Abstract
Objectives
Constipation is a common complaint in cats presenting to the emergency room and can become a frustrating recurrent condition. Despite widespread anecdotal reports of risk factors for constipation, at the time of writing there have been no studies supporting these associations or assessing treatment outcomes. The aim of this study was to identify risk factors in the signalment, history, physical examination and clinicopathologic findings of cats presenting to the emergency room for constipation. In addition, we aimed to assess factors contributing to the success or failure of enemas administered to these cats.
Methods
A medical record search identified 189 cats with a diagnosis of constipation/obstipation that were treated and discharged by the emergency service at an academic veterinary hospital. Data regarding signalment, medical history, physical examination and clinicopathologic findings, as well as treatments performed, were recorded. Ninety-nine cats presenting to the emergency room for other reasons were identified as controls. Statistical analysis was performed to assess risk factors for constipation, as well as success/failure of enema treatments.
Results
Older, overweight cats and cats with chronic kidney disease or previous episodes of constipation were found to be at increased risk of constipation (P <0.0001, P = 0.0004, P = 0.0046 and P <0.0001, respectively). Ionized calcium levels were significantly higher in constipated cats, though varied significantly within the cohort (P = 0.0133). Cats noted to be painful on abdominal palpation were less likely to defecate following an enema. Adjunctive treatments (fluids, laxatives) increased the likelihood of a successful enema but were not statistically significant.
Conclusions and relevance
Older, overweight cats with a history of constipation or chronic kidney disease are more likely to present for constipation. Further studies are needed to determine the most appropriate treatment protocol in an urgent care setting.
Keywords: Constipation, obstipation, enema, risk factors
Introduction
Constipation and obstipation are common and frustrating complaints in cats presenting to emergency rooms. Constipation is defined as decreased frequency or difficulty in passing stool, while obstipation refers to a loss of function of the ability to defecate normally and, clinically, denotes multiple prior treatment failures. 1 Historically, constipation in cats has been associated with comorbidities that can lead to dehydration, such as chronic kidney disease (CKD), diabetes mellitus and hyperthyroidism. 2 Other risk factors discussed in the existing literature include pelvic fractures, neuropathies (as with feline dysautonomia syndrome), sacral spinal cord disease or injury, and megacolon (both idiopathic and secondary) – all conditions that alter the normal mechanical and/or physiologic function of the colon and rectum. 2 In human medicine, risk factors for constipation have largely been assessed within discrete populations; however, certain demographics have been widely identified as at risk for chronic constipation (specifically, females, the elderly, and overweight individuals). In addition, constipation is a known side effect of many anticonvulsants, calcium channel blockers, diuretics, antihistamines, iron supplements and other drug classes.3,4
Despite the aforementioned risk factors for constipation in cats, to our knowledge at the time of writing there have been no studies supporting these associations. Existing literature focuses on risk factors and treatment options for megacolon, and while there is likely significant overlap between these conditions, the dearth in primary literature precludes the use of evidence-based medicine to treat and prevent constipation. The aim of this study was to identify risk factors in the signalment, history, physical examination and clinicopathologic findings of cats diagnosed with constipation and/or obstipation at an urban, tertiary referral center emergency room. In addition, we aimed to identify factors that may predict the success or failure of enemas given in hospital to constipated cats.
Materials and methods
A retrospective, case-control study of cats treated at the Matthew J Ryan Veterinary Hospital of the University of Pennsylvania was designed. A database search of all cats discharged by the emergency service between 1 January 2011 and 31 July 2017 was performed. Cats discharged from the emergency service with a diagnosis of constipation and/or obstipation were included in the study. Medical records were reviewed to ensure the coded diagnosis was appropriate. Inclusion criteria included clinical signs of straining to defecate, owner-reported decrease in or absence of stool production, physical examination findings of firm stool in the colon or radiographic evidence of fecal impaction. Cats were excluded from the study if a review of their medical record did not support a confirmed diagnosis of constipation/obstipation, or if significant parts of the record were missing. Cases that were discharged by services other than emergency were not reviewed.
