Abstract
Objectives
The main aim of the study was to establish response, disease-free interval (DFI) and overall survival of cats with nasal planum squamous cell carcinoma (SCC) treated with Sr90 plesiotherapy. A secondary aim was to determine whether a fractionated protocol is more effective than a single-dose protocol in terms of response, DFI and overall survival. The third aim was to evaluate whether we can identify prognostic factors that influence overall survival.
Methods
This was a retrospective study that included cats with a diagnosis of nasal planum SCC treated with Sr90 plesiotherapy at a single institution.
Results
Seventy-four cats were included in the study. Thirty-two were treated with a fractionated protocol and 42 with a single-dose treatment. Sr90 plesiotherapy was able to induce complete response in 74% of cats with nasal planum SCC. The median DFI was 780 days (95% confidence interval [CI] 383–1177), with 17% of cats experiencing local recurrence. The overall survival for all cats was 1039 days (95% CI 55–1528). The DFI of cats treated with the fractionated Sr90 was significantly longer compared with the single-dose treatment, whereas response and overall survival were not statistically different. Other prognostic factors that influenced the overall survival were early-stage disease, absence of concurrent problems and complete response to the treatment. Acute and long-term toxicity associated with the treatment were minimal and the aesthetic outcome was pleasing in almost all cases.
Conclusions and relevance
Strontium plesiotherapy is a safe and effective treatment of nasal planum SCC in cats.
Introduction
Squamous cell carcinoma (SCC) is one of the most common malignant skin tumours in cats, and accounts for between 15% and 25% of cutaneous tumours in this species.1,2 Solar exposure is important in its development and SCCs of this aetiology are seen almost exclusively in non-pigmented areas of the head, such as nasal planum, eyelids and pinnae, with white cats or coloured cats with white areas being at greater risk.3,4
The stage of cutaneous SCC (T-stage) is defined by the depth of invasion and by the size of the lesion (Table 1). 5 Feline cutaneous SCCs are relatively slow to metastasise and are reported to spread to the draining lymph nodes and the lungs. 4
Table 1.
World Health Organization classification of feline tumours of epidermal origin
| Tis | Pre-invasive carcinoma |
| T0 | No evidence of tumour |
| T1 | Tumour <2 cm maximum diameter, superficial or exophytic |
| T2 | Tumour 2–5 cm maximum diameter, or with minimal invasion, irrespective of size |
| T3 | Tumour >5 cm maximum diameter, or with invasion of the subcutis, irrespective of size |
| T4 | Tumour invading other structures such as fascia, muscle, bone or cartilage |
Strontium plesiotherapy (Sr90) is an effective treatment of early-stage nasal planum SCC in cats (Tis, T1 and T2), whereas it is considered ineffective for advanced-stage SCC (T3 and T4). 4 Currently, there are two radiation protocols published in the veterinary literature. The first consists of a total dose of 200 Gy administered in five fractions on an alternate-day basis; 6 the second consists of a total dose of 97–195 Gy administered in a single treatment. 7 Both protocols are reported to induce complete remission in ~85% of cats. For cases that achieve complete remission, recurrence has not been reported with the fractionated protocol and a low recurrence rate is expected, whereas local recurrence has been reported in 20% of cats treated with a single fraction.6,7
The aim of this retrospective study was to establish response, disease-free interval (DFI) and overall survival (OS) of a large cohort of cats with nasal planum SCC treated with Sr90. A secondary aim was to determine whether the fractionated protocol (5-Sr) is more effective than the single-dose protocol (1-Sr) in terms of response, DFI and OS. Finally, we evaluated whether prognostic factors that influence OS could be identified.
