Abstract
We have previously shown that prophylactic administration of the liposome-encapsulated immunomodulating agents muramyl tripeptide phosphatidylethanolamine (MTPPE) and gamma interferon (IFN-γ) results in strongly increased survival of mice from a normally lethal septicemia with Klebsiella pneumoniae. It was anticipated that the treatment acts on macrophages and nonspecifically augments host resistance to various infections. In the present study, we provide evidence for a key role for T cells in host defense potentiation by the liposomal immunomodulators toward K. pneumoniae septicemia. It is shown that both CD4 and CD8 cells are important in immunomodulation, most likely due to production of IFN-γ. Depletion of circulating IFN-γ resulted in strong reduction of the antimicrobial host defense activation. Administration of interleukin-10 resulted in decreased antimicrobial host defense activation by liposomal immunomodulators. Moreover, administration of liposomal immunomodulators was shown to induce predominantly T-helper type 1 (Th1) cell populations in the spleen. These findings indicate that immunomodulation with liposomal MTPPE and IFN-γ favors Th1 and NK cell activation.
In several models of infection or tumor therapy, nonspecific stimulation of the host defense system has been convincingly shown to be effective. We have previously demonstrated that prophylactic treatment of mice exhibiting a lethal Klebsiella pneumoniae septicemia with liposome-encapsulated muramyl tripeptide phosphatidylethanolamine (LE-MTPPE) or gamma interferon (LE-IFN-γ), or both immunomodulators coencapsulated in liposomes (LE-MTPPE/IFN-γ), could substantially augment survival (25). As the liposomes used in these studies are taken up primarily by cells of the mononuclear phagocyte system (MPS), it is suggested that these cells are the primary target for the liposomal immunomodulators (15). Macrophages were shown to exhibit intracellular receptors for both immunomodulators (9, 14, 24), and we demonstrated that these cells are of major importance in the observed host defense potentiation with LE-MTPPE in vivo (16).
Stimulation of purified macrophages in vitro with LE-MTPPE, LE-IFN-γ, or LE-MTPPE/IFN-γ resulted in enhanced production of oxygen and nitrogen metabolites but not in increased phagocytic activity when K. pneumoniae was added to the cells (25). These data suggest that activation of MPS cells is not sufficient to obtain effective killing of this microorganism. It appeared that these cells lacked important costimulating signals in vitro. Therefore, it is hypothesized that T cells are involved in activation of macrophage or MPS as a whole. This view is supported by studies with mice treated with cyclosporin A before treatment with liposome-encapsulated immunomodulators. Cyclosporin A pretreatment resulted in a dramatic reduction of antimicrobial host defense potentiation toward K. pneumoniae infection induced by immunomodulators (27): the 100% survival rate effected by LE-MTPPE/IFN-γ was reduced to 0%.
In this work, the contribution of T cells and their cytokines to the efficacy of immunomodulation with LE-MTPPE, LE-IFN-γ, or LE-MTPPE/IFN-γ to K. pneumoniae septicemia was studied in vivo. To this end, mice were depleted of T cells or T-cell subsets, or the production of specific cytokines (e.g., IFN-γ and tumor necrosis factor alpha [TNF-α]) was modulated by treatment with neutralizing antibodies. Here we demonstrate, for the first time, an important role for T cells, and in particular T-helper type 1 (Th1) cells, in nonspecific immunomodulation.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free female C57BL/Ka mice, 11 to 13 weeks of age (ITRI-TNO, Rijswijk, The Netherlands), were used.
Bacteria.
K. pneumoniae capsular serotype 2 (ATCC 43816) was grown for 16 h at 37°C in Todd-Hewitt broth (Oxoid Ltd., Basingstoke, England). The bacteria were preserved on ice and washed three times in phosphate-buffered saline (PBS) directly before use. The 50% lethal dose in C57BL/Ka mice was 2 × 102 bacteria after intraperitoneal (i.p.) injection.
Reagents.
LE-MTPPE was kindly provided by Ciba-Geigy (Basel, Switzerland). Placebo liposomes (PL) were prepared from a dry lyophilysate composed of phosphatidylcholine and phosphatidylserine in a molar ratio of 7:3 as previously described (25). Recombinant murine IFN-γ was derived from supernatant fluid of a CHO cell line that carries and expresses an amplified murine IFN-γ cDNA (8). Murine IFN-γ was used at a concentration of 2.5 × 105 U/ml. IFN-γ was purified by affinity chromatography on an anti-rat IFN-γ monoclonal antibody (MAb DB-1) to a specific activity of 6 × 106 U/mg. Liposomes containing MTPPE, IFN-γ, and MTPPE plus IFN-γ were prepared as described previously (25).
