Abstract
Objectives
The aim of this study was to compile an overview of the clinical features of intracranial complication of otitis media/interna (OMI) in cats managed across five veterinary referral hospitals. Of additional interest were culture results that could inform empirical antibiotic selection, as well as outcome with both medical and surgical management.
Methods
A retrospective medical record review was conducted at five veterinary referral practices to identify cats with a diagnosis of intracranial complication secondary to OMI between 2009 and 2017. Clinical features, diagnostic findings, treatment and outcome were recorded.
Results
At total of 19 cats were identified. Sixty-three percent had no previous history of ear infection. Otoscopic examination was normal in 47% of cases. The most common bacterial isolate was Pasteurella multocida, which was identified in 24% of cases. Outcome was successful for 83% of cats managed with ventral bulla osteotomy (VBO) and in 66% of cats managed without surgical intervention.
Conclusions and relevance
Clinical suspicion of intracranial complications of OMI should remain high in cats with central vestibular disease even if otoscopic examination is normal. Antibiotic selection should be based on a culture and sensitivity; however, initial antibiotic therapy should include broad-spectrum coverage with special consideration for P multocida. Cats with intracranial complications of OMI can have a good outcome with either surgical or medical management and prospective studies are needed to assess the role of VBO in enhancing recovery.
Introduction
Intracranial complications such as intracranial abscess or meningoencephalomyelitis are important, life-threating and relatively uncommon sequelae associated with otitis media/interna (OMI). These abnormalities may be apparent on MRI or CT as evidence of fluid accumulation within the tympanic cavity in conjunction with abnormalities of the adjacent intracranial structures such as meningitis, encephalitis or overt abscess formation.1,2 Involvement of the brain may be limited to structures adjacent to the inner ear, or may be more widespread throughout the cerebellum, brainstem and thalamocortex. 3 Several case reports and one small case series describe the phenomenon in dogs and cats;3 –8 however, the veterinary literature is sparse with respect to descriptive studies of this rare complication of OMI, particularly related to feline-specific clinical and outcome data.
While similarly uncommon in people, a more substantial body of literature exists related to intracranial complications associated with human OMI. Documented pathways for spread of infection from the tympanic bulla into the cranial vault include osteothrombophlebitis, bone erosion due to pressure or enzymatic actions of pathogens, extension through pre-formed channels or defects in the skull, or hematogenous spread. 9 Regardless of species, failure to recognize this rare but severe consequence of OMI can lead to increased morbidity and mortality owing to a delay in diagnosis and treatment. The goal of this retrospective study was to provide information to enhance clinician awareness and recognition of this problem by compiling an overview of clinical features of intracranial complication of OMI in cats managed across five veterinary referral hospitals. Of additional interest were culture results that could inform empirical antibiotic selection, as well as outcome with both medical and surgical management.
Materials and methods
A retrospective search of medical records was conducted at four veterinary academic institutions (The Ohio State University, Purdue University, University of Illinois and University of Florida) and one private referral practice (VCA West Los Angeles) to identify cats with a diagnosis of intracranial complication secondary to OMI between 2009 and 2017. Intracranial complications of OMI included either suppurative meningitis or intracranial abscess in association with OMI. Diagnosis was based on cross-sectional imaging or autopsy findings.
Medical records were reviewed and clinical data was collected relating to signalment, owner-reported history of ear disease or allergies, presenting complaint, duration and type of neurologic abnormalities, physical and otoscopic examination findings, results of hematologic and biochemical profiles, diagnostic imaging and cerebrospinal fluid (CSF) analysis findings, treatment, outcome, and duration of available follow-up. Presenting complaint was further categorized as acute (0–48 h), subacute (3–7 days) or chronic (>7 days) with respect to duration of neurologic signs prior to presentation. 3 Neurologic outcome was considered successful in cats in which neurologic signs had improved or resolved at the time of last follow-up and was considered unsuccessful if signs were static, worsened, or if the cat was ultimately euthanized because of intracranial complications of OMI.
Summary data are presented as median and range, with descriptive statistics provided where appropriate based on the number of cases evaluated.
