Abstract
The L1 stage of the parasitic nematode Trichinella spiralis displays on its surface glycoproteins that are immunologically cross-reactive with several larval excretory-secretory (ES) products. The basis for the cross-reactivity is tyvelose, the terminal residue on the complex glycans shared by these surface and ES glycoproteins. In neonatal rats, tyvelose-specific monoclonal antibodies mediate the expulsion of larvae from the intestine. The aim of the studies described in this report was to determine how antibody binding to larval surfaces contributes to expulsion. In these experiments, which involve an in vitro assay of epithelial cell invasion, surface proteins on living larvae were biotinylated to distinguish them from ES products. Biotinylated and nonbiotinylated larvae were cocultured with avidin, biotin-specific antibodies, or anti-tyvelose monoclonal antibodies. Biotinylated larvae cultured with avidin or biotin-specific antibodies invaded Madin-Darby canine kidney (MDCK) cells equally as well as biotinylated larvae cultured with medium alone. Anti-tyvelose monoclonal antibodies were highly protective in this assay; however, biotinylation of larval surfaces hindered the ability of anti-tyvelose monoclonal antibodies to prevent larval invasion of epithelial cells. This correlated with a reduction in the binding of anti-tyvelose antibody to biotinylated larval surfaces. Our results indicate that antibody binding to surface glycoproteins contributes to protection against T. spiralis invasion but that surface binding alone is not sufficient for protection. Our findings support the notion that protection is effected by cross-linking of ES products to surface antigens.
Trichinosis is acquired by the ingestion of animal muscle tissue containing viable mature L1 Trichinella spiralis larvae (11, 15). Larvae molt to adulthood, mate, and reproduce in the host small intestine. The T. spiralis life cycle is completed when newborn larvae invade and mature in striated muscle cells of the new host (11). During the intestinal phase of infection, larval and adult parasites localize to the crypt-villus junction, establishing an intramulticellular niche composed of numerous epithelial cells (21). The parasites are mobile in the epithelium, continually invading and occupying the cytoplasm of new cells (22).
Rat pups suckling previously infected dams expel up to 99% of a challenge dose of infective larvae (1, 9). A major component of this dramatic protection, called rapid expulsion, is mediated by antibodies specific for a dideoxyhexose called tyvelose (2, 4, 12). Tyvelose residues cap antennae of complex glycans shared by several glycoproteins expressed on the surfaces and in the ES products of L1 larvae (10, 19). Anti-tyvelose antibodies appear to protect in two ways: by excluding larvae from the epithelium and by dislodging them from that site. Exclusion may occur with or without entrapment of larvae in mucus (5). Mucus entrapment occurs as early as 30 min after a challenge of immune rat pups, retaining larvae in the intestinal lumen and preventing invasion (5, 6). Mucus-trapped larvae are coated with antibody, suggesting that binding of antibodies to the surface promotes entrapment or exclusion. Mucus entrapment is reversible and is insufficient to effect protection (6). Alternate mechanisms by which larvae are excluded from epithelia have not been elucidated.
In this paper, we describe experiments designed to assess the protection afforded by specific antibody binding to larval surface glycoproteins. We inoculated cultured epithelial cells with surface-tagged larvae in the presence of surface binding (tag-specific) antibodies or surface and excretory-secretory product (ES) binding antibodies (anti-tyvelose). We report evidence that surface tyvelose-bearing glycoproteins are secondary targets in antibody-mediated exclusion of larvae from epithelia.
MATERIALS AND METHODS
Tissue culture.
The AA7 clone (strain 1) of the Madin-Darby canine kidney (MDCK) cell line was a gift from William Young (University of Kentucky) (16). Cells were maintained in minimal essential medium (Earle’s salts) supplemented with l-glutamine, nonessential amino acids, and 10% fetal bovine serum. The cells were dispersed with 0.5% trypsin–0.65 mM EDTA and passaged no more than 15 times before being used in experiments.
Parasite.
T. spiralis (pig strain) infectious larvae were recovered from infected AO rats by digestion of carcasses in acidified pepsin (8). Pepsin-digested L1 larvae were activated by incubation in 25% rat intestinal contents in 0.85% saline for 2 h at 37°C (13). They were then washed four times in saline and incubated in saline at 37°C for an additional 1 h (13).
MAbs.
