Abstract
Objectives
The aim of this study was to establish ultrasound criteria for the diagnosis of autosomal dominant polycystic kidney disease (ADPKD) in Persian cats.
Methods
Eighty-two Persian cats were assessed using renal ultrasound and genotyped for the C→A transversion in exon 29 of PKD1. The animals were also submitted to hematological characterization, serum biochemistry analyses and urinalysis.
Results
Age, sex and neutering status did not differ between ADPKD (n = 12) and non-ADPKD (n = 70) cats. After integrated molecular genetics/ultrasonographic analysis, the presence of at least one renal cyst was sufficient to establish a diagnosis of ADPKD in animals up to 15 months of age. Two or more cysts were required for diagnosis in cats aged 16–32 months, and at least three cysts warranted diagnosis of ADPKD in animals aged 33–49 months. Finally, four or more cysts led to diagnosis in cats aged 50–66 months. Although cats with ADPKD exhibited higher serum calcium levels than non-affected cats, hematological, urinalysis and other biochemical parameters did not differ between the two groups.
Conclusions and relevance
Integrated analyses of imaging and molecular genetics data enabled, for the first time, the establishment of age-based ultrasonographic criteria for the diagnosis of ADPKD in Persian cats. The development of imaging criteria is particularly relevant and useful in the clinical setting given the current limitations to access and the cost of molecular genetics-based diagnostic tests.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common life-threatening inherited disease in humans, affecting 1 in 500–1000 of the general population. 1 It is characterized by focal development and progressive enlargement of renal cysts, typically leading to gradual increase in kidney size and, after decades of relatively preserved renal function, a steady decline in glomerular filtration rate. 2 ADPKD is responsible for 4.4–10.0% of end-stage kidney disease cases worldwide, representing a major burden on public health. 3
ADPKD is caused by a mutation in the polycystic kidney disease (PKD) genes, PKD1 or PKD2. Given the complex, large transcript size and extensive distribution of PKD1 and PKD2 germline mutations in ADPKD families, access to direct gene testing and its high costs significantly limit the use of this tool to establish the diagnosis of disease in conventional clinical practice. 4 Ultrasonography (US), however, is widely available, and is a safe, inexpensive, and effective imaging method to detect and characterize renal cysts. In this context, Ravine et al 5 conducted an integrated linkage-ultrasonographic analysis in PKD1-linked families, establishing an imaging criterion for pre-symptomatic ADPKD diagnosis in at-risk family members. More recently, Pei et al performed a genotype-ultrasound analysis including PKD1- and PKD2-linked families. 6 This study enabled the establishment of ultrasound diagnostic criteria applicable to asymptomatic at-risk members of ADPKD families of unknown genotype.
The prevalence of PKD in Persian cats is extremely high, reaching 25.9–50% of tested cats in the UK, France, Italy, Slovenia and Taiwan.7–16 The clinical manifestations and course of this disease in Persian and Persian-related cats closely resemble the features of the human illness.17,18 Interestingly, all known cases of ADPKD in cats have been linked to a single germline mutation in the feline ortholog of the human PKD1 gene, also named PKD1. This variant consists of a single nucleotide transversion (C→A) located at position 3284 of exon 29, which introduces a premature stop codon into the corresponding mRNA transcript. 19
While to date the single mutation makes molecular testing an adequate tool for diagnosis of ADPKD in Persian and Persian-related cats, its limited access and high cost, especially in developing countries, restricts its application in cats. US, however, has been demonstrated to be the most cost-effective imaging modality for large-scale renal phenotypical evaluation of potentially affected cats.9,10,18 Notably, a previous study involving affected Persian cats reported a US sensitivity and specificity for renal cyst detection of 91% and 100%, respectively, at 36 weeks of life compared with kidney histopathology. 18
Cysts can be identified as anechoic structures within the renal parenchyma by US.20,21 The criterion of at least one anechoic cyst in at least one kidney has been previously used to diagnose PKD in 13-week–10-year-old cats, 9 and in Persian cats >10 months of age. 10 However, these criteria did not take into account the genetic molecular basis of the disease, which was unknown at the time of the studies. Moreover, the development of renal cysts secondary to other clinical circumstances, such as obstruction due to nephrolithiasis, lymphoma and chronic kidney disease with interstitial nephritis, is well recognized, especially in old cats.10,22
Previous reports have illustrated how problematic it is to use the one-cyst criterion to diagnose ADPKD in Persian cats. In fact, Kappe et al described three Persian cats with a renal cyst and a negative genetic test. 22 Bonnazzi et al reported four cats with at least one renal cyst in only one kidney that were negative according to PCR-restriction fragment length polymorphism (PCR-RFLP) and sequencing for feline ADPKD, 14 and one cat negative at ultrasound that was positive in the genetic test. Finally, in a study by Lee et al, 16 three cats that were diagnosed with PKD using US did not harbor the mutation within exon 29.