Results
A summary of signalment, historical factors, culture results, medical or surgical treatment, and outcome of all cats is presented in Table 1.
Table 1.
Age, duration of neurologic signs, culture results, treatment and outcome of 19 cats diagnosed with intracranial complications of otitis media/interna (OMI) across five veterinary referral centers
| Case | Age (years) | Duration | Culture results | VBO | Steroids | Antibiotics | Outcome | Follow-up |
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | Chronic | Pasteurella multocida | Y | Y | Ampicillin-sulbactam; then amoxicillin-clavulanic acid | Unsuccessful | 1 month |
| 2 | 2 | Subacute | Staphylococcus species | Y | Y | Clindamycin and marbofloxacin | Successful | 9 months |
| 3 | 0 | Chronic | No growth | N | Y | Cefotaxime; then cefpodoxime | Successful | 1 month |
| 4 | 5 | Acute | Streptococcus species | Y | Y | Ampicillin-sulbactam, enrofloxacin, and clindamycin; then trimethoprim-sulfa | Successful | 8 months |
| 5 | 4 | Chronic | No growth | Y | N | Clindamycin and marbofloxacin | Successful | 1 month |
| 6 | 4 | NA | Actinomyces, Corynebacterium, Staphlococcus species | N | Y | Clindamycin | Unsuccessful | 3 days |
| 7 | 3 | Chronic | No growth | Y | Y | Clindamycin | Successful | 4 months |
| 8 | 5 | Acute | No growth | Y | N | Clindamycin | Successful | 1 month |
| 9 | 12 | Chronic | Pseudomonas aeruginosa | Y | Y | Cefpodoxime; then amoxicillin-clavulanic acid | Successful | 4 months |
| 10 | 12 | Subacute | No growth | N | N | Clindamycin | Successful | 1 month |
| 11 | 7 | Unknown | Enterobacter aerogenes, Pseudomonas aeruginosa, Escherichia coli | N | Y | Trimethoprim-sulfa | Successful | 2 months |
| 12 | 6 | Subacute | Pasteurella multocida | N | Y | Amoxicillin-clavulanic acid | Unsuccessful | 18 h |
| 13 | 3 | Subacute | No growth | Y | Y | Clindamycin and amoxicillin-clavulanic acid | Successful | 2 months |
| 14 | 7 | Unknown | Escherichia coli | N | Y | Marbofloxacin | Successful | 36 months |
| 15 | 6 | Subacute | Streptococcus species | Y | N | Enrofloxacin; then, amoxicillin-clavulanic acid | Successful | 4 months |
| 16 | 10 | Acute | Pasteurella multocida, Clostridium perfringens, Peptostreptococcus species, Bacteroides vulgatus | N | Y | Not administered | Unsuccessful | 0 h |
| 17 | 12 | Unknown | No growth | Y | N | Enrofloxacin | Successful | 2 months |
| 18 | 10 | Acute | Not performed | Y | Y | Amoxicillin-clavulanic acid | Successful | 12 months |
| 19 | 13 | Subacute | Propionibacterium acnes, Pasteurella multocida | Y | Y | Clindamycin; then, amoxicillin-clavulanic acid | Unsuccessful | 3 weeks |
Presenting complaint was categorized as acute (0–48 h), subacute (3–7 days) or chronic (>7 days) with respect to duration of neurologic signs prior to presentation. Neurologic outcome was considered successful in cats in which neurologic signs had improved or resolved at the time of last follow-up and was considered unsuccessful if signs were static, worsened or if the cat was ultimately euthanized because of intracranial complications of OMI
VBO = ventral bulla osteotomy; Y = yes; N = no; NA = not applicable
Signalment
At total of 19 cats with a diagnosis of intracranial complications associated with OMI were identified on retrospective medical records searches from participating veterinary institutions. Eleven cases were identified from The Ohio State University College of Veterinary Medicine, and two cases each were identified from University of Florida, Purdue University, University of Illinois College of Veterinary Medicine and VCA West Los Angeles. Cats ranged in age from 2 months to 13 years, with a median age at presentation of 4.9 years. Included breeds were as follows: domestic mixed breed (n = 16), Sphinx (n = 1), Japanese Bobtail (n = 1), Cornish Rex (n = 1). There were eight spayed females, one sexually intact male and 10 castrated males.
