Abstract
Objectives
Intradermal allergy testing can be difficult to interpret in cats. Studies have shown that intradermal testing leads to elevations in blood cortisol, which may be an explanation for weak wheal reactions in cats. The primary objective of this study was to determine whether utilizing pre-appointment gabapentin will alter stress before and during intradermal testing, as determined by cortisol/glucose concentrations.
Methods
This was a randomized, single-blinded, crossover clinical trial of 16 privately owned healthy cats. Cats were scheduled two veterinary visits and randomly assigned to receive either gabapentin (25.0–30.5 mg/kg) or no treatment prior to the first visit and the opposite treatment prior to the second visit. Blood samples were obtained to measure cortisol/glucose concentrations at three time points: directly after physical examination; directly after sedation; and 10 mins after the second blood sample. A limited intradermal test was performed after the second blood sample. The primary author also recorded which visit they believed gabapentin was administered with low/high confidence. A non-blinded owner assessment survey documenting stress levels in their cats was also obtained.
Results
Mean cortisol concentrations were calculated to be 0.30 μg/dl lower in the gabapentin group but this reduction was not significant. Mean glucose concentrations were calculated to be 18 mg/dl higher in the gabapentin group. Gabapentin had no negative effect on intradermal histamine readings. The author was able to correctly identify when 14/16 cats received gabapentin. Non-blinded owners (n = 14/16) believed their cats were less stressed when gabapentin was administered.
Conclusions and relevance
Gabapentin did not significantly decrease cortisol/glucose concentrations. A sedative effect, rather than suppression of the pituitary–adrenocortical axis, may have led to the lower stress assessment. It is unlikely that pre-appointment gabapentin will alter intradermal testing in a majority of cats. This study supports recent clinical trials demonstrating that administration of gabapentin can lower veterinarian/owner assessment of stress in cats.
Keywords: Stress, cortisol, glucose, gabapentin, intradermal testing, allergy
Introduction
Countless cats are brought into veterinary clinics daily under extreme stress, which can begin at home and intensify at the hospital. Owners’ perceived stress level in their cats, due to transportation and veterinary examination, is thought to be one of the major deterrents that led to a 14% decrease in feline veterinary visits from 2001 to 2011. 1 The fractious nature of these stressed cats can make the veterinary visit stressful for the cats and their owners, and can be stressful and dangerous for veterinary staff and doctors. This can lead to difficulties in performing routine examinations, diagnostics and procedures.
The effects of stress on cats can be seen on routine diagnostic testing, including hyperglycemia and changes in the complete blood count.2–5 Glucose concentrations as high as 613 mg/dl have been reported as a result of stress. 2 Both an epinephrine- and cortisol-induced neutrophilia have been documented in cats due to stress/fear.3,4 Stress also leads to an increased concentration of serum cortisol in cats.2,6 Stressors leading to a rise in cortisol could include transportation, physical examination and procedures varying from a venipuncture to intradermal testing (IDT). A previous study demonstrated that handling and performing IDT activates pituitary–adrenocortical activity in cats, leading to an increase in cortisol. 6
IDT is considered by some the gold standard in feline allergy medicine for feline atopic dermatitis. 7 However, interpretation of skin testing can be difficult in the cat.7,8 A previous study concluded that increases in cortisol, corticotropin and alpha-melanocyte-stimulating hormone after handling and performing IDT may be a reasonable explanation for weak skin testing reactions in cats as cortisol suppresses synthesis, secretion or the action of inflammatory mediators. 6 If the stress-induced rise in serum cortisol can be decreased, it is possible that we may be able to improve IDT in cats. This may improve allergen selection and thus allergen-specific immunotherapy success.