Treatment of mice with immunomodulators.
Mice were injected iv with LE-MTPPE, LE-IFN-γ, or LE-MTPPE/IFN-γ as previously described (25). Briefly, mice received a total of five injections, at 48-h intervals, of 25 μg of LE-MTPPE, 7,500 U of LE-IFN-γ, or 25 μg of MTPPE and 7,500 U IFN-γ coencapsulated in 6.25 mg of lipid. Twelve hours after the last dose, mice were infected i.p. with K. pneumoniae or mock infected with PBS.
Depletion of T cells and T-cell subsets.
Mice were depleted of T cells by a single i.p. injection of 100 μg of 17A2, a mouse CD3-specific rat immunoglobulin G2b (IgG2b) MAb (17), at 24 h before the first dose with immunomodulator. Depletion of CD4- or CD8-positive cells was obtained by three i.p. injections of 100 μg of YTS-191 or YTS-169, a CD4- or CD8-specific rat IgG2b MAb, respectively (5). These antibodies were administered at 72, 48, and 24 h before the first dose with immunomodulator. Depletion of T cells was evaluated in the blood and spleen by flow cytometry after staining with anti-CD3 MAb KT3 (28). Flow cytometry demonstrated a more than 85% depletion of the T-cell fractions in blood 1 day after injection of the T-cell-specific antibodies. T cells only slowly returned thereafter. Also in spleen, a pronounced depletion with 17A2 was obtained, with a maximum of 60% at 5 days after injection. Depletion of CD4- or CD8-positive cells in spleen was even more pronounced, with maximum levels of depletion of 85 and 95%, respectively. Controls for injection of IgG2b MAb were injected with equal amounts of a rat IgG2b anti-β-galactosidase MAb (PH2-49; a kind gift of W. Van Ewijk, Department of Immunology, Erasmus University, Rotterdam, The Netherlands).
Depletion of endogenous IFN-γ.
Endogenous IFN-γ was depleted by two i.p. injections of 100 μg of F3, a mouse IFN-γ-specific rat IgG2a MAb (11), at 48 h before the first dose and 12 h after the last dose with immunomodulator. This dose and treatment scheme was shown to neutralize all bioactive IFN-γ in vivo. F3 was given as plain ascites fluid (in vitro neutralizing titer of 105 against 30 U of murine IFN-γ per ml; rat Ig content, 3.5 mg/ml). Side effects induced by injections with IgG2a antibody F3 were evaluated by using a control GL117 rat IgG2a anti-β-galactosidase MAb (obtained by the courtesy of J. Abrams, DNAX Research Institute, Palo Alto, Calif.) in control ascites fluid at an equal dose.
Administration of IL-10.
Mice were injected i.p. 24 h before the first dose of immunomodulator with 2 × 106 murine interleukin-10 (IL-10) cDNA-transfected J558 cells encapsulated in alginate as previously described, resulting in elevated cytokine levels in vivo (23). This cell dose has been widely shown for several cytokine-transfected cell lines to be effective in vivo. Side effects of alginate–IL-10 injections were evaluated in mock-transfected J558 cells encapsulated in alginate. These cell lines were the kind gift of K. Moore (DNAX Research Institute).
Depletion of TNF-α.
Endogenous TNF-α was depleted by a single i.p. injection, at 48 h before first dose of immunomodulator, of an optimally titrated dose of 300 μg of TNF-α-specific rabbit IgG isolated from hyperimmune serum. Controls were injected with the same amount of rabbit IgG isolated from normal serum. The rabbit anti-murine TNF-α was obtained by immunizing rabbits with recombinant murine TNF-α. The capacity to inhibit the biological activity of recombinant TNF-α was ascertained by assessing its capacity to inhibit the cytolytic effect of murine TNF-α by purified immune rabbit IgG. The antibodies did not inhibit the biological activity of murine TNF-β.
Experimental infection with K. pneumoniae.
Acquired septicemia was induced by i.p. inoculation of 103 CFU of K. pneumoniae in mice as described previously (25). In this model, multiplication of bacteria resulted in delayed but continuous appearance of bacteria in the blood, eventually leading to septicemia and resulting in death. Mice were monitored daily for survival until 28 days after bacterial inoculation. All mice that died during the experiment were examined for the presence of K. pneumoniae in liver and blood. Each group contained 20 mice.
Evaluation of major histocompatibility complex class II (Ia) antigen expression of spleen macrophages by flow cytometry.