Protective rat monoclonal antibodies (MAbs) used in these experiments were anti-tyvelose 18H (immunoglobulin G2a [IgG2a]), and 9E (IgG2c) (2, 6). MAb 16H (IgG1) has an alternate specificity and is not protective (2, 6). All antibodies were concentrated from ascites by (NH4)2SO4 precipitation as described previously (6).
Biotinylation of larval surface proteins.
Activated larvae were washed twice in saline and then twice in 50 mM carbonate buffer (pH 8.5). The larvae were incubated at room temperature in sulfo-NHS-LC-biotin (Pierce, Rockford, Ill.) diluted to 1 mg/ml in 50 mM carbonate buffer (pH 8.5) for 1.5 h. Control larvae were incubated in carbonate buffer alone. The larvae were washed four times in saline before being used in assays. The viability of the larvae was estimated to be greater than 95%.
Fluorescence microscopy.
Biotinylated and control larvae were washed twice in ice-cold Dulbecco’s phosphate-buffered saline (DPBS) and then incubated with 10 μg of MAb 18H per ml in DPBS for 30 min on ice. Following four 5-min washes with cold DPBS, larvae were incubated on ice for 30 min in goat anti-rat IgG conjugated with fluorescein isothiocyanate (FITC; Organon Teknika Corp., Durham, N.C.) diluted to 200 μg/ml in DPBS. The larvae were washed as above, suspended in mounting medium (Vectashield; Vector Laboratories, Inc., Burlingame, Calif.), placed on a slide, and covered with a coverslip. They were examined with an inverted microscope equipped for epifluorescence (Nikon Diaphot; Opti-quip, Highland Mills, N.Y.). Images were captured with a charge-coupled device camera (Hamamatsu Photonics K. K.) and NIH Image 1.58. To monitor the quality of labeling, biotinylated and control larvae were routinely incubated with FITC-streptavidin (Pierce) and examined by fluorescence microscopy as described above.
Western blots.
Biotinylated or control larvae were washed in 15 ml of ice-cold DPBS seven times and then homogenized in DPBS containing 1.5% N-octylglucopyranoside, 1 mM EDTA/1 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, and 25 μg of tolysulfonyl phenylalanyl chloromethyl ketone (TPCK) (3). Homogenates were centrifuged at 10,000 × g for 10 min at 4°C, and the soluble portion (lysate) was stored at −20°C. Proteins from lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and then subjected to Western blotting (2). The blots were washed in 0.1% Nonidet P-40 (NP-40) in DPBS for 45 min at room temperature and then probed with streptavidin-horseradish peroxidase (Pierce) diluted 1:5,000 in 0.1% NP-40–1% bovine serum albumin in DPBS for 45 min. Following four 5-min washes in 0.1% NP-40 in DPBS, the blots were developed with a chemiluminescent substrate (Amersham Life Science, Inc., Cleveland, Ohio) and exposed to X-Omat AR film (Kodak, Rochester, N.Y.).
Invasion assay.
The invasion assay has been described elsewhere (13). Briefly, MDCK cells were grown to confluence in eight-well glass chamber slides (Nunc, Naperville, Ill.). Monolayers were overlaid with larvae suspended in minimal essential medium–15 mM HEPES–1.75% agarose. For protection assays, larvae were suspended in culture medium containing avidin or antibodies. Avidin (Neutravidin; Pierce) was included in the assay mixtures at concentrations ranging from 1.0 to 0.125 mg/ml; affinity-purified goat anti-biotin (Sigma, St. Louis, Mo.) was included at 0.25 mg/ml. After incubation of the cultures for 2 h at 37°C under 5% CO2, the chamber housing, gasket, and medium were removed from the slides. Monolayers were submerged for 2 min in 0.4% trypan blue solution (Sigma), rinsed in DPBS (with MgCl2 and CaCl2), and fixed in 10% buffered formalin for 20 min. Coverslips were mounted on slides with glycergel (DAKO Corp., Carpenteria, Calif.). The area of dead (trypan blue-stained) cells in monolayers was quantified by computer-assisted image capture analysis (NIH Image 1.58). At least 25 fields per monolayer were captured by video microscopy with a 4× objective (Labophot; Nikon and COHU, Inc., San Diego, Calif.). The mean area of dead cells per field was estimated for at least three monolayers per treatment group. Differences between groups were determined by analysis of variance and Scheffés mean separation test.
