Abstract
Objectives
Our objectives were, first, to determine if therapeutic serum theophylline concentrations could be achieved using long-term, once-daily dosing of transdermal theophylline and, secondarily, to evaluate the difference between two transdermal theophylline formulations.
Methods
Seven healthy cats, 1–10 years of age, were evaluated in a two-way, randomized, double-blinded, crossover study. Participants received transdermal theophylline at 15 mg/kg for 21 days in either pluronic lecithin organogel (PLO) or Lipoderm formulation. On day 22, blood was collected 2, 6, 14 and 24 h after dosing. After a 14 day washout period, blood was collected to verify non-detectible theophylline concentrations. The alternate formulation was administered for 21 days, and sampling was repeated. Serum theophylline concentrations were determined using an automated immunoassay. Serum concentrations were compared between formulations using a two-way random-measures ANOVA and over time within a formulation using a repeated-measures ANOVA.
Results
Therapeutic serum theophylline concentrations were achieved for 2/7 cats in each group. Of 56 serum theophylline measurements obtained, only seven (13%) were within the therapeutic range. No significant difference was detected in drug concentrations achieved by the transdermal formulations at any time point. In addition, no significant difference in serum theophylline concentrations was noted between time points for PLO (P = 0.751) or Lipoderm (P = 0.107).
Conclusions and relevance
Once-daily transdermal dosing of theophylline does not reliably achieve therapeutic concentrations. Individual cats may achieve therapeutic concentrations. No significant difference was noted between PLO and Lipoderm formulations. Therefore, transdermal theophylline formulations should not be considered as a first-line therapy in feline asthma patients.
Introduction
Feline asthma is a significant respiratory disease that affects as much as 5% of the pet feline population. 1 Mainstays of therapy for feline asthma involve administration of glucocorticoids and bronchodilators. While inhaled bronchodilators may be an attractive option for long-term management to avoid oral dosing of cats, long-term use of the commonly prescribed racemic form of the inhaled bronchodilator albuterol can increase airway inflammation. 2 Thus, the oral bronchodilator theophylline may be a better option for providing therapy without inciting airway inflammation during long-term management of feline asthmatic patients.
For efficacious therapy, most asthmatic cats must be dosed with a bronchodilator at least once daily. 3 Owner compliance can decrease if the pet is uncooperative during medication administration. Transdermal application is easier and improves owner compliance; 4 however, very little scientific evidence exists regarding the bioavailability and pharmacokinetics of transdermal medications. The pharmacokinetics of transdermal delivery of glipizide, 5 fluoxetine, 6 phenobarbital, 7 amlodipine, 8 methimazole, 9 amitriptyline, 10 buspirone, 10 dexamethasone, 11 ondansetron 12 and mirtazapine 13 have been published. With the exception of methimazole and, more recently, mirtazapine, transdermal formulations have limited success in achieving therapeutic blood levels or achieving the clinically desired effect. Although a commercial transdermal theophylline product is not currently available, it can be readily obtained through commercial compounding facilities. Owing to the potential life-threatening consequences of failing to provide adequate therapy in asthmatic cats, it is vital that the ability of transdermal theophylline formulations to achieve therapeutic serum drug concentrations be evaluated.
Administration of oral theophylline formulations achieve plasma concentrations in cats within the established human therapeutic concentration range of 5–20 µg/ml. 3 In addition, feline bronchiolar smooth muscle dilation is achieved when plasma theophylline levels are within the human therapeutic range. 14 Based on its chemical characteristics, theophylline has the potential to be well absorbed transdermally. It has a LogP (partition coefficient) of −0.02, is non-ionic and has a relatively low molecular weight of 180 g/mol. In humans, depending on the formulation, theophylline reaches therapeutic blood levels in infants when delivered transdermally, 15 and can have significant flux through the skin in vitro. 16 Thus, although theophylline may also work transdermally in cats, to our knowledge, transdermal efficacy of theophylline in cats has not been previously evaluated.
