Abstract
Simple Summary
Head and neck cancer (HNC) is an aggressive form of cancer that affects hundreds of thousands of people worldwide and has a relatively poor prognosis. In the last decade, new therapeutics designed to enhance a patient’s immune system have been approved for use, but HNC has developed many different methods that help it escape the immune system. The existing immunotherapies target only one of these mechanisms, allowing HNC to utilize others to continue to elude the immune system. This review details the various strategies used by HNC to escape the immune response, dividing them into four general categories: evade, resist, inhibit, and recruit. Each of the immune escape mechanisms represents a potential immunotherapy target that could be used to treat HNC.
Abstract
Head and neck cancers (HNCs) arise from the mucosal lining of the aerodigestive tract and are often associated with alcohol use, tobacco use, and/or human papillomavirus (HPV) infection. Over 600,000 new cases of HNC are diagnosed each year, making it the sixth most common cancer worldwide. Historically, treatments have included surgery, radiation, and chemotherapy, and while these treatments are still the backbone of current therapy, several immunotherapies have recently been approved by the Food and Drug Administration (FDA) for use in HNC. The role of the immune system in tumorigenesis and cancer progression has been explored since the early 20th century, eventually coalescing into the current three-phase model of cancer immunoediting. During each of the three phases—elimination, equilibrium, and escape—cancer cells develop and utilize multiple strategies to either reach or remain in the final phase, escape, at which point the tumor is able to grow and metastasize with little to no detrimental interference from the immune system. In this review, we summarize the many strategies used by HNC to escape the immune system, which include ways to evade immune detection, resist immune cell attacks, inhibit immune cell functions, and recruit pro-tumor immune cells.
Keywords: head and neck cancer, immune escape, immunotherapy, PDL1, cancer immunoediting
1. Introduction
Head and neck cancer (HNC) is the sixth most common cancer worldwide, with approximately 66,500 new cases in the United States each year and over 16,000 associated deaths [1]. Often associated with alcohol use, tobacco use, and/or human papillomavirus (HPV) [2], HNC tumors arise from a variety of sites in the mucosal lining of the aerodigestive tract, such as the oral cavity, larynx, pharynx, and salivary glands. The prognosis for advanced stage HNC remains relatively poor, with five-year relative survival rates of less than 50% [1].
Clinical trials continue to refine optimal HNC treatment strategies, though only modest increases in overall survival have been achieved over the past decades. Early-stage HNC (stage I/II) can be effectively managed with surgery or radiation alone. However, the majority of HNC patients present with advanced disease (stage III/IV) for which multimodal treatment is required to achieve the highest probability of long-term survival.
In the 1990s–2000s, preclinical data from in vitro and in vivo studies [3,4,5] was validated in a landmark randomized trial using the epidermal growth factor receptor (EGFR)-targeting monoclonal antibody cetuximab. Combined with high-dose radiation, treatment with cetuximab increased survival for advanced-stage HNC patients over that achieved with radiation alone [6,7]. During the mid-2000s, improved understanding of significantly higher survival rates for HPV-associated HNC prompted efforts to reduce the toxicity burden in these patients. However, randomized trials (RTOG 1016 and De-ESCALaTE HPV) in p16-positive oropharynx cancer patients showed that radiation combined with cisplatin was superior to radiation combined with cetuximab with respect to both progression-free and overall survival [8,9]. Many other molecular targeting agents, including the anti-angiogenic agent bevacizumab and vascular endothelial growth factor receptor (VEGFR)2 inhibitors, showed potential in HNC when combined with radiation, but they have not altered the current clinical treatment landscape [10,11,12].
Immunotherapeutic agents came of age in the 2010s, when nivolumab and pembrolizumab demonstrated improved median survival rates in the metastatic/recurrent setting versus conventional chemotherapeutic agents, making a significant clinical impact in HNC [13,14,15]. Despite the hope that this benefit might be readily translated into the curative setting for HNC patients, the first major randomized trial using avelumab combined with chemoradiation (Javelin 100) showed no benefit of immunotherapy over chemoradiation alone in either progression-free or overall survival [16].
These early results emphasize the importance of investigating immune checkpoint inhibitors in the treatment of HNC and understanding how these agents interact with radiation and the immune system in the curative treatment setting. As noted, several immune checkpoint inhibitors (ICIs) have been approved by the Food and Drug Administration (FDA) for the treatment of HNC [13,17]. Unfortunately, only about 15% of HNC patients are initially responsive to ICI treatment [18], and those patients typically acquire resistance over time.
Immune checkpoints were first discovered in the late 1980s, when Brunet et al. found cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) in a screen of mouse cytotoxic T cell complementary deoxyribonucleic acid (cDNA) [19]. However, CTLA4’s function as a negative immune regulator was not understood until several years later [20,21]. Following closely behind, programmed cell death protein 1 (PD1) was discovered in 1992 [22], and its similar function as an inhibitory checkpoint was uncovered shortly thereafter [23,24]. In 1996, Leach and colleagues reported the first evidence of ICI efficacy, demonstrating that CTLA4 blockade had significant anti-tumor effect in murine cancer models [25]. Likewise, Dong et al. demonstrated efficacy of anti-PD1 therapeutics in the treatment of murine tumors in 2002 [26]. These studies and others eventually led to the first FDA approval of an ICI for cancer treatment—ipilimumab was approved for the treatment of melanoma in 2011 [27].
The field of immune checkpoints and ICIs is only 40 years old, but scientists have been attempting to harness the immune system of cancer patients for over a century [28]. While experiments with novel therapeutics have met with mixed success, the understanding of the immune system’s role in tumorigenesis and cancer progression has continually moved forward since the early 20th century. In 1908, Paul Ehrlich first posited that the immune system can and does inhibit the growth of cancer cells [29]. Decades of study followed, and in the 1950s, F. Macfarlane Burnet and Lewis Thomas contributed to what eventually became known as the cancer immunosurveillance hypothesis [30,31,32,33]. In this model, the immune system acts as a sentinel, constantly surveying the body for newly formed tumor cells and targeting any such cells for destruction [34]. While further study validated the immunosurveillance model [35], the early 2000s brought the recognition that immunosurveillance is only one part of the immune system’s interaction with cancer [36,37]. The umbrella model that arose as a result of these findings, called cancer immunoediting [36], is still utilized today to describe the relationship between the immune system and cancer.
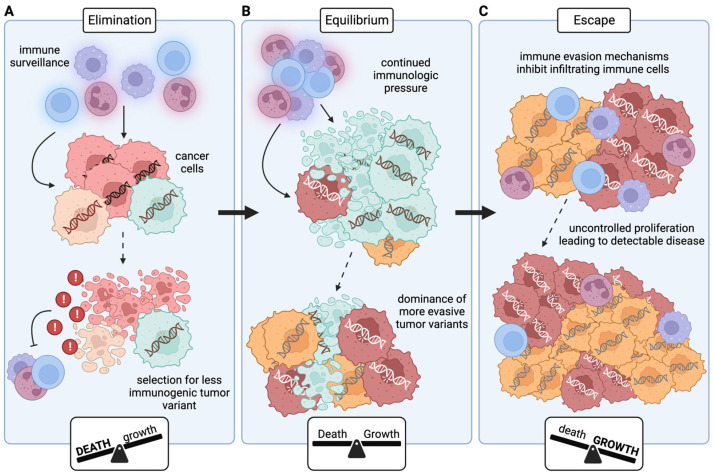
Cancer immunoediting (Figure 1) consists of three phases: elimination, equilibrium, and escape [37]. The first phase, elimination, is what was previously referred to as cancer immunosurveillance, where effector cells continually survey the host and destroy any nascent cancer cells [38] (Figure 1A). However, the continual release of danger signals from dying cancer cells can eventually stimulate secretion of immunosuppressive molecules, hampering the ability of immune cells to eliminate cancer cells [39]. Further, the hallmark genetic instability of cancer cells [40] combined with high levels of host immune scrutiny results in selective pressure for less immunogenic tumor cells [41]. The decrease in immune cytotoxic potential mixed with decreased cancer immunogenicity can allow the cancer to move to the next phase: equilibrium (Figure 1B). In the equilibrium phase, the cancer is ‘dormant’, with the rate of cancer cell proliferation being matched by the rate of cancer cell death. This state can result from angiogenic dormancy, where the lack of vasculature slows proliferation [42], or from immune dormancy, where the rate of proliferation matches the rate of immune-mediated death [43]. Again, the genetic instability of cancer cells combined with continued immunologic pressure can result in selection for tumor variants that can elude the immune system, eventually leading to clinically detectable disease and complete immune escape, the final phase of cancer immunoediting (Figure 1C).
Figure 1.
Three E’s of cancer immunoediting. (A) During the elimination phase, immune cells survey the body and kill any newly developed cancer cells; the rate of cancer cell death is much higher than the rate of cancer cell proliferation. However, the death of cancer cells can result in the release of molecules that inhibit immune cell function, and the immunologic pressure selects for tumor variants that employ one or more immune escape mechanisms. (B) The surviving, less immunogenic variants are able to proliferate at a sufficient rate to maintain their population, but their growth is kept in check by immune cells (or by a lack of nutrients resulting from insufficient vasculature). In the equilibrium phase, the rate of cancer cell death is approximately equal to the rate of cancer cell growth. Immunologic attacks again provide selection pressure, resulting in the dominance of even more immune evasive tumor variants. (C) The now-dominant immunosuppressive variants employ mechanisms to completely escape immune cells, allowing the cancer cells to proliferate uncontrollably and develop into clinically detectable tumors. The rate of cancer cell growth is much higher than the rate of cancer cell death in the escape phase.
There are four major strategies used by cancer cells to overcome targeting by the immune system: evade, resist, inhibit, and recruit. Cancer cells can evade detection altogether, resist destruction by those immune cells they could not evade, inhibit those immune cells they could not resist, and/or recruit immune cells that create a pro-tumor environment [44]. In this review, we will summarize the combination of these four strategies used by HNC to escape the immune system.
2. Evade
Evading the immune system can be accomplished in a few ways. Cancer cells can prevent immune infiltration altogether through disruption of chemoattractant pathways, and, if that strategy fails, they can impair antigen-presentation (AP) machinery to escape detection.
Activation of the β-catenin/Wnt pathway has been shown to decrease levels of dendritic cell (DC) and T cell infiltration via downregulation of C-C motif chemokine ligand (CCL)4 [45]. In HNC, members of the Wnt pathway are highly overexpressed [46,47], and activation of the pathway has been correlated with lower levels of immune infiltrate [48]. Additionally, overexpression of hypoxia inducible factor (HIF)1α, HIF2α, and carbonic anhydrase 9 (CA9) is similarly associated with poor infiltration of immune cells [49]. Downregulation of the micro-ribonucleic acid (miRNA) 34a (miR-34a), associated with cell proliferation and angiogenesis in HNC [50], leads to decreased levels of pro-B cells, naïve cluster of differentiation (CD)8 T cells, and T helper (TH)1 cells [51].
Genomic aberrations can also impact immune infiltration. The phosphoinositide 3-kinase (PI3K) pathway has long been implicated in cancer progression [52], and mutations in PI3K pathway components are found in about a third of HNCs [53]. One downstream target of the PI3K pathway is phosphatase and tensin homolog (PTEN), loss of which results in increased expression of CCL2 and vascular endothelial growth factor (VEGF), which in turn can block T cell infiltration [54]. CUB And Sushi Multiple Domains 1 (CSMD1), another gene with a role in cancer progression, is lost in approximately 40% of HNCs; reduced or absent CSMD1 is associated with decreased T cell infiltration [55]. Tumor protein P53 (TP53) is one of the most commonly mutated genes in HNC, with approximately 70% of patients harboring one or more mutations [47,56]. Tumors with mutated TP53 have been shown to have decreased levels of B cells, CD8 T cells, and natural killer (NK) cells [57]. Finally, genomic cyclin-dependent kinase inhibitor 2A (CDKN2A) disruptions occur in nearly 80% of HNCs [56], and loss of heterozygosity in this gene is associated with lower levels of CD8 T cells, regulatory T cells (Tregs), and B cells [58].
To successfully evade detection by those immune cells that manage to infiltrate the tumor immune microenvironment (TIME), HNC cells can downregulate, mutate, or otherwise impair AP components [44,59,60]. Manipulation of these molecules is a delicate operation. NK cells will attack any cell lacking major histocompatibility complex class I (MHCI) expression, but having MHCI molecules puts tumor-associated antigens (TAAs)/tumor-specific antigens (TSAs) on display and leaves tumor cells vulnerable to T cell detection.
Despite the risk of NK cell attack, some HNC cells downregulate MHCI directly [61]. This decrease in expression can sometimes result from genomic defects, including loss of heterozygosity or loss of allospecificity [56,61,62,63]. For example, loss of heterozygosity on chromosome 6, which encodes the heavy chains for MHCI, is found in ~40% of HNC tumors [62,64,65]. MHCI expression can also be affected by epigenetic changes [62]. Hypermethylation of CpG islands in MHCI promoter regions can result in expression loss; while minimal methylation of those regions can be detected in normal epithelial tissue, ~66% of HNC samples exhibit hypermethylation [66]. Yet another mechanism for MHCI downregulation is via the EGFR signaling pathway. EGFR, which is overexpressed in nearly 80% of HNCs [65], can alter the expression levels of MHCI by affecting the MHCI transcriptional regulator class II major histocompatibility complex transactivator (CIITA) [67]. Irrespective of cause, partial or total loss of MHCI expression has been detected in anywhere from 30 to 81% of HNCs [63,64,68,69,70,71], though most HNC cells express MHCI in sufficient quantities for antigen presentation, suggesting that immune evasion is likely achieved by alterations in other AP components [61,72,73].
The AP pathway involves many different molecules beyond MHCI itself, and loss of expression of even a single pathway component is sufficient to alter antigen presentation [65,74]. Low levels of transporter associated with antigen processing (TAP)1, TAP2, and tapasin are associated with lack of cytotoxic T lymphocyte (CTL) recognition [75], though they are certainly not the only AP components HNC downregulates to evade the immune system. Latent membrane protein (LMP)2 and LMP7 are commonly downregulated in HNC, as are beta-2-microglobulin (β2M), calnexin, calreticulin, and endoplasmic reticulum protein 57 (ERp57), though at varying frequencies (Table 1). Interestingly, while perturbation of any AP molecule can impair antigen presentation, Lopez-Albaitero et al. showed that reintroduction of TAP1 was sufficient to restore CTL recognition [73].
Table 1.
Antigen-processing (AP) components are downregulated with varying frequency in head and neck cancer (HNC). Studies on AP component expression alterations were performed on primary HNC samples and/or on HNC cell lines.
Expression of AP pathway components appears to be primarily regulated at the epigenetic and/or transcriptional level. In many cancers, processes like DNA methylation and histone hypoacetylation can decrease expression of AP molecules [62,76,77,78]. The methyltransferase enhancer of zeste homolog 2 (EZH2), part of a complex that catalyzes the methylation of histone 3 lysine 27 (H3K27), is associated with decreased AP component expression in HNC [79]. Zhou et al. found that EZH2 inhibition results in upregulation of AP molecules due to a reduction in the H3K27 methylation mark on the β2M promoter [80]. Transcriptionally, the interferon (IFN)γ-signal transducer and activator of transcription (STAT)1 pathway regulates AP component expression [81,82]. Lack of IFNγ or loss of IFNγ-response pathway components can result in downregulation or loss of AP molecule expression [74]. Some HNC cells harbor mutations in the IFNγ receptor gene [8], while others express decreased levels of phosphorylated STAT1 [81]. HNC cells may also overexpress Src homology-2 domain-containing protein tyrosine phosphatase (SHP)-2, which inhibits IFNγ-mediated STAT1 phosphorylation and therefore AP component expression [82]. Studies have shown that AP molecule downregulation can be overcome by exposure to IFNγ, such as that secreted by tumor-infiltrating immune cells, if the loss of AP component expression was not due to a genetic defect [65,70,73,75].
Finally, if tumor cells cannot prevent infiltration of or detection by immune cells, they can employ the evolutionary strategy of immunoediting. HNC tumors, on average, contain 2–6 distinct subclones [83], some of which may be more immunogenic than others. Clones with immunogenic epitopes are vulnerable to detection and killing by infiltrating immune cells, while clones without the now immune-dominant epitope escape unharmed and continue to proliferate [60,74,84]. However, few proteins that are overexpressed or mutated in HNC make for effective, immunogenic TAAs/TSAs able to trigger efficient immune responses [44,85]. Tumor-specific CTLs are often found in HNC patients independent of an effective immune response [84,85], suggesting that HNC tumors are generally unsuccessful in their attempts to evade the immune system entirely and therefore must be employing alternate immune escape strategies.
3. Resist
If cancer cells cannot evade immune detection, they may attempt to resist the immune system by preventing immune cell activation and/or preventing the immune cells from killing them.
To prevent immune cell activation, HNC cells often downregulate the B7 family of molecules [86,87,88] via autocrine signaling with interleukin (IL)-6 and granulocyte-macrophage colony-stimulating factor (GMCSF) [89]. Loss of these B7 molecules, which normally provide a critical costimulatory signal to T cells during activation, can help prevent T cell-mediated cell death [44]. Cytokines can also stimulate immune activation, but the expression levels of pro-inflammatory TH1 cytokines are reduced in HNC [90,91], further promoting immune escape.
If cancer cells cannot prevent immune activation, they can attempt to prevent immune-mediated cell death via manipulation of apoptotic and cell cycle pathways. Downregulation of Fas [92] and CSMD1 [55], as well as overexpression of secreted phosphoprotein 1 (SPP1) [93,94], Toll-like receptor 4 (TLR4) [84,95], serine protease inhibitor (SERPIN) B1/4/9 [96,97], Fas associated phosphatase 1 (FAP-1) [98], FLICE-like inhibitory protein (c-FLIP) [99], decoy receptor (DcR)-1/2 [100,101], transient receptor potential cation channel (TRP)M2 [102], IL-2 [103], and IL-1α [104,105], has been associated with HNC resistance to apoptosis.
Some of these molecules act by initiating or promoting survival signals. SPP1 activation resists apoptosis by triggering the mammalian target of rapamycin (mTOR)/PI3K/Akt and Janus kinase 2 (JAK2)/STAT3 [93,94] survival pathways, and TLR4 activation acts similarly via the PI3K/Akt and nuclear factor kappa B (NFκB) pathways [84,95]. TLR4 is activated by lipopolysaccharide (LPS), a component of Gram-negative bacterial cell walls, and, interestingly, HNC tumors are often found to be colonized by Gram-negative bacteria [95]. IL-1α overexpression induces activation of the activator protein 1 (AP-1) pathway, which in turn triggers B-cell lymphoma-2 (Bcl-2) expression and thus suppression of apoptosis [104,105].
HNC cells also perturb receptor-mediated apoptotic pathways. In normal head and neck tissues, expression of the death receptor Fas is found in ~85% of cells, but in HNC tissues, only ~1% of cells express Fas [92]. Without surface expression of Fas, immune cells expressing Fas ligand (FasL) cannot initiate Fas-mediated apoptosis. HNC cells also overexpress FAP-1 and c-FLIP to further protect against Fas-mediated apoptosis. FAP-1 binds directly to the negative regulatory domain of Fas itself and also offers protection via activation of the NFκB pathway [98], while c-FLIP acts as a decoy for caspase-8, resembling caspase-8 structurally but being unable to propagate the apoptotic signal [65,98]. Caspase-8 itself is mutated in 9% of HNCs [56]; these mutated versions remain constitutively bound to the adaptor molecule Fas-associated death domain protein (FADD) and thereby prevent proper recruitment of death-inducing signaling complex (DISC) components [106]. Also affected by caspase-8 mutations is the tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-death receptor mediated apoptotic pathway, which is caspase-8 dependent. The TRAIL receptors themselves can also be altered, with polymorphisms in DR4 (TRAIL-R1) found in ~90% of HNCs [107]. Further, TRAIL decoy receptors DcR-1 and DcR-2 are overexpressed in HNC, providing another method of resistance to TRAIL-death receptor mediated apoptosis [100,101].
