Abstract
Transcription factors are pivotal regulators in the cellular life process. Activating transcription factor 3 (ATF3), a member of the ATF/CREB (cAMP response element-binding protein) family, plays a crucial role as cells respond to various stresses and damage. As a transcription factor, ATF3 significantly influences signal transduction regulation, orchestrating a variety of signaling pathways, including apoptosis, ferroptosis, and cellular differentiation. In addition, ATF3 serves as an essential link between inflammation, oxidative stress, and immune responses. This review summarizes the recent advances in research on ATF3 activation and its role in regulating inflammatory responses, cell apoptosis, and ferroptosis while exploring the dual functions of ATF3 in these processes. Additionally, this article discusses the role of ATF3 in diseases related to pathogenic microbial infections. Our review may be helpful to better understand the role of ATF3 in cellular responses and disease progression, thus promoting advancements in clinical treatments for inflammation and oxidative stress-related diseases.
Keywords: ATF3, inflammatory, apoptosis, ferroptosis, microorganism
1. Introduction
Transcription factors (TFs) play a crucial role in the regulation of cellular function and the development of diseases due to their direct influence on gene expression, thus becoming a focal point of research in recent years. TFs regulate a variety of cellular physiological activities and immune responses by initiating or inhibiting the transcription of specific genes. Serving as key regulatory factors in the coordination of immune responses, TFs are essential for the activation of immune cells and their direct involvement in the production of inflammatory cytokines [1,2,3]. However, in the prolonged struggle between pathogens and hosts, some pathogenic microorganisms have evolved strategies to manipulate host TFs, thereby promoting their survival and replication within the host. Studies have elucidated that specific pathogenic organisms manipulate the activation of the NF-κB pathway as a survival strategy, which can effectively protect the host cell from apoptosis and allow bacterial survival within host cells [2]. Therefore, TFs are not only central to the host immune response but are also a vital strategy for some pathogens to establish infection. Increasing evidence suggests that TFs, such as STAT1, STAT2, JunB, CHOP, ATF3, and NF-κB, are involved in the interaction between host and pathogen and the regulation of host immune responses [2,4,5,6,7]. Thus, understanding the specific roles of TF in different infectious environments can provide insight for the development of targeted therapies to enhance host defense capability.
Activating transcription factor 3 (ATF3) is a stress-induced transcription factor that belongs to the activating transcription factor/cAMP response element-binding protein (ATF/CREB) family [8,9], whose members include ATF1, CREB, CREM, ATF2, ATF3, ATF4, ATF5, ATF6, ATF7, and B-ATF [10]. ATF3 regulates gene transcription by forming homodimers or heterodimers through its basic-region leucine zipper (bZIP) domain, thus modulating the biological functions of genes. ATF3 expression is relatively stable under normal physiological conditions, but changes in its expression are associated with various pathophysiological responses such as inflammation, oxidative stress, stress of the endoplasmic reticulum, and cell death [8,9,11]. ATF3 expression is induced by a variety of signals, including those initiated by cytokines, genotoxic agents, or physiological stressors [10]. ATF3 is upregulated under multiple stress conditions, regulating the interaction between cellular metabolism and immune and inflammatory responses, thus maintaining cellular homeostasis. Interestingly, unlike other members of the ATF family, increasing evidence suggests the involvement of ATF3 in the regulation of the host–pathogen interaction process [4,12,13,14,15,16,17]. Therefore, this review discusses the role of ATF3 in the regulation of cellular apoptosis, ferroptosis, and inflammatory responses, with a particular focus on the complex regulatory role of ATF3 in pathogen infections, and explains its bidirectional regulatory role in these processes.
2. Literature Screening
We conducted a literature search using the following search items on PubMed and Web of Science (all databases, topic): ATF3, ATF3 inflammation, ATF3 apoptosis, ATF3 ferroptosis, ATF3 virus, ATF3 bacteria, ATF3 fungi, and ATF3 parasite. References from all sources were reviewed to identify relevant articles. The gathered literature underwent a first round of screening, with exclusion criteria including duplicate articles, non-English manuscripts, and those without full-text availability. This was followed by a second round of screening, where articles were manually evaluated based on their titles, abstracts, or full texts to assess the relevance of ATF3 to inflammation, cell apoptosis, cell ferroptosis, and pathogenic microbes. During this screening process, a ‘citation within a citation’ or snowballing method was also employed to discover additional papers that may have been overlooked in the initial literature screening process. High-quality research findings published in authoritative journals were summarized and given priority in citations in the review.
3. Mechanisms of ATF3 Induction under Stressful Physiological Conditions
ATF3 shares the same binding site, 5′-TGACGTCA-3′, found in other transcription factors of the ATF/CREB family [10]. They interact with target DNA by binding to the entire region within the bZIP domain [8,10]. Several genes have been identified to possess this recognition sequence, including Nrf2, JunD, c-Jun, and IL-6 [18,19]. Intriguingly, some promoters of target genes regulated by ATF3 have binding sequences that differ from these common motifs (Table S1). ATF3 expression is relatively stable under normal physiological conditions; however, it rapidly changes in response to disturbances in the internal environment or external stimuli [20]. Changes in ATF3 expression are induced by inflammatory responses, cell death, cytokines, and cellular stresses (oxidative stress, DNA damage, or endoplasmic reticulum stress (ERS)). The specific induction mechanism may vary depending on the type of stress but usually involves the activation of stress-responsive kinases and upstream transcription factors, which then bind to the ATF3 promoter and stimulate its transcription [21,22]. Due to its induction in response to various stress signals, ATF3 is considered a stress-induced gene [23,24,25], which participates in cellular growth, tissue remodeling, cytoskeletal reorganization, and inflammation [21]. NF-E2-related factor 2 (Nrf2) transcriptionally upregulates ATF3 expression in astrocytes, thus promoting antioxidant and cytoprotective functions [26]. Naringin (Nar) activates ATF3 and inhibits PINK1 transcription by suppressing ERS and mitochondrial autophagy-related genes, as well as the expression of downstream ERS proteins [27]. Studies have shown that erastin can induce upregulation of ATF3 expression, which then strongly binds to the SLC7A11 promoter, promoting ferroptosis in cells [28]. ATF3 is also involved in the signaling pathways of the response to DNA damage. Following damage to DNA, the ATF3 promoter can be activated by MEKK1, suggesting that the involvement of the MAPK pathway is likely in the induction of ATF3 after DNA damage [29]. Furthermore, studies have shown that the JNK/SAPK signaling pathway can also induce ATF3 expression [30,31]. In particular, in studies using hydrogen peroxide as a stress signal, ATF3 induction was almost entirely inhibited by the antioxidant agent N-acetyl-L-cysteine, indicating that the induction of ATF3 requires oxidative stress [32,33].
ATF3 interacts with the cAMP response element (CRE) sequences through its basic region and forms homodimers or heterodimers with other CREB family members through its bZip domain [21,34]. Heterodimers, such as those formed by ATF3 with C-jun, ATF2, and JunB, have been demonstrated to possess transcriptional activation capabilities, enhancing the transcriptional activity of downstream target genes [32,35,36]. Currently, it is widely accepted that ATF3 might occur through stabilizing inhibitory cofactors on the promoter, thereby suppressing the transcriptional activity of downstream genes [19,34]. ATF3 recruits histone deacetylase 1 (HDAC1) to promoters containing ATF3 binding sites. Subsequently, HDAC1 causes histone deacetylation, leading to chromatin condensation and transcriptional repression [19,37]. Histone acetylation is a crucial physiological process that opens the chromatin structure, allowing transcription factors to bind to gene promoters and activate transcription [19,38]. Additionally, the various transcripts of ATF3 may represent another potential mechanism for its transcriptional activation capabilities [37]. The splice variant of ATF3, ATF3ΔZip2, inhibits the formation of the histone acetyltransferase (HAT) complex, thereby indirectly preventing histone acetylation [19,39,40]. Interestingly, the transcriptional inhibitory function of the ATF3 transcription factor might also be achieved through its binding to specific combinations of cis-acting elements. The homodimers of ATF3 possess the ability to inhibit the transcription of promoters containing its binding sites [21,37]. Recent studies have identified two adjacent ATF3 binding sites on the SLC7A11 promoter sequence, suggesting that binding to these two sites may facilitate the formation of ATF3 homodimers, thereby repressing gene transcription [28]. Notably, the SLC7A11 promoter possesses BS-1/BS-2 sequences and C/EBP-ATF response elements, allowing binding using many activators from the C/EBP and ATF/CREB transcription factor families [28]. This also implies that ATF3 might inhibit the expression of SLC7A11 by competing with transcription activators/co-activators for promoter binding. A similar phenomenon has been observed in the transcriptional regulation of ATF3 itself [41]. Two adjacent ATF3 binding sites have been found in its own promoter, leading to transcriptional autorepression by ATF3 [41]. This indicates that the presence of multiple adjacent ATF3 binding sites within a promoter sequence could lead to the formation of homodimers, potentially resulting in the inhibition of transcription. However, further experimental data are required to substantiate this hypothesis.
4. Implications of PAMPs and PRRs Activation on the Expression of ATF3
The Toll-like receptor (TLR) family consists of pattern recognition receptors (PRRs) involved in the detection of pathogen-associated molecular patterns (PAMPs) [10,42]. TLR1, 2, 4, 5, and 6 are expressed on the cell surface membrane and recognize bacterial and fungal products, while TLR3, 7, 8, and 9 are located in intracellular endosomes, specializing in the detection of pathogen-associated nucleic acids [10,42]. Upon recognition of their ligands, TLRs initiate complex cell signaling pathways, conferring antiviral and antibacterial states to the cell and promoting the expression of inflammatory cytokines, chemokines, and co-stimulatory molecules, which are crucial for the activation of adaptive immune responses [10,42]. Studies have shown that murine bone marrow macrophages treated with multiple PAMPs recognized by TLRs such as LPS, pIC, CpG-ODN, pIC/CpG-ODN, and zymosan significantly increase ATF3 protein expression [43], indicating that ATF3 expression is induced by various TLR responses. According to this, ATF3 is a potential transcriptional regulator in the TLR signaling cascade in macrophages. Furthermore, TLR4 mediates ATF3 expression in RAW 264.7 cells infected with Streptococcus pneumoniae through the JNK/p38 pathway [17]. This further implies the significant role of ATF3 in the recognition of PAMPs and the activation response of PRRs.
5. Modulatory Role of ATF3 in Inflammatory Cytokine
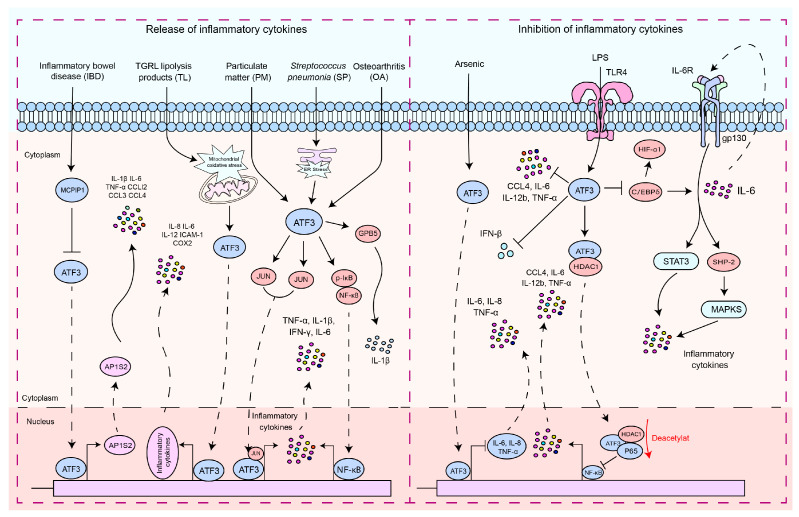
As a pivotal hub in the network of cellular adaptive responses and a transcription factor regulating immune response genes, ATF3 is a key regulator of both local and systemic inflammation, aiding cells in responding to disruptions in internal homeostasis. This is due to its ability to positively or negatively modulate the functional status or bioactivities of immune and nonimmune cells. The role of ATF3 has been studied in different contexts. In macrophages, ATF3 is a crucial regulator of innate immune responses. It is a product of TLR signaling and modulates inflammation in lipopolysaccharide (LPS)-stimulated cells by returning to this pathway [43,44,45,46]. Monocyte chemotactic protein-1-induced protein 1 (MCPIP1) inhibits macrophage polarization of M1 and promotes polarization of M2 by regulating the ATF3-AP1S2 signaling pathway, limiting the expression of pro-inflammatory cytokines in monocytes from patients with active inflammatory bowel disease (IBD). ATF3 can bind to the AP1S2 promoter and induce inflammation [47]. Postprandial triglyceride-rich lipoprotein (TGRL) lipolysis products (TL) induce the expression of pro-inflammatory factors in human brain microvascular endothelial cells by upregulating ATF3 through the activation of mitochondrial oxidative stress [48]. NF-κB regulates its expression by binding to the IL-6 promoter [49,50], while ATF3 has been shown to modulate NF-κB-dependent transcription by altering the phosphorylation of IκBα [34]. In osteoarthritis (OA), ATF3 modulates phosphorylation of p65 by altering IκB phosphorylation, which regulates the NF-κB signaling pathway, thus regulating the expression of IL-6 in chondrocytes [51]. ATF3 mediates particulate matter (PM)-induced inflammatory cytokine expression through the NF-κB and AP-1 pathways [52]. After infection with Streptococcus pneumoniae (S. pneumoniae), ATF3 regulates GPB5 or forms a complex with JUN, thus promoting the production of inflammatory cytokines (TNF-α, IL-1β, IL-6, and IFN-γ) [4,17].