The primary objective of this study was to determine if administration of once-daily dosing of transdermal theophylline for 21 days could achieve serum theophylline concentrations considered to be therapeutic (5–20 µg/ml). A secondary objective was to compare two different transdermal formulations and determine which, if either, vehicle is able to achieve higher serum drug concentrations. The two formulations use a different vehicle, Lipoderm (LD; Professional Compounding Centers of America) or pluronic lecithin organogel (PLO), for theophylline solubilization and facilitation of drug absorption. These two formulations were utilized in a recent study of transdermal phenobarbital; the results showed a potential for superiority of drug delivery with PLO, although the difference did not reach statistical significance. 7 Our hypothesis was that after multiple dosing, the LD, but not the PLO gel, would achieve serum theophylline concentrations considered to be in the therapeutic range in at least 50% of the cats treated.
Materials and methods
This investigation was designed as a double-blinded, crossover, randomized controlled trial. Healthy cats were recruited from students and staff of the Auburn University College of Veterinary Medicine. A power calculation was performed based on pharmacokinetic data that were available regarding oral extended-release in cats that had been previously published. 3 Based on this power calculation, 12 cats would be needed to detect a difference of 1 µg/ml between formulations with 80% confidence. Cats were included in the study if they were between 1 and 10 years of age, weighed <6 kg, had not received any medications in the preceding 12 weeks other than routine parasite preventatives, had no clinical signs of illness and had a temperament that would facilitate handling and sample collection.
Once cats were identified for inclusion into the study, a physical examination, complete blood count, serum biochemistry profile and urinalysis were performed, as well as measurement of serum total thyroxine (T4) and free T4 concentrations. Cats were excluded from entry into the study if their temperament did not allow for sample collection or if significant abnormalities were noted on physical examination or screening laboratory work. The owners of all study participants provided written, informed consent. The study was approved by the Auburn University Institutional Animal Care and Use Committee.
Two different transdermal formulations of theophylline were evaluated during this study. One formulation contained PLO as the transdermal vehicle, whereas the other formulation contained LD. Theophylline concentrations in each formulation varied between individual participants, and were based on weight in order to facilitate similar dose volumes for each participant while maintaining a consistent daily dose of 15 mg/kg/day. The target dose volume was 0.15 ml applied to each ear, giving a total volume of 0.3 ml of gel per dose. The two different formulations were prepared by a commercial veterinary compounding pharmacy (Wedgewood Pharmacy) and packaged into identical syringes for administration. The theophylline used was from United States Pharmacopeia (USP)-grade material purchased from US Food and Drug Administration-registered chemical suppliers. The beyond-use date was 30 days from compounding, and the formulations were labeled for storage at room temperature. The compounding pharmacy is an accredited Pharmacy Compounding Accreditation Board member. The excipients used in the PLO formulation were Lipoil (lecithin/isopropyl palmitate) liquid, poloxamer 20% gel, polysorbate 80 and sorbitan monooleate. The excipients used in the LD formulation include LD base vehicle and ethoxy diglycol liquid.
The transdermal theophylline required for each arm of the study was shipped immediately before that portion of the study began. For both investigators and participants to remain blinded, the two formulations were shipped to the Auburn University Veterinary Teaching Hospital (AUVTH) pharmacy and randomly assigned the names ‘drug A’ and ‘drug B’ by the pharmacy staff. Upon receipt, the AUVTH pharmacy staff coded each syringe with the participant’s name, the formulation it contained (drug A or drug B) and the dosing instructions.
Cats were randomly assigned to one of two groups using a random-number generator. Group 1 was assigned to initially receive 15 mg/kg theophylline formulated in PLO once daily applied to the inner pinnae for 21 days. Group 2 was assigned to initially receive the same dose of theophylline formulated in LD once daily applied to the inner pinnae for 21 days. On day 22, 1 ml blood samples were collected from a medial saphenous vein in each participant at 2, 6, 14 and 24 h post-dosing. Time points were chosen in order to determine if serum drug concentrations could be maintained within the therapeutic range during a 24 h dosing interval. The blood samples were allowed to clot, and serum was separated from the samples after centrifugation at 4000 revolutions per minute for 10 mins. The serum samples were stored at −80ºC until further analysis. All participants then completed a minimum 2 week washout period, during which no medication was administered. After the washout period, a single serum sample was collected and analyzed to ensure that serum theophylline concentrations had fallen below the assay’s limit of detection. Once this was confirmed, cats were crossed over and were administered the alternate theophylline formulation for 21 days as previously described. On day 22, serum samples were again collected at 2, 6, 14 and 24 h post-dosing. Serum samples were again stored at −80ºC until further analysis.