The establishment of ultrasound criteria for ADPKD diagnosis based on appropriate age ranges in Persian cats is highly desirable for large-scale application in clinical practice. The present study, comprising an integrated molecular genetics–ultrasonographic analysis, achieved this goal. The development of such a criterion is therefore anticipated to have a significant impact on conventional feline clinical evaluation.
Materials and methods
Study population
Eighty-two Persian cats belonging to 33 distinct pedigrees were included in the current study. Persian cats of both sexes and >3 months of age were selected from catteries of accredited breeders. All cats were privately owned and the owners were thoroughly informed of the research aims and protocols. The study was approved by the institution’s ethics committee (protocol 1812010514). This study was performed at the Department of Clinical Medicine and Department of Pathology, School of Veterinary Medicine and Animal Science, University of São Paulo, São Paulo, Brazil.
The animals were submitted to routine physical examination (including thyroid gland palpation), abdominal US, genotype analyses, complete blood count (CBC), serum biochemistry profile (including total thyroxine concentration) and urinalysis.
Genotype analyses
The presence or absence of the C→A transversion at position 3284 of exon 29 in the feline PKD1 gene was investigated in all cats included in this study using a PCR-RFLP assay. Blood samples were collected by venipuncture, enabling genomic DNA extraction from whole blood. This procedure was performed using a commercially available kit (Illustra Blood Genomic Prep Mini Spin Kit; GE Healthcare) according to the manufacturer’s instructions.
A 559 base pair (bp) PCR fragment containing exon 29 was amplified using the same pair of primers previously reported by Lyons et al: 19 PKD1F1 (5′–CAGGTAGACGGGATAGACGA–3′) and PKD1R1 (5′–TTCTTCCTGGTCAACGACTG–3′). The reaction included genomic DNA template, 2.5 mM of each dNTP, 50 mM MgCl2, 10 μM PKD1F1, 10 μM PKD1R1, PCR buffer and 5 U/μl Taq DNA Polymerase (Thermo Fisher Scientific), and was run on a thermal cycler (Eppendorf). The PCR conditions included an initial 3 mins of denaturation at 94°C; 35 amplification cycles, including 1 min denaturation at 94°C, 1 min primer annealing at 58°C and 1 min extension at 72°C; and a final 1 min extension at 72°C. The amplified product was digested with 10 U of MLY1 (New England Biolabs) and analyzed by electrophoresis on 2% agarose gels and subsequent documentation using the Chemidoc system (BioRad). Product digestion resulting in 316 bp and 243 bp fragments indicated the presence of the C→A transversion, whereas observation of only the non-digested 559 bp fragment revealed the absence of this genetic variant. Positive and negative controls were used in all reactions.
All positive results were confirmed by direct automated Sanger sequencing of the respective amplified PCR products. Cats with this pathogenic mutation received definitive diagnosis of ADPKD, whereas cats without such a variant had the diagnosis of ADPKD excluded.
Ultrasonographic analysis
The sonographic assessments were performed by a veterinary specialist in US using a multifrequency (7.5–12 MHz) linear transducer (Logiq 7; GE Healthcare), with harmonic and Doppler image features (color, spectral and amplitude). The resolution was sufficient to detect 0.3 cm (diameter) renal cysts. The cats were not sedated for examination. Hair clipping was not performed or, when necessary, was reduced to a small window on each side of the abdomen. The kidneys, liver and pancreas were scanned for evidence of anechoic cysts within the parenchyma. Renal length was measured according to the longitudinal axis. Cysts were quantified and measured.
Laboratory evaluation
A CBC was obtained within 1 h of blood collection using a hematology analyzer (BC-2800Vet; Shenzhen Mindray Bio-Medical Electronics). Evaluated parameters included red blood cell count, hemoglobin concentration, hematocrit, mean corpuscular volume, mean cell hemoglobin, concentration, platelet count, and white blood cell count and differential. Air-dried Wright–Giemsa-stained blood films were performed immediately after blood collection. Differential white blood cell counts were performed manually by counting 100 nucleated cells per smear.
Selected biochemical analytes in the serum were quantified using an automated spectrophotometer clinical wet chemistry analyzer (Liasys; Analyzer Medical System). The analytes included serum urea nitrogen, creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), albumin, total protein (TP), phosphate and calcium. Sodium, potassium and chloride were measured using an ion-specific electrolyte analyzer (AVL-OMNI4-Roche), whereas thyroxin concentration was quantified using radioimmunoassay (Abbot Laboratories). The analyzers were calibrated before each sample run, the runs followed the manufacturers’ instructions, and the measurements were appropriately performed using control samples. Grossly hemolyzed or lipemic samples were discarded.
Laboratory urine evaluation
All urine samples were collected via cystocentesis and sample volumes ranged from 3.5–12 ml. Urinalysis consisted of a urinary dipstick test, determination of urine-specific gravity (USG) with a manual refractometer, measurement of urinary pH and urinary protein-to-creatinine ratio (UPC) using an analyzer (Liasys), and sediment examination. The sediment was prepared as described previously,23,24 and evaluated using microscopy within 1 h of urine collection.