At 12 h and 4 days after the end of treatment, spleens were excised, treated with collagenase, and minced into cell suspensions as described elsewhere (26). Cells were stained with MAb M5/114 (13) directed against Ia antigen, and expression was measured by flow cytometry on a FACScan (Becton Dickinson, San Jose, Calif.). During analysis, lymphocytes were gated out. Expression was calculated as molecules equivalent to soluble fluorescein isothiocyanate as described by Leenen et al. (13). Three mice were used per treatment.
Examination of activity of Th1 and Th2 cells in spleen after immunomodulation.
T-cell pulse-chase experiments were performed as described previously (3). Briefly, to examine the effect of immunomodulation on the activity of Th1 and Th2 cells, spleens were excised from treated mice at 12 h and 4 days after end of treatment. The spleens were minced into a cell suspension, washed, resuspended at 200,000 cells per well, and cultured on anti-CD3 MAb-coated 96-well culture plates. Cells were stimulated with medium or IL-2 (200 U/ml) alone (controls), with IL-2 (200 U/ml) and IFN-γ (400 U/ml) accompanied by anti-IL-4 MAb 11B11 (3) (40 μg/ml) (Th1 pulse), or with IL-2 (200 U/ml) and IL-4 (140 ng/ml) accompanied by anti-IFN-γ MAb XMG1.2 (4) (20 μg/ml) (Th2 pulse). After 4 days of culture at 37°C, cells were washed two times, transferred to 96-well plates freshly coated with anti-CD3 MAb, and chased with IL-2 (100 U/ml) to induce production of cytokines. After 4 days of culture at 37°C, supernatants were harvested and IFN-γ and IL-4 levels were measured by sandwich enzyme-linked immunosorbent assay as described previously (3, 4).
Statistical analysis.
Differences in survival curves among groups of mice were evaluated by log rank test. P values below 0.05 were considered significant.
RESULTS
Effect of depletion of T cells on augmentation of antimicrobial host defense by liposomal immunomodulators.
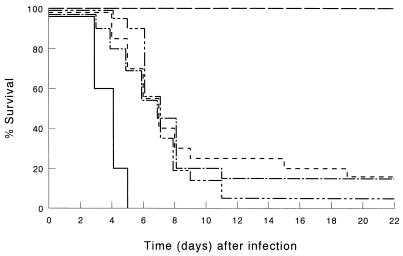
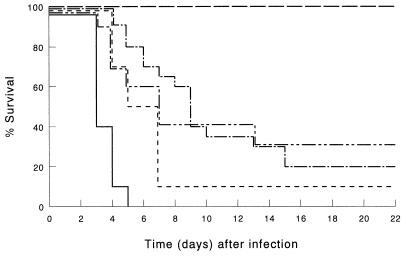
It was shown before that treatment of immunocompetent mice with LE-MTPPE or LE-IFN-γ resulted in a dramatic increase in host defense; 65% of the mice survived, whereas all control mice, treated with PL, died within 5 days after inoculation; treatment with immunomodulators combined by coencapsulation enhanced survival to 100% (25). Control experiments performed during the present study also demonstrated that LE-MTPPE (n = 9) or LE-IFN-γ (n = 9) treatment resulted in 65 or 60% survival, respectively, 21 days after infection (data not shown). Confirmatory experiments on treatment with LE-MTPPE/IFN-γ performed in this study also demonstrated 100% survival of the mice (Fig. 1). Depletion of T cells with anti-CD3 MAb 17A2 dramatically reduced the potency of the immunomodulators (Fig. 1). In LE-MTPPE/IFN-γ-treated mice, survival decreased from 100% in immunocompetent mice to 15% in T-cell-depleted mice (P < 0.001 compared with the immunocompetent controls). Survival of LE-MTPPE-treated mice or LE-IFN-γ-treated mice was reduced from 65% in immunocompetent mice to 15 or 5%, respectively, in T-cell-depleted mice. Because LE-MTPPE/IFN-γ treatment is superior to treatment with single immunomodulators, the remainder of this study focuses on the combination.
FIG. 1.
Survival of T-cell-depleted mice infected by i.p. inoculation of 103 CFU of K. pneumoniae. T cells were depleted by injection of CD3-specific IgG2b MAb. Mice were treated before infection with LE-MTPPE/IFN-γ (——–), LE-MTPPE (––––), LE-IFN-γ (—––), or PL (———). Control mice were injected with rat IgG2b anti-β-galactosidase MAb and treated with LE-MTPPE/IFN-γ (——). Each group contained 20 mice.
Differentiation between contribution of CD4- and CD8-positive cells in augmentation of antimicrobial host defense by liposomal immunomodulators.