ATF3 plays a dual role in the regulation of inflammatory responses (Figure 1). In endotoxin-stimulated monocytes, the stress associated with reactive oxygen species (ROS) leads to the induction of ATF3 expression and inhibits IL-6 production, making mice highly susceptible to secondary bacterial and fungal infections [53]. Negative regulation of transcription by ATF3 may be achieved through the inhibition of CCAAT/enhancer binding protein δ (C/EBPδ), a positive regulator of cytokine gene induction [54,55]. C/EBPδ enhances the expression of IL-6 [54], and in turn, IL-6, through activation of IL-6R, can activate the STAT3, SHP-2, and MAPK pathways, thus promoting cytokine secretion [54,56,57]. It is reported that ATF3 recruits HDAC1 to the ATF3/p65 complex and promotes the deacetylation of p65 to inhibit the production of pro-inflammatory cytokines [58]. The role of ATF3 in macrophages is not limited to the regulation of pro-inflammatory cytokines. It is also essential for regulating the production of interferon (IFN)-β downstream of innate immune receptors. ATF3 acts as a transcriptional repressor by binding to regulatory sites on the Ifnb1 promoter [59]. The expression of macrophage inflammatory protein 1β (CCL4) leads to the onset of inflammatory diseases [44,60]. ATF3 inhibits the expression of CCL4 in mouse macrophages induced by LPS [44]. ATF3 negatively regulates the gene expression of IL-6 and IL-12 in macrophages by altering the structure of chromatin [19,43]. LPS activation of TLR4 induces ATF3 expression, which in turn suppresses the expression of various inflammatory genes induced by TLR4 signaling, including IL-6, IL-12b, and TNF-α [19,20]. Arsenic exposure-induced upregulation of ATF3 inhibits inflammatory responses by suppressing the production of cytokines such as IL-6, IL-8, and TNF-α [61]. ATF3, as a high-density lipoprotein (HDL)-inducible target gene in macrophages, has been reported to downregulate the expression of TLR-induced pro-inflammatory cytokines [62]. This suggests that ATF3 can negatively regulate the transcription of pro-inflammatory cytokines. By inhibiting the expression of pro-inflammatory genes, ATF3 can prevent an overactive immune response and potentially serve as a key regulator in the fight against invading pathogens and inflammatory diseases.
Figure 1.
The signaling pathways by which ATF3 regulates the inflammatory response. These signaling pathways by which ATF3 regulates the inflammatory response can be divided into two types: (1) pro-inflammatory response—ATF3 increased the production of pro-inflammatory cytokines and chemokines by enhancing AP1S2 expression by binding to AP1S2 promoters in inflammation of the intestine (IBD). S. pneumoniae (SP) stimulates the formation of an ATF3 complex with c-Jun, and this complex binds to cytokine promoters of cytokines (TNF-α, IL-1β, and IFN-γ), resulting in increased cytokine production. During an infection, lung macrophages quickly phagocytose invasive S. pneumoniae, resulting in ER stress and ATF3 activation. ATF3 then promotes GBP5 activation, triggering IL-1β secretion. TGRL lipolysis products (TL) potentiate ROS in mitochondria, activating mitochondrial oxidative stress and ATF3 signaling. Furthermore, ATF3 regulates TL-induced inflammation. ATF3 may positively regulate IL-6 expression in osteoarthritis (OA) chondrocytes through modulation of NF-κB-dependent transcription by modifying IκB phosphorylation. ATF3 may heterodimerize with c-JUN and activate IL-6 transcription in HBE cells induced by PM. (2) Anti-inflammatory response, including regulation of the ATF3/HDAC1/NF-κB axis and ATF3/C-EBPδ axis—ATF3 inhibits the production of inflammatory cytokines by suppressing C/EBPδ. ATF3 inhibits the production of pro-inflammatory cytokines by recruiting HDAC1 into the ATF3/p65 complex and facilitating the deacetylation of p65. ATF3 acted as a transcriptional repressor and regulated IFN-β. LPS activates ATF3 by stimulating TLRs, thus inhibiting the production of inflammatory cytokines. Solid arrows indicate promotion, dashed arrows denote translocation, horizontal arrows represent inhibition, and red arrows signify the processes of modification undergone.
6. The Role of ATF3 in the Regulation of Cell Death
6.1. The Regulation of Apoptosis
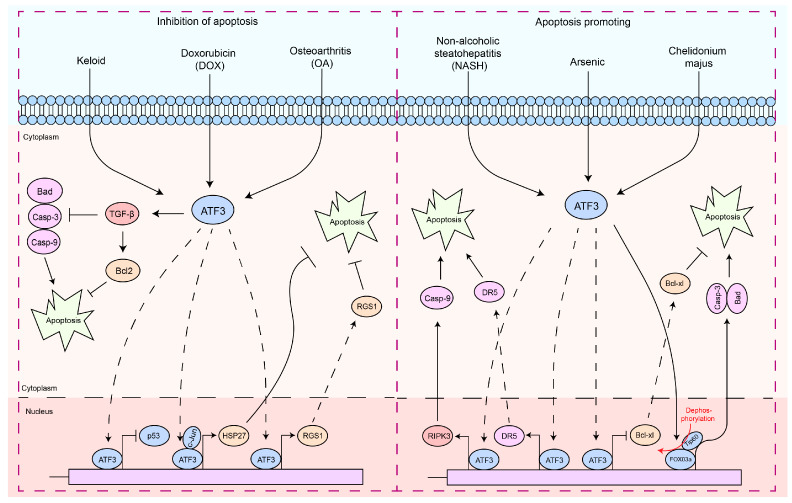
Apoptosis is a finely regulated process of programmed cell death that plays a crucial role in the maintenance of physiological functions in organisms, as well as in interactions between pathogens and hosts [63,64,65,66]. Apoptosis is a form of cell death by which the body maintains the homeostasis of the internal environment [5,67]. In addition, it plays an important role in the regulation of the immune system, especially in autoreactive immune cells [5,68,69,70]. In the interaction between pathogens and hosts, apoptosis can serve as a host defense mechanism to limit the replication and spread of pathogens [5,71,72]. In contrast, certain pathogens may exploit host defense mechanisms to evade host immune responses or induce excessive inflammation and tissue damage during infection [5,71,72]. Therefore, the diverse and complex functions of apoptosis are essential to understanding cellular behavior in physiological and pathological states. In particular, ATF3 induction appears to be consistently associated with cellular damage, as most of the signals that induce ATF3 also induce cellular injury [21,32,73]. Interestingly, ATF3 exhibits a dual role in the regulation of apoptosis (Figure 2). In cardiomyocytes, ATF3 effectively inhibits doxorubicin-induced apoptosis [74]. Similarly, adenovirus-mediated expression of ATF3 protects neuronal cells from apoptosis induced by mitogen-activated kinase kinase kinase 1 (MEKK1)–c-Jun N-terminal kinase (JNK) [36]. The combination of ATF3 and c-Jun induces the antiapoptotic factor Hsp27, which activates Akt directly or indirectly, potentially inhibiting apoptosis and inducing neurite outgrowth [36]. ATF3 inhibits fibroblast apoptosis through the TGF-β/Smad pathway [75]. In TGF-β1-induced synovial fibroblasts, ATF3 binds to the RGS1 promoter, enhancing RGS1 expression, accelerating cell proliferation, and blocking apoptosis [76]. ATF3 plays a crucial role in injuries induced by ischemia/reperfusion [77,78]. In renal ischemia–reperfusion injury, overexpression of ATF3 protects HK2 cells from H2O2-induced cell death by inhibiting p53 and enhancing the expression of p21 [79]. The protein kinase p21 is involved in cellular apoptosis through its regulation of the cell cycle process [80]. Concurrently, p53 possesses the capacity to promote apoptosis in cells [81]. Research has reported that ATF3 inhibits the transcription of p53 by binding to the PF-1 site, subsequently suppressing cardiomyocyte apoptosis induced by doxorubicin, thereby exerting a protective effect on cardiomyocytes [74]. Thus, the inhibition of p53 transcription by ATF3 is a vital pathway in its anti-apoptotic function. In a study on brain injury following transient focal cerebral ischemia, ATF3 knockout mice exhibited significantly higher infarct volumes, worsened neurological functions, and upregulation of neuronal apoptosis, inflammatory gene expression, and cellular inflammatory responses [82]. This indicates that ATF3 may be an essential protective regulatory factor in cerebral ischemic injury. Additionally, ATF3 can inhibit apoptosis mediated by cerebral ischemic injury through the downregulation of carboxy-terminal modulatory protein (CTMP), a pro-apoptotic factor that inhibits the anti-apoptotic Akt/PKB cascade [83].
Figure 2.
The functions of ATF3 in the response to apoptosis. These signaling pathways by which ATF3 regulates the apoptosis response can be divided into two types: (1) antiapoptotic response—ATF3 can regulate apoptosis of cells by upregulating the expression of the anti-apoptotic gene (HSP27, RGS1, and Bcl2) by binding to promoters, preventing p53 expression, which inhibits Caspases-3/9 activities. In addition, ATF3 inhibits Bad expression via TGF-β. (2) Pro-apoptosis response—ATF3 triggers the apoptotic pathway by upregulating RIPK3, DR5, and Caspase-9 by binding to their promoter, while concurrently inhibiting BCL-XL by binding to its promoter. Furthermore, ATF3 can also promote Caspase-3 and Bad transcription by activating FOXO3a, thereby regulating cell apoptosis. Solid arrows indicate promotion, dashed arrows denote translocation, horizontal arrows represent inhibition, and red arrows signify the processes of modification undergone.
These findings suggest a role for ATF3 in inhibiting apoptosis. However, ectopic expression of ATF3 improves the apoptotic capacity of topotecan-induced HeLa cells or camptothecin-induced HeLa cells [84]. ATF3 may act as a downstream target of the NF-κB and JNK/SAPK signaling pathways, promoting β-cell apoptosis [73]. ATF3 expression intensifies t-butyl hydroperoxide (TBHP)-induced apoptosis in nucleus pulposus cells (NPC) [85]. ATF3-dependent induction of RIPK3 causes a shift from apoptosis to necroptosis in hepatocytes [86]. The opposing regulation of DR5 and Bcl-xL expression by ATF3 promotes arsenic-induced apoptosis [87]. Forkhead transcription factors (FOXO3a) are a key molecule that promotes apoptosis, primarily functioning by facilitating the transcription of apoptosis-related factors, thereby mediating cell apoptosis [88,89,90,91]. The PI3K/Akt pathway inhibits apoptosis by phosphorylating FOXO3a, which prevents its nuclear translocation [90,91,92]. Chelidonium majus induces apoptosis in SKOV-3 cells by increasing the expression levels of ATF3 and its downstream protein, Tip60 [93]. Further studies have found that under Chelidonium majus stimulation, ATF3 enhances the expression of Tip60, which then promotes the dephosphorylation and nuclear translocation of FOXO3a, leading to apoptosis [93]. Tip60 is reported to acetylate p53 at K120, promoting the expression of the p53 target gene PUMA, thereby inducing apoptosis [94,95]. Research also reports that p53 promotes apoptosis by inhibiting the phosphorylation of PI3K/Akt [96]. Notably, it has been reported that Tip60 expression may be inversely correlated with PI3K/Akt activation [97,98]. Consequently, this may indicate that Tip60 acetylates p53, which in turn inhibits the phosphorylation of PI3K/Akt, subsequently leading to the dephosphorylation of FOXO3a. This sequence of molecular events mediates cellular apoptosis. This evidence highlights the role of ATF3 in promoting apoptosis.