Affinity chromatography.
Antibody 18H was conjugated to cyanogen-bromide activated Sepharose 4B beads (Sigma) as previously described (3). Lysates from biotinylated larvae were applied to the affinity column (1.5 by 7.5 cm), which had been equilibrated with wash buffer (0.01 M Tris-Cl, 0.14 M NaCl, 0.5% Triton X-100, 0.5% deoxycholate [pH 8.0]) (20). The column was washed with 0.05 M Tris-Cl–0.5 M NaCl (pH 8.0) and then with 0.05 M Tris-Cl–0.5 M NaCl (pH 9.0). Bound glycoproteins were eluted with 50 mM triethanolamine–0.5 M NaCl–0.1% Triton X-100 (pH 11.5). Eluted fractions were neutralized with 1 M Tris-Cl (pH 6.7) and stored at 4°C (20).
RESULTS
Biotinylation of larval surface proteins.
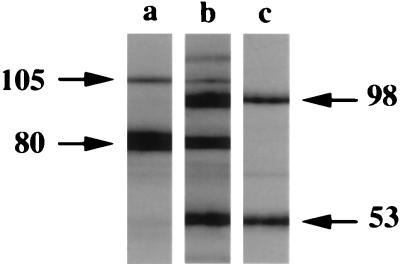
In these experiments, we wanted to identify the protective effects associated with antibody binding to larval surface glycoproteins. Our strategy was to provide a distinct surface binding target. Membrane-impermeant biotin was used to label surface proteins, and FITC-streptavidin staining revealed that the biotin was evenly distributed over the larval surfaces (data not shown). Biotinylation of larval proteins was confirmed by immunoblot analysis with streptavidin-horseradish peroxidase. Streptavidin bound to proteins (estimated molecular masses of 115, 105, 98, 80, and 53 kDa) in lysates of biotinylated larvae (Fig. 1, lane b). Two of these proteins (105 and 80 kDa) were also present in lysates of control larvae (lane a), indicating that these larval proteins are inherently reactive with avidin. Affinity chromatography revealed that MAb 18H bound two of the biotinylated proteins (98 and 53 kDa) but not the native avidin binding proteins (lane c).
FIG. 1.
Western blot of T. spiralis larval lysates analyzed with streptavidin-horseradish peroxidase as described in Materials and Methods. Lanes: a, proteins from lysate of control larvae; b, proteins from lysate of biotinylated larvae; c, proteins from lane b affinity purified with MAb 18H. Estimated molecular masses (in kilodaltons) of dominant proteins are indicated by arrows. A Power Macintosh 720/120 computer and Adobe Photoshop 4.0 was used to label the figure. The image from the autoradiograph was imported into Adobe Photoshop 4.0 with a UMAX PowerLook II scanner.
Effect of anti-biotin in invasion assays.
Avidin binding to biotinylated surface proteins of infectious larvae was used to mimic the binding of MAb to tyvelose in surface glycoproteins. Damage to monolayers infected with either biotinylated or control larvae was at least threefold greater than that in treatment-matched, uninfected controls (Table 1) (Note that all values were elevated in this assay in comparison with other experiments reported here. We believe that this is due to stain variation, and it does not alter conclusions we have drawn on the basis of statistical analysis). The presence of avidin (Table 1) or streptavidin (data not shown) did not protect monolayers infected with biotinylated or control larvae. To more closely match the size of anti-tyvelose MAbs, we tested anti-biotin antibodies. Affinity-purified goat anti-biotin antibodies were tested in assays at 0.25 mg/ml, a concentration at which both anti-tyvelose MAb 18H (Table 2) and 9E (Fig. 2) were protective. The presence of biotin-specific antibodies did not reduce damage to monolayers infected with biotinylated larvae (Table 2). These findings demonstrate that the specific binding of biotinylated surface proteins on T. spiralis larvae by either avidin or anti-biotin antibodies does not prevent invasion of epithelia in vitro.
TABLE 1.