Gabapentin is an anticonvulsant that has been used as an at-home sedation option to reduce fear, anxiety and stress in healthy cats.9,10 Single pre-appointment use of gabapentin has been shown to cause a significant reduction in stress-related behaviors during transportation and examination of cats. 10 It has also been shown to decrease fear response in community cats. 11 The mechanism of action is not fully understood, but it is believed to involve a decrease of calcium influx and release of excitatory neurotransmitters. 12 Peak plasma levels occur about 2 h post-dose. 12 Doses as high as 47.6 mg/kg have been well tolerated in cats to reduce stress. 11 The main side effect of a single pre-appointment dose of gabapentin is sedation. 9 It may also cause ataxia, hypersalivation and vomiting. 10 Utilizing a single pre-appointment dose of gabapentin may provide a better opportunity for stress-free hospital visits for cats, allow for a safer work environment, and improve IDT in cats by decreasing stress-induced cortisol release.
The goal of this study was to determine if a single pre-appointment dose of gabapentin will decrease stress in healthy cats as measured by cortisol and glucose concentrations. We hypothesize that stress indicator concentrations will decrease when gabapentin is given prior to the appointment.
Materials and methods
The study protocol was approved by the Animal Dermatology Clinic Research Committee. The procedure below was described to the owners. The owners were provided with and signed consent forms prior to each procedure. A copy of the owner consent form can be seen in Appendix 1 in the supplementary material
Animals
Sixteen privately owned healthy cats of at least 1 year of age were recruited. Any cat with a history of skin or gastrointestinal disease was excluded. Cats were also excluded if there was use of injectable steroids within 6 months, oral steroids within 2 months or antihistamines within 1 month. The above exclusion criteria were for the IDT portion of the study to limit false-negative reactions due to the effects of drugs on IDT. Any cat on current medications with the exception of flea, tick and heartworm preventative were also excluded. All cats were owned by a veterinarian, fourth-year veterinary student or a veterinary technician.
Procedure
This was a prospective, investigator-blinded crossover design using 16 cats. Cats were randomly divided into two groups (n = 8 each). Group 1 cats received gabapentin (Actavis Pharma) at the first visit while receiving nothing prior to the second visit. Group 2 cats received gabapentin at the second visit while receiving nothing prior to the first visit.
Cats were scheduled two visits 1–2 weeks apart. Gabapentin was given 2 h prior to their scheduled visit. The gabapentin capsule was opened and the gabapentin was administered in 1 tablespoon of wet food. The dose of gabapentin was assigned by weight and could range from 25.0 to 35.7 mg/kg (see Table 1). All cats were fasted from the previous night. Temperature, pulse and respiration were obtained by the owner the night before each visit to ensure no abnormalities were found prior to drug administration.
Table 1.
Gabapentin dosing chart
| Cat weight (kg) | Gabapentin dose (mg) |
|---|---|
| 1.4–2.0 | 50 |
| 2.1–3.0 | 75 |
| 3.1–4.0 | 100 |
| 4.1–5.0 | 125 |
| 5.1–6.0 | 150 |
| 6.1–7.0 | 175 |
On arrival at the clinic, the cat was brought directly to an examination room. The same doctor/technician team oversaw all visits. A physical examination was completed with the exception of obtaining a temperature. Immediately after the physical examination, a blood sample was collected. This was directly followed by intravenous catheterization of a cephalic vein. Each cat was then sedated with 0.02 mg/kg of dexmedetomidine (Dexdomitor; Zoetis) intravenously through the catheter. Once the cat was sedated, a second blood sample was obtained. After the second blood sampling, an IDT was performed (see Figure 1). A third blood sample was obtained 10 mins after the second blood sample (range 10–24 mins; median 12 mins). This time frame is based on the peak cortisol concentrations reported in cats undergoing IDT. 6 Approximately 2 ml of blood was collected from each cat at each blood sampling and placed in a red-top tube clot activator with gel (VACUTTE; Greiner). The red-top tube was centrifuged 10 mins after blood sampling. The tube was refrigerated (35–40ºF) until sample pick-up by the laboratory (ANTECH Diagnostics) the same day as the procedure. Serum cortisol concentrations were measured via the IMMULITE chemiluminescent immunoassay. The Beckman enzymatic method was used to measure serum glucose concentrations.
Figure 1.