6.2. The Regulation of Ferroptosis
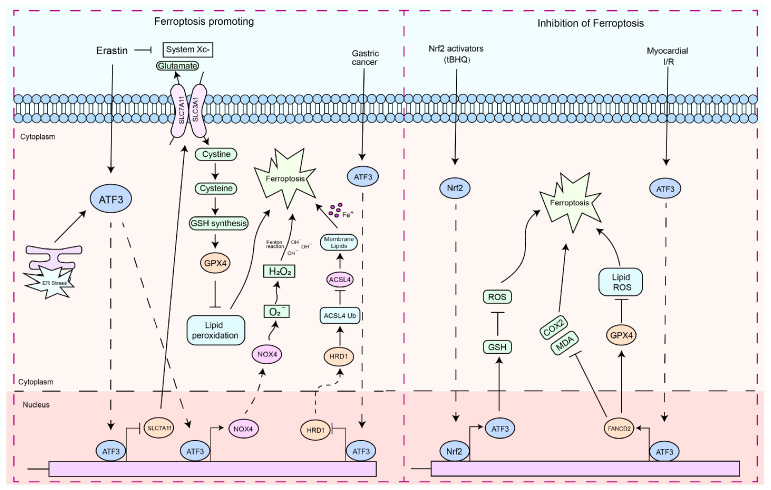
Ferroptosis is a nonapoptotic form of cell death that can be induced by metabolic stress, such as glutathione (GSH) depletion [28,99,100]. Recently defined as a newly discovered form of cell death, ferroptosis is different from apoptosis in that it does not involve caspase activation [99]. Ferroptosis leads to an increase in ROS and malondialdehyde (MDA), which ultimately causes overwhelming lipid peroxidation and results in cell death [99,101,102]. ATF3 is often involved in vital cellular activities such as metabolism. Currently, a large amount of research data indicates that ATF3 plays a significant role in the regulation of ferroptosis [28,31,102,103,104,105,106,107]. Nuclear factor erythroid 2 related factor 2 (Nrf2) can promote the expression of SLC7A11 and GPX4 under oxidative stress, which is crucial to mediate the onset of ferroptosis [108,109,110]. As an endogenous inhibitor of SLC7A11, ATF3 promotes erastin-induced ferroptosis by inhibiting the cystine/glutamate antiporter (system Xc-) [28]. Furthermore, ATF3 has been reported to facilitate ferroptosis in strychnine-induced glioblastoma cells by increasing H2O2 and suppressing SLC7A11 transcription [103]. In acute renal injury, an increase in ATF3 expression is observed, and ATF3 knockout markedly increases the expression of SLC7A11 and GPX4, which consequently improves the vitality of proximal tubular epithelial cells in the kidney [107]. ATF3 mediates osteoblast ferroptosis by inhibiting the expression of GPX4 and promoting the accumulation of lipid peroxides [105]. ATF3 induces ferroptosis in GC cells by transcriptionally repressing GPX4 and HRD1 [111]. HRD1 mediates the ubiquitination and degradation of ACSL4 to inhibit ferroptosis [111]. ACSL4 is a key molecule that promotes the ferroptosis process [112,113]. Interestingly, ATF3 also exhibits a dual role in the regulation of cellular ferroptosis (Figure 3). Nrf2 plays a key role in the defense system against oxidative stress [114]. To counter oxidative stress, the transcription factor Nrf2 binds to the antioxidant response element (ARE), mediating the transcriptional activation of its responsive genes and regulating the body’s defense mechanisms against oxidative damage [114,115]. Research has discovered that the promoter sequence of ATF3 contains ARE binding sites [26]. Furthermore, Nrf2 enhances its protective function against oxidative damage in astrocytes by upregulating the transcription of ATF3 [26]. ATF3 upregulates GSH levels to protect astrocytes from oxidative stress [26], and negatively regulates TLR4, which has been reported to promote ferroptosis [19,116,117]. ATF3 can inhibit the ferroptosis response induced by myocardial ischemia/reperfusion (I/R) by regulating the transcription of a series of ferroptosis-responsive genes, including GPX4, Ptgs2, Fth1, FANCD2, and Nox1 [118]. GPX4 and FANCD2 are considered two important molecules for suppressing the ferroptotic response [119]. The absence of ATF3 leads to reduced expression of GPX4 during I/R and H/R injuries, while its re-expression upregulates GPX4 expression, accompanied by a reduction in Fe2+ accumulation, ROS production, and MDA release [118]. In erastin- and RSL3-treated cardiomyocytes, overexpression of ATF3 lowers the levels of Fe2+, ROS, and MDA associated with ferroptosis and reduces cell death [118]. This suggests that, in certain contexts, ATF3 can also act as an inhibitory factor for ferroptosis.
Figure 3.
The functions of ATF3 in the ferroptosis response. These signaling pathways by which ATF3 regulates the ferroptosis response can be divided into two types: (1) pro-ferroptosis response—ATF3 triggers the ferroptosis pathway through the downregulation of SLC7A11. Stress from erastin and ER can induce ATF3 expression. ATF3 then binds to the promoters of the SLC7A11 genes, downregulating their expression. ATF3 suppressed the Xc−, depleted the intracellular GSH, and thus promoted erastin-induced lipid peroxidation. ATF3 regulates ferroptosis by mediating transcription inhibition of GPX4 and HRD1. ATF3 contributes to cell ferroptosis by increasing NOX4 and H2O2. (2) Nrf2-dependent ATF3 contributes to the antioxidant functions of Nrf2. ATF3 inhibits ferroptosis by regulating the expression of FANCD2. Solid arrows indicate promotion, dashed arrows denote translocation, and horizontal arrows represent inhibition.
In general, the pathways of cellular apoptosis and ferroptosis are mediated by the downstream targets of ATF3. ATF3 mediates cell death by regulating the expression of genes related to apoptosis and ferroptosis, either directly or indirectly. However, the molecular mechanisms underlying the regulation of these cell death processes remain to be elucidated.
7. The Functions of ATF3 in the Pathogenic Microbial Infection Process
The interaction between hosts and pathogens represents a pivotal issue in the realm of infectious biology. During this intricate interplay, the response mechanisms of host cells are crucial for controlling infection and disease progression. In recent years, the function of ATF3 in the regulation of immune responses has been extensively studied, especially with regard to its expression and regulatory mechanism in host cells after pathogen infection. The expression of ATF3 can be induced by various pathogen infections, including bacteria and viruses (Table 1), indicating its universality on host defense mechanisms. Here, we summarize the role of ATF3 during pathogen infection and discuss its potential impact within host defense mechanisms.
7.1. The Functions of ATF3 in Viral Infection
Recent research has revealed the dual role of ATF3 in viral infections, where it can inhibit viral replication and can also be exploited by viruses to enhance their replication [120,121,122]. Therefore, the precise function of ATF3 depends on the specific host–virus interaction environment, as well as the type of infected cell and the species of virus involved.
7.1.1. DNA Virus
The HBx protein, one of the seven proteins made by the Hepatitis B virus (HBV), is very toxic and can activate various genes in cells [122,123]. This affects many cell processes, such as the regulation of intracellular gene transcription, signal transduction, protein degradation, the cell cycle, and apoptosis [122,123]. Furthermore, HBx is a protein with a short half-life, primarily degraded in the cell via the ubiquitin-dependent proteasome pathway. Inhibiting HBx degradation and increasing the level of intracellular expression of HBx is one way HBV can cause liver cancer [123]. IL-1β/ATF3 promotes HBx mRNA degradation by mediating the expression of Ski2, which helps prevent complications mediated by HBx [122]. In particular, the level of Ski2 is also regulated by the HBx protein, forming a significant negative feedback loop to suppress HBx levels [122]. This mechanism is likely crucial for the virus to maintain an optimal concentration of HBx to support viral replication without triggering apoptosis [122,124]. Interestingly, HBx appears to induce Ski2 promoter activity by interacting with ATF3 [121,122].
Murine cytomegalovirus (MCMV) is a virus particularly susceptible to control by IFN-γ, produced by NK cells. The viral load of MCMV in the liver is regulated by IFN-γ, which is secreted by NK cells in response to IL-12 [125,126,127]. Previous studies have shown that depletion of IFN-γ exacerbates infection, leading to an increase in liver MCMV viral load and the appearance of hepatitis [126,128,129]. Concurrently, IFN-γ derived from NK cells restricts MCMV replication and liver damage [126,130]. Mice with a knockout of the ATF3 gene have been reported to exhibit a reduced liver viral load and less hepatic pathology after MCMV infection [126]. This is attributed to the role of ATF3 as a negative regulator of IFN-γ expression in NK cells rather than in T cells, thus facilitating MCMV infection [126].
A key characteristic of the Herpes Simplex Virus (HSV) is its ability to establish latent infection in the autonomic ganglia and reactivate under physical, hormonal, or emotional stress [131,132]. ATF3 is currently recognized as a significant stress-induced factor associated with the suppression of latent viral activation [132]. ATF3 has been reported to play a role in maintaining the latent state of HSV [132]. ATF3 is specifically expressed after neuronal injury, and it acts in synergy with STAT3 to induce the expression of downstream genes [133]. The main function of ATF3 in cells infected with HSV-1 has been reported to be to maintain neuronal integrity [132].
Human Papillomavirus (HPV) infection is a primary risk factor for cervical cancer [134,135]. HPV facilitates the proteolytic degradation and inactivation of p53 through the expression of the E6 protein [136,137]. Therefore, inhibiting the E6-promoted degradation of p53 appears to be an effective intervention against HPV-induced cervical cancer [137,138,139]. Research has found that ATF3 inhibits the E6-promoted degradation of p53 by directly binding to the HPV protein [138]. This interaction prevents the binding of E6AP to E6, thereby obstructing the recruitment of ubiquitin ligase to p53, reducing its ubiquitination and proteolytic degradation [138]. Unlike E6, ATF3 does not bind to E6AP, but it competes with E6AP to form a complex with E6 and p53 [137,138]. Further studies have found that ATF3 plays a significant role in inducing apoptosis in HeLa cells with depleted levels of p53 caused by HPV18 E6 activity [139]. They indicate that ATF3 plays a key role in a mechanism defending against HPV-induced carcinogenesis.
Table 1.
The functions of ATF3 in the process of pathogenic microbial infections.
| Microbial Name | Microbial Type | Functions of ATF3 | Research Models | References |
|---|---|---|---|---|
| Virus | ||||
| Hepatitis B Virus | DNA virus | ATF3 increases HBx mRNA degradation by regulating Ski2 expression. | HepG2, PXB, and AML12 cells. | [121,122] |
| Murine CytoMegalovirus | DNA virus | ATF3 regulates anti-MCMV responses by controlling the production of IFN-γ in NK cells. | C57BL/6, Rag1−/−, BALB/c, and ATF3−/− mice. | [126] |
| Herpes Simplex Virus 1 | DNA virus | ATF3 maintains the integrity of the neurons harboring latent virus. | HEp-2, Vero cell, HEK 293T, and CBA/J mice. | [132] |
| Human Papillomavirus | DNA virus | ATF3 plays a significant role in inducing apoptosis in HeLa cells. | HeLa cells. | [138,139] |
| Japanese Encephalitis Virus | RNA virus | ATF3 as a negative regulator of antiviral response and autophagy in mammalian cells during JEV infection. | Neuro2a, HEK, HeLa, and MEF cells. | [120] |
| Zika Virus | RNA virus | ATF3 acts to limit ZIKV infection by regulating autophagy and, thus, also ZIKV replication. | Wild-type and ATF3 knockout A549 cell lines. | [140] |
| Coxsackievirus B3 | RNA virus | -ATF3 regulates cell death induced by CVB3 infection. | HeLa cells. | [141] |
| Dengue Virus | RNA virus | Dengue virus degrades USP33-ATF3 axis via extracellular vesicles to activate microglial cells. | THP1 and HEK293T cells. | [142] |
| Human Immunodeficiency Virus | RNA virus | ATF3 orchestrates a recruitment of chromatin-modifying proteins. | Cervical carcinoma cell line C33A. | [143] |
| Bacteria | ||||
| Staphylococcus aureus | Gram-positive bacteria | ATF3 regulates antibacterial genes for antimicrobial processes. | Wild-type and ATF3 knockout mice. RAW 264.7 cell lines. | [14,15] |
| Streptococcus pneumoniae | Gram-positive bacteria | ATF3 promotes cytokine production (IL-17A TNF-α, IL-1β, and IFN-γ) in response to S. pneumoniae infection. | C57BL/6 WT and ATF3 KO mice. RAW 264.7 cells. | [4,13,17] |
| Listeria monocytogenes | Gram-positive bacteria | ATF3 provides protection from L. monocytogenes infections. | ATF3 knockout and wild-type mice. A549, HEp2, and RAW 264.7 cells. | [15] |
| Neisseria gonorrhoeae | Gram-negative bacteria | ATF3 negatively regulates IL-6 expression during N. gonorrhoeae infection. | T84 colorectal epithelial cells, End 1 endocervical cells, nasopharyngeal cells, and bronchial epithelial cell line 16HBE14. | [144] |
| Escherichia coli | Gram-negative bacteria | ATF3-mediated suppression of the innate cytokine storm abrogated the control of bacteria and causes high susceptibility to secondary infections. | C57BL/6 WT and ATF3 KO mice. A549, HEp2, and RAW 264.7 cells. | [15,53] |
| Pseudomonas aeruginosa | Gram-negative bacteria | ATF3 suppresses the progression of PA infection in hosts by inhibiting the activity of NF/κB. | AW264.7 and C57BL/6 ATF3 KO mice. | [145,146] |
| Mycobacterium tuberculosis | Other bacteria | ATF3 promotes cell autophagy and suppresses inflammatory response in Mycobacterium-tuberculosis-infected A549 cells. | A549 cells and RAW264.7 cells. BALB/c mice. | [12,147] |
| Mycoplasma pneumoniae | Other bacteria | ATF3 inhibits the expression and release of TNF-α, IL-1β, IL-6, and IL-18 induced by Mycoplasma pneumoniae in vitro and in vivo. | BALB/c mice, C57BL/6 mice, and RAW264.7 cells. | [148] |
| Fungi and Parasite | ||||
| Patulin | Fungal toxin | Patulin enhances ATF3 expression and promotes apoptosis in colorectal cancer cells. | HCT116 cells. | [149] |
| Deoxynivalenol | Fungal toxin | Deoxynivalenol induces G2/M cell cycle arrest in HepG2 cells by ATF3ΔZip2a/2b. | HepG2 cells. | [150] |
| Leishmania | Parasite | ATF3 promotes the survival of the Leishmania by regulating inflammatory response. | RAW 264.7 and BMDM cells. | [151,152] |
7.1.2. RNA Virus
The binding of type I interferons (IFN) to their receptors leads to receptor dimerization, subsequently activating the IRF and STAT families of TF [120,153]. STAT1 and STAT2 undergo dimerization and interact with IRF9, resulting in the formation of the interferon-stimulated gene factor 3 (ISGF3) complex [120,153]. This complex then translocates to the nucleus and binds to the conserved interferon-stimulated response element, thus inducing a range of interferon-sensitive genes (ISGs) that inhibit the replication of the Japanese Encephalitis Virus (JEV) [120]. In addition to cellular antiviral signaling, autophagy has also been demonstrated to inhibit JEV replication [154]. ATF3 regulates viral infection by stimulating and inhibiting immune responses [20,140,155]. ATF3 has been reported to inhibit cellular antiviral signaling and autophagy by binding to the promoter regions of STAT1, IRF9, ISG15, and ATG5, thereby promoting JEV virus replication [120].