Statistical analysis
Continuous variables were tested for normality using the Kolmogorov–Smirnov and Shapiro–Wilk tests. The values were expressed as mean and SD for parametric data, and as median and quartiles for non-parametric data. Categorical data were presented as absolute (n) values and percentages (%), and were tested using Pearson’s χ2 test and Fisher’s exact test, if applicable. Non-parametric data were compared using the Mann–Whitney U-test for two independent samples. Parametric data were compared using the Student’s t-test for two independent samples. Discrimination of variables was calculated using receiver–operator characteristic (ROC) curve analysis with area under the curve and asymptotic significance. Some continuous variables were categorized via ROC curve analysis. 25 The cut-off points were calculated using the value associated with the best sensitivity and specificity. Correlations were calculated using the Spearman rank test and predicted associations by linear regression. Values with an r2 ⩾0.7 were determined to have good performance or linearity. An alpha risk ⩽5% for committing type I error and beta risk ⩽20% for type II error were accepted.26,27 Analyses were performed using SPSS version 19.0 (IBM) and GraphPad Prism 5 (GraphPad).
Results
Feline population and determination of molecular diagnostic status
Twelve of 82 (14.6%) Persian cats were heterozygous for the C→A mutation in exon 29 of PKD1. The presence of this allele established a definitive diagnosis of ADPKD in such cats. The remaining 70 (85.4%) animals were homozygous for the wild-type PKD1 allele. Because no other genetic variant in PKD1 or other genes have been linked to ADPKD in cats to date, the absence of the C→A transversion in PKD1 was considered to be sufficient to exclude the diagnosis of ADPKD in such cats. The ADPKD group consisted of seven intact females, two neutered females, two intact males and one neutered male. Among the non-ADPKD cats, 43 were intact females, two were neutered males and 25 were intact males. The age range was 11–162 months in the ADPKD group and 5–168 months among non-ADPKD animals. The ADPKD and non-ADPKD groups did not differ with regard to sex (P = 0.531) and age (87.4 ± 52.6 vs 75.8 ± 35.8 months, respectively; P = 0.345). Of a total of 33 pedigrees, 11 pedigrees including 12 cats had at least one affected animal, whereas in 22 pedigrees comprising 70 cats, no animal was diagnosed with ADPKD.
Ultrasonographic characterization and imaging criteria for diagnosis of ADPKD
All 12 genetically confirmed ADPKD cats exhibited renal cysts (Table 1). The lowest number of cysts was detected in the affected group – two in each kidney – observed in an 11-month-old animal (Table 2). The diameter of the cystic lesions ranged from 0.3–1.9 cm. The renal longitudinal length was higher in ADPKD than in non-ADPKD cats (3.848 ± 0.528 cm vs 3.542 ± 0.496 cm; P <0.01) (Table 1). Affected kidneys also exhibited increased cortical echogenicity, irregular contour and corticomedullary blurring (75% vs 38.57% [P <0.01]; 50% vs 3.57% [P <0.001]; 62.5% vs 6.43% [P < 0.001], respectively) (Table 1). Additionally, 33.3% (n = 4/12) of ADPKD animals had cysts in the liver (Table 2). No pancreatic cysts were observed.
Table 1.
Descriptive statistics for ultrasonography variables in the right kidney (RK) and left kidney (LK) from autosomal dominant polycystic kidney disease (ADPKD) and control (non-ADPKD) Persian cats
| ADPKD |
Control |
P value | |
|---|---|---|---|
| n = 24 (RK + LK) | n = 140 (RK + LK) | ||
| Number of cysts per kidney (n) | |||
| Mean ± SD | 6.20 ± 2.17 | 0 | |
| Median | 6 | 0 | |
| Range | 2–10 | 0 | |
| Renal longitudinal length (cm) | <0.01* | ||
| Mean ± SD | 3.848 ± 0.528 | 3.542 ± 0.496 | |
| Median | 0.279 | 0.246 | |
| Range | 2.53–4.61 | 1.75–4.95 | |
| Renal echogenicity, n (%) | <0.01 † | ||
| Normal | 6 (25) | 86 (61.4) | |
| Increased | 18 (75) | 54 (38.6) | |
| Renal contour, n (%) | <0.0001 † | ||
| Regular | 12 (50) | 135 (96.4) | |
| Irregular | 12 (50) | 5 (3.6) | |
| Corticomedullary junction, n (%) | <0.000001 † | ||
| Preserved | 9 (37.5) | 131 (93.6) | |
| Blurring | 15 (62.5) | 9 (6.4) |
Bold denotes significance
Student’s t-test for equal variances
Fisher’s exact test
Table 2.
