Abstract
Cytolethal distending toxin (CDT) from the diarrheagenic bacterium Campylobacter jejuni was shown to cause a rapid and specific cell cycle arrest in HeLa and Caco-2 cells. Within 24 h of treatment, CDT caused HeLa cells to arrest with a 4N DNA content, indicative of cells in G2 or early M phase. Immunofluorescence studies indicated that the arrested cells had not entered M phase, since no evidence of tubulin reorganization or chromatin condensation was visible. CDT treatment was also shown to cause HeLa cells to accumulate the inactive, tyrosine-phosphorylated form of CDC2. These results indicated that CDT treatment results in a failure to activate CDC2, which leads to cell cycle arrest in G2. This mechanism of action is novel for a bacterial toxin and provides a model for the generation of diarrheal disease by C. jejuni and other diarrheagenic bacteria that produce CDT.
Campylobacter jejuni is the most common bacterial cause of diarrheal disease in humans in the United States (33). In 1988 Johnson and Lior (13) reported that C. jejuni makes an unusual toxin, which they named cytolethal distending toxin (CDT). CDT causes certain cultured cells, including HeLa cells, to become slowly distended and then die (11–13). In addition to being produced by C. jejuni strains, CDT is made by other diarrheagenic bacterial species, including closely related Campylobacter spp., such as C. coli and C. fetus (13, 29), as well as by some Escherichia coli (12) and Shigella spp. (11) isolates. The CDT produced by Shigella dysenteriae was recently shown to cause diarrheal symptoms in a suckling mouse model (23). Recently, CDT was reported to be made by the nondiarrheagenic pathogen Haemophilus ducreyi (6). The specific contribution of CDT to H. ducreyi disease has not yet been determined.
The cdt genes have been cloned and sequenced from E. coli (25, 28, 31), S. dysenteriae (24), H. ducreyi (6), and C. jejuni (29). CDT production has been shown to depend upon the expression of three adjacent genes, cdtA, cdtB, and cdtC, and expression of the cdt genes in nontoxic E. coli strains indicates that the three genes are sufficient for CDT production (23, 28, 29). The biochemical function of the individual Cdt proteins is not known. The predicted amino acid sequences of CdtA, CdtB, and CdtC are not homologous to other, non-CDT sequences, indicating that CDT is novel.
Aragon et al. (2) recently reported that E. coli CDT blocked proliferation of Chinese hamster ovary cells, and Pérès et al. (25) recently reported on DNA content experiments that showed that E. coli CDT causes HeLa cells to become blocked in G2/M. We report here on experiments which indicate that C. jejuni CDT causes HeLa cells to accumulate the inactive, phosphorylated form of CDC2 and thus to become rapidly and irreversibly blocked in the G2 phase of the cell cycle.
(This research was presented in part at the 97th General Meeting of the American Society for Microbiology, Miami Beach, Fla., May 1997 [27].)
MATERIALS AND METHODS
Bacterial strains, media, and cell culture.
C. jejuni 81-176 was isolated from a human with diarrheal disease and has been described previously (4, 15). C. jejuni was routinely grown on brucella agar in a microaerobic atmosphere consisting of 5% O2, 10% CO2, and 85% N2 at 42°C for 24 h. When necessary, kanamycin or chloramphenicol was added to the medium to a final concentration of 30 or 20 μg per ml, respectively. E. coli DH5αMCR (Gibco/BRL, Gaithersburg, Md.) was grown in L medium (22) at 37°C. When necessary, L medium was supplemented with ampicillin to a final concentration of 50 μg per ml. HeLa cells were grown as described previously (29) in Eagle’s minimal essential medium (EMEM) supplemented with 10% fetal bovine serum. Caco-2 cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum, 25 mM HEPES, and 2 mM glutamine.
Isolation of a C. jejuni 81-176 cdt mutant.
A derivative of C. jejuni 81-176 that has the DNA between nucleotide 56 of cdtA and nucleotide 155 of cdtC deleted (29) was constructed. A kanamycin resistance gene capable of expression in C. jejuni was inserted into the site of the deletion. Actual construction of the mutant was carried out in a manner analogous to that described by Miller et al. (21) for construction of a C. jejuni flhA mutant. Briefly, a plasmid containing all three C. jejuni cdt genes was cut with NsiI and BclI, generating the desired deletion, into which was inserted the kanamycin resistance gene. After further insertion of an oriT region (16) into this plasmid at an ScaI site which is outside of the cdt genes, the plasmid was mobilized into C. jejuni via conjugation (21). These kanamycin-resistant conjugants are isolates in which recombination has occurred in the chromosome, since the plasmid cannot replicate in C. jejuni. A putative mutant was shown in Southern hybridization and PCR experiments to be the result of a double-cross-over recombination event so that it contained the version of the cdt locus with the expected deletion and no wild-type copies of the cdt genes. The inability of the mutant to make active CDT was verified in a HeLa cell assay. Western blots of extracts from the mutant confirmed that no Cdt proteins were made by this strain (26).