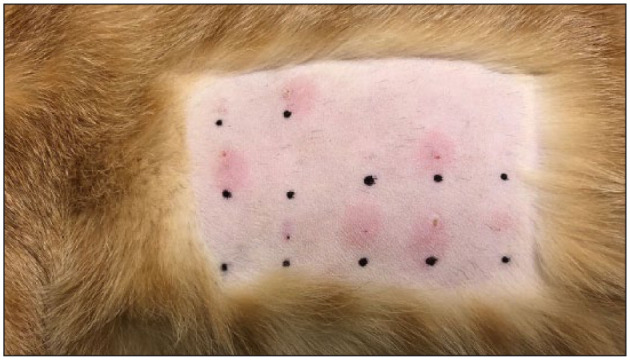
Example of the limited intradermal test (IDT). Saline (Greer Laboratories) and histamine 0.1 mg/ml (Histatrol; ALK) were used as the known negative and positive controls (top row). The cat was injected with 0.1 mg/ml of histamine, 0.05 mg/ml of histamine, saline, 8000 protein nitrogen units (PNU)/ml) of Schinus species (Pepper tree; Greer Laboratories) and 8000 PNU/ml of Cynodon dactylon (Bermuda grass; Greer Laboratories). Each of the five items were duplicated and injection sites randomized for each cat with the investigator blinded (middle and bottom rows). A total of 0.05 ml was delivered for each intradermal injection via a 27 G needle. The IDT was read 15 mins after the first injection. A subjective wheal score of 0–4 was given to each wheal based on erythema, size, turgor and steepness of the wheal. An objective wheal score was determined by measuring the longest and shortest diameter of the wheal
Upon completion of the IDT, dexmedetomidine was reversed with 0.2 mg/kg IM atipamezole (Antisedan; Zoetis). Mucous membrane color, capillary refill time, temperature and pulse were monitored every 5 mins throughout the time the cat was sedated. A detailed timing log was also kept that documented all the major steps of the visit from time out of the carrier to time reversed with atipamezole. The number of attempts for each blood sampling was also logged.
Assessments
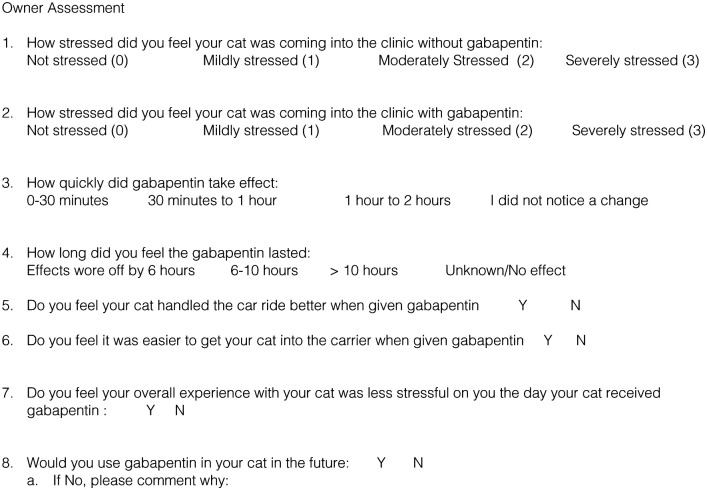
An ease-of-catheterization scale for each visit was obtained. This was scored 0–2 via a non-validated grading scale used for this study. Easily placed (0) consisted of no scruffing, no attempts to withdraw the forelimb and mild-to-absent vocalization. Placed with some objection (1) consisted of light scruffing, attempts to withdraw the limb or increased vocalization. Difficulty placed (2) consisted of the use of a muzzle or towel to place a catheter. At the end of the second visit for each cat, the investigator logged which visit he believed that cat received gabapentin with low or high confidence. The owners also filled out a questionnaire the day after the second visit to assess the overall experience of the visits (see Figure 2).
Figure 2.
Copy of the owner assessment form. Y = yes; N = no
Statistics
All analyses were performed using SAS version 9.3. A significance threshold of 0.05 was used.
Ease of catheter placement (score 0–2) was compared between visits when cats received gabapentin and visits when cats did not receive gabapentin using a generalized linear mixed model to account for both within cat correlation and ordinal data. Each generalized linear mixed model had a fixed factor of treatment and a random intercept for each cat. A multinomial distribution with a cumulative link function was assumed.