Cats discharged by the emergency service for any reason over the same time period were identified and 99 were randomly selected to be used as controls. Ran-domization was performed by selecting the first 15 cases discharged in a representative month for each year included in the study (ie, 15 cases from December 2011, 15 from May 2016, etc). Of the 105 cases identified, six were excluded for incomplete medical records or presenting for euthanasia only. The admission dates of control cats were distributed evenly throughout the year, to eliminate any confounding factors secondary to seasonality.
Signalment, medical history, diet, physical examination findings, venous blood gas results (on presentation), abdominal imaging findings and all in-hospital treatments were recorded for both constipated and control cats. Diets were classified as ‘wet’ or ‘dry’; specific commercial diets were not compared. Some physical examination findings were based on subjective evaluation by the clinician on record (ie, hydration status, pain on abdominal palpation, body condition score [BCS], heart murmur).
Hydration was assessed based on classifications found on the standardized physical examination form used within the hospital, which uses the terms ‘good’, ‘fair’ and ‘poor’. No specific guidelines for percentage dehydration are used to define these categories. Cats considered to have ‘fair’ or ‘poor’ hydration statuses were considered dehydrated for statistical analysis. BCS was available for most, but not all, cats in the study, and was based on the World Small Animal Veterinary Association Global Nutrition Committee’s BCS system. Ideal body condition was considered 4–5/9, in line with this scoring system. Comorbidities (constipation, CKD, diabetes mellitus, hyperthyroidism, inflammatory bowel disease [IBD] or small-cell lymphoma) were determined based on available prior medical records, or as reported by the owner at the time of the emergency room visit. Venous blood gas data included packed cell volume, serum total solids, blood urea nitrogen (BUN), creatinine, ionized calcium, potassium and magnesium (samples analyzed on Stat Profile pHOx Ultra machine [Nova Biomedical]). Statistical analysis for each variable omitted any cats for which relevant data were unavailable.
Treatment variables included administration of enemas, intravenous (IV) or subcutaneous (SC) fluids, or oral lactulose while in the hospital. Specific data (ie, fluid type, volumes) were recorded when applicable. Treatment success was defined by any notation in the medical record indicating defecation following enema administration during the emergency service stay. Manual de-obstipation was not considered an adjunct treatment (as it was presumably used following enema failure rather than before). As most cats were treated as outpatients, the success of treatment reflected defecation in hospital only. Treatment outcome was omitted if information regarding defecation was not available.
Continuous variables were assessed for normality using the Shapiro–Wilks test. Mean ± SD were used to described normally distributed variables, while median (range) was used for those not normally distributed. Categorical variables were described using proportions (%). Fisher’s exact test (for counts <5 in any cell) or χ2 tests (counts >5) were used to compare variables between these groups. Univariate logistical regression was used to generate odds ratio (OR) and Woolf’s method was used to calculate the 95% confidence intervals (CIs). Backward stepwise multiple logistic regression was performed on the physical examination and clinicopathologic findings that were statistically significant on univariate analysis. For all analyses, a P value <0.05 was considered significant. All analyses were performed using a statistical software program (Stata 14.0 for MAC).
Results
Two hundred and fifty-one cases were initially identified in a medical record search. Sixty-two were eliminated owing to incomplete medical records, records that did not support a diagnosis of constipation or duplicate entries (due to redundant diagnosis coding). Ultimately, 189 cats were included in the constipated cohort of this study. Admission dates were relatively evenly distributed throughout the year (Figure 1). It was not possible to determine which cats were truly obstipated, as, by definition, this would require multiple consecutive treatment failures, which could not be determined based on a single visit to the hospital.
Figure 1.