Historical factors
Seven cats (37%) were noted by their owners to have a history of previous ear infection, ranging in one case from a single previous infection diagnosed as a kitten to a history of multiple infections managed chronically by a either a family veterinarian or a board-certified veterinary dermatologist in five cases. Twelve cats (63%) had no previous known history of ear infection as reported by their owner. Seven cats (37%) had been diagnosed previously with other allergic or dermatologic diseases. Of cats with a previous diagnosis of allergic or dermatologic disease, four had been diagnosed historically with upper airway disease, cough or rhinitis, two had been previously diagnosed with atopy and one with food allergies.
Presenting complaint
Duration of neurologic signs prior to presentation could be determined from the medical record in 16 cases. Of these, four cats presented acutely (<48 h) for the development of neurologic signs, whereas six presented subacutely (3–7 days). In 4/6 cats with a subacute duration of neurologic signs, systemic signs such as lethargy, decreased appetite or fever were present chronically (range 2 weeks–4 months). Five cats presented with a chronic duration of neurologic signs (>7 days). One cat was not presented for neurologic signs and an intracranial abscess associated with OMI was identified incidentally during imaging of the middle ear for evaluation of chronic otitis externa. This cat was not evaluated by a board-certified veterinary neurologist at the time of initial diagnosis.
Physical and otoscopic examination
A description of the external ear canal and tympanic membrane was reported in the medical record of 15 cats. Of these, mild-to-moderate ceruminous discharge within the external ear canal was noted in five cats (33%), and erythema or pronounced otic discharge suggestive of otitis externa was noted in four cats (27%). The tympanic membrane was observed and specifically reported to be normal in seven cats (47%). Abnormalities of the tympanic membrane were noted in two cats, one of which was intact but opaque, and one of which was overtly ruptured. For six cats, the tympanic membrane could not be visualized owing to excessive debris in the external ear canal.
Neurologic examination
Neuroanatomic localization for the 18 cats evaluated by a board-certified veterinary neurologist was recorded as central nervous system in 15 cats and peripheral nervous system in three cats. Specific localizations were as follows: unilateral peripheral vestibular (n = 3), central vestibular/rostral medulla (n = 9), multifocal central nervous system (rostral medulla and cerebellum or rostral medulla and thalamocortex; n = 6). Clinical signs of Horner syndrome were present in six cats (33%); three had an apparent complete Horner syndrome, whereas three had partial Horner syndrome, with pupillary miosis being the only clinical sign. Facial paralysis was not reported in any cat in the present study. Decreased level of consciousness was reported in eight cats (44%).
Hematologic and biochemical profiles
A complete blood count and biochemical profile were performed in 18 cats and revealed no significant abnormalities in seven cats (39%). A stress leukogram, consisting of a mild mature neutrophilia (less than twice the upper end of the reference interval [RI] with or without concurrent lymphopenia) was observed in seven cats (39%). Four cats (22%) had an inflammatory leukogram consisting of a pronounced increase in mature neutrophil populations (greater than twice the upper end of the RI) with or without the presence of a left shift. One cat had a mild normocytic, normochromic non-regenerative anemia. Three cats (17%) had mild-to-moderate hyperglobulinemia (range 4.8–5.7 g/dl; RI 2.2–2.9 g/dl).
Imaging results
Cross-sectional imaging of the brain and middle ear was performed in 18 cats (five imaged via CT and 13 imaged via MRI). Unilateral (n = 11; 61%) or bilateral (n = 7; 39%) OMI was observed in all cases. For cats imaged via CT, significant findings noted in addition to unilateral or bilateral OMI ranged from lysis of the petrous temporal bone (Figure 1a) and meningeal enhancement within the caudal fossa adjacent to the bulla (Figure 1b,c) to, in one case, a contrast-enhancing mass causing displacement of the rostal medulla adjacent to the tympanic bulla (Figure 1d). One cat imaged via CT had evidence of OMI but no evidence of intracranial extension noted on imaging; however, CSF analysis revealed marked neutrophilic inflammation, and clinical signs resolved with treatment of OMI.
