Abstract
Objectives
Alpha(α)2-agonist administration has been documented to increase blood glucose concentrations in many species. The aim of this study was to further describe the effect of dexmedetomidine on glucose and its regulatory hormones in healthy cats.
Methods
A randomized crossover study using eight healthy cats with a 14 day washout period was used to assess the effect of dexmedetomidine (10 μg/kg IV) and saline on glucose, cortisol, insulin, glucagon and non-esterified fatty acid (NEFA) concentrations at 0, 20, 60, 120 and 180 mins post-administration. Glucose:insulin ratios were calculated for each time point.
Results
Within the dexmedetomidine group, significant differences (P <0.05) were detected: increased median (range) blood glucose concentrations at 60 mins (11.55 mmol/l [5.9–16.6 mmol/l]) and 120 mins (12.0 mmol/l [6.1–13.8 mmol/l]) compared with baseline (6.05 mmol/l [4.8–13.3 mmol/l]); decreased glucagon concentrations at 120 mins (3.8 pmol/l [2.7–8.8 pmol/l]) and 180 mins (4.7 pmol/l [2.1–8.2 pmol/l]) compared with baseline (11.85 pmol/l [8.3–17.2 pmol/l]); decreased NEFA concentrations at 60 mins (0.281 mmol/l [0.041–1.357 mmol/l]) and 120 mins (0.415 mmol/l [0.035–1.356 mmol/l]) compared with baseline (0.937 mmol/l [0.677–1.482 mmol/l]); and significantly larger (P <0.05) glucose:insulin ratios at 60 mins compared with baseline. Insulin and cortisol concentrations were not significantly changed after dexmedetomidine administration.
Conclusions and relevance
Feline practitioners should be aware of the endocrine effects associated with the use of α2-agonists, particularly when interpreting blood glucose concentrations. The transient effects of dexmedetomidine on glucose homeostasis are unlikely to significantly affect clinical practice.
Keywords: Dexmedetomidine, glucose homeostasis, glucose, insulin, glucagon, cortisol, non-esterified fatty acids, α2-agonists
Introduction
Dexmedetomidine has become increasingly popular in veterinary practice as a sedative and pre-anesthetic medication.1,2 Additional uses of dexmedetomidine are based upon its analgesic, muscle-relaxing and emetic properties.3,4 The most well-known side effects of dexmedetomidine are centered around its cardiovascular effects that lead to peripheral vasoconstriction, bradycardia and decreased cardiac output.5–7 Dexmedetomidine also has effects on the endocrine system and glucose homeostasis.8–15
Alpha(α)2-adrenoceptors are associated with modulation of the sympathoadrenal system. 9 Subtypes of α2-adrenoceptors (α2A, α2B and α2C) mediate distinct physiologic functions. 9 α2A-adrenoceptors constitute the most common subtype in murine pancreatic islets, 16 and are responsible for inhibition of insulin secretion in both mice and rats.16,17 In addition, research in transgenic mice demonstrated that overexpression of the α2A-adrenoreceptor in pancreatic beta cells led to impaired glucose tolerance and type 2 diabetes.18,19
Dexmedetomidine has been shown to significantly decrease the plasma insulin concentration and increase the plasma glucose concentration in dogs. 8 To our knowledge, the effects of xylazine and racemic medetomidine on glucose homeostasis have been investigated in five healthy cats, 15 but not the isolated dextrorotatory isomer of medetomidine, dexmedetomidine. One study in rat pancreatic islets showed that dexmedetomidine is 49 times more potent than medetomidine for inhibition of insulin secretion. 13 These findings suggest that glucose homeostasis may be significantly more affected by dexmedetomidine than medetomidine.
The objective of this study is to describe the feline metabolic response to dexmedetomidine by measuring glucose, cortisol, insulin, glucagon and non-esterified fatty acids (NEFAs), as well as calculating glucose:insulin ratios following intravenous (IV) administration of dexmedetomidine compared with saline in a crossover study with eight healthy cats. We hypothesized that dexmedetomidine significantly modifies glucose homeostasis in healthy cats, in particular decreasing insulin concentration and increasing blood glucose concentration.