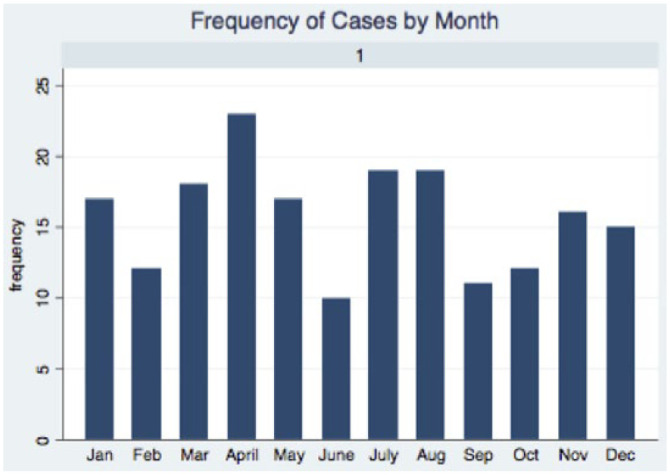
Case distribution by month
The breed and sex distribution of all cats is summarized in Table 1 and was similar for both cohorts. Sex was not found to be a risk factor for constipation. The sample sizes for each breed were too small to perform meaningful analysis. Constipated cats were significantly (P <0.0001) older (median age 10 years, range 3 weeks to 21 years) than control cats (median 6 years, range 8 weeks to 26 years). Increased age remained a significant finding following multiple logistic regression. The proportion of cats fed an exclusively dry diet was similar for both constipated (n = 57/163 [35%]) and control (n = 26/72 [36%]) groups, and was not found to be a significant risk factor for the development of constipation (OR 0.95, 95% CI 0.5–1.8; P = 0.8659).
Table 1.
Summary of signalment, age and body condition score (BCS) characteristics for constipated and control groups
| Constipated cats | Control cats | P value (if significant) | |
|---|---|---|---|
| Median (range) age (years) | 10 (3 weeks to 21 years) | 6 (8 weeks to 26 years) | <0.0001 |
| Sex (intact and castrated)* | 0.9105 | ||
| Female | 72 (38) | 38 (39) | |
| Male | 117 (62) | 60 (61) | |
| Breed † | |||
| DSH | 166 (88) | 86 (87) | – |
| DLH | 12 (6) | 6 (6) | – |
| Manx | 2 (1) | 0 | – |
| Ragdoll | 2 (1) | 0 | – |
| Siamese | 2 (1) | 2 (2) | – |
| Persian | 3 (2) | 1 (1) | – |
| Maine Coon | 1 (1) | 2 (2) | – |
| Other | 1 (1) | 2 (2) | – |
| BCS (range) | 6 (1.5–9) | 5 (1–9) | 0.0004 |
Data are n (%) unless otherwise indicated
The sex of one control cat was unavailable
Too many categories to compare groups
DSH = domestic shorthair; DLH = domestic longhair
Constipated cats had a significantly (P = 0.0004) higher BCS (median 6, range 1.5–9) than controls (median 5, range 1–9), though, as noted by the ranges, cats of all body conditions were found in both groups. No other physical examination finding was significantly different between cohorts (Table 2). Backward stepwise logistic regression was performed on signalment, body weight and BCS. Sex and weight were not significant, leaving age (OR 1.2, 95% CI 1.1–1.2) and BCS (OR 1.4, 95% CI 1.2–1.7) as the only significant variables left in the model (P <0001, R2 = 0.1618).
Table 2.