Serum theophylline concentrations were measured by the AVUTH Clinical Pharmacology Laboratory Therapeutic Drug Monitoring service using a commercial immunoassay (Siemens Dimension) that has been previously validated for the detection of theophylline in cats. The intra-assay coefficients of variation at 5, 10, 20 and 40 µg/ml were 1.7%, 3.0%, 0.0% and 0.1%, respectively. The inter-assay coefficients of variation at 5, 10, 20 and 40 µg/ml were 1.9%, 2.8%, 1.2% and 2.6%, respectively. Assay accuracy was determined by testing samples of feline serum spiked with theophylline to create low (7.5 µg/ml), medium (15 µg/ml) and high (30 µg/ml) concentration samples. Accuracy for these samples was determined to be 106.9%, 108.6% and 108.7%, respectively. The lower and upper limits of quantitation are 2 and 40 µg/ml, respectively. The limit of detection of the assay is 1 µg/ml. Theophylline concentrations below the limit of detection were assigned a value of 0 µg/ml. In order to confirm the drug concentrations present in the compounded formulations, five syringes were randomly selected for analysis by the Auburn University Therapeutic Drug Monitoring service. Samples were collected from each syringe and were analyzed using the same commercial immunoassay (Siemens Dimension). Prior to analysis, samples were diluted by a factor of 10 in water in order for the measured concentration to fall within the upper limits of detection of the assay. Water was used to dilute the samples collected from the syringes, as this technique has previously been validated, and water is used by the machine itself as a diluent.
During the study period, owners were instructed to monitor their cat for any potential adverse effects and to discontinue medication administration if any concerns arose. Potential adverse effects that owners were specifically instructed to monitor included increased activity level, vomiting, reddening of the skin on the pinnae and the development of pruritus of the pinnae. Owners were provided with a typed instruction sheet outlining the procedure for applying the transdermal gels, and were provided with examination gloves to wear during administration. Owners were observed administering a dose of transdermal gel in order to confirm they understood the dosing instructions. As part of these instructions, owners were told to wipe away any gel remaining on the inner pinnae from a previous dose prior to administering the next dose.
For statistical analysis, median serum theophylline concentrations were compared at each time point for each formulation using a repeated-measures ANOVA in order to look for differences in concentrations based on time. In addition, a two-way random-measures ANOVA was performed to detect any differences in serum concentrations between formulations at each time point. A commercially available statistical software package (SigmaPlot 12) was used for all analyses, and statistical significance was set at P <0.05.
Results
Fourteen cats were screened for inclusion. One cat was excluded from the study owing to a persistent neutropenia noted on complete blood count. Another cat was excluded from study participation after completion of the screening process but before the initiation of the first treatment period owing to the development of an allergic condition that required medical intervention. Of the remaining 12 cats, six were randomly assigned to each treatment group. After the initiation of the treatment phases, five additional cats were excluded from the study. Three cats were excluded owing to the inability to obtain serum samples after the first treatment phase. One cat was excluded after successful completion of the first treatment phase and sample collection owing to the development of an unrelated illness during the washout period. Lastly, one cat was excluded during the second phase of treatment owing to the owner’s inability to medicate the cat consistently. Thus, a total of seven cats completed both arms of the study and had complete data sets available for statistical analysis. No serious adverse events were reported. One cat developed hair thinning on the outer pinnae; however, no other dermatologic abnormalities were present.
All seven cats that completed both arms of the treatment period were American domestic shorthairs. Three were spayed females and four were castrated males. The median age of the study population was 3.5 years (range 1–9 years). The median weight was 4.8 kg (range 3.7–5.9 kg).
Of the 56 total serum theophylline concentrations measured, only seven (13%) were ⩾5 µg/ml, ie, within the range considered to be therapeutic (Figure 1a,b). Six of the seven measurements occurred in two cats; each had three measurements ⩾5 µg/ml. Nine of the 56 total measurements (16%) were <2 µg/ml, ie, the limit of quantification of the assay. All 56 serum samples had theophylline concentrations detectable by the assay (>1 µg/ml). Drug concentration measurements performed on the five randomly selected syringes revealed a deviation from the labeled concentration that ranged from −6.4% to +16.2%.
Figure 1.