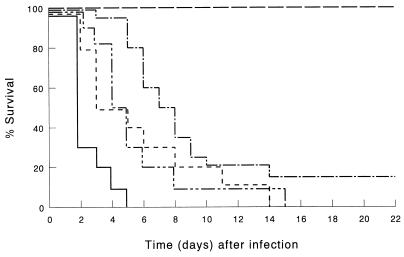
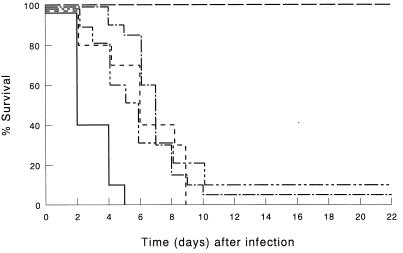
Next we investigated which subset of T cells is of major importance in the observed reduction induced by anti-CD3 MAb 17A2 in host defense potentiation after immunomodulation. To this end, mice were depleted of CD4- or CD8-positive cells by using published dosages and treatment schemes (28). Depletion of CD4+ cells resulted in a dramatic decrease of host defense potentiation by immunomodulators compared with immunocompetent controls (P < 0.001) (Fig. 2). In mice treated with LE-MTPPE/IFN-γ, survival was reduced from 100 to 15%. Also, depletion of CD8+ cells resulted in a dramatic decrease of host defense potentiation by immunomodulators (P < 0.001) (Fig. 3). In mice treated with LE-MTPPE/IFN-γ, survival was reduced from 100% in immunocompetent to 5% in CD8-depleted mice. Depletion of CD3+ T cells as well as depletion of CD4 or CD8 subsets of T cells had no significant influence on survival of mice treated with PL. In mice treated with LE-MTPPE or LE-IFN-γ depletion of CD4+ or CD8+ cells also resulted in a dramatic reduction of survival (P < 0.001 for both [Fig. 2 and 3, respectively]). Taken together, these results indicate that both CD4+ and CD8+ T cells are of importance in activation of the host defense by liposomal immunomodulators.
FIG. 2.
Survival of CD4+ cell-depleted mice infected by i.p. inoculation of 103 CFU of K. pneumoniae. CD4+ cells were depleted by injection of CD4-specific IgG2b MAb. Mice were treated before infection with LE-MTPPE/IFN-γ (——–), LE-MTPPE (––––), LE-IFN-γ (—––), or PL (——). Control mice were injected with rat IgG2b anti-β-galactosidase MAb and treated with LE-MTPPE/IFN-γ (——). Each group contained 20 mice.
FIG. 3.
Survival of CD8+ cell-depleted mice infected by i.p. inoculation of 103 CFU of K. pneumoniae. CD8+ cells were depleted by injection of CD8-specific IgG2b MAb. Mice were treated before infection with LE-MTPPE/IFN-γ (—–), LE-MTPPE (––––), LE-IFN-γ (——––), or PL (——). Control mice were injected with rat IgG2a anti-β-galactosidase MAb and treated with LE-MTPPE/IFN-γ (——). Each group contained 20 mice.
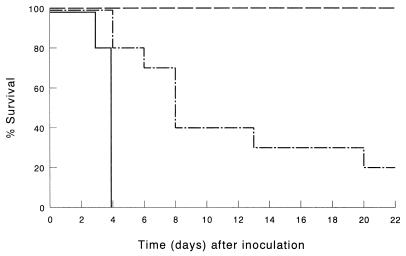
Effect of depletion of endogenous IFN-γ on augmentation of antimicrobial host defense by liposomal immunomodulators.
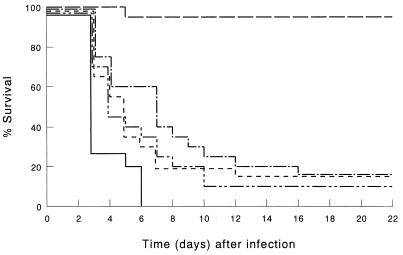
Depletion of endogenous IFN-γ by treatment with MAb F3 resulted in a diminished host defense augmentation by the immunomodulators compared with immunocompetent mice (P < 0.001) (Fig. 4). In LE-MTPPE/IFN-γ-treated mice, depletion of endogenous IFN-γ resulted in a decline of survival from 100 to 15%. Mice treated with LE-MTPPE or LE-IFN-γ showed a more rapid decline in survival by IFN-γ depletion, resulting in survival of 15 or 10%, respectively.
FIG. 4.