Figure 1.
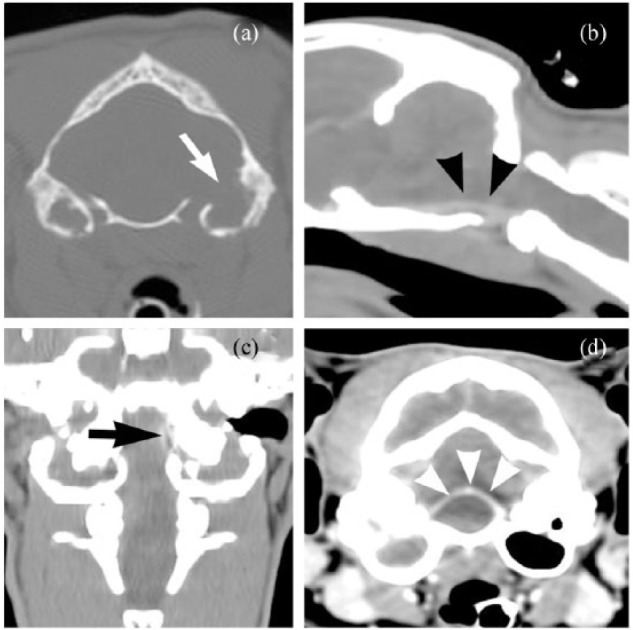
CT findings in cats with intracranial complications of otitis media/internal (OMI). In addition to unilateral or bilateral OMI, abnormalities ranged from lysis of the petrous temporal bone ([a] white arrow) and meningeal enhancement within the caudal fossa adjacent to the bulla ([b] black arrowheads; [c] black arrow) to a contrast-enhancing mass causing displacement of the rostral medulla adjacent to the tympanic bulla ([d] white arrowheads)
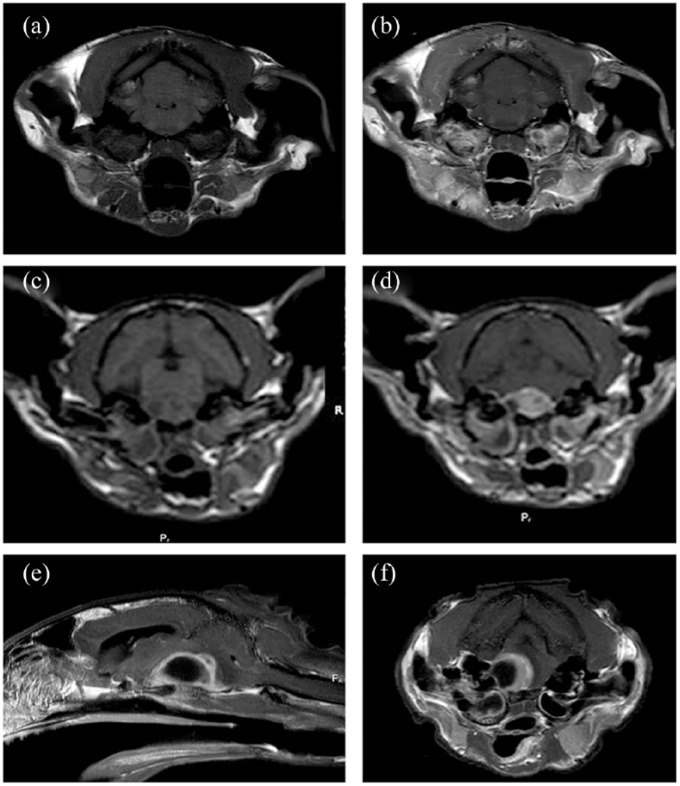
For cats imaged via MRI, significant findings noted in addition to unilateral or bilateral middle-ear effusion included moderate or marked meningeal and/or brainstem parenchymal enhancement adjacent to the petrous temporal bone (n = 6) (Figure 2a,b) or presence of a contrast-enhancing mass in the caudal fossa suggestive of an intracranial abscess (n = 7) (Figure 2c–f).