Summary of the effect of comorbidities on risk of constipation
| Comorbidity | Cohort | Affected | Unaffected | Percentage affected | OR | 95% CI | P value |
|---|---|---|---|---|---|---|---|
| History of constipation | Constipated | 74 | 115 | 39 | 20.4 | 6.3, 103 | <0.0001 |
| Control | 3 | 95 | 3 | ||||
| CKD | Constipated | 32 | 157 | 17 | 3.8 | 1.4, 12.9 | 0.0046 |
| Control | 5 | 93 | 5 | ||||
| DM | Constipated | 9 | 180 | 5 | 1.6 | 0.4, 9.4 | 0.4849 |
| Control | 3 | 96 | 3 | ||||
| Hyperthyroidism | Constipated | 16 | 173 | 8 | 1.1 | 0.4, 2.6 | 0.9106 |
| Control | 8 | 91 | 8 | ||||
| IBD/lymphoma | Constipated | 27 | 162 | 14 | 1.5 | 0.7, 3.6 | 0.3249 |
| Control | 10 | 89 | 10 | ||||
| Heart murmur | Constipated | 51 | 133 | 28 | 1.1 | 0.6, 1.9 | 0.7741 |
| Control | 24 | 68 | 35 | ||||
| Gallop rhythm | Constipated | 10 | 179 | 5 | 0.9 | 0.3, 3.0 | 0.7710 |
| Control | 6 | 92 | 6 |
OR = odds ratio; CI = confidence interval; CKD = chronic kidney disease; DM = diabetes mellitus; IBD = inflammatory bowel disease
Comorbidity data are summarized in Table 2. Seventy-four of the constipated cats (39%) had a history of constipation vs three (3%) of the control cases. The control cats with a history of constipation were presenting for reasons unrelated to defecation, and details regarding history were not available (as each was owner-reported). Constipated cats were 20 × more likely to have a history of constipation than controls (95% CI 6.3–103; P <0.0001), and 3.8 × more likely to have CKD (OR 3.8, 95% CI 1.4–12.9; P = 0.0046). They were also more likely to have diabetes mellitus (OR 1.6, 95% CI 0.4–9.4), and IBD or small-cell lymphoma (OR 1.5, 95% CI 0.7–3.6), but neither reached significance.
Venous blood gas results were available for 119 (63%) of the constipated cats and 44 (45%) of the controls and are summarized in Table 3. Total solids (median 7.75 g/dl, range 5.8–10 [7.4 g/dl, 4.8–10.8) was significantly higher (P = 0.0487) in constipated cats. Ionized calcium was also significantly higher in constipated cats (P = 0.0133), though the range varied widely, and the median values remained at the low end of the reference interval (RI; median 1.15 mmol/l, range 0.96–1.47 mmol/l [1.12 mmol/l, 0.59–1.81]; RI 1.11–1.38). Constipated cats had lower potassium levels (median 3.945 mmol/l, range 2.92–5.17 [4.16 mmol/l, 2.98–8.64 mmol/l]; P = 0.0406). Packed cell volume and creatinine were not significantly different between groups, though magnesium and BUN levels were both lower in constipated cats and trending towards significance (P = 0.0646 and P = 0.0744, respectively). Backward stepwise multiple logistic regression was performed on the statistically significant univariate venous blood gas variables. Both total solids and potassium were dropped from the model owing to statistical insignificance (P >0.05), leaving only ionized calcium (OR 45, 95% CI 1.8–1133; P = 0.021).
Table 3.