Survival of mice depleted of endogenous IFN-γ and infected by i.p. inoculation of 103 CFU of K. pneumoniae. IFN-γ was depleted by injection of IFN-γ-specific IgG2a MAb. Mice were treated before infection with LE-MTPPE/IFN-γ (——–), LE-MTPPE (––––), LE-IFN-γ (——––), or PL (——). Control mice were injected with rat IgG2a anti-β-galactosidase MAb and treated with LE-MTPPE/IFN-γ (——). Each group contained 20 mice.
Effect of administration of IL-10 on augmentation of antimicrobial host defense by liposomal immunomodulators.
Also IL-10, produced by i.p.-implanted alginate-encapsulated IL-10-transfected cells in mice treated with LE-MTPPE/IFN-γ, induced a decreased host defense: survival of 20% in IL-10-treated mice, compared with 100% in immunocompetent LE-MTPPE/IFN-γ-treated mice (P < 0.001) (Fig. 5). Administration of IL-10-producing cells in mice treated with LE-IFN-γ resulted in a comparable decreased survival, whereas a more rapid effect was seen in LE-MTPPE-treated mice.
FIG. 5.
Survival of mice injected i.p. with IL-10-producing cells in alginate and infected by i.p. inoculation of 103 CFU of K. pneumoniae. Mice were treated before infection with LE-MTPPE/IFN-γ (——–), LE-MTPPE (––––), LE-IFN-γ (——–), or PL (——). Control mice were injected with mock-transfected alginate-encapsulated cells and treated with LE-MTPPE/IFN-γ (——). Each group contained 20 mice.
Effect of depletion of endogenous TNF-α on augmentation of antimicrobial host defense by liposomal immunomodulators.
Administration of TNF-α-specific rabbit IgG to LE-MTPPE/IFN-γ-treated mice resulted in reduced survival of mice after infection with K. pneumoniae (P < 0.001), indicating that stimulation of TNF-α production by macrophages and/or T cells is also of importance in the described immunomodulation (Fig. 6).
FIG. 6.
Survival of mice depleted of endogenous TNF-α and infected by i.p. inoculation of 103 CFU of K. pneumoniae. TNF-α was depleted by injection of TNF-α-specific polyclonal rabbit IgG. Mice were treated before infection with LE-MTPPE/IFN-γ (——–) or PL (——). Control mice were injected with an equal amount of rabbit IgG and treated with LE-MTPPE/IFN-γ (——). Each group contained 20 mice.
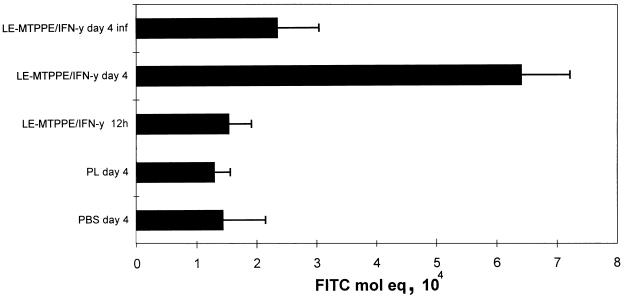
Effect of immunomodulation on Ia antigen expression of splenic macrophages.
The spleen is an important organ in the communication between macrophages and T cells, and a large proportion of the liposomal immunomodulator localizes in the spleen. Moreover, macrophages are considered to be the targets for immunomodulation, and the liposomes used target mainly these cells. We therefore examined macrophage activation after immunomodulation in the spleen. Treatment of mice with LE-MTPPE/IFN-γ resulted in enhanced expression of Ia antigen at 4 days after the end of treatment (Fig. 7). In addition, complement receptor 3 antigen expression was enhanced upon treatment with immunomodulator at 12 h after the end of treatment (data not shown).
FIG. 7.
Ia antigen expression on spleen cells measured by flow cytometry in mice treated with LE-MTPPE/IFN-γ, PL, or PBS. One group of mice was also infected by i.p. inoculation of 103 CFU of K. pneumoniae (LE-MTPPE/IFN-γ day 4 inf) 12 h after the end of treatment. Spleen cells were examined 12 h and 4 days (day 4) after the end of treatment. Each bar represents the average + standard deviation of three mice. FITC, fluorescein isothiocyanate.
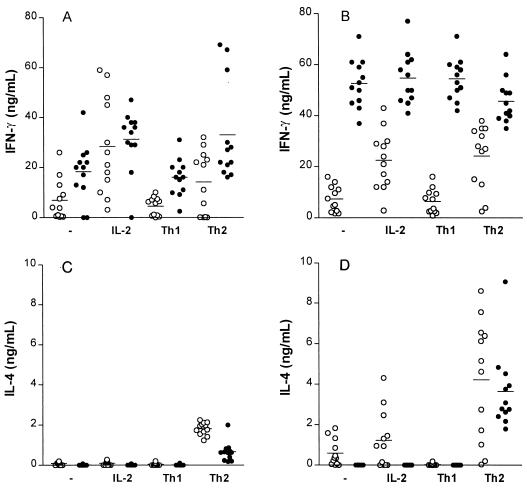
Effect of immunomodulation on Th1 and Th2 cell activity in the spleen.