Interestingly, compared to its response to JEV infection, ATF3 exhibits a different function in hosts infected with the Zika virus (ZIKV). Research found that ATF3 inhibits ZIKV infection by differentially regulating the transcription of specific innate immune response and autophagy genes [140]. During the infection with ZIKV of A549 cells, ATF3 promotes the transcription of the RIG-I, STAT1, IRF9, and ISG15 genes while simultaneously inhibiting the transcription levels of IFNβ and IFIT2 [140]. In addition to modulating ER stress and innate immune responses, ZIKV also disrupts the autophagy pathway in the early stages of infection, thus facilitating viral replication [156,157]. ATF3 binds to the promoter sequences of the autophagy-related genes Beclin-1 and ATG5 [120,158] and inhibits their expression during ZIKV infection [140].
Viruses often need to inhibit host cell death in the early stages of infection to allow sufficient replication time for the production of adequate viral progeny [159]. Later in the infection, promoting host cell death or utilizing budding mechanisms can facilitate viral dissemination [159]. Research has found that after infecting HeLa cells, Coxsackievirus B3 (CVB3) regulates the downregulation of ATF3 to inhibit cell death, thereby ensuring enough replication time for generating sufficient viral progeny [141]. This suggests that the downregulation of ATF3 may act as a mechanism to attenuate cell death induced by CVB3 infection, enhancing the virus’s ability to infect the host.
Infection with the Dengue virus (DENV) can induce a robust cytokine storm in the brain, leading to neurological symptoms or death in the host [160]. An upregulation of ATF3 expression has been observed in blood samples of patients infected with DENV [161]. Research has discovered that monocytes infected with DENV secrete extracellular vesicles (EV), which are internalized by microglia [142]. The miR-148a carried within these EVs inhibits the expression level of the ubiquitin-specific peptidase 33 (USP33) protein. The reduction in USP33, in turn, decreases the stability of cellular ATF3 protein through deubiquitination, thereby promoting the expression of pro-inflammatory genes such as TNF-α, NF-κB, and IFN-β [142]. This indicates that DENV manipulates the EV pathway to transfer miR-148a, thereby regulating the levels of USP33 and downstream ATF3 in human microglia and leading to neuroinflammation within the central nervous system.
In the context of the pathogenesis of integrated viruses, a pivotal aspect involves the exploitation of host cellular machinery for the expression of the viral genome during host infection. Specifically, the viral genome’s integration into the host’s chromatin architecture necessitates a strategic utilization of the host’s gene regulatory systems [143,162]. This phenomenon is exemplified in the case of Human Immunodeficiency Virus Type 1 (HIV-1), where, post-infection, the viral genome becomes assimilated into the host genome as a component of chromatin [143,162]. Central to this process is Nuc-1, a nucleosome situated immediately downstream of the HIV-1 transcription initiation site, which inherently inhibits the activity of the long-terminal repeat (LTR) [143,162,163]. The initiation of LTR-driven transcription and consequent viral expression are contingent upon both epigenetic modifications and the disruption of nuc-1 [162]. Within this nucleosome, the presence of three AP1 sites is critical for the facilitation of viral transcription and replication [164,165,166]. Notably, the disruption of nuc-1 is rapidly induced following the treatment of latently infected cells with agents such as TNF-α or phorbol myristate acetate (PMA), both of which activate the AP1 and ATF/CREB pathways [163]. ATF3, a constituent of the ATF/CREB family, is instrumental in modulating gene translation. Some research suggests a potential role for ATF3 in HIV infection [143,167,168], postulating that the formation of a stable ATF3/JunB/HMGA1 complex at the periphery of nuc-1 orchestrates a sequential recruitment of chromatin-modifying enzymes, culminating in the disruption of nuc-1 [143]. These observations infer a broader regulatory capacity of ATF3, particularly in modulating the transcriptional expression of pathogenic microorganisms, with an emphasis on integrated viruses.
7.2. The Functions of ATF3 in Bacterial Infection
Cell death modes, inflammatory responses, and immune regulation play crucial roles in the interactions between bacteria and their hosts. ATF3 differentially regulates these physiological processes in both Gram-positive bacteria and Gram-negative bacteria.
7.2.1. Gram-Positive Bacteria
During the infection process of Staphylococcus aureus (S. aureus), the host mediates the secretion of immune factors such as cytokines and chemokines through TLR-2, subsequently promoting the production of IL-17 to coordinate the host immune response [14,169]. IL-17, together with IL-22/IL-23, modulates macrophage function, thus inducing the expression of antimicrobial peptides (AMP) that kill or inactivate the pathogen [170,171]. Inactivation of IL-17, IL-22, and IL-23 leads to an increased S. aureus load and exacerbates the disease [170,172]. ATF3 has been reported to promote bacterial clearance by regulating the production of inflammatory cytokines, thus alleviating lethal S. aureus pneumonia [14]. ATF3 positively regulates the host’s resistance to S. aureus infection by modulating macrophage Reg3 expression and AMPs gene-mediated bacterial clearance, as well as the recruitment of macrophages, thus playing a significant role in the early stages of S. aureus infection [14].
IL-17A is critical in the early defense against Streptococcus pneumoniae (S. pneumoniae), as mice lacking IL-17A and IL-17RA show increased vulnerability to bacterial pathogens that incite lung diseases [173,174,175]. ATF3 has been reported to facilitate the generation of IL-17A in γδ T cells through macrophage-mediated secretion of IL-1β, thus modulating the response to infection [4]. ATF3 regulates the immune response by maintaining the intracellular balance of ROS and calcium ions (Ca2+), influencing macrophage production of IL-1β and IL-23p19 [4]. This process is essential for stimulating the secretion of IL-17A, which is necessary for early defense against infections and crucial to eradicating S. pneumoniae [4]. Mice deficient in ATF3 exhibit reduced survival rates and an increased bacterial load in the lungs after infection with S. pneumoniae [4,13]. During infection, ATF3 actively adjusts innate immunity by enhancing the expression of TNF-α, IL-1β, and IFN-γ, thereby facilitating bacterial clearance [13]. The pneumococcal toxin pneumolysin (PLY) interacts with TLR4 to activate the MAPK pathway [17]. The activation of ATF3 depends on MAPK signaling. Upon activation, ATF3 enters the nucleus and interacts with c-Jun, promoting the production of cytokines, which in turn suppresses S. pneumoniae infection [17].
Wild-type mice demonstrate more effective bacterial clearance than ATF3-null mice during Listeria monocytogenes (L. monocytogenes) infection [15]. This suggests that ATF3 plays a critical role in resisting L. monocytogenes infection. PLY induces ATF3 expression through the TLR4/MAPK pathway [17]. Listeriolysin O (LLO), a member of the cytolysins released by L. monocytogenes, is known to stimulate TLR4-dependent cytokine expression and acts as a TLR4 agonist [176]. Furthermore, ATF3 significantly improves the expression levels of TNF-α, IL-1β, and IFN-γ during L. monocytogenes infection [15].
7.2.2. Gram-Negative Bacteria
During episodes of bacterial sepsis, the host modulates its response to infection by upregulating or suppressing cytokines through ATF3 [13,53]. LPS significantly induces ATF3, which functions as a negative regulator in the production of inflammatory cytokines [19,53]. For example, in the context of Escherichia coli (E. coli) and Neisseria gonorrhoeae invasions, ATF3 acts as a negative regulator, suppressing the production of inflammatory cytokines [53,144]. ATF3 expression is stimulated in a MAPK-dependent manner during Neisseria gonorrhoeae infection [144]. In addition, the contraction of Tfp (Type IV pilus) further amplifies ATF3 expression. Subsequently, activated ATF3 then inhibits the transcription of the pro-inflammatory cytokine IL-6, thus promoting the progression of the infection [144].
E. coli sepsis is currently one of the most important types of sepsis [177]. ATF3 facilitates the progression of E. coli sepsis by suppressing IL-6 transcription [53]. Due to immunosuppression associated with ATF3-mediated sepsis, ATF3 knockout mice exhibit longer survival than wild-type mice after infection with E. coli [53]. Post-infection with uropathogenic Escherichia coli (UPEC), cytokines such as IL-1β, IL-6, and IFN-γ are significantly suppressed, while the bacterial load in the lungs and spleens of wild-type mice is substantially higher than in ATF3 knockout mice [15]. This indicates that ATF3 exhibits a divergent mechanism in Gram-positive and Gram-negative bacterial infections. This contradictory result may be caused by the following reasons: (1) LPS-induced ATF3 competes with NF-κB for binding to the promoters of target cytokines, thus inhibiting the production of inflammatory cytokines [15,19]. (2) LPS-induced ATF3 binds to cytokine promoters, and its interaction with HDAC leads to histone deacetylation. This process results in chromatin condensation, which suppresses cytokine gene transcription [15,19]. (3) The regulation of TLR4 differs; in Gram-positive bacterial infections, ATF3 may positively regulate TLR4 expression and stimulate cytokine production [13,15,17]. In contrast, LPS-induced activation of TLR4 enhances ATF3 expression, which acts to suppress the generation of inflammatory cytokines [19,20].
Pseudomonas aeruginosa (PA) induces acute lung injury in infected hosts through the release of inflammatory cytokines [178,179]. Thus, suppressing the host’s inflammatory response is crucial for resisting acute inflammation caused by PA. ATF3, a stress transcription factor known to regulate inflammatory responses, has also been reported to play a role in PA infection [145,146]. Research has found that ATF3 can bind with lipopolysaccharide-binding protein (LBP) to inhibit the activity of NF-κB and the inflammatory response, thereby protecting mice from acute lung injury induced by PA [146]. Another study indicates that during PA infection, ATF3 negatively regulates the translocation and Ser-536 phosphorylation of NF-κB p65, thus inhibiting the inflammatory response [145]. This suggests that ATF3 suppresses the progression of PA infection in hosts by inhibiting the activity of NF-κB.
In summary, the regulation of cytokines through the LPS/Toll/ATF3 axis has become a common phenomenon in Gram-negative bacteria.
7.2.3. Other Bacteria
ATF3 expression is significantly upregulated during Mycobacterium tuberculosis (Mtb) infection [147]. ATF3 cooperates with BRG1 to activate the expression of the inflammatory cytokines IL-6, TNF-α, and IL-12p40 and increase the production of nitric oxide [12,147]. Foamy macrophages, a subpopulation of macrophages, play a pivotal role in the pathogenesis of tuberculosis [12]. They are characterized by an abundance of liposomes (LB), which may provide a survival environment for mycobacteria in granulomas [12,180]. Mtb stimulates the formation of LB-rich foamy macrophages [180,181]. ATF3 has been reported to inhibit liposome formation by regulating the expression of genes related to lipid metabolism [12]. This suggests that ATF3 limits Mtb survival by inhibiting LB formation.
ATF3 reduces the inflammatory response in the lungs of Mtb-infected mice by inhibiting IL-6 and IL-8 induced by the NF-κB pathway [147,182]. Mtb inhibits autophagy by impeding phagosome maturation [147,183,184]. Hence, facilitating the onset of autophagy is beneficial for the clearance of intracellular Mtb [147,183,184]. ATF3 has been reported to protect A549 cells from the inflammatory response induced by mycobacterial infection [12]. ATF3 induces autophagy and facilitates the clearance of mycobacteria by activating TIMP2 and inhibiting NF-κB [12].