Figure 2.
MRI findings in cats with intracranial complications of otitis media/internal. Significant findings ranged from (a,b) moderate or marked meningeal and/or brainstem parenchymal enhancement adjacent to the petrous temporal bone to (c–f) the presence of a contrast-enhancing mass in the caudal fossa suggestive of an intracranial abscess. (e,f) Note the peripheral contrast enhancement. Panels (a,b) and (d–f) are T1-weighted images acquired after intravenous administration of gadolinium. (c) T1-weighted image acquired prior to administration of gadolinium
CSF results
CSF analysis was performed in 10 cases (53%). Median total nucleated cell count was 73 cells/μl (range 1–2952/μl; normal ⩽5 cells/μl) and median protein concentration was 30.3 mg/dl (range 11.2–267.7; normal <25 mg/dl). In all 10 cases, the predominant cell type was neutrophils. Cytologic evaluation described neutrophils as non-degenerate (n = 9) or non-degenerate to mildly degenerate (n = 1). Bacterial or other organisms were not observed on cytology in any case.
Culture results
A total of 21 samples were submitted for culture from 18 cats. Samples submitted were CSF (n = 3), myringotomy samples (n = 12), or from tissue or fluid samples obtained during ventral bulla osteotomy (VBO; n = 5). In one cat, a sample of fluid was obtained from the middle ear at the time of autopsy. Bacterial isolates included Pasteurella multocida (n = 4), Staphylococcus species (n = 2), Streptococcus species (n = 2), Actinomyces species (n = 1), Corynebacterium species (n = 1), Pseudomonas aeruginosa (n = 2), Enterobacter aerogenes (n = 1), Escherichia coli (n = 2), Clostridium perfringens (n = 1), Peptostreptococcus species (n = 1), Propionibacterium acnes (n = 1) and Bacteroides vulgatus (n = 1). In three cats, more than one distinct bacterial population was cultured from myringotomy fluid. In one cat, two different bacterial populations were cultured: one from CSF and another from fluid obtained from the middle ear.
Treatment
Craniotomy for surgical debridement of intracranial infection was not performed in any case in the present study. VBO of the affected ear(s) was performed in 12 cats, whereas six were managed medically with a combination of myringotomy/ear flush and antibiotics (n = 3), or antibiotics with no myringotomy/ear flush (n = 5). One cat was euthanized immediately on presentation and ultimate diagnosis of OMI with intracranial complication was made via autopsy; therefore, outcome for this cat was not included in either the VBO or medical management category.
Eighteen cats were prescribed antibiotic therapy for treatment of their OMI with intracranial complications. Initial antibiotic choice was highly variable, with clindamycin (n = 9) and potentiated amoxicillin/clavulanic acid (n = 5) representing the most common choices. Four cats were placed on more than one antibiotic at the time of initial diagnosis: two on a combination of clindamycin and marbofloxacin, and two on a combination of clindamycin and ampicillin-sulbactam. Duration of antibiotic therapy was also highly variable.
Fourteen cats were prescribed steroids in conjunction with antibiotic therapy. In five cases, a single anti-inflammatory dose of corticosteroids (0.1–0.15 mg/kg dexamethasone sodium phosphate) was administered at the time of diagnosis and not repeated. Four cats received an anti-inflammatory dose of steroids (0.1–0.15 mg/kg dexamethasone sodium phosphate) for 3 days following diagnosis. An additional five cats received an initial anti-inflammatory dose of oral steroids (0.5–1.0 mg/kg/day prednisolone) which was tapered over 7–14 days. One cat, with a large brainstem abscess originally diagnosed on CT, was managed initially with oral antibiotics and a 3 day course of anti-inflammatory steroids. One week after initial imaging, CT was repeated for surgical planning of VBO and showed apparent resolution of the intracranial abscess with medical treatment alone (Figure 3).