Materials and methods
Animals
Eight healthy cats (one domestic shorthair castrated male and seven domestic shorthair spayed females, mean age 30.4 months [range 9–65 months], mean weight 4.5 kg [range 3.4–6.3]) were used in this study. The cats were housed according to the requirements of the Canadian Council for Animal Care in a licensed facility. They were fed a standard commercial dry cat food. Prior to inclusion in the study, unremarkable complete blood count, biochemistry, urinalysis and fructosamine concentrations confirmed the health status of all cats as performed by Prairie Diagnostic Services (data not included). The experimental protocol was approved by the University Animal Care Committee at the University of Saskatchewan.
Study preparation
Food, but not water, was withheld for at least 12 h prior to the procedure.
General anesthesia was induced with sevoflurane (SevoFlo; Zoetis) in a clear-walled induction chamber and maintained by a facemask to facilitate placement of a jugular (19 G × 15 cm polyurethane catheter; MILA International) and cephalic catheter (22 G 0.9 × 25 mm IV Catheter; BD Insyte) vein using an aseptic technique. A minimum of 60 mins was allowed for full recovery from sevoflurane prior to initiation of the experiment.
Experimental protocol
A randomized crossover design study with a 14 day washout period was used to assess the effect of 10 μg/kg IV dexmedetomidine (Dexdomitor; Zoetis) made up to a total volume of 1 ml with 0.9% saline vs 1 ml 0.9% saline (placebo). Randomization of cats into each group was performed by a computer-generated random number spreadsheet (Microsoft Excel). The cephalic catheter was used to administer these two treatments. Blood sampling (4 ml) was performed from the jugular catheter prior to treatment (baseline), and at 20, 60, 120 and 180 mins after treatment. During the saline arm of the experiment, the jugular catheter did not remain patent in one cat, necessitating sampling from the cephalic catheter at all time points.
Analytic methods
The blood sample was processed at each time point as follows. Glucose (AlphaTRAK2; Zoetis) and lactate (Lactate pro; Arkray) concentrations were immediately measured using a drop of whole blood on specific hand-held commercial analyzers. The lactate values below the detection limit of the analyzer were considered to be equal to the detection limit (ie, 0.8 mmol/l). The remaining sample was kept on ice until clotted (20 mins). Within 30 mins of sample collection, the tubes were centrifuged at 2200 g and 4ºC for 12 mins. Aliquots of the serum were stored at –80ºC and analyzed within 1 month.
Serum cortisol, insulin, glucagon and NEFA were measured using commercially available diagnostic assays (Table 1). Cortisol and glucagon assays were previously validated in cats.20,21 Spike and recovery assessment were run using a pooled feline serum sample for each plate. Glucose:insulin ratios were calculated for each time point.
Table 1.
Characteristics of the assays used to measure cortisol, insulin, glucagon and non-esterified fatty acid (NEFA) in eight healthy cats
| Parameter | Assay | Limit of detection | Intra-assay variation (COV) for given parameter concentrations |
|---|---|---|---|
| Cortisol | Immulite/Immulite 1000 Cortisol (Siemens) | 5.5 nmol/l | 5.6% and 4.0% for 103.1 and 844.7 nmol/l, respectively |
| Insulin | Feline Insulin Elisa kit 10-1233-01 (Mercodia AB) | 9.2 ng/l | 2.4% and 15% for 534.9 and 1221.4 ng/l, respectively |
| Glucagon | Glucagon Elisa Kit 10-1281-01 (Mercodia AB) | 1.5 pmol/l | 8.0% and 15.2% for 7.7 and 55.6 pmol/l, respectively |
| NEFA | Enzymatic colorimetric method assay (Randox) | 0.072 mmol/l | 2.30% |
There was no inter-assay variation as all samples could be run on a single ELISA plate for each analyte measured
COV = coefficient of variation
Statistical analysis
Prior to conducting this study, a power analysis on existing canine data suggested that eight cats would be sufficient (power = 0.8, alpha = 0.05) to detect a 15.1 µU/ml, and a 2.3 mmol/l difference in serum insulin and whole blood glucose concentrations, respectively (GraphPad StatMate 2.00). 8
The blood glucose, lactate and hormone analysis data collected was determined to be non-parametric based on the violation of the Shapiro–Wilk normality test. A Friedman test was performed for each variable within groups (dexmedetomidine or saline). If significant differences were identified, then post-hoc analysis was performed using a Dunn’s multiple comparisons test to compare treatment time points to baseline. A Wilcoxon matched pair signed rank test was performed for each variable between groups (dexmedetomidine and saline) at each time point. All statistical analyses were performed using commercially available software (Graph Pad Prism version 7.0). P values <0.05 were considered significant.