Materials and methods
Case selection
The database of a single institution was searched for cats treated with Sr90 between 1992 and 2017. Cats were included in the study if there was a histological diagnosis of nasal planum SCC. Information collected for each cat included signalment (breed, age, sex), concurrent diseases, clinical stage, staging investigations, protocol used (5-Sr or 1-Sr, total dose, number of treatment fields) and toxicity (acute and late using the Veterinary Radiology Therapy Oncology Group [VRTOG] criteria). 8
Procedures
The strontium applicator has a 0.7 cm2 active area and is attached to a hand-held probe with a Perspex guard. All cats received Sr90 under general anaesthesia in a designated ‘radiation-controlled area’. All treatments were administered by an oncologist and consisted of the application of a variable number of overlapping fields to cover the entire lesion with margins of at least 2 mm around the tumour. The total dose prescribed and the fractionation (5-Sr or 1-Sr) depended on the clinical judgement of the oncologist in charge of the case or on the owner’s preference. 5-Sr was delivered in five fractions on a Monday–Wednesday–Friday schedule (total dose range 200–260 Gy), whereas 1-Sr was delivered as a single treatment (total dose range 85–140 Gy). The duration of exposure to deliver the prescribed dose was calculated on the day of the first treatment using an internally developed electronic spread sheet taking into account the source decay over the time. The dose reported is the dose delivered to the surface, whereas the dose at 2 mm depth is ~30% of the surface dose.
Statistical analysis
The outcome measures evaluated are response, DFI and OS. The response assessment (complete response [CR], partial response [PR], stable disease [SD] or progressive disease [PD]) was based on RECIST criteria, 9 and evaluated 6–8 weeks after treatment. When the owner could not come to the hospital for a revisit, the response was assessed with a digital image sent via email. DFI was defined as the period from the date of the first Sr90 until the date of recurrence (local in the nasal planum within or without the radiation field, loco-regional to the draining lymph nodes or systemic metastasis) and OS was defined as the period from the date of the first Sr90 until death from any cause. When the exact date of an event was unknown (eg, a cat died in November 2015) we approximated the date to the first day of the month. If partial or incomplete information was available from our records, we contacted the referring veterinary practice for an update. Cats without follow-up after the first Sr90 were excluded.
A χ2 or Fisher’s exact test was used to compare categorical variables. The Student’s t-test was used to assess normal continuous variables. Survival analysis (Kaplan–Meier and log-rank) was used to compare outcomes of the two different protocols (5-Sr and 1-Sr) and to evaluate other prognostic factors. The following categories were used for the statistical analyses: age (divided into quartiles), sex (male neutered, male entire, female neutered), concurrent diseases (present or absent), T-stage (early stage [Tis, T1 and T2]; late stage [T3 and T4]), total dose (<135 Gy or ⩾135 Gy [135 Gy was the median dose]), the number of treatment fields (<3 or ⩾3 [3 was the median number of fields]), recurrence for cats that achieved CR (occurred or did not occur) and response to the first Sr90 (CR, PR, PD). Factors with a P value <0.10 were included in the Cox multivariate regression analysis. Results of the statistical tests were considered significant at P <0.05.
Commercial software was used for the statistical analysis (IBM SPSS Statistic for Windows, Version 21.0).
Results
Patients
In total, 123 cats treated with Sr90 between 1992 and 2017 were found in our database (Figure 1). Seventy-four cats were included in the study (mean age 11.5 years; median age 11.1 years [range 3.1–20.1 years]). There were 49 neutered males, 22 neutered females and one entire male; the gender was unknown in two cats. Sixty-six cats were domestic shorthairs, five were domestic longhairs and one was a ragdoll; the breed for two cats was unknown. Sixty-four cats (86%) had a coloured coat with white areas or white coat, while four had a solid colour (5%); hair colour was unknown for six cats.
Figure 1.