Figure 3.
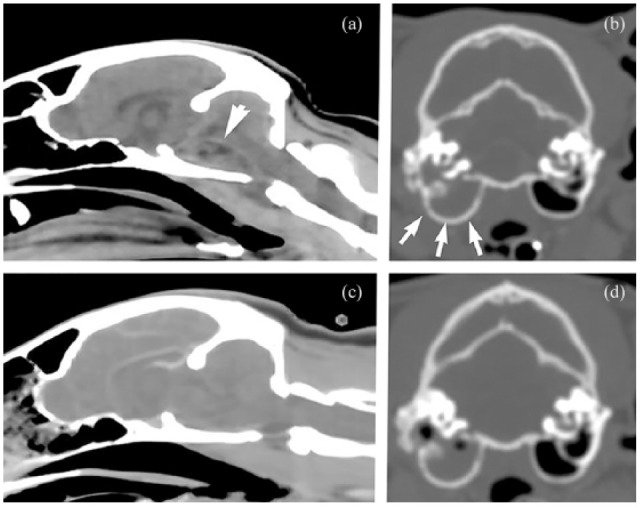
CT images (a,b) before and (c,d) after antibiotic therapy for presumptive intracranial complications of otitis media/interna with a presumptive (a) intracranial abscess (arrow) and (b) middle-ear effusion (arrows). This cat (shown also in [d]) was managed initially with oral antibiotics and a 3 day course of anti-inflammatory corticosteroids. One week after initial imaging, CT was repeated for surgical planning of ventral bulla osteotomy and showed apparent resolution of the intracranial abscess with medical treatment
Outcome and follow-up
Median duration of follow-up for the 19 cats included in the current study was 4 months (range 0–36 months). Neurologic outcome was considered successful (improved or resolved) in 14 cats (74%) and unsuccessful (static, declined or euthanized) in five cats (26%). For cats with a successful neurologic outcome, median duration of follow-up was 8 weeks (range 4 weeks–36 months). For cats with an unsuccessful outcome, median duration of follow up was 0.3 weeks (range 0–4 weeks). For cats that underwent VBO (n = 12), neurologic outcome was considered successful in 83% of cases. For those managed medically (n = 6), outcome was considered successful in 66% of cases.
Discussion
This retrospective study constitutes the largest published series of cats with intracranial complications associated with OMI. While a life-threatening complication of OMI, the apparent low prevalence of this problem may hinder or prevent recognition of the condition in cats, making a firm understanding of clinical features of the disease vital in order to raise awareness so that appropriate treatment can be initiated in a timely fashion in cats with compatible signs. This is particularly important given the fact that more that 63% of cats in the present study had no previous history of ear disease. These results are in contrast to those reported by Sturges et al, 3 where 8/11 cats with intracranial complications of OMI (73%) had a reported history of pre-existing ear disease.
In the present study, 53% of cats lacked evidence of external or middle-ear disease on otoscope examination at the time of presentation. Because of the retrospective nature of this study, the type of otoscopic examination performed (routine vs video) was not standardized in all cases or recorded, and the nature of the examination may have influenced the ability to identify middle-ear pathology. Results of otoscopic examination were not reported for the two previously published case series of cats with vestibular signs due to OMI with or without intracranial complication,3,6 so comparison of results is not possible. In the face of a normal otoscopic examination, a clinician may be inclined to discount otogenic complications of OMI from the list of differential diagnoses for a cat with signs of central nervous system disease; however, our results suggest this should not be the case. Bacterial extension from the nasopharynx to the middle ear is anecdotally a more common putative cause of OMI in cats than in dogs and therefore may explain the lack of external signs of ear disease in cats in the present study. 10
Most cats in the current study were presented with clinical signs referable to disease of the central vestibular system/rostral medulla with or without other signs of brain disease. This is in keeping with previous veterinary studies that suggest the most common neurologic signs of intracranial complication of OMI are vestibular signs, decreased level of consciousness, paresis and seizures.3,6 Interestingly, a small number of cats in the current study had no clear indications that their vestibular signs were central in nature, despite ultimately being diagnosed with an intracranial complication of OMI. This suggests that intracranial extension of OMI should always be considered a possibility in cats with middle-ear infection and vestibular signs, regardless of whether the signs appear peripheral or central in nature.