Results
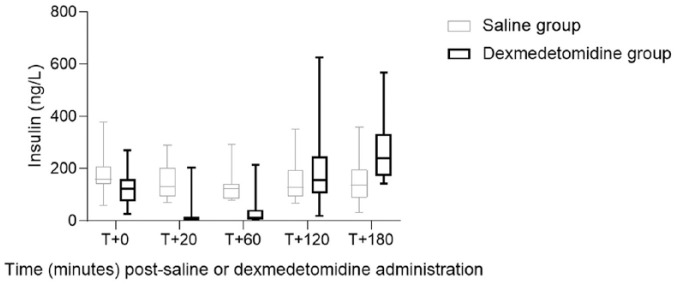
Within the dexmedetomidine group, significant differences (P <0.05) (median [range]) were detected: increased blood glucose concentrations at 60 mins (11.55 mmol/l [5.9–16.6 mmol/l]) and 120 mins (12.0 mmol/l [6.1–13.8 mmol/l]) compared with baseline (6.05 mmol/l [4.8–13.3 mmol/l]) (Figure 1); decreased glucagon concentrations at 120 mins (3.8 pmol/l [2.7–8.8 pmol/l]) and 180 mins (4.7 pmol/l [2.1–8.2 pmol/l]) compared with baseline (11.85 pmol/l [8.3–17.2 pmol/l]) (Table 2); and decreased NEFA concentrations at 60 mins (0.281 mmol/l [0.041–1.357 mmol/l]) and 120 mins (0.415 mmol/l [0.035–1.356 mmol/l]) compared with baseline (0.937 mmol/l [0.677–1.482 mmol/l]) (Table 2). Insulin and cortisol concentrations were not significantly changed after dexmedetomidine administration (Figure 2; Table 2).
Figure 1.
Box plot comparison of glucose concentration after dexmedetomidine or saline administration in eight healthy cats. Error bars represent range, interquartile range and median glucose concentration within a treatment group. A significant difference (P <0.05) was noted at T+60 (*) and T+120 (**) minutes within the dexmedetomidine group
Table 2.
Mean concentrations of glucagon, cortisol and non-esterified fatty acid (NEFA) after administration of saline or dexmedetomidine in eight healthy cats
| Time points after administration of saline or dexmedetomidine (mins) | |||||
|---|---|---|---|---|---|
| T+0 | T+20 | T+60 | T+120 | T+180 | |
| Glucagon (pmol/l) | |||||
| Saline | 11.68 | 9.88 | 9.30 | 9.05 | 8.08 |
| Dexmedetomidine | 12.59 | 11.99 | 7.10 | 4.39* | 4.48* |
| Cortisol (nmol/l) | |||||
| Saline | 139.95 | 147.68 | 115.03 | 124.11 | 100.84 |
| Dexmedetomidine | 108.01 | 85.04 | 69.04 | 94.13 | 81.65 |
| NEFA (mmol/l) | |||||
| Saline | 0.98 | 0.91 | 0.84 | 0.89 | 0.85 |
| Dexmedetomidine | 0.98 | 0.59 | 0.39 | 0.52* | 0.51 |
Significant differences (P <0.05) of glucagon and NEFA concentrations were noted at T+120 and T+180 mins, and T+120 mins, respectively, within the dexmedetomidine group
Figure 2.
