the study had an assessed pain score meeting the rescue analgesia threshold of moderate pain.
Discussion
In this study, discrete injections of bupivacaine targeted to potentially painful areas reduced acute postoperative pain scores in routine, elective feline ovariohysterectomies for cats weighing >2.7 kg. Acute pain scores for the bupivacaine group were lower than the two control groups at both time points, but only attained significance in the higher weight stratum. The technique used to administer the tested drugs was simple, requiring an average of just over 1 min. The cost of bupivacaine for the dosages used was minimal and added little to the overall cost of the surgery.
Previous studies using local anesthetics as an anesthesia/analgesia adjunct have shown mixed results depending upon the agent used, administration techniques and anatomical sites treated. This study attempted to refine the interventions and capture lessons learned from previous studies. As the predominate site of origination of ovariohysterectomy pain remains unclear, the technique used for this study discretely infiltrated bupivacaine in small volumes at the four specific anatomical sites where the viscera and abdominal wall were mechanically disrupted.13–15 This technique was developed to keep bupivacaine near the specifically affected area longer, in contrast to studies where the local anesthetic was sprayed, loosely disseminated, infused or splashed around the peritoneum adjacent to the ovarian pedicles and the uterine body.
There appears to be little to equivocal reported benefit with superficial use of local anesthetics perioperatively to reduce acute postoperative pain in dogs and cats. Fitzpatrick et al looked at the effects of incision site infiltration with bupivacaine as a part of a multimodal analgesia protocol on postoperative pain and incisional healing in dogs undergoing ovariohysterectomy. 16 They reported no additional analgesic benefit with local administration, and a higher number of complications in dogs having pre-incisional infiltration. 16 Similarly, Merema et al showed no additional analgesic benefit with the use of transdermal lidocaine in dogs treated concurrently with recommended doses of morphine and carprofen following ovariohysterectomy. 15 Even given the lack of evidence in cats, the incisional layer was included in this study for completeness as tissues were mechanically disrupted by the surgical approach.
Intraperitoneal use of local anesthetics is safe and offers better results than incisional use alone, albeit not without some conflicting studies. In a study of intraperitoneal bupivacaine in cats undergoing ovariohysterectomy, Benito et al reported that plasma concentrations did not cause observable toxicosis. 17 Bubalo et al used a local infiltration of the mesovarium with lidocaine and found neither an isoflurane sparing effect nor a difference in autonomic response to surgery in 20 dogs. 13 Kim et al showed sprayed intraperitoneal bupivacaine could be used as part of a multimodal approach for pain management after laparoscopic spays in 16 dogs. 18 They reported lower pain scores postoperatively, and no increase in cortisol concentrations after 1 h postoperatively. 18 Benito et al investigated the analgesic efficacy of intraperitoneal bupivacaine in cats undergoing ovariohysterectomy. 19 Similar to the present study, Benito et al aimed their application of bupivacaine into the peritoneal space over the right and left ovarian pedicle and caudal aspect of the uterus before ovariohysterectomy. Although they saw no difference in pain scores among groups, based on the need for rescue analgesia, intraperitoneal bupivacaine was considered to provide analgesia in cats after ovariohysterectomy. 19 The targeted use of intraperitoneal bupivacaine in this study was an attempt to infiltrate bupivacaine at discrete locations where pain could potentially be elicited thereby optimizing and prolonging its analgesic effect.
Studies using both incisional and intraperitoneal local anesthetics reported somewhat better results. Zilberstein et al administered lidocaine as a skin infiltration, topical application (splash block) on both ovaries, and on abdominal muscular layers in 56 cats. 20 They reported lidocaine significantly reduced the need for supplementary injectable anesthetic in response to movements during surgery. 20 Carpenter et al investigated intraperitoneal and incisional lidocaine or bupivacaine for analgesia following ovariohysterectomy in 30 dogs. 21 They reported dogs receiving intraperitoneal and incisional bupivacaine had lower visual analogue scale pain scores than their control group with no adverse effects observed. 21 Of note, they found no differences observed with a composite pain scale. Less rescue analgesia was required with their bupivacaine group (2/10) than their lidocaine (4/10) or control (7/10) groups. Additionally, they reported peak postoperative pain scores for all their groups occurred at 0.5 h and returned to baseline by 18 h. 21 Their observation of early peak post-surgical pain scores is consistent with this study where pain scores 1 h post-recovery were significantly higher than scores at discharge.