As initial antibiotic selection in cats with intracranial complications of OMI is extremely important but typically empirical, culture results were of particular interest in the present study. P multocida was the most common bacteria identified, isolated in 24% of cases. P multocida is a gram-negative facultative anaerobic organism that is a common bacterial inhabitant of the feline oral cavity, is frequently isolated from infected feline bite wounds, and has been reported to cause spinal empyema and meningoencephalomyelitis.11–13 Ascending infection from the mouth to the middle ear via the Eustachian tube is a putative mechanism for the development of feline primary OMI. 10 This may explain the prevalence of Pasteurella-positive samples in the current study, as well as the lack of external ear pathology in many cases.
Excepting the commonality of Pasteurella species in the current study, there was substantial variability in the type, number and features of bacterial isolates from cats with intracranial complications of OMI. These results support the recommendations of previous studies, which suggest that given the variability in culture results of cats with intracranial complications of OMI, empirical therapy with broad-spectrum antibiotic coverage for gram-positive, gram-negative, aerobic and anaerobic organisms is most rational while awaiting culture results. While Cryptococcus neoformans has been previously reported as a cause of OMI in cats, 14 it was not observed as a cause of intracranial complications associated with OMI in the present study. 8
Corticosteroids, provided at an assortment of doses and for variable lengths of time, were used in the management of approximately half of the cats in the present study. The most common dosing regimen for steroids was a single anti-inflammatory dose of dexamethasone sodium phosphate given immediately after diagnostic imaging was performed.
The use of corticosteroids in the management of intracranial infection in people is somewhat controversial and there are no well-controlled, randomized clinical studies evaluating their use in otogenic intracranial infection. However, a recent systematic review evaluating the use of a short course (2–4 days) of an anti-inflammatory dose of corticosteroids for bacterial meningitis in people suggests that use was associated with fewer residual neurologic deficits and did not increase risk of adverse events. 15 The rationale for the use of corticosteroids comes from experimental animal studies, which suggest that the outcome for infectious meningitis is worsened with increased severity of the inflammatory response, and that use of corticosteroids reduces the inflammatory response, decreases vasogenic brain edema and improves outcome.16,17 Owing to the retrospective nature of the present study, it is impossible to evaluate the effect of corticosteroid therapy on outcome in cats with intracranial complications of OMI; however, the use of corticosteroids appears common among veterinary clinicians managing these cases.
Overall, 74% of cats in the current study had a successful neurologic outcome (defined as improved or resolved neurologic signs), regardless of whether medical or surgical management of their disease was pursued. This finding is in agreement with a previous study that reported a good prognosis for cats with intracranial complications of OMI; 3 however, few cats in that case series were managed solely with medical therapy and the authors recommended surgical drainage of the tympanic bulla and cranial vault as standard therapy. Twelve of 18 cats diagnosed ante-mortem in the current study underwent VBO. Outcome in these cats was good to excellent, with 83% of them having improved or resolved neurologic signs at a median follow-up of 4 months after diagnosis. The remaining six cats were managed medically without VBO and 66% of these had a successful outcome, suggesting that medical management can be considered in some cases of feline OMI with intracranial extension. Follow-up was substantially shorter in the cats managed without VBO. This coupled with the retrospective nature of the study make it difficult to reliably assess the association between VBO and outcome.