The effect of number of venipunctures and timing on cortisol and glucose concentrations were tested using linear mixed models to account for within cat correlation. The models had fixed factors of treatment and a fixed covariate of number of venipunctures or timing and a treatment by number of venipunctures or timing interaction effect (to allow for different slopes for each treatment) and a random intercept for each cat.
Serum cortisol and glucose concentrations were compared between gabapentin and non-gabapentin groups using a linear mixed models. The cortisol or glucose model had fixed factors for treatment, time and a treatment-by-time interaction and a random intercept for each cat. Simple effects of treatment at each time were constructed.
Results
Animals
Eighteen cats were enrolled into the study. Two cats were excluded owing to vomiting within 30–60 mins of receiving the gabapentin. The 16 remaining cats completed the study. There were 10 male neutered and six female spayed cats. Age ranged from 1 to 12 years (median 4 years; mean 4.13 years). Breeds included domestic shorthair (n = 11), domestic longhair (n = 4) and Turkish Angora (n = 1). Body weights ranged from 3.3 to 6.9 kg. The gabapentin dose ranged from 25.0 to 30.5 mg/kg (mean 27.9 mg/kg; median 28.1 mg/kg). Two owners reported excessive sedation after one visit. Both cats had received 30.5 mg/kg gabapentin at that visit.
Cortisol and glucose results
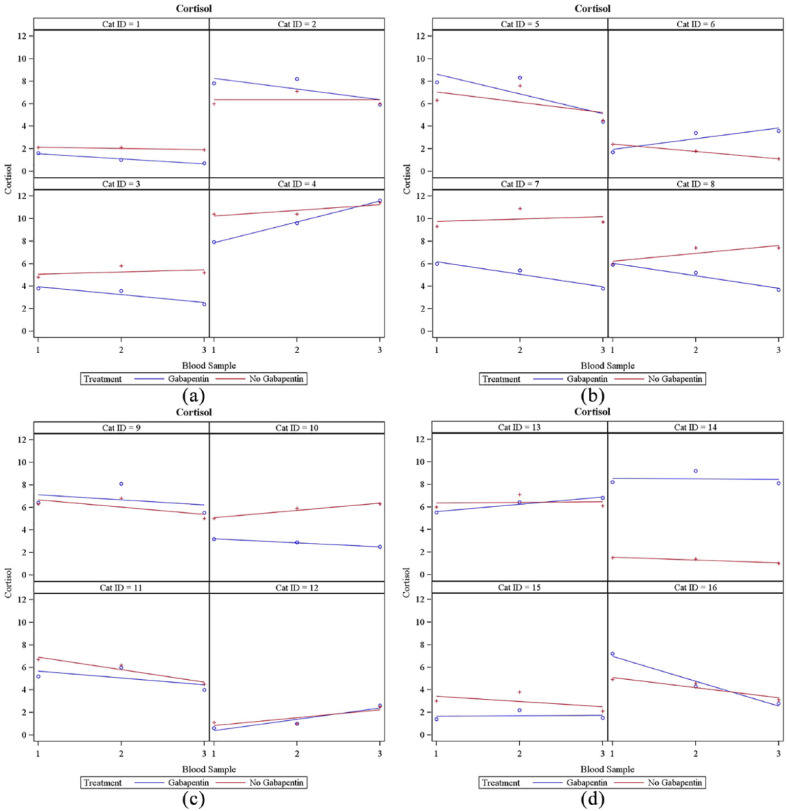
The mean serum cortisol concentration was calculated to be 0.30 μg/dl lower in the gabapentin group, but this value was not significant (95% confidence interval [CI] −0.97 to 0.37; P = 0.3729). Cortisol concentrations of both groups can be seen in Table 2. Results of each cat’s cortisol concentrations at each visit can be seen in Figure 3. Cats receiving gabapentin had 24/48 of the total cortisol readings above the reference interval (RI; 1–5 μg/dl). Cats that did not receive gabapentin had 25/48 of the total cortisol readings above the RI.
Table 2.