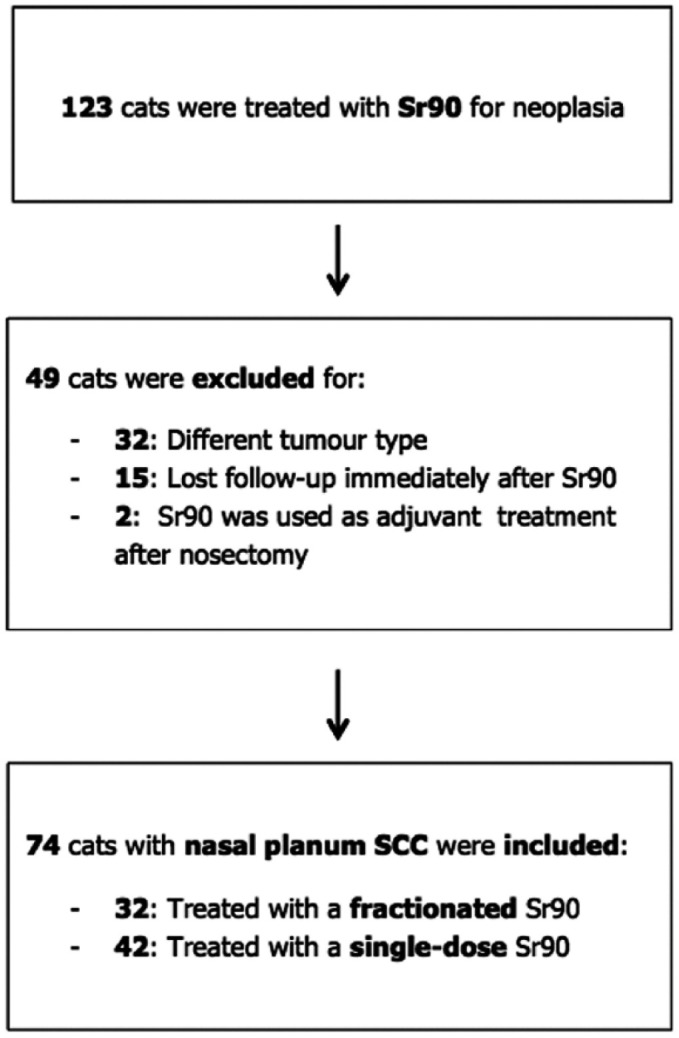
Flow-chart representing the inclusion process of the cases in the present study. SCC = squamous cell carinoma
Tumour staging and concurrent problems
Local stage was evaluated in all cats. The SCC was staged as in situ (Tis) in nine (12%), T1 in 17 (23%), T2 in 42 (57%), T3 in four (5%) and T4 in two cats (3%). There was a significant difference in the distribution of stages between the two treatment groups (Pearson’s χ2 P = 0.018); 1-Sr had more Tis and T1 than 5-Sr (50% vs 16%). All cats had pre-anaesthetic blood tests. Other investigations to evaluate local invasion, local and distant spread, or concurrent problems were performed in 44 cats (59%). Local invasion was evaluated in five cats (CT of the head), local spread in 12 cats (lymph node cytology), thoracic imaging was performed in 45 cats (38 thoracic radiographs, five thoracic CT and two echocardiography) and abdominal imaging in nine cats (seven abdominal radiographs and two abdominal ultrasound). None of the cats presented with local or distant metastases. Twenty-three cats (31%) had concurrent problems, including eight with heart murmurs and/or cardiac disease, five with SCCs affecting either pinnae or eyelids, four with chronic kidney disease (CKD) and hyperthyroidism, three with CKD, one with hyperthyroidism, one with epilepsy and one with pancreatic carcinoma. Four cats with concurrent problems were treated with 5-Sr and 19 with 1-Sr. There was a significant statistical difference in the distribution of concurrent diseases between the two treatments groups (χ2 P = 0.003).
Treatment
Thirty-two cats (43%) were treated with 5-Sr and 42 (57%) with 1-Sr. Mean and median dose for 5-Sr was 233 Gy and 235 Gy, respectively (range 200–260 Gy), whereas mean and median for 1-Sr was 120 Gy (range 85–140 Gy). There was a significant statistical difference between the mean total dose of the two treatment groups (t-test P <0.001). For the overall population mean and median number of treatment fields was 2.7 and 3.0, respectively, and there was no statistical difference between 5-Sr and 1-Sr (t-test P = 0.30).