In addition, we were able to complement the mutation with a specially constructed clone which carried only the C. jejuni cdt genes. A 2.4-kb region of DNA encompassing the cdt genes, 206 bp of upstream DNA, and 106 bp of downstream DNA was amplified in a PCR, and the resulting product was inserted into pUOA18 (36). Plasmid pUOA18 carries a chloramphenicol resistance gene which is expressed in C. jejuni and E. coli, an oriT region, and genes for replication functions for both C. jejuni and E. coli hosts. Subsequent to verification of production of active CDT, the construct was mobilized into the C. jejuni 81-176 cdt mutant in a manner analogous to that described above for construction of the mutant, except that both chloramphenicol and kanamycin were present in the selection medium. Colonies that appeared on the selection medium were subcultured once and tested for CDT production; all produced active CDT in the HeLa cell assay. Plasmid DNAs of the expected size were isolated from all of these isolates. In summary, these results confirm that the inability to make CDT in the C. jejuni 81-176 cdt mutant was the result of the mutation in the cdt genes and was not the result of polar effects on upstream or downstream coding regions.
Construction of a recombinant-CDT-overproducing strain.
The cdt genes from C. jejuni 81-176 (29) were cloned into the expression vector pTrc99A (1) to make the construct pTrc18CDT. The entire cdt coding region, as well as 696 bp of upstream DNA and 654 bp of downstream DNA, was included in this construct. The downstream DNA includes 181 noncoding base pairs immediately following cdtC, as well as the first 473 nucleotides of the putative lctP (lactate permease) gene of C. jejuni (29). Immediately upstream of cdtA are 102 noncoding base pairs. Upstream of this noncoding region, and on the strand opposite from that of the cdt genes, is a putative open reading frame (ORF) of 230 nucleotides with no homology to anything in GenBank, followed immediately by a second ORF which appears to encode the large subunit of the C. jejuni cytochrome d oxidase (26). Expression driven by the pTrc tac promoter does not transcribe these upstream genes, since they are on the opposite strand. Plasmid pTrc18CDT, when it was transformed into E. coli DH5αMCR, produced active CDT. (E. coli DH5αMCR does not contain cdt genes and thus does not produce CDT.) Isopropyl-β-d-thiogalactopyranoside (IPTG) increased CDT expression 40-fold over the level seen without IPTG, indicating that the pTrc tac promoter was successfully driving expression of the cdt genes in this construct. Coupled transcription-translation experiments indicated that the C. jejuni DNA in pTrc18CDT produced apparently normal-sized CdtA, CdtB, and CdtC proteins; the only other proteins seen in this system were vector encoded (26). It is clear from both the mutant complementation described above and the construction of additional clones (26) that the cdt genes are sufficient to produce active CDT; the upstream and downstream ORFs do not appear to express products which are involved in CDT production.
CDT preparations.
CDT was prepared from C. jejuni 81-176 cells grown overnight on brucella agar. The cells were harvested into 10 mM phosphate buffer, pH 7.0, and subsequently lysed by two sequential passages through a three-eighths-in.-diameter French pressure cell (1,000 lb/in2). The lysate was centrifuged at 3,600 × g to remove unbroken cells, and the supernatant fraction was centrifuged at 267,000 × g at 4°C for 60 min to pellet membrane fragments. The membranes were washed twice in 10 mM phosphate buffer, pH 7.0, by incubation of suspended pellets at room temperature with gentle agitation for 30 min. After each wash, the material was recentrifuged at 267,000 × g at 20°C for 60 min. The extracts applied in all assays were aliquots of the supernatant fraction from the first phosphate buffer wash. This partially purified preparation typically contained 1 to 2.5 U of CDT activity per μl. A unit of CDT activity is the reciprocal of the highest dilution of a preparation that causes 50 to 75% of the HeLa cells in an assay well to become distended within 48 h (29). The extract prepared from the cdt mutant derivative of C. jejuni 81-176 was prepared in an analogous fashion, except that kanamycin was present in the agar medium. This cdt mutant extract contained no detectable CDT activity in the HeLa cell assay or in DNA content analysis. Twenty-five micrograms of both C. jejuni extracts were added to 35-mm-diameter culture dishes for DNA content experiments.
Recombinant CDT from E. coli (pTrc18CDT) and the vector control extract from E. coli (pTrc99A) were prepared as described for the C. jejuni extracts, except that the cells were grown differently. The E. coli strains were grown overnight in L broth supplemented with ampicillin. Induction of expression of the cdt genes was accomplished by addition of IPTG to a final concentration of 1 mM. The extract from E. coli (pTrc18CDT) typically contained 5 to 10 U of recombinant CDT activity per μl. The vector control extract, which was prepared in a manner identical to that for the recombinant CDT extract, contained no detectable CDT activity. Identical amounts of protein (40 μg per 35-mm-diameter dish; 6 μg per well of a chamber slide) from the two extracts were used in the experiments described in this work. The vector control extract and the recombinant CDT extract can be considered the same except for the fact that the vector control extract contains no Cdt proteins.
DNA content analysis.
For DNA content analysis, either 3.0 ml of 1.0 × 105 HeLa cells per ml of medium or 3.0 ml of 1.9 × 105 Caco-2 cells per ml of medium were seeded into 35-mm-diameter petri dishes. After 18 h of incubation, CDT-containing preparations or appropriate control materials were added to the dishes. Forty units of CDT activity from C. jejuni 81-176 was added per 35-mm-diameter dish; 100 U of recombinant CDT activity from the overexpressing E. coli strain was added per 35-mm-diameter dish. Control extracts, isolated from either the C. jejuni cdt mutant derivative or the vector control strain, were added in microgram amounts equal to those of their respective CDT-containing extracts. The dishes were then incubated for an additional 1, 2, or 3 days, after which the HeLa cells were harvested and stained with propidium iodide and their DNA contents were determined by flow cytometry (34). The means and standard deviations for values from three separate experiments were calculated for all DNA content experiments.