Other proteins work to directly counter immune-derived cytotoxins. SERPINs inactivate the effects of granzymes (molecules that trigger a caspase-independent apoptotic pathway) and are not typically expressed in normal head and neck tissues [96,97]. However, SERPINB9, which inactivates granzyme B, is found in 4% of HNCs; SERPINB4, which inactivates granzyme M, is found in 60% of HNCs; and SERPINB1, which inactivates granzyme H, is found in 30% of HNCs [97].
If immune-derived cytotoxins cannot be inactivated, HNC cells can alter the cell cycle to resist cytotoxic damage. HNCs often have mutations in proteins that regulate the G1/S cell cycle checkpoint, like p53, p21, and Rb [56,65], leading them to be more dependent on the G2/M cell cycle checkpoint for DNA repair [108]. This dependency gives cells more time to repair any damage sustained from immune cell attacks and helps prevent segregation of damaged DNA during mitosis [108]. Wee1 kinase has been found to be a key driver of G2/M cell cycle arrest in HNC, able to rescue cells from granzyme B-induced apoptosis [109,110,111].
4. Inhibit
While mechanisms for evading immune cell detection or resisting immune cell attacks can be effective, cancer cells can also inhibit infiltrating immune cells directly as another line of defense. HNC cells can inhibit immune cell activation/function, can disrupt immune cell maturation/differentiation, and can even induce immune cell apoptosis.
HNC-derived exosomes containing cyclooxygenase-2 (COX2), transforming growth factor beta (TGFβ), PD1, and CTLA4 can promote CD8 T cell death and inhibit NK cell/CD4 T cell function [112,113]. Additionally, HNC cells expressing proteins like FasL [114,115,116,117], TRAIL [118], TNFα [118], galectin-1 [119], and programmed death ligand (PDL)1 [26,120] are able to induce apoptosis in tumor-infiltrating immune cells. Beyond direct immune cell killing, galectin-1 can prevent further T cell infiltration into the tumor via upregulation of galectin-9 and PDL1 [121].
PDL1 is overexpressed in 50–60% of HNCs [122,123], and expression leads to both immune cell apoptosis [26,120] and T cell dysfunction [124,125]. Extracellular factors, including HNC-derived exosomes [126] and cytokines (such as IFNγ, TNFα, GMCSF, and IL-4) [24,127,128,129], can induce PDL1 upregulation in HNC. Specifically, IFNγ regulates phosphorylation of STAT1/STAT3 through polycystic kidney disease 3 (PKD3), thus resulting in increased PDL1 expression [130,131,132]. In HNC, intracellular pathways also play a role in upregulating PDL1, including EGFR-mediated JAK2/STAT1 signaling [122] and the Axl/PI3K/Akt pathway [133,134]. Additionally, the cation channel TRPM8, which is overexpressed in HNC, increases PDL1 expression via the calcineurin-nuclear factor of activated T cells 3 (NFATc3) pathway [135]. The epithelial-to-mesenchymal transition (EMT) can also upregulate PDL1 [136]. The transcription factor zinc finger E-box binding homeobox 1 (ZEB1), activated during EMT, suppresses the miRNA miR-200, an inhibitor of PDL1 expression, thus allowing upregulation of PDL1 [137,138,139]. PDL2, an alternate ligand for PD1, is also expressed in HNC [140,141], where it plays a similar role in inhibiting T cell activation [142]. Post-translational modification, specifically glycosylation, enhances PDL1 and PDL2 activity via regulation of stability, translocation, and protein-protein interactions [143,144,145,146]. Stt3a is responsible for PDL1 glycosylation [147], while fucosyltransferase 8 (FUT8) glycosylates PDL2 [146]. As mentioned previously, the EGFR pathway is often aberrantly hyperactivated in HNC [65]; EGFR signaling via STAT3 can increase levels of FUT8 and thereby result in enhanced PDL2 activity [146]. Similarly, the cytokine TGFβ (overexpressed in HNC [148,149]) upregulates Stt3a expression via c-Jun activation, thus enhancing PDL1 activity [147].
Outside of the cancer cell, secreted TGFβ can directly affect immune cells such as DCs, T cells, and NK cells, suppressing maturation, activation, and/or cytotoxicity [150,151,152,153]. In NK cells, TGFβ reduces expression of activating receptor NK group 2 member D (NKG2D) and Fc receptor CD16 [154,155]. Levels of NKG2D can also be impacted by other HNC-derived cytokines. IL-6 and IL-8 are two cytokines that are highly overexpressed in HNC [90,156,157], and secreted IL-6/IL-8 can activate STAT3 in NK cells, resulting in downregulation of activating receptors NKG2D and NKp30 [158]. STAT3 signaling can also inhibit activation of T cells and DCs [159,160,161,162,163]. Increased STAT3 signaling can impair antigen-specific T cell responses, decreasing the amount of IL-2 secreted from the T cells and promoting a tolerogenic phenotype [159]. In DCs, STAT3 activation downregulates IL-12, MHC class II (MHCII), and CD40, impairing critical pro-inflammatory functions [161,162,163].
IL-6-mediated STAT3 activity in tumor cells can stimulate the release of factors, such as VEGF and IL-10, that inhibit DC maturation [150,159,163,164]. VEGF is overexpressed in most HNCs [165,166] and is known to inhibit DC maturation and antigen-presentation capabilities [44,59,61,167,168]. VEGF expression correlates with a reduced number of mature DCs and an increased number of immature DCs [169,170], possibly due to the inhibition of fms-like tyrosine kinase 3 (FLT3) ligand activity [171]. IL-10 has also been reported to interfere with DC maturation [163,172,173,174,175] and critical DC functions [172,175]. IL-10 decreases expression of costimulatory molecules [176], production of IL-12 [175,177], and antigen-presentation capabilities [175].
Another cytokine, IL-1α, is overexpressed in HNC and can induce the overexpression of IL-6 and C-X-C motif chemokine ligand (CXCL)8 in tumor cells; IL-6 and CXCL8 are known to inhibit macrophage functions [105,178,179,180]. HNC cells also overproduce the hormone prostaglandin E2 (PGE2) [90], which can interfere with monocyte functions such as migration and adherence to endothelial cells [181,182].
Membrane-associated gangliosides, produced by HNC cells and shed into the TIME [183], are another vehicle by which HNCs inhibit the immune system. These gangliosides inhibit the ability of monocytes and DCs to activate T cells by downregulating costimulatory molecules, MHC components, IL-12, and TNFα; they can also inhibit DC maturation and even DC differentiation from monocytes [184,185,186,187,188].
HNC cells secrete many different factors into the TIME to inhibit immune cells, but they also display various factors on their surface to achieve immune escape, including high mobility group box 1 protein (HMGB1) [189], carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) [190], CD47 [191], CTLA4 [192,193], and CD276 [194,195]. HMGB1 and CEACAM1, ligands for T cell immunoglobulin- and mucin-domain containing 3 (TIM3), bind to TIM3 on NK cells and cause downregulation of mixed lineage leukemia (MLL)T1, resulting in decreased expression of IFNγ/perforin and therefore impaired NK cytotoxicity [189,190,196]. CD47 is a ‘do not eat me’ signal that interacts with the protein signal regulatory protein alpha (SIRPα) on macrophages and DCs [197]. When CD47 binds to SIRPα, SHP-1 and/or SHP-2 are recruited to dephosphorylate motor protein myosin IIA, thus inhibiting the phagocytic process [198]. CTLA4 is a well-characterized immune checkpoint molecule; it competes with CD28 for binding to CD80/CD86 ligands on DCs [199]. CD28 binding to CD80/CD86 provides a critical costimulatory signal for T cell activation, but CTLA4 binding can prevent this process and thereby inhibit T cell activation [199,200]. Another immune checkpoint molecule, CD276 (B7-H3) has been found on the invasive front of HNC tumors, serving as a ‘shield’ against lymphocyte interference/infiltration by acting to suppress antigen-specific T cell activation and proliferation [194,195,201].
Finally, HNC cells can manipulate the surrounding environment itself, both directly and indirectly, to inhibit immune cell activation/function. Indirectly, HNC cells create regions of hypoxia with their rapid growth and abnormal angiogenesis [202,203], resulting in the secretion of adenosine and galectin-1, molecules known to be inhibitory to T cells [204,205,206,207]. Additionally, high rates of anaerobic glycolysis in HNC cells leads to the accumulation of extracellular lactate [74]. This lactate buildup inhibits the export of lactate from T cells and NK cells, which causes decreased IFNγ production and thus impairs cytotoxicity [208,209]. Further, extracellular lactate accumulation causes acidosis [210], which can lead to loss of T cell function [209,211]. HNC cells can also directly influence the environment through nutrient deprivation and production of inhibitory compounds. For example, HNC cells often overexpress glucose metabolism genes, thus depriving infiltrating T cells of the fuel needed for activation and expansion; overexpression of glucose transporter 1 has been correlated with decreased T cell infiltration in HNC [212,213]. Additionally, between 20 and 95% of HNCs overexpress the enzyme indoleamine 2,3-dioxygenase (IDO), which depletes the amino acid tryptophan [214,215]. Lack of available tryptophan causes T cells to arrest in the G1 phase of the cell cycle, and neither restoration of tryptophan nor costimulatory signaling through CD28 is able to restart cell cycle progression [216,217]. HNC cells also upregulate the enzymes CD39 and CD73, responsible for converting adenosine triphosphate (ATP) into adenosine [218,219,220,221], a molecule inhibitory towards T cells, DCs, and NK cells [222,223,224].
5. Recruit
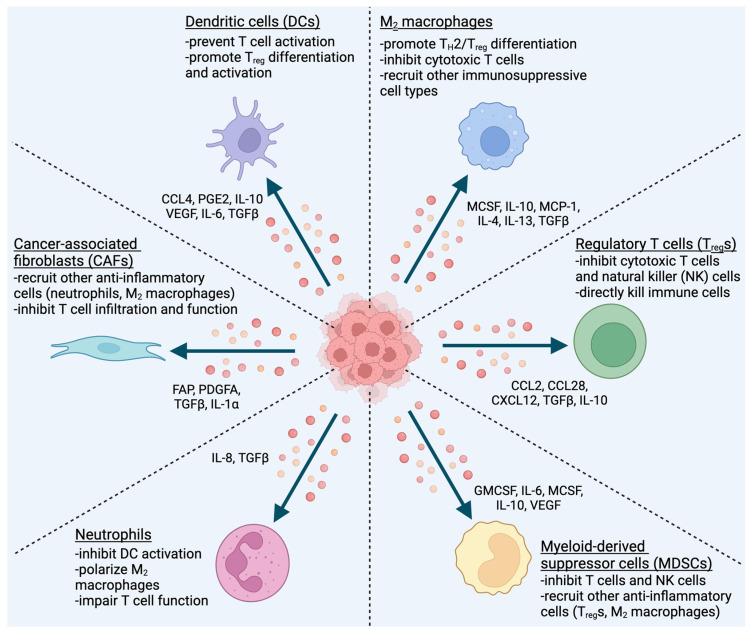
Cancer cells employ a variety of methods for evading, resisting, or inhibiting immune cells. These strategies for immune escape all involve direct interaction between the cancer cell (or a cancer cell-derived product) and the immune system. The final strategy for immune escape instead relies on the recruitment of intermediary cells, which then inhibit immune cells and create a pro-tumor environment. HNC cells recruit many different cell types to serve as these intermediaries, including DCs, macrophages, Tregs, myeloid-derived suppressor cells (MDSCs), neutrophils, and cancer-associated fibroblasts (CAFs) (Figure 2).
Figure 2.
HNC cells secrete cytokines, chemokines, and other molecules to recruit immunosuppressive, pro-tumor cell types. The soluble factors released by cancer cells can also polarize, activate, and/or differentiate the recruited cells to enhance their anti-inflammatory effects.
5.1. Dendritic Cells (DCs)
Conventional DCs are recruited to the tumor by CCL4, CCL5, and X-C motif chemokine ligand (XCL)1 [225]; CCL5 and XCL1 are typically produced by NK cells [225], while CCL4 is produced by tumor cells [45]. Once at the tumor, HNC-derived cytokines manipulate DC function and maturation to create an immunosuppressive environment. HNC-derived PGE2 [90,226] and IL-10 [90,91,227] decrease the amount of IL-12 produced by DCs, which then results in an increase in TH2 differentiation [228]. The loss of IL-12 also inhibits proper antigen-presentation in DCs, thus preventing DC-mediated T cell activation [175,229]. In addition to HNC-derived IL-10, DCs can themselves be induced by HNC cells to secrete IL-10, thereby resulting in IL-12 loss, increased TH2 differentiation, and inhibition of T cell activation [175,228,229].
As discussed above, HNC cells overexpress IL-6 [156] and VEGF [165,166], suppressing DC maturation via STAT3 activation [230,231] and FLT3 ligand inhibition [171], respectively. These immature DCs express low levels of MHCII and the costimulatory molecules CD80/CD86, thereby impairing antigen presentation and preventing T cell activation [167,169,232,233]. Further, HNC-derived IL-10, VEGF, and TGFβ convert immature DCs into tolerogenic DCs, which can induce T cell tolerance by promoting the activation and differentiation of Tregs [175,233,234,235,236,237,238,239].
Besides conventional DCs, HNC cells also recruit plasmacytoid DCs (pDCs) via CXCL12 and CXCL14 overexpression [240,241,242]. While some pDCs are anti-tumor [243] (typically in HPV + HNC [244]), the majority are pro-tumor [131,243], having reduced expression of IFNα and costimulatory molecules [242] and stimulating the recruitment of Tregs [241].
5.2. Macrophages
Macrophages are another immune cell type recruited by HNC to promote an immunosuppressive TIME. Many cytokines and chemokines have been implicated in the recruitment of macrophages to the TIME, including CCL3/4/5/8 [245,246,247], VEGF [245,246,247,248], macrophage colony stimulating factor (MCSF) [245,246,247,248], IL-10 [248], platelet-derived growth factor (PDGF) [248], CCL18/20 [249], CXCL12 [249], TGFβ [155,250,251], and monocyte chemoattractant protein 1 (MCP-1) [250,251,252,253]. Tumor-associated macrophages (TAMs) as a whole are linked to poor clinical outcomes in HNC [254], though TAMs are generally divided into two subtypes, M1 and M2, that each have distinct biological characteristics [255]. Activated M1 macrophages stimulate a TH1-like, pro-inflammatory immune response, while M2 macrophages stimulate a TH2-like, anti-inflammatory response [256,257]. Typically, the majority of TAMs are M2s [258], and these tumor-infiltrating M2 macrophages have been associated with a poor prognosis in HNC patients [259,260,261].
M2 polarization is induced by cells in the TIME secreting cytokines and other factors, including IL-4 and IL-13 [262,263,264,265,266,267], MCSF [268,269], IL-10 and TGFβ [270,271], and IL-8 [262,272]. HNC cells overexpress TH2 cytokines IL-14, IL-13, IL-10, and TGFβ [89,91,227,273,274], and HNC stromal cells also produce significantly increased levels of TGFβ [275,276]. Environmental conditions in the tumor can further stimulate M2 polarization: production of IL-10, TGFβ, and MCSF is increased under hypoxic conditions [270,271,277,278], and the acidosis caused by high rates of anerobic glycolysis in tumor cells [74] can enhance M2 differentiation [279]. Additionally, macrophage expression of C-C motif chemokine receptor (CCR)2 and IL-4Rα is essential for M2 polarization and survival [280,281,282]. Macrophages can also be polarized to the M2 state following engulfment of apoptotic tumor cells [283]. In the non-cancer setting, this transition promotes wound-healing and tissue regeneration, but in tumors, it instead fosters an immunosuppressive TIME [278,283].
M2 macrophage polarization results in the expression and secretion of factors that create an immunosuppressive environment, stimulating TH2 and Treg differentiation and inhibiting cytotoxic T cells, NK cells, and DCs [245,246,284,285,286]. M2 macrophages upregulate TGFβ, PGE2, IL-10, arginase 1 (ARG1), peroxisome proliferation activated receptor gamma (PPARγ), IL-1Rα, VEGF, TNFα, IL-1, IL-6, IL-8, and GMCSF, all of which contribute to immune suppression [245,246,247,287,288,289,290,291,292,293].
Secretion of TGFβ plays a major role in promoting an immunosuppressive environment by (1) inducing Treg differentiation/activation [152,153,278,294,295]; (2) inhibiting the differentiation, maturation, activation, and proliferation of CTLs [278,291,295]; (3) inhibiting the maturation and function of DCs [237,296]; (4) inhibiting M1 macrophages [287,290]; and (5) recruiting (and polarizing) other immunosuppressive cells, such as CAFs [153], M2 macrophages [250,251,270,271], and N2 neutrophils [297,298,299,300]. Similarly, IL-10 production results in (1) induction of Treg differentiation/activation [301,302], (2) inhibition of DC maturation and function [175,238,296], and (3) recruitment of MDSCs [303,304]. In HNC, secretion of TGFβ and IL-10 are associated with reduced survival time [259]. Further, upregulation of ARG1 contributes to T cell dysfunction by depleting the TIME of arginine, a required metabolite for T cell proliferation [291,305,306]. Lack of arginine prevents T cells from utilizing oxidative phosphorylation, thus decreasing survival capacity and anti-tumor activity [307,308,309].
M2 macrophages also express CD39 and CD73, ectonucleotidases that convert ATP into adenosine [218,278,310,311,312,313]. As mentioned previously, adenosine is an immunosuppressive molecule, inhibiting NK cells, T cells, and DCs [222,223,224]. More specifically, adenosine inhibits the antigen presentation function of DCs and other antigen-presenting cells (APCs), thereby preventing T cell activation [314]. Adenosine also promotes the proliferation and activation of M2 macrophages and Tregs, thus contributing to an immunosuppressive TIME [222,223,315].
Another way M2 macrophages promote immune suppression and escape is through the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) [305,306,316]. High levels of ROS can negatively impact T cell proliferation and activation [317], as well as promote differentiation of anti-inflammatory TH2 cells [318,319]. Macrophages also release RNS after infiltrating the tumor, resulting in nitration of CCL2 and thereby preventing CCL2-mediated recruitment of CTLs [316].
Finally, M2 macrophages express immune checkpoint ligands like PDL1, PDL2, and CTLA4, which suppress T cell responses [305,306,320,321]. HNC cells induce PDL1 expression in macrophages via an IL-10-dependent process [322], while PDL2 overexpression is mediated by the CCL2/CCR2 pathway [282]. As discussed previously, PDL1/PDL2 signaling results in T cell dysfunction [124,125,142] and can induce apoptosis in immune cells [26,120], and CTLA4 impairs T cell activation through competition with the critical costimulatory molecule CD28 [199,200].
5.3. Regulatory T Cells (Tregs)
In addition to macrophages and DCs, HNC cells also recruit Tregs to help themselves escape the immune system [323,324]. Tregs are an extremely immunosuppressive cell type that supports tumor progression through the inhibition of NK cells, DCs, B cells, and even other T cells [325,326]. They can be recruited either as ‘natural’ Tregs or as naïve CD4 T cells that are polarized into Tregs once inside the tumor [327,328]. Those Tregs that are polarized in the tumor are more immunosuppressive than ‘natural’, circulating Tregs, typically expressing higher levels of immune checkpoint molecules [329]. HNC cells recruit Tregs through several chemokines and their associated receptors, including CCL2/CCR4, CCL28/CCR10, and CXCL12/C-X-C motif chemokine receptor (CXCR)4 [330,331,332,333].
Once Tregs reach the tumor, differentiation/polarization and activation is induced by a variety of tumor- and immune-derived factors, including TGFβ [113,152,153,294], IL-10 [301,302], PGE2/COX2 [112,113,155], IL-35 [301,302], adenosine [222,223,315], IL-6/STAT3 [334], and even has_circ_0069313, a tumor-derived circular RNA that helps Tregs maintain FoxP3 levels [335]. Further, Tregs function through the expression of immunosuppressive cytokines and immunomodulatory receptors, including TGFβ, IL-10, IL-35, and other TH2 cytokines [296,323,324,336,337,338], as well as CD39/CD73 [256,313,339,340] and immune checkpoint molecules like CTLA4, TIM3, lymphocyte activation gene 3 (LAG3), and PD1 [329,341,342].