Unlike Mtb, ATF3 inhibits cytokine production in macrophages infected with Mycoplasma pneumoniae (M. pneumoniae) [148]. Overexpression of ATF3 inhibits the expression and release of TNF-α, IL-1β, IL-6, and IL-18 from macrophages infected with M. pneumoniae [148]. Expression of ATF3 reduced the release of inflammatory cytokines in lung tissue and bronchoalveolar lavage (BALF) of mice infected with M. pneumoniae-infected mice [148]. This seems to imply that ATF3 also exhibits different roles in special pathogenic bacteria. This may be due to the fact that ATF3 plays different regulatory functions in response to different stimulation factors, different cell types, and different stress states. This undeniably enhances the intrigue surrounding research into the regulatory mechanisms of the ATF3 molecule.
7.3. The Functions of ATF3 in Fungal and Parasite Infections
Patulin is a fungal toxin primarily released by Aspergillus and Penicillium species [185]. Patulin exerts its toxic effect by covalently binding to reactive sulfhydryl groups in cellular proteins and by depleting glutathione, resulting in oxidative damage and the generation of reactive oxygen species (ROS) [186,187]. Research has found that Patulin induces transcription factor EGR-1 phosphorylation through increased oxidative stress, thereby enhancing ATF3 expression and promoting apoptosis in colorectal cancer cells [149]. Deoxynivalenol (DON), commonly known as vomitoxin, is a type B trichothecene mycotoxin predominantly produced by Fusarium species, such as F. culmorum and F. graminearum [188]. Interestingly, unlike Patulin, Deoxynivalenol induces G2/M cell cycle arrest in HepG2 cells by inducing the expression of EGR1 and p21 through the induction of ATF3’s splice variant, ATF3ΔZip2a/2b [150]. This once again highlights the complexity and significance of ATF3’s regulatory mechanisms. Notably, Candida albicans, a fungal pathogen that infects humans, significantly upregulates ATF3 after infecting cells [189,190]. This may suggest that ATF3 plays a role in Candida albicans infections.
The lifestyle of Leishmania is characterized by its role as an obligate intracellular pathogen, infecting the monocyte/macrophage lineage, which it enters through phagocytosis [191]. Consequently, Leishmania exhibits exceptional survival and replication capabilities in adverse environments [191]. Regulating the host’s anti-inflammatory environment and suppressing the production of superoxides are crucial for its persistent infection [192]. Research has found that ATF3 expression is upregulated in macrophages infected with Leishmania [151,152], and the survival rate of Leishmania in macrophages lacking ATF3 is reduced [152]. Studies have discovered that Leishmania upregulates the transcriptional activity of ATF3 via NRF2 [152]. Subsequently, ATF3 recruits HDAC1 to inhibit the transcriptional activity of NF-κB and IL-12b, thus promoting the survival of the parasite [152]. This suggests that Leishmania can manipulate the host’s inflammatory response through ATF3 to facilitate its own survival.
8. Prospects for Clinical Applications
Considering the multifaceted role of ATF3 in the regulation of physiological functions, it presents a broad prospect as a clinical pharmacological target: (1) Treatments that target ATF3 to control inflammation, useful in autoimmune and other inflammatory diseases. (2) Exploiting ATF3 to develop treatments for neurological disorders and nerve damage. (3) Utilizing ATF3 expression levels as a diagnostic and prognostic tool, especially in cancer. However, we need to carefully consider ATF3’s dual functions to avoid adverse effects in treatment. Targeting ATF3 presents significant opportunities for novel therapeutic developments and improved disease management in clinical medicine. Its potential as a drug target and a biomarker can lead to advances in personalized medicine. However, the complexity of ATF3’s roles necessitates careful research and development to ensure the efficacy and safety of these new approaches.
9. Conclusions and Future Perspectives
TFs are a significant class of protein factors involved in cellular activities that virtually participate in the regulation of all cellular life processes. ATF3, a stress-responsive transcription factor, is upregulated in response to cellular stressors such as oxidative stress, DNA damage, and inflammation, thus modulating the interactions between cellular metabolism, immunity, and inflammatory responses [193,194,195,196,197]. Consequently, ATF3 has emerged as a promising target for the treatment of certain inflammatory diseases. From our review of the literature, we conclude that ATF3 plays a complex and critical role in multiple biological processes. The function of ATF3 is dual, which is reflected in its response to inflammation, apoptosis, ferroptosis, and infection by pathogenic microorganisms. In terms of inflammation regulation, ATF3 has the ability to both promote and suppress inflammatory responses, with its specific action dependent on the type of stimulus encountered by the cell. This indicates that ATF3 is involved in the precise modulation of cellular responses to inflammatory signals. This dual functionality helps to maintain the balance of the immune system and prevent excessive inflammatory responses. In regulating apoptosis and ferroptosis, ATF3 similarly exhibits the capacity to promote and inhibit cell death. This underscores the key role of ATF3 in determining cell fate, further highlighting the diversity and complexity of ATF3 in cell death mechanisms. In response to pathogen infection, ATF3 can enhance or inhibit pathogen infection, potentially related to host cell defense mechanisms and pathogen evasion strategies. This finding suggests that ATF3 plays an important role in the development and prevention of infectious diseases.
Consequently, under the influence of varying cellular physiological states, ATF3 exhibits different regulatory functions. Furthermore, numerous questions merit further exploration. For example, why does ATF3 exhibit dual regulatory functions? Does ATF3 possess the capability to transition between its dual roles? What are the factors that induce this transformation? Is there an intrinsic link between ATF3 in inflammation, cell death, and responses to pathogenic microbial infections? For example, it is pertinent to investigate under which conditions ATF3-induced cell death is advantageous or detrimental to pathogen infection and propagation, or whether these processes trigger or inhibit more robust inflammatory immune responses. How pathogens exploit ATF3 to facilitate their replication and spread, or how host cells use ATF3-induced immune responses to limit the replication and dissemination of pathogens, are critical aspects warranting further research. As a key transcription factor in the host stress response, could ATF3 potentially influence the expression of pathogen-related genes, thereby regulating the process of pathogen infection? Therefore, the intricate internal balance among these factors deserves further study. Elucidating these mechanisms in detail could contribute to innovative breakthroughs in the prevention and treatment of inflammatory diseases and microbial infections.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms25020824/s1.
Author Contributions
S.L. (Shuang Liu) and Z.L. are responsible for writing the whole manuscript. S.L. (Shimei Lan), H.H., and A.A.B. are in charge of drawing the pictures in the manuscript. X.Y. and P.G. organized the literature. S.C. and Y.C. are in charge of checking and revision. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No data were used for the research described in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was supported by grants from the National Key Research and Development Program of China (2022YFD1800704), The Key Program of the Natural Science Foundation of Gansu Province (22JR5RA409), and the Chinese Academy of Agricultural Science and Technology Innovation Program (CAAS-ASTIP-2021-LVRI).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Wang W., Chang X., Yao W., Wei N., Huo N., Wang Y., Wei Q., Liu H., Wang X., Zhang S., et al. Host CARD11 Inhibits Newcastle Disease Virus Replication by Suppressing Viral Polymerase Activity in Neurons. J. Virol. 2019;93:e01499-19. doi: 10.1128/JVI.01499-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caamano J., Hunter C.A. NF-kappaB family of transcription factors: Central regulators of innate and adaptive immune functions. Clin. Microbiol. Rev. 2002;15:414–429. doi: 10.1128/CMR.15.3.414-429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Negrate G. Subversion of innate immune responses by bacterial hindrance of NF-kappaB pathway. Cell Microbiol. 2012;14:155–167. doi: 10.1111/j.1462-5822.2011.01719.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee S., Kim G.L., Kim N.Y., Kim S.J., Ghosh P., Rhee D.K. ATF3 Stimulates IL-17A by Regulating Intracellular Ca2+/ROS-Dependent IL-1beta Activation During Streptococcus pneumoniae Infection. Front. Immunol. 2018;9:1954. doi: 10.3389/fimmu.2018.01954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu H., Tian M., Ding C., Yu S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2018;9:3083. doi: 10.3389/fimmu.2018.03083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang K., Yang B., Shen C., Zhang T., Hao Y., Zhang D., Liu H., Shi X., Li G., Yang J., et al. MGF360-9L Is a Major Virulence Factor Associated with the African Swine Fever Virus by Antagonizing the JAK/STAT Signaling Pathway. mBio. 2022;13:e0233021. doi: 10.1128/mbio.02330-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ariffianto A., Deng L., Harada S., Liang Y., Matsui C., Abe T., Shoji I. Transcription Factor JunB Suppresses Hepatitis C Virus Replication. Kobe J. Med. Sci. 2023;69:E86–E95. [PMC free article] [PubMed] [Google Scholar]
- 8.Li D., Jing J., Dong X., Zhang C., Wang J., Wan X. Activating transcription factor 3: A potential therapeutic target for inflammatory pulmonary diseases. Immun. Inflamm. Dis. 2023;11:e1028. doi: 10.1002/iid3.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohini M., Haritha Menon A., Selvamurugan N. Role of activating transcription factor 3 and its interacting proteins under physiological and pathological conditions. Int. J. Biol. Macromol. 2018;120:310–317. doi: 10.1016/j.ijbiomac.2018.08.107. [DOI] [PubMed] [Google Scholar]
- 10.Thompson M.R., Xu D., Williams B.R. ATF3 transcription factor and its emerging roles in immunity and cancer. J. Mol. Med. 2009;87:1053–1060. doi: 10.1007/s00109-009-0520-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou H., Li N., Yuan Y., Jin Y.G., Guo H., Deng W., Tang Q.Z. Activating transcription factor 3 in cardiovascular diseases: A potential therapeutic target. Basic. Res. Cardiol. 2018;113:37. doi: 10.1007/s00395-018-0698-6. [DOI] [PubMed] [Google Scholar]
- 12.Kumar M., Majumder D., Mal S., Chakraborty S., Gupta P., Jana K., Gupta U.D., Ghosh Z., Kundu M., Basu J. Activating transcription factor 3 modulates the macrophage immune response to Mycobacterium tuberculosis infection via reciprocal regulation of inflammatory genes and lipid body formation. Cell Microbiol. 2020;22:e13142. doi: 10.1111/cmi.13142. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen C.T., Kim E.H., Luong T.T., Pyo S., Rhee D.K. ATF3 confers resistance to pneumococcal infection through positive regulation of cytokine production. J. Infect. Dis. 2014;210:1745–1754. doi: 10.1093/infdis/jiu352. [DOI] [PubMed] [Google Scholar]