Comparison of venous blood gas results between constipated and control cats
| Variable | Constipated cats (median; range) | Percentage of constipated cats outside of the RI (%) | Control cats (median; range) | Percentage of control cats outside of the RI (%) | RI | P value |
|---|---|---|---|---|---|---|
| PCV (%) | 38; 20–56 | Above: 3 | 40; 16–58 | Above: 13 | 31–48 | 0.2501 |
| Below:14 | Below: 18 | |||||
| TS (g/dl) | 7.75; 5.8–10 | Above: 14 | 7.4; 4.8–10.8 | Above: 11 | 6.6–8.4 | 0.0487 |
| Below: 8 | Below: 20 | |||||
| Ionized calcium (mmol/l) | 1.15; 0.96–1.47 | Above: 6 | 1.12; 0.59–1.81 | Above: 2 | 1.11–1.38 | 0.0133 |
| Below: 30 | Below: 41 | |||||
| Potassium (mmol/l) | 3.945; 2.92–5.17 | Above: 6 | 4.16; 2.98–8.64 | Above: 6 | 3.5–4.8 | 0.0406 |
| Below: 10 | Below: 7 | |||||
| Ionized magnesium (mmol/l) | 0.47; 0.34–0.73 | Above: <1 | 0.49; 0.35–1.01 | Above: 12 | 0.42–0.65 | 0.0646 |
| Below: 16 | Below: 12 | |||||
| BUN (mg/dl) | 25; 12–253 | Above: 31 | 29; 11–257 | Above: 45 | 15–32 | 0.0744 |
| Below: 8 | Below: 11 | |||||
| Creatinine (mg/dl) | 1.3; 0.6–12.3 | Above: 17 | 1.55; 0.8–9.9 | Above: 21 | 0.8–2.1 | 0.3559 |
| Below: 3 | Below: 0 |
RI = reference interval; PCV = packed cell volume; TS = total solids; BUN = blood urea nitrogen
One hundred and twenty-four of the 189 constipated cats (66%) received at least one enema while hospitalized, with 45 of the cats (24% of total, 36% of cats receiving enemas) receiving two or more. Information on the use of sedation or anesthesia for enema administration was not recorded. Outcome of enema (defecation/no defecation) was recorded for 85 (69%) of the cats, with 45 (53%) resulting in defecation in hospital. Other treatments administered (either alone or in addition to enemas) included SC or IV fluids, and oral lactulose administration. Fifty cats (40%) received an enema as the sole treatment, while the remaining 74 cats received an enema in conjunction with one or more ancillary treatment (Table 4). Ten of the 74 cats received more than one adjunctive treatment.
Table 4.
Analysis of adjunct treatment effect on enema success
| Treatment | Total number treated | Treatment + enema* | Defecation † | OR | 95% CI | P value |
|---|---|---|---|---|---|---|
| Enema only | 50/124 (40%) | NA | 15/29 (52%) | NA | NA | NA |
| SC fluids | 47/189 (25%) | 18/47 (49%) | 3/5 (60%) | 1.39 | 0.31–6.1 | 0.314 |
| IV fluids | 58/189 (31%) | 20/58 (34%) | 13/20 (65%) | 1.52 | 0.52–4.4 | 0.439 |
| Oral lactulose | 25/189 (13%) | 2/25 (8%) | 0/2 (0%) | 1.06 | 0.64–3.0 | 0.304 |
Does not include 10 cats that received an enema and more than one adjunctive treatment
Only includes cats for which treatment outcome (defecation vs no defecation) was specifically recorded in the medical record
OR = odds ratio; CI = confidence interval; NA = not applicable; SC = subcutaneous; IV = intravenous
Almost all of the enemas given were a warm water and lubricant solution (brands unavailable, though the hospital in this study currently uses Lubricating Jelly distributed by McKesson Medical-Surgical), with a mode of 60 ml (combined volume). The number of enemas given ranged from one to five per cat, with a median of two. The cumulative volume of fluid administered via enema ranged from 20 to 300 ml, with a mean of 100 ml. Receiving >60 ml (cumulative volume) was not found to increase the chance of defecation significantly, nor was receiving multiple enemas.
Cats with a history of constipation (39/85 cats with known treatment outcomes) were more likely to defecate following enemas (26/39 [67%; P = 0.011]). Cats that were subjectively painful on abdominal palpation were less likely to defecate following enema administration (painful cats 13/33 [39%] vs non-painful 20/26 [77%]; OR 4.47, 95% CI 2.3–8.7 [P = 0.004]). Ten (8%) of the cats that received enemas also received methadone and 24 (19%) received buprenorphine while hospitalized. The use of methadone or buprenorphine was not found to affect treatment outcome, though cats were not reassessed to determine if they were still painful following administration of analgesics. No other comorbidity, physical examination or clinicopathologic finding was found to significantly effect defecation rates.