Response, recurrence rate and DFI
After treatment with Sr90, 55 (74%) tumours achieved CR, 16 (22%) PR and three (4%) PD. There was a significant association between the T-stage before Sr90 and response (χ2 P = 0.002). CR was achieved in 89% of cats with Tis, 94% of T1, 68% of T2, 50% of T3 and 0% of T4. Of cats that received 5-Sr, 23 (72%) achieved CR, eight (25%) PR and one (3%) PD, whereas of the cats that received 1-Sr, 32 (76%) achieved CR, eight (19%) PR and two (5%) PD. There was no significant statistical difference in the response between 5-Sr and 1-Sr (χ2 P = 0.79).
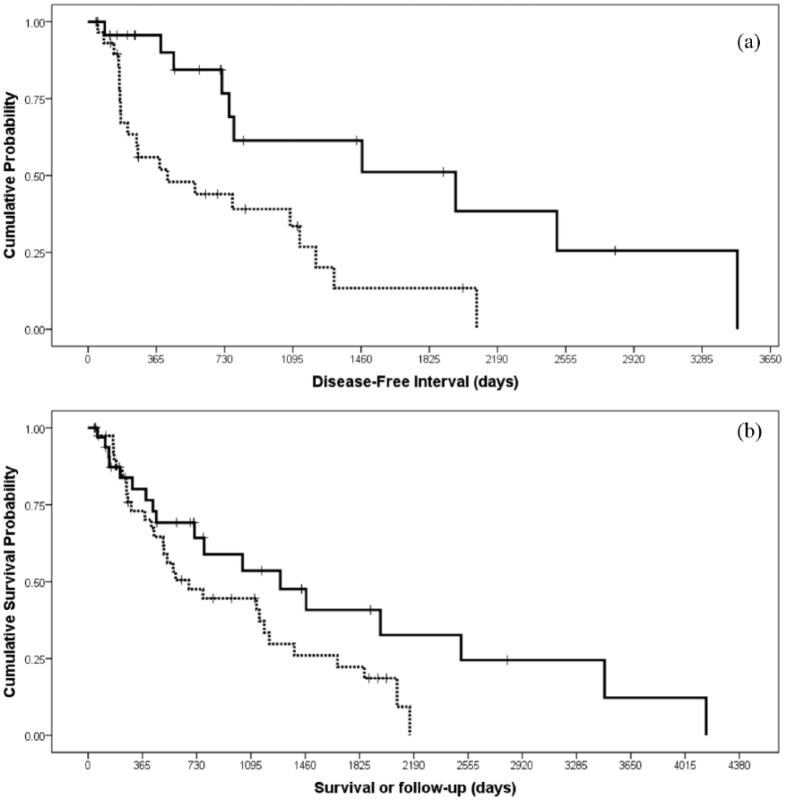
Of the 55 cats achieving CR, the overall DFI was 780 days (95% CI 383–1177). The DFI was significantly longer in cats that received 5-Sr (1966 days; 95% CI 413–3518) compared with 1-Sr (248 days; 95% CI 0–911) (log-rank P = 0.004; Figure 2). Recurrence occurred in the nasal planum in 17 (31%) cats and it was within the radiation field in four cats, marginal to the field in two cats and outside the radiation field in five cats. Among cats that experienced recurrence, six received 5-Sr and 11 received 1-Sr. There was no significant difference in recurrence rate between the two protocols (χ2 P = 0.74). The median time of recurrence was 251 days (95% CI 48–454) and there was no significant difference in time to recurrence between 5-Sr and 1-Sr (log-rank P = 0.41). The distribution of the total dose between SCC that recurred and that did not recur was not statistically different (Mann–Whitney-U-test P = 0.34). After recurrence, 12 cats received a second Sr90 (all 1-Sr) and then four cats went on to receive further treatment (two nosectomies, one external-beam radiotherapy and one topical imiquimod). The median survival of these 17 cats after recurrence was 974 days (95% CI 367–1581).
Figure 2.
