Abstract
Objectives
Vaccination against feline herpesvirus-1 (FHV-1) is recommended for all cats. However, it is unknown how adult healthy cats with different pre-vaccination antibodies respond to FHV-1 vaccination in the field. The aim of the study was to determine the prevalence of neutralising antibodies against FHV-1 in healthy adult cats and the response to FHV-1 vaccination within 28 days of vaccination.
Methods
One hundred and ten cats (⩾1 year of age) that had not received a vaccination within the past 12 months were vaccinated with a combined FHV-1 vaccine. Antibodies against FHV-1 were determined before vaccination (day 0), on day 7 and day 28 by serum neutralisation test. Uni- and multivariate statistical analyses were used to determine factors associated with the presence of pre-vaccination antibodies and response to vaccination.
Results
Pre-vaccination neutralising antibody titres (⩾10) were present in 40.9% of cats (45/110; 95% confidence interval [CI] 32.2–50.3); titres were generally low (range 10–640). Antibody response to vaccination (⩾four-fold titre increase) was observed in 8.3% (9/109; 95% CI 4.2–15.1). Cats ⩾2 years of age were more likely to have pre-vaccination neutralising antibodies than cats aged between 1 and 2 years (odds ratio [OR] 24.619; P = 0.005). Cats from breeders were more likely to have pre-vaccination neutralising antibodies than privately owned cats (OR 7.070; P = 0.007). Domestic shorthair cats were more likely to have an at least four-fold titre increase vs purebred cats (OR 11.22; P = 0.027).
Conclusions and relevance
Many cats have no detectable neutralising antibodies against FHV-1 despite previous vaccinations and fail to develop a ⩾four-fold titre increase after vaccination. This is likely because older cats and cats with a higher FHV-1 exposure risk are more likely to get infected with FHV-1 and thus to have FHV-1 neutralizing antibodies. Purebred cats more often fail to develop a ⩾four-fold titre increase after vaccination.
Keywords: FHV-1, active immunization, protection, MLV
Introduction
Feline herpesvirus-1 (FHV-1) is an enveloped virus with linear, double-stranded DNA. It is assigned to the family Herpesviridae, subfamily Alphaherpesvirinae. 1 FHV-1 commonly leads to acute and subsequent chronic infection with intermittent reactivation, 2 potentially resulting in inflammation of the upper respiratory tract and eyes. 3 Although vaccination against FHV-1 cannot prevent infection, it can reduce severity of disease and virus excretion upon infection if applied before the first contact with FHV-1, and it has been relatively successful in controlling disease and recrudescence. 4 All cats are at risk for infection and thus FHV-1 vaccination is considered a core vaccine. 5
FHV-1 vaccines act by inducing both antibodies and cellular immunity. 4 The presence of neutralising antibodies acquired through previous vaccination is linked with protection against FHV-1 challenge.6,7 Although quality of protection 1 or 3 years after vaccination is not as good as 1 month after vaccination, 8 the duration of FHV-1 immunity has been shown to last for at least 3 years,9,10 indicating that revaccinations in short intervals are not always necessary.
All vaccinations, including those against FHV-1, might be associated with the development of feline injection-site sarcomas (FISSs). 11 In addition, recent studies indicated that cats with frequent vaccinations might be at risk of developing chronic kidney disease, 12 and it has been discussed that antibodies against Crandell Rees feline kidney cell line antigen, which is often used for vaccine production, are developed after vaccination. 13 Thus, vaccination should only be performed if a beneficial effect can be expected. 5 In certain diseases, such as feline panleukopenia, a beneficial effect of revaccination is unlikely if antibodies are already present. 14 Data on FHV-1 antibodies, however, are limited. So far, it is unknown how adult, healthy cats with pre-existing FHV-1 neutralising antibodies respond to vaccination and which factors are associated with antibody increase after vaccination.
Therefore, the aims of the study were to: (1) determine the prevalence of neutralising antibodies against FHV-1 in healthy adult cats by serum neutralisation test (SN); (2) evaluate the antibody response after vaccination with a FHV-1 modified-live vaccine (MLV) by measuring antibodies within a period of 28 days after vaccination; and (3) evaluate factors that are associated with the presence of pre-vaccination neutralising antibodies, as well as with response to vaccination.
Materials and methods
Study population
Samples from 110 cats were included in the study. A necessary minimum sample size of at least 96 cats had been estimated in a power analysis, based on an assumed FHV-1 antibody prevalence of 50%, with a 95% confidence interval (CI) and a 10% margin of error. All cats were presented for routine vaccination. The study protocol was approved by the ethical committee of the Centre of Clinical Veterinary Medicine, LMU Munich (reference number 55.2-1-54-2532.3-62-11).
The inclusion criteria were: (1) unremarkable physical examination; (2) ⩾1 year of age; and (3) last vaccination against FHV-1 >1 year ago. Exclusion criteria were: (1) immunosuppressive drugs or passive immunisation <4 weeks; and (2) infection with feline immunodeficiency virus (FIV) and/or feline leukaemia virus (FeLV) determined using a commercial ELISA (SNAP Kombi Plus FeLV/FIV Antibody Test; IDEXX). Signalment of the cats is shown in Table 1.
Table 1.