Descriptive data for sex, age and renal and hepatic cysts from Persian cats with autosomal dominant polycystic kidney disease (ADPKD)
| Animal | Sex | Age (months) | Number of renal cysts |
Presence of hepatic cysts | ||
|---|---|---|---|---|---|---|
| RK | LK | Total | ||||
| 1 | Female | 18 | 10 | 10 | 20 | No |
| 2 | Male | 18 | 5 | 5 | 10 | No |
| 3 | Female | 120 | 6 | 4 | 10 | No |
| 4 | Female | 60 | 5 | 5 | 10 | No |
| 5 | Male | 72 | 3 | 2 | 5 | No |
| 6 | Female | 138 | 6 | 6 | 12 | Yes |
| 7 | Female | 123 | 7 | 8 | 15 | Yes |
| 8 | Female | 162 | 10 | 6 | 16 | Yes |
| 9 | Female | 99 | 4 | 6 | 10 | No |
| 10 | Female | 11 | 2 | 2 | 4 | No |
| 11 | Male | 144 | 10 | 10 | 20 | No |
| 12 | Female | 84 | 7 | 10 | 17 | Yes |
RK = right kidney; LK = left kidney
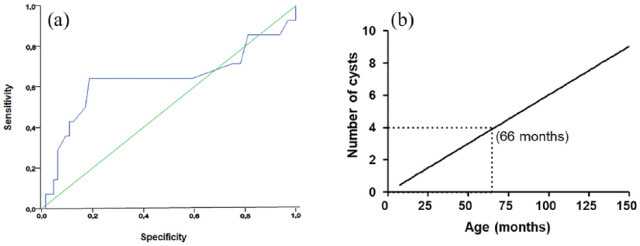
The area under the ROC curve was 0.636 for age (95% confidence interval [CI] 43.7–83.5%; P <0.05) and 1 for the number of cysts (95% CI 100–100%; P <0.05). The cut-off value for the number of cysts to discriminate between ADPKD and non-ADPKD animals associated with the highest sensitivity and specificity was three cysts in one or both kidneys (Figure 1a). Sensitivity and specificity were calculated using the positive differential rates, yielding values of 64.3% and 81.3%, respectively. The best cut-off for age, in turn, was 66 months (100% sensitivity and 100% specificity).
Figure 1.
(a) Receiver–operating characteristic (ROC) curves for age and number of cysts detected in Persian cats with autosomal dominant polycystic kidney disease (ADPKD). The area under the ROC curve for age was 0.636 (95% confidence interval [CI] 0.437–0.835; P <0.05) and 1 for number of cysts (95% CI 100–100%; P <0.05) for ADPKD diagnosis. (b) Linear regression curve trend line for age and number of cysts detected in Persian cats with ADPKD
Linear regression analysis was performed only for cats with ADPKD because the non-ADPKD animals did not have cysts and therefore did not constitute a measurable group for this statistical method. The lower limit value of the 95% CI was the minimum number of cysts in ADPKD animals over the animal’s life (in months). A separate analysis of the animals’ age groups was not performed for the calculation of minimum number of cysts, but rather a trend line that accounted for all evaluated animals was used. The most central region of the curve was used to select the age at which the cut-off should be made.
Linear regression analysis revealed a strong linear correlation (R2 = 0.74; P <0.0001) between age and the number of kidney cysts. The trend curve was used to extrapolate the number of cysts vs age according to the CI, enabling the establishment of specific thresholds for the diagnosis of ADPKD in Persian cats (Figure 1b). This analysis confirmed the data yielded by the ROC curve assessment, supporting a cut-off point of four cysts in animals at 66 months of age.
Based on the integrated analysis, it was possible to determine statistically supported ultrasonographic criteria for the diagnosis of ADPKD in Persian cats. The risk was determined based on the probability of a positive diagnosis as a function of the expected number of cysts for a given age. The presence of at least one renal cyst was sufficient to establish the diagnosis of ADPKD in animals aged 15 months or younger; two or more cysts in one or both kidneys are required for this diagnosis in cats aged 16–32 months; at least three cysts in one or both kidneys warrant the diagnosis of ADPKD in animals aged 33–49 months; and four or more cysts in one or both kidneys resulted in the diagnosis in cats aged 50–66 months (Table 3). The absence of renal cysts in all 70 non-affected cats at all evaluated ages, including ADPKD-containing and no ADPKD-containing pedigrees, associated with the high prevalence of ADPKD in Persian cats, enables the use of the proposed diagnostic criteria in all Persian cats, not restricting its application to animals that belong to ADPKD-linked pedigrees.
Table 3.
Diagnostic criteria for age-dependent kidney ultrasound in Persian cats with autosomal dominant polycystic kidney disease
| Age (months) | Diagnostic criteria for kidney ultrasound |
|---|---|
| ⩽15 | ⩾1 cysts |
| 16–32 | ⩾2 cysts |
| 33–49 | ⩾3 cysts |
| 50–66 | ⩾4 cysts |
Blood and urine analyses
None of the blood-based samples was discarded as a result of gross hemolysis or significant hyperlipidemia. No differences in hematological parameters were observed between ADPKD and non-ADPKD cats (see Table 1 in the supplementary material). ADPKD animals, however, exhibited higher serum calcium levels than non-affected cats (10.56 and 9.46 mg/dl, respectively; P <0.05) (Table 4). Serum creatinine concentration, as well as the proportion of cats with serum creatinine exceeding the reference intervals, did not significantly differ between the disease and non-disease groups. The levels of ALT, AST, GGT, ALP, TP, phosphate, sodium, potassium, chloride, and thyroxine also did not differ between the two groups (Table 4).