A potential confounding factor of similar studies was surgeon and technician experience. In most previously published studies, surgery was performed by veterinary students. This study is in stark contrast. All three surgeons had several years of experience in high volume spay surgeries. Each surgeon had performed several thousand procedures. Surgical times were short, incision sites were small and visceral manipulation was minimal. Technicians were exceptionally well experienced in feline ovariohysterectomy recovery. Given these factors, the expected pain scores would be relatively low making it more difficult to show significant differences among the study groups. With less experienced surgeons, perhaps greater positive effects would be elicited with targeted applications of bupivacaine.
Owing to the challenges of a high-volume practice, the anesthetic protocol used for this study provided some variation in the mg/kg dosages of ketamine (3.75–16.5 mg/kg) and dexmedetomidine (0.02–0.08 mg/kg). Significant differences in pain scores were only seen in the highest weight stratum where the mg/kg doses of ketamine (3.75–7.4 mg/kg) and dexmedetomidine (0.02–0.04 mg/kg) were the lowest. In this stratum, the cats administered bupivacaine had significantly lower acute pain scores than the other two control groups. Although not measured, it is reasonable that heavier, older cats receiving less ketamine and dexmedetomidine could have recovered relatively earlier from anesthesia. This earlier recovery and responsiveness to evaluators by cats during assessments could have biased evaluators towards lower pain scores. It cannot be discounted that rather than just drug doses alone, the effect observed may also be due to size (larger cats) or age, as older cats tend to be larger. Polson et al showed that kittens aged <4 months have less affective pain than adult cats post-ovariohysterectomy at 4 h and 24 h. 22
Numerous pain intensity measures have been developed and are commonly used in human medicine. Several of these provide a subjective assessment of pain intensity (eg, visual analog scale [VAS], NRS). Other scales are more dynamic and require close interaction with the patient. Although they are commonly used and provide a structured format for assessing pain, none of the three pain scales in this study, as used, is validated for cats. 23 Different scales offer different strengths and weaknesses. To account for potential differences among various scales, two different scales were used to evaluate cats in each of the study’s two phases.
In the first phase of this study, the MCPS and NRS were used. The multidimensional structure of the MCPS has the evaluator observe pain expression, psychomotor changes and physiological variables separately. A total score describing the overall pain intensity is calculated from item scores within each dimension. The NRS is a segmented version of the VAS where the evaluator selects a whole number that best reflects the intensity of the acute pain. Scores range from 0 to 10, with higher scores indicating greater pain intensity. In this study, the MCPS proved difficult to use and time consuming, especially with feral and fractious cats. Often the evaluator was unable to effectively assess cats and could only determine a partial score given the inability to palpate some cats. Time required for complete MCPS evaluations took as long as 10 mins per cat. In a high-volume spay environment, this was considered too long to be practical.
Subsequently, the MCPS was replaced with the mCSU scale. Like the NRS, the CSU scale ranks the intensity of pain on a number scale from 0 to 4 with higher scores indicating greater pain intensity. Illustrations and brief word descriptions are provided to assist in selecting the best score. This scale proved much easier to use and took considerably less time than the MCPS. Additionally, assessed pain scores correlated well between evaluators making it a better choice for the study.
It is possible that the pain scales used were not sensitive enough to accurately describe the pain experienced in study cats. Numeric reference pain scales are unidimensional, only reflecting pain intensity. They do not completely reveal the overall complexity and idiosyncratic nature of a patient’s experience with pain. Pain can be an emotional and multidimensional experience. The International Association for the Study of Pain defines pain as an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage. 24 In veterinary medicine, the score is assigned by an observer with their own unique biases for postoperative pain assessment. However, they are simple to use and have shown validity and reliability in human patients who self-report pain intensity. 25 They require minimal translation across language, age, sex and culture. In veterinary patients, they are similarly simple to use, require a short amount of time, and are safe to use with fearful and aggressive animals. One significant drawback is that these scales may exclude palpation of the surgical site, an important domain in pain assessment in animals.