Characteristics of cats and association with the presence of antibodies to feline herpesvirus-1 (FHV-1) in uni- and multivariate analyses
| Variable | Number of cats | Category | Number of cats per group | Cats with pre-vaccination FHV-1 antibodies | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|---|---|---|
| P value | OR | 95% CI | P value | |||||
| Age | 110 | 1<2 years | 17 | 2 | 0.008 | Ref. value | NA | NA |
| ⩾2 years | 93 | 43 | 24.619 | 2.600–233.162 | 0.005 | |||
| Breed | 110 | DSH | 73 | 27 | 0.305 | – | ||
| Purebred | 37 | 18 | ||||||
| Sex | 110 | Female | 60 | 31 | 0.019 | 2.160 | 0.879–5.291 | 0.093 |
| Male | 50 | 14 | Ref. value | NA | NA | |||
| Weight | 110 | <2 kg | 15 | 8 | 0.497 | – | ||
| 2–4 kg | 42 | 14 | ||||||
| 4–6 kg | 47 | 20 | ||||||
| >6 kg | 6 | 3 | ||||||
| Neutering status | 110 | Intact | 32 | 10 | 0.207 | – | ||
| Neutered | 78 | 35 | ||||||
| Origin | 110 | Breeder | 20 | 13 | 0.012 | 7.070 | 1.694–29.517 | 0.007 |
| Shelter | 34 | 16 | 2.560 | 0.970–6.754 | 0.058 | |||
| Private | 56 | 16 | Ref. value | NA | NA | |||
| Environment | 110 | Urban | 88 | 34 | 0.344 | – | ||
| Rural | 22 | 11 | ||||||
| Lifestyle | 110 | Indoor cat | 90 | 40 | 0.135 | – | ||
| Outdoor cat | 20 | 5 | ||||||
| Cohabitation with dogs | 110 | Yes | 28 | 10 | 0.657 | – | ||
| No | 82 | 35 | ||||||
| Housing conditions | 110 | Multi-cat household | 92 | 36 | 0.438 | – | ||
| Single-cat household | 18 | 9 | ||||||
| Time since last vaccination | 110 | 1–3 years | 59 | 30 | 0.049 | Eliminated* | ||
| >3 years | 13 | 5 | ||||||
| Never | 38 | 10 | ||||||
| Vaccination status | 106 | Vaccinated | 72 | 35 | 0.019 | Eliminated* | ||
| Not vaccinated | 34 | 8 | ||||||
| Complete vaccination series | 110 | Yes | 20 | 11 | 0.209 | – | ||
| No | 90 | 34 | ||||||
Bold values indicate statistically significant results
The factor was eliminated by the backward stepwise variable selection within logistic regression model and was thus not associated with the presence of pre-vaccination antibodies in the multivariate analysis
OR = odds ratio; CI = confidence interval; Ref. value = reference value indicating that this category was used as baseline for comparison for each variable; NA = not applicable; DSH = domestic shorthair
Study protocol
Each cat received a single dose of an MLV vaccine (Purevax RCP; Merial) on day 0, containing attenuated FHV-1 strain F2 with a viral titre of at least 104.9 cell culture infective dose 50% (CCID50), as well as a subunit feline calicivirus (FCV) component and a MLV feline parvovirus (FPV) component; FCV and FPV were not subject of the present study.
For the detection of pre- and post-vaccination FHV-1 neutralising antibodies, serum samples were collected on days 0, 7 and 28, and frozen at ‒20°C until analysed. In 25 cats, no serum was available on day 7 and in one of these 25 cats also on day 28.
Data on signalment (age, breed, sex, neutering status, body weight), origin (breeder, private household, animal shelter, foreign country), environment (urban, rural), housing conditions (multi-, single-cat household), lifestyle (indoor, outdoor), cohabitation with dogs and vaccination status (previous vaccination; complete vaccination series; time since last vaccination) were collected from the owners on day 0. Besides obtaining a detailed history, health status of the cats was evaluated by physical examination on days 0, 7 and 28. Vaccine-associated adverse events (VAAEs) were recorded on days 7 and 28.
Most of the cats (n = 72/106; 67.9%) had been vaccinated in the past. Only 27.8% (n = 20/72) of the cats had received a complete vaccination series according to current guidelines. 5 A complete vaccination against FHV-1 was defined as a primary FHV-1 vaccination series starting at an age of 6–8 weeks with subsequent booster vaccinations at 3–4 week intervals and the last vaccination with at least 16 weeks. A booster vaccination had to be given 11–13 months later. In cats >12 weeks, vaccination was considered complete if they had received two vaccinations in a 3–4 week interval with a final booster after 11–13 months. After the primary vaccination series, cats had to have been revaccinated subsequently in at least 3 year intervals.
Detection of neutralising antibodies by SN
Serum samples were heat-treated at 56°C for 30 mins and aliquots were stored at ‒20°C. Samples of each serum (100 µl) were pre-diluted (1:5) in phosphate-buffered saline (pH 7.2) and further serially diluted at steps of 1:2. Each dilution was mixed with an equal volume of FHV-1 isolate KS 285 (200 median tissue culture infective dose per 0.1 ml) and incubated at 37°C for 90 mins. Subsequently, feline renal kidney cells seeded in 96-well microtitre plates were inoculated with 100 μl of these serum/virus mixtures. Plates were incubated for 3–5 days at 37°C, 5% CO2. All samples from an individual cat were run in duplicate in the same test. Sera were end point-diluted, which was at least a dilution of up to 1:5120. The determined titre corresponded to the dilution step. Virus titre was confirmed by back titration for each batch of samples tested at the same day. TCID50 was calculated according to Spaermann (1908) and Kaerber (1921).15,16 The positive control serum was obtained from the MegaFLUO FHV test-kit (MEGACOR Diagnostic) and had a titre of 1:320 and was stored in small aliquots at ‒20°C. It was tested in duplicate for each run. Variation of one dilution step was accepted as within and between-assay variation. A titre <10 was considered negative. A positive antibody response to vaccination was defined as four-fold titre increase on day 28.
Statistical analysis
Statistical analysis was performed with SPSS version 22 (IBM). Fisher’s exact test was used to assess factors associated with (1) the presence of pre-vaccination neutralising antibodies (Table 1) and with (2) a ⩾four-fold titre increase after vaccination (Table 2). Multivariate logistic regression analysis was performed for significant factors in univariate analysis with backward stepwise variable selection based on the Wald statistic. Levels of significance were set at P <0.05.