Table 4.
Descriptive statistics for serum biochemical parameters in autosomal dominant polycystic kidney disease (ADPKD) and control (non-ADPKD) Persian cats
| Analyte | Group | Mean ± SE | Median | Range | n | P value |
|---|---|---|---|---|---|---|
| BUN (mg/dl) | ADPKD | 23.04 ± 6.78 | 21.54 | 13.41–35.00 | 12 | 0.212* |
| Control | 20.87 ± 4.40 | 20.05 | 14.58–37.76 | 70 | ||
| Creatinine (mg/dl) | ADPKD | 1.283 ± 0.350 | 1.24 | 0.78–2.10 | 12 | 0.269* |
| Control | 1.084 ± 0.199 | 1.05 | 0.70–1.61 | 70 | ||
| ALT (U/dl) |
ADPKD | 45.76 ± 21.18 | 34.7 | 24.6–83.8 | 10 | 0.160* |
| Control | 64.22 ± 47.74 | 46.25 | 19.2–241.8 | 70 | ||
| AST (U/dl) |
ADPKD | 15.83 ± 4.615 | 15.25 | 9.3–24.77 | 10 | 0.667* |
| Control | 15.03 ± 6.303 | 14.25 | 7.1–43.0 | 70 | ||
| ALP (U/dl) |
ADPKD | 18.70 ± 9.32 | 16.65 | 9.2–35.82 | 10 | 0.133* |
| Control | 27.66 ± 21.21 | 22.75 | 6.0–139.9 | 68 | ||
| GGT (U/dl) |
ADPKD | 0.667 ± 1.385 | 0.667 | 0.0–4.2 | 10 | 1.00* |
| Control | 0.33 ± 0.64 | 0.329 | 0.0–2.9 | 70 | ||
| TP (g/dl) |
ADPKD | 7.289 ± 0.40 | 7.285 | 6.65–8.00 | 12 | 0.615* |
| Control | 7.05 ± 0.70 | 7.160 | 5.23–9.05 | 70 | ||
| ALB (g/dl) |
ADPKD | 3.063 ± 0.16 | 3.03 | 2.80–3.38 | 10 | 0.969 † |
| Control | 3.06 ± 0.30 | 3.12 | 2.13–3.60 | 70 | ||
| Calcium (mg/dl) | ADPKD | 10.55 ± 1.43 | 10.56 | 8.88–12.71 | 09 | 0.021 * |
| Control | 9.45 ± 1.00 | 9.46 | 7.45–13.28 | 69 | ||
| Phosphorus (mg/dl) | ADPKD | 4.63 ± 0.868 | 4.76 | 2.65–5.62 | 09 | 0.133* |
| Control | 5.34 ± 1.09 | 5.21 | 3.80–8.88 | 69 | ||
| Sodium (mEq/l) | ADPKD | 152.92 ± 2.16 | 152.2 | 150.8–156.0 | 09 | 0.484* |
| Control | 154.64 ± 4.89 | 153.1 | 147.3–172.3 | 67 | ||
| Potassium (mEq/l) | ADPKD | 4.60 ± 0.408 | 4.61 | 3.90–5.21 | 09 | 0.557* |
| Control | 4.74 ± 0.46 | 4.70 | 3.72–6.02 | 67 | ||
| Chloride (mEq/l) | ADPKD | 117.82 ± 1.68 | 117.30 | 115.5–120.4 | 09 | 0.910* |
| Control | 118.36 ± 3.89 | 117.40 | 112.3–129.2 | 67 | ||
| Total T4 (μg/dl) | ADPKD | 1.561 ± 0.185 | 1.64 | 1.24–1.74 | 07 | 0.725* |
| Control | 1.684 ± 0.496 | 1.58 | 0.89–2.50 | 39 |
Mann–Whitney U-test
Student’s t-test for different variances
Bold indicates P < 0.05
BUN = blood urea nitrogen; ALT = alanine aminotransferase; AST = aspartate aminotransferase; ALP = alkaline phosphatase; GGT = gamma-glutamyl transferase; TP = total protein; ALB = albumin; calcium = total serum calcium; phosphorus = serum inorganic phosphorus; sodium = serum sodium; potassium = serum potassium; chloride = serum chloride; T4 = thyroxine
Urine samples were not available for one ADPKD and 29 non-ADPKD cats owing to empty bladder or pregnancy. In addition, the amount of collected urine was insufficient for all analyses in three ADPKD and one control cat (<4 ml). The determination of USG, UPC and the proportion of cats with borderline proteinuria (UPC 0.2–0.4 [4/11 ADPKD cats, 11/41 non-ADPKD cats]) 28 or overt proteinuria (UPC >0.4 [1/11 ADPKD cats, 10/41 non-ADPKD cats]) did not differ significantly between affected and non-affected animals. Urinary dipstick testing, however, did not reveal significant differences in hemoglobin, acetone, protein, urobilinogen, glucose, bilirubin and nitrites between the ADPKD and non-ADPKD animals. The amount and type of urinary crystals and the proportion of cats with casts also did not differ between the two groups (Table 2 in the supplementary material).