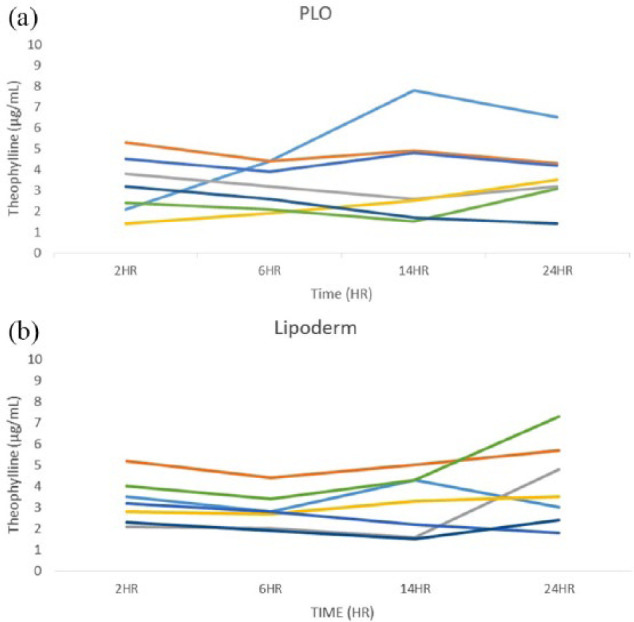
Theophylline serum concentrations at all sampled time points. Each line represents an individual cat. (a) Theophylline serum concentrations achieved after pluronic lecithin organogel (PLO) transdermal theophylline administration. Two cats (three time points in total) achieved concentrations in the therapeutic window (>5 μg/ml). (b) Theophylline serum concentrations achieved after Lipoderm transdermal theophylline administration. Two cats (four time points in total) achieved concentrations in the therapeutic window
No significant differences were detected over time for either formulation (repeated-measures ANOVA; P = 0.751 and P = 0.107 for PLO and Lipoderm, respectively) (Figure 2). A two-way repeated-measures ANOVA found no significant effect of time (P = 0.258) or treatment (P = 0.839) on serum theophylline concentrations nor an interaction between them (P = 0.624).
Figure 2.
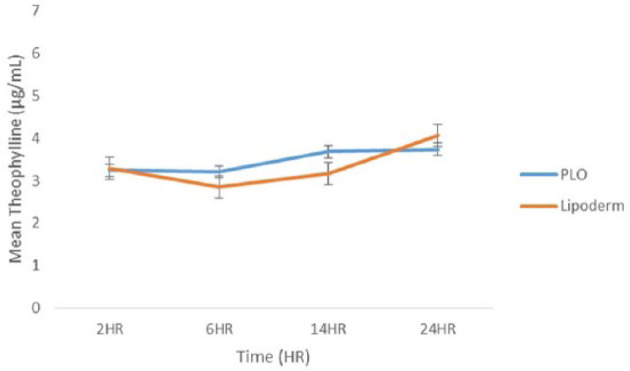
Mean theophylline serum concentrations achieved after 21 days of once-daily administration of pluronic lecithin organogel (PLO) and Lipoderm transdermal theophylline taken 2, 6, 14 and 24 h after the final dose
Discussion
Transdermal theophylline dosed once daily at 15 mg/kg failed to reach therapeutic concentrations for the majority of the time points measured in this study, making it an unreliable method for routine theophylline administration. Only 13% of the measured concentrations were ⩾5 µg/ml, the lowest level considered to be potentially therapeutic in a previous publication. 3 Interestingly, the majority of those measurements (6/7) occurred in two individual cats. Although it is difficult to reach a conclusion based on such a small number of cats, it appears that some cats may absorb transdermal formulations of theophylline more readily than others. Similarly, previous studies evaluating the pharmacokinetics of transdermally delivered medications in cats showed a wide range in individual absorption variability.6,8,9 Although it is difficult to prove, this phenomenon of some cats seeming to absorb transdermal medications more readily may be a product of their individual grooming behaviors leading to oral ingestion of the medication.
Although use of transdermal formulations for daily administered medications remains appealing, their unreliable absorption has prevented their use as a first-line option in a clinical setting.5,7,9 –11 Perhaps the only exceptions are transdermal formulations of methimazole and mirtazapine, which effectively lower serum total T4 levels and significantly improve appetite and food consumption, respectively.4,13 However, as was observed in this investigation, there appears to be individual cats that will more readily absorb transdermal medications, and thereby achieve higher concentrations of the drug in circulation. The reason for individual variability is uncertain, but may relate to dissimilarities in skin thickness and vascular supply. 9 Additionally, the degree of variation of theophylline concentration in each syringe may have also played a role in the variability of serum theophylline concentrations noted among individuals in this investigation, as some syringes had a percent error that fell outside of the USP standards (>10%).