- 14.Du Y., Ma Z., Zheng J., Huang S., Yang X., Song Y., Dong D., Shi L., Xu D. ATF3 Positively Regulates Antibacterial Immunity by Modulating Macrophage Killing and Migration Functions. Front. Immunol. 2022;13:839502. doi: 10.3389/fimmu.2022.839502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen C.T., Luong T.T., Lee S., Kim G.L., Pyo S., Rhee D.K. ATF3 provides protection from Staphylococcus aureus and Listeria monocytogenes infections. FEMS Microbiol. Lett. 2016;363:fnw062. doi: 10.1093/femsle/fnw062. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., Takeuchi H., Nishioka M., Morimoto N., Kamioka M., Kumon Y., Sugiura T. Relationship of IL-8 production and the CagA status in AGS cells infected with Helicobacter pylori exposed to low pH and activating transcription factor 3 (ATF3) Microbiol. Res. 2009;164:180–190. doi: 10.1016/j.micres.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen C.T., Kim E.H., Luong T.T., Pyo S., Rhee D.K. TLR4 mediates pneumolysin-induced ATF3 expression through the JNK/p38 pathway in Streptococcus pneumoniae-infected RAW 264.7 cells. Mol. Cells. 2015;38:58–64. doi: 10.14348/molcells.2015.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J., Li X., Guo M., Yu J., Yan C. The common stress responsive transcription factor ATF3 binds genomic sites enriched with p300 and H3K27ac for transcriptional regulation. BMC Genom. 2016;17:335. doi: 10.1186/s12864-016-2664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilchrist M., Thorsson V., Li B., Rust A.G., Korb M., Roach J.C., Kennedy K., Hai T., Bolouri H., Aderem A. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature. 2006;441:173–178. doi: 10.1038/nature04768. [DOI] [PubMed] [Google Scholar]
- 20.Hai T., Wolford C.C., Chang Y.S. ATF3, a hub of the cellular adaptive-response network, in the pathogenesis of diseases: Is modulation of inflammation a unifying component? Gene Expr. 2010;15:1–11. doi: 10.3727/105221610X12819686555015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hai T., Wolfgang C.D., Marsee D.K., Allen A.E., Sivaprasad U. ATF3 and stress responses. Gene Expr. 1999;7:321–335. [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H., Luo S., Chen H., Zhang C. ATF3 regulates SPHK1 in cardiomyocyte injury via endoplasmic reticulum stress. Immun. Inflamm. Dis. 2023;11:e998. doi: 10.1002/iid3.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu R., Shtil A.A., Tan T.H., Roninson I.B., Kong A.N. Adriamycin activates c-jun N-terminal kinase in human leukemia cells: A relevance to apoptosis. Cancer Lett. 1996;107:73–81. doi: 10.1016/0304-3835(96)04345-5. [DOI] [PubMed] [Google Scholar]
- 24.Drysdale B.E., Howard D.L., Johnson R.J. Identification of a lipopolysaccharide inducible transcription factor in murine macrophages. Mol. Immunol. 1996;33:989–998. doi: 10.1016/S0161-5890(96)00043-0. [DOI] [PubMed] [Google Scholar]
- 25.Weir E., Chen Q., DeFrances M.C., Bell A., Taub R., Zarnegar R. Rapid induction of mRNAs for liver regeneration factor and insulin-like growth factor binding protein-1 in primary cultures of rat hepatocytes by hepatocyte growth factor and epidermal growth factor. Hepatology. 1994;20:955–960. doi: 10.1002/hep.1840200426. [DOI] [PubMed] [Google Scholar]
- 26.Kim K.H., Jeong J.Y., Surh Y.J., Kim K.W. Expression of stress-response ATF3 is mediated by Nrf2 in astrocytes. Nucleic Acids Res. 2010;38:48–59. doi: 10.1093/nar/gkp865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei Y., Sun L., Liu C., Li L. Naringin regulates endoplasmic reticulum stress and mitophagy through the ATF3/PINK1 signaling axis to alleviate pulmonary fibrosis. Naunyn Schmiedebergs Arch. Pharmacol. 2023;396:1155–1169. doi: 10.1007/s00210-023-02390-z. [DOI] [PubMed] [Google Scholar]
- 28.Wang L., Liu Y., Du T., Yang H., Lei L., Guo M., Ding H.F., Zhang J., Wang H., Chen X., et al. ATF3 promotes erastin-induced ferroptosis by suppressing system Xc−. Cell Death Differ. 2020;27:662–675. doi: 10.1038/s41418-019-0380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan F., Jin S., Amundson S.A., Tong T., Fan W., Zhao H., Zhu X., Mazzacurati L., Li X., Petrik K.L., et al. ATF3 induction following DNA damage is regulated by distinct signaling pathways and over-expression of ATF3 protein suppresses cells growth. Oncogene. 2002;21:7488–7496. doi: 10.1038/sj.onc.1205896. [DOI] [PubMed] [Google Scholar]
- 30.Liang G., Wolfgang C.D., Chen B.P., Chen T.H., Hai T. ATF3 gene. Genomic organization, promoter, and regulation. J. Biol. Chem. 1996;271:1695–1701. doi: 10.1074/jbc.271.3.1695. [DOI] [PubMed] [Google Scholar]
- 31.American Association of Neurological Surgeons (AANS) American Society of Neuroradiology (ASNR) Cardiovascular and Interventional Radiology Society of Europe (CIRSE) Canadian Interventional Radiology Association (CIRA) Sacks D., Baxter B., Campbell B.C.V., Carpenter J.S., Cognard C., Dippel D., et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke. 2018;13:612–632. doi: 10.1016/j.jvir.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 32.Hai T., Hartman M.G. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: Activating transcription factor proteins and homeostasis. Gene. 2001;273:1–11. doi: 10.1016/S0378-1119(01)00551-0. [DOI] [PubMed] [Google Scholar]
- 33.Allen-Jennings A.E., Hartman M.G., Kociba G.J., Hai T. The roles of ATF3 in glucose homeostasis. A transgenic mouse model with liver dysfunction and defects in endocrine pancreas. J. Biol. Chem. 2001;276:29507–29514. doi: 10.1074/jbc.M100986200. [DOI] [PubMed] [Google Scholar]
- 34.Kim E.Y., Shin H.Y., Kim J.Y., Kim D.G., Choi Y.M., Kwon H.K., Rhee D.K., Kim Y.S., Choi S. ATF3 plays a key role in Kdo2-lipid A-induced TLR4-dependent gene expression via NF-kappaB activation. PLoS ONE. 2010;5:e14181. doi: 10.1371/journal.pone.0014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nilsson M., Toftgard R., Bohm S. Activated Ha-Ras but not TPA induces transcription through binding sites for activating transcription factor 3/Jun and a novel nuclear factor. J. Biol. Chem. 1995;270:12210–12218. doi: 10.1074/jbc.270.20.12210. [DOI] [PubMed] [Google Scholar]
- 36.Nakagomi S., Suzuki Y., Namikawa K., Kiryu-Seo S., Kiyama H. Expression of the activating transcription factor 3 prevents c-Jun N-terminal kinase-induced neuronal death by promoting heat shock protein 27 expression and Akt activation. J. Neurosci. 2003;23:5187–5196. doi: 10.1523/JNEUROSCI.23-12-05187.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen B.P., Liang G., Whelan J., Hai T. ATF3 and ATF3 delta Zip. Transcriptional repression versus activation by alternatively spliced isoforms. J. Biol. Chem. 1994;269:15819–15826. doi: 10.1016/S0021-9258(17)40754-X. [DOI] [PubMed] [Google Scholar]
- 38.Shvedunova M., Akhtar A. Modulation of cellular processes by histone and non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 2022;23:329–349. doi: 10.1038/s41580-021-00441-y. [DOI] [PubMed] [Google Scholar]
- 39.Furumatsu T., Tsuda M., Yoshida K., Taniguchi N., Ito T., Hashimoto M., Ito T., Asahara H. Sox9 and p300 cooperatively regulate chromatin-mediated transcription. J. Biol. Chem. 2005;280:35203–35208. doi: 10.1074/jbc.M502409200. [DOI] [PubMed] [Google Scholar]
- 40.Hua B., Tamamori-Adachi M., Luo Y., Tamura K., Morioka M., Fukuda M., Tanaka Y., Kitajima S. A splice variant of stress response gene ATF3 counteracts NF-kappaB-dependent anti-apoptosis through inhibiting recruitment of CREB-binding protein/p300 coactivator. J. Biol. Chem. 2006;281:1620–1629. doi: 10.1074/jbc.M508471200. [DOI] [PubMed] [Google Scholar]
- 41.Wolfgang C.D., Liang G., Okamoto Y., Allen A.E., Hai T. Transcriptional autorepression of the stress-inducible gene ATF3. J. Biol. Chem. 2000;275:16865–16870. doi: 10.1074/jbc.M909637199. [DOI] [PubMed] [Google Scholar]
- 42.Kawai T., Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 43.Whitmore M.M., Iparraguirre A., Kubelka L., Weninger W., Hai T., Williams B.R. Negative regulation of TLR-signaling pathways by activating transcription factor-3. J. Immunol. 2007;179:3622–3630. doi: 10.4049/jimmunol.179.6.3622. [DOI] [PubMed] [Google Scholar]
- 44.Khuu C.H., Barrozo R.M., Hai T., Weinstein S.L. Activating transcription factor 3 (ATF3) represses the expression of CCL4 in murine macrophages. Mol. Immunol. 2007;44:1598–1605. doi: 10.1016/j.molimm.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Suganami T., Yuan X., Shimoda Y., Uchio-Yamada K., Nakagawa N., Shirakawa I., Usami T., Tsukahara T., Nakayama K., Miyamoto Y., et al. Activating transcription factor 3 constitutes a negative feedback mechanism that attenuates saturated Fatty acid/toll-like receptor 4 signaling and macrophage activation in obese adipose tissue. Circ. Res. 2009;105:25–32. doi: 10.1161/CIRCRESAHA.109.196261. [DOI] [PubMed] [Google Scholar]
- 46.Yuan X., Yu L., Li J., Xie G., Rong T., Zhang L., Chen J., Meng Q., Irving A.T., Wang D., et al. ATF3 suppresses metastasis of bladder cancer by regulating gelsolin-mediated remodeling of the actin cytoskeleton. Cancer Res. 2013;73:3625–3637. doi: 10.1158/0008-5472.CAN-12-3879. [DOI] [PubMed] [Google Scholar]
- 47.Lu H., Zhang C., Wu W., Chen H., Lin R., Sun R., Gao X., Li G., He Q., Gao H., et al. MCPIP1 restrains mucosal inflammation by orchestrating the intestinal monocyte to macrophage maturation via an ATF3-AP1S2 axis. Gut. 2023;72:882–895. doi: 10.1136/gutjnl-2022-327183. [DOI] [PubMed] [Google Scholar]
- 48.Nyunt T., Britton M., Wanichthanarak K., Budamagunta M., Voss J.C., Wilson D.W., Rutledge J.C., Aung H.H. Mitochondrial oxidative stress-induced transcript variants of ATF3 mediate lipotoxic brain microvascular injury. Free Radic. Biol. Med. 2019;143:25–46. doi: 10.1016/j.freeradbiomed.2019.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dendorfer U., Oettgen P., Libermann T.A. Multiple regulatory elements in the interleukin-6 gene mediate induction by prostaglandins, cyclic AMP, and lipopolysaccharide. Mol. Cell Biol. 1994;14:4443–4454. doi: 10.1128/mcb.14.7.4443-4454.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Haij S., Bakker A.C., van der Geest R.N., Haegeman G., Vanden Berghe W., Aarbiou J., Daha M.R., van Kooten C. NF-kappaB mediated IL-6 production by renal epithelial cells is regulated by c-jun NH2-terminal kinase. J. Am. Soc. Nephrol. 2005;16:1603–1611. doi: 10.1681/ASN.2004090781. [DOI] [PubMed] [Google Scholar]
- 51.Iezaki T., Ozaki K., Fukasawa K., Inoue M., Kitajima S., Muneta T., Takeda S., Fujita H., Onishi Y., Horie T., et al. ATF3 deficiency in chondrocytes alleviates osteoarthritis development. J. Pathol. 2016;239:426–437. doi: 10.1002/path.4739. [DOI] [PubMed] [Google Scholar]
- 52.Yan F., Wu Y., Liu H., Wu Y., Shen H., Li W. ATF3 is positively involved in particulate matter-induced airway inflammation in vitro and in vivo. Toxicol. Lett. 2018;287:113–121. doi: 10.1016/j.toxlet.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 53.Hoetzenecker W., Echtenacher B., Guenova E., Hoetzenecker K., Woelbing F., Bruck J., Teske A., Valtcheva N., Fuchs K., Kneilling M., et al. ROS-induced ATF3 causes susceptibility to secondary infections during sepsis-associated immunosuppression. Nat. Med. 2011;18:128–134. doi: 10.1038/nm.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balamurugan K., Mendoza-Villanueva D., Sharan S., Summers G.H., Dobrolecki L.E., Lewis M.T., Sterneck E. C/EBPdelta links IL-6 and HIF-1 signaling to promote breast cancer stem cell-associated phenotypes. Oncogene. 2019;38:3765–3780. doi: 10.1038/s41388-018-0516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Litvak V., Ramsey S.A., Rust A.G., Zak D.E., Kennedy K.A., Lampano A.E., Nykter M., Shmulevich I., Aderem A. Function of C/EBPdelta in a regulatory circuit that discriminates between transient and persistent TLR4-induced signals. Nat. Immunol. 2009;10:437–443. doi: 10.1038/ni.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu X., Wei S., Chen M., Li J., Wei Y., Zhang J., Dong W. P2RY13 Exacerbates Intestinal Inflammation by Damaging the Intestinal Mucosal Barrier via Activating IL-6/STAT3 Pathway. Int. J. Biol. Sci. 2022;18:5056–5069. doi: 10.7150/ijbs.74304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kwon J.W., Kwon H.K., Shin H.J., Choi Y.M., Anwar M.A., Choi S. Activating transcription factor 3 represses inflammatory responses by binding to the p65 subunit of NF-kappaB. Sci. Rep. 2015;5:14470. doi: 10.1038/srep14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Labzin L.I., Schmidt S.V., Masters S.L., Beyer M., Krebs W., Klee K., Stahl R., Lutjohann D., Schultze J.L., Latz E., et al. ATF3 Is a Key Regulator of Macrophage IFN Responses. J. Immunol. 2015;195:4446–4455. doi: 10.4049/jimmunol.1500204. [DOI] [PubMed] [Google Scholar]
- 60.Ma J.Q., Li Z., Xie W.R., Liu C.M., Liu S.S. Quercetin protects mouse liver against CCl4-induced inflammation by the TLR2/4 and MAPK/NF-kappaB pathway. Int. Immunopharmacol. 2015;28:531–539. doi: 10.1016/j.intimp.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 61.Shi Q., Hu B., Yang C., Deng S., Cheng X., Wu J., Qi N. ATF3 inhibits arsenic-induced malignant transformation of human bronchial epithelial cells by attenuating inflammation. Toxicology. 2021;460:152890. doi: 10.1016/j.tox.2021.152890. [DOI] [PubMed] [Google Scholar]