To determine whether washing CDT from the HeLa cells would reverse the effect of CDT, either 3 ml of medium or 40 μg (100 U in 3 ml of medium) of the recombinant CDT extract was added to nonconfluent, 18-h HeLa cells at time zero. At 20, 40, and 60 min after addition of CDT, the HeLa cell supernatant was removed and the HeLa cells were then washed four times with fresh medium. Twenty-four hours later, the HeLa cells were harvested and stained with propidium iodide and their DNA contents were analyzed by flow cytometry.
DNA content analysis experiments examining the effect of caffeine on CDT-treated HeLa cells were carried out based on the protocols of Bartz et al. (3) and Lock et al. (18). Etoposide was prepared (19) and used at a final concentration of 50 μM (18); caffeine was used at final concentrations of 1, 2, and 4 mM. Nonconfluent HeLa cells were treated with CDT and incubated for 18 h, after which time caffeine was added and incubation continued for either 6 or 12 h. Etoposide was added to nonconfluent HeLa cells for 1 h, after which the reagent was removed and the cells were washed twice with fresh medium and then reincubated for 16 h. Caffeine was then added, and incubation continued for 6 or 12 h.
Immunofluorescence.
HeLa cells, in 400 μl of EMEM, were seeded at 1.6 × 104 cells per ml, into the chambers of eight-well chamber slides and incubated for 18 h at 37°C. The HeLa cells were then treated with either fresh medium (400 μl), vector control extracts (6 μg in 400 μl), or the recombinant CDT extract (6 μg containing 15 U of activity in 400 μl). One, 2, 3, or 4 days after additions, the cells were washed with TBS-azide (10 mM Tris [pH 7.4], 200 mM NaCl, 1% bovine serum albumin, 0.02% sodium azide), permeabilized with PEM–Triton X-100 (100 mM PIPES [pH 6.8], 1 mM EGTA, 1 mM MgCl2, 0.1% Triton X-100) for 1 min at 25°C, and then rapidly fixed in 3% paraformaldehyde in PEM for 3 min at 25°C. Cells were then postfixed in −20°C methanol for 2 min and washed twice with TBS-azide (1 min, 25°C). The cells were stained with a 1:100 dilution of the monoclonal anti-α-tubulin antibody DM1A (Sigma) and then with a 1:500 dilution of Cy3-labeled anti-mouse immunoglobulin G (Jackson ImmunoResearch, West Grove, Pa.). Antibodies were diluted in TBS-azide–1% bovine serum albumin. Antibody incubations were for 30 min at 37°C and were followed by three washes with TBS-azide. The cells were then stained with 4′,6-diamidino-2-phenylindole (DAPI; 5 min in 0.7 μg of DAPI per ml in TBS), washed twice with TBS-azide, air-dried, and mounted in mounting medium (TBS, 90% glycerol, 0.1% p-phenylenediamine).
Western blot analysis of HeLa cell proteins.
HeLa cells were grown for 18 h in 35-mm-diameter dishes and then treated with either fresh EMEM, 40 μg of the vector control extract, or 40 μg of recombinant CDT extract. The cells were reincubated for 1, 2, 3, or 4 days, after which they were harvested directly into sodium dodecyl sulfate-lysis buffer (17). Fifty micrograms of total HeLa cell proteins from each sample was separated on a sodium dodecyl sulfate–14% polyacrylamide gel (20), and the gel was subsequently blotted onto Immobilon P (Millipore, Bedford, Mass.). Blots were reacted with antibody and developed with ProtoBlot II AP (Promega, Madison, Wis.) according to the manufacturer’s instructions. Anti-CDC2 antibody and anti-CDC2 phosphotyrosine antibody were obtained from New England Biolabs (Beverly, Mass.).
RESULTS
CDT action on HeLa cells.
When HeLa cells are treated with CDT preparations, the cells do not undergo any dramatic changes in gross morphology within the first 24 h. However, by 48 h after toxin addition, the cells are noticeably enlarged, although they otherwise appear healthy. Careful observation of the toxin-treated cells suggested that very few, if any, cells appeared to be dividing 24 h after toxin treatment.
We therefore tested the ability of CDT to block cell cycle progression by analyzing HeLa cell DNA content by flow cytometry (34). Nonconfluent HeLa cells were treated either with medium, with a CDT-containing extract from C. jejuni 81-176, or with an extract prepared from an isogenic cdt mutant derivative of C. jejuni 81-176 (Table 1). HeLa cell DNA content was analyzed at 24-h intervals after the additions. The CDT-containing preparation from wild-type C. jejuni caused 64% of the HeLa cells to be in G2/M within 24 h, compared to only 10% of the HeLa cells treated with an analogous preparation from the isogenic cdt mutant C. jejuni strain. Forty-eight hours after addition of CDT, 97% of the HeLa cells were in G2/M, while only 9% of the mutant extract-treated HeLa cells were in G2/M. DNA content analysis of HeLa cells incubated for up to 3 days continued to give the same result (Table 1), although by the third day of incubation a small percentage of the HeLa cells were rounded and appeared to be disintegrating.
TABLE 1.