Owing to the high degree of variation in absorption of transdermal medications, different vehicles have been developed with the hopes of improving drug delivery across the epidermis. Few studies have objectively compared transdermal drug delivery vehicles. Joy et al compared the absorption of phenobarbital in cats between PLO and LD formulations. Although a significant difference was not detected between serum concentrations achieved with the two formulations, PLO appeared to outperform the LD vehicle, based on the overall increased mean maximum drug concentrations achieved. 7 The secondary aim of our study was to compare the serum concentrations of theophylline achieved using these two drug delivery vehicles. We detected no significant difference in serum concentrations achieved at any time point between these two formulations, although the possibility of a type II statistical error exists based on the study being underpowered.
Our results support the need for therapeutic drug monitoring, if using transdermal theophylline. Although a direct comparison to oral dosing of theophylline was not included in this investigation, previous publications indicate that oral theophylline administration achieves much higher serum drug concentrations than the serum concentrations achieved in this investigation using an equivalent dose of transdermal theophylline, and does so in a much more reliable fashion. As such, we strongly recommend that oral administration remains the first choice when long-term use of theophylline is indicated. In cats that cannot tolerate oral medication, transdermal delivery should not be considered as an alternative because of inconsistency in attaining therapeutic targets and wide variation in the quality of compounded products.
One limitation of this study is the small population. Although other veterinary studies evaluating the use of transdermal formulation have utilized similar numbers of participants, the ability to extrapolate the results of this investigation to a larger population is limited. In addition, the study was significantly underpowered for the purposes of determining a difference between the two different transdermal vehicles. A power calculation performed after initial data was acquired using transdermal theophylline delivery revealed that a population of 34 cats would be needed to detect a difference of 1 µg/ml in serum theophylline concentrations between the two vehicles with 80% confidence. Upon evaluating our initial data set, it was determined that adequate data had been gathered to address our initial objective of determining whether therapeutic blood concentrations could be achieved with once-daily dosing of theophylline at 15 mg/kg. Additionally, with the significant variability in theophylline concentrations that was observed between individuals at various time points, attaining additional samples to determine differences between formulations was deemed unnecessary.
Ingestion during clinical use of transdermal medications is a consistent constraint for their use in veterinary medicine. The possibility of oral ingestion cannot be ruled out entirely as a contributing factor for the cats in this study that achieved a therapeutic concentration. However, this does not alter our conclusion that there was significant variability in theophylline concentrations that would result in unreliability of transdermal theophylline for routine clinical use.
Another limitation of this investigation was the inability to increase the theophylline dose. Previous veterinary transdermal drug delivery publications have indicated the need to increase transdermal doses to approximately three-fold or more vs the oral dose to achieve the same serum drug concentrations.5,7,13 Unfortunately, the highest available concentration of transdermal theophylline is 300 mg/ml. Thus, the highest dose that could be delivered in the targeted 0.3 ml dose is 90 mg, which equals the recommended oral dose of 15 mg/kg in a 6 kg cat. Based on the current compounding limitations, doses higher than 15 mg/kg would only be achievable in smaller cats, or by applying more than 0.3 ml daily. Given the surface area of the inner pinna of an average-sized cat, applying more than 0.15 ml per ear is likely to result in excessive accumulation of transdermal gel that is not contributing to additional drug absorption owing to limited contact with the stratum corneum.
Conclusions
Our results indicate that chronic once-daily dosing of two transdermal theophylline formulations does not reliably achieve therapeutic serum concentrations at numerous time points during the dosing interval. As such, transdermal theophylline should not be considered a first-line treatment option for cats requiring long-term treatment with a bronchodilator. Although all cats within the investigation achieved detectable serum theophylline concentrations at all measured points, there is very little potential for the development of a reasonable dosing protocol that can reliably achieve therapeutic serum concentrations in the majority of patients. Further studies are indicated looking at the effect of a shortened dosing interval, as well as an increase in the dose administered based on body weight. Given the currently available concentrations, once-daily dosing of TD theophylline is unacceptable for the management of the majority of cats in need of chronic bronchodilator therapy.
Acknowledgments
The authors thank Janeva Cole LVT, Callie Anselmo RVT and Maureen Henderson LVT for assistance with sample collection.