Kaplan–Meier survival plots comparing (a) disease-free interval (DFI) and (b) overall survival (OS) of cats treated with a fractionated (continuous line) or a single-dose Sr90 protocol (dotted line). There was a statistically significant difference in DFI (P = 0.004) but not in OS (P = 0.07)
Of the 16 cats achieving PR after Sr90, six cats received a second Sr90 treatment (all 1-Sr), whereas two were treated with external-beam radiotherapy. After the second Sr90, three cats went on to receive further treatment (one nosectomy, one external-beam radiotherapy and one third treatment of Sr90). The median survival of these 16 cats after the first Sr90 was 435 days (95% CI 166–704).
Three cats developed progressive disease after Sr90; the survival after Sr90 was 63, 65 and 290 days, respectively. The cat that lived longer was treated with external-beam radiotherapy as rescue treatment.
Overall survival and prognostic factors
During the follow-up period, 47 (64%) cats died and 27 (36%) were still alive at the time of data collection. The median OS of the 74 cats included in the study was 1039 days (95% CI 55–1528). Cats treated with 5-Sr had an OS of 1293 days (95% CI 491–2095) and cats treated with 1-Sr of 678 days (95% CI 338–1018). There was no difference in survival between the two protocols (log-rank P = 0.07; Figure 2).
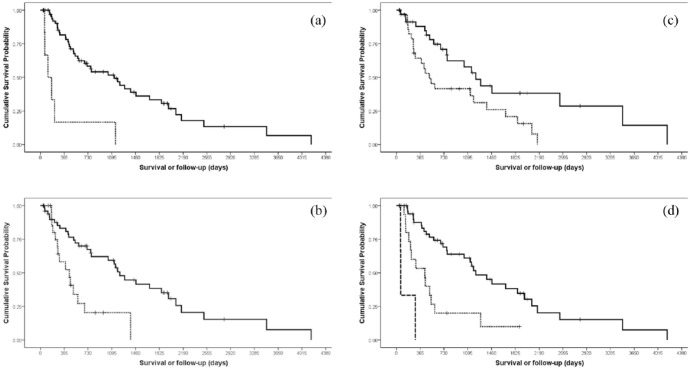
Among the prognostic factors evaluated with the log-rank, age (P = 0.11), sex (P = 0.29), number of treatment fields (P = 0.33) and recurrence for cats that achieved CR (P = 0.24) did not significantly affect the OS, whereas T-stage (P <0.001), presence of concurrent diseases (P = 0.001), total dose (P = 0.01) and response to the first Sr90 (P <0.001) were significant. Results are summarised in Table 2 and Figure 3.
Table 2.
Median survival times (MSTs) and significance of prognostic factors affecting overall survival
| Variable | Category | Number of cats | Number of events | MST in days (95% CI) | P value |
|---|---|---|---|---|---|
| T-stage | Early (Tis, T1 and T2) | 68 | 41 | 1132 (633–1631) | <0.001 |
| Advanced (T3 and T4) | 6 | 6 | 115 (0–242) | ||
| Concurrent diseases | Absent | 51 | 32 | 1218 (1004–1432) | 0.001 |
| Present | 23 | 15 | 443 (222–664) | ||
| Total dose | ⩾135 Gy | 34 | 19 | 1218 (864–1572) | 0.01 |
| <135 Gy | 33 | 23 | 505 (302–708) | ||
| Response | CR | 55 | 31 | 1218 (886–1550) | <0.001 |
| PR | 16 | 13 | 435 (166–704) | ||
| PD | 3 | 4 | 65 (62–68) |
T-stage = stage of cutaneous squamous cell carcinoma; CI = confidence interval; CR = complete response; PR = partial response; PD = progressive disease
Figure 3.
Kaplan–Meier survival plots of significant prognostic factors affecting overall survival. (a) Stage of cutaneous squamous cell carcinoma (early stage shown by continuous line; advanced stage shown by dotted line) (P <0.001). (b) Concurrent diseases (absent shown by continuous line; present shown by dotted line) (P = 0.001). (c) Total dose divided by the median value (⩾135 Gy shown by continuous line; <135 Gy shown by dotted line) (P = 0.01). (d) Response to the first Sr90 (complete response shown by continuous line; partial response by dotted line; and progressive disease by dashed line) (P <0.001)
The multivariate analysis (Table 3) confirmed the significance of the T-stage (P <0.001), the presence of concurrent diseases (P = 0.003) and the response to the first Sr90 (P <0.001), whereas the total dose lost its significance (P = 0.10). The risk associated with individual factors was high for concurrent disease (hazard ratio [HR] 3.72) and response to the first Sr90 (HR for PR 4.76; HR for PD 8.66), and low for the T-stage at presentation (HR for advanced stage 0.08).