DNA content analysis of HeLa cells treated with CDT
| Time after addition (h) | Additiona | %b of HeLa cells in cell cycle phase:
|
||
|---|---|---|---|---|
| G0/G1 | S | G2/M | ||
| 24 | Medium | 60.3 ± 2.1 | 28.6 ± 8.6 | 11.2 ± 6.7 |
| C. jejuni 81-176 | 8.3 ± 1.4 | 27.8 ± 19.2 | 63.9 ± 18.1 | |
| C. jejuni cdt mutant | 62.0 ± 4.3 | 27.7 ± 6.8 | 10.3 ± 5.5 | |
| 48 | Medium | 68.5 ± 3.2 | 24.8 ± 3.5 | 6.8 ± 0.4 |
| C. jejuni 81-176 | 2.2 ± 2.9 | 0.5 ± 0.8 | 97.3 ± 2.4 | |
| C. jejuni cdt mutant | 64.3 ± 8.2 | 27.0 ± 2.6 | 8.6 ± 5.7 | |
| 72 | Medium | 67.9 ± 4.7 | 26.0 ± 4.0 | 6.0 ± 1.1 |
| C. jejuni 81-176 | 1.1 ± 1.8 | 0.0 ± 0.0 | 98.9 ± 1.8 | |
| C. jejuni cdt mutant | 62.2 ± 7.9 | 24.7 ± 2.2 | 13.2 ± 6.5 | |
Additions consisted of either EMEM (medium), a CDT-containing extract from wild-type C. jejuni 81-176, or a homologous extract from the cdt mutant derivative of 81-176.
Values are means ± standard deviations.
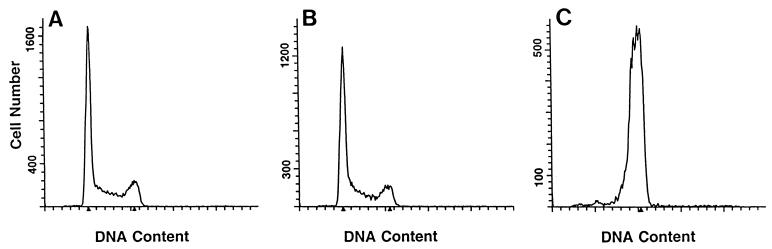
A similar experiment was done with extracts prepared from E. coli DH5αMCR containing either the expression vector pTrc99A or a derivative of pTrc99A containing the C. jejuni cdtABC genes. HeLa cells treated with medium alone or with the vector control extract [from E. coli (pTrc99A)] had nearly identical DNA content profiles (Fig. 1A and B, respectively). However, by 24 h after addition of recombinant CDT, 95% of the HeLa cells were in G2/M (Fig. 1C). Results for 48 and 72 h were essentially identical; about 98% of the HeLa cells treated with CDT remained in G2/M. Since it was easier to grow these E. coli strains than the C. jejuni strains and since the recombinant-CDT preparation led to a greater percentage of HeLa cells in G2/M within 24 h than the C. jejuni CDT preparation, we chose to do the following experiments using the recombinant preparation.
FIG. 1.
DNA contents of HeLa cells treated with CDT. (A) HeLa cells treated with EMEM; (B) HeLa cells treated with vector control extract; (C) HeLa cells treated with recombinant extract containing 100 U of CDT activity. Twenty-four hours after addition of control medium or extracts, the HeLa cells were harvested and their DNA contents were determined. Arrowheads indicate 2N and 4N DNA content.
We performed experiments which indicated that washing the HeLa cells, even as soon as 20 min after addition of CDT, did not substantially reverse the effect of CDT (Table 2). In summary, the results from all of these DNA content experiments indicated that CDT caused HeLa cells to become blocked in their cell cycle so that they were unable to progress past G2 or early M phase.
TABLE 2.
Effects of washing on CDT action
| Treatmenta | %b of HeLa cells in cell cycle phase:
|
||
|---|---|---|---|
| G0/G1 | S | G2/M | |
| Not washed | 0.1 ± 0.1 | 7.4 ± 6.5 | 92.5 ± 6.5 |
| Washed at 60 min | 2.6 ± 1.2 | 7.5 ± 7.1 | 89.9 ± 8.0 |
| Washed at 40 min | 5.8 ± 1.9 | 10.0 ± 4.8 | 84.2 ± 6.4 |
| Washed at 20 min | 12.4 ± 2.7 | 14.0 ± 4.2 | 73.6 ± 6.9 |
| Medium control | 57.2 ± 5.4 | 26.8 ± 4.8 | 16.0 ± 1.8 |
HeLa cells were treated with either recombinant CDT or EMEM (medium control). Times reflect times after addition of CDT.
Values are means ± standard deviations.
CDT-mediated arrest is in G2.