Footnotes
Accepted: 16 April 2018
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Funding for this study was provided by the Auburn University Department of Clinical Sciences. Transdermal theophylline was graciously provided by Wedgewood Pharmacy.
ORCID iD: Tekla Lee-Fowler  https://orcid.org/0000-0002-2965-2841
https://orcid.org/0000-0002-2965-2841
References
- 1. Trzil JE, Reinero CR. Update on feline asthma. Vet Clin North Am Small Anim Pract 2014; 44: 91–105. [DOI] [PubMed] [Google Scholar]
- 2. Reinero CR, Delgado C, Spinka C, et al. Enantiomer-specific effects of albuterol on airway inflammation in healthy and asthmatic cats. Int Arch Allergy Immunol 2009; 150: 43–50. [DOI] [PubMed] [Google Scholar]
- 3. Guenther-Yenke CL, McKiernan BC, Papich MG, et al. Pharmacokinetics of an extended-release theophylline product in cats. J Am Vet Med Assoc 2007; 231: 900–906. [DOI] [PubMed] [Google Scholar]
- 4. Hill KE, Gieseg MA, Kingsbury D, et al. The efficacy and safety of a novel lipophilic formulation of methimazole for the once daily transdermal treatment of cats with hyperthyroidism. J Vet Intern Med 2011; 25: 1357–1365. [DOI] [PubMed] [Google Scholar]
- 5. Bennett N, Papich MG, Hoenig M, et al. Evaluation of transdermal application of glipizide in a pluronic lecithin gel to healthy cats. Am J Vet Res 2005; 66: 581–588. [DOI] [PubMed] [Google Scholar]
- 6. Ciribassi J, Luescher A, Pasloske KS, et al. Comparative bioavailability of fluoxetine after transdermal and oral administration to healthy cats. Am J Vet Res 2003; 64: 994–998. [DOI] [PubMed] [Google Scholar]
- 7. Delamaide Gasper JA, Barnes Heller HL, Robertson M, et al. Therapeutic serum phenobarbital concentrations obtained using chronic transdermal administration of phenobarbital in healthy cats. J Feline Med Surg 2015; 17: 359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Helms SR. Treatment of feline hypertension with transdermal amlodipine: a pilot study. J Am Anim Hosp Assoc 2007; 43: 149–156. [DOI] [PubMed] [Google Scholar]
- 9. Hoffman SB, Yoder AR, Trepanier LA. Bioavailability of transdermal methimazole in a pluronic lecithin organogel (PLO) in healthy cats. J Vet Pharmacol Ther 2002; 25: 189–193. [DOI] [PubMed] [Google Scholar]
- 10. Mealey KL, Peck KE, Bennett BS, et al. Systemic absorption of amitriptyline and buspirone after oral and transdermal administration to healthy cats. J Vet Intern Med 2004; 18: 43–46. [DOI] [PubMed] [Google Scholar]
- 11. Willis-Goulet HS, Schmidt BA, Nicklin CF, et al. Comparison of serum dexamethasone concentrations in cats after oral or transdermal administration using pluronic lecithin organogel (PLO): a pilot study. Vet Dermatol 2003; 14: 83–89. [DOI] [PubMed] [Google Scholar]
- 12. Zajic LB, Herndon AK, Sieberg LG, et al. Assessment of absorption of transdermal ondansetron in normal research cats. J Feline Med Surg 2017; 19: 1245–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Benson KK, Zajic LB, Morgan PK, et al. Drug exposure and clinical effect of transdermal mirtazapine in healthy young cats: a pilot study. J Feline Med Surg 2017; 19: 998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leemans J, Kirschvink N, Gustin P. A comparison of in vitro relaxant responses to ipratropium bromide, beta-adrenoceptor agonists and theophylline in feline bronchial smooth muscle. Vet J 2012; 193: 228–233. [DOI] [PubMed] [Google Scholar]
- 15. Cartwright RG, Cartlidge PH, Rutter N, et al. Transdermal delivery of theophylline to premature infants using a hydrogel disc system. Br J Clin Pharmacol 1990; 29: 533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thakur RA, Michniak BB, Meidan VM. Transdermal and buccal delivery of methylxanthines through human tissue in vitro. Drug Dev Ind Pharm 2007; 33: 513–521. [DOI] [PubMed] [Google Scholar]