As described above, TGFβ and IL-10 have immunosuppressive effects on the TIME, where they inhibit polarization/activation/maturation/function of M1 macrophages [287,290], DCs [175,237,238,296], and CTLs [153,295,323,324,336] and recruit/activate/polarize M2 macrophages [250,251,270,271], MDSCs [303,304], N2 neutrophils [297,298,299,300], CAFs [153], and more Tregs [152,294,301,302]. Similarly, IL-35 functions to suppress T cell proliferation and activity while also promoting the differentiation/function of more Tregs [343,344,345,346].
Just like M2 macrophages, Tregs express CD39 and CD73, enzymes responsible for metabolizing ATP and generating adenosine [218,311]. As mentioned above, adenosine has immunosuppressive effects on the TIME, inhibiting the functions of NK cells, M1 macrophages, T cells, and DCs [224,314,340,347] and promoting the proliferation/activation of M2 macrophages and Tregs [222,223,315].
CTLA4 and PD1, as discussed above, function to inhibit T cell responses. CTLA4 binds to CD80/CD86 on APCs, thus preventing interaction with the T cell costimulatory molecule CD28 [340]. Further, the binding of CTLA4 to CD80/CD86 on DCs promotes the generation of tolerogenic IDO+ DCs [233,348]; IDO depletes the TIME of tryptophan, which irreversibly causes T cells to arrest in the G1 phase of the cell cycle [214,216,217]. Likewise, LAG3 promotes immune tolerance via suppression of CTL recruitment and activity [341,342], and TIM3 expression promotes Treg function and CTL inhibition [349,350,351].
Tregs can also directly kill other immune cells; through the secretion of granzyme B and perforin, they can kill NK cells and T cells [337,338,352]. In addition, Tregs can induce Fas-mediated apoptosis in CD8 T cells [353].
5.4. Myeloid-Derived Suppressor Cells (MDSCs)
Another cell type, MDSCs, are recruited by HNC to promote an immunosuppressive TIME and thus escape the immune system. HNC cells produce GMCSF [89,90,91], IL-6 [89,90,91,180], MCSF [354,355], IL-10 [90,91,301,302], VEGF [90,165,166], PGE2/COX2 [182,226,356,357], IDO [214,215], MCP-1 [250,251], and IL-8 [90,180], all of which play a role in MDSC recruitment [303,358,359]. The hypoxic conditions typically found in tumors can also recruit MDSCs via the interaction of the HIF-1α/macrophage migration inhibitory factor (MIF) and NFκB/IL-6 axes [303,358,359] as well as the stimulation of MCSF production [277,278]. Though MDSCs are naturally anti-inflammatory, they can be differentiated further to an even more immunosuppressive phenotype by MCSF [360,361,362] and PGE2 [363].
MDSCs create an immunosuppressive TIME through the secretion of IL-10 [364,365,366], TGFβ [278,295], PGE2/COX2 [155,367], and MCSF [368,369], as well as the expression of ARG1 [370,371,372,373], IDO [374], PDL1 [375,376], and inducible nitric oxide synthase (iNOS) [370,373,377,378]. The secretion of IL-10 and TGFβ results in recruitment/activation of Tregs [278,295,332,379], while M2 macrophages are recruited and polarized by the release of IL-10 and MCSF [368,369,380]. Similarly, MDSC-derived PGE2/COX2 can induce Treg production [155] as well as induce STAT1/STAT3 phosphorylation [367], which in turn results in the upregulation of ARG1 [309] and IDO [374].
As discussed above, the enzyme IDO is responsible for metabolizing tryptophan, a critical nutrient for T cell function [214,215]. In addition to simply depleting tryptophan, IDO also converts tryptophan into immunosuppressive kynurenine metabolites [374]. Cysteine, another nutrient critical for T cell activation and function, is similarly depleted from the local environment by MDSC-mediated sequestration [381].
MDSC expression of ARG1 and iNOS inhibits T cell and NK cell responses, both through nutrient depletion and through production of immunosuppressive molecules [370,373,377,382]. ARG1 and iNOS both metabolize arginine, leading to the production of ornithine and nitric oxide (NO), respectively, as well as simply depleting arginine from the local environment [364,383,384]. Depletion of arginine from the TIME results in T cell inhibition, as arginine is essential for correct CD3 expression [385]. Additionally, arginine deprivation results in T cells being shunted away from oxidative phosphorylation, decreasing survival capacity through the inhibition of cell cycle regulators cyclin D3 and CDK4 [307,308,309,386].
Nitric oxide inhibits the immune response through a variety of mechanisms. Production of NO inhibits MHCII expression, thus preventing antigen presentation and activation of T cells [387]. Further, NO impairs Fc receptor binding on NK cells, reducing cytotoxicity and impairing signal transduction [388]. Nitric oxide also inhibits T cells and NK cells by rendering cells unresponsive to IL-2 via nitrosylation of cysteine residues on proteins in IL-2 response pathways [389]. Lack of response to IL-2 inhibits T cell proliferation/activation [390] and NK cell activation/cytotoxic potential [391].
Beyond direct effects on the immune system, NO can react further with superoxide to produce peroxynitrite, an immunosuppressive RNS molecule [392]. Peroxynitrite can induce apoptosis [393] or anergy [394] in activated T cells, and it can also cause nitration of the TCR/CD8 complex, altering the specific peptide binding and thus rendering T cells unresponsive to antigen-specific stimulation [394,395].
5.5. Neutrophils
Neutrophils are another immune cell type associated with HNC progression and immune escape. In HNC patients, high neutrophil infiltration is positively associated with tumor stage and negatively associated with overall survival [396]. Neutrophils are recruited by HNC-derived IL-8 [397] and are polarized to an immunosuppressive, N2 phenotype by TGFβ [298,299,300]. Tumor-associated neutrophils (TANs) can inhibit T cell responses through expression of ARG1, PDL1, IL-10, and ROS [371,372,398,399], and TANs can actually induce CD8 T cell apoptosis through NO production and the TNFα pathway [400]. Additionally, TANs can polarize macrophages to the M2 phenotype through MCSF production [368,369]. TANs also produce CCL4 and CCL5, which recruit DCs to the TIME [401], as well as myeloperoxidase and elastase, which inhibit DC activation [402].
5.6. Cancer-Associated Fibroblasts (CAFs)
Finally, HNC cells recruit CAFs [194], which, while not immune cells, contribute to the development of an immunosuppressive TIME through their effects on immune cells such as macrophages, T cells, MDSCs, and DCs [403]. In HNC, CAFs are strongly correlated with invasion, recurrence, treatment resistance, and ultimately poor patient outcomes [404,405].
HNC expression of fibroblast-associated protein (FAP) [406], platelet-derived growth factor A (PDGFA) [407], and TGFβ [275,276] all contribute to the recruitment of CAFs [153,408,409], while HNC-derived IL-1α promotes CAF proliferation [410]. Once they reach the TIME, CAFs regulate an immunosuppressive environment via the secretion of cytokines, chemokines, and growth factors, such as VEGF, epidermal growth factor (EGF), IL-6, TGFβ, TNF, and CXCL8 [276,411,412,413], though there is some evidence suggesting that the immunosuppressive effects of CAFs are mediated through FAP and CXCL12 [414,415]. Neutrophils and macrophages can be recruited by CAF-derived CXCL12 [298,416], while T cells can be excluded from the tumor in a CXCL12-dependent manner [414,415].
CAFs can then induce M2 macrophage and N2 neutrophil polarization both through direct secretion of TGFβ [275,276] and through secretion of cardiotrophin-like cytokine factor 1 (CLCF1), which can induce expression of TGFβ in tumor cells [417]. Further, CAF-derived IL-6 activates STAT3 in neutrophils, resulting in upregulation of PDL1 and thus T cell inhibition [417,418].
T cells are also affected by CAFs, through direct and indirect mechanisms. In HNC, CAFs inhibit T cell proliferation via the PDL1/PDL2 axis and can even induce effector T cell apoptosis [413]. Additionally, CAF-derived CCR4 and IL-1β induce CCL22 overexpression in HNC cells, thus promoting Treg recruitment and activation [413,419].
6. Conclusions and Future Directions
Head and neck cancer is a major global health issue, impacting hundreds of thousands of lives each year. The classic treatments of surgery, radiation, and chemotherapy have been recently joined by immunotherapies, though only a small subset of patients respond to these new therapeutics [18]. Understanding the mechanisms of primary and acquired resistance to ICIs is therefore an area of extensive research [420,421,422,423]. Of note, however, is that the only two ICIs approved for use in HNC target the same immune checkpoint, PD1/PDL1.
In this review, we discussed some of the many mechanisms by which HNC can escape the immune system. HNC cells can evade immune detection altogether, resist attacks from immune cells, inhibit immune cells directly, or recruit immunosuppressive, pro-tumor immune cells. While the PD1/PDL1 immune checkpoint is one of these mechanisms, it is certainly not the only mechanism utilized by HNC to escape the immune system. Each of the discussed immune escape strategies represents a potential immunotherapeutic target that could be used to improve treatment outcomes in HNC.
Acknowledgments
The figures were created with Biorender.com (accessed on 4 January 2024).
Author Contributions
Conceptualization, K.L.K., M.I., B.E.C., R.S., P.M.H., J.Y.B. and D.L.W.; resources, D.L.W.; writing—original draft preparation, K.L.K.; writing—review and editing, K.L.K., M.I., B.E.C., P.M.H. and D.L.W.; supervision, D.L.W.; funding acquisition, D.L.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts.
Funding Statement
This work was supported by the NCI under the Wisconsin Head and Neck Cancer SPORE grant (D.L.W., P.M.H., J.Y.B., P50CA278595) and the NIH (D.L.W., 1R01CA262292-01A1). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Gillison M.L., Koch W.M., Capone R.B., Spafford M., Westra W.H., Wu L., Zahurak M.L., Daniel R.W., Viglione M., Symer D.E., et al. Evidence for a Causal Association Between Human Papillomavirus and a Subset of Head and Neck Cancers. JNCI. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 3.Harari P.M., Huang S. Radiation combined with EGFR signal inhibitors: Head and neck cancer focus. Semin. Radiat. Oncol. 2006;16:38–44. doi: 10.1016/j.semradonc.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Harari P.M., Wheeler D.L., Grandis J.R. Molecular target approaches in head and neck cancer: Epidermal growth factor receptor and beyond. Semin. Radiat. Oncol. 2009;19:63–68. doi: 10.1016/j.semradonc.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang S.-M., Bock J.M., Harari P.M. Epidermal Growth Factor Receptor Blockade with C225 Modulates Proliferation, Apoptosis, and Radiosensitivity in Squamous Cell Carcinomas of the Head and Neck1. Cancer Res. 1999;59:1935–1940. [PubMed] [Google Scholar]
- 6.Bonner J.A., Harari P.M., Giralt J., Azarnia N., Shin D.M., Cohen R.B., Jones C.U., Sur R., Raben D., Jassem J., et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 7.Bonner J.A., Harari P.M., Giralt J., Cohen R.B., Jones C.U., Sur R.K., Raben D., Baselga J., Spencer S.A., Zhu J., et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 8.Gillison M.L., Akagi K., Xiao W., Jiang B., Pickard R.K.L., Li J., Swanson B.J., Agrawal A.D., Zucker M., Stache-Crain B., et al. Human papillomavirus and the landscape of secondary genetic alterations in oral cancers. Genome Res. 2019;29:1–17. doi: 10.1101/gr.241141.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehanna H., Robinson M., Hartley A., Kong A., Foran B., Fulton-Lieuw T., Dalby M., Mistry P., Sen M., O’Toole L., et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet. 2019;393:51–60. doi: 10.1016/S0140-6736(18)32752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoang T., Huang S., Armstrong E., Eickhoff J.C., Harari P.M. Enhancement of radiation response with bevacizumab. J. Exp. Clin. Cancer Res. 2012;31:37. doi: 10.1186/1756-9966-31-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee N.Y., Harris J., Kim J., Garden A., Mechalakos J., Pfister D.G., Chan A.T.C., Hu K., Colevas A.D., Frank S., et al. Long-term Outcomes of Bevacizumab and Chemoradiation for Locoregionally Advanced Nasopharyngeal Carcinoma: A Nonrandomized Controlled Trial. JAMA Netw. Open. 2023;6:e2316094. doi: 10.1001/jamanetworkopen.2023.16094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J., Huang S., Armstrong E.A., Fowler J.F., Harari P.M. Angiogenesis and radiation response modulation after vascular endothelial growth factor receptor-2 (VEGFR2) blockade. Int. J. Radiat. Oncol. Biol. Phys. 2005;62:1477–1485. doi: 10.1016/j.ijrobp.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 13.Burtness B., Harrington K.J., Greil R., Soulieres D., Tahara M., de Castro G., Jr., Psyrri A., Baste N., Neupane P., Bratland A., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 14.Cohen E.E.W., Soulières D., Le Tourneau C., Dinis J., Licitra L., Ahn M.J., Soria A., Machiels J.P., Mach N., Mehra R., et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet. 2019;393:156–167. doi: 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- 15.Ferris R.L., Blumenschein G., Jr., Fayette J., Guigay J., Colevas A.D., Licitra L., Harrington K., Kasper S., Vokes E.E., Even C., et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee N.Y., Ferris R.L., Psyrri A., Haddad R.I., Tahara M., Bourhis J., Harrington K., Chang P.M., Lin J.C., Razaq M.A., et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: A randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22:450–462. doi: 10.1016/S1470-2045(20)30737-3. [DOI] [PubMed] [Google Scholar]
- 17.Ferris R., Gillison M.L. Nivolumab for Squamous-Cell Cancer of Head and Neck. N. Engl. J. Med. 2017;376:596. doi: 10.1056/NEJMc1615565. [DOI] [PubMed] [Google Scholar]
- 18.Ran X., Yang K. Inhibitors of the PD-1/PD-L1 axis for the treatment of head and neck cancer: Current status and future perspectives. Drug Des. Devel Ther. 2017;11:2007–2014. doi: 10.2147/DDDT.S140687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunet J.F., Denizot F., Luciani M.F., Roux-Dosseto M., Suzan M., Mattei M.G., Golstein P. A new member of the immunoglobulin superfamily—CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 20.Krummel M.F., Allison J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walunas T.L., Lenschow D.J., Bakker C.Y., Linsley P.S., Freeman G.J., Green J.M., Thompson C.B., Bluestone J.A. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-X. [DOI] [PubMed] [Google Scholar]
- 22.Ishida Y., Agata Y., Shibahara K., Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. Embo J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong H., Zhu G., Tamada K., Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 24.Freeman G.J., Long A.J., Iwai Y., Bourque K., Chernova T., Nishimura H., Fitz L.J., Malenkovich N., Okazaki T., Byrne M.C., et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leach D.R., Krummel M.F., Allison J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 26.Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B., Roche P.C., Lu J., Zhu G., Tamada K., et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 27.Traynor K. Ipilimumab approved for metastatic melanoma. Am. J. Health Syst. Pharm. 2011;68:768. doi: 10.2146/news110025. [DOI] [PubMed] [Google Scholar]
- 28.Dobosz P., Dzieciątkowski T. The Intriguing History of Cancer Immunotherapy. Front. Immunol. 2019;10:2965. doi: 10.3389/fimmu.2019.02965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrlich P. Über den jetzigen Stand der Karzinomforschung. Ned. Tijdschr. Geneeskd. 1909;5:273–290. [Google Scholar]
- 30.Burnet F.M. Immunological Surveillance in Neoplasia. Immunol. Rev. 1971;7:3–25. doi: 10.1111/j.1600-065X.1971.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 31.Burnet M. Cancer—A Biological Approach. Br. Med. J. 1957;1:841–847. doi: 10.1136/bmj.1.5023.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burnet M. Immunological factors in the process of carcinogenesis. Br. Med. Bull. 1964;20:154–158. doi: 10.1093/oxfordjournals.bmb.a070310. [DOI] [PubMed] [Google Scholar]
- 33.Verdonck E., Schaap K., Thomas L.C. A discussion of the principles and applications of Modulated Temperature DSC (MTDSC) Int. J. Pharm. 1999;192:3–20. doi: 10.1016/S0378-5173(99)00267-7. [DOI] [PubMed] [Google Scholar]
- 34.Burnet F. The concept of immunological surveillance. Immunol. Asp. Neoplasia. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 35.Smyth M.J., Godfrey D.I., Trapani J.A. A fresh look at tumor immunosurveillance and immunotherapy. Nat. Immunol. 2001;2:293–299. doi: 10.1038/86297. [DOI] [PubMed] [Google Scholar]
- 36.Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 37.Dunn G.P., Old L.J., Schreiber R.D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 38.Smyth M.J., Thia K.Y., Street S.E., MacGregor D., Godfrey D.I., Trapani J.A. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J. Exp. Med. 2000;192:755–760. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilczynski J.R., Nowak M. Interaction of Immune and Cancer Cells. Springer; Vienna, Austria: 2014. Cancer immunoediting: Elimination, equilibrium, and immune escape in solid tumors; pp. 143–205. [DOI] [PubMed] [Google Scholar]
- 40.Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 41.Whiteside T.L. Immune responses to malignancies. J. Allergy Clin. Immunol. 2010;125:S272–S283. doi: 10.1016/j.jaci.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naumov G.N., Akslen L.A., Folkman J. Role of angiogenesis in human tumor dormancy: Animal models of the angiogenic switch. Cell Cycle. 2006;5:1779–1787. doi: 10.4161/cc.5.16.3018. [DOI] [PubMed] [Google Scholar]
- 43.Teng M.W., Swann J.B., Koebel C.M., Schreiber R.D., Smyth M.J. Immune-mediated dormancy: An equilibrium with cancer. J. Leukoc. Biol. 2008;84:988–993. doi: 10.1189/jlb.1107774. [DOI] [PubMed] [Google Scholar]
- 44.Allen C.T., Judd N.P., Bui J.D., Uppaluri R. The clinical implications of antitumor immunity in head and neck cancer. Laryngoscope. 2012;122:144–157. doi: 10.1002/lary.21913. [DOI] [PubMed] [Google Scholar]
- 45.Spranger S., Bao R., Gajewski T.F. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 46.Leethanakul C., Patel V., Gillespie J., Pallente M., Ensley J.F., Koontongkaew S., Liotta L.A., Emmert-Buck M., Gutkind J.S. Distinct pattern of expression of differentiation and growth-related genes in squamous cell carcinomas of the head and neck revealed by the use of laser capture microdissection and cDNA arrays. Oncogene. 2000;19:3220–3224. doi: 10.1038/sj.onc.1203703. [DOI] [PubMed] [Google Scholar]
- 47.Wijetunga N.A., Yu Y., Morris L.G., Lee N., Riaz N. The head and neck cancer genome in the era of immunotherapy. Oral Oncol. 2021;112:105040. doi: 10.1016/j.oraloncology.2020.105040. [DOI] [PubMed] [Google Scholar]