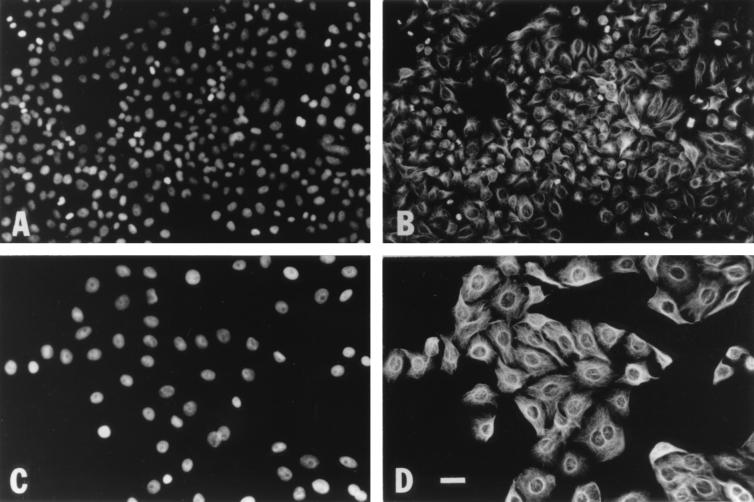
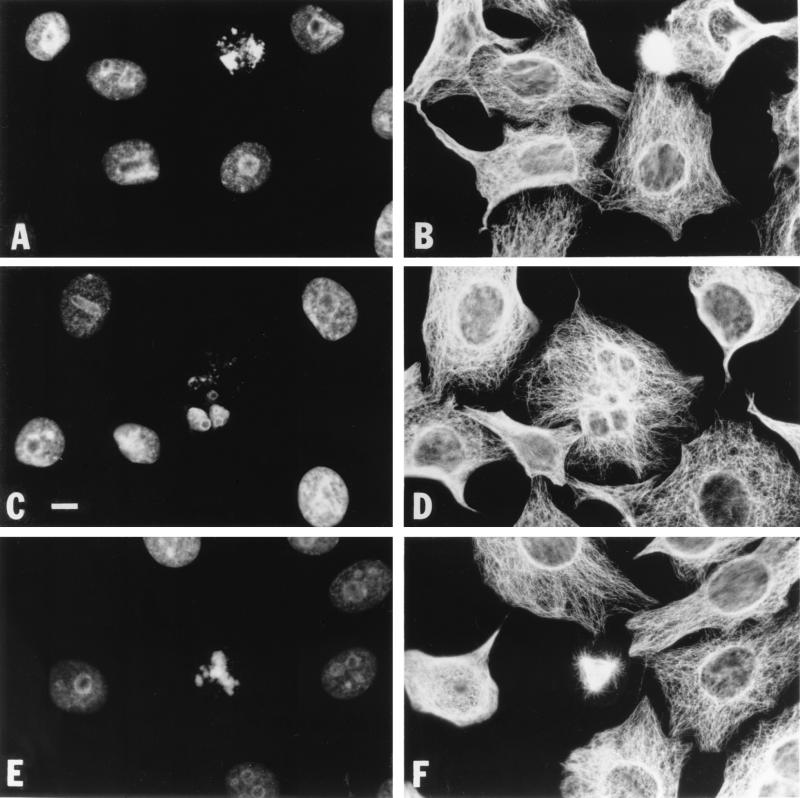
In order to determine whether CDT arrests HeLa cells in G2 or M phase, we examined the microtubule and chromatin organizations of CDT-treated and control cells. HeLa cells were treated with medium, the vector control extract, or the recombinant-CDT extract and analyzed 1, 2, 3, or 4 days after treatment. Medium- and vector control extract-treated cells continued to divide, showing 5 to 10% mitotic cells with condensed chromatin and mitotic spindles (Fig. 2A and B). In contrast, CDT-treated cells stopped dividing and, by day two, were comprised entirely of cells with typical interphase microtubule arrays and decondensed chromatin (Fig. 2C and D). The day 2, CDT-treated cells had enlarged to approximately twice the size of control cells (Fig. 2, compare panels A and B to panels C and D). CDT-treated cells continued to enlarge until they ultimately disintegrated. The tubulin and chromatin staining patterns of cells on days 3 and 4 revealed several types of aberrant morphologies that may represent intermediates in the cell disintegration process (Fig. 3). Cells of one class were rounded, generally had decondensed chromatin, and appeared to have tubulin bundles predominantly adjacent to their nuclei, creating a ring appearance; 18% of the cells on both days 3 and 4 had this ring morphology (data not shown). Other rounded cells contained an irregular distribution of chromatin and had disorganized microtubules that appeared as slightly fuzzy balls (Fig. 3A and B). Few of these cells were apparent on day 3, but by day 4 they represented 28% of the cells (89 of 315 cells). Cells of a less frequently occurring (2%) class were flat and contained multiple fragmented nuclei on day 3 (Fig. 3C); by day 4, 7% of the cells had this appearance. These cells contained interphase microtubule arrays, and each nuclear fragment was surrounded by cytoplasmic microtubules (Fig. 3D). One percent of the cells on day 3 contained distorted spindle structures resembling mitotic asters and irregular chromatin (Fig. 3E and F), potentially representing cells that have tried to overcome their G2 arrest with an abnormal mitotic event. By day 4, there were even fewer of these abnormal mitotic structures visible, suggesting that either the chromatin had decondensed or these cells were now completely disintegrated. Overall, these results clearly show that CDT treatment blocked cells in G2 phase, not in M phase. Furthermore, these data illustrate the nuclear disintegration that appears to be associated with HeLa cell death following CDT treatment.
FIG. 2.
HeLa cells treated with CDT are blocked in G2. (A and B) Vector control-treated cells; (C and D) recombinant CDT-treated cells; (A and C) DAPI staining; (B and D) tubulin staining. The control-treated cells continued to divide, as was evidenced by the numerous mitotic cells shown in panels A and B. In contrast, CDT-treated cells stopped dividing, enlarged, and were devoid of obvious mitotic cells. The size bar in panel D represents 31 μm; all photographs were taken at a magnification of ×63.