One of the limitations of this study was the observation period. There were only two postoperative observations within an average of 4.2 ± 0.05 h of recovery (range 1.7–7 h). One attractive property of bupivacaine is its long duration of action. In veterinary medicine, bupivacaine’s duration of action is commonly reported to be 4–6 h. 7 In a recent report in dogs, the median time to return of full recovery from sensory blockage was up to 15 h. 26 There are numerous reports in humans of bupivacaine lasting ⩾8 h, and, although equivocal, there is a reported period of analgesia persisting after the return of sensation.7,27,28 With this long duration of action, bupivacaine potentially reduces the need for opioid analgesics postoperatively. A potential area for future study would be to assess at home pain and discomfort of cats given bupivacaine as part of their analgesia management during the days following surgery.
Conclusions
Targeted bupivacaine reduced early postoperative pain scores in cats >2.7 kg undergoing routine ovariohysterectomies, with a similar trend observed in cats in the lowest weight strata (0.9–1.5 kg). The technique was simple and inexpensive. Currently validated pain-scoring systems for cats that involve palpating surgical incisions postoperatively, like the MCPS, can be problematic in high-volume surgical settings, especially with feral and/or fractious cats.
Acknowledgments
The authors thank Faith Northcutt and Devin Angelucci for their technical support, especially with postoperative recovery and pain assessment. The authors also thank Shayne Palowski for technical assistance with operating room procedures. Further, the authors extend their gratitude to Bernadette Vogel for administrative support.
Footnotes
Accepted: 6 January 2019
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: James Mack Fudge  https://orcid.org/0000-0001-8945-0371
https://orcid.org/0000-0001-8945-0371
References
- 1. Griffin B, Bushby PA, McCobb E, et al. The Association of Shelter Veterinarians’ 2016 veterinary medical care guidelines for spay-neuter programs. J Am Vet Med Assoc 2016; 249: 165–188. [DOI] [PubMed] [Google Scholar]
- 2. Hansen BD. Analgesia and sedation in the critically ill. J Vet Emerg Crit Care 2005; 15: 285–294. [Google Scholar]
- 3. Epstein M, Rodan I, Griffenhagen G, et al. 2015 AAHA/AAFP pain management guidelines for dogs and cats. J Am Anim Hosp Assoc 2015; 51: 67–84. [DOI] [PubMed] [Google Scholar]
- 4. Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res 2017; 10: 2287–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murauski JD, Gonzalez KR. Peripheral nerve blocks for postoperative analgesia. AORN J 2002; 75: 136–147. [DOI] [PubMed] [Google Scholar]
- 6. Campoy L, Read M. Local anesthetics. In: Gaynor JS, Muir WW. (eds). Handbook of veterinary pain management. 3rd ed. St Louis, MO: Elsevier, 2015, pp 216–223. [Google Scholar]
- 7. Lemke KA, Dawson SD. Local and regional anesthesia. Vet Clin North Am Small Anim Pract 2000. 30: 839–857. [DOI] [PubMed] [Google Scholar]
- 8. Balakrishnan K, Ebenezer V, Dakir A, et al. Bupivacaine versus lignocaine as the choice of local anesthetic agent for impacted third molar surgery a review. J Pharm Bioallied Sci 2015; Suppl 1: S230–S233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brondani JT, Khursheed RM, Luna SPL, et al. Validation of the English version of the UNESP-Botucatu multidimensional composite pain scale for assessing postoperative pain in cats. BMC Vet Res 2013; 9: 143. DOI: 10.1186/1746-6148-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oliveira JP, Mencalha R, Santos Sousa CA, et al. Pain assessment in cats undergoing ovariohysterectomy by midline or lateral celiotomy through use of a previously validated multidimensional composite pain scale. Acta Cir Bras 2014; 29: 633–638. [DOI] [PubMed] [Google Scholar]
- 11. McCaffery M. Pain (0–10 numeric pain rating scale). In: McCaffery M, Pasero C. (eds). Pain: clinical manual. St Louis, MO: Mosby, 1999, p 16. [Google Scholar]
- 12. Hellyer PW, Uhrig SR, Robinson NG. Feline acute pain scale. http://csu-cvmbs.colostate.edu/Documents/anesthesia-pain-management-pain-score-feline.pdf (2006, accessed February 12, 2018).