The use and effect of adjunctive treatments are summarized in Table 4. It should be noted that cats that received multiple adjunctive treatments (ie, both lactulose and SC fluids) were not included when assessing effect on defecation. The use of oral lactulose, SC or IV fluids was not found to have a statistically significantly impact on the chance of defecation, though the OR was >1 for cats receiving either SC (OR 1.39, 95% CI 0.31–6.1) or IV fluids (OR 1.52, 95% CI 0.52–4.4). Of the 47 cats that received SC fluids, all were administered PlasmaLyte A, at total volumes ranging from 6.25 to 60 ml/kg (median 33.1 ml/kg). Of the 58 cats receiving IV fluids, 49 received PlasmaLyte A alone, eight received PlasmaLyte A with potassium chloride supplementation, one received PlasmaLyte A and 0.45% sodium chloride, one received PlasmaLyte A with potassium chloride and 0.45% sodium chloride, and one received both PlasmaLyte A with 2.5% dextrose and Hetastarch. The total time on IV fluids was not recorded. Twenty-five cats received oral lactulose in hospital. The number of doses ranged from one to five, and the median total volume administered was 3.8 ml (range 1–9 ml).
Discussion
While the results of this study confirm some of the anecdotally reported and physiologically intuitive causes of constipation, other presumed risk factors were not statistically supported by the findings. It should be emphasized that data for many of these variables were in agreement with anecdotal reports, but the study was underpowered to detect them statistically (specifically, a history of diabetes mellitus, IBD/small-cell lymphoma and clinicopathologic evidence of dehydration [eg, increased total solids]). Larger future studies may statistically support these anecdotal reports.
With regard to populations at risk of constipation, older cats and those with a higher BCSs were significantly more likely to present for constipation. The increased risk with age could theoretically coincide with a higher incidence of other comorbidities that can cause constipation, such as CKD, diabetes, arthritis and other systemic diseases. However, multiple logistic regression was performed and both age and BCS remained significant, suggesting that they are independently associated with constipation. Obesity is also correlated with constipation in humans; however, a causative relationship has not been established. 5 Obesity can exacerbate orthopedic or neurologic disease that may make posturing to defecate difficult. It has also been suggested that obesity influences gastrointestinal motility, as gastroesophageal reflux and IBD are also more prevalent in obese humans. 5 However, multiple logistic regression was performed and both risk factors remained significant, suggesting that they are independently associated with constipation.
No increased risk of constipation was found in cats fed a dry diet in our study population. Anecdotally, wet food diets are often recommended for cats with a history of constipation in an effort to increase water intake. This study did not control for differences in moisture or fiber content, so some dry diets may still increase the risk of constipation. It is likely that diet choice may be more relevant depending on the underlying cause of constipation (ie, cats with CKD may benefit from a wet diet as they have greater fluid outputs). Additionally, new commercial diets for cats with chronic constipation have recently come on the market and may affect long-term management recommendations in the future.
CKD was the only pre-existing comorbidity that was found to be significantly more common in constipated cats. This association is likely multifactorial, with water imbalance, electrolyte disturbances and pharmacologic management of the condition all contributing. 6 Cats with CKD do not concentrate their urine appropriately, leading to increased water loss and dehydration. Water is subsequently reabsorbed through the colon to help correct this imbalance, leading to constipation. 6 Hypokalemia, which alters gastrointestinal smooth muscle motility (as described below), can also be seen in cats with CKD and may be a contributing factor. 6 In addition, the use of aluminum phosphate binders to control hyperphosphatemia can cause constipation. 7 Only one cat in this study was noted to be on aluminum hydroxide (as reported in the medical record), so conclusions about the effect of these drugs on constipation could not be made. Despite the increased prevalence of CKD in constipated cats in this study, BUN and creatinine of affected cats was not significantly higher than that of controls, and median values were within normal limits for both groups. It is possible that there was selection bias, as more azotemic cats may have been transferred to other services within the hospital for more intensive treatment.