Table 2.
Cats (n = 9/109) with a ⩾four-fold increase in antibodies to vaccination against feline herpesvirus-1 (FHV-1) and their respective titres on days 0, 7 and 28
| Cat | FHV-1 antibody titre |
||
|---|---|---|---|
| Day 0 | Day 7 | Day 28 | |
| 1 | <10 | NA | 40 |
| 2 | <10 | <10 | 40 |
| 3 | <10 | 80 | 320 |
| 4 | <10 | NA | 80 |
| 5 | 80 | 80 | 320 |
| 6 | 20 | 80 | 160 |
| 7 | <10 | <10 | 40 |
| 8 | <10 | 80 | 160 |
| 9 | 20 | 20 | 80 |
NA = not applicable
Results
Presence of pre-vaccination neutralising antibodies
Neutralising antibody titres of ⩾10 against FHV-1 on day 0 were present in 45/110 (40.9%; 95% CI 32.2–50.3) cats; 35 of these 45 cats had received previous vaccinations; eight had not received previous vaccinations; the vaccination status of two cats was unknown beyond the previous 12 months.
In univariate analysis, the factors age, sex, origin, time since last vaccination and vaccination status were significantly associated with the presence of pre-vaccination neutralising antibodies (Table 1). However, in multivariate analysis, only the factors age and origin proved to be significant. Cats ⩾2 years of age were more likely to have pre-vaccination neutralising antibodies than cats between 1 and 2 years of age (odds ratio [OR] 8.97; P = 0.047). Cats from breeders were more likely to have pre-vaccination neutralising antibodies than cats from private households (OR 7.070; P = 0.007). The factors ‘time since last vaccination’ and ‘vaccination status’ were eliminated by the backwards stepwise variable selection within the logistic regression model and thus were not associated with presence of pre-vaccination neutralising antibodies in the multivariate context.
Titre increase after vaccination
A titre increase was observed in 22.0% (24/109 cats; 95% CI 16.8–23.7) of the cats. Only 8.3% of cats (n = 9/109) had a four-fold or higher titre increase (Table 2).
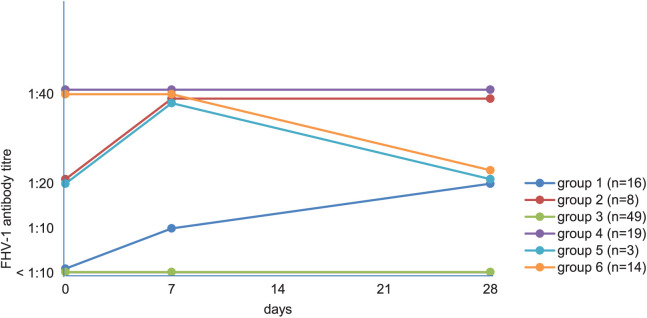
Cats were categorised into six different groups according to their antibody response to vaccination (Figures 1 and 2). Cats in group 1 (n = 16; 14.6%) had neutralising antibodies <10 on day 0 and showed a titre increase (median titre day 0: <10 [range <10–<10]; median titre day 7: 10 [range <10–80]; median titre day 28: 20 [range 10–320]), which was four-fold or higher (6/16 cats). In group 2 (n = 8; 7.3%), cats already had neutralising antibodies ⩾10 on day 0 and showed an increase of their antibody titre after vaccination (median titre day 0: 20 [range 10–80]; median titre day 7: 40 [range 10–160]; median titre day 28: 40 [range 20–320]), which was four-fold or higher (3/8 cats). Cats in group 3 (n = 49; 45.0%) had no neutralising antibodies pre- and post-vaccination. Group 4 consisted of 19 cats (17.4%) with a pre-vaccination neutralising antibody titre ⩾10 on day 0 and showed no change in antibody titre after vaccination (median titre day 0, day 7 and day 28: 40 [range 10–640]). In group 5 (n = 3; 2.8%), cats showed an increase in their neutralising antibody titre on day 7, but a decrease on day 28 (median titre day 0: 20 [range 10–40]; median titre day 7: 40 [range 10–80]; median titre day 28: 20 [range 20–40]). In group 6 (n = 14; 12.8%), cats had neutralising antibodies ⩾10 and showed a decrease on day 28 (median titre day 0: 40 [range 20–640]; median titre day 7: 40 [range 20–640]; median titre day 28: 20 [range <10–320]).
Figure 1.
Categorisation of cats depending on median feline herpesvirus-1 (FHV-1) antibody titres and on antibody reaction after vaccination against FHV-1. Vertical axis shows cats’ median antibody titre against FHV-1; horizontal axis shows course of the study, at day 0 (before vaccination), at day 7 and at day 28 (after vaccination). Herpesvirus-1 titre could not be determined in 23 cats on day 7. Group 1 = cats without antibodies <10 on day 0 and an antibody titre increase (n = 16; 14.6%); group 2 = cats with antibodies ⩾10 and an antibody titre increase (n = 8; 7.3%); group 3 = cats with an antibody titre remaining <10 pre- and post-vaccination (n = 49; 45.0%); group 4 = cats with pre-vaccination antibody titre ⩾10 on day 0 and no change in titre after vaccination (n = 19; 17.4%); group 5 = cats with antibody titre increase on day 7 but decrease on day 28 (n = 3; 2.8%); group 6 = cats with antibody titre decrease on day 28 (n = 14; 12.8%)
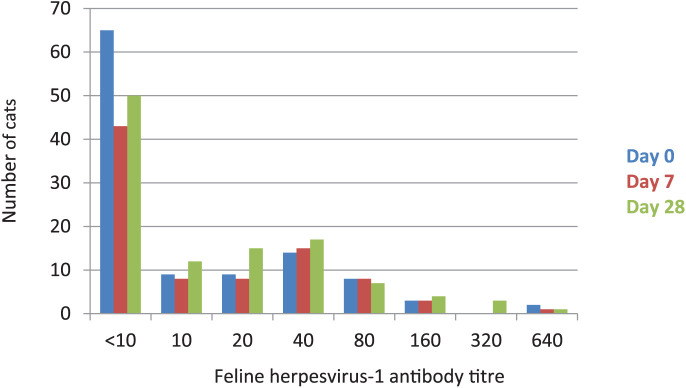
Figure 2.