FIG. 3.
HeLa cells 72 h after treatment with CDT. All panels show HeLa cells treated with recombinant CDT. (A, C, and E) DAPI staining, (B, D, and F) tubulin staining. The size bar in panel C represents 17 μm; all photographs were taken at a magnification of ×92.
Activation state of CDC2 in CDT-treated HeLa cells.
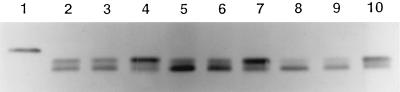
Since our results indicated that CDT caused a G2-phase block, we tested whether CDC2, the catalytic subunit of the cyclin-dependent kinase required for entry into M phase, is predominantly phosphorylated (inactive) or dephosphorylated (active) in CDT-treated HeLa cells (10). Total HeLa cell proteins from control and CDT-treated HeLa cells were reacted with anti-CDC2 antiserum, and the results are shown in Fig. 4. The slowest-migrating band in lanes 2 to 10 is fully phosphorylated inactive CDC2 and is clearly predominant in the CDT-treated HeLa cells (Fig. 4, lanes 4, 7, and 10). However, in the control lanes, the fastest-migrating band, which is CDC2 that has been dephosphorylated at both Tyr-15 and Thr-14, is the predominant band. An identical blot was reacted with an antibody to CDC2-phosphorylated Tyr-15 and confirmed that the slowest-migrating band was CDC2 phosphorylated at Tyr-15 (data not shown). These results indicate that CDT caused the HeLa cells to become blocked in G2 due to some action that led to a failure to dephosphorylate CDC2.
FIG. 4.
CDT leads to accumulation of inactive CDC2. Shown is a Western blot of total HeLa cell proteins isolated from cells treated with either fresh medium (lanes 2, 5, and 8), the vector control extract (lanes 3, 6, and 9), or the recombinant CDT extract (lanes 4, 7, and 10). Lane 1 contains a positive control protein, a CDC2 fusion protein tagged with a hemagglutinin epitope, produced by the baculovirus-Sf9 expression system (gift of Michael Mendenhall, University of Kentucky, Lexington, Ky.). Lanes 2, 3, and 4 contain proteins from HeLa cells harvested 24 h after treatment with medium, vector only extract, and recombinant CDT, respectively. Lanes 5 through 10 represent total proteins from HeLa cells treated for 48 h (lanes 5, 6, and 7) and 72 h (lanes 8, 9, and 10).
Effect of caffeine on the CDT-induced G2 block.
Methylxanthines, such as caffeine, have the ability to override a G2 block brought about by topoisomerase II inhibitors or by a variety of agents which damage DNA. These agents all act through a DNA damage checkpoint pathway which can lead to a G2 cell cycle block; therefore, it seemed possible that CDT acts on a component of this pathway. To test this hypothesis, we tested the ability of caffeine to abrogate the effect of CDT on HeLa cells. We tested caffeine at the levels (1, 2, and 4 mM) reported by Lock et al. (18) to have the ability to override a G2 block caused by the topoisomerase II inhibitor, etoposide. While all three concentrations of caffeine clearly started to push etoposide-treated HeLa cells blocked in G2 past the G2/M boundary, caffeine had no effect on CDT-treated HeLa cells in repeated experiments (data not shown).
Effect of CDT on Caco-2 cells.
Since C. jejuni causes an enteritis in humans, we tested whether CDT caused a similar cell cycle block in Caco-2 cells, a cell line derived from human intestinal epithelial cells (9). Our DNA content results showed that CDT caused Caco-2 cells to become blocked in G2 (Table 3), although it took almost 48 h for this effect to be seen in this cell line, probably because the doubling time of these cells is longer than that of HeLa cells.
TABLE 3.
DNA content analysis of Caco-2 cells treated with CDT
| Time after addition (h) | Additiona | %b of Caco-2 cells in cell cycle phase:
|
||
|---|---|---|---|---|
| G0/G1 | S | G2/M | ||
| 24 | Medium | 41.8 ± 0.6 | 36.6 ± 6.3 | 21.6 ± 6.2 |
| Vector | 41.9 ± 0.8 | 37.8 ± 6.9 | 20.3 ± 7.6 | |
| CDT | 19.6 ± 4.2 | 44.3 ± 6.4 | 36.1 ± 10.6 | |
| 48 | Medium | 48.0 ± 2.6 | 37.8 ± 6.1 | 14.2 ± 4.4 |
| Vector | 43.0 ± 3.4 | 43.7 ± 8.0 | 13.3 ± 5.0 | |
| CDT | 5.7 ± 2.6 | 4.6 ± 5.3 | 89.7 ± 7.9 | |
| 72 | Medium | 53.1 ± 2.7 | 37.1 ± 1.4 | 9.8 ± 1.5 |
| Vector | 44.3 ± 1.9 | 49.4 ± 1.4 | 6.3 ± 2.1 | |
| CDT | 0.9 ± 1.2 | 6.1 ± 7.1 | 93.0 ± 6.1 | |
Additions consisted of either RPMI 1640 (medium), an extract from the vector control strain (vector), or the recombinant CDT extract (CDT).
Values are means ± standard deviations.