Mean serum cortisol/glucose concentrations when cats received gabapentin vs when cats did not receive gabapentin
| Mean cortisol concentration with gabapentin (µg/dl) | Mean cortisol concentration without gabapentin (µg/dl) | Mean glucose concentration with gabapentin (mg/dl) | Mean glucose concentration without gabapentin (mg/dl) | |
|---|---|---|---|---|
| Blood sample 1 | 5.02 | 5.11 | 122.13 | 104.25 |
| Blood sample 2 | 5.30 | 5.62 | 121.94 | 95.56 |
| Blood sample 3 | 4.37 | 4.86 | 170.06 | 159.31 |
| Combined blood sample (1, 2, 3) |
4.90 | 5.20 | 138.04 | 119.71 |
For the visits where cats received gabapentin, the mean cortisol concentration was 4.90 µg/dl (range 0.60–11.60). For the visits where cats did not receive gabapentin, mean cortisol concentration was 5.20 µg/dl (range 1.00–11.40). For the visits where cats received gabapentin, mean glucose concentration was 138.04 mg/dl (range 78.00–297.00 mg/dl). For the visits where cats did not receive gabapentin, mean glucose concentration was 119.71 mg/dl (range 44.00–258.00 mg/dl)
Figure 3.
Results of each cat’s serum cortisol concentrations at each visit. (a) Cats 1–4; (b) cats 5–8; (c) cats 9–12; (d) cats 13–16. y-axis = serum cortisol concentration (µg/dl); x-axis = blood sample. The blue ○ corresponds to cortisol concentrations at blood samples 1, 2 and 3 when gabapentin was administered. The red + corresponds to cortisol concentrations at blood samples 1, 2 and 3 when gabapentin was not administered. The line indicates linear regression
Increasing number of venipunctures did not cause an increase in serum cortisol concentration when cats received gabapentin. Increasing number of venipunctures significantly increased serum cortisol concentrations in the group that did not receive gabapentin by 0.16 μg/dl per additional venipuncture (95% CI 0.04–0.27; P = 0.0103). Increasing time out of carrier to first blood sample (P = 0.0141), catheter placement (P = 0.0004) and third blood sample (P = 0.0006) significantly increased serum cortisol concentrations in the group that did not receive gabapentin. However, increasing time out of carrier to first blood sample, catheter placement and third blood sample did not significantly increase the cortisol concentrations in the gabapentin group.
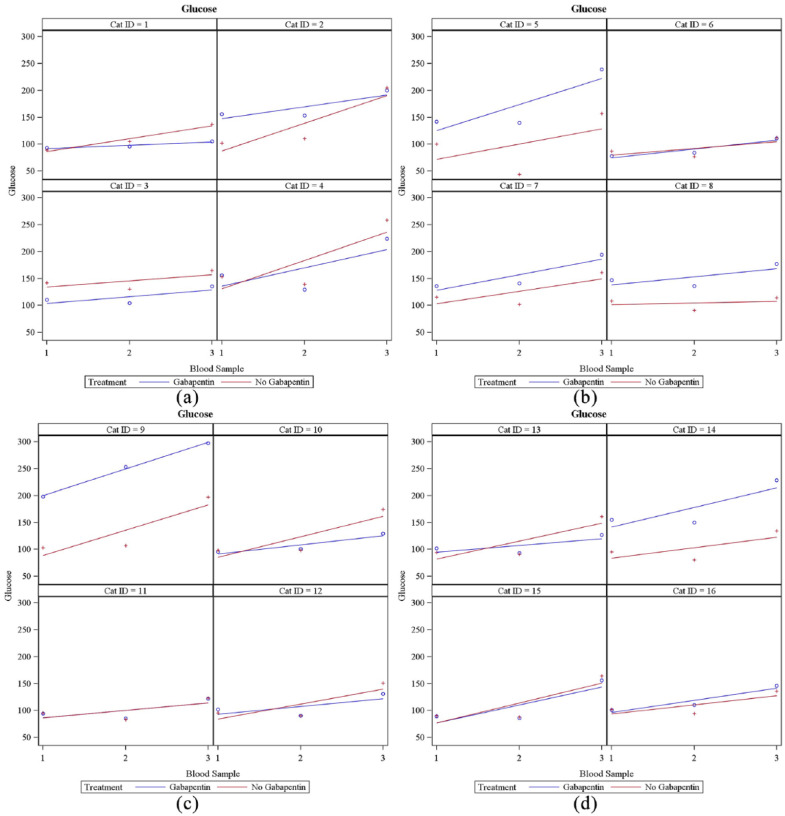
The mean serum glucose concentration was calculated to be 18 mg/dl higher in the gabapentin group (95% CI 7–29; P = 0.0014). Glucose concentrations can be seen in Table 2. Results of each cat’s glucose values at each visit can be seen in Figure 4. Increasing number of venipunctures did not significantly increase the serum glucose concentrations in either group. Increasing time out of carrier to first blood sample, catheter placement and third blood sample did not significantly increase glucose concentrations in either group.