Number of cats with the respective feline herpesvirus-1 (FHV-1) antibody titres on day 0 (before vaccination), day 7 and day 28 (after vaccination). Vertical axis shows the number of cats with the respective antibody titre against FHV-1; horizontal axis shows the FHV-1 antibody titres on day 0 (before vaccination), day 7 and day 28 (after vaccination). Herpesvirus-1 titre could not be determined in 25 cats on day 7, and in one of these 25 cats also on day 28
In univariate analysis, only one factor (breed) was significantly associated with a ⩾four-fold titre increase after vaccination (Table 3); thus, multivariate analysis was not performed. Domestic shorthairs were more likely to have a ⩾four-fold titre increase than purebred cats (OR 11.22; P = 0.027).
Table 3.
Factors associated with a ⩾four-fold increase in antibodies to vaccination against feline herpesvirus-1 in univariate analysis
| Variable | Number of cats | Category | Number of cats per group | Cats with ⩾four-fold titre increase | Univariate analysis |
||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | |||||
| Age | 109 | 1<2 years | 16 | 0 | Ref. value | NA | NA |
| ⩾2 years | 93 | 9 | 3.710 | 0.206–66.96 | 0.351 | ||
| Breed | 109 | DSH | 72 | 9 | 11.22 | 0.634–198.5 | 0.027 |
| Purebred | 37 | 0 | Ref. value | NA | NA | ||
| Sex | 109 | Female | 60 | 6 | 1.704 | 0.430–6.200 | 0.511 |
| Male | 49 | 3 | Ref. value | NA | NA | ||
| Weight | 109 | <2 kg | 15 | 0 | Ref. value | NA | NA |
| 2–4 kg | 41 | 2 | 1.962 | 0.089–43.270 | 1.000 | ||
| 4–6 kg | 47 | 7 | 5.741 | 0.309–106.7 | 0.180 | ||
| >6 kg | 6 | 0 | * | * | * | ||
| Neutering status | 109 | Intact | 31 | 1 | Ref. value | NA | NA |
| Neutered | 78 | 8 | 3.429 | 0.410–28.65 | 0.441 | ||
| Origin | 109 | Breeder | 20 | 0 | Ref. value | NA | NA |
| Shelter | 33 | 4 | 6.254 | 0.319–122.7 | 0.285 | ||
| Private | 56 | 5 | 4.379 | 0.231–82.880 | 0.317 | ||
| Environment | 109 | Urban | 88 | 9 | 5.138 | 0.287–91.92 | 0.202 |
| Rural | 21 | 0 | Ref. value | NA | NA | ||
| Lifestyle | 109 | Indoor cat | 89 | 7 | 1.302 | 0.249–6.795 | 0.669 |
| Outdoor cat | 20 | 2 | Ref. value | NA | NA | ||
| Cohabitation with dogs | 109 | Yes | 28 | 2 | Ref. value | NA | NA |
| No | 81 | 7 | 1.230 | 0.240–6.303 | 1.000 | ||
| Housing conditions | 109 | Multi-cat household | 91 | 8 | 1.639 | 0.192–13.98 | 1.000 |
| Single-cat household | 18 | 1 | Ref. value | NA | NA | ||
| Time since last vaccination | 109 | 1–3 years | 59 | 6 | 1.981 | 0.378–10.38 | 0.480 |
| >3 years | 13 | 1 | 1.458 | 0.121–17.570 | 1.000 | ||
| Never | 37 | 2 | Ref. value | NA | NA | ||
| Vaccination status | 106 | Vaccinated | 72 | 7 | 1.723 | 0.338–8.776 | 0.715 |
| Not vaccinated | 34 | 2 | Ref. value | NA | NA | ||
| Complete vaccination series | 109 | Yes | 20 | 2 | 1.302 | 0.249–6.795 | 0.668 |
| No | 89 | 7 | Ref. value | NA | NA | ||
| Pre-vaccination antibodies | 109 | <1:10 | 64 | 6 | 1.447 | 0.342–6.135 | 0.734 |
| ⩾1:10 | 45 | 3 | Ref. value | NA | NA | ||
| Side effects | 109 | Mild lethargy | 9 | 1 | 1.438 | 0.159–12.99 | 0.554 |
| None | 100 | 8 | Ref. value | NA | NA | ||
Analysis not possible as one row is filled with zeros
OR = odds ratio; CI = confidence interval; Ref. value = reference value indicating that this category was used as baseline for comparison for each variable; NA = not applicable; DSH = domestic shorthair
VAAEs were not associated with a ⩾four-fold titre increase. They were recorded in nine cats and limited to a slightly reduced general condition after vaccination for a few days.