The T-cell depletion studies indicated an important role for T cells, in particular cell populations producing IFN-γ. This led us to examine the effect of immunomodulation on Th1 and Th2 cell development in the spleen. The activity of T-cell subsets was assessed ex vivo as the amount of IFN-γ (Th1) and IL-4 (Th2) produced after immunomodulation. Treatment of mice with LE-MTPPE/IFN-γ resulted in T cells predominantly producing IFN-γ, independently of the culture conditions (Fig. 8). Spleen cells from mice treated with PL produced low levels of INF-γ. However, spleen cells from LE-MTPPE/IFN-γ-treated mice produced high IFN-γ levels when cultured in unsupplemented medium or in medium with IL-2 alone, but also when stimulated in the Th1 or Th2 direction (P < 0.001), indicating a strong effect of the immunomodulation on the Th1 population. Whereas T cells from the spleen produced IFN-γ in high amounts under all culture conditions, production of IL-4 was in most cases below the detection level or even apparently suppressed after immunomodulation. T-cell proliferation was induced by addition of IFN-γ (Th1) or IL-4 (Th2), which explains the high titers, especially in the Th2 group represented in Fig. 8D. Taken together, these results indicate a predominant activation by the liposomal immunomodulators of NK cells and of CD8 and Th1 cells in the spleen, cells known to produce IFN-γ, and an absence of a significant activation of Th2 cells, which produce IL-4.
FIG. 8.
Presence of Th1 and Th2 cells in spleens of mice treated with LE-MTPPE/IFN-γ, measured by production of IFN-γ and IL-4. Three mice were used per treatment group, and cytokine production was determined in quadruplicate. T cells were isolated 12 h (A and C and 4 days (B and D after the end of treatment. Cells were cultured in anti-CD3-coated plates in the presence of IL-2 (IL-2), IL-2, IFN-γ, and anti-IL-4 (Th1), IL-2, IL-4, and anti-IFN-γ (Th2), or medium alone (-). Horizontal lines represent averages of the determinations. ○, PL-treated controls; •, LE-MTPPE/IFN-γ-treated mice.
DISCUSSION
We have examined the role of T cells in immunomodulation by liposomal MTPPE and IFN-γ in K. pneumoniae septicemia in mice. We demonstrate for the first time a key role for T cells in immunomodulation to gram-negative septicemia. CD4 and CD8 T cells were shown to be important. Furthermore, IFN-γ was shown to be crucial in enhancement of host defense by immunomodulators. Therefore, we hypothesize that especially Th1 cells, CD8-positive cells, and NK cells, all known to produce IFN-γ upon stimulation, are activated in this process by liposomal immunomodulator-stimulated macrophages. From experiments with exogenous IL-10, demonstrating that IL-10 can indeed counteract the stimulation of the host defense by the immunomodulators, it is concluded that Th2 cells do not play a significant role in the immunomodulation. Th2 cells may not be stimulated or may be adequately suppressed by IFN-γ. This possibility is supported by our observation that IFN-γ production is increased in the spleen after immunomodulation, indicating a shift to Th1 cells in the spleen upon treatment with LE-MTPPE/IFN-γ.
In mice, two types of T-helper cells can be distinguished: Th1, producing IL-2, IFN-γ, granulocyte-macrophage colony-stimulating factor, but little or no IL-4 and IL-5; and Th2, producing IL-3, IL-4, IL-5, and IL-10 (21). One aspect of the Th1 response is macrophage activation, which enhances its capacity to kill intracellular microorganisms. The Th2 response, on the other hand, leads to macrophage deactivation (reviewed in references 19 and 22). The present data indicate an important role for IFN-γ in activation of nonspecific host defense by liposomal immunomodulators. In immunocompetent mice, NK cells and T cells (Th1 and CD8+) produce IFN-γ upon stimulation (29). The presumed cell type responsible for the IFN-γ expression in T-cell-deficient mice is therefore the NK cell, the only non T cell type reported to produce IFN-γ in vivo (1). However, depletion of T cells, both CD4 and CD8, was demonstrated to be detrimental for immunomodulation in mice in our septicemia model, which was shown to depend strongly on IFN-γ. In our model, NK cells appeared not able to substantially induce host defense activation in T-cell-depleted mice. Taken together, our data show that T cells are essential for the production of IFN-γ to enhance the host defense upon immunomodulation.