Avidin does not protect MDCK monolayers from invasion by biotinylated T. spiralis L1 larvae
| Treatment | Damage to monolayer (mm2, 103)a by:
|
||
|---|---|---|---|
| Uninfected control | Biotinylated larvae | Control larvae | |
| Medium | 9 ± 2 | 56 ± 17b | 30 ± 9c |
| Avidin | |||
| 1.0 mg/ml | 8 ± 1 | 27 ± 4 | 32 ± 6 |
| 0.5 mg/ml | 34 ± 3 | 32 ± 1 | |
| 0.25 mg/ml | 40 ± 6 | 26 ± 4 | |
| 0.125 mg/ml | 38 ± 11 | 28 ± 11 | |
Damage to monolayer expressed as mean ± 1SD; n = 3 or 4 monolayers.
Mean was not significantly different from means of damage by avidin-treated biotinylated larvae.
Mean was not significantly different from means of damage by avidin-treated control larvae.
TABLE 2.
Goat anti-biotin antibody does not prevent invasion of MDCK cells by biotinylated T. spiralis larvae
| Treatmenta | Damage to monolayer (mm2, 103)b by:
|
||
|---|---|---|---|
| Uninfected control | Biotinylated larvae | Control larvae | |
| 16H | 2 ± 1 | 13 ± 1c | 14 ± 5c |
| 18H | 1 ± 1 | 5 ± 1 | 3 ± 1 |
| Goat anti-biotin antibody | 2 ± 1 | 21 ± 5c | 22 ± 5c |
Antibodies were all used at 0.25 mg/ml.
Damage to monolayer expressed as mean ± 1SD; n = 3 or 4 monolayers.
Mean was significantly greater than that for the treatment-matched, noninfected control (p ≤ 0.003).
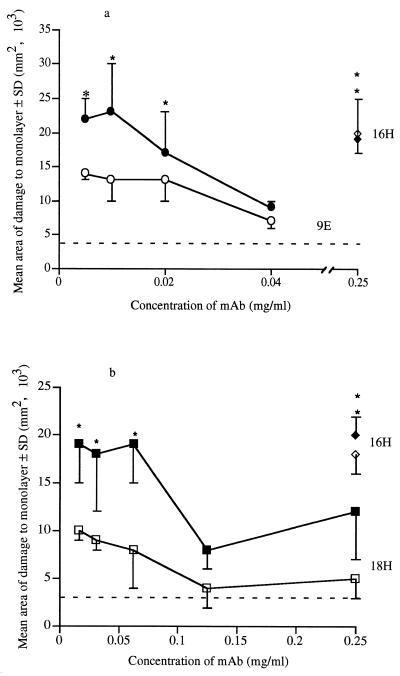
FIG. 2.
Titration of anti-tyvelose MAb on MDCK monolayers inoculated with biotinylated and control T. spiralis larvae. Biotinylated and control larvae were cocultured with various concentrations of MAb 9E (a) or MAb 18H (b) in overlays of confluent MDCK monolayers as described in the text. Data points are means of areas of damage to three or four monolayers. Solid symbols indicate mean area of damage caused by biotinylated larvae; open symbols indicate mean areas of damage caused by control larvae. Dashed lines show mean damage to uninfected control monolayers. Asterisks denote areas of damage which were significantly greater than those in noninfected treatment-matched control (P ≤ 0.05).
Protection by anti-tyvelose MAbs.
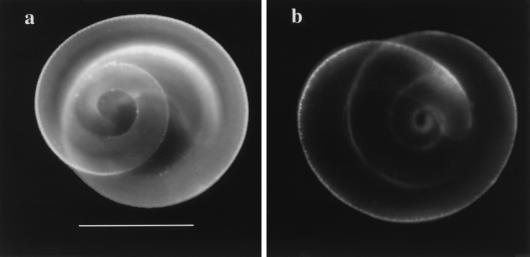
MAb 18H at 0.25 μg/ml protected MDCK cells from invasion by T. spiralis infectious larvae (Table 2). We assayed the protective abilities of MAbs 9E and 18H at more physiologic concentrations. Monolayer damage caused by larvae in the presence of MAb 16H (0.25 mg/ml) was significantly greater than that in treatment-matched, uninfected controls (Fig. 2) and approximated the level of damage caused by larvae in the presence of medium alone (Table 1). Damage in monolayers infected with control larvae increased with decreasing concentrations of MAb 9E; however, protection was evident at all concentrations tested (Fig. 2a). In contrast, damage in monolayers infected with biotinylated larvae increased more sharply with decreasing concentrations of 9E (Fig. 2a): protection was lost at 9E concentrations of ≤20 μg/ml (Fig. 2a). This trend was repeated when larvae were cultured with anti-tyvelose MAb 18H (Fig. 2b). Protection against control larvae was provided by all concentrations of 18H tested. By comparison, protection against biotinylated larvae was lost at 18H concentrations of ≤62 μg/ml (P = 0.002) (Fig. 2b). Biotinylated and control larvae were equally invasive in the presence of MAb 16H. Therefore, the differential protection by anti-tyvelose MAbs does not appear to be due to an inherent increase in activity of biotinylated larvae. Rather, these data suggest that anti-tyvelose MAbs are less effective in protecting MDCK cells from invasion by biotinylated larvae than by control larvae. The reduced protective efficacy of anti-tyvelose MAbs against biotinylated larvae correlated with reduced binding of these antibodies to the surfaces of biotinylated larvae (Fig. 3).