- 62.De Nardo D., Labzin L.I., Kono H., Seki R., Schmidt S.V., Beyer M., Xu D., Zimmer S., Lahrmann C., Schildberg F.A., et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat. Immunol. 2014;15:152–160. doi: 10.1038/ni.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yue J., Lopez J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020;21:2346. doi: 10.3390/ijms21072346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morana O., Wood W., Gregory C.D. The Apoptosis Paradox in Cancer. Int. J. Mol. Sci. 2022;23:1328. doi: 10.3390/ijms23031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.D’Arcy M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019;43:582–592. doi: 10.1002/cbin.11137. [DOI] [PubMed] [Google Scholar]
- 66.Bertheloot D., Latz E., Franklin B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell Mol. Immunol. 2021;18:1106–1121. doi: 10.1038/s41423-020-00630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kitakata H., Endo J., Ikura H., Moriyama H., Shirakawa K., Katsumata Y., Sano M. Therapeutic Targets for DOX-Induced Cardiomyopathy: Role of Apoptosis vs. Ferroptosis. Int. J. Mol. Sci. 2022;23:1414. doi: 10.3390/ijms23031414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williams G.T. Role of apoptosis in the immune system. Biochem. Cell Biol. 1994;72:447–450. doi: 10.1139/o94-059. [DOI] [PubMed] [Google Scholar]
- 69.Nagata S., Tanaka M. Programmed cell death and the immune system. Nat. Rev. Immunol. 2017;17:333–340. doi: 10.1038/nri.2016.153. [DOI] [PubMed] [Google Scholar]
- 70.Ekert P.G., Vaux D.L. Apoptosis and the immune system. Br. Med. Bull. 1997;53:591–603. doi: 10.1093/oxfordjournals.bmb.a011632. [DOI] [PubMed] [Google Scholar]
- 71.Place D.E., Lee S., Kanneganti T.D. PANoptosis in microbial infection. Curr. Opin. Microbiol. 2021;59:42–49. doi: 10.1016/j.mib.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Y., Zychlinsky A. Apoptosis induced by bacterial pathogens. Microb. Pathog. 1994;17:203–212. doi: 10.1006/mpat.1994.1066. [DOI] [PubMed] [Google Scholar]
- 73.Hartman M.G., Lu D., Kim M.L., Kociba G.J., Shukri T., Buteau J., Wang X., Frankel W.L., Guttridge D., Prentki M., et al. Role for activating transcription factor 3 in stress-induced beta-cell apoptosis. Mol. Cell Biol. 2004;24:5721–5732. doi: 10.1128/MCB.24.13.5721-5732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nobori K., Ito H., Tamamori-Adachi M., Adachi S., Ono Y., Kawauchi J., Kitajima S., Marumo F., Isobe M. ATF3 inhibits doxorubicin-induced apoptosis in cardiac myocytes: A novel cardioprotective role of ATF3. J. Mol. Cell Cardiol. 2002;34:1387–1397. doi: 10.1006/jmcc.2002.2091. [DOI] [PubMed] [Google Scholar]
- 75.Wang X.M., Liu X.M., Wang Y., Chen Z.Y. Activating transcription factor 3 (ATF3) regulates cell growth, apoptosis, invasion and collagen synthesis in keloid fibroblast through transforming growth factor beta (TGF-beta)/SMAD signaling pathway. Bioengineered. 2021;12:117–126. doi: 10.1080/21655979.2020.1860491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He X., Deng L., Zou K., Tian Y., Tang X. ATF3 Modulates the Proliferation, Migration, and Apoptosis of Synovial Fibroblasts after Arthroscopy by Promoting RGS1 Transcription. Curr. Mol. Med. 2023;23:981–990. doi: 10.2174/1566524023666230417084150. [DOI] [PubMed] [Google Scholar]
- 77.Lin H., Cheng C.F. Activating transcription factor 3, an early cellular adaptive responder in ischemia/reperfusion-induced injury. Ci Ji Yi Xue Za Zhi. 2018;30:61–65. doi: 10.4103/tcmj.tcmj_37_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheng C.F., Lin H. Acute kidney injury and the potential for ATF3-regulated epigenetic therapy. Toxicol. Mech. Methods. 2011;21:362–366. doi: 10.3109/15376516.2011.557876. [DOI] [PubMed] [Google Scholar]
- 79.Yoshida T., Sugiura H., Mitobe M., Tsuchiya K., Shirota S., Nishimura S., Shiohira S., Ito H., Nobori K., Gullans S.R., et al. ATF3 protects against renal ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2008;19:217–224. doi: 10.1681/ASN.2005111155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thiel D.A., Reeder M.K., Pfaff A., Coleman T.R., Sells M.A., Chernoff J. Cell cycle-regulated phosphorylation of p21-activated kinase 1. Curr. Biol. 2002;12:1227–1232. doi: 10.1016/S0960-9822(02)00931-4. [DOI] [PubMed] [Google Scholar]
- 81.Meulmeester E., Jochemsen A.G. p53: A guide to apoptosis. Curr. Cancer Drug Targets. 2008;8:87–97. doi: 10.2174/156800908783769337. [DOI] [PubMed] [Google Scholar]
- 82.Wang L., Deng S., Lu Y., Zhang Y., Yang L., Guan Y., Jiang H., Li H. Increased inflammation and brain injury after transient focal cerebral ischemia in activating transcription factor 3 knockout mice. Neuroscience. 2012;220:100–108. doi: 10.1016/j.neuroscience.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 83.Huang C.Y., Chen J.J., Wu J.S., Tsai H.D., Lin H., Yan Y.T., Hsu C.Y., Ho Y.S., Lin T.N. Novel link of anti-apoptotic ATF3 with pro-apoptotic CTMP in the ischemic brain. Mol. Neurobiol. 2015;51:543–557. doi: 10.1007/s12035-014-8710-0. [DOI] [PubMed] [Google Scholar]
- 84.Mashima T., Udagawa S., Tsuruo T. Involvement of transcriptional repressor ATF3 in acceleration of caspase protease activation during DNA damaging agent-induced apoptosis. J. Cell Physiol. 2001;188:352–358. doi: 10.1002/jcp.1130. [DOI] [PubMed] [Google Scholar]
- 85.Li Y., Pan D., Wang X., Huo Z., Wu X., Li J., Cao J., Xu H., Du L., Xu B. Silencing ATF3 Might Delay TBHP-Induced Intervertebral Disc Degeneration by Repressing NPC Ferroptosis, Apoptosis, and ECM Degradation. Oxid. Med. Cell Longev. 2022;2022:4235126. doi: 10.1155/2022/4235126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Inaba Y., Hashiuchi E., Watanabe H., Kimura K., Oshima Y., Tsuchiya K., Murai S., Takahashi C., Matsumoto M., Kitajima S., et al. The transcription factor ATF3 switches cell death from apoptosis to necroptosis in hepatic steatosis in male mice. Nat. Commun. 2023;14:167. doi: 10.1038/s41467-023-35804-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi Q., Hu B., Yang C., Zhao L., Wu J., Qi N. ATF3 Promotes Arsenic-Induced Apoptosis and Oppositely Regulates DR5 and Bcl-xL Expression in Human Bronchial Epithelial Cells. Int. J. Mol. Sci. 2021;22:4223. doi: 10.3390/ijms22084223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang J.Y., Zong C.S., Xia W., Yamaguchi H., Ding Q., Xie X., Lang J.Y., Lai C.C., Chang C.J., Huang W.C., et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat. Cell Biol. 2008;10:138–148. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maiese A., Spina F., Visi G., Del Duca F., De Matteis A., La Russa R., Di Paolo M., Frati P., Fineschi V. The Expression of FOXO3a as a Forensic Diagnostic Tool in Cases of Traumatic Brain Injury: An Immunohistochemical Study. Int. J. Mol. Sci. 2023;24:2584. doi: 10.3390/ijms24032584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maiese A., Manetti A.C., Santoro P., Del Duca F., De Matteis A., Turillazzi E., Frati P., Fineschi V. FOXO3 Depletion as a Marker of Compression-Induced Apoptosis in the Ligature Mark: An Immunohistochemical Study. Int. J. Mol. Sci. 2023;24:1396. doi: 10.3390/ijms24021396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li D., Qu Y., Mao M., Zhang X., Li J., Ferriero D., Mu D. Involvement of the PTEN-AKT-FOXO3a pathway in neuronal apoptosis in developing rat brain after hypoxia-ischemia. J. Cereb. Blood Flow. Metab. 2009;29:1903–1913. doi: 10.1038/jcbfm.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Van Der Heide L.P., Hoekman M.F., Smidt M.P. The ins and outs of FoxO shuttling: Mechanisms of FoxO translocation and transcriptional regulation. Biochem. J. 2004;380:297–309. doi: 10.1042/bj20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shen L., Lee S., Joo J.C., Hong E., Cui Z.Y., Jo E., Park S.J., Jang H.J. Chelidonium majus Induces Apoptosis of Human Ovarian Cancer Cells via ATF3-Mediated Regulation of Foxo3a by Tip60. J. Microbiol. Biotechnol. 2022;32:493–503. doi: 10.4014/jmb.2109.09030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fang X., Lu G., Ha K., Lin H., Du Y., Zuo Q., Fu Y., Zou C., Zhang P. Acetylation of TIP60 at K104 is essential for metabolic stress-induced apoptosis in cells of hepatocellular cancer. Exp. Cell Res. 2018;362:279–286. doi: 10.1016/j.yexcr.2017.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang Y., Luo J., Zhang W., Gu W. Tip60-dependent acetylation of p53 modulates the decision between cell-cycle arrest and apoptosis. Mol. Cell. 2006;24:827–839. doi: 10.1016/j.molcel.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 96.Qiu M., Liu J., Su Y., Guo R., Zhao B., Liu J. Diosmetin Induces Apoptosis by Downregulating AKT Phosphorylation via P53 Activation in Human Renal Carcinoma ACHN Cells. Protein Pept. Lett. 2020;27:1022–1028. doi: 10.2174/0929866527666200330172646. [DOI] [PubMed] [Google Scholar]
- 97.Yang Y., Sun J., Chen T., Tao Z., Zhang X., Tian F., Zhou X., Lu D. Tat-interactive Protein-60KDA (TIP60) Regulates the Tumorigenesis of Lung Cancer In Vitro. J. Cancer. 2017;8:2277–2281. doi: 10.7150/jca.19677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Y., Ji G., Han S., Shao Z., Lu Z., Huo L., Zhang J., Yang R., Feng Q., Shen H., et al. Tip60 Suppresses Cholangiocarcinoma Proliferation and Metastasis via PI3k-AKT. Cell Physiol. Biochem. 2018;50:612–628. doi: 10.1159/000494183. [DOI] [PubMed] [Google Scholar]
- 99.Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., Fulda S., Gascon S., Hatzios S.K., Kagan V.E., et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shimada K., Skouta R., Kaplan A., Yang W.S., Hayano M., Dixon S.J., Brown L.M., Valenzuela C.A., Wolpaw A.J., Stockwell B.R. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat. Chem. Biol. 2016;12:497–503. doi: 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xie Y., Hou W., Song X., Yu Y., Huang J., Sun X., Kang R., Tang D. Ferroptosis: Process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fu D., Wang C., Yu L., Yu R. Induction of ferroptosis by ATF3 elevation alleviates cisplatin resistance in gastric cancer by restraining Nrf2/Keap1/xCT signaling. Cell Mol. Biol. Lett. 2021;26:26. doi: 10.1186/s11658-021-00271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lu S., Wang X.Z., He C., Wang L., Liang S.P., Wang C.C., Li C., Luo T.F., Feng C.S., Wang Z.C., et al. ATF3 contributes to brucine-triggered glioma cell ferroptosis via promotion of hydrogen peroxide and iron. Acta Pharmacol. Sin. 2021;42:1690–1702. doi: 10.1038/s41401-021-00700-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Y., Yan J., Zhao Q., Zhang Y., Zhang Y. ATF3 promotes ferroptosis in sorafenib-induced cardiotoxicity by suppressing Slc7a11 expression. Front. Pharmacol. 2022;13:904314. doi: 10.3389/fphar.2022.904314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao Y., Du Y., Gao Y., Xu Z., Zhao D., Yang M. ATF3 Regulates Osteogenic Function by Mediating Osteoblast Ferroptosis in Type 2 Diabetic Osteoporosis. Dis. Markers. 2022;2022:9872243. doi: 10.1155/2022/9872243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Y., Chen D., Xie H., Jia M., Sun X., Peng F., Guo F., Tang D. AUF1 protects against ferroptosis to alleviate sepsis-induced acute lung injury by regulating NRF2 and ATF3. Cell Mol. Life Sci. 2022;79:228. doi: 10.1007/s00018-022-04248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Y., Quan F., Cao Q., Lin Y., Yue C., Bi R., Cui X., Yang H., Yang Y., Birnbaumer L., et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2021;28:231–243. doi: 10.1016/j.jare.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu B., Ni Y., Gong Y., Kang X., Guo H., Liu X., Li J., Wang L. Formononetin ameliorates ferroptosis-associated fibrosis in renal tubular epithelial cells and in mice with chronic kidney disease by suppressing the Smad3/ATF3/SLC7A11 signaling. Life Sci. 2023;315:121331. doi: 10.1016/j.lfs.2022.121331. [DOI] [PubMed] [Google Scholar]