- 48.Luke J.J., Bao R., Sweis R.F., Spranger S., Gajewski T.F. WNT/beta-catenin Pathway Activation Correlates with Immune Exclusion across Human Cancers. Clin. Cancer Res. 2019;25:3074–3083. doi: 10.1158/1078-0432.CCR-18-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koukourakis I.M., Gkegka A.G., Xanthopoulou E., Nanos C., Giatromanolaki A., Koukourakis M.I. Prognostic and Predictive Relevance of Tumor-Infiltrating Lymphocytes in Squamous Cell Head-Neck Cancer Patients Treated with Radical Radiotherapy/Chemo-Radiotherapy. Curr. Oncol. 2022;29:4274–4284. doi: 10.3390/curroncol29060342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y., Chen J., Chen X., Jiang F., Sun Y., Pan Y., Zhang W., Zhang J. MiR-34a suppresses HNSCC growth through modulating cell cycle arrest and senescence. Neoplasma. 2017;64:543–553. doi: 10.4149/neo_2017_408. [DOI] [PubMed] [Google Scholar]
- 51.Wu X., Cheng Y.L., Matthen M., Yoon A., Schwartz G.K., Bala S., Taylor A.M., Momen-Heravi F. Down-regulation of the tumor suppressor miR-34a contributes to head and neck cancer by up-regulating the MET oncogene and modulating tumor immune evasion. J. Exp. Clin. Cancer Res. 2021;40:70. doi: 10.1186/s13046-021-01865-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karakas B., Bachman K.E., Park B.H. Mutation of the PIK3CA oncogene in human cancers. Br. J. Cancer. 2006;94:455–459. doi: 10.1038/sj.bjc.6602970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lui V.W., Hedberg M.L., Li H., Vangara B.S., Pendleton K., Zeng Y., Lu Y., Zhang Q., Du Y., Gilbert B.R., et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov. 2013;3:761–769. doi: 10.1158/2159-8290.CD-13-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng W., Chen J.Q., Liu C., Malu S., Creasy C., Tetzlaff M.T., Xu C., McKenzie J.A., Zhang C., Liang X., et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016;6:202–216. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X., Chen X., Liu Y., Huang S., Ding J., Wang B., Dong P., Sun Z., Chen L. CSMD1 suppresses cancer progression by inhibiting proliferation, epithelial-mesenchymal transition, chemotherapy-resistance and inducing immunosuppression in esophageal squamous cell carcinoma. Exp. Cell Res. 2022;417:113220. doi: 10.1016/j.yexcr.2022.113220. [DOI] [PubMed] [Google Scholar]
- 56.Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lyu H., Li M., Jiang Z., Liu Z., Wang X. Correlate the TP53 Mutation and the HRAS Mutation with Immune Signatures in Head and Neck Squamous Cell Cancer. Comput. Struct. Biotechnol. J. 2019;17:1020–1030. doi: 10.1016/j.csbj.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siemers N.O., Holloway J.L., Chang H., Chasalow S.D., Ross-MacDonald P.B., Voliva C.F., Szustakowski J.D. Genome-wide association analysis identifies genetic correlates of immune infiltrates in solid tumors. PLoS ONE. 2017;12:e0179726. doi: 10.1371/journal.pone.0179726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duray A., Demoulin S., Hubert P., Delvenne P., Saussez S. Immune suppression in head and neck cancers: A review. Clin. Dev. Immunol. 2010;2010:701657. doi: 10.1155/2010/701657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moy J.D., Moskovitz J.M., Ferris R.L. Biological mechanisms of immune escape and implications for immunotherapy in head and neck squamous cell carcinoma. Eur. J. Cancer. 2017;76:152–166. doi: 10.1016/j.ejca.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferris R.L. Immunology and Immunotherapy of Head and Neck Cancer. J. Clin. Oncol. 2015;33:3293–3304. doi: 10.1200/JCO.2015.61.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campoli M., Ferrone S. HLA antigen changes in malignant cells: Epigenetic mechanisms and biologic significance. Oncogene. 2008;27:5869–5885. doi: 10.1038/onc.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grandis J.R., Falkner D.M., Melhem M.F., Gooding W.E., Drenning S.D., Morel P.A. Human Leukocyte Antigen Class I Allelic and Haplotype Loss in Squamous Cell Carcinoma of the Head and Neck: Clinical and Immunogenetic Consequences. Clin. Cancer Res. 2000;6:2794–2802. [PubMed] [Google Scholar]
- 64.Ferris R.L., Hunt J.L., Ferrone S. Human Leukocyte Antigen (HLA) Class I Defects in Head and Neck Cancer. Immunol. Res. 2005;33:113–133. doi: 10.1385/IR:33:2:113. [DOI] [PubMed] [Google Scholar]
- 65.Greene S., Patel P., Allen C.T. How patients with an intact immune system develop head and neck cancer. Oral Oncol. 2019;92:26–32. doi: 10.1016/j.oraloncology.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nie Y., Yang G.-y., Song Y., Zhao X., So C., Liao J., Wang L.-D., Yang C.S. DNA hypermethylation is a mechanism for loss of expression of the HLA class I genes in human esophageal squamous cell carcinomas. Carcinogenesis. 2001;22:1615–1623. doi: 10.1093/carcin/22.10.1615. [DOI] [PubMed] [Google Scholar]
- 67.Pollack B.P., Sapkota B., Cartee T.V. Epidermal growth factor receptor inhibition augments the expression of MHC class I and II genes. Clin. Cancer Res. 2011;17:4400–4413. doi: 10.1158/1078-0432.CCR-10-3283. [DOI] [PubMed] [Google Scholar]
- 68.Bandoh N., Ogino T., Katayama A., Takahara M., Katada A., Hayashi T., Harabuchi Y. HLA class I antigen and transporter associated with antigen processing downregulation in metastatic lesions of head and neck squamous cell carcinoma as a marker of poor prognosis. Oncol. Rep. 2010;23:933–939. doi: 10.3892/or_00000717. [DOI] [PubMed] [Google Scholar]
- 69.Chang C.C., Campoli M., Ferrone S. Classical and nonclassical HLA class I antigen and NK Cell-activating ligand changes in malignant cells: Current challenges and future directions. Adv. Cancer Res. 2005;93:189–234. doi: 10.1016/S0065-230X(05)93006-6. [DOI] [PubMed] [Google Scholar]
- 70.Meissner M., Reichert T.E., Kunkel M., Gooding W., Whiteside T.L., Ferrone S., Seliger B. Defects in the Human Leukocyte Antigen Class I Antigen-Processing Machinery in Head and Neck Squamous Cell Carcinoma: Association with Clinical Outcome. Clin. Cancer Res. 2005;11:2552–2560. doi: 10.1158/1078-0432.CCR-04-2146. [DOI] [PubMed] [Google Scholar]
- 71.Ogino T., Shigyo H., Ishii H., Katayama A., Miyokawa N., Harabuchi Y., Ferrone S. HLA class I antigen down-regulation in primary laryngeal squamous cell carcinoma lesions as a poor prognostic marker. Cancer Res. 2006;66:9281–9289. doi: 10.1158/0008-5472.CAN-06-0488. [DOI] [PubMed] [Google Scholar]
- 72.Horton J.D., Knochelmann H.M., Day T.A., Paulos C.M., Neskey D.M. Immune Evasion by Head and Neck Cancer: Foundations for Combination Therapy. Trends Cancer. 2019;5:208–232. doi: 10.1016/j.trecan.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lopez-Albaitero A., Nayak J.V., Ogino T., Machandia A., Gooding W., DeLeo A.B., Ferrone S., Ferris R.L. Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J. Immunol. 2006;176:3402–3409. doi: 10.4049/jimmunol.176.6.3402. [DOI] [PubMed] [Google Scholar]
- 74.Lee M.Y., Allen C.T. Mechanisms of resistance to T cell-based immunotherapy in head and neck cancer. Head. Neck. 2020;42:2722–2733. doi: 10.1002/hed.26158. [DOI] [PubMed] [Google Scholar]
- 75.Ferris R.L., Whiteside T.L., Ferrone S. Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin. Cancer Res. 2006;12:3890–3895. doi: 10.1158/1078-0432.CCR-05-2750. [DOI] [PubMed] [Google Scholar]
- 76.Seliger B. Molecular mechanisms of MHC class I abnormalities and APM components in human tumors. Cancer Immunol. Immunother. 2008;57:1719–1726. doi: 10.1007/s00262-008-0515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Setiadi A.F., David M.D., Seipp R.P., Hartikainen J.A., Gopaul R., Jefferies W.A. Epigenetic control of the immune escape mechanisms in malignant carcinomas. Mol. Cell Biol. 2007;27:7886–7894. doi: 10.1128/MCB.01547-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tomasi T.B., Magner W.J., Khan A.N. Epigenetic regulation of immune escape genes in cancer. Cancer Immunol. Immunother. 2006;55:1159–1184. doi: 10.1007/s00262-006-0164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Campbell J.D., Yau C., Bowlby R., Liu Y., Brennan K., Fan H., Taylor A.M., Wang C., Walter V., Akbani R., et al. Genomic, Pathway Network, and Immunologic Features Distinguishing Squamous Carcinomas. Cell Rep. 2018;23:194–212.e196. doi: 10.1016/j.celrep.2018.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou L., Mudianto T., Ma X., Riley R., Uppaluri R. Targeting EZH2 Enhances Antigen Presentation, Antitumor Immunity, and Circumvents Anti-PD-1 Resistance in Head and Neck Cancer. Clin. Cancer Res. 2020;26:290–300. doi: 10.1158/1078-0432.CCR-19-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leibowitz M.S., Andrade Filho P.A., Ferrone S., Ferris R.L. Deficiency of activated STAT1 in head and neck cancer cells mediates TAP1-dependent escape from cytotoxic T lymphocytes. Cancer Immunol. Immunother. 2011;60:525–535. doi: 10.1007/s00262-010-0961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leibowitz M.S., Srivastava R.M., Andrade Filho P.A., Egloff A.M., Wang L., Seethala R.R., Ferrone S., Ferris R.L. SHP2 is overexpressed and inhibits pSTAT1-mediated APM component expression, T-cell attracting chemokine secretion, and CTL recognition in head and neck cancer cells. Clin. Cancer Res. 2013;19:798–808. doi: 10.1158/1078-0432.CCR-12-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morris L.G.T., Chandramohan R., West L., Zehir A., Chakravarty D., Pfister D.G., Wong R.J., Lee N.Y., Sherman E.J., Baxi S.S., et al. The Molecular Landscape of Recurrent and Metastatic Head and Neck Cancers: Insights From a Precision Oncology Sequencing Platform. JAMA Oncol. 2017;3:244–255. doi: 10.1001/jamaoncol.2016.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Badoual C., Sandoval F., Pere H., Hans S., Gey A., Merillon N., Van Ryswick C., Quintin-Colonna F., Bruneval P., Brasnu D., et al. Better understanding tumor-host interaction in head and neck cancer to improve the design and development of immunotherapeutic strategies. Head. Neck. 2010;32:946–958. doi: 10.1002/hed.21346. [DOI] [PubMed] [Google Scholar]
- 85.Freiser M.E., Serafini P., Weed D.T. The immune system and head and neck squamous cell carcinoma: From carcinogenesis to new therapeutic opportunities. Immunol. Res. 2013;57:52–69. doi: 10.1007/s12026-013-8462-3. [DOI] [PubMed] [Google Scholar]
- 86.Lang S., Whiteside T.L., Lebeau A., Zeidler R., Mack B., Wollenberg B. Impairment of T-Cell Activation in Head and Neck Cancer In Situ and In Vitro. Arch. Otolaryngol. Head. Neck Surg. 1999;125:82–88. doi: 10.1001/archotol.125.1.82. [DOI] [PubMed] [Google Scholar]
- 87.Wollenberg B., Zeidler R., Lebeau A., Mack B., Lang S. Lack of B7.1 and B7.2 on head and neck cancer cells and possible significance for gene therapy. Int. J. Mol. Med. 1998;2:167–338. doi: 10.3892/ijmm.2.2.167. [DOI] [PubMed] [Google Scholar]
- 88.Yang W.F., Yu J.M., Zuo W.S., Wang S.Z. Expression of CD80, CD86, TGF-beta1 and IL-10 mRNA in the esophageal carcinoma. Zhonghua Zhong Liu Za Zhi. 2006;28:762–765. [PubMed] [Google Scholar]
- 89.Thomas G.R., Chen Z., Leukinova E., Van Waes C., Wen J. Cytokines IL-1 alpha, IL-6, and GM-CSF constitutively secreted by oral squamous carcinoma induce down-regulation of CD80 costimulatory molecule expression: Restoration by interferon gamma. Cancer Immunol. Immunother. 2004;53:33–40. doi: 10.1007/s00262-003-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Z., Malhotra P.S., Thomas G.R., Ondrey F.G., Duffey D.C., Smith C.W., Enamorado I., Yeh N.T., Kroog G.S., Rudy S., et al. Expression of Proinflammatory and Proangiogenic Cytokines in Patients with Head and Neck Cancer1. Clin. Cancer Res. 1999;5:1369–1379. [PubMed] [Google Scholar]
- 91.Lathers D.M., Young M.R. Increased aberrance of cytokine expression in plasma of patients with more advanced squamous cell carcinoma of the head and neck. Cytokine. 2004;25:220–228. doi: 10.1016/j.cyto.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 92.Loro L.L., Vintermyr O.K., Johannessen A.C., Liavaag P.G., Jonsson R. Suppression of Fas receptor and negative correlation of Fas ligand with differentiation and apoptosis in oral squamous cell carcinoma. J. Oral Pathol. Med. 1999;28:82–87. doi: 10.1111/j.1600-0714.1999.tb02001.x. [DOI] [PubMed] [Google Scholar]
- 93.Bie T., Zhang X. Higher Expression of SPP1 Predicts Poorer Survival Outcomes in Head and Neck Cancer. J. Immunol. Res. 2021;2021:8569575. doi: 10.1155/2021/8569575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cao D.X., Li Z.J., Jiang X.O., Lum Y.L., Khin E., Lee N.P., Wu G.H., Luk J.M. Osteopontin as potential biomarker and therapeutic target in gastric and liver cancers. World J. Gastroenterol. 2012;18:3923–3930. doi: 10.3748/wjg.v18.i30.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Szczepanski M.J., Czystowska M., Szajnik M., Harasymczuk M., Boyiadzis M., Kruk-Zagajewska A., Szyfter W., Zeromski J., Whiteside T.L. Triggering of Toll-like receptor 4 expressed on human head and neck squamous cell carcinoma promotes tumor development and protects the tumor from immune attack. Cancer Res. 2009;69:3105–3113. doi: 10.1158/0008-5472.CAN-08-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Medema J.P., De Jong J., Peltenburg L.T.C., Verdegaal E.M.E., Gorter A., Bres S.A., Franken K.L.M.C., Hahne M., Albar J.P., Melief C.J.M., et al. Blockade of the granzyme B/perforin pathway through overexpression of the serine protease inhibitor PI-9/SPI-6 constitutes a mechanism for immune escape by tumors. Proc. Natl. Acad. Sci. USA. 2001;98:11515–11520. doi: 10.1073/pnas.201398198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Kempen P.M., Noorlag R., Swartz J.E., Bovenschen N., Braunius W.W., Vermeulen J.F., Van Cann E.M., Grolman W., Willems S.M. Oropharyngeal squamous cell carcinomas differentially express granzyme inhibitors. Cancer Immunol. Immunother. 2016;65:575–585. doi: 10.1007/s00262-016-1819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wieckowski E., Atarashi Y., Stanson J., Sato T.A., Whiteside T.L. FAP-1-mediated activation of NF-kappaB induces resistance of head and neck cancer to Fas-induced apoptosis. J. Cell Biochem. 2007;100:16–28. doi: 10.1002/jcb.20922. [DOI] [PubMed] [Google Scholar]
- 99.Li X., Pan X., Zhang H., Lei D., Liu D., Xu F., Luan X. Overexpression of cFLIP in head and neck squamous cell carcinoma and its clinicopathologic correlations. J. Cancer Res. Clin. Oncol. 2008;134:609–615. doi: 10.1007/s00432-007-0325-7. [DOI] [PubMed] [Google Scholar]
- 100.Nguyen P.T., Nguyen D., Chea C., Miyauchi M., Fujii M., Takata T. Interaction between N-cadherin and decoy receptor-2 regulates apoptosis in head and neck cancer. Oncotarget. 2018;9:31516–31530. doi: 10.18632/oncotarget.25846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yoldas B., Ozer C., Ozen O., Canpolat T., Dogan I., Griffith T.S., Sanlioglu S., Ozluoglu L.N. Clinical significance of TRAIL and TRAIL receptors in patients with head and neck cancer. Head. Neck. 2011;33:1278–1284. doi: 10.1002/hed.21598. [DOI] [PubMed] [Google Scholar]
- 102.Zhao L.Y., Xu W.L., Xu Z.Q., Qi C., Li Y., Cheng J., Liu L.K., Wu Y.N., Gao J., Ye J.H. The overexpressed functional transient receptor potential channel TRPM2 in oral squamous cell carcinoma. Sci. Rep. 2016;6:38471. doi: 10.1038/srep38471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reichert T.E., Kashii Y., Stanson J., Zeevi A., Whiteside T.L. The role of endogenous interleukin-2 in proliferation of human carcinoma cell lines. Br. J. Cancer. 1999;81:822–831. doi: 10.1038/sj.bjc.6690770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alam M., Kashyap T., Pramanik K.K., Singh A.K., Nagini S., Mishra R. The elevated activation of NFκB and AP-1 is correlated with differential regulation of Bcl-2 and associated with oral squamous cell carcinoma progression and resistance. Clin. Oral Investig. 2017;21:2721–2731. doi: 10.1007/s00784-017-2074-6. [DOI] [PubMed] [Google Scholar]
- 105.Niklander S.E., Murdoch C., Hunter K.D. IL-1/IL-1R Signaling in Head and Neck Cancer. Front. Oral Health. 2021;2:722676. doi: 10.3389/froh.2021.722676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li C., Egloff A.M., Sen M., Grandis J.R., Johnson D.E. Caspase-8 mutations in head and neck cancer confer resistance to death receptor-mediated apoptosis and enhance migration, invasion, and tumor growth. Mol. Oncol. 2014;8:1220–1230. doi: 10.1016/j.molonc.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Teng M.S., Brandwein-Gensler M.S., Teixeira M.S., Martignetti J.A., Duffey D.C. A Study of TRAIL Receptors in Squamous Cell Carcinoma of the Head and Neck. Arch. Otolaryngol. Head. Neck Surg. 2005;131:407–412. doi: 10.1001/archotol.131.5.407. [DOI] [PubMed] [Google Scholar]
- 108.Vitale I., Galluzzi L., Castedo M., Kroemer G. Mitotic catastrophe: A mechanism for avoiding genomic instability. Nat. Rev. Mol. Cell Biol. 2011;12:385–392. doi: 10.1038/nrm3115. [DOI] [PubMed] [Google Scholar]
- 109.Chen G., Shi L., Litchfield D.W., Greenberg A.H. Rescue from Granzyme B-induced Apoptosis by Wee1 Kinase. J. Exp. Med. 1995;181:2295–2300. doi: 10.1084/jem.181.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Friedman J., Morisada M., Sun L., Moore E.C., Padget M., Hodge J.W., Schlom J., Gameiro S.R., Allen C.T. Inhibition of WEE1 kinase and cell cycle checkpoint activation sensitizes head and neck cancers to natural killer cell therapies. J. Immunother. Cancer. 2018;6:59. doi: 10.1186/s40425-018-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sun L., Moore E., Berman R., Clavijo P.E., Saleh A., Chen Z., Van Waes C., Davies J., Friedman J., Allen C.T. WEE1 kinase inhibition reverses G2/M cell cycle checkpoint activation to sensitize cancer cells to immunotherapy. Oncoimmunology. 2018;7:e1488359. doi: 10.1080/2162402X.2018.1488359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ludwig S., Floros T., Theodoraki M.N., Hong C.S., Jackson E.K., Lang S., Whiteside T.L. Suppression of Lymphocyte Functions by Plasma Exosomes Correlates with Disease Activity in Patients with Head and Neck Cancer. Clin. Cancer Res. 2017;23:4843–4854. doi: 10.1158/1078-0432.CCR-16-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Theodoraki M.N., Hoffmann T.K., Jackson E.K., Whiteside T.L. Exosomes in HNSCC plasma as surrogate markers of tumour progression and immune competence. Clin. Exp. Immunol. 2018;194:67–78. doi: 10.1111/cei.13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gastman B.R., Atarashi Y., Reichert T.E., Saito T., Balkir L., Rabinowich H., Whiteside T.L. Fas Ligand Is Expressed on Human Squamous Cell Carcinomas of the Head and Neck, and It Promotes Apoptosis of T Lymphocytes1. Cancer Res. 1999;59:5356–5364. [PubMed] [Google Scholar]
- 115.Gastman B.R., Johnson D.E., Whiteside T.L., Rabinowich H. Tumor-induced apoptosis of T lymphocytes: Elucidation of intracellular apoptotic events. Blood. 2000;95:2015–2023. doi: 10.1182/blood.V95.6.2015. [DOI] [PubMed] [Google Scholar]
- 116.Hoffmann T.K., Dworacki G., Tsukihiro T., Meidenbauer N., Gooding W., Johnson J.T., Whiteside T.L. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin. Cancer Res. 2002;8:2553–2562. [PubMed] [Google Scholar]