Conclusions
Clinical suspicion of intracranial complications of OMI should remain high in cats with central vestibular or multifocal central nervous system signs, regardless of negative findings on otoscopic examination. Medical treatment should be based on a culture and sensitivity, given the broad range of bacterial isolates obtained from cats in the current study; however, initial antibiotic therapy should include four-quadrant coverage with special consideration for P multocida. Cats with intracranial complications of OMI can have a good outcome with either surgical or medical management, and prospective studies are needed to assess the role of VBO in enhancing recovery.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Accepted: 16 February 2018
References
- 1. Wanna GB, Dharamsi LM, Moss JR, et al. Contemporary management of intracranial complications of otitis media. Otol Neurotol 2009; 31: 111–117. [DOI] [PubMed] [Google Scholar]
- 2. Wu J-F, Jin Z, Yang J-M, et al. Extracranial and intracranial complications of otitis media: 22-year clinical experience and analysis. Acta Otolaryngol 2012; 132: 261–265. [DOI] [PubMed] [Google Scholar]
- 3. Sturges BK, Dickinson PJ, Kortz GD, et al. Clinical signs, magnetic resonance imaging features, and outcome after surgical and medical treatment of otogenic intracranial infection in 11 cats and 4 dogs. J Vet Intern Med 2006; 20: 648–656. [DOI] [PubMed] [Google Scholar]
- 4. Spangler ES, Dewey CW. Meningoencephalitis secondary to bacterial otitis media/interna in a dog. J Am Anim Hosp Assoc 2000; 36: 239–243. [DOI] [PubMed] [Google Scholar]
- 5. Cook LB, Bergman RL, Bahr A, et al. Inflammatory polyp in the middle ear with secondary suppurative meningoencephalitis in a cat. Vet Rad Ultrasound 2003; 44: 648–651. [DOI] [PubMed] [Google Scholar]
- 6. Negrin A, Cherubini GB, Lamb C, et al. Clinical signs, magnetic resonance imaging findings and outcome in 77 cats with vestibular disease: a retrospective study. J Feline Med Surg 2010; 12: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martin-Vaquero P, da Costa RC, Daniels JB. Presumptive meningoencephalitis secondary to extension of otitis media/interna caused by Streptococcus equi subspecies zooepidemicus in a cat. J Feline Med Surg 2011; 13: 606–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siak MK, Paul A, Drees R, et al. Otogenic meningoencephalomyelitis due to Cryptococcus gattii (VGII) infection in a cat from Western Australia. JFMS Open Rep 2015; 1: DOI: 20155116915585022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dubey SP, Larawin V, Molumi CP. Intracranial spread of chronic middle ear suppuration. Am J Otoloaryngol 2010; 31: 73–77. [DOI] [PubMed] [Google Scholar]
- 10. Gotthelf LN. Diagnosis and management of otitis media in dogs and cats. Vet Clin North Am Small Anim Pract 2004; 34: 469–487. [DOI] [PubMed] [Google Scholar]
- 11. Messer JS, Kegge SJ, Cooper ES, et al. Meningoencephalomyelitis caused by Pasteurella multocida in a cat. J Vet Intern Med 2006; 20: 1033–1036. [DOI] [PubMed] [Google Scholar]
- 12. Granger N, Hidalgo A, Leperlier D, et al. Successful treatment of cervical spine epidural empyema secondary to grass awn migration in a cat. J Feline Med Surg 2007; 9: 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dolieslager SM, Riggio MP, Lennon A, et al. Identification of bacteria associated with feline chronic gingivostomatitis using culture-dependent and culture-independent methods. Vet Microbiol 2011; 148: 93–98. [DOI] [PubMed] [Google Scholar]
- 14. Beatty JA, Barrs VR, Swinney GR, et al. Peripheral vestibular disease associated with Cryptococcus in three cats. J Feline Med Surg 2000; 2: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brouwer MC, McIntyre P, Prasad K, et al. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev 2015; 9: CD004405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scheld WM, Dacey RG, Winn HR, et al. Cerebrospinal fluid outflow resistance in rabbits with experimental meningitis. Alterations with penicillin and methylprednisolone. J Clin Invest 1980; 66: 243–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tauber MG, Khayam-Bashi H, Sande MA. Effects of ampicillin and corticosteroids on brain water content, cerebrospinal fluid pressure, and cerebrospinal lactate levels in experimental pneumococcal meningitis. J Infect Disease 1985; 151: 528–534. [DOI] [PubMed] [Google Scholar]