Discussion
ADPKD is the most common inherited renal disease of domestic cats but primarily affects Persian cats and Persian-derived exotic breeds such as Himalayans, British Shorthairs and Scottish Folds. 19 The disease is characterized by the slow development of multiple renal and, occasionally, hepatic and pancreatic cysts, leading to end-stage renal disease late in life (>7 years of age). 17 Owing to the high morbidity/mortality of the disease, an early diagnosis of mutation-carrying animals is essential for a sustainable and healthy breeding program. Moreover, in symptomatic animals, complementary tests are necessary for the evaluation and clinical follow-up of the animal.
The only current molecular diagnosis with adequate accuracy is a PCR-RFLP, 19 or a real time-PCR assay. 12 However, because these molecular-genetic tests are available in so few international clinical veterinary centers, which limits access, and are relatively expensive, such tests are seldom performed in the clinical setting in developing countries. Consequently, US continues to be used routinely for disease investigation because it is an inexpensive and widely available modality in veterinary clinics. 14 However, the risk for false-positives compromises the quality of this test for the diagnosis of the disease. In this scenario, we propose here an ultrasonographic, age-based inclusion criterion supported by the certainty of genetic-molecular diagnosis for ADPKD in Persian cats, similar to the strategy used for the development of US diagnostic criteria for ADPKD in humans.5,6
Despite the occurrence of some cases of Persian cats with renal cystic disease not associated with the C→A transversion in exon 29 of PKD1, for which the diagnosis of ADPKD could not be excluded,14,16,22,29 this is the only pathogenic mutation to be linked to ADPKD in cats to date.19,30 The C→A transversion introduces a stop codon in the PKD1 transcript, leading to the truncation of polycystin-1, the protein product encoded by this gene. 19 In this context, other pathogenic mutations in PKD1 or other genes, such as PKD2, cannot be completely discarded as potential causes of feline ADPKD; however, heterozygosity for the C→A variant should be considered the cause of this disease in the absolute majority of – if not all – ADPKD-affected cats. 19 Based on these data, Persian cats without the C→A mutation can be virtually excluded to be affected by the disease.
Renal US remains not only the most practical non-invasive diagnostic method used in feline clinical practice, but also enables the assessment of severity and monitoring disease progression in affected cats. 15 Our study showed several non-cystic structural alterations in the kidneys of most cats diagnosed with ADPKD, from early adulthood onward. Such abnormalities included higher renal longitudinal length, irregular contour, increased cortical echogenicity and corticomedullary blurring. Increased cortical or cortical and medullary echogenicity can be a normal finding in cats, but is also one of the most common signs of chronic or acute kidney disease in this species. 31 In this study, therefore, this finding was not considered to be a reliable biomarker of renal injury; nevertheless, its prevalence was higher in ADPKD vs non-ADPKD animals. Renal cysts were not found in the genetically unaffected Persian cats, revealing that ultrasound specificity for cyst detection in our study was extremely high.
This finding is consistent with previous data for at-risk PKD cats.14,16 In addition, Gerwing et al examined the kidneys of 1527 non-Persian cats and found renal cysts in only 0.5%, 32 indicating a very low incidence of both PKD and acquired renal cysts in such cats.
The mean number of cysts in ADPKD cats was 7.0 ± 2.6, which were distributed in one or both kidneys. Ultrasonographic evaluation of severely affected cats is usually simple given the large number of renal cysts. However, small cysts in limited numbers can be more difficult to identify with certainty, especially when they are located in the medulla. This can occur as a result of low contrast between the anechoic cystic fluid and medullary echogenicity. 15 Ultrasound sensitivity for ADPKD diagnosis is expected to increase with age and to be associated with high specificity.9–11,18 A previous study comparing ultrasound results obtained at 3 and 12 months of age reported a diagnostic sensitivity of 100%; however, only a small number of cats were evaluated, the diagnosis was not anchored in genetic testing, and animal assessment was not performed. 33 An additional study, appropriately controlled by genetic testing, reported US sensitivity and specificity for ADPKD diagnosis of 96.2% and 91%, respectively, in Persian and Persian-related cats >10 months of age. 14 No analyses and criteria based on age and number of cysts, however, were proposed.
The age-specific positive and negative predictive values obtained in our analysis are only approximations of the post-test predictive values. However, the trends enable the generation of age-specific diagnostic criteria for ADPKD in Persian cats. The presence of at least one renal cyst in cats ⩽15 months of age is sufficient to establish the diagnosis; among those aged 16–32 months, ⩾2 uni- or bilateral cysts are needed to make this diagnosis; for animals aged 33–49 months, at least three cysts in one or both kidneys are required for diagnosis; and in cats 50–66 months of age, ⩾4 uni- or bilateral cysts are sufficient for the diagnosis of ADPKD. It must be noted that the absence of renal cysts in all non-ADPKD analyzed animals, the inclusion of all evaluated ages and pedigrees with and without cases of this disease, and the high prevalence of this disorder in Persian cats, do not limit the proposed diagnostic criteria to at-risk cats, but instead extends such criteria to the evaluation of all Persian cats. It is also important to note that these diagnostic criteria were developed for, and therefore should be applied to, ultrasound analysis performed at a renal cyst resolution of 0.3 cm.