- 117.Whiteside T.L. Tumor-induced death of immune cells: Its mechanisms and consequences. Semin. Cancer Biol. 2002;12:43–50. doi: 10.1006/scbi.2001.0402. [DOI] [PubMed] [Google Scholar]
- 118.Kassouf N., Thornhill M.H. Oral cancer cell lines can use multiple ligands, including Fas-L, TRAIL and TNF-alpha, to induce apoptosis in Jurkat T cells: Possible mechanisms for immune escape by head and neck cancers. Oral Oncol. 2008;44:672–682. doi: 10.1016/j.oraloncology.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 119.Saussez S., Camby I., Toubeau G., Kiss R. Galectins as modulators of tumor progression in head and neck squamous cell carcinomas. Head. Neck. 2007;29:874–884. doi: 10.1002/hed.20559. [DOI] [PubMed] [Google Scholar]
- 120.Strome S.E., Dong H., Tamura H., Voss S.G., Flies D.B., Tamada K., Salomao D., Cheville J., Hirano F., Lin W., et al. B7-H1 Blockade Augments Adoptive T-Cell Immunotherapy for Squamous Cell Carcinoma1. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 121.Nambiar D.K., Aguilera T., Cao H., Kwok S., Kong C., Bloomstein J., Wang Z., Rangan V.S., Jiang D., von Eyben R., et al. Galectin-1-driven T cell exclusion in the tumor endothelium promotes immunotherapy resistance. J. Clin. Investig. 2019;129:5553–5567. doi: 10.1172/JCI129025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Concha-Benavente F., Srivastava R.M., Trivedi S., Lei Y., Chandran U., Seethala R.R., Freeman G.J., Ferris R.L. Identification of the Cell-Intrinsic and -Extrinsic Pathways Downstream of EGFR and IFNγ That Induce PD-L1 Expression in Head and Neck Cancer. Cancer Res. 2016;76:1031–1043. doi: 10.1158/0008-5472.CAN-15-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Seiwert T.Y., Burtness B., Mehra R., Weiss J., Berger R., Eder J.P., Heath K., McClanahan T., Lunceford J., Gause C., et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–965. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 124.Zandberg D.P., Strome S.E. The role of the PD-L1:PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2014;50:627–632. doi: 10.1016/j.oraloncology.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 125.Zou W., Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 126.Bellmunt À.M., López-Puerto L., Lorente J., Closa D. Involvement of extracellular vesicles in the macrophage-tumor cell communication in head and neck squamous cell carcinoma. PLoS ONE. 2019;14:e0224710. doi: 10.1371/journal.pone.0224710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Butte M.J., Keir M.E., Phamduy T.B., Sharpe A.H., Freeman G.J. Programmed Death-1 Ligand 1 Interacts Specifically with the B7-1 Costimulatory Molecule to Inhibit T Cell Responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Eppihimer M.J., Gunn J., Freeman G.J., Greenfield E.A., Chernova T., Erickson J., Leonard J.P. Expression and regulation of the PD-L1 immunoinhibitory molecule on micro vascular endothelial cells. Microcirculation. 2002;9:133–145. doi: 10.1080/713774061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen S., Crabill G.A., Pritchard T.S., McMiller T.L., Wei P., Pardoll D.M., Pan F., Topalian S.L. Mechanisms regulating PD-L1 expression on tumor and immune cells. J. Immunother. Cancer. 2019;7:305. doi: 10.1186/s40425-019-0770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cui B., Chen J., Luo M., Wang L., Chen H., Kang Y., Wang J., Zhou X., Feng Y., Zhang P. Protein kinase D3 regulates the expression of the immunosuppressive protein, PD-L1, through STAT1/STAT3 signaling. Int. J. Oncol. 2020;56:909–920. doi: 10.3892/ijo.2020.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kondoh N., Mizuno-Kamiya M. The Role of Immune Modulatory Cytokines in the Tumor Microenvironments of Head and Neck Squamous Cell Carcinomas. Cancers. 2022;14:2884. doi: 10.3390/cancers14122884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kuo C.-S., Yang C.-Y., Lin C.-K., Lin G.-J., Sytwu H.-K., Chen Y.-W. Triptolide suppresses oral cancer cell PD-L1 expression in the interferon-γ-modulated microenvironment in vitro, in vivo, and in clinical patients. Biomed. Pharmacother. 2021;133:111057. doi: 10.1016/j.biopha.2020.111057. [DOI] [PubMed] [Google Scholar]
- 133.Parsa A.T., Waldron J.S., Panner A., Crane C.A., Parney I.F., Barry J.J., Cachola K.E., Murray J.C., Tihan T., Jensen M.C., et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 134.Skinner H.D., Giri U., Yang L.P., Kumar M., Liu Y., Story M.D., Pickering C.R., Byers L.A., Williams M.D., Wang J., et al. Integrative Analysis Identifies a Novel AXL-PI3 Kinase-PD-L1 Signaling Axis Associated with Radiation Resistance in Head and Neck Cancer. Clin. Cancer Res. 2017;23:2713–2722. doi: 10.1158/1078-0432.CCR-16-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lan X., Zhao J., Song C., Yuan Q., Liu X. TRPM8 facilitates proliferation and immune evasion of esophageal cancer cells. Biosci. Rep. 2019;39:BSR20191878. doi: 10.1042/BSR20191878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mullins R.D.Z., Pal A., Barrett T.F., Heft Neal M.E., Puram S.V. Epithelial-Mesenchymal Plasticity in Tumor Immune Evasion. Cancer Res. 2022;82:2329–2343. doi: 10.1158/0008-5472.CAN-21-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen L., Gibbons D.L., Goswami S., Cortez M.A., Ahn Y.H., Byers L.A., Zhang X., Yi X., Dwyer D., Lin W., et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun. 2014;5:5241. doi: 10.1038/ncomms6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Noman M.Z., Janji B., Abdou A., Hasmim M., Terry S., Tan T.Z., Mami-Chouaib F., Thiery J.P., Chouaib S. The immune checkpoint ligand PD-L1 is upregulated in EMT-activated human breast cancer cells by a mechanism involving ZEB-1 and miR-200. Oncoimmunology. 2017;6:e1263412. doi: 10.1080/2162402X.2016.1263412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sahoo S., Nayak S.P., Hari K., Purkait P., Mandal S., Kishore A., Levine H., Jolly M.K. Immunosuppressive Traits of the Hybrid Epithelial/Mesenchymal Phenotype. Front. Immunol. 2021;12:797261. doi: 10.3389/fimmu.2021.797261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dhupar R., Van Der Kraak L., Pennathur A., Schuchert M.J., Nason K.S., Luketich J.D., Lotze M.T. Targeting Immune Checkpoints in Esophageal Cancer: A High Mutational Load Tumor. Ann. Thorac. Surg. 2017;103:1340–1349. doi: 10.1016/j.athoracsur.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 141.Yearley J.H., Gibson C., Yu N., Moon C., Murphy E., Juco J., Lunceford J., Cheng J., Chow L.Q.M., Seiwert T.Y., et al. PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clin. Cancer Res. 2017;23:3158–3167. doi: 10.1158/1078-0432.CCR-16-1761. [DOI] [PubMed] [Google Scholar]
- 142.Latchman Y., Wood C.R., Chernova T., Chaudhary D., Borde M., Chernova I., Iwai Y., Long A.J., Brown J.A., Nunes R., et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 143.Dai X., Gao Y., Wei W. Post-translational regulations of PD-L1 and PD-1: Mechanisms and opportunities for combined immunotherapy. Semin. Cancer Biol. 2022;85:246–252. doi: 10.1016/j.semcancer.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hu X., Lin Z., Wang Z., Zhou Q. Emerging role of PD-L1 modification in cancer immunotherapy. Am. J. Cancer Res. 2021;11:3832–3840. [PMC free article] [PubMed] [Google Scholar]
- 145.Hu X., Wang J., Chu M., Liu Y., Wang Z.-w., Zhu X. Emerging Role of Ubiquitination in the Regulation of PD-1/PD-L1 in Cancer Immunotherapy. Mol. Ther. 2021;29:908–919. doi: 10.1016/j.ymthe.2020.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu Y., Gao Z., Hu R., Wang Y., Wang Y., Su Z., Zhang X., Yang J., Mei M., Ren Y., et al. PD-L2 glycosylation promotes immune evasion and predicts anti-EGFR efficacy. J. Immunother. Cancer. 2021;9:e002699. doi: 10.1136/jitc-2021-002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ma X.M., Luo Y.F., Zeng F.F., Su C., Liu X., Li X.P., Lu J. TGF-beta1-Mediated PD-L1 Glycosylation Contributes to Immune Escape via c-Jun/STT3A Pathway in Nasopharyngeal Carcinoma. Front. Oncol. 2022;12:815437. doi: 10.3389/fonc.2022.815437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Blum A.E., Venkitachalam S., Ravillah D., Chelluboyina A.K., Kieber-Emmons A.M., Ravi L., Kresak A., Chandar A.K., Markowitz S.D., Canto M.I., et al. Systems Biology Analyses Show Hyperactivation of Transforming Growth Factor-β and JNK Signaling Pathways in Esophageal Cancer. Gastroenterology. 2019;156:1761–1774. doi: 10.1053/j.gastro.2019.01.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gholamin M., Moaven O., Memar B., Farshchian M., Naseh H., Malekzadeh R., Sotoudeh M., Rajabi-Mashhadi M.T., Forghani M.N., Farrokhi F., et al. Overexpression and interactions of interleukin-10, transforming growth factor beta, and vascular endothelial growth factor in esophageal squamous cell carcinoma. World J. Surg. 2009;33:1439–1445. doi: 10.1007/s00268-009-0070-y. [DOI] [PubMed] [Google Scholar]
- 150.Hargadon K.M. Tumor-altered dendritic cell function: Implications for anti-tumor immunity. Front. Immunol. 2013;4:192. doi: 10.3389/fimmu.2013.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen Y., Di C., Zhang X., Wang J., Wang F., Yan J.-f., Xu C., Zhang J., Zhang Q., Li H., et al. Transforming growth factor β signaling pathway: A promising therapeutic target for cancer. J. Cell. Physiol. 2020;235:1903–1914. doi: 10.1002/jcp.29108. [DOI] [PubMed] [Google Scholar]
- 152.Moutsopoulos N.M., Wen J., Wahl S.M. TGF-beta and tumors—An ill-fated alliance. Curr. Opin. Immunol. 2008;20:234–240. doi: 10.1016/j.coi.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kondo Y., Suzuki S., Takahara T., Ono S., Goto M., Miyabe S., Sugita Y., Ogawa T., Ito H., Satou A., et al. Improving function of cytotoxic T-lymphocytes by transforming growth factor-β inhibitor in oral squamous cell carcinoma. Cancer Sci. 2021;112:4037–4049. doi: 10.1111/cas.15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Duffey D.C., Chen Z., Dong G., Ondrey F.G., Wolf J.S., Brown K., Siebenlist U., Van Waes C. Expression of a dominant-negative mutant inhibitor-kappaBalpha of nuclear factor-kappaB in human head and neck squamous cell carcinoma inhibits survival, proinflammatory cytokine expression, and tumor growth in vivo. Cancer Res. 1999;59:3468–3474. [PubMed] [Google Scholar]
- 155.Varilla V., Atienza J., Dasanu C.A. Immune alterations and immunotherapy prospects in head and neck cancer. Expert Opin. Biol. Ther. 2013;13:1241–1256. doi: 10.1517/14712598.2013.810716. [DOI] [PubMed] [Google Scholar]
- 156.Riedel F., Zaiss I., Herzog D., Götte K., Naim R., Hörmann K. Serum levels of interleukin-6 in patients with primary head and neck squamous cell carcinoma. Anticancer Res. 2005;25:2761–2765. [PubMed] [Google Scholar]
- 157.Vinocha A., Grover R.K., Deepak R. Clinical significance of interleukin-6 in diagnosis of lung, oral, esophageal, and gall bladder carcinomas. J. Cancer Res. Ther. 2018;14:S758–S760. doi: 10.4103/0973-1482.183217. [DOI] [PubMed] [Google Scholar]
- 158.Wu J., Gao F.X., Wang C., Qin M., Han F., Xu T., Hu Z., Long Y., He X.M., Deng X., et al. IL-6 and IL-8 secreted by tumour cells impair the function of NK cells via the STAT3 pathway in oesophageal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2019;38:321. doi: 10.1186/s13046-019-1310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cheng F., Wang H.W., Cuenca A., Huang M., Ghansah T., Brayer J., Kerr W.G., Takeda K., Akira S., Schoenberger S.P., et al. A critical role for Stat3 signaling in immune tolerance. Immunity. 2003;19:425–436. doi: 10.1016/S1074-7613(03)00232-2. [DOI] [PubMed] [Google Scholar]
- 160.Johnson D.E., O’Keefe R.A., Grandis J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018;15:234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sun Y., Chin Y.E., Weisiger E., Malter C., Tawara I., Toubai T., Gatza E., Mascagni P., Dinarello C.A., Reddy P. Cutting edge: Negative regulation of dendritic cells through acetylation of the nonhistone protein STAT-3. J. Immunol. 2009;182:5899–5903. doi: 10.4049/jimmunol.0804388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Barton B.E. STAT3: A potential therapeutic target in dendritic cells for the induction of transplant tolerance. Expert. Opin. Ther. Targets. 2006;10:459–470. doi: 10.1517/14728222.10.3.459. [DOI] [PubMed] [Google Scholar]
- 163.Wang T., Niu G., Kortylewski M., Burdelya L., Shain K., Zhang S., Bhattacharya R., Gabrilovich D., Heller R., Coppola D., et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat. Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 164.Park S.J., Nakagawa T., Kitamura H., Atsumi T., Kamon H., Sawa S., Kamimura D., Ueda N., Iwakura Y., Ishihara K., et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J. Immunol. 2004;173:3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 165.Seiwert T.Y., Cohen E.E. Targeting angiogenesis in head and neck cancer. Semin. Oncol. 2008;35:274–285. doi: 10.1053/j.seminoncol.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 166.Vassilakopoulou M., Psyrri A., Argiris A. Targeting angiogenesis in head and neck cancer. Oral Oncol. 2015;51:409–415. doi: 10.1016/j.oraloncology.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 167.Gabrilovich D., Ishida T., Oyama T., Ran S., Kravtsov V., Nadaf S., Carbone D.P. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. doi: 10.1182/blood.V92.11.4150. [DOI] [PubMed] [Google Scholar]
- 168.Gabrilovich D.I., Chen H.L., Girgis K.R., Cunningham H.T., Meny G.M., Nadaf S., Kavanaugh D., Carbone D.P. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 169.Johnson B.F., Clay T.M., Hobeika A.C., Lyerly H.K., Morse M.A. Vascular endothelial growth factor and immunosuppression in cancer: Current knowledge and potential for new therapy. Expert. Opin. Biol. Ther. 2007;7:449–460. doi: 10.1517/14712598.7.4.449. [DOI] [PubMed] [Google Scholar]
- 170.Strauss L., Volland D., Kunkel M., Reichert T.E. Dual role of VEGF family members in the pathogenesis of head and neck cancer (HNSCC): Possible link between angiogenesis and immune tolerance. Med. Sci. Monit. 2005;11:Br280–Br292. [PubMed] [Google Scholar]
- 171.Ohm J.E., Shurin M.R., Esche C., Lotze M.T., Carbone D.P., Gabrilovich D.I. Effect of vascular endothelial growth factor and FLT3 ligand on dendritic cell generation in vivo. J. Immunol. 1999;163:3260–3268. doi: 10.4049/jimmunol.163.6.3260. [DOI] [PubMed] [Google Scholar]
- 172.Brossart P., Zobywalski A., Grünebach F., Behnke L., Stuhler G., Reichardt V.L., Kanz L., Brugger W. Tumor necrosis factor alpha and CD40 ligand antagonize the inhibitory effects of interleukin 10 on T-cell stimulatory capacity of dendritic cells. Cancer Res. 2000;60:4485–4492. [PubMed] [Google Scholar]
- 173.Hadjigol S., Shah B.A., O’Brien-Simpson N.M. The ‘Danse Macabre’-Neutrophils the Interactive Partner Affecting Oral Cancer Outcomes. Front. Immunol. 2022;13:894021. doi: 10.3389/fimmu.2022.894021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Morse M.A., Mosca P.J., Clay T.M., Lyerly H.K. Dendritic cell maturation in active immunotherapy strategies. Expert. Opin. Biol. Ther. 2002;2:35–43. doi: 10.1517/14712598.2.1.35. [DOI] [PubMed] [Google Scholar]
- 175.Yang A.S., Lattime E.C. Tumor-induced interleukin 10 suppresses the ability of splenic dendritic cells to stimulate CD4 and CD8 T-cell responses. Cancer Res. 2003;63:2150–2157. [PubMed] [Google Scholar]
- 176.Steinbrink K., Wölfl M., Jonuleit H., Knop J., Enk A.H. Induction of tolerance by IL-10-treated dendritic cells. J. Immunol. 1997;159:4772–4780. doi: 10.4049/jimmunol.159.10.4772. [DOI] [PubMed] [Google Scholar]
- 177.Zhou L., Nazarian A.A., Smale S.T. Interleukin-10 inhibits interleukin-12 p40 gene transcription by targeting a late event in the activation pathway. Mol. Cell Biol. 2004;24:2385–2396. doi: 10.1128/MCB.24.6.2385-2396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Loaiza N., Demaria M. Cellular senescence and tumor promotion: Is aging the key? Biochim. Biophys. Acta (BBA) Rev. Cancer. 2016;1865:155–167. doi: 10.1016/j.bbcan.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 179.Wolf J.S., Chen Z., Dong G., Sunwoo J.B., Bancroft C.C., Capo D.E., Yeh N.T., Mukaida N., Van Waes C. IL (interleukin)-1alpha promotes nuclear factor-kappaB and AP-1-induced IL-8 expression, cell survival, and proliferation in head and neck squamous cell carcinomas. Clin. Cancer Res. 2001;7:1812–1820. [PubMed] [Google Scholar]
- 180.Woods K.V., Adler-Storthz K., Clayman G.L., Francis G.M., Grimm E.A. Interleukin-1 regulates interleukin-6 secretion in human oral squamous cell carcinoma in vitro: Possible influence of p53 but not human papillomavirus E6/E7. Cancer Res. 1998;58:3142–3149. [PubMed] [Google Scholar]
- 181.Pries R., Wollenberg B. Cytokines in head and neck cancer. Cytokine Growth Factor. Rev. 2006;17:141–146. doi: 10.1016/j.cytogfr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 182.Zeidler R., Csanady M., Gires O., Lang S., Schmitt B., Wollenberg B. Tumor cell-derived prostaglandin E2 inhibits monocyte function by interfering with CCR5 and Mac-1. FASEB J. 2000;14:661–668. doi: 10.1096/fasebj.14.5.661. [DOI] [PubMed] [Google Scholar]
- 183.Tourkova I.L., Shurin G.V., Chatta G.S., Perez L., Finke J., Whiteside T.L., Ferrone S., Shurin M.R. Restoration by IL-15 of MHC class I antigen-processing machinery in human dendritic cells inhibited by tumor-derived gangliosides. J. Immunol. 2005;175:3045–3052. doi: 10.4049/jimmunol.175.5.3045. [DOI] [PubMed] [Google Scholar]
- 184.Caldwell S., Heitger A., Shen W., Liu Y., Taylor B., Ladisch S. Mechanisms of ganglioside inhibition of APC function. J. Immunol. 2003;171:1676–1683. doi: 10.4049/jimmunol.171.4.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shen W., Ladisch S. Ganglioside GD1a impedes lipopolysaccharide-induced maturation of human dendritic cells. Cell Immunol. 2002;220:125–133. doi: 10.1016/S0008-8749(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 186.Shurin G.V., Shurin M.R., Bykovskaia S., Shogan J., Lotze M.T., Barksdale E.M., Jr. Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function. Cancer Res. 2001;61:363–369. [PubMed] [Google Scholar]
- 187.Whiteside T.L., Stanson J., Shurin M.R., Ferrone S. Antigen-processing machinery in human dendritic cells: Up-regulation by maturation and down-regulation by tumor cells. J. Immunol. 2004;173:1526–1534. doi: 10.4049/jimmunol.173.3.1526. [DOI] [PubMed] [Google Scholar]