FIG. 3.
Immunofluorescent staining of control (a) and biotinylated (b) larvae. Activated control and biotinylated larvae were prepared as described in the text. Larvae were incubated with 20 μg of MAb 18H per ml before being stained with FITC-conjugated goat anti-rat IgG. Bar, 0.1 mm. The figure was prepared as described for Fig. 1.
Formation of anterior caps.
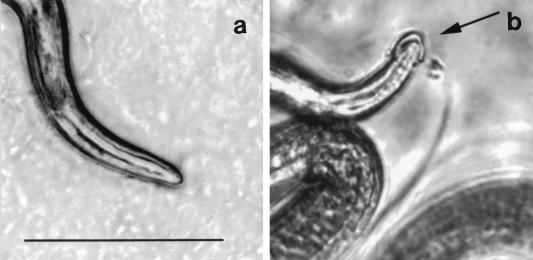
When inoculated onto MDCK cells, larvae probe the surface of the monolayer with their heads. This browsing behavior precedes invasion of the monolayer (13). When cocultured with tyvelose-specific MAbs, larvae acquired cephalic caps (Fig. 4). Caps also formed when larvae were cultured with anti-tyvelose antibody in liquid medium in the absence of cells (data not shown), indicating that they were composed of immune complexes rather than cell debris or agarose. The presence of a cap did not noticeably alter the movement of the larva; however, capped larvae were excluded from monolayers. Larvae were able to dislodge the caps.
FIG. 4.
Antibody-mediated formation of cephalic caps. Larvae were included in overlays of MDCK cells that were grown to confluence in single-chamber slides and incubated at 37°C under 5% CO2 for 15 min. The images were captured as described in Materials and Methods. (a) Anterior of an infectious larva cultured in medium alone. (b) Cephalic cap on a larva cultured with medium containing 1.0 mg of MAb 18H per ml. Bar, 0.1 mm. The figure was prepared as described for Fig. 1.
DISCUSSION
Neonatal rats passively immunized with anti-tyvelose MAbs exhibit rapid expulsion after challenge with T. spiralis larvae (2). Evidence suggests that rapid expulsion results from direct interaction of specific antibody with tyvelose in glycoproteins on larval surfaces and in ES antigens (5, 7). The purpose of this study was to assess how specific antibody binding to larval surface glycoproteins contributes to protection.
T. spiralis L1 larvae invade and move through MDCK cells, leaving serpentine trails of dead and damaged cells (13). In this study, anti-tyvelose MAbs 18H and 9E prevented larval invasion of MDCK cells. This establishes that tyvelose-specific antibodies are protective in vitro, validating the invasion assay as a tool for investigating protective immunity and confirming the central role of antibody in rapid expulsion. Furthermore, our results demonstrate that antibodies can protect epithelia without the assistance of inflammatory cells, soluble cofactors, or mucus.
Four major surface proteins of L1 larvae have been identified. Surface iodination of larvae with chloramine-T labels proteins of approximately 105, 97, 55, and 51 kDa as measured by SDS-PAGE (3, 18). All four of these proteins are precipitated by anti-tyvelose MAbs (3). Our aim in this study was to generate binding targets on larval surfaces distinct from tyvelose residues. We used sulfosuccinimidyl-6-(biotinamido)hexanoate to selectively label surface proteins of living larvae. SDS-PAGE analysis of biotin-labeled larval proteins revealed major bands at 98 and 55 kDa. While we did not make a direct comparison, it appears that only a subset of the surface proteins revealed by chloramine-T iodination are accessible to the biotin label. This would agree with earlier reports that surface proteins are not equally exposed on the larva (17) and that IODOGEN labels only two of four larval surface proteins labeled by the chloramine-T method (3).