Figure 4.
Results of each cat’s serum glucose concentrations at each visit. (a) Cats 1–4; (b) cats 5–8; (c) cats 9–12; (d) cats 13–16. y-axis = serum glucose concentration (mg/dl); x-axis = blood sample. The blue ○ corresponds to glucose concentrations at blood samples 1, 2 and 3 when gabapentin was administered. The red + corresponds to glucose concentrations at blood samples 1, 2 and 3 when gabapentin was not administered. The line indicates linear regression
Ease of catheterization and investigator assessment
For the visits where cats received gabapentin, 4/16 cats were easier to catheterize. For the visits where cats did not receive gabapentin, 2/16 cats were easier to catheterize. Only one cat’s ease of catheterization score differed by 2 points. This cat’s catheter was easily placed the day it received gabapentin and placed with difficulty the day it did not receive gabapentin. There were no significant differences in ease of catheter placement between groups (P = 0.3558).
The investigator was able to identify correctly when a cat received gabapentin in 14/16 cats. The investigator had high certainty a cat received gabapentin in 7/16 cases and correctly identified when all seven of these cats received gabapentin. The investigator had low certainty a cat received gabapentin in 9/16 cases and correctly identified when 7/9 of these cats received gabapentin.
Intradermal skin testing results
Statistics were not done on the IDT owing to results being similar. All blinded saline subjective readings were 0 in both groups. All blinded histamine readings of both concentrations (0.05 and 0.1 mg/ml) were subjectively graded as 4, except for 1 histamine (0.1 mg/ml) was graded a 3 in the gabapentin group. The duplicate on this skin test was graded a 4.
Owner questionnaire
Non-blinded owner assessment of stress levels in their cats were lower in 14/16 cats on the visit they received gabapentin. Other findings included that 13/16 owners thought it was easier to get their cat into the carrier the day it received gabapentin; 15/16 owners believed their cats were less stressed in the car the day it received gabapentin; 15/16 owners themselves were less stressed on the day their cat received gabapentin; 16/16 owners stated they would use gabapentin in the future. However, 2/16 owners stated they would like to use a lower dose of gabapentin. Owner questionnaire results can be found in Appendix 2 in the supplementary material.
Discussion
To our knowledge, this is the first study to report the effects of a single pre-appointment dose of gabapentin on cortisol and glucose concentrations in cats. Although cortisol concentrations were lower on visits when cats received gabapentin, this reduction was not statistically significant. The lack of a significant decrease in serum cortisol concentrations suggest that pretreatment with gabapentin is unlikely to change IDT results blunted by endogenously released cortisol. Furthermore, in 24/48 venipuncture episodes, serum cortisol concentrations were elevated in cats receiving gabapentin. These findings indicate that these procedures were still stressful in many of these cats, despite the use of gabapentin and that a stress-induced rise in cortisol could lead to difficulty interpreting IDT. However, Table 3 shows that cats receiving gabapentin were more likely to have a 25% decrease in cortisol concentration. These results are more promising and suggest that gabapentin could improve IDT results in selected cats.
Table 3.