- 188.Wölfl M., Batten W.Y., Posovszky C., Bernhard H., Berthold F. Gangliosides inhibit the development from monocytes to dendritic cells. Clin. Exp. Immunol. 2002;130:441–448. doi: 10.1046/j.1365-2249.2002.02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li B., Song T.-N., Wang F.-R., Yin C., Li Z., Lin J.-P., Meng Y.-Q., Feng H.-M., Jing T. Tumor-derived exosomal HMGB1 promotes esophageal squamous cell carcinoma progression through inducing PD1+ TAM expansion. Oncogenesis. 2019;8:17. doi: 10.1038/s41389-019-0126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Qian W., Huang P., Liang X., Chen Y., Guan B. High expression of carcinoembryonic antigen-associated cell adhesion molecule 1 is associated with microangiogenesis in esophageal squamous cell carcinoma. Transl. Cancer Res. 2020;9:4762. doi: 10.21037/tcr-19-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vermeer D.W., Spanos W.C., Vermeer P.D., Bruns A.M., Lee K.M., Lee J.H. Radiation-induced loss of cell surface CD47 enhances immune-mediated clearance of human papillomavirus-positive cancer. Int. J. Cancer. 2013;133:120–129. doi: 10.1002/ijc.28015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yu G.T., Bu L.L., Zhao Y.Y., Mao L., Deng W.W., Wu T.F., Zhang W.F., Sun Z.J. CTLA4 blockade reduces immature myeloid cells in head and neck squamous cell carcinoma. Oncoimmunology. 2016;5:e1151594. doi: 10.1080/2162402X.2016.1151594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang X.F., Pan K., Weng D.S., Chen C.L., Wang Q.J., Zhao J.J., Pan Q.Z., Liu Q., Jiang S.S., Li Y.Q., et al. Cytotoxic T lymphocyte antigen-4 expression in esophageal carcinoma: Implications for prognosis. Oncotarget. 2016;7:26670–26679. doi: 10.18632/oncotarget.8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Elmusrati A., Wang J., Wang C.Y. Tumor microenvironment and immune evasion in head and neck squamous cell carcinoma. Int. J. Oral Sci. 2021;13:24. doi: 10.1038/s41368-021-00131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang C., Li Y., Jia L., Kim J.k., Li J., Deng P., Zhang W., Krebsbach P.H., Wang C.-Y. CD276 expression enables squamous cell carcinoma stem cells to evade immune surveillance. Cell Stem Cell. 2021;28:1597–1613.e7. doi: 10.1016/j.stem.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu C., Li X., Xiong F., Wang L., Chen K., Wu P., Hua L., Zhang Z. Down-regulation of MLLT1 super elongation complex subunit impairs the anti-tumor activity of natural killer cells in esophageal cancer. Immunobiology. 2022;227:152238. doi: 10.1016/j.imbio.2022.152238. [DOI] [PubMed] [Google Scholar]
- 197.Barclay A.N., Berg T.K.v.d. The Interaction Between Signal Regulatory Protein Alpha (SIRPα) and CD47: Structure, Function, and Therapeutic Target. Annu. Rev. Immunol. 2014;32:25–50. doi: 10.1146/annurev-immunol-032713-120142. [DOI] [PubMed] [Google Scholar]
- 198.Blazar B.R., Lindberg F.P., Ingulli E., Panoskaltsis-Mortari A., Oldenborg P.-A., Iizuka K., Yokoyama W.M., Taylor P.A. Cd47 (Integrin-Associated Protein) Engagement of Dendritic Cell and Macrophage Counterreceptors Is Required to Prevent the Clearance of Donor Lymphohematopoietic Cells. J. Exp. Med. 2001;194:541–550. doi: 10.1084/jem.194.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Engelhardt J.J., Sullivan T.J., Allison J.P. CTLA-4 Overexpression Inhibits T Cell Responses through a CD28-B7-Dependent Mechanism1. J. Immunol. 2006;177:1052–1061. doi: 10.4049/jimmunol.177.2.1052. [DOI] [PubMed] [Google Scholar]
- 200.Collins A.V., Brodie D.W., Gilbert R.J., Iaboni A., Manso-Sancho R., Walse B., Stuart D.I., van der Merwe P.A., Davis S.J. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17:201–210. doi: 10.1016/S1074-7613(02)00362-X. [DOI] [PubMed] [Google Scholar]
- 201.Kontos F., Michelakos T., Kurokawa T., Sadagopan A., Schwab J.H., Ferrone C.R., Ferrone S. B7-H3: An Attractive Target for Antibody-based Immunotherapy. Clin. Cancer Res. 2021;27:1227–1235. doi: 10.1158/1078-0432.CCR-20-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ackerman D., Simon M.C. Hypoxia, lipids, and cancer: Surviving the harsh tumor microenvironment. Trends Cell Biol. 2014;24:472–478. doi: 10.1016/j.tcb.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pouysségur J., Dayan F., Mazure N.M. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 204.Ariffin A.B., Forde P.F., Jahangeer S., Soden D.M., Hinchion J. Releasing pressure in tumors: What do we know so far and where do we go from here? A review. Cancer Res. 2014;74:2655–2662. doi: 10.1158/0008-5472.CAN-13-3696. [DOI] [PubMed] [Google Scholar]
- 205.Le Q.T., Shi G., Cao H., Nelson D.W., Wang Y., Chen E.Y., Zhao S., Kong C., Richardson D., O’Byrne K.J., et al. Galectin-1: A link between tumor hypoxia and tumor immune privilege. J. Clin. Oncol. 2005;23:8932–8941. doi: 10.1200/JCO.2005.02.0206. [DOI] [PubMed] [Google Scholar]
- 206.Sitkovsky M., Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat. Rev. Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 207.Sitkovsky M.V., Kjaergaard J., Lukashev D., Ohta A. Hypoxia-adenosinergic immunosuppression: Tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin. Cancer Res. 2008;14:5947–5952. doi: 10.1158/1078-0432.CCR-08-0229. [DOI] [PubMed] [Google Scholar]
- 208.Brand A., Singer K., Koehl G.E., Kolitzus M., Schoenhammer G., Thiel A., Matos C., Bruss C., Klobuch S., Peter K., et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016;24:657–671. doi: 10.1016/j.cmet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 209.Pilon-Thomas S., Kodumudi K.N., El-Kenawi A.E., Russell S., Weber A.M., Luddy K., Damaghi M., Wojtkowiak J.W., Mulé J.J., Ibrahim-Hashim A., et al. Neutralization of Tumor Acidity Improves Antitumor Responses to Immunotherapy. Cancer Res. 2016;76:1381–1390. doi: 10.1158/0008-5472.CAN-15-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Xie H., Hanai J., Ren J.G., Kats L., Burgess K., Bhargava P., Signoretti S., Billiard J., Duffy K.J., Grant A., et al. Targeting lactate dehydrogenase—A inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metab. 2014;19:795–809. doi: 10.1016/j.cmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fischer K., Hoffmann P., Voelkl S., Meidenbauer N., Ammer J., Edinger M., Gottfried E., Schwarz S., Rothe G., Hoves S., et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 212.Ho P.C., Bihuniak J.D., Macintyre A.N., Staron M., Liu X., Amezquita R., Tsui Y.C., Cui G., Micevic G., Perales J.C., et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 2015;162:1217–1228. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ottensmeier C.H., Perry K.L., Harden E.L., Stasakova J., Jenei V., Fleming J., Wood O., Woo J., Woelk C.H., Thomas G.J., et al. Upregulated Glucose Metabolism Correlates Inversely with CD8+ T-cell Infiltration and Survival in Squamous Cell Carcinoma. Cancer Res. 2016;76:4136–4148. doi: 10.1158/0008-5472.CAN-15-3121. [DOI] [PubMed] [Google Scholar]
- 214.Kiyozumi Y., Baba Y., Okadome K., Yagi T., Ishimoto T., Iwatsuki M., Miyamoto Y., Yoshida N., Watanabe M., Komohara Y., et al. IDO1 Expression Is Associated With Immune Tolerance and Poor Prognosis in Patients With Surgically Resected Esophageal Cancer. Ann. Surg. 2019;269:1101–1108. doi: 10.1097/SLA.0000000000002754. [DOI] [PubMed] [Google Scholar]
- 215.Lin D.J., Ng J.C.K., Huang L., Robinson M., O’Hara J., Wilson J.A., Mellor A.L. The immunotherapeutic role of indoleamine 2,3-dioxygenase in head and neck squamous cell carcinoma: A systematic review. Clin. Otolaryngol. 2021;46:919–934. doi: 10.1111/coa.13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Munn D.H., Shafizadeh E., Attwood J.T., Bondarev I., Pashine A., Mellor A.L. Inhibition of T Cell Proliferation by Macrophage Tryptophan Catabolism. J. Exp. Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Uyttenhove C., Pilotte L., Théate I., Stroobant V., Colau D., Parmentier N., Boon T., Van den Eynde B.J. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 218.Deaglio S., Robson S.C. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Adv. Pharmacol. 2011;61:301–332. doi: 10.1016/b978-0-12-385526-8.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ren Z.H., Yuan Y.X., Ji T., Zhang C.P. CD73 as a novel marker for poor prognosis of oral squamous cell carcinoma. Oncol. Lett. 2016;12:556–562. doi: 10.3892/ol.2016.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Xue F., Wang T., Shi H., Feng H., Feng G., Wang R., Yao Y., Yuan H. CD73 facilitates invadopodia formation and boosts malignancy of head and neck squamous cell carcinoma via the MAPK signaling pathway. Cancer Sci. 2022;113:2704–2715. doi: 10.1111/cas.15452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Marafon F., Bonadiman B., de Rocco Donassolo S., Marins K., Zanchi M.M., Kosvosky G.C., Basso H.F., Zamoner A., Bagatini M.D. Deregulation of purinergic ectoenzyme activity in head and neck cancer promotes immunosuppression. Mol. Biol. Rep. 2022;49:7687–7695. doi: 10.1007/s11033-022-07586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Allard B., Beavis P.A., Darcy P.K., Stagg J. Immunosuppressive activities of adenosine in cancer. Curr. Opin. Pharmacol. 2016;29:7–16. doi: 10.1016/j.coph.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 223.Cekic C., Day Y.J., Sag D., Linden J. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res. 2014;74:7250–7259. doi: 10.1158/0008-5472.CAN-13-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hoskin D.W., Mader J.S., Furlong S.J., Conrad D.M., Blay J. Inhibition of T cell and natural killer cell function by adenosine and its contribution to immune evasion by tumor cells (Review) Int. J. Oncol. 2008;32:527–535. doi: 10.3892/ijo.32.3.527. [DOI] [PubMed] [Google Scholar]
- 225.Böttcher J.P., Reis e Sousa C. The Role of Type 1 Conventional Dendritic Cells in Cancer Immunity. Trends Cancer. 2018;4:784–792. doi: 10.1016/j.trecan.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Schuon R., Brieger J., Franke R.L., Jakob R., Mann W.J. Increased PGE2 levels in nonmalignant mucosa adjacent to squamous cell carcinoma of the head and neck. ORL J. Otorhinolaryngol. Relat. Spec. 2005;67:96–100. doi: 10.1159/000084996. [DOI] [PubMed] [Google Scholar]
- 227.Young M.R. Trials and tribulations of immunotherapy as a treatment option for patients with squamous cell carcinoma of the head and neck. Cancer Immunol. Immunother. 2004;53:375–382. doi: 10.1007/s00262-003-0456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kaliński P., Smits H.H., Schuitemaker J.H., Vieira P.L., van Eijk M., de Jong E.C., Wierenga E.A., Kapsenberg M.L. IL-4 is a mediator of IL-12p70 induction by human Th2 cells: Reversal of polarized Th2 phenotype by dendritic cells. J. Immunol. 2000;165:1877–1881. doi: 10.4049/jimmunol.165.4.1877. [DOI] [PubMed] [Google Scholar]
- 229.Brocks C.P., Pries R., Frenzel H., Ernst M., Schlenke P., Wollenberg B. Functional alteration of myeloid dendritic cells through head and neck cancer. Anticancer Res. 2007;27:817–824. [PubMed] [Google Scholar]
- 230.Pahne-Zeppenfeld J., Schröer N., Walch-Rückheim B., Oldak M., Gorter A., Hegde S., Smola S. Cervical cancer cell-derived interleukin-6 impairs CCR7-dependent migration of MMP-9-expressing dendritic cells. Int. J. Cancer. 2014;134:2061–2073. doi: 10.1002/ijc.28549. [DOI] [PubMed] [Google Scholar]
- 231.Ratta M., Fagnoni F., Curti A., Vescovini R., Sansoni P., Oliviero B., Fogli M., Ferri E., Della Cuna G.R., Tura S., et al. Dendritic cells are functionally defective in multiple myeloma: The role of interleukin-6. Blood. 2002;100:230–237. doi: 10.1182/blood.V100.1.230. [DOI] [PubMed] [Google Scholar]
- 232.Chaux P., Favre N., Martin M., Martin F. Tumor-infiltrating dendritic cells are defective in their antigen-presenting function and inducible B7 expression in rats. Int. J. Cancer. 1997;72:619–624. doi: 10.1002/(SICI)1097-0215(19970807)72:4<619::AID-IJC12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 233.French J.D. Revisiting immune-based therapies for aggressive follicular cell-derived thyroid cancers. Thyroid. 2013;23:529–542. doi: 10.1089/thy.2012.0566. [DOI] [PubMed] [Google Scholar]
- 234.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat. Rev. Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 235.Hawiger D., Inaba K., Dorsett Y., Guo M., Mahnke K., Rivera M., Ravetch J.V., Steinman R.M., Nussenzweig M.C. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jonuleit H., Schmitt E., Schuler G., Knop J., Enk A.H. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kel J.M., Girard-Madoux M.J., Reizis B., Clausen B.E. TGF-beta is required to maintain the pool of immature Langerhans cells in the epidermis. J. Immunol. 2010;185:3248–3255. doi: 10.4049/jimmunol.1000981. [DOI] [PubMed] [Google Scholar]
- 238.Steinbrink K., Jonuleit H., Müller G., Schuler G., Knop J., Enk A.H. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8(+) T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634–1642. doi: 10.1182/blood.V93.5.1634. [DOI] [PubMed] [Google Scholar]
- 239.Yamazaki S., Inaba K., Tarbell K.V., Steinman R.M. Dendritic cells expand antigen-specific Foxp3+ CD25+ CD4+ regulatory T cells including suppressors of alloreactivity. Immunol. Rev. 2006;212:314–329. doi: 10.1111/j.0105-2896.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- 240.Oliveira-Neto H.H., Silva E.T., Leles C.R., Mendonça E.F., Alencar Rde C., Silva T.A., Batista A.C. Involvement of CXCL12 and CXCR4 in lymph node metastases and development of oral squamous cell carcinomas. Tumour Biol. 2008;29:262–271. doi: 10.1159/000152944. [DOI] [PubMed] [Google Scholar]
- 241.Parikh A., Shin J., Faquin W., Lin D.T., Tirosh I., Sunwoo J.B., Puram S.V. Malignant cell-specific CXCL14 promotes tumor lymphocyte infiltration in oral cavity squamous cell carcinoma. J. Immunother. Cancer. 2020;8:e001048. doi: 10.1136/jitc-2020-001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Thiel A., Pries R., Jeske S., Trenkle T., Wollenberg B. Effect of head and neck cancer supernatant and CpG-oligonucleotides on migration and IFN-alpha production of plasmacytoid dendritic cells. Anticancer Res. 2009;29:3019–3025. [PubMed] [Google Scholar]
- 243.Zhou B., Lawrence T., Liang Y. The Role of Plasmacytoid Dendritic Cells in Cancers. Front. Immunol. 2021;12:749190. doi: 10.3389/fimmu.2021.749190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Poropatich K., Dominguez D., Chan W.C., Andrade J., Zha Y., Wray B., Miska J., Qin L., Cole L., Coates S., et al. OX40+ plasmacytoid dendritic cells in the tumor microenvironment promote antitumor immunity. J. Clin. Investig. 2020;130:3528–3542. doi: 10.1172/JCI131992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Coffelt S.B., Hughes R., Lewis C.E. Tumor-associated macrophages: Effectors of angiogenesis and tumor progression. Biochim. Biophys. Acta. 2009;1796:11–18. doi: 10.1016/j.bbcan.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 246.Lamagna C., Aurrand-Lions M., Imhof B.A. Dual role of macrophages in tumor growth and angiogenesis. J. Leukoc. Biol. 2006;80:705–713. doi: 10.1189/jlb.1105656. [DOI] [PubMed] [Google Scholar]
- 247.Lewis C.E., Pollard J.W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 248.Sawa-Wejksza K., Kandefer-Szerszeń M. Tumor-Associated Macrophages as Target for Antitumor Therapy. Arch. Immunol. Ther. Exp. 2018;66:97–111. doi: 10.1007/s00005-017-0480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Franklin R.A., Liao W., Sarkar A., Kim M.V., Bivona M.R., Liu K., Pamer E.G., Li M.O. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Liss C., Fekete M.J., Hasina R., Lam C.D., Lingen M.W. Paracrine angiogenic loop between head-and-neck squamous-cell carcinomas and macrophages. Int. J. Cancer. 2001;93:781–785. doi: 10.1002/ijc.1407. [DOI] [PubMed] [Google Scholar]
- 251.Liss C., Fekete M.J., Hasina R., Lingen M.W. Retinoic acid modulates the ability of macrophages to participate in the induction of the angiogenic phenotype in head and neck squamous cell carcinoma. Int. J. Cancer. 2002;100:283–289. doi: 10.1002/ijc.10507. [DOI] [PubMed] [Google Scholar]
- 252.Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Qian B.Z., Li J., Zhang H., Kitamura T., Zhang J., Campion L.R., Kaiser E.A., Snyder L.A., Pollard J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ni Y.H., Ding L., Huang X.F., Dong Y.C., Hu Q.G., Hou Y.Y. Microlocalization of CD68+ tumor-associated macrophages in tumor stroma correlated with poor clinical outcomes in oral squamous cell carcinoma patients. Tumour Biol. 2015;36:5291–5298. doi: 10.1007/s13277-015-3189-5. [DOI] [PubMed] [Google Scholar]
- 255.Murray P.J., Allen J.E., Biswas S.K., Fisher E.A., Gilroy D.W., Goerdt S., Gordon S., Hamilton J.A., Ivashkiv L.B., Lawrence T., et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mantovani A., Biswas S.K., Galdiero M.R., Sica A., Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 257.Weber M., Büttner-Herold M., Hyckel P., Moebius P., Distel L., Ries J., Amann K., Neukam F.W., Wehrhan F. Small oral squamous cell carcinomas with nodal lymphogenic metastasis show increased infiltration of M2 polarized macrophages—An immunohistochemical analysis. J. Craniomaxillofac Surg. 2014;42:1087–1094. doi: 10.1016/j.jcms.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 258.Mantovani A., Sozzani S., Locati M., Allavena P., Sica A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 259.Costa N.L., Valadares M.C., Souza P.P., Mendonça E.F., Oliveira J.C., Silva T.A., Batista A.C. Tumor-associated macrophages and the profile of inflammatory cytokines in oral squamous cell carcinoma. Oral Oncol. 2013;49:216–223. doi: 10.1016/j.oraloncology.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 260.Marcus B., Arenberg D., Lee J., Kleer C., Chepeha D.B., Schmalbach C.E., Islam M., Paul S., Pan Q., Hanash S., et al. Prognostic factors in oral cavity and oropharyngeal squamous cell carcinoma. Cancer. 2004;101:2779–2787. doi: 10.1002/cncr.20701. [DOI] [PubMed] [Google Scholar]
- 261.Wolf G.T., Chepeha D.B., Bellile E., Nguyen A., Thomas D., McHugh J. Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: A preliminary study. Oral Oncol. 2015;51:90–95. doi: 10.1016/j.oraloncology.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Fujita Y., Okamoto M., Goda H., Tano T., Nakashiro K., Sugita A., Fujita T., Koido S., Homma S., Kawakami Y., et al. Prognostic significance of interleukin-8 and CD163-positive cell-infiltration in tumor tissues in patients with oral squamous cell carcinoma. PLoS ONE. 2014;9:e110378. doi: 10.1371/journal.pone.0110378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Joyce J.A., Pollard J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Laoui D., Movahedi K., Van Overmeire E., Van den Bossche J., Schouppe E., Mommer C., Nikolaou A., Morias Y., De Baetselier P., Van Ginderachter J.A. Tumor-associated macrophages in breast cancer: Distinct subsets, distinct functions. Int. J. Dev. Biol. 2011;55:861–867. doi: 10.1387/ijdb.113371dl. [DOI] [PubMed] [Google Scholar]
- 265.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 266.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Petruzzi M.N., Cherubini K., Salum F.G., de Figueiredo M.A. Role of tumour-associated macrophages in oral squamous cells carcinoma progression: An update on current knowledge. Diagn. Pathol. 2017;12:32. doi: 10.1186/s13000-017-0623-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Tymoszuk P., Evens H., Marzola V., Wachowicz K., Wasmer M.H., Datta S., Müller-Holzner E., Fiegl H., Böck G., van Rooijen N., et al. In situ proliferation contributes to accumulation of tumor-associated macrophages in spontaneous mammary tumors. Eur. J. Immunol. 2014;44:2247–2262. doi: 10.1002/eji.201344304. [DOI] [PubMed] [Google Scholar]