Box plot comparison of insulin concentration after dexmedetomidine or saline administration in eight healthy cats. Error bars represent range, interquartile range and median insulin concentration within a treatment group. No significant difference (P <0.05) was noted within or between groups
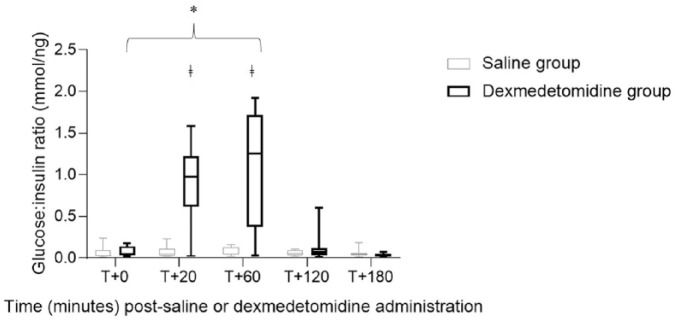
Glucose:insulin ratios were significantly different (P <0.05) between the dexmedetomidine group and the saline group at 20 and 60 mins (Figure 3). Within the dexmedetomidine group, the median glucose:insulin ratio was significantly larger (P <0.05) at 60 mins compared with baseline (Figure 3).
Figure 3.
Box plot comparison of glucose:insulin ratio after dexmedetomidine or saline administration in eight healthy cats. Error bars represent range, interquartile range and median glucose:insulin ratio within a treatment group. A significant difference (P <0.05) was noted at T+20 and T+60 mins between groups (ǂ) and at T+60 mins within the dexmedetomidine group (*)
Lactate concentrations were measured at each time point for all cats and groups, with no significant differences observed (data not shown).
Discussion
This study documents the metabolic effects of dexmedetomidine on glucose homeostasis in healthy cats. Significant elevations in blood glucose concentrations were observed within the dexmedetomidine group. We believe that acute stress associated with inhalational anesthetic, sampling catheter placement and repeated sampling do not account for the elevated blood glucose concentrations observed in this study as there were no significant changes over time in the control (saline) group with any variable. α2-agonists are known to increase blood glucose concentrations in rats, 22 mice, 14 dogs8,10,11 and cats. 15 Studies in rats17,22,23 and mice14,24 suggested that α2-agonists inhibit insulin secretion via the α2A-adrenoreceptor subtype on pancreatic beta-cells.
The inhibition of insulin release from pancreatic beta cells was hypothesized to be responsible for the increase in blood glucose concentrations after dexmedetomidine administration. Despite decreasing trends in serum insulin concentrations at 20 mins and 60 mins (Figure 2), there were no statistically significant changes observed in this study. We believe that a type II error was committed, despite performing a power analysis during study design. Similar non-significant trends were observed in a previous study in which plasma insulin concentrations were decreased after medetomidine and xylazine administration. 15 Statistical significance was only observed at a non-clinical high-dose of medetomidine (320 μg/kg IM), which is in contrast to the clinically relevant 10 μg/kg dose used in this study. 15 It is our opinion that the decreasing trends in serum insulin concentration are real changes, but limitations with sample size and variability preclude the detection of statistical significance.
To our knowledge, this study is the first to describe glucose:insulin ratios after the administration of an α2 agonist in cats. Glucose:insulin ratios were significantly larger at 20 mins and 60 mins in the dexmedetomidine group compared with the saline group. We chose to depict the data using glucose:insulin ratios primarily to demonstrate the trends observed in both blood glucose and insulin concentrations. Although the glucose:insulin ratio is most often used as a simplified indicator of insulin sensitivity, 25 we caution interpreting the results of this study as such.