Electrolyte disturbances including hypercalcemia, hypokalemia and hypomagnesemia have been associated with constipation in humans, and reported to be a clinical finding in cats.1,8 Both hypokalemia and hypomagnesemia are known to cause paralytic ileus, though the precise mechanism has not been elucidated, to our knowledge. 9 Hypercalcemia decreases the excitability of gastrointestinal smooth muscle, thus decreasing motility and causing constipation. 10 Some of these metabolic disturbances can occur with other systemic conditions (eg, CKD and diabetes), so the cause of constipation may be multifactorial, and not a direct result of these electrolyte changes. A larger study with a greater number of patients may help elucidate some of these correlations that were not significant in this study. In addition, a minority of cats received IV fluids with supplemental potassium, which may have affected the outcome of their enema by helping to normalize their gastrointestinal motility. This study did not examine whether hypokalemic cats that received potassium supplementation had a greater chance of defecation following enemas than those that did not have their hypokalemia corrected. Larger studies may be considered to better assess electrolyte disturbances in constipated cats.
Unsurprisingly, constipated cats were more likely to be painful on abdominal palpation. Painful cats were less likely to have a successful enema, possibly owing to contractions/spasms of the abdominal musculature preventing defecation. Alternatively, more painful cats may be more severely constipated and/or obstipated, thus refractory to treatment. The subjectivity of assessing both pain and degree of constipation in animals makes this theory difficult to prove, and this study did not look at specific pain scores or further qualify discomfort. Opioid analgesics were used frequently in this population but did not increase the chance of defecation following enemas. Opioids themselves are known to induce constipation in humans due to binding of mu-opioid receptors in the enteric nervous system. 11 This results in delayed gut transit time, decreased chloride secretion, increased anal sphincter tone, and increased non-propulsive motility, each of which can contribute to constipation and, in themselves, cause abdominal discomfort. 10 The degree to which this occurs in cats is not known, however. Addressing abdominal tension and discomfort may improve the efficacy of enemas. Future studies are needed to determine the effect of opioids on gastrointestinal motility in cats, as well as what analgesic is most appropriate for constipated cats.
The use of oral lactulose or SC/IV fluids was not found to significantly improve defecation rates; however, each adjunctive treatment was associated with a greater chance of defecation than enemas alone. As the sample sizes for cats receiving each individual adjunctive treatment were low (many cats received multiple treatments), the statistical power of these findings is low, and future studies with larger cohort sizes and longer follow-up times may support the benefit of these treatments. Additionally, these treatments may act synergistically if administered together, which should also be explored.
This study was limited by a number of factors. Most importantly, there was significant selection bias given the exclusion of cats that were transferred to other services within the hospital. It is likely that cats requiring transfer were more systemically ill, thus their physical examination and clinicopathologic findings may be different to that of our population. Additionally, many of the parameters examined were subjective (ie, hydration status, BCS), and likely varied between clinicians. The inability to standardize physical examination findings and descriptions between clinicians is a problem encountered quite broadly in medical literature and is difficult to avoid. Furthermore, this study was not able to conclusively differentiate between constipated and obstipated cats (which, by definition, are refractory to treatment). Treatment outcomes were also difficult to assess, given how many cats were treated as outpatients, for which longer follow-up time points were not available. Similarly, the length of time cats were kept in the hospital following treatment was not available, and likely varied significantly. Finally, while incomplete records were excluded from the study, there were varying degrees of detail between records, making it possible that relevant information was omitted.