Table 3.
Multivariate Cox regression analysis of prognostic factors affecting survival
| Variable | Category | Multivariate analysis HR (95% CI) | P value |
|---|---|---|---|
| T-stage | Early | Reference | <0.001 |
| Advanced | 0.08 (0.02–0.30) | ||
| Concurrent diseases | Absent | Reference | 0.003 |
| Present | 3.72 (1.58–8.76) | ||
| Total dose | ⩾135 Gy | Reference | 0.10 |
| <135 Gy | 2.43 (0.83–7.14) | ||
| Response | CR | Reference | <0.001 |
| PR | 4.76 (2.14–10.59) | ||
| PD | 8.66 (2.19–34.29) | ||
| Protocol | Fractionated | Reference | 0.53 |
| Single-dose | 0.70 (0.22–2.19) |
T-stage = stage of cutaneous squamous cell carcinoma; HR = hazard ratio; CI = confidence interval; CR = complete response; PR = partial response; PD = progressive disease
Toxicity and aesthetic outcome
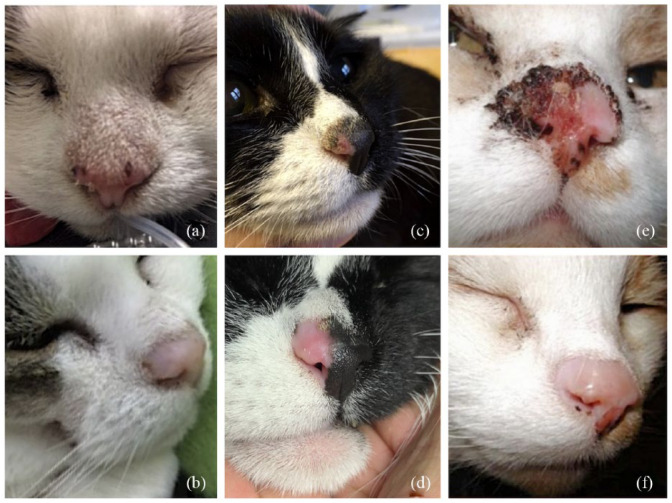
Acute toxicity was described in 19 cases. Dermatitis was classified as mild (VRTOG grade 1) in 15 cats, moderate (VRTOG grade 2) in one cat and severe (VRTOG grade 3) in three cats. Long-term side effects were consistent with alopecia in all cases. Three cats developed epidermal hyperplasia and hyperkeratosis confirmed on histopathology. The cosmetic outcome was described by the attending clinician in 21 cats and judged as excellent or very good in 18 cats or pleasing with a mild deformation of the cartilage in three cats (Figure 4).
Figure 4.