- 269.Van Overmeire E., Stijlemans B., Heymann F., Keirsse J., Morias Y., Elkrim Y., Brys L., Abels C., Lahmar Q., Ergen C., et al. M-CSF and GM-CSF Receptor Signaling Differentially Regulate Monocyte Maturation and Macrophage Polarization in the Tumor Microenvironment. Cancer Res. 2016;76:35–42. doi: 10.1158/0008-5472.CAN-15-0869. [DOI] [PubMed] [Google Scholar]
- 270.Barsoum I.B., Koti M., Siemens D.R., Graham C.H. Mechanisms of hypoxia-mediated immune escape in cancer. Cancer Res. 2014;74:7185–7190. doi: 10.1158/0008-5472.CAN-14-2598. [DOI] [PubMed] [Google Scholar]
- 271.Barsoum I.B., Smallwood C.A., Siemens D.R., Graham C.H. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-13-0992. [DOI] [PubMed] [Google Scholar]
- 272.Kai K., Moriyama M., Haque A., Hattori T., Chinju A., Hu C., Kubota K., Miyahara Y., Kakizoe-Ishiguro N., Kawano S., et al. Oral Squamous Cell Carcinoma Contributes to Differentiation of Monocyte-Derived Tumor-Associated Macrophages via PAI-1 and IL-8 Production. Int. J. Mol. Sci. 2021;22:9475. doi: 10.3390/ijms22179475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Pries R., Thiel A., Brocks C., Wollenberg B. Secretion of tumor-promoting and immune suppressive cytokines by cell lines of head and neck squamous cell carcinoma. In Vivo. 2006;20:45–48. [PubMed] [Google Scholar]
- 274.Squarize C.H., Castilho R.M., Sriuranpong V., Pinto D.S., Jr., Gutkind J.S. Molecular cross-talk between the NFkappaB and STAT3 signaling pathways in head and neck squamous cell carcinoma. Neoplasia. 2006;8:733–746. doi: 10.1593/neo.06274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Logullo A.F., Nonogaki S., Miguel R.E., Kowalski L.P., Nishimoto I.N., Pasini F.S., Federico M.H., Brentani R.R., Brentani M.M. Transforming growth factor beta1 (TGFbeta1) expression in head and neck squamous cell carcinoma patients as related to prognosis. J. Oral Pathol. Med. 2003;32:139–145. doi: 10.1034/j.1600-0714.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 276.Rosenthal E., McCrory A., Talbert M., Young G., Murphy-Ullrich J., Gladson C. Elevated expression of TGF-beta1 in head and neck cancer-associated fibroblasts. Mol. Carcinog. 2004;40:116–121. doi: 10.1002/mc.20024. [DOI] [PubMed] [Google Scholar]
- 277.Lewis C., Murdoch C. Macrophage responses to hypoxia: Implications for tumor progression and anti-cancer therapies. Am. J. Pathol. 2005;167:627–635. doi: 10.1016/S0002-9440(10)62038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Wennerberg E., Lhuillier C., Vanpouille-Box C., Pilones K.A., García-Martínez E., Rudqvist N.P., Formenti S.C., Demaria S. Barriers to Radiation-Induced In Situ Tumor Vaccination. Front. Immunol. 2017;8:229. doi: 10.3389/fimmu.2017.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Chen P., Zuo H., Xiong H., Kolar M.J., Chu Q., Saghatelian A., Siegwart D.J., Wan Y. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc. Natl. Acad. Sci. USA. 2017;114:580–585. doi: 10.1073/pnas.1614035114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Gallina G., Dolcetti L., Serafini P., De Santo C., Marigo I., Colombo M.P., Basso G., Brombacher F., Borrello I., Zanovello P., et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J. Clin. Investig. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Roth F., De La Fuente A.C., Vella J.L., Zoso A., Inverardi L., Serafini P. Aptamer-mediated blockade of IL4Rα triggers apoptosis of MDSCs and limits tumor progression. Cancer Res. 2012;72:1373–1383. doi: 10.1158/0008-5472.CAN-11-2772. [DOI] [PubMed] [Google Scholar]
- 282.Yang H., Zhang Q., Xu M., Wang L., Chen X., Feng Y., Li Y., Zhang X., Cui W., Jia X. CCL2-CCR2 axis recruits tumor associated macrophages to induce immune evasion through PD-1 signaling in esophageal carcinogenesis. Mol. Cancer. 2020;19:41. doi: 10.1186/s12943-020-01165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Willems J.J.L.P., Arnold B.P., Gregory C.D. Sinister Self-Sacrifice: The Contribution of Apoptosis to Malignancy. Front. Immunol. 2014;5:299. doi: 10.3389/fimmu.2014.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Siveen K.S., Kuttan G. Role of macrophages in tumour progression. Immunol. Lett. 2009;123:97–102. doi: 10.1016/j.imlet.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 285.Kouketsu A., Sato I., Oikawa M., Shimizu Y., Saito H., Tashiro K., Yamashita Y., Takahashi T., Kumamoto H. Regulatory T cells and M2-polarized tumour-associated macrophages are associated with the oncogenesis and progression of oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2019;48:1279–1288. doi: 10.1016/j.ijom.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 286.Laviron M., Boissonnas A. Ontogeny of Tumor-Associated Macrophages. Front. Immunol. 2019;10:1799. doi: 10.3389/fimmu.2019.01799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.El-Rouby D.H. Association of macrophages with angiogenesis in oral verrucous and squamous cell carcinomas. J. Oral Pathol. Med. 2010;39:559–564. doi: 10.1111/j.1600-0714.2010.00879.x. [DOI] [PubMed] [Google Scholar]
- 288.Qian B.Z., Pollard J.W. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Rodriguez P.C., Hernandez C.P., Quiceno D., Dubinett S.M., Zabaleta J., Ochoa J.B., Gilbert J., Ochoa A.C. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J. Exp. Med. 2005;202:931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Sica A., Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Takahashi H., Sakakura K., Kudo T., Toyoda M., Kaira K., Oyama T., Chikamatsu K. Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget. 2017;8:8633–8647. doi: 10.18632/oncotarget.14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Van Ginderachter J.A., Meerschaut S., Liu Y., Brys L., De Groeve K., Hassanzadeh Ghassabeh G., Raes G., De Baetselier P. Peroxisome proliferator-activated receptor gamma (PPARgamma) ligands reverse CTL suppression by alternatively activated (M2) macrophages in cancer. Blood. 2006;108:525–535. doi: 10.1182/blood-2005-09-3777. [DOI] [PubMed] [Google Scholar]
- 293.Vaupel P., Multhoff G. Hypoxia-/HIF-1α-Driven Factors of the Tumor Microenvironment Impeding Antitumor Immune Responses and Promoting Malignant Progression. Adv. Exp. Med. Biol. 2018;1072:171–175. doi: 10.1007/978-3-319-91287-5_27. [DOI] [PubMed] [Google Scholar]
- 294.Pang X., Tang Y.L., Liang X.H. Transforming growth factor-β signaling in head and neck squamous cell carcinoma: Insights into cellular responses. Oncol. Lett. 2018;16:4799–4806. doi: 10.3892/ol.2018.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Rückert M., Deloch L., Fietkau R., Frey B., Hecht M., Gaipl U.S. Immune modulatory effects of radiotherapy as basis for well-reasoned radioimmunotherapies. Strahlenther. Onkol. 2018;194:509–519. doi: 10.1007/s00066-018-1287-1. [DOI] [PubMed] [Google Scholar]
- 296.Larmonier N., Marron M., Zeng Y., Cantrell J., Romanoski A., Sepassi M., Thompson S., Chen X., Andreansky S., Katsanis E. Tumor-derived CD4(+)CD25(+) regulatory T cell suppression of dendritic cell function involves TGF-beta and IL-10. Cancer Immunol. Immunother. 2007;56:48–59. doi: 10.1007/s00262-006-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Egeblad M., Nakasone E.S., Werb Z. Tumors as organs: Complex tissues that interface with the entire organism. Dev. Cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Fridlender Z.G., Albelda S.M. Tumor-associated neutrophils: Friend or foe? Carcinogenesis. 2012;33:949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- 299.Fridlender Z.G., Sun J., Kim S., Kapoor V., Cheng G., Ling L., Worthen G.S., Albelda S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Kaffenberger W., Clasen B.P., van Beuningen D. The respiratory burst of neutrophils, a prognostic parameter in head and neck cancer? Clin. Immunol. Immunopathol. 1992;64:57–62. doi: 10.1016/0090-1229(92)90059-W. [DOI] [PubMed] [Google Scholar]
- 301.Gasparoto T.H., de Souza Malaspina T.S., Benevides L., de Melo E.J., Jr., Costa M.R., Damante J.H., Ikoma M.R., Garlet G.P., Cavassani K.A., da Silva J.S., et al. Patients with oral squamous cell carcinoma are characterized by increased frequency of suppressive regulatory T cells in the blood and tumor microenvironment. Cancer Immunol. Immunother. 2010;59:819–828. doi: 10.1007/s00262-009-0803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Maggioni D., Pignataro L., Garavello W. T-helper and T-regulatory cells modulation in head and neck squamous cell carcinoma. Oncoimmunology. 2017;6:e1325066. doi: 10.1080/2162402X.2017.1325066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Davis R.J., Van Waes C., Allen C.T. Overcoming barriers to effective immunotherapy: MDSCs, TAMs, and Tregs as mediators of the immunosuppressive microenvironment in head and neck cancer. Oral Oncol. 2016;58:59–70. doi: 10.1016/j.oraloncology.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Umansky V., Blattner C., Gebhardt C., Utikal J. The Role of Myeloid-Derived Suppressor Cells (MDSC) in Cancer Progression. Vaccines. 2016;4:36. doi: 10.3390/vaccines4040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.DeNardo D.G., Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019;19:369–382. doi: 10.1038/s41577-019-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Poh A.R., Ernst M. Targeting Macrophages in Cancer: From Bench to Bedside. Front. Oncol. 2018;8:49. doi: 10.3389/fonc.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Geiger R., Rieckmann J.C., Wolf T., Basso C., Feng Y., Fuhrer T., Kogadeeva M., Picotti P., Meissner F., Mann M., et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell. 2016;167:829–842.e813. doi: 10.1016/j.cell.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Vasquez-Dunddel D., Pan F., Zeng Q., Gorbounov M., Albesiano E., Fu J., Blosser R.L., Tam A.J., Bruno T., Zhang H., et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J. Clin. Investig. 2013;123:1580–1589. doi: 10.1172/JCI60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Hyman M.C., Petrovic-Djergovic D., Visovatti S.H., Liao H., Yanamadala S., Bouïs D., Su E.J., Lawrence D.A., Broekman M.J., Marcus A.J., et al. Self-regulation of inflammatory cell trafficking in mice by the leukocyte surface apyrase CD39. J. Clin. Investig. 2009;119:1136–1149. doi: 10.1172/JCI36433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Knapp K., Zebisch M., Pippel J., El-Tayeb A., Müller C.E., Sträter N. Crystal structure of the human ecto-5′-nucleotidase (CD73): Insights into the regulation of purinergic signaling. Structure. 2012;20:2161–2173. doi: 10.1016/j.str.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 312.Mandapathil M., Hilldorfer B., Szczepanski M.J., Czystowska M., Szajnik M., Ren J., Lang S., Jackson E.K., Gorelik E., Whiteside T.L. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J. Biol. Chem. 2010;285:7176–7186. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Sitkovsky M., Lukashev D., Deaglio S., Dwyer K., Robson S.C., Ohta A. Adenosine A2A receptor antagonists: Blockade of adenosinergic effects and T regulatory cells. Br. J. Pharmacol. 2008;153((Suppl. S1)):S457–S464. doi: 10.1038/bjp.2008.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.de Oliveira Bravo M., Carvalho J.L., Saldanha-Araujo F. Adenosine production: A common path for mesenchymal stem-cell and regulatory T-cell-mediated immunosuppression. Purinergic Signal. 2016;12:595–609. doi: 10.1007/s11302-016-9529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Ohta A., Kini R., Ohta A., Subramanian M., Madasu M., Sitkovsky M. The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front. Immunol. 2012;3:190. doi: 10.3389/fimmu.2012.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Molon B., Ugel S., Del Pozzo F., Soldani C., Zilio S., Avella D., De Palma A., Mauri P., Monegal A., Rescigno M., et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J. Exp. Med. 2011;208:1949–1962. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Yarosz E.L., Chang C.H. The Role of Reactive Oxygen Species in Regulating T Cell-mediated Immunity and Disease. Immune Netw. 2018;18:e14. doi: 10.4110/in.2018.18.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Frossi B., De Carli M., Piemonte M., Pucillo C. Oxidative microenvironment exerts an opposite regulatory effect on cytokine production by Th1 and Th2 cells. Mol. Immunol. 2008;45:58–64. doi: 10.1016/j.molimm.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 319.Kaminski M.M., Sauer S.W., Klemke C.D., Süss D., Okun J.G., Krammer P.H., Gülow K. Mitochondrial reactive oxygen species control T cell activation by regulating IL-2 and IL-4 expression: Mechanism of ciprofloxacin-mediated immunosuppression. J. Immunol. 2010;184:4827–4841. doi: 10.4049/jimmunol.0901662. [DOI] [PubMed] [Google Scholar]
- 320.Lyford-Pike S., Peng S., Young G.D., Taube J.M., Westra W.H., Akpeng B., Bruno T.C., Richmon J.D., Wang H., Bishop J.A., et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–1741. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Parry R.V., Chemnitz J.M., Frauwirth K.A., Lanfranco A.R., Braunstein I., Kobayashi S.V., Linsley P.S., Thompson C.B., Riley J.L. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Kubota K., Moriyama M., Furukawa S., Rafiul H., Maruse Y., Jinno T., Tanaka A., Ohta M., Ishiguro N., Yamauchi M., et al. CD163(+)CD204(+) tumor-associated macrophages contribute to T cell regulation via interleukin-10 and PD-L1 production in oral squamous cell carcinoma. Sci. Rep. 2017;7:1755. doi: 10.1038/s41598-017-01661-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Bergmann C., Strauss L., Wang Y., Szczepanski M.J., Lang S., Johnson J.T., Whiteside T.L. T regulatory type 1 cells in squamous cell carcinoma of the head and neck: Mechanisms of suppression and expansion in advanced disease. Clin. Cancer Res. 2008;14:3706–3715. doi: 10.1158/1078-0432.CCR-07-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Strauss L., Bergmann C., Szczepanski M., Gooding W., Johnson J.T., Whiteside T.L. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin. Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 325.Ralainirina N., Poli A., Michel T., Poos L., Andrès E., Hentges F., Zimmer J. Control of NK cell functions by CD4+CD25+ regulatory T cells. J. Leukoc. Biol. 2007;81:144–153. doi: 10.1189/jlb.0606409. [DOI] [PubMed] [Google Scholar]
- 326.Viguier M., Lemaître F., Verola O., Cho M.S., Gorochov G., Dubertret L., Bachelez H., Kourilsky P., Ferradini L. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J. Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 327.Valzasina B., Piconese S., Guiducci C., Colombo M.P. Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25- lymphocytes is thymus and proliferation independent. Cancer Res. 2006;66:4488–4495. doi: 10.1158/0008-5472.CAN-05-4217. [DOI] [PubMed] [Google Scholar]
- 328.Zhou G., Levitsky H.I. Natural regulatory T cells and de novo-induced regulatory T cells contribute independently to tumor-specific tolerance. J. Immunol. 2007;178:2155–2162. doi: 10.4049/jimmunol.178.4.2155. [DOI] [PubMed] [Google Scholar]
- 329.Jie H.B., Gildener-Leapman N., Li J., Srivastava R.M., Gibson S.P., Whiteside T.L., Ferris R.L. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br. J. Cancer. 2013;109:2629–2635. doi: 10.1038/bjc.2013.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Facciabene A., Peng X., Hagemann I.S., Balint K., Barchetti A., Wang L.P., Gimotty P.A., Gilks C.B., Lal P., Zhang L., et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 331.Lippitz B.E. Cytokine patterns in patients with cancer: A systematic review. Lancet Oncol. 2013;14:e218–e228. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- 332.Nishikawa H., Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr. Opin. Immunol. 2014;27:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 333.Zou L., Barnett B., Safah H., Larussa V.F., Evdemon-Hogan M., Mottram P., Wei S., David O., Curiel T.J., Zou W. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 334.Pallandre J.R., Brillard E., Créhange G., Radlovic A., Remy-Martin J.P., Saas P., Rohrlich P.S., Pivot X., Ling X., Tiberghien P., et al. Role of STAT3 in CD4+CD25+FOXP3+ regulatory lymphocyte generation: Implications in graft-versus-host disease and antitumor immunity. J. Immunol. 2007;179:7593–7604. doi: 10.4049/jimmunol.179.11.7593. [DOI] [PubMed] [Google Scholar]
- 335.Chen Y., Li Z., Liang J., Liu J., Hao J., Wan Q., Liu J., Luo C., Lu Z. CircRNA has_circ_0069313 induced OSCC immunity escape by miR-325-3p-Foxp3 axes in both OSCC cells and Treg cells. Aging. 2022;14:4376–4389. doi: 10.18632/aging.204068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Chikamatsu K., Sakakura K., Whiteside T.L., Furuya N. Relationships between regulatory T cells and CD8+ effector populations in patients with squamous cell carcinoma of the head and neck. Head. Neck. 2007;29:120–127. doi: 10.1002/hed.20490. [DOI] [PubMed] [Google Scholar]
- 337.Jarnicki A.G., Lysaght J., Todryk S., Mills K.H. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: Influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J. Immunol. 2006;177:896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- 338.Togashi Y., Shitara K., Nishikawa H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019;16:356–371. doi: 10.1038/s41571-019-0175-7. [DOI] [PubMed] [Google Scholar]
- 339.Mandapathil M., Szczepanski M.J., Szajnik M., Ren J., Lenzner D.E., Jackson E.K., Gorelik E., Lang S., Johnson J.T., Whiteside T.L. Increased ectonucleotidase expression and activity in regulatory T cells of patients with head and neck cancer. Clin. Cancer Res. 2009;15:6348–6357. doi: 10.1158/1078-0432.CCR-09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Perri F., Ionna F., Longo F., Della Vittoria Scarpati G., De Angelis C., Ottaiano A., Botti G., Caponigro F. Immune Response Against Head and Neck Cancer: Biological Mechanisms and Implication on Therapy. Transl. Oncol. 2020;13:262–274. doi: 10.1016/j.tranon.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Grosso J.F., Kelleher C.C., Harris T.J., Maris C.H., Hipkiss E.L., De Marzo A., Anders R., Netto G., Getnet D., Bruno T.C., et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J. Clin. Investig. 2007;117:3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Mishra A.K., Kadoishi T., Wang X., Driver E., Chen Z., Wang X.J., Wang J.H. Squamous cell carcinomas escape immune surveillance via inducing chronic activation and exhaustion of CD8+ T Cells co-expressing PD-1 and LAG-3 inhibitory receptors. Oncotarget. 2016;7:81341–81356. doi: 10.18632/oncotarget.13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Collison L.W., Chaturvedi V., Henderson A.L., Giacomin P.R., Guy C., Bankoti J., Finkelstein D., Forbes K., Workman C.J., Brown S.A., et al. IL-35-mediated induction of a potent regulatory T cell population. Nat. Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Collison L.W., Workman C.J., Kuo T.T., Boyd K., Wang Y., Vignali K.M., Cross R., Sehy D., Blumberg R.S., Vignali D.A.A. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 345.Olson B., Sullivan J., Burlingham W. Interleukin 35: A Key Mediator of Suppression and the Propagation of Infectious Tolerance. Front. Immunol. 2013;4:315. doi: 10.3389/fimmu.2013.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Olson B.M., Jankowska-Gan E., Becker J.T., Vignali D.A.A., Burlingham W.J., McNeel D.G. Human Prostate Tumor Antigen–Specific CD8+ Regulatory T Cells Are Inhibited by CTLA-4 or IL-35 Blockade. J. Immunol. 2012;189:5590–5601. doi: 10.4049/jimmunol.1201744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Schmidt A., Oberle N., Krammer P.H. Molecular mechanisms of treg-mediated T cell suppression. Front. Immunol. 2012;3:51. doi: 10.3389/fimmu.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 349.Banerjee H., Nieves-Rosado H., Kulkarni A., Murter B., McGrath K.V., Chandran U.R., Chang A., Szymczak-Workman A.L., Vujanovic L., Delgoffe G.M., et al. Expression of Tim-3 drives phenotypic and functional changes in Treg cells in secondary lymphoid organs and the tumor microenvironment. Cell Rep. 2021;36:109699. doi: 10.1016/j.celrep.2021.109699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Sheng C.C., Han F.Y. Immunoregulation effects of TIM-3 on tumors. Neoplasma. 2019;66:167–175. doi: 10.4149/neo_2018_180610N385. [DOI] [PubMed] [Google Scholar]