A potent inhibitor of monocyte/macrophage activation is IL-10, produced by Th2 cells and macrophages. IL-10 inhibits production of TNF-α, IL-1, and IFN-γ by lymphocytes. Moreover, IL-10 is a potent inhibitor of IL-12 production by activated mononuclear cells (reviewed in references 18 and 20). IL-12, produced by monocytes/macrophages and B cells, is required for IFN-γ production in synergy with IL-2 (2, 6), and activation of macrophages by liposomal immunomodulators is expected to induce release of IL-12, resulting in IFN-γ production by activated Th1 and CD8+ cells. Experiments on the role of IL-12 in our model are in progress. Others claim that inhibition of IFN-γ production is due primarily to blocking of the production from accessory cells of IL-12 as well as the costimulating cytokine IL-1 (7). These results support those of earlier studies showing that IL-10 does not suppress IFN-γ production by activated lymphocytes directly (10). The present study shows that exogenous IL-10 has a profound inhibitory effect on survival of mice treated with liposomal immunomodulators, indicating that activation of macrophages most likely results in activation of Th1 cells and NK cells, probably through IL-12, resulting in IFN-γ production (7, 12).
Macrophages are activated by immunomodulators, as indicated by increased Ia antigen expression on these cells upon immunomodulation. The immunomodulators are known to induce TNF-α, presumably produced by activated macrophages, which was shown to be essential for the host defense augmentation by LE-MTPPE/IFN-γ. Both macrophages and T cells appeared to play a role in the augmented resistance to K. pneumoniae upon immunomodulation. As the placebo-treated mice all died within 5 days after challenge, the role of specific antibodies might be limited. However, studies revealed that injections with LE-MTPPE/IFN-γ resulted in a dramatic increase of phagocytic cells, which could explain the augmented resistance to infections (unpublished data).
Although a dramatic reduction of host defense potentiation by immunomodulators was noticed after T-cell depletion, indicating a key role function of these cells in this process, significant enhancement of the host defense was still seen in most of the T-cell-depleted groups upon treatment with immunomodulators. It appears that in addition to LE-MTPPE/IFN-γ, other, yet unknown T-cell factors must be given to obtain sufficient host defense activation in T-cell-deficient hosts.
ACKNOWLEDGMENTS
This work was financially supported in part by Ciba Geigy (Basel, Switzerland).
We thank Astrid Vredendaal for excellent technical assistance with the in vitro T-cell experiments.
REFERENCES
- 1.Arase H, Arase N, Saito T. Interferon gamma production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J Exp Med. 1996;183:2391–2396. doi: 10.1084/jem.183.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan S H, Kobayashi M, Santoli D, Perussia B, Tranchieri G. Mechanism of IFN-γ induction by natural killer cell stimulatory factor (NKSF/IL-12) J Immunol. 1992;148:92–98. [PubMed] [Google Scholar]
- 3.Chatelain R, Verkila K, Coffman R L. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992;148:1182–1187. [PubMed] [Google Scholar]
- 4.Cherwinski H M, Schumacher J H, Brown K D, Mosmann T R. Two types mouse helper T cell clones. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays and monoclonal antibodies. J Exp Med. 1987;166:1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cobbold S P, Jayasuriya A, Nash A, Prospero T D, Waldman H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984;312:548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 6.D’Andrea A, Rengaraju M, Valiante N M, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Andrea A, Aste-Amezaga M, Valiante N M, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL10) inhibits human lymphocyte interferon γ-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dijkmans R, Heremans H, Billiau A. Heterogeneity of chinese hamster ovaria cell-produced recombinant murine interferon-γ. J Biol Chem. 1987;262:2528–2535. [PubMed] [Google Scholar]
- 9.Fidler I J, Fogler W E, Kleinerman E S, Saiki I. Abrogation of species specificity for activation of tumoricidal properties in macrophages by recombinant mouse or human interferon-γ encapsulated in liposomes. J Immunol. 1985;135:4289–4296. [PubMed] [Google Scholar]