Three cats with nasal planum squamous cell carcinoma (SCC) before and after Sr90 plesiotherapy. (a,b) Complete response of two small superficial SCCs (stage Tis). (c,d) Complete response of an early-stage SCC (stage T1). (e,f) Partial response of an advanced SCC (stage T3). The nasal philtrum was treated with a second Sr90 treatment
Discussion
Signalment and hair colour of cats included in this study was similar to that described in previous literature.6,7,10,11 The SCC was at an early local stage in the majority of cats (92%) and advanced in few cases (8%). Staging for systemic disease was not complete in all cats, but in none of the cases was evidence of local or distant metastasis at presentation found. 10 Concurrent problems were present in 31% of cats, which is similar to what has been reported. 7
The main aim of this retrospective study was to establish response and outcomes of cats with nasal planum SCC treated with two different protocols of Sr90. We found that Sr90 is able to induce CR in 76% of cats. The DFI for cats that achieved CR was 780 days and was significantly longer with 5-Sr compared with 1-Sr. These results are similar to a previous study describing the use of a fractionated protocol; after the first Sr90, CR was achieved in 73% of cats and the DFI was 652 days. 6 A higher response rate and DFI was seen in a study describing a single-dose protocol, 7 in which CR was achieved in 88% of cats and the DFI was 1710 days. The better response rate could be explained with the inclusion of cats with advanced disease and a lower percentage of cats with SCC in situ in our study. The difference in DFI was also conspicuous considering that the DFI of cats treated with 1-Sr in our study was only 248 days. The main reason for such disparity lies within the different definition of DFI: in our study we defined DFI as the time between the first Sr90 and the occurrence of another nasal planum SCC (within or outside the radiation field), whereas in the study by Hammond et al they only considered SCC recurring within the radiation field as recurrence. 7 In our opinion, the definition of DFI that we adopted is more representative of the progression of the disease accounting for the ‘field carcinogenesis’ and provides more relevant information about the chances of another SCC occurring.
From previous studies, we were expecting a low recurrence rate with 5-Sr, 6 and a high recurrence rate with 1-Sr. 7 The overall recurrence rate in this study was 31%, but we could not demonstrate a significant difference between 5-Sr and 1-Sr (26% vs 34%). Interestingly, tumour recurrence did not significantly influence OS and cats lived for a long time after recurrence, even if additional treatment was not pursued.
The OS in this study was 1039 days and there was no significant difference between patients receiving 5-Sr and 1-Sr. Cats treated with 5-Sr had an OS of 1293 days, which was slightly longer than the 780 days reported in the study by Goodfellow et al, 6 whereas cats treated with 1-Sr had an OS of 678 days. Unfortunately, the study by Hammond et al could not be used as comparison because the authors reported the tumour-specific survival (all cats that died for a cause unrelated to the SCC were censored) and not the OS. 7
A secondary aim was to evaluate whether 5-Sr was more effective than 1-Sr. In this study population, the only significant difference between the two treatments groups was the DFI; response rate and OS were not different. These findings suggest that cats achieving CR after Sr90 enjoyed a longer DFI if they were treated with 5-Sr than with 1-Sr. However, the OS of cats treated with 5-Sr or 1-Sr was not different, implying that the time to recurrence does not impact on OS. From this retrospective study is not possible to establish which protocol is superior and a controlled study should be prospectively designed to address this aspect.
Our study found few prognostic factors that were significantly associated with the OS in the univariate and multivariate analysis. The first one was T-stage at presentation, which made perfect biological sense considering that the higher the T-stage the deeper the infiltration of the tumour, and given that one of the main limitations of Sr90 is the poor penetration of the β-particles into the tissue. In the multivariate analysis, cats with advanced stage had an 8% increased risk of dying vs cats at an early stage.
The second factor was the presence of concurrent health conditions. It is intuitive that cats with extension of the SCC to other sites or with systemic conditions are likely to succumb earlier than cats with only the nasal planum SCC. Cats with a concurrent problem were more likely to be treated with 1-Sr because the aim of the treatment was palliative rather than curative intent. During the follow-up time cats with concurrent disease were 3.72 times more likely to die than cats without any other condition.
The third factor was the response to the first Sr90. Cats with CR of the tumour survived longer than cats that had a PR or PD. Cats that achieved PR were 4.76 more at risk of dying than cats that achieved CR; the risk was 8.66 times higher for cats that achieved PD. This prognostic factor was also described in the study by Hammond et al. 7
Finally, the total dose, as a categorical variable dichotomised by the median value, was significant in the univariate analysis but lost its significance in the multivariate analysis. The only explanation we can provide for this finding is that the dose prescribed was, in part, biased by the judgement of the attending clinician (eg, small and superficial tumours were prescribed a lower total dose than large and deep tumours; cats with concurrent problems that could not be anaesthetised many times received a higher dose, etc). This hypothesis is supported by the fact that the total dose administered to cats that experienced recurrence was not statistically different from cats that did not.