There were no significant increases in serum glucagon concentrations at any time point during this study to suggest that glucagon secretion was responsible for the elevation in blood glucose level. Instead, a significant decrease in serum glucagon concentration was observed. This finding most likely reflects normal physiology as the serum glucagon concentration decreased in response to the increase in blood glucose concentration.
Previous studies in cats 15 and dogs8,26 did not demonstrate a significant effect of α2-agonists on serum cortisol concentration. There was also no significant effect of dexmedetomidine upon serum cortisol concentrations in this study. Cortisol is unlikely to be a major factor in acute blood glucose regulation, as observed in this study.
The lactate concentration remained within the normal range, except for three cats (one cat in the dexmedetomidine group and two cats in the control group) and there was no significant difference between the two treatment groups at all time points. Therefore, it is unlikely that lactate release is the mechanism for hyperglycemia, as demonstrated in a previous study evaluating acute stress hyperglycemia in healthy cats. 27
Hormone-sensitive lipase (HSL) liberates NEFAs from adipocytes, resulting in lipolysis. HSL activity is upregulated by catecholamines and downregulated by insulin. Dexmedetomidine significantly decreased serum NEFA concentrations in this study, as previously observed in cats 15 and dogs8,28 after the administration of α2-agonists. Although not analyzed, we speculate that decreasing catecholamine concentrations from dexmedetomidine’s sympatholytic activity resulted in a modified insulin:catecholamine ratio, which may account for the decreased serum NEFA concentrations. 15 A direct effect of the α2-agonist on fatty cell α2-receptors could also be possible. 29
There are some important limitations to recognize with this study. Linearity of diffusion assessment was not performed on each assay for measurement of glucagon and cortisol. Consideration should be given to the role of other glucoregulatory hormones not measured, such as adrenaline (epinephrine), noradrenaline (norepinephrine) and growth hormone. Future research into the effect of α2-agonists on these glucoregulatory hormones is warranted. In addition, sedation and hypothermia can also affect blood glucose concentrations. None of the cats were profoundly sedated by the dexmedetomidine, but serial body temperatures and sedation scoring were not performed. Finally, the predominance of female spayed cats in this study may affect the results as drugs can be metabolized differently between the sexes.
The clinical significance of a transient increase in blood glucose post-dexmedetomidine administration remains unclear. A false-positive diagnosis of diabetes mellitus might arise if hyperglycemia and glucosuria are concurrently identified. Unfortunately, urine dipsticks were not evaluated to identify possible glucosuria. Interestingly, 25% of dexmedetomidine-treated cats vs 17% of saline-treated cats had blood glucose concentrations above the renal threshold of 12 mmol/l. 30 Clinicians should continue to interpret laboratory findings in the context of clinical signs and consider running a serum fructosamine to differentiate between transient and long-standing hyperglycemia. Finally, the role of α2-adrenoceptor agonists in cats developing insulin resistance during the prediabetic state remains to be elucidated.
Conclusions
The short-term effect of dexmedetomidine on glucose regulation was described in this study. Elucidating the underlying mechanisms of hyperglycemia associated with dexmedetomidine, and its implications in clinical practice, may warrant further study.
Acknowledgments
The authors would like to thank Dr Suraj Unniappan and Terri Osborn for their respective support with the design and execution of this study.
Footnotes
Accepted: 8 April 2019
Author note: This paper was presented as an abstract at the 2018 ACVIM Forum.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Financial support for the study was provided by the Companion Animal Health Fund at the Western College of Veterinary Medicine. Financial support for a student stipend was provided by Zoetis.
Ethical approval: This work involved the use of experimental animals; or involved the use of client-owned animal(s) outside of established internationally recognised high standards (‘best practice’) of individual veterinary clinical patient care. The study therefore had ethical approval from an established committee as stated in the manuscript.