Comparison of each cat’s individual blood samples
| Gabapentin group | Non-gabapentin group | |
|---|---|---|
| Total number of blood samples | 48 (16) | 48 (16) |
| Number of blood samples with ⩾25% decrease in cortisol concentration | 16/48 (7) | 8/48 (4) |
| Number of blood samples with ⩾50% decrease in cortisol concentration | 9/48 (6) | 4/48 (2) |
For each cat, the first, second and third blood samples when gabapentin was administered were individually compared with the first, second and third blood samples when gabapentin was not administered, respectively, for a total of 48 blood sample comparisons. For example, cat 1, blood sample 1, with pre-appointment gabapentin was compared with cat 1, blood sample 1, without pre-appointment gabapentin. In parenthesis, the number of cats that met the criteria is noted
Statistics were not performed on the IDT results owing to the similarity in objective/subjective findings between both groups. It does not appear that a single pre-appointment dose of gabapentin will negatively affect the positive control of histamine in healthy cats. However, future studies on the effects of gabapentin on histamine in allergic cats still need to be performed.
Pre-appointment gabapentin has been used for several years to decrease stress responses in cats with reported success. 9 Recently, other controlled studies have reported that owners and veterinarians observed a significant reduction in stress-related behaviors in cats after a single dose of pre-appointment gabapentin.10,11 The results of this study are consistent with the findings of the previous studies. The primary author of this study was able to correctly identify the visit in which cats received gabapentin in 14/16 cats. Also, owners in this study perceived that their cats were less stressed with gabapentin. However, during this study the owners were not blinded, which could have led to biased results. A placebo could have been used, but as the primary purpose was to evaluate serum cortisol and glucose concentrations as indicators of stress, a placebo was not necessary.
Thorough documentation of the number of venipunctures for each blood sample, as well as timing of the entire procedure, were performed. On the visits cats received gabapentin, these variables did not significantly alter the cortisol concentrations. However, increasing the number of venipunctures/time out of the carrier significantly increased the cortisol concentrations in the cats that did not receive gabapentin. This could indicate that gabapentin allowed for increased handling without an elevation in stress. If the cats were stressed longer, it is possible a significant difference in cortisol concentrations could have occurred.
Glucose concentrations were significantly higher in cats that received gabapentin. However, the clinical importance of the elevation is believed to be minimal. One cat that did not receive gabapentin had a glucose level of 44 mg/dl, which led to concern of a possible laboratory error. Although this may have altered the results slightly, the conclusion that gabapentin did not lead to a decrease in glucose remained the same. With the glucose readings it is important to note that for all 32 visits, the third glucose was the highest concentration for all cats. The authors conclude that this finding indicates that the overall procedure was stressful to cats in both groups.
Two cats were excluded from the study owing to vomiting shortly after the administration of gabapentin. These cats received 25.4 mg/kg and 27.3 mg/kg of gabapentin, respectively. Two cats were also reported to have excessive sedation the visit for which they received gabapentin. Both of these cats received 30.5 mg/kg of gabapentin, which was the highest dose received during this study. Sedation is a side effect of a single pre-appointment dose of gabapentin and the sedative effects can increase with higher doses. 10 However, reports of doses higher than 30.5 mg/kg not causing excessive sedation have been documented. 11 In this study cats also received 0.02 mg/kg dexmedetomidine, which can also cause reversible sedation. 13 It is believed that gabapentin contributed to the excessive sedation as both cats had excessive sedation only on the visits during which they received gabapentin. Pharmacokinetic data suggest a wide range of individual variation in peak plasma concentrations with oral gabapentin. 14 The authors have recommended lowering the dose of gabapentin in these two cats when used in the future, and that dosing of gabapentin should be tailored to the individual cat.
Conclusions
Pre-appointment gabapentin did not significantly decrease serum cortisol and glucose concentrations in this group of cats. The decrease in stress-induced outward response noted by owners/veterinarians, in this study and previous studies, is likely due to a sedative effect. However, gabapentin does not appear to suppress the pituitary–adrenocortical axis. It is unlikely that pre-appointment gabapentin would improve interpretation of IDT in a majority of cats based on these results. Higher-powered studies are recommended in the future. Pre-appointment gabapentin appears to be a safe and effective option to decrease stress-induced outward responses in cats.