Our data do not underplay the relevance of other imaging information for the potential diagnosis of ADPKD, including the presence of hepatic or pancreatic cysts, and at very young ages and renal enlargement. 18 However, our study revealed that most ADPKD-affected cats develop multiple bilateral renal cysts, indicating that equivocal results are exceptions rather than common events. This information is particularly important because our data were obtained from affected animals with serum creatinine levels not significantly different from control cats and therefore in ADPKD animals that, in large part, had not reached advanced stages of chronic kidney disease.
We currently have no robust explanations, however, for the increased serum calcium levels observed in ADPKD animals. The lack of other significant differences in hematological, serum biochemical and urine parameters between the ADPKD and non-ADPKD groups were consistent with previously reported data, given that most of the evaluated affected cats had not reached advanced phases of chronic kidney disease.17,18
Conclusions
Our study established ultrasonographic criteria for the diagnosis of ADPKD in Persian cats, supporting and guiding the use of this imaging technique for this purpose. The development of accurate age-based US criteria for this diagnosis is particularly relevant and useful in the clinical setting, given the current limitations to access and the cost of molecular genetics-based diagnostic tests.
Supplemental Material
Descriptive statistics for haematological parameters and urinary protein-to-creatinine ratio in autosomal dominant polycystic kidney disease (ADPKD) and control (non-ADPKD) Persian cats
Descriptive statistics for urinary parameters in autosomal dominant polycystic kidney disease (ADPKD) and control (non-ADPKD) Persian cats
Acknowledgments
The authors thank Associação dos Criadores de Gato Persa (São Paulo, SP, Brazil) for facilitating communication with the owners of the cats.
Footnotes
Supplementary material: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Supplementary Table 1: Descriptive statistics for hematological parameters and urinary protein-to-creatinine ratio in autosomal dominant polycystic kidney disease (ADPKD) and control (non-ADPKD) Persian cats.
Supplementary Table 2: Descriptive statistics for urinary parameters in autosomal dominant polycystic kidney disease (ADPKD) and control (non-ADPKD) Persian cats.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The study was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP grant numbers 12/19614-6 and 13/06471-5).
Accepted: 16 February 2018
References
- 1. Spithoven E, Kramer A, Meijer E, et al. Renal replacement therapy for autosomal dominant polycystic kidney disease (ADPKD) in Europe: prevalence and survival – an analysis of data from the ERA-EDTA Registry. Nephrol Dial Transplant 2014; 29: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet 2007; 369: 1287–1301. [DOI] [PubMed] [Google Scholar]
- 3. Chapman AB, Devuyst O, Eckardt KU, et al. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies conference. Kidney Int 2015; 88: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harris PC, Torres VE. Polycystic kidney disease, autosomal dominant. In: Pagon RA, Adam MP, Ardinger HH, et al. (eds). GeneReviews [Internet]. Seattle, WA: University of Washington, Seattle, 1993–2017. https://www.ncbi.nlm.nih.gov/books/NBK1246/ (updated June 11, 2015). [PubMed] [Google Scholar]
- 5. Ravine D, Gibson RN, Walker RG, et al. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 1994; 343: 824–827. [DOI] [PubMed] [Google Scholar]
- 6. Pei Y, Obaji J, Dupuis A, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 2009; 20: 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DiBartola S. Autosomal dominant polycystic kidney disease. Proceedings of the 18th Annual Veterinary Medical Forum of the American College of Veterinary Internal Medicine; 2000, May 25–28; Seattle, WA, USA: American College of Veterinary Internal Medicine, 2000, pp 438–440. [Google Scholar]
- 8. Barrs VR, Gunew M, Foster SF, et al. Prevalence of autosomal dominant polycystic kidney disease in Persian and related-breeds in Sydney and Brisbane. Aust Vet J 2001; 79: 257–259. [DOI] [PubMed] [Google Scholar]
- 9. Beck C, Lavelle RB. Feline polycystic kidney disease in Persian and other cats: a prospective study using ultrasonography. Aust Vet J 2001; 79: 181–184. [DOI] [PubMed] [Google Scholar]
- 10. Cannon MJ, Barr FJ, Rudorf H, et al. Prevalence of polycystic kidney disease in Persian cats in the United Kingdom. Vet Rec 2001; 149: 409–411. [DOI] [PubMed] [Google Scholar]