Discussion
Overall, 59.1% (n = 65/110) of cats from the present study had no detectable pre-vaccination neutralising antibodies (<10), although 37/65 cats without pre-vaccination antibodies had been vaccinated before. A study in Florida including cats entering an animal shelter found that even more cats (89.0%) had no detectable neutralising antibodies; the vaccination status of these cats was unknown. 17 Another study in Florida found that 79.0% of trapped feral cats had no detectable neutralising antibodies. 18 The presence of neutralising antibodies indicates previous exposure or vaccination, but after experimental infection, FHV-1 neutralising antibodies are generally low and rise slowly. In an old experimental study, only 6/15 cats (40%) developed FHV-1 neutralising antibodies within 16–20 days post-infection (p.i.) and even 30–34 days p.i., only 11/15 cats (73%) had developed antibodies, and titres were generally low (between 4 and 64). 19 This could be explained by the fact that the spread of FHV-1 from infected cells is restricted to the adjacent cells, producing only foci of virus infection and no viraemia.4,20,21 Antigen exposure to immune cells is therefore limited. The reason for differences in FHV-1 neutralising antibody responses between different individual cats is unknown.
Similar to FHV-1 infection, development of FHV-1 neutralising antibodies after vaccination also only occurs in a small number of cats and titres are generally low, especially if MLV vaccines are used. 4 However, results of different studies vary and comparison is difficult owing to different protocols, type of vaccine and type of antibody test.In one experimental study, only 2/11 specific pathogen-free (SPF) kittens that were vaccinated once against FHV-1 (with Purevax RCPCh-FeLV [Merial]; which contained the same FHV-1 strain and doses as used in the present study) developed neutralising antibodies against FHV-1 within 4 weeks of vaccination. Nevertheless, protection was observed after challenge at 1 and 4 weeks after vaccination, despite the absence of pre-challenge antibodies. 22 In a similar experimental study evaluating the antibody response of 15 SPF kittens after a primary vaccination series by a blocking ELISA (two vaccinations 4 weeks apart and one final booster vaccination 1 year later also with Purevax RCPCh-FeLV [Merial]) only few cats developed antibodies after the first two vaccinations, while 14/15 cats developed antibodies after the final booster after 1 year. 10 In contrast, in another study using an inactivated vaccine, all 41 vaccinated cats developed neutralising antibodies, whereas unvaccinated controls had no detectable antibodies. 23 Thus, there is likely a difference between non-adjuvanted and adjuvanted vaccines; killed adjuvanted vaccines might induce a stronger response than MLV vaccines. Also, different methods of antibody testing make comparison between studies difficult. In the present study, SN was performed because it is the only test that detects antibodies that are able to neutralise infectious particles and prevent infection.
In the present study, 40.9% of the cats had neutralising pre-vaccination antibodies either due to previous infection or vaccination. As the presence of antibodies was significantly associated with age (higher likelihood of infection with age) and origin of the cats (higher risk of getting infected by other cats) but not with the cats’ vaccination status, it is more likely that cats’ antibodies were present owing to previous infection and not vaccination.
A titre <10 does not necessarily mean that the cats from the present study were not protected. Cats without detectable neutralising antibodies might still be protected due to rapid activation of immunity by memory cells. 24 As with other alphaherpesviruses (eg equine herpesvirus-1 [EHV-1]), other immunity mechanisms are crucial for the prevention of FHV-1 infections, not only antibodies.4,25 In horses, T cells seem to be most important for clearance of replicating EHV-1 because: (1) EHV-1 rapidly becomes intracellular and evades the neutralising effects of antibodies;26,27 and (2) primarily activity of CD8+ lymphocytes, which can limit the spread of infectious agents by recognising and killing infected cells or secreting specific antiviral cytokines and CD4+ lymphocytes, 28 increases in blood during EHV-1 viraemia. 29
Nevertheless, the presence of neutralising antibodies is a surrogate of protection against disease, at least in cats that had been vaccinated before. In one experimental study, 72 cats were vaccinated against FHV-1 and challenged with virulent virus between 9 and 36 months later. Most cats that possessed neutralising antibodies after vaccination were resistant to FHV-1 challenge. The predictive value of FHV-1 presence of antibodies in SN was 91%; the predictive value of a negative result was 67%. 6
Correlation of several parameters with the presence of pre-vaccination neutralising antibodies was evaluated. The significant association with the cats’ age was an interesting finding. Cats ⩾2 years were 25 times more likely to have pre-vaccination antibodies than cats between 1 and 2 years, independent of the cats’ vaccination history. Older cats are more likely to have been exposed to the virus, and all exposed cats likely become FHV-1 carriers. Antibodies tend to rise to moderate levels after repeated exposure to FHV-1. 4 Thus, antibodies against FHV-1 are more common in older cats. An association between age and presence of herpesvirus antibodies has been reported previously.17,30, 31 DiGangi et al found that cats aged ⩾6 months were more likely to have FHV-1 neutralising antibodies than cats aged <6 months. 17 In the present study, cats from breeders were also more likely to have antibodies and this is also most likely due to a higher risk of infection and reinfection in such households. 32