- 10.Fiorentino D F, Zlotnik A, Vieira P, Mosmann T R, Howard M, Moore K W, O’Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 11.Heremans H, Dijkmans R, Sobis H, Vandekerckhove F, Billiau A. Regulation by interferons of the local inflammatory response to bacterial lipopolysaccharide. J Immunol. 1987;138:4175–4179. [PubMed] [Google Scholar]
- 12.Hsieh C-S, Macatonia S E, Tripp C S, Wolf S F, O’Garra A, Murphy K M. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 13.Leenen P J M, de Bruijn M F T R, Voerman J S A, Campbell P A, van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 14.Mehta K, Juliano R L, Lopez-Berestein G. Stimulation of macrophage protease secretion via liposomal delivery of muramyl dipeptide derivatives to intracellular sites. Immunology. 1984;51:517–527. [PMC free article] [PubMed] [Google Scholar]
- 15.Melissen P M B, van Vianen W, Leenen P J M, Bakker-Woudenberg I A J M. Tissue distribution and cellular distribution of liposomes encapsulating muramyltripeptide phosphatidyl ethanolamide. Biotherapy. 1994;7:71–78. doi: 10.1007/BF01878157. [DOI] [PubMed] [Google Scholar]
- 16.Melissen P M B, van Vianen W, Bakker-Woudenberg I A J M. Roles of peripheral leukocytes and tissue macrophages in antimicrobial resistance induced by free or liposome-encapsulated muramyl tripeptide phosphatidylethanolamide. Infect Immun. 1992;60:2891–2897. doi: 10.1128/iai.60.11.4891-4897.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miescher G C, Schreyer M, MacDonald H R. Production and characterization of a rat monoclonal antibody against the murine CD3 molecular complex. Immunol Lett. 1989;23:113–118. doi: 10.1016/0165-2478(89)90122-3. [DOI] [PubMed] [Google Scholar]
- 18.Moore K W, O’Gara A, de Waal Malefyt R, Vieira P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann T R, Cherwinski H, Bond M W, Giedlin M A, Coffman R L. Two types of murine helper T cell clone I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 20.Mosmann T R. Properties and functions of interleukin-10. Adv Immunol. 1994;56:1–26. [PubMed] [Google Scholar]
- 21.Openshaw P, Murphy E E, Hosken N A, Maino V, Davis K, Murphy K, O’Gara A. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romagnani S. Induction of Th1 and Th2 responses: a key role for the ‘natural’ immune response. Immunol Today. 1992;13:379–381. doi: 10.1016/0167-5699(92)90083-J. [DOI] [PubMed] [Google Scholar]
- 23.Savelkoul H F J, van Ommen R, Vossen A C T M, Breedlend E G, Coffman R L, van Oudenaren A. Modulation of systemic cytokine levels by implantation of alginate encapsulated cells. J Immunol Methods. 1994;170:185–196. doi: 10.1016/0022-1759(94)90394-8. [DOI] [PubMed] [Google Scholar]
- 24.Smith M R, Muegge K, Keller J R, Kung H-F, Young H A, Durum S K. Direct evidence for an intracellular role for IFN-γ: microinjection of human IFN-γ induces Ia expression on murine macrophages. J Immunol. 1990;144:1777–1782. [PubMed] [Google Scholar]
- 25.ten Hagen T L M, van Vianen W, Bakker-Woudenberg I A J M. Modulation of the non-specific antimicrobial resistance of mice by separate or combined liposome-encapsulated muramyl tripeptide phosphatidylethanolamine and interferon-γ towards Klebsiella pneumoniae septicemia. J Infect Dis. 1995;171:385–392. doi: 10.1093/infdis/171.2.385. [DOI] [PubMed] [Google Scholar]
- 26.ten Hagen T L M, van Vianen W, Bakker-Woudenberg I A J M. Isolation and characterization of murine Kupffer cells and splenic macrophages. J Immunol Methods. 1996;193:81–91. doi: 10.1016/0022-1759(96)00045-2. [DOI] [PubMed] [Google Scholar]
- 27.ten Hagen T L M, van Vianen W, Straathof E A T, Bakker-Woudenberg I A J M. Effect of liposome-encapsulated muramyl tripeptide phosphatidylethanolamine and interferon gamma on Klebsiella pneumoniae infection in T-cell-deficient mice and in vitro. In: Masihi K N, editor. Immunotherapy in infections. New York, N.Y: Marcel Dekker Inc.; 1994. pp. 225–234. [Google Scholar]
- 28.Tomonari K. A rat antibody against a structure functionally related to the mouse T-cell receptor/T3 complex. Immunogenetics. 1988;28:455–458. doi: 10.1007/BF00355379. [DOI] [PubMed] [Google Scholar]
- 29.Young H A, Hardy K J. Interferon-γ: producer cells, activation stimuli, and molecular genetic regulation. Pharmacol Ther. 1989;45:137–151. doi: 10.1016/0163-7258(90)90012-q. [DOI] [PubMed] [Google Scholar]