None of the larval surface proteins has been cloned; therefore, we cannot estimate the number of primary amines available for biotinylation. For the same reason, we are not able to estimate the number of tyvelose residues in larval surface glycoproteins available for binding. Thus, a comparison of the number of available biotin residues with the number of tyvelose residues on larval surfaces is not possible. However, indirect immunofluorescence demonstrated that biotin, like tyvelose, is uniformly distributed over larval surfaces (reference 5 and data not shown). Further, the biotin label was retained on larval surfaces throughout the invasion assay (data not shown). Our data show that the major biotinylated proteins (98 and 55 kDa) were bound by MAb 18H. Taken together, these findings suggest that the biotinylated proteins are relevant larval surface binding targets.
In this study, anti-tyvelose MAbs protected MDCK cells from invasion by T. spiralis larvae. In contrast, neither avidin nor antibody binding of biotin on the surfaces of infectious larvae prevented their invasion of MDCK cells. These findings differ from results of experiments wherein we surface labeled larvae with trinitrophenyl (TNP) and fed them to rat pups together with anti-TNP antibodies. A moderate reduction (42%) in worm burden was observed in those rats (3a). Moderate protection was also observed when infectious larvae were coated with anti-tyvelose antibodies before being inoculated into suckling rat pups (6). The difference between results obtained in vivo and in vitro may lie in the contribution of larval mucus entrapment to exclusion in vivo. At present we are conducting experiments to test a role for mucus in the in vitro model of protection.
Investigation of more subtle devices of immune defense is possible by using the cell culture assay of invasion. Indeed, results of the present study indicate that a mucus-independent mechanism of exclusion exists. We speculate that antibodies complex disgorged tyvelose-bearing glycoproteins and, further, cross-link the complexed material to surface glycoproteins, promoting the formation and retention of cephalic immune complexes. The presence of these affixed complexes would physically block sensory receptors of larvae and thus impede their invasion of epithelial cells (14). Our evidence for this mechanism is indirect and includes the observation that anti-tyvelose MAbs were less able to protect MDCK cells from invasion by biotinylated than by unlabeled larvae. This reduced protection correlated with a reduced binding of tyvelose-specific MAb to biotinylated larval surfaces. Although we have not proven that sensory reception is compromised, our results clearly show that specific binding of anti-tyvelose antibody to larval surface glycoproteins contributes to the exclusion of T. spiralis from epithelia and that this exclusion is independent of mucus entrapment.
ACKNOWLEDGMENTS
We thank Lucille Gagliardo and Arin Betchen for their technical assistance. We are also grateful to Barbara Butcher for helpful discussions and suggestions and to Alan Sher for suggesting the hapten approach.
This research was supported by U.S. PHS grant AI 14490 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Appleton J A, McGregor D D. Rapid expulsion of Trichinella spiralis in suckling rats. Science. 1984;226:70–72. doi: 10.1126/science.6474191. [DOI] [PubMed] [Google Scholar]
- 2.Appleton J A, Schain L R, McGregor D D. Rapid expulsion of Trichinella spiralis in suckling rats: mediation by monoclonal antibodies. Immunology. 1988;65:487–492. [PMC free article] [PubMed] [Google Scholar]
- 3.Appleton J A, Usack L. Identification of potential antigenic targets for rapid expulsion of Trichinella spiralis. Mol Biochem Parasitol. 1993;58:53–62. doi: 10.1016/0166-6851(93)90090-k. [DOI] [PubMed] [Google Scholar]
- 3a.Appleton, J. A. Unpublished data.