Response to vaccination followed different reaction schemes. Only 22.0% of cats had a titre increase after vaccination, and titres after vaccination were generally low (range <10–640). Only 8.3% of cats had a four-fold or higher titre increase. Similar results have been reported in experimental studies. 33 However, in contrast to the present study, Mouzin et al stated that 97.8% of cats either had neutralising antibodies ⩾16 before non-adjuvanted MLV vaccination or a titre increase ⩾four-fold after revaccination; 7 thus, their number of responder cats was much higher than in the present study.A reason for this could be that all cats in the study of Mouzin et al had received a complete primary vaccination series with the same vaccine prior to entering the study. 7 Previous vaccination and complete primary vaccination series likely resulted in higher titres. 33 However, in the present study, there was no correlation with the cats’ vaccination history and neutralising antibodies either pre- or post-vaccination.Differences in the viral titre of the vaccine or the vaccine strain could also be responsible for different antibody responses. The manufacturer of the vaccine used in the Mouzin et al study reported a higher FHV-1 dose (107.3 CCID50; strain not specified) than the vaccine from the present study (104.9 CCID50). Thus, higher antigen doses used as a booster might induce stronger neutralising antibody titres, presumably by recruiting more memory B cells into the response.Further, the cats’ age is likely the reason for the high antibody titres in the study of Mouzin et al. 7 In the present study, cats aged ⩾2 years were more likely to have pre-vaccination neutralising antibodies than cats between 1 and 2 years of age. In the study of Mouzin et al, 7 only cats aged >2 years were included. 7 The likelihood of having a higher titre with higher age can also be explained by a higher previous FHV-1 exposure rate of older cats. It has been shown that after re-exposure, FHV-1 antibodies produced by memory cells generated during primary responses tend to rise.19,34
In the present study, all cats were vaccinated with a MLV vaccine. Interestingly, some studies found increases in neutralising antibody more often following vaccination with inactivated herpesvirus vaccines when compared with MLV vaccines in horses 27 and in feral cats. 18 While 81.0% of cats receiving inactivated FHV-1 vaccines developed antibodies, only 16.0% of cats receiving a MLV FHV-1 vaccine developed antibodies 10 weeks after vaccination. 18 However, efficacy of MLV FHV-1 vaccines seems to be similar to that of inactivated vaccines. 35 Only one study found significantly fewer signs of respiratory diseases in cats vaccinated with an inactivated FHV-1 vaccine compared with cats receiving a MLV vaccine; however, the total clinical score was similar in both groups. 36 A reason for the weaker antibody response after vaccination with MLV could be that, similarly to natural infection, MLV becomes intracellular soon after application, inducing predominantly T-cell responses. However, MLV vaccines are generally preferred for vaccination in cats, as inactivated vaccines usually contain adjuvants that might be associated with FISS development, although there is still no clear evidence for the causal relationship. 11
An association between a ⩾four-fold titre increase after vaccination and the cats’ breed was found in the present study. Something similar has also been reported for feline panleukopenia. 14 Owing to the small number of cats with a ⩾four-fold titre increase, no conclusion on specific breeds is possible and further studies should be performed to evaluate breed predisposition towards vaccination failure in different cat breeds.
Interestingly, the cats’ pre-vaccination neutralising antibody titre was not associated with a ⩾four-fold titre increase after vaccination. High antibodies have been proposed to neutralise the vaccine virus before it stimulates the immune system, at least in FPV vaccination; 14 however, this could not be confirmed for FHV-1, likely owing to the fact that systemic antibodies are generally low compared with antibody titres against parvoviruses.14,37 Many veterinarians today choose to test for parvovirus antibodies to determine whether adult cats and dogs require re-vaccination.14,37,38 A semiquantitative in-house test for the detection of FPV antibodies is available. This test also measures FCV and FHV-1 antibodies. However, the value of measuring antibodies to determine cats’ immunity to FHV-1 instead of administering regular re-vaccinations is still not determined, and, unfortunately, the present study was not able to answer this question fully. A definitive answer could only be given through challenge experiments that cannot be performed in privately owned cats.
Current efforts in human medicine aim at reducing reactivation of herpes simplex through therapeutic vaccination. 39 However, whether vaccination in latently FHV-1-infected cats is of any use has not been determined yet. Sussman et al have shown that the latency load was lower in cats previously vaccinated than in non-vaccinated cats. 40 It would be interesting to see whether a booster vaccines would have a similar effect in infected cats.In bovine herpesvirus-1 (BHV-1) infection, virus excretion of already infected cattle after vaccination was compared with that in BHV-1-infected unvaccinated controls. All cattle, controls and vaccinees excreted virus after reactivation. However, inactivated vaccines reduced virus excretion more efficiently than the MLV vaccine. 41 In EHV-1 infection, clinical signs of already infected horses were unaffected by vaccination with either MLV or inactivated vaccines; however, after vaccination with inactivated vaccines against EHV-1, virus excretion was reduced during reactivation. 42 Thus, vaccination of latently infected cats might be meaningful when risk of transmission has to be minimised, such as in cats from shelters or catteries, and it could be discussed if inactivated vaccines would be preferable.41,42 However, the benefits and risks have to be considered as no data exist and the use of inactivated (adjuvants-based) vaccines should be avoided until further studies have been conducted.
A limitation of the study is that pre-dilution of serum samples might have led to an underestimation of the true FHV-1 neutralising antibody prevalence. Furthermore, a lack of antibody titre increase is not equivalent to a lack of development of protection against disease, as antibodies are not the only source of protection. Cell-mediated immunity is likely to play a key role, especially with MLV vaccines. However, challenge studies would be needed to prove this hypothesis, which cannot be done in client-owned cats. Other mechanisms, such as non-neutralising antibody-dependent cell-mediated cytotoxicity 43 as well as local immunity 44 might also play a role in protection.
Conclusions
Many cats have no detectable neutralising antibodies against FHV-1 despite previous vaccinations and fail to develop a ⩾four-fold titre increase after vaccination. Older cats and cats with a higher risk of FHV-1 exposure are more likely to be infected and thus to have FHV-1 neutralising antibodies. Purebred cats more often fail to develop a ⩾four-fold titre increase after vaccination. Further studies should be performed to evaluate breed predispositions towards vaccination failure in cats.
Acknowledgments
We thank Mrs Nadja Leinecker from the Institute of Animal Hygiene and Veterinary Public Health, University of Leipzig, for her advice, expertise and processing of the samples. We thank Boehringer Ingelheim for partially supporting the study and especially Jean-Christophe Thibault and Hervé Poulet for their input. We thank Prof Göhring from the Equine Hospital–Division of Medicine and Reproduction, LMU, Munich for his valuable input.