Acute toxicity after completing Sr90 was described in 19 cats. In the majority of cases the toxicity was mild (79%), but in a few cases it was classified as moderate (5%) or severe (16%). In our experience, the treatment area becomes initially mildly erythematous in the periphery and then a thick scab forms over the neoplastic ulcer. Over the following 4–8 weeks, the scab becomes gradually smaller and then falls off. Given that most nasal planum SCCs present clinically as non-healing ulcers, in some case it is difficult to distinguish between neoplastic ulceration and radiation toxicity, and the only way is to evaluate the lesion progression over the time. Long-term side effects included alopecia and, in a few cases, epidermal hyperplasia and/or hyperkeratosis. The latter presents clinically as scaling dermatitis or as a non-healing ulcer, and both these presentations can be misinterpreted as recurrence. Thus, a biopsy should always be performed when recurrence is suspected before recommending additional treatment. According to the attending clinician and with all the limitations of retrospective studies, the cosmetic outcome of most patients was pleasing, but occasionally a small deformation of the underlying cartilage remained.
Conclusions
Sr90 is a safe and effective treatment of nasal planum SCC in cats. In this study, 5-Sr90 was associated with a longer DFI than 1-Sr but did not affect response or OS. Other important prognostic factors that affected OS are T-stage at presentation, presence of concurrent diseases and response to the first Sr90.
Footnotes
Accepted: 3 April 2018
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Davide Berlato  https://orcid.org/0000-0001-7951-428X
https://orcid.org/0000-0001-7951-428X
References
- 1. Bostock DE. Neoplasms of the skin and subcutaneous tissues in dogs and cats. Br Vet J 1986; 142: 1–19. [DOI] [PubMed] [Google Scholar]
- 2. Miller MA, Nelson SL, Turk JR, et al. Cutaneous neoplasia in 340 cats. Vet Pathol 1991; 28: 389–395. [DOI] [PubMed] [Google Scholar]
- 3. Dorn CR, Taylor DO, Schneider R. Sunlight exposure and risk of developing cutaneous and oral squamous cell carcinomas in white cats. J Natl Cancer Inst 1971; 46: 1073–1078. [PubMed] [Google Scholar]
- 4. Murphy S. Cutaneous squamous cell carcinoma in the cat: current understanding and treatment approaches. J Feline Med Surg 2013; 15: 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Owen LN. TNM classification of tumours in domestic animal. Geneva: World Health Organization, 1980. [Google Scholar]
- 6. Goodfellow MR, Hayes AM, Murphy S, et al. A retrospective study of 90Strontium plesiotherapy for feline squamous cell carcinoma of the nasal planum. J Feline Med Surg 2006; 8: 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hammond GM, Gordon IK, Theon AP, et al. Evaluation of strontium Sr 90 for the treatment of superficial squamous cell carcinoma of the nasal planum in cats: 49 cases (1990–2006). J Am Vet Med Assoc 2007; 231: 736–741. [DOI] [PubMed] [Google Scholar]
- 8. LaDue TA, Klein MK. Toxicity criteria of the veterinary radiation therapy oncology group. Vet Radiol Ultrasound 2001; 42: 475–476. [DOI] [PubMed] [Google Scholar]
- 9. Nguyen SM, Thamm DH, Vail DM, et al. Response evaluation criteria for solid tumours in dogs (v1.0): a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol 2015; 13: 176–183. [DOI] [PubMed] [Google Scholar]
- 10. Lana SE, Ogilvie GK, Withrow SJ, et al. Feline cutaneous squamous cell carcinoma of the nasal planum and the pinnae: 61 cases. J Am Anim Hosp Assoc 1997; 33: 329–332. [DOI] [PubMed] [Google Scholar]
- 11. Théon AP, Madewell BR, Shearn VI, et al. Prognostic factors associated with radiotherapy of squamous cell carcinoma of the nasal plane in cats. J Am Vet Med Assoc 1995; 206: 991–996. [PubMed] [Google Scholar]