- 4.Bell R G, McGregor D D. Trichinella spiralis: role of different life cycle phases in induction, maintenance, and expression of rapid expulsion in rats. Exp Parasitol. 1979;48:51–60. doi: 10.1016/0014-4894(79)90054-7. [DOI] [PubMed] [Google Scholar]
- 5.Carlisle M S, McGregor D D, Appleton J A. The role of mucus in antibody-mediated rapid expulsion of Trichinella spiralis in suckling rats. Immunology. 1990;70:126–132. [PMC free article] [PubMed] [Google Scholar]
- 6.Carlisle M S, McGregor D D, Appleton J A. Intestinal mucus entrapment of Trichinella spiralis larvae induced by specific antibodies. Immunology. 1991;74:546–554. [PMC free article] [PubMed] [Google Scholar]
- 7.Carlisle M S, McGregor D D, Appleton J A. The role of the antibody Fc region in rapid expulsion of Trichinella spiralis in suckling rats. Immunology. 1991;74:552–558. [PMC free article] [PubMed] [Google Scholar]
- 8.Crum E D, Despommier D D, McGregor D D. Immunity to Trichinella spiralis. I. Transfer of resistance by two classes of lymphocytes. Immunology. 1977;33:787–795. [PMC free article] [PubMed] [Google Scholar]
- 9.Culbertson J T. Natural transmission of immunity against Trichinella spiralis from mother rats to their offspring. J Parasitol. 1943;29:114–116. [Google Scholar]
- 10.Denkers E Y, Wassom D L, Hayes C E. Characterization of Trichinella spiralis antigens sharing an immunodominant, carbohydrate-associated determinant distinct from phosphorylcholine. Mol Biochem Parasitol. 1990;41:241–250. doi: 10.1016/0166-6851(90)90187-q. [DOI] [PubMed] [Google Scholar]
- 11.Despommier D D. Biology. In: Campbell W C, editor. Trichinella and trichinosis. New York, N.Y: Plenum Press; 1983. p. 75. [Google Scholar]
- 12.Ellis L A, Reason A J, Morris H R, Dell A, Iglesias R, Ubeira F M, Appleton J A. Glycans as targets for monoclonal antibodies that protect rats against Trichinella spiralis. Glycobiology. 1994;4:585–592. doi: 10.1093/glycob/4.5.585. [DOI] [PubMed] [Google Scholar]
- 13.ManWarren T, Gagliardo L, Geyer J, McVay C, Pearce-Kelling S, Appleton J. Invasion of intestinal epithelia in vitro by the parasitic nematode Trichinella spiralis. Infect Immun. 1997;11:4806–4812. doi: 10.1128/iai.65.11.4806-4812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaren D J. Nematode sense organs. Adv Parasitol. 1976;14:195–265. doi: 10.1016/s0065-308x(08)60515-1. [DOI] [PubMed] [Google Scholar]
- 15.Neva F A, Brown H W. Basic clinical parasitology. 6th ed. Norwalk, Conn: Appleton & Lange; 1994. Intestinal nematodes of human beings; p. 113. [Google Scholar]
- 16.Nichols G E, Lovejoy J C, Borgman C A, Sanders J M, Young W J. Isolation and characterization of two types of MDCK epithelial cell clones based on glycosphingolipid pattern. Biochim Biophys Acta. 1986;887:1–12. doi: 10.1016/0167-4889(86)90115-1. [DOI] [PubMed] [Google Scholar]
- 17.Ortega-Pierres G, Chayen A, Clark N W, Parkhouse R M. The occurrence of antibodies to hidden and exposed determinants of surface antigens of Trichinella spiralis. Parasitology. 1984;88:359–369. [PubMed] [Google Scholar]
- 18.Philipp M, Parkhouse R M, Ogilvie B M. Changing proteins on the surface of a parasitic nematode. Nature. 1980;287:538–540. doi: 10.1038/287538a0. [DOI] [PubMed] [Google Scholar]
- 19.Reason A J, Ellis L A, Appleton J A, Wisnewski N, Grieve R B, McNeil M, Wassom D L, Morris H R, Dell A. Novel tyvelose-containing tri- and tetra-antennary N-glycans in the immunodominant antigens of the intracellular parasite Trichinella spiralis. Glycobiology. 1994;4:593–603. doi: 10.1093/glycob/4.5.593. [DOI] [PubMed] [Google Scholar]
- 20.Springer T A. Isolation and analysis of proteins. In: Coligan J E, Kruisbeek A M, Marguiles D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1991. pp. 8.2.1–8.2.8. [Google Scholar]
- 21.Wright K A. Trichinella spiralis: an intracellular parasite in the intestinal phase. J Parasitol. 1979;65:441–445. [PubMed] [Google Scholar]
- 22.Wright K A, Weidman E, Hong H. The distribution of cells killed by Trichinella spiralis in the mucosal epithelium of two strains of mice. J Parasitol. 1987;73:935–939. [PubMed] [Google Scholar]