Footnotes
Accepted: 1 April 2019
Author note: Part of the results were presented as an abstract (<250 words) and oral presentation at the 5th ISCAID/IFRRS Symposium in Portland, OR, USA, 2018.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: A part of the study was funded by Boehringer Ingelheim. Boehringer Ingelheim played no role in the interpretation of data, or in the decision to submit the manuscript for publication. Dr Michèle Bergmann and Prof Katrin Hartmann have performed collaborative research and given presentations sponsored by Boehringer Ingelheim. There is no commercial conflict of interest as the information generated here is solely for scientific dissemination.
Ethical approval: This work involved the use of experimental animals; or involved the use of client-owned animal(s) outside of established internationally recognised high standards (‘best practice’) of individual veterinary clinical patient care. The study therefore had ethical approval from an established committee as stated in the manuscript.
Informed consent: Informed consent (either verbal or written) was obtained from the owners described in this work for the procedure(s) undertaken. No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Michèle Bergmann  https://orcid.org/0000-0002-8856-3849
https://orcid.org/0000-0002-8856-3849
References
- 1. Rota PA, Maes RK, Ruyechan WT. Physical characterization of the genome of feline herpesvirus-1. Virology 1986; 154: 168–179. [DOI] [PubMed] [Google Scholar]
- 2. Nasisse MP, Davis BJ, Guy JS, et al. Isolation of feline herpesvirus 1 from the trigeminal ganglia of acutely and chronically infected cats. J Vet Intern Med 1992; 6: 102–103. [DOI] [PubMed] [Google Scholar]
- 3. Maggs DJ. Update on pathogenesis, diagnosis, and treatment of feline herpesvirus type 1. Clin Tech Small Anim Pract 2005; 20: 94–101. [DOI] [PubMed] [Google Scholar]
- 4. Gaskell R, Dawson S, Radford A, et al. Feline herpesvirus. Vet Res 2007; 38: 337–354. [DOI] [PubMed] [Google Scholar]
- 5. Hosie MJ, Addie D, Belak S, et al. Matrix vaccination guidelines: ABCD recommendations for indoor/outdoor cats, rescue shelter cats and breeding catteries. J Feline Med Surg 2013; 15: 540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lappin MR, Andrews J, Simpson D, et al. Use of serologic tests to predict resistance to feline herpesvirus 1, feline calicivirus, and feline parvovirus infection in cats. J Am Vet Med Assoc 2002; 220: 38–42. [DOI] [PubMed] [Google Scholar]
- 7. Mouzin DE, Lorenzen MJ, Haworth JD, et al. Duration of serologic response to three viral antigens in cats. J Am Vet Med Assoc 2004; 224: 61–66. [DOI] [PubMed] [Google Scholar]
- 8. Poulet H. Alternative early life vaccination programs for companion animals. J Comp Pathol 2007; 137 Suppl 1: S67–S71. [DOI] [PubMed] [Google Scholar]
- 9. Scott FW, Geissinger CM. Long-term immunity in cats vaccinated with an inactivated trivalent vaccine. Am J Vet Res 1999; 60: 652–658. [PubMed] [Google Scholar]
- 10. Jas D, Frances-Duvert V, Vernes D, et al. Three-year duration of immunity for feline herpesvirus and calicivirus evaluated in a controlled vaccination-challenge laboratory trial. Vet Microbiol 2015; 177: 123–131. [DOI] [PubMed] [Google Scholar]
- 11. Hartmann K, Day MJ, Thiry E, et al. Feline injection-site sarcoma: ABCD guidelines on prevention and management. J Feline Med Surg 2015; 17: 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finch NC, Syme HM, Elliott J. Risk factors for development of chronic kidney disease in cats. J Vet Intern Med 2016; 30: 602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lappin MR, Jensen WA, Jensen TD, et al. Investigation of the induction of antibodies against Crandell-Rees feline kidney cell lysates and feline renal cell lysates after parenteral administration of vaccines against feline viral rhinotracheitis, calicivirus, and panleukopenia in cats. Am J Vet Res 2005; 66: 506–511. [DOI] [PubMed] [Google Scholar]
- 14. Bergmann M, Schwertler S, Reese S, et al. Antibody response to feline panleukopenia virus vaccination in healthy adult cats. J Feline Med Surg 2018; 20: 1087–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spaerman C. The method of “right and wrong cases” (“constant stimuli”) without Gauss’s formulae. Br J Psychol 1908; 2: 227–242. [Google Scholar]
- 16. Kaerber G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch Exp Path Pharma 1921; 162: 480–487. [Google Scholar]
- 17. DiGangi BA, Levy JK, Griffin B, et al. Prevalence of serum antibody titers against feline panleukopenia virus, feline herpesvirus 1, and feline calicivirus in cats entering a Florida animal shelter. J Am Vet Med Assoc 2012; 241: 1320–1325. [DOI] [PubMed] [Google Scholar]
- 18. Fischer SM, Quest CM, Dubovi EJ, et al. Response of feral cats to vaccination at the time of neutering. J Am Vet Med Assoc 2007; 230: 52–58. [DOI] [PubMed] [Google Scholar]
- 19. Gaskell RM, Povey RC. The dose response of cats to experimental infection with feline viral rhinotracheitis virus. J Comp Pathol 1979; 89: 179–191. [DOI] [PubMed] [Google Scholar]
- 20. Gaskell RM, Povey RC. Feline viral rhinotracheitis: sites of virus replication and persistence in acutely and persistently infected cats. Res Vet Sci 1979; 27: 167–174. [PubMed] [Google Scholar]
- 21. York IA, Jhonson DC. Viroceptors, virokines and related immune modulators encoded by DNA viruses. Boca Raton, FL: CRC Press, 1995. [Google Scholar]