- 351.Yu J., Zhang H., Sun S., Sun S., Li L. The effects of Tim-3 activation on T-cells in gastric cancer progression. Oncol. Lett. 2019;17:1461–1466. doi: 10.3892/ol.2018.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Cao X., Cai S.F., Fehniger T.A., Song J., Collins L.I., Piwnica-Worms D.R., Ley T.J. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 353.Strauss L., Bergmann C., Whiteside T.L. Human circulating CD4+CD25highFoxp3+ regulatory T cells kill autologous CD8+ but not CD4+ responder cells by Fas-mediated apoptosis. J. Immunol. 2009;182:1469–1480. doi: 10.4049/jimmunol.182.3.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Kuropkat C., Duenne A.A., Herz U., Renz H., Werner J.A. Significant correlation of matrix metalloproteinase and macrophage colony-stimulating factor serum concentrations in patients with head and neck cancer. Neoplasma. 2004;51:375–378. [PubMed] [Google Scholar]
- 355.McDermott R.S., Deneux L., Mosseri V., Védrenne J., Clough K., Fourquet A., Rodriguez J., Cosset J.M., Sastre X., Beuzeboc P., et al. Circulating macrophage colony stimulating factor as a marker of tumour progression. Eur. Cytokine Netw. 2002;13:121–127. [PubMed] [Google Scholar]
- 356.Itoh S., Matsui K., Furuta I., Takano Y. Immunohistochemical study on overexpression of cyclooxygenase-2 in squamous cell carcinoma of the oral cavity: Its importance as a prognostic predictor. Oral Oncol. 2003;39:829–835. doi: 10.1016/S1368-8375(03)00105-2. [DOI] [PubMed] [Google Scholar]
- 357.Nathan C.O., Leskov I.L., Lin M., Abreo F.W., Shi R., Hartman G.H., Glass J. COX-2 expression in dysplasia of the head and neck: Correlation with elF4E. Cancer. 2001;92:1888–1895. doi: 10.1002/1097-0142(20011001)92:7<1888::AID-CNCR1706>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 358.Katoh H., Wang D., Daikoku T., Sun H., Dey S.K., Dubois R.N. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 2013;24:631–644. doi: 10.1016/j.ccr.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Zhu Y., Knolhoff B.L., Meyer M.A., Nywening T.M., West B.L., Luo J., Wang-Gillam A., Goedegebuure S.P., Linehan D.C., DeNardo D.G. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Hamilton J.A. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 361.Priceman S.J., Sung J.L., Shaposhnik Z., Burton J.B., Torres-Collado A.X., Moughon D.L., Johnson M., Lusis A.J., Cohen D.A., Iruela-Arispe M.L., et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: Combating tumor evasion of antiangiogenic therapy. Blood. 2010;115:1461–1471. doi: 10.1182/blood-2009-08-237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Xu J., Escamilla J., Mok S., David J., Priceman S., West B., Bollag G., McBride W., Wu L. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Serafini P. Editorial: PGE2-producing MDSC: A role in tumor progression? J. Leukoc. Biol. 2010;88:827–829. doi: 10.1189/jlb.0510303. [DOI] [PubMed] [Google Scholar]
- 364.Ostrand-Rosenberg S., Sinha P. Myeloid-derived suppressor cells: Linking inflammation and cancer. J. Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Serafini P., Meckel K., Kelso M., Noonan K., Califano J., Koch W., Dolcetti L., Bronte V., Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J. Exp. Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Whiteside T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Rapidis A.D., Wolf G.T. Immunotherapy of head and neck cancer: Current and future considerations. J. Oncol. 2009;2009:346345. doi: 10.1155/2009/346345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Braza M.S., Conde P., Garcia M., Cortegano I., Brahmachary M., Pothula V., Fay F., Boros P., Werner S.A., Ginhoux F., et al. Neutrophil derived CSF1 induces macrophage polarization and promotes transplantation tolerance. Am. J. Transplant. 2018;18:1247–1255. doi: 10.1111/ajt.14645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Condamine T., Dominguez G.A., Youn J.I., Kossenkov A.V., Mony S., Alicea-Torres K., Tcyganov E., Hashimoto A., Nefedova Y., Lin C., et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci. Immunol. 2016;1:aaf8943. doi: 10.1126/sciimmunol.aaf8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Ostrand-Rosenberg S. Immune surveillance: A balance between protumor and antitumor immunity. Curr. Opin. Genet. Dev. 2008;18:11–18. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Rodriguez P.C., Ernstoff M.S., Hernandez C., Atkins M., Zabaleta J., Sierra R., Ochoa A.C. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Rotondo R., Barisione G., Mastracci L., Grossi F., Orengo A.M., Costa R., Truini M., Fabbi M., Ferrini S., Barbieri O. IL-8 induces exocytosis of arginase 1 by neutrophil polymorphonuclears in nonsmall cell lung cancer. Int. J. Cancer. 2009;125:887–893. doi: 10.1002/ijc.24448. [DOI] [PubMed] [Google Scholar]
- 373.Wu G., Morris S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Pt 1Biochem. J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Yu J., Du W., Yan F., Wang Y., Li H., Cao S., Yu W., Shen C., Liu J., Ren X. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J. Immunol. 2013;190:3783–3797. doi: 10.4049/jimmunol.1201449. [DOI] [PubMed] [Google Scholar]
- 375.Noman M.Z., Desantis G., Janji B., Hasmim M., Karray S., Dessen P., Bronte V., Chouaib S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Weber R., Fleming V., Hu X., Nagibin V., Groth C., Altevogt P., Utikal J., Umansky V. Myeloid-Derived Suppressor Cells Hinder the Anti-Cancer Activity of Immune Checkpoint Inhibitors. Front. Immunol. 2018;9:1310. doi: 10.3389/fimmu.2018.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Bogdan C. Nitric oxide and the immune response. Nat. Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 378.Rosbe K.W., Prazma J., Petrusz P., Mims W., Ball S.S., Weissler M.C. Immunohistochemical characterization of nitric oxide synthase activity in squamous cell carcinoma of the head and neck. Otolaryngol. Head Neck Surg. 1995;113:541–549. doi: 10.1177/019459989511300504. [DOI] [PubMed] [Google Scholar]
- 379.Huang B., Pan P.Y., Li Q., Sato A.I., Levy D.E., Bromberg J., Divino C.M., Chen S.H. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 380.Sinha P., Clements V.K., Bunt S.K., Albelda S.M., Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J. Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 381.Srivastava M.K., Sinha P., Clements V.K., Rodriguez P., Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Greene S., Robbins Y., Mydlarz W.K., Huynh A.P., Schmitt N.C., Friedman J., Horn L.A., Palena C., Schlom J., Maeda D.Y., et al. Inhibition of MDSC Trafficking with SX-682, a CXCR1/2 Inhibitor, Enhances NK-Cell Immunotherapy in Head and Neck Cancer Models. Clin. Cancer Res. 2020;26:1420–1431. doi: 10.1158/1078-0432.CCR-19-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Pak A.S., Wright M.A., Matthews J.P., Collins S.L., Petruzzelli G.J., Young M.R. Mechanisms of immune suppression in patients with head and neck cancer: Presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin. Cancer Res. 1995;1:95–103. [PubMed] [Google Scholar]
- 384.Parker K.H., Beury D.W., Ostrand-Rosenberg S. Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Suppression in the Tumor Microenvironment. Adv. Cancer Res. 2015;128:95–139. doi: 10.1016/bs.acr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Rodriguez P.C., Quiceno D.G., Zabaleta J., Ortiz B., Zea A.H., Piazuelo M.B., Delgado A., Correa P., Brayer J., Sotomayor E.M., et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 386.Rodriguez P.C., Quiceno D.G., Ochoa A.C. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Harari O., Liao J.K. Inhibition of MHC II gene transcription by nitric oxide and antioxidants. Curr. Pharm. Des. 2004;10:893–898. doi: 10.2174/1381612043452893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Stiff A., Trikha P., Mundy-Bosse B., McMichael E., Mace T.A., Benner B., Kendra K., Campbell A., Gautam S., Abood D., et al. Nitric Oxide Production by Myeloid-Derived Suppressor Cells Plays a Role in Impairing Fc Receptor-Mediated Natural Killer Cell Function. Clin. Cancer Res. 2018;24:1891–1904. doi: 10.1158/1078-0432.CCR-17-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Mazzoni A., Bronte V., Visintin A., Spitzer J.H., Apolloni E., Serafini P., Zanovello P., Segal D.M. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 2002;168:689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 390.Bingisser R.M., Tilbrook P.A., Holt P.G., Kees U.R. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J. Immunol. 1998;160:5729–5734. doi: 10.4049/jimmunol.160.12.5729. [DOI] [PubMed] [Google Scholar]
- 391.Liu C., Yu S., Kappes J., Wang J., Grizzle W.E., Zinn K.R., Zhang H.G. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109:4336–4342. doi: 10.1182/blood-2006-09-046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Bentz B.G., Haines G.K., 3rd, Radosevich J.A. Increased protein nitrosylation in head and neck squamous cell carcinogenesis. Head. Neck. 2000;22:64–70. doi: 10.1002/(SICI)1097-0347(200001)22:1<64::AID-HED10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 393.Bronte V., Serafini P., De Santo C., Marigo I., Tosello V., Mazzoni A., Segal D.M., Staib C., Lowel M., Sutter G., et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J. Immunol. 2003;170:270–278. doi: 10.4049/jimmunol.170.1.270. [DOI] [PubMed] [Google Scholar]
- 394.Nagaraj S., Gupta K., Pisarev V., Kinarsky L., Sherman S., Kang L., Herber D.L., Schneck J., Gabrilovich D.I. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Nagaraj S., Schrum A.G., Cho H.I., Celis E., Gabrilovich D.I. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J. Immunol. 2010;184:3106–3116. doi: 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Trellakis S., Bruderek K., Dumitru C.A., Gholaman H., Gu X., Bankfalvi A., Scherag A., Hütte J., Dominas N., Lehnerdt G.F., et al. Polymorphonuclear granulocytes in human head and neck cancer: Enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int. J. Cancer. 2011;129:2183–2193. doi: 10.1002/ijc.25892. [DOI] [PubMed] [Google Scholar]
- 397.Haqqani A.S., Sandhu J.K., Birnboim H.C. Expression of interleukin-8 promotes neutrophil infiltration and genetic instability in mutatect tumors. Neoplasia. 2000;2:561–568. doi: 10.1038/sj.neo.7900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Castell S.D., Harman M.F., Morón G., Maletto B.A., Pistoresi-Palencia M.C. Neutrophils Which Migrate to Lymph Nodes Modulate CD4(+) T Cell Response by a PD-L1 Dependent Mechanism. Front. Immunol. 2019;10:105. doi: 10.3389/fimmu.2019.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Sun R., Xiong Y., Liu H., Gao C., Su L., Weng J., Yuan X., Zhang D., Feng J. Tumor-associated neutrophils suppress antitumor immunity of NK cells through the PD-L1/PD-1 axis. Transl. Oncol. 2020;13:100825. doi: 10.1016/j.tranon.2020.100825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Michaeli J., Shaul M.E., Mishalian I., Hovav A.H., Levy L., Zolotriov L., Granot Z., Fridlender Z.G. Tumor-associated neutrophils induce apoptosis of non-activated CD8 T-cells in a TNFα and NO-dependent mechanism, promoting a tumor-supportive environment. Oncoimmunology. 2017;6:e1356965. doi: 10.1080/2162402X.2017.1356965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Tecchio C., Cassatella M.A. Neutrophil-derived chemokines on the road to immunity. Semin. Immunol. 2016;28:119–128. doi: 10.1016/j.smim.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Odobasic D., Kitching A.R., Yang Y., O’Sullivan K.M., Muljadi R.C., Edgtton K.L., Tan D.S., Summers S.A., Morand E.F., Holdsworth S.R. Neutrophil myeloperoxidase regulates T-cell-driven tissue inflammation in mice by inhibiting dendritic cell function. Blood. 2013;121:4195–4204. doi: 10.1182/blood-2012-09-456483. [DOI] [PubMed] [Google Scholar]
- 403.Monteran L., Erez N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front. Immunol. 2019;10:1835. doi: 10.3389/fimmu.2019.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.New J., Arnold L., Ananth M., Alvi S., Thornton M., Werner L., Tawfik O., Dai H., Shnayder Y., Kakarala K., et al. Secretory Autophagy in Cancer-Associated Fibroblasts Promotes Head and Neck Cancer Progression and Offers a Novel Therapeutic Target. Cancer Res. 2017;77:6679–6691. doi: 10.1158/0008-5472.CAN-17-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Yu B., Wu K., Wang X., Zhang J., Wang L., Jiang Y., Zhu X., Chen W., Yan M. Periostin secreted by cancer-associated fibroblasts promotes cancer stemness in head and neck cancer by activating protein tyrosine kinase 7. Cell Death Dis. 2018;9:1082. doi: 10.1038/s41419-018-1116-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 406.Kratochwil C., Flechsig P., Lindner T., Abderrahim L., Altmann A., Mier W., Adeberg S., Rathke H., Röhrich M., Winter H., et al. (68)Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019;60:801–805. doi: 10.2967/jnumed.119.227967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Gleich L.L., Srivastava L., Gluckman J.L. Plasma Platelet-Derived Growth Factor: Preliminary Study of a Potential Marker in Head and Neck Cancer. Ann. Otol. Rhinol. Laryngol. 1996;105:710–712. doi: 10.1177/000348949610500907. [DOI] [PubMed] [Google Scholar]
- 408.Liu T., Zhou L., Li D., Andl T., Zhang Y. Cancer-Associated Fibroblasts Build and Secure the Tumor Microenvironment. Front. Cell Dev. Biol. 2019;7:60. doi: 10.3389/fcell.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Qin X., Yan M., Wang X., Xu Q., Wang X., Zhu X., Shi J., Li Z., Zhang J., Chen W. Cancer-associated Fibroblast-derived IL-6 Promotes Head and Neck Cancer Progression via the Osteopontin-NF-kappa B Signaling Pathway. Theranostics. 2018;8:921–940. doi: 10.7150/thno.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Bae J.Y., Kim E.K., Yang D.H., Zhang X., Park Y.J., Lee D.Y., Che C.M., Kim J. Reciprocal interaction between carcinoma-associated fibroblasts and squamous carcinoma cells through interleukin-1α induces cancer progression. Neoplasia. 2014;16:928–938. doi: 10.1016/j.neo.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Bello I.O., Vered M., Dayan D., Dobriyan A., Yahalom R., Alanen K., Nieminen P., Kantola S., Läärä E., Salo T. Cancer-associated fibroblasts, a parameter of the tumor microenvironment, overcomes carcinoma-associated parameters in the prognosis of patients with mobile tongue cancer. Oral Oncol. 2011;47:33–38. doi: 10.1016/j.oraloncology.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 412.Lotti F., Jarrar A.M., Pai R.K., Hitomi M., Lathia J., Mace A., Gantt G.A., Jr., Sukhdeo K., DeVecchio J., Vasanji A., et al. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. J. Exp. Med. 2013;210:2851–2872. doi: 10.1084/jem.20131195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Takahashi H., Sakakura K., Kawabata-Iwakawa R., Rokudai S., Toyoda M., Nishiyama M., Chikamatsu K. Immunosuppressive activity of cancer-associated fibroblasts in head and neck squamous cell carcinoma. Cancer Immunol. Immunother. 2015;64:1407–1417. doi: 10.1007/s00262-015-1742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Fearon D.T. The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol. Res. 2014;2:187–193. doi: 10.1158/2326-6066.CIR-14-0002. [DOI] [PubMed] [Google Scholar]
- 415.Feig C., Jones J.O., Kraman M., Wells R.J., Deonarine A., Chan D.S., Connell C.M., Roberts E.W., Zhao Q., Caballero O.L., et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Li X., Bu W., Meng L., Liu X., Wang S., Jiang L., Ren M., Fan Y., Sun H. CXCL12/CXCR4 pathway orchestrates CSC-like properties by CAF recruited tumor associated macrophage in OSCC. Exp. Cell Res. 2019;378:131–138. doi: 10.1016/j.yexcr.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 417.Song M., He J., Pan Q.Z., Yang J., Zhao J., Zhang Y.J., Huang Y., Tang Y., Wang Q., He J., et al. Cancer-Associated Fibroblast-Mediated Cellular Crosstalk Supports Hepatocellular Carcinoma Progression. Hepatology. 2021;73:1717–1735. doi: 10.1002/hep.31792. [DOI] [PubMed] [Google Scholar]
- 418.Cheng Y., Li H., Deng Y., Tai Y., Zeng K., Zhang Y., Liu W., Zhang Q., Yang Y. Cancer-associated fibroblasts induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 2018;9:422. doi: 10.1038/s41419-018-0458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Huang T.X., Fu L. The immune landscape of esophageal cancer. Cancer Commun. 2019;39:79. doi: 10.1186/s40880-019-0427-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Jenkins R.W., Barbie D.A., Flaherty K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer. 2018;118:9–16. doi: 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Karasarides M., Cogdill A.P., Robbins P.B., Bowden M., Burton E.M., Butterfield L.H., Cesano A., Hammer C., Haymaker C.L., Horak C.E., et al. Hallmarks of Resistance to Immune-Checkpoint Inhibitors. Cancer Immunol. Res. 2022;10:372–383. doi: 10.1158/2326-6066.CIR-20-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Marei H.E., Hasan A., Pozzoli G., Cenciarelli C. Cancer immunotherapy with immune checkpoint inhibitors (ICIs): Potential, mechanisms of resistance, and strategies for reinvigorating T cell responsiveness when resistance is acquired. Cancer Cell Int. 2023;23:64. doi: 10.1186/s12935-023-02902-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Wang B., Han Y., Zhang Y., Zhao Q., Wang H., Wei J., Meng L., Xin Y., Jiang X. Overcoming acquired resistance to cancer immune checkpoint therapy: Potential strategies based on molecular mechanisms. Cell Biosci. 2023;13:120. doi: 10.1186/s13578-023-01073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.