- 13. Bubalo V, Moens YP, Holzmann A, et al. Anaesthetic sparing effect of local anaesthesia of the ovarian pedicle during ovariohysterectomy in dogs. Vet Anaesth Analg 2008; 35: 537–542. [DOI] [PubMed] [Google Scholar]
- 14. Campagnol D, Teixeira-Neto FJ, Monteiro ER, et al. Effect of intraperitoneal or incisional bupivacaine on pain and the analgesic requirement after ovariohysterectomy in dogs: intraperitoneal or incisional bupivacaine in dogs. Vet Anaesth Analg 2012; 39: 426–430. [DOI] [PubMed] [Google Scholar]
- 15. Merema DK, Schoenrock EK, Boedec KL, et al. Effects of a transdermal lidocaine patch on indicators of postoperative pain in dogs undergoing midline ovariohysterectomy. J Am Vet Med Assoc 2017; 250: 1140–1147. [DOI] [PubMed] [Google Scholar]
- 16. Fitzpatrick CL, Weir HL, Monnet E. Effects of infiltration of the incision site with bupivacaine on postoperative pain and incisional healing in dogs undergoing ovariohysterectomy. J Am Vet Med Assoc 2010; 237: 395–401. [DOI] [PubMed] [Google Scholar]
- 17. Benito J, Monteiro BP, Beaudry F, et al. Pharmacokinetics of bupivacaine after intraperitoneal administration to cats undergoing ovariohysterectomy. Am J Vet Res 2016; 77: 641–645. [DOI] [PubMed] [Google Scholar]
- 18. Kim YK, Lee SS, Suh EH, et al. Sprayed intraperitoneal bupivacaine reduced early postoperative pain behavior and biochemical stress response after laparoscopic ovariohysterectomy in dogs. Vet J 2012; 191: 188–192. [DOI] [PubMed] [Google Scholar]
- 19. Benito J, Monteiro B, Lavoie AM, et al. Analgesia efficacy of intraperitoneal administration of bupivacaine in cats. J Feline Med Surg 2016; 18: 906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zilberstein LF, Moens YP, Leterrier E. The effect of local anaesthesia on anaesthetic requirements for feline ovariectomy. Vet J 2008; 178: 214–218. [DOI] [PubMed] [Google Scholar]
- 21. Carpenter RE, Wilson DV, Evans AT. Evaluation of intraperitoneal and incisional lidocaine or bupivacaine for analgesia following ovariohysterectomy in the dog. Vet Anaesth Analg 2004; 31: 46–52. [DOI] [PubMed] [Google Scholar]
- 22. Polson S, Taylor PM, Yates D. Effects of age and reproductive status on postoperative pain after routine ovariohysterectomy in cats. J Feline Med Surg 2014; 16: 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shipley H, Guedes A, Graham L, et al. Preliminary appraisal of the reliability and validity of the Colorado State University Feline Acute Pain Scale. J Feline Med Surg 2019; 21: 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Merskey H, Albe FD, Bonica JJ, et al. Pain terms: a list with definitions and notes on usage. Recommended by the IASP subcommittee on taxonomy. Pain 1979; 6: 249–252. [PubMed] [Google Scholar]
- 25. Haefeli M, Elfering A. Pain assessment. Eur Spine J 2006; 15 Suppl 1: S17–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cathasaigh M, Read MR, Atilla A, et al. Blood concentration of bupivacaine and duration of sensory and motor block following ultrasound-guided femoral and sciatic nerve blocks in dogs. PLoS One 2018; 13: e0193400. DOI: 10.1371/journal.pone.0193400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alhelail M, Al-Salamah M, Al-Mulhim M, et al. Comparison of bupivacaine and lidocaine with epinephrine for digital nerve blocks. Emerg Med J 2009; 26: 347–350. [DOI] [PubMed] [Google Scholar]
- 28. Calder K, Chung B, O’Brien C, et al. Bupivacaine digital blocks: how long is the pain relief and temperature elevation? Plast Reconstr Surg 2013; 131: 1098–1104. [DOI] [PubMed] [Google Scholar]