ORCID iD: Juliette Bouillon  https://orcid.org/0000-0003-2926-4926
https://orcid.org/0000-0003-2926-4926
References
- 1. McSweeney PM, Martin DD, Ramsey DS, et al. Clinical efficacy and safety of dexmedetomidine used as a preanesthetic prior to general anesthesia in cats. J Am Vet Med Assoc 2012; 240: 404–412. [DOI] [PubMed] [Google Scholar]
- 2. Rodan I, Sundahl E, Carney H, et al. AAFP and ISFM feline-friendly handling guidelines. J Feline Med Surg 2011; 13: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thawley VJ, Drobatz KJ. Assessment of dexmedetomidine and other agents for emesis induction in cats: 43 cases (2009–2014). J Am Vet Med Assoc 2015; 247: 1415–1418. [DOI] [PubMed] [Google Scholar]
- 4. Scrollavezza P, Tambella AM, Vullo C, et al. Evaluation of the muscular relaxant effect of dexmedetomidine or medetomidine in cats. Vet Res Commun 2009; 33: S213–S215. [DOI] [PubMed] [Google Scholar]
- 5. Pypendop BH, Honkavaara J, Ilkiw JE. Pharmacokinetics of dexmedetomidine, MK-467, and their combination following intravenous administration in male cats. J Vet Pharmacol Ther 2016; 39: 460–468. [DOI] [PubMed] [Google Scholar]
- 6. Pypendop BH, Barter LS, Stanley SD, et al. Hemodynamic effects of dexmedetomidine in isoflurane-anesthetized cats. Vet Anaesth Analg 2011; 38: 555–567. [DOI] [PubMed] [Google Scholar]
- 7. Kallio A, Scheinin M, Koulu M, et al. Effects of dexmedetomidine, a selective α2-adrenoceptor agonist, on hemodynamic control mechanisms. Clin Pharmacol Ther 1989; 46: 33–42. [DOI] [PubMed] [Google Scholar]
- 8. Restitutti F, Raekallio M, Vainionpää M, et al. Plasma glucose, insulin, free fatty acids, lactate and cortisol concentrations in dexmedetomidine-sedated dogs with or without MK-467: a peripheral α-2 adrenoceptor antagonist. Vet J 2012; 193: 481–485. [DOI] [PubMed] [Google Scholar]
- 9. Fagerholm V, Haaparanta M, Scheinin M. α2-adrenoceptor regulation of blood glucose homeostasis. Basic Clin Pharmacol Toxicol 2011; 108: 365–370. [DOI] [PubMed] [Google Scholar]
- 10. Burton SA, Lemke KA, Ihle SL, et al. Effects of medetomidine on serum insulin and plasma glucose concentrations in clinically normal dogs. Am J Vet Res 1997; 58: 1440–1442. [PubMed] [Google Scholar]
- 11. Guedes AG, Rude EP. Effects of pre-operative administration of medetomidine on plasma insulin and glucose concentrations in healthy dogs and dogs with insulinoma. Vet Anaesth Analg 2013; 40: 472–481. [DOI] [PubMed] [Google Scholar]
- 12. Yamazaki S, Katada T, Ui M. Alpha2-adrenergic inhibition of insulin secretion via interference with cyclic AMP generation in rat pancreatic islets. Mol Pharmacol 1982; 21: 648–653. [PubMed] [Google Scholar]
- 13. Yamato-Kodera S, Yoshida M, Dezaki K, et al. Inhibition of insulin secretion from rat pancreatic islets by dexmedetomidine and medetomidine, two sedatives frequently used in clinical settings. Endocr J 2013; 60: 337–346. [DOI] [PubMed] [Google Scholar]
- 14. Angel I, Bidet S, Langer SZ. Pharmacological characterization of the hyperglycemia induced by alpha-2 adrenoceptor agonists. J Pharmacol Exp Ther 1988; 246: 1098–1103. [PubMed] [Google Scholar]
- 15. Kanda T, Hikasa Y. Neurohormonal and metabolic effects of medetomidine compared with xylazine in healthy cats. Can J Vet Res 2008; 72: 278–286. [PMC free article] [PubMed] [Google Scholar]