DISCUSSION
Our results indicate that CDT is a novel bacterial toxin, with an activity unlike that of any other known bacterial toxin. The direct target of CDT action has not yet been identified, but the ultimate effect of CDT is to cause a rapid and apparently irreversible block in G2. There are a number of ways in which CDT might cause HeLa cells to become blocked in G2. For example, CDT might act directly on the CDC2 kinase or on proteins that interact directly with CDC2, such as the CDC25 phosphatase which carries out the reaction which activates CDC2 for entry into mitosis (10). Additional proteins also interact directly with CDC2, including the Wee-1 tyrosine kinase, which phosphorylates CDC2 at Tyr-15 (10), and CKShs1 (p13), a protein which physically associates with CDC2 and may help regulate CDC2 but whose function is not completely understood (7, 30). Whether these or other cell cycle proteins are targets of CDT remains to be determined.
Alternatively, CDT might act on a target that causes the DNA damage checkpoint pathway to operate and thereby lead to the observed block in G2. Our results indicating that caffeine does not override the G2 block caused by CDT, however, suggest either that CDT does not cause the G2 block via the DNA damage checkpoint pathway or that CDT acts downstream of the point at which caffeine operates to override the checkpoint.
Our results clearly document that although the CDT-treated HeLa cells were able to grow for 2 to 3 days, they ultimately died. The DAPI staining of CDT-treated HeLa cell chromatin revealed the nuclear fragmentation and abnormal chromatin condensation observed during the cell death period. Whether any or all of the CDT-treated cells undergo apoptosis is the subject of current research. However, the fragmented nuclei resemble structures called micronuclei seen in fibroblasts injected with p13 protein or anti-p13 antibodies (30). This similarity between the results of CDT treatment and perturbation of the function of p13, which binds to and regulates CDC2 (7), suggests that loss of normal CDC2 function, or failure to timely activate CDC2, ultimately causes CDT-induced cell death. It may be then that CDT leads to cell death as a consequence of disturbing the cell cycle.
The effect of CDT on Caco-2 cells confirms that CDT can act on human cell lines which are derived from intestinal epithelial cells. It will be of interest to determine the range of cell types upon which CDT is active. It may be that CDT is limited in its scope of activity by species boundaries or by cell type. It is certainly likely that CDT affects only cells that are actively proliferating, if indeed cell death results only after the G2 block is obtained.
Our results thus suggest a novel hypothesis for generation of diarrheal disease by C. jejuni and other diarrheagenic bacteria that make CDT. C. jejuni CDT is likely produced in proximity to intestinal epithelial cells, particularly cells in the intestinal crypts (35), and may cause these rapidly proliferating cells to become blocked in G2 and consequently no longer able to divide and differentiate. Thus, CDT may have profound effects upon the ability of the crypt cells to survive and/or mature into functional villus epithelial cells and may therefore be responsible for a temporary erosion of the villus erosion of the villus epithelium and loss of absorptive functions (8, 14, 32).
Finally, our results confirm and extend the results of Comayras et al. (5) which showed that CDTs from three different E. coli isolates cause a G2 block in HeLa cells. It seems likely that all of the CDTs have similar modes of action, although given the degree of variation in sequence of some of the Cdt subunits, there may be differences in specific activities, in sensitivities of different cell types, or in species sensitivities.
ACKNOWLEDGMENTS
We thank Michael Mendenhall for his interest and advice. Plasmid pUOA18 was kindly provided by Diane E. Taylor.
REFERENCES
- 1.Amann E, Ochs B, Able K J. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988;69:301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- 2.Aragon V, Chao K, Dreyfus L A. Effect of cytolethal distending toxin on F-actin assembly and cell division in Chinese hamster ovary cells. Infect Immun. 1997;65:3774–3780. doi: 10.1128/iai.65.9.3774-3780.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartz S R, Rogel M E, Emerman M. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J Virol. 1996;70:2324–2331. doi: 10.1128/jvi.70.4.2324-2331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black R E, Levine M M, Clements M L, Hughs T P, Blaser M J. Experimental Campylobacter jejuni infection in humans. J Infect Dis. 1988;157:472–480. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- 5.Comayras C, Tasca C, Pérès S Y, Ducommun B, Oswald E, Rycke J D. Escherichia coli cytolethal distending toxin blocks the HeLa cell cycle at the G2/M transition by preventing cdc2 protein kinase dephosphorylation and activation. Infect Immun. 1997;65:5088–5095. doi: 10.1128/iai.65.12.5088-5095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cope L D, Lumbley S, Latimer J L, Klesney-Tait J, Stevens M K, Johnson L S, Purven M, Munson R S, Langergard T, Radoff J D. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endicott J A, Nurse P. The cell cycle and suc1: from structure to function? Structure. 1995;3:321–325. doi: 10.1016/s0969-2126(01)00162-9. [DOI] [PubMed] [Google Scholar]