- 11. Barthez P, Rivier P, Begon D. Prevalence of polycystic kidney disease in Persian and Persian related cats in France. J Feline Med Surg 2003; 5: 345–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Helps C, Tasker S, Harley R. Correlation of the feline PKD1 genetic mutation with cases of PKD diagnosed by pathological examination. Exp Mol Pathol 2007; 83: 264–268. [DOI] [PubMed] [Google Scholar]
- 13. Domanjko-Petric A, Cernec D, Cotman M. Polycystic kidney disease: a review and occurrence in Slovenia with comparison between ultrasound and genetic testing. J Feline Med Surg 2008; 10: 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonazzi M, Volta A, Gnudi G, et al. Comparison between ultrasound and genetic testing for the early diagnosis of polycystic kidney disease in Persian and Exotic Shorthair cats. J Feline Med Surg 2009; 11: 430–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wills SJ, Barrett EL, Barr FJ, et al. Evaluation of the repeatability of ultrasound scanning for detection of feline polycystic kidney disease. J Feline Med Surg 2009; 11: 993–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee Y-J, Chen H-Y, Hsu W-L, et al. Diagnosis of feline polycystic kidney disease by a combination of ultrasonographic examination and PKD1 gene analysis. Vet Rec 2010; 167: 614–618. [DOI] [PubMed] [Google Scholar]
- 17. Eaton KA, Biller DS, DiBartola SP, et al. Autosomal dominant polycystic kidney disease in Persian and Persian-cross cats. Vet Pathol 1997; 34: 117–126. [DOI] [PubMed] [Google Scholar]
- 18. Biller DS, DiBartola SP, Eaton KA, et al. Inheritance of polycystic kidney disease in Persian cats. J Hered 1996; 87: 1–5. [DOI] [PubMed] [Google Scholar]
- 19. Lyons LA, Biller DS, Erdman CA, et al. Feline polycystic kidney disease mutation identified in PKD1. J Am Soc Nephrol 2004; 15: 2548–2555. [DOI] [PubMed] [Google Scholar]
- 20. Biller DS, Chew DJ, DiBartola SP. Polycystic kidney disease in a family of Persian cats. J Am Vet Med Assoc 1990; 196: 1288–1290. [PubMed] [Google Scholar]
- 21. Reichle JK, DiBartola SP, Léveillé R. Renal ultrasonographic and computed tomographic appearance, volume, and function of cats with autosomal dominant polycystic kidney disease. Vet Radiol Ultrasound 2002; 43: 368–373. [DOI] [PubMed] [Google Scholar]
- 22. Kappe EC, Hecht W, Gerwing M, et al. Polycystic kidney disease in the German population of Persian cats. A comparative study of ultrasonographical examination and genetic testing. Tierarztl Prax Ausg K 2005; 33: 413–418. [Google Scholar]
- 23. Paepe D, Verjans G, Duchateau L, et al. Routine health screening of apparently healthy middle-aged and old cats. J Feline Med Surg 2013; 15: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meyer DJ. Microscopic examination of the urinary sediment. In: Raskin RE, Meyer DJ. (eds). Atlas of canine and feline cytology. Philadelphia, PA: WB Saunders, 2001, pp 261–276. [Google Scholar]
- 25. Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr 2007; 96: 644e7. [DOI] [PubMed] [Google Scholar]
- 26. Ward CD. The differential positive rate, a derivative of receiver operating characteristic curves useful in comparing tests and determining decision levels. Clin Chem 1986; 32: 1427e8. [PubMed] [Google Scholar]
- 27. Deeks JJ, Altman DG. Education and debate. Statistics notes. Diagnostic tests 4: likelihood ratios. BMJ 2004; 329: 168e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lees GE, Brown SA, Elliott J, et al. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM forum consensus statement (small animal). J Vet Intern Med 2005; 19: 377–385. [DOI] [PubMed] [Google Scholar]
- 29. Guerra JM, Daniel AGT, Cardoso NC, et al. Congenital hepatic fibrosis and polycystic kidney disease not linked to C >A mutation in exon 29 of PKD1 in a Persian cat. JFMS Open Rep 2015; 1: DOI: 10.1177/2055116915619191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Young AE, Biller DS, Herrgesell EJ, et al. Feline polycystic kidney disease is linked to the PKD1 region. Mamm Genome 2005; 16: 59–65. [DOI] [PubMed] [Google Scholar]
- 31. Debruyn K, Haers H, Combes A, et al. Ultrasonography of the feline kidney: technique, anatomy and changes associated with disease. J Feline Med Surg 2012; 14: 794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gerwing M, Michele U, Kramer M, et al. PKD (polycystic kidney disease) – Polyzystisches syndrome. Tierarztl Prax Ausg K 1999; 80: 374–396 [Google Scholar]
- 33. Ottesen N. Polycystic kidney disease in Persian cats: comparison of renal ultrasonography at the age of 3 and 12 months [abstract]. Vet Radiol Ultrasound 2004; 45: 600. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Descriptive statistics for haematological parameters and urinary protein-to-creatinine ratio in autosomal dominant polycystic kidney disease (ADPKD) and control (non-ADPKD) Persian cats
Descriptive statistics for urinary parameters in autosomal dominant polycystic kidney disease (ADPKD) and control (non-ADPKD) Persian cats