- 16. Fagerholm V, Grönroos T, Marjamäki P, et al. Altered glucose homeostasis in α2A-adrenoceptor knockout mice. Eur J Pharmacol 2004; 505: 243–252. [DOI] [PubMed] [Google Scholar]
- 17. Niddam R, Angel I, Bidet S, et al. Pharmacological characterization of alpha-2 adrenergic receptor subtype involved in the release of insulin from isolated rat pancreatic islets. J Pharmacol Exp Ther 1990; 3: 883–887. [PubMed] [Google Scholar]
- 18. Devedjian JC, Pujol A, Cayla C, et al. Transgenic mice overexpressing α2A-adrenoceptors in pancreatic beta-cells show altered regulation of glucose homeostasis. Diabetologia 2000; 43: 899–906. [DOI] [PubMed] [Google Scholar]
- 19. Davani B, Portwood N, Bryzgalova G, et al. Aged transgenic mice with increased glucocorticoid sensitivity in pancreatic beta-cells develop diabetes. Diabetes 2004; 53: S51–59. [DOI] [PubMed] [Google Scholar]
- 20. Singh AK, Jiang Y, White T, et al. Validation of nonradioactive chemiluminescent immunoassay methods for the analysis of thyroxine and cortisol in blood samples obtained from dogs, cats, and horses. J Vet Diagn Invest 1997; 9: 261–268. [DOI] [PubMed] [Google Scholar]
- 21. Hall MJ, Adin CA, Borin-Crivellenti S, et al. Pharmacokinetics and pharmacodynamics of the glucagon-like peptide-1 analog liraglutide in healthy cats. Domest Anim Endocrinol 2015; 51: 114–121. [DOI] [PubMed] [Google Scholar]
- 22. Angel I, Langer SZ. Adrenergic-induced hyperglycemia in anaesthetized rats: involvement of peripheral alpha2-adrenoreceptors. Eur J Pharmacol 1988; 154: 191–196. [DOI] [PubMed] [Google Scholar]
- 23. Hirose H, Maruyama H, Ito K, et al. Glucose-induced insulin secretion and alpha 2-adrenergic receptor subtypes. J Lab Clin Med 1993; 121: 32–37. [PubMed] [Google Scholar]
- 24. Angel I, Niddam R, Langer SZ. Involvement of alpha-2 adrenergic receptor subtypes in hyperglycemia. J Pharmacol Exp Ther 1990; 254: 877–882. [PubMed] [Google Scholar]
- 25. Appleton D, Rand J, Sunvold G. Basal plasma insulin and homeostasis model assessment (HOMA) are indicators of insulin sensitivity in cats. J Feline Med Surg 2005; 7: 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raekallio M, Kuusela E, Lehtinen M, et al. Effects of exercise-induced stress and dexamethasone on plasma hormone and glucose concentrations and sedation in dogs treated with dexmedetomidine. Am J Vet Res 2005; 66: 260–265. [DOI] [PubMed] [Google Scholar]
- 27. Rand JS, Kinnaird E, Baglioni A, et al. Acute stress hyperglycemia in cats is associated with struggling and increased concentrations of lactate and norepinephrine. J Vet Intern Med 2002; 16: 123–132. [DOI] [PubMed] [Google Scholar]
- 28. Ambrisko TD, Hikasa Y. Neurohormonal and metabolic effects of medetomidine compared with xylazine in beagle dogs. Can J Vet Res 2002; 66: 42–49. [PMC free article] [PubMed] [Google Scholar]
- 29. Lafontan M, Berlan M. Evidence for the α2 nature of the α-adrenergic receptor inhibiting lipolysis in human fat cells. Eur J Pharmacol 1980; 66: 87–93. [DOI] [PubMed] [Google Scholar]
- 30. Rand J. Current understanding of feline diabetes: part 1, pathogenesis. J Feline Med Surg 1999; 1: 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]