- 22. Jas D, Aeberle C, Lacombe V, et al. Onset of immunity in kittens after vaccination with a non-adjuvanted vaccine against feline panleucopenia, feline calicivirus and feline herpesvirus. Vet J 2009; 182: 86–93. [DOI] [PubMed] [Google Scholar]
- 23. Povey RC, Koonse H, Hays MB. Immunogenicity and safety of an inactivated vaccine for the prevention of rhinotracheitis, caliciviral disease, and panleukopenia in cats. J Am Vet Med Assoc 1980; 177: 347–350. [PubMed] [Google Scholar]
- 24. Tizard I, Ni Y. Use of serologic testing to assess immune status of companion animals. J Am Vet Med Assoc 1998; 213: 54–60. [PubMed] [Google Scholar]
- 25. Zhang J, Liu H, Wei B. Immune response of T cells during herpes simplex virus type 1 (HSV-1) infection. J Zhejiang Univ Sci B 2017; 18: 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kydd JH, Smith KC, Hannant D, et al. Distribution of equid herpesvirus-1 (EHV-1) in respiratory tract associated lymphoid tissue: implications for cellular immunity. Equine Vet J 1994; 26: 470–473. [DOI] [PubMed] [Google Scholar]
- 27. Kydd JH, Smith KC, Hannant D, et al. Distribution of equid herpesvirus-1 (EHV-1) in the respiratory tract of ponies: implications for vaccination strategies. Equine Vet J 1994; 26: 466–469. [DOI] [PubMed] [Google Scholar]
- 28. Allen RG, Carey C, Parker JD, et al. Targeted ablation of pituitary pre-proopiomelanocortin cells by herpes simplex virus-1 thymidine kinase differentially regulates mRNAs encoding the adrenocorticotropin receptor and aldosterone synthase in the mouse adrenal gland. Mol Endocrinol 1995; 9: 1005–1016. [DOI] [PubMed] [Google Scholar]
- 29. Crabb BS, Studdert MJ. Equine herpesviruses 4 (equine rhinopneumonitis virus) and 1 (equine abortion virus). Adv Virus Res 1995; 45: 153–190. [DOI] [PubMed] [Google Scholar]
- 30. Munks MW, Montoya AM, Pywell CM, et al. The domestic cat antibody response to feline herpesvirus-1 increases with age. Vet Immunol Immunopathol 2017; 188: 65–70. [DOI] [PubMed] [Google Scholar]
- 31. Scepanovic P, Alanio C, Hammer C, et al. Human genetic variants and age are the strongest predictors of humoral immune responses to common pathogens and vaccines. Genome Med 2018; 10: 59. DOI: 10.1186/s13073-018-0568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gastón JZ, Stengel C, Harbour D, et al. Vergleich zwischen Mehrkatzenbeständen mit und ohne Katzenschnupfen. Tierarztl Prax Ausg K Kleintiere Heimtiere 2005; 33: 351–358. [Google Scholar]
- 33. Lappin MR, Veir J, Hawley J. Feline panleukopenia virus, feline herpesvirus-1, and feline calicivirus antibody responses in seronegative specific pathogen-free cats after a single administration of two different modified live FVRCP vaccines. J Feline Med Surg 2009; 11: 159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Walton TE, Gillespie JH. Feline viruses. VII. Immunity to the feline herpesvirus in kittens inoculated experimentally by the aerosol method. Cornell Vet 1970; 60: 232–239. [PubMed] [Google Scholar]
- 35. Povey RC, Wilson MR. A comparison of inactivated feline viral rhinotracheitis and feline caliciviral disease vaccines with live viral vaccines. Feline Pract 1978; 8: 35–42. [Google Scholar]
- 36. Summers SC, Ruch-Gallie R, Hawley JR, et al. Effect of modified live or inactivated feline herpesvirus-1 parenteral vaccines on clinical and laboratory findings following viral challenge. J Feline Med Surg 2017; 19: 824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Riedl M, Truyen U, Reese S, et al. Prevalence of antibodies to canine parvovirus and reaction to vaccination in client-owned, healthy dogs. Vet Rec 2015; 177: 597. [DOI] [PubMed] [Google Scholar]
- 38. Day MJ, Karkare U, Schultz RD, et al. Recommendations on vaccination for Asian small animal practitioners: a report of the WSAVA Vaccination Guidelines Group. J Small Anim Pract 2015; 56: 77–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cohen JI. Vaccination to reduce reactivation of herpes simplex virus type 2. J Infect Dis 2017; 215: 844–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sussman MD, Maes RK, Kruger JM. Vaccination of cats for feline rhinotracheitis results in a quantitative reduction of virulent feline herpesvirus-1 latency load after challenge. Virology 1997; 228: 379–382. [DOI] [PubMed] [Google Scholar]
- 41. Bosch JC, Kaashoek MJ, van Oirschot JT. Inactivated bovine herpesvirus 1 marker vaccines are more efficacious in reducing virus excretion after reactivation than a live marker vaccine. Vaccine 1997; 15: 1512–1517. [DOI] [PubMed] [Google Scholar]
- 42. Heldens JG, Hannant D, Cullinane AA, et al. Clinical and virological evaluation of the efficacy of an inactivated EHV1 and EHV4 whole virus vaccine (Duvaxyn EHV1,4). Vaccination/challenge experiments in foals and pregnant mares. Vaccine 2001; 19: 4307–4317. [DOI] [PubMed] [Google Scholar]
- 43. Wardley RC, Rouse BT, Babiuk LA. Observations on recovery mechanisms from feline viral rhinotracheitis. Can J Comp Med 1976; 40: 257–264. [PMC free article] [PubMed] [Google Scholar]
- 44. Lappin MR, Sebring RW, Porter M, et al. Effects of a single dose of an intranasal feline herpesvirus 1, calicivirus, and panleukopenia vaccine on clinical signs and virus shedding after challenge with virulent feline herpesvirus 1. J Feline Med Surg 2006; 8: 158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]