Supplemental Material
Owner consent form
Owner assessment survey results
Acknowledgments
We thank Deborah Keys for her assistance with statistical analysis, and Whitney Dickerson for all her organizational/technical assistance.
Footnotes
Accepted: 21 January 2019
Supplementary material: The following files are available online:
Appendix 1: Owner consent form.
Appendix 2: Owner assessment survey results.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors would like to thank the American College of Veterinary Dermatology for their support in funding the study.
ORCID iD: Christopher P Hudec  https://orcid.org/0000-0002-0324-0793
https://orcid.org/0000-0002-0324-0793
References
- 1. Volk JO, Thomas JG, Colleran EJ, et al. Executive summary of phase 3 of the Bayer veterinary care usage study. J Am Vet Med Assoc 2014; 244: 799–802. [DOI] [PubMed] [Google Scholar]
- 2. Rand JS, Kinnaird E, Baglioni A, et al. Acute stress hyperglycemia in cats is associated with struggling and increased concentrations of lactate and norepinephrine. J Vet Intern Med 2002; 16: 123–132. [DOI] [PubMed] [Google Scholar]
- 3. DeClue AE, Spann DR. Leukopenia, leukocytosis. In: Ettinger SJ, Feldman EC, Cote E. (eds). Textbook of veterinary internal medicine expert consult. 8th ed. St Louis, MO: Elsevier Mosby, 2017, pp 235–237. [Google Scholar]
- 4. Irizarry-Rovira AR. Neutrophilia and neutropenia. In: Lappin MR. (ed). Feline internal medicine secrets. Philadelphia, PA: Hanley & Belfus, 2001, pp 356–362. [Google Scholar]
- 5. Nibblett BM, Ketzis JK, Grigg EK. Comparison of stress exhibited by cats examined in the clinic versus a home setting. Appl Anim Behav Sci 2015; 173: 68–75. [Google Scholar]
- 6. Willemse T, Vroom MW, Mol JA, et al. Changes in plasma cortisol, corticotropin, and alpha melanocyte-stimulating hormone concentrations in cats before and after physical restraint and intradermal testing. Am J Vet Res 1993; 54: 69–72. [PubMed] [Google Scholar]
- 7. Prost C. Feline atopic dermatitis: clinical signs and diagnosis. Eur J Comp Anim Pract 2009; 19: 223–229. [Google Scholar]
- 8. Miller WH, Griffin CE, Campbell KL. Hypersensitivity disorders. In: Muller and Kirk’s small animal dermatology. St Louis, MO: Elsevier Mosby, 2013, pp 363–431. [Google Scholar]
- 9. Shafford H. Serenity now: practical sedation option for cats. vetanesthesiaspecialists.com/wp-content/uploads/2015/11/SerenityNowSedationOptions_Feline_ABVP2015_HeidiLShafford.pdf (2015, accessed May 17, 2017).
- 10. van Haaften KA, Forsythe LRE, Stelow EA, et al. Effects of a single preappointment dose of gabapentin on signs of stress in cats during transportation and veterinary examination. J Am Vet Med Assoc 2017; 251: 1175–1181. [DOI] [PubMed] [Google Scholar]
- 11. Pankratz KE, Ferris KK, Griffith EH, et al. Use of single-dose oral gabapentin to attenuate fear responses in cage-trap confined community cats: a double blind, placebo-controlled field trial. J Feline Med Surg 2018; 20: 535–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Plumb DC. Gabapentin. In: Plumb’s veterinary drug handbook. 8th ed. Ames, IA: PharmaVet, 2015, pp 478–480. [Google Scholar]
- 13. Murrell JC, Hellebrekers LJ. Medetomidine and dexmedetomidine: a review of cardiovascular effects and antino-ciceptive properties in the dog. Vet Anaesth Analg 2005; 32: 117–127. [DOI] [PubMed] [Google Scholar]
- 14. Siao KT, Pypendop BH, Ilkiw JE. Pharmacokinetics of gabapentin in cats. Am J Vet Res 2010; 71: 817–821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Owner consent form
Owner assessment survey results