- 8.Gordon J I. Intestinal epithelial differentiation: new insights from chimeric and transgenic mice. J Cell Biol. 1989;180:1187–1194. doi: 10.1083/jcb.108.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grasset E, Bernabeu J, Pinto M. Epithelial properties of human colonic carcinoma cell line Caco-2: effect of secretagogues. Am J Physiol. 1985;248:C410–C418. doi: 10.1152/ajpcell.1985.248.5.C410. [DOI] [PubMed] [Google Scholar]
- 10.Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 11.Johnson W M, Lior H. Production of shiga toxin and a cytolethal distending toxin (CLDT) by serogroups of Shigella spp. FEMS Microbiol Lett. 1987;48:235–238. [Google Scholar]
- 12.Johnson W M, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Escherichia coli isolates from clinical material. Microb Pathog. 1988;4:103–113. doi: 10.1016/0882-4010(88)90052-6. [DOI] [PubMed] [Google Scholar]
- 13.Johnson W M, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter spp. Microb Pathog. 1988;4:115–126. doi: 10.1016/0882-4010(88)90053-8. [DOI] [PubMed] [Google Scholar]
- 14.Ketley J M. Pathogenesis of enteric infection by Campylobacter. Microbiology. 1997;143:5–21. doi: 10.1099/00221287-143-1-5. [DOI] [PubMed] [Google Scholar]
- 15.Korlath J A, Osterholm M T, Judy L A, Forfang F C, Robinson R A. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. J Infect Dis. 1985;152:592–596. doi: 10.1093/infdis/152.3.592. [DOI] [PubMed] [Google Scholar]
- 16.Labigne-Roussel A, Harel J, Tompkins L. Gene transfer from Escherichia coli to Campylobacter species: development of shuttle vectors for genetic analysis of Campylobacter jejuni. J Bacteriol. 1987;169:5320–5323. doi: 10.1128/jb.169.11.5320-5323.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Lock R B, Galperina O V, Feldhoff R C, Rhodes L J. Concentration-dependent differences in the mechanisms by which caffeine potentiates etoposide cytotoxicity in HeLa cells. Cancer Res. 1994;54:4933–4939. [PubMed] [Google Scholar]
- 19.Lock R B, Ross W E. Inhibition of p34cdc2 kinase activity by etoposide or irradiation as a mechanism of G2 arrest in Chinese hamster ovary cells. Cancer Res. 1990;50:3761–3766. [PubMed] [Google Scholar]
- 20.Lugtenberg B, Meijers J, Peters R, van der Hoek P, van Alphen L. Electrophoretic resolution of the major outer membrane protein of Escherichia coli K-12 into 4 bands. FEBS Lett. 1975;58:254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- 21.Miller S, Pesci E C, Pickett C L. A Campylobacter jejuni homolog of the LcrD/FlbF family of proteins is necessary for flagellar biogenesis. Infect Immun. 1993;61:2930–2936. doi: 10.1128/iai.61.7.2930-2936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagel de Zwaig R, Luria S E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967;94:1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okuda J, Fukumoto M, Takeda Y, Nishibuchi M. Examination of diarrheagenicity of cytolethal distending toxin: suckling mouse response to the products of the cdtABC genes of Shigella dysenteriae. Infect Immun. 1997;65:428–433. doi: 10.1128/iai.65.2.428-433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okuda J, Kurazono H, Takeda Y. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb Pathog. 1995;18:167–172. doi: 10.1016/s0882-4010(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 25.Pérès S Y, Marchès O, Daigle F, Nougayrède J-P, Hérault F, Tasca C, De Rycke J, Oswald E. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol Microbiol. 1997;24:1095–1107. doi: 10.1046/j.1365-2958.1997.4181785.x. [DOI] [PubMed] [Google Scholar]
- 26.Pickett, C. L. Unpublished data.
- 27.Pickett C L. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Cytolethal distending toxin causes a G2 phase cell cycle block, abstr. B-259; p. 73. [Google Scholar]
- 28.Pickett C L, Cottle D L, Pesci E C, Bikah G. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect Immun. 1994;62:1046–1051. doi: 10.1128/iai.62.3.1046-1051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pickett C L, Pesci E C, Cottle D L, Russell G, Erdem A N, Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB genes. Infect Immun. 1996;64:2070–2078. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riabowol K, Draetta G, Brizuela L, Vandre D, Beach D. The cdc2 kinase is a nuclear protein that is essential for mitosis in mammalian cells. Cell. 1989;57:393–401. doi: 10.1016/0092-8674(89)90914-8. [DOI] [PubMed] [Google Scholar]
- 31.Scott D A, Kaper J B. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect Immun. 1994;62:244–251. doi: 10.1128/iai.62.1.244-251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sears C L, Kaper J B. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol Rev. 1996;60:167–215. doi: 10.1128/mr.60.1.167-215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tauxe R V. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni current status and future trends. Washington, D.C: American Society for Microbiology; 1992. pp. 9–19. [Google Scholar]
- 34.Vindelov L, Christensen I J, Nissen N I. Limits of detection of nuclear DNA abnormalities by flow cytometric DNA analysis. Results obtained by a set of methods for sample-storage, staining and internal standardization. Cytometry. 1983;3:323–329. doi: 10.1002/cyto.990030505. [DOI] [PubMed] [Google Scholar]
- 35.Walker R I, Caldwell M B, Lee E C, Guerry P, Trust T J, Ruiz-Palacios G M. Pathophysiology of Campylobacter enteritis. Microbiol Rev. 1986;50:81–94. doi: 10.1128/mr.50.1.81-94.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Taylor D E. Chloramphenicol resistance in Campylobacter coli: nucleotide sequence, expression, and cloning vector construction. Gene. 1990;94:23–28. doi: 10.1016/0378-1119(90)90463-2. [DOI] [PubMed] [Google Scholar]