It should also be noted that this study did not look at the incidence of megacolon in cats. Megacolon, which refers to ‘persistent, irreversible distension of the colon diameter’, is a significant cause of chronic constipation, and can be idiopathic (in two-thirds of cases), or secondary to chronic colonic distension (as from neurologic dysfunction, narrowing of the pelvic canal or constipation). 1 Megacolon can be radiographically indistinguishable from acute obstipation, so diagnosis is usually made through a combination of clinical signs, repeated radiographic evidence of fecal impaction and a history of intractable constipation. 11 While megacolon was reported radiographically for some cats in the study, repeatability was not documented, preventing confirmation of the diagnosis. Further studies with multiple imaging time points would be needed to assess the incidence or development of chronic megacolon in constipated cats.
Although our study did look at some risk factors for enema success or failure, further work is needed to determine the most effective treatment for acute constipation. While water/lubricant-based enemas were the most common first-line treatments at this hospital, the use of glycerin, dicotyl sodium sulfosuccinate or lactulose may increase efficacy and decrease the need for multiple enemas. Additionally, the use of suppository laxatives may be more effective than oral lactulose (in the acute setting) and is another treatment option that should be examined.
Conclusions
Constipation is a significant cause of morbidity in cats, and a relatively common cause of presentation to the emergency room. This study found increasing age, obesity and a history of constipation or CKD to be risk factors for constipation. The use of water/lubricant enemas only led to defecation in 52% of the cats treated in this study, indicating significant treatment failure. Refinement of treatment protocols, as well as prophylactic lifestyle changes to cats at risk, could likely improve outcomes and prevent re-presentation for constipation.
Footnotes
Accepted: 30 January 2019
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This study involved the use of client-owned animal(s) only, and followed internationally recognized high standards (‘best practice’) of individual veterinary clinical patient care. Ethical approval from a committee was not therefore needed.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal guardian of all animal(s) described in this study for the procedure(s) undertaken. No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Sarah E Benjamin  https://orcid.org/0000-0001-9475-4492
https://orcid.org/0000-0001-9475-4492
References
- 1. Washabau R, Holt D. Pathogenesis, diagnosis, and therapy of feline idiopathic megacolon. Vet Clin North Am Small Anim Pract 1999; 29: 589–603. [PubMed] [Google Scholar]
- 2. Bertoy R. Megacolon in the cat. Vet Clin North Am Small Anim Pract 2002; 32: 901–915. [DOI] [PubMed] [Google Scholar]
- 3. Ueki T, Nagai K, Ooe N, et al. Case-controlled study on risk factors for the development of constipation in hospitalized patients. Yakugaku Zasshi 2011; 131: 469–476. [DOI] [PubMed] [Google Scholar]
- 4. Talley N. Risk factors for chronic constipation based on a general practice sample. Am J Gastroenterol 2003; 98: 1107–1111. [DOI] [PubMed] [Google Scholar]
- 5. Ho W, Spiegel BMR. The relationship between obesity and functional gastrointestinal disorders: causation, association, or neither? Gastroenterol Hepatol 2008; 4: 572–578. [PMC free article] [PubMed] [Google Scholar]
- 6. Quimby J. Update on medical management of clinical manifestations of chronic kidney disease. Vet Clin North Am Small Anim Pract 2016; 46: 1163–1181. [DOI] [PubMed] [Google Scholar]
- 7. Kidder A, Chew D. Treatment options for hyperphosphatemia in feline CKD: what’s out there? J Feline Med Surg 2009; 11: 913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Behm B, Stollman N. Postoperative ileus: etiologies and interventions. Clin Gastroenterol Hepatol 2003; 1: 71–80. [DOI] [PubMed] [Google Scholar]
- 9. Sanders K. Regulation of smooth muscle excitation and contraction. Neurogastroenterol Motil 2008; 20: 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Panchal S, Müller-Schwefe P, Wurzelmann J. Opioid-induced bowel dysfunction: prevalence, pathophysiology and burden. Int J Clin Pract 2007; 61: 1181–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trevail T, Gunn-Moore D, Carrera I, et al. Radiographic diameter of the colon in normal and constipated cats and in cats with megacolon. Vet Radiol Ultrasound 2011; 52: 516–520. [DOI] [PubMed] [Google Scholar]