- 109.Dong H., Qiang Z., Chai D., Peng J., Xia Y., Hu R., Jiang H. Nrf2 inhibits ferroptosis and protects against acute lung injury due to intestinal ischemia reperfusion via regulating SLC7A11 and HO-1. Aging. 2020;12:12943–12959. doi: 10.18632/aging.103378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ge M.H., Tian H., Mao L., Li D.Y., Lin J.Q., Hu H.S., Huang S.C., Zhang C.J., Mei X.F. Zinc attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury by activating Nrf2/GPX4 defense pathway. CNS Neurosci. Ther. 2021;27:1023–1040. doi: 10.1111/cns.13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shao C.J., Zhou H.L., Gao X.Z., Xu C.F. Downregulation of miR-221-3p promotes the ferroptosis in gastric cancer cells via upregulation of ATF3 to mediate the transcription inhibition of GPX4 and HRD1. Transl. Oncol. 2023;32:101649. doi: 10.1016/j.tranon.2023.101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Y., Zhang M., Bi R., Su Y., Quan F., Lin Y., Yue C., Cui X., Zhao Q., Liu S., et al. ACSL4 deficiency confers protection against ferroptosis-mediated acute kidney injury. Redox Biol. 2022;51:102262. doi: 10.1016/j.redox.2022.102262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miao Z., Tian W., Ye Y., Gu W., Bao Z., Xu L., Sun G., Li C., Tu Y., Chao H., et al. Hsp90 induces Acsl4-dependent glioma ferroptosis via dephosphorylating Ser637 at Drp1. Cell Death Dis. 2022;13:548. doi: 10.1038/s41419-022-04997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang X., Tomso D.J., Chorley B.N., Cho H.Y., Cheung V.G., Kleeberger S.R., Bell D.A. Identification of polymorphic antioxidant response elements in the human genome. Hum. Mol. Genet. 2007;16:1188–1200. doi: 10.1093/hmg/ddm066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pajares M., Rojo A.I., Arias E., Diaz-Carretero A., Cuervo A.M., Cuadrado A. Transcription factor NFE2L2/NRF2 modulates chaperone-mediated autophagy through the regulation of LAMP2A. Autophagy. 2018;14:1310–1322. doi: 10.1080/15548627.2018.1474992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Feng R., Xiong Y., Lei Y., Huang Q., Liu H., Zhao X., Chen Z., Chen H., Liu X., Wang L., et al. Lysine-specific demethylase 1 aggravated oxidative stress and ferroptosis induced by renal ischemia and reperfusion injury through activation of TLR4/NOX4 pathway in mice. J. Cell Mol. Med. 2022;26:4254–4267. doi: 10.1111/jcmm.17444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu L., Jia M., Xiao L., Wang Z., Yao R., Zhang Y., Gao L. TRIM-containing 44 aggravates cardiac hypertrophy via TLR4/NOX4-induced ferroptosis. J. Mol. Med. 2023;101:685–697. doi: 10.1007/s00109-023-02318-3. [DOI] [PubMed] [Google Scholar]
- 118.Liu H., Mo H., Yang C., Mei X., Song X., Lu W., Xiao H., Yan J., Wang X., Yan J., et al. A novel function of ATF3 in suppression of ferroptosis in mouse heart suffered ischemia/reperfusion. Free Radic. Biol. Med. 2022;189:122–135. doi: 10.1016/j.freeradbiomed.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 119.Park T.J., Park J.H., Lee G.S., Lee J.Y., Shin J.H., Kim M.W., Kim Y.S., Kim J.Y., Oh K.J., Han B.S., et al. Quantitative proteomic analyses reveal that GPX4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes. Cell Death Dis. 2019;10:835. doi: 10.1038/s41419-019-2061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sood V., Sharma K.B., Gupta V., Saha D., Dhapola P., Sharma M., Sen U., Kitajima S., Chowdhury S., Kalia M., et al. ATF3 negatively regulates cellular antiviral signaling and autophagy in the absence of type I interferons. Sci. Rep. 2017;7:8789. doi: 10.1038/s41598-017-08584-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barnabas S., Hai T., Andrisani O.M. The hepatitis B virus X protein enhances the DNA binding potential and transcription efficacy of bZip transcription factors. J. Biol. Chem. 1997;272:20684–20690. doi: 10.1074/jbc.272.33.20684. [DOI] [PubMed] [Google Scholar]
- 122.Shiromoto F., Aly H.H., Kudo H., Watashi K., Murayama A., Watanabe N., Zheng X., Kato T., Chayama K., Muramatsu M., et al. IL-1beta/ATF3-mediated induction of Ski2 expression enhances hepatitis B virus x mRNA degradation. Biochem. Biophys. Res. Commun. 2018;503:1854–1860. doi: 10.1016/j.bbrc.2018.07.126. [DOI] [PubMed] [Google Scholar]
- 123.Chen H., Zhang Y., Ye S., Wu Q., Lin Y., Sheng K., Chen W., Lin X., Lin X. Chromatin remodelling factor BAF155 protects hepatitis B virus X protein (HBx) from ubiquitin-independent proteasomal degradation. Emerg. Microbes Infect. 2019;8:1393–1405. doi: 10.1080/22221751.2019.1666661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim K.H., Seong B.L. Pro-apoptotic function of HBV X protein is mediated by interaction with c-FLIP and enhancement of death-inducing signal. EMBO J. 2003;22:2104–2116. doi: 10.1093/emboj/cdg210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Orange J.S., Biron C.A. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J. Immunol. 1996;156:1138–1142. doi: 10.4049/jimmunol.156.3.1138. [DOI] [PubMed] [Google Scholar]
- 126.Rosenberger C.M., Clark A.E., Treuting P.M., Johnson C.D., Aderem A. ATF3 regulates MCMV infection in mice by modulating IFN-gamma expression in natural killer cells. Proc. Natl. Acad. Sci. USA. 2008;105:2544–2549. doi: 10.1073/pnas.0712182105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pien G.C., Satoskar A.R., Takeda K., Akira S., Biron C.A. Cutting edge: Selective IL-18 requirements for induction of compartmental IFN-gamma responses during viral infection. J. Immunol. 2000;165:4787–4791. doi: 10.4049/jimmunol.165.9.4787. [DOI] [PubMed] [Google Scholar]
- 128.Orange J.S., Salazar-Mather T.P., Opal S.M., Biron C.A. Mechanisms for virus-induced liver disease: Tumor necrosis factor-mediated pathology independent of natural killer and T cells during murine cytomegalovirus infection. J. Virol. 1997;71:9248–9258. doi: 10.1128/jvi.71.12.9248-9258.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Orange J.S., Wang B., Terhorst C., Biron C.A. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J. Exp. Med. 1995;182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tay C.H., Welsh R.M. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J. Virol. 1997;71:267–275. doi: 10.1128/jvi.71.1.267-275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kang W., Mukerjee R., Fraser N.W. Establishment and maintenance of HSV latent infection is mediated through correct splicing of the LAT primary transcript. Virology. 2003;312:233–244. doi: 10.1016/S0042-6822(03)00201-0. [DOI] [PubMed] [Google Scholar]
- 132.Shu M., Du T., Zhou G., Roizman B. Role of activating transcription factor 3 in the synthesis of latency-associated transcript and maintenance of herpes simplex virus 1 in latent state in ganglia. Proc. Natl. Acad. Sci. USA. 2015;112:E5420–E5426. doi: 10.1073/pnas.1515369112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kiryu-Seo S., Kato R., Ogawa T., Nakagomi S., Nagata K., Kiyama H. Neuronal injury-inducible gene is synergistically regulated by ATF3, c-Jun, and STAT3 through the interaction with Sp1 in damaged neurons. J. Biol. Chem. 2008;283:6988–6996. doi: 10.1074/jbc.M707514200. [DOI] [PubMed] [Google Scholar]
- 134.Schiffman M., Castle P.E., Jeronimo J., Rodriguez A.C., Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 135.zur Hausen H. Papillomaviruses causing cancer: Evasion from host-cell control in early events in carcinogenesis. J. Natl. Cancer Inst. 2000;92:690–698. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]
- 136.Scheffner M., Werness B.A., Huibregtse J.M., Levine A.J., Howley P.M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 137.Martinez-Zapien D., Ruiz F.X., Poirson J., Mitschler A., Ramirez J., Forster A., Cousido-Siah A., Masson M., Vande Pol S., Podjarny A., et al. Structure of the E6/E6AP/p53 complex required for HPV-mediated degradation of p53. Nature. 2016;529:541–545. doi: 10.1038/nature16481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang H., Mo P., Ren S., Yan C. Activating transcription factor 3 activates p53 by preventing E6-associated protein from binding to E6. J. Biol. Chem. 2010;285:13201–13210. doi: 10.1074/jbc.M109.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kooti A., Abuei H., Farhadi A., Behzad-Behbahani A., Zarrabi M. Activating transcription factor 3 mediates apoptotic functions through a p53-independent pathway in human papillomavirus 18 infected HeLa cells. Virus Genes. 2022;58:88–97. doi: 10.1007/s11262-022-01887-8. [DOI] [PubMed] [Google Scholar]
- 140.Badu P., Pager C.T. Activation of ATF3 via the Integrated Stress Response Pathway Regulates Innate Immune and Autophagy Processes to Restrict Zika Virus. bioRxiv. 2023 doi: 10.1101/2023.07.26.550716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hwang H.Y., Kim J.Y., Lim J.Y., Chung S.K., Nam J.H., Park S.I. Coxsackievirus B3 modulates cell death by downregulating activating transcription factor 3 in HeLa cells. Virus Res. 2007;130:10–17. doi: 10.1016/j.virusres.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 142.Mishra R., Lahon A., Banerjea A.C. Dengue Virus Degrades USP33-ATF3 Axis via Extracellular Vesicles to Activate Human Microglial Cells. J. Immunol. 2020;205:1787–1798. doi: 10.4049/jimmunol.2000411. [DOI] [PubMed] [Google Scholar]
- 143.Henderson A., Holloway A., Reeves R., Tremethick D.J. Recruitment of SWI/SNF to the human immunodeficiency virus type 1 promoter. Mol. Cell Biol. 2004;24:389–397. doi: 10.1128/MCB.24.1.389-397.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Calton C.M., Wade L.K., So M. Upregulation of ATF3 inhibits expression of the pro-inflammatory cytokine IL-6 during Neisseria gonorrhoeae infection. Cell Microbiol. 2013;15:1837–1850. doi: 10.1111/cmi.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhao Q., Luo Y.F., Tian M., Xiao Y.L., Cai H.R., Li H. Activating transcription factor 3 involved in Pseudomonas aeruginosa PAO1-induced macrophage senescence. Mol. Immunol. 2021;133:122–127. doi: 10.1016/j.molimm.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 146.Zhao Y., Wu X., Qian L., Guo L., Liao J., Wu X. Activating transcription factor 3 protects mice against pseudomonas aeruginosa-induced acute lung injury by interacting with lipopolysaccharide binding protein. Mol. Immunol. 2017;90:27–32. doi: 10.1016/j.molimm.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 147.Zhang B., Li H., Zhang J., Hang Y., Xu Y. Activating transcription factor 3 protects alveolar epithelial type II cells from Mycobacterium tuberculosis infection-induced inflammation. Tuberculosis. 2022;135:102227. doi: 10.1016/j.tube.2022.102227. [DOI] [PubMed] [Google Scholar]
- 148.Wang J., Cheng W., Wang Z., Xin L., Zhang W. ATF3 inhibits the inflammation induced by Mycoplasma pneumonia in vitro and in vivo. Pediatr. Pulmonol. 2017;52:1163–1170. doi: 10.1002/ppul.23705. [DOI] [PubMed] [Google Scholar]
- 149.Kwon O., Soung N.K., Thimmegowda N.R., Jeong S.J., Jang J.H., Moon D.O., Chung J.K., Lee K.S., Kwon Y.T., Erikson R.L., et al. Patulin induces colorectal cancer cells apoptosis through EGR-1 dependent ATF3 up-regulation. Cell Signal. 2012;24:943–950. doi: 10.1016/j.cellsig.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yuan L., Mu P., Huang B., Li H., Mu H., Deng Y. EGR1 is essential for deoxynivalenol-induced G2/M cell cycle arrest in HepG2 cells via the ATF3DeltaZip2a/2b-EGR1-p21 pathway. Toxicol. Lett. 2018;299:95–103. doi: 10.1016/j.toxlet.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 151.Abhishek K., Das S., Kumar A., Kumar A., Kumar V., Saini S., Mandal A., Verma S., Kumar M., Das P. Leishmania donovani induced Unfolded Protein Response delays host cell apoptosis in PERK dependent manner. PLoS Negl. Trop. Dis. 2018;12:e0006646. doi: 10.1371/journal.pntd.0006646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Saha S., Roy S., Dutta A., Jana K., Ukil A. Leishmania donovani Targets Host Transcription Factor NRF2 To Activate Antioxidant Enzyme HO-1 and Transcriptional Repressor ATF3 for Establishing Infection. Infect. Immun. 2021;89:e0076420. doi: 10.1128/IAI.00764-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schindler C., Levy D.E., Decker T. JAK-STAT signaling: From interferons to cytokines. J. Biol. Chem. 2007;282:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 154.Sharma M., Bhattacharyya S., Nain M., Kaur M., Sood V., Gupta V., Khasa R., Abdin M.Z., Vrati S., Kalia M. Japanese encephalitis virus replication is negatively regulated by autophagy and occurs on LC3-I- and EDEM1-containing membranes. Autophagy. 2014;10:1637–1651. doi: 10.4161/auto.29455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.McFadden M.J., Gokhale N.S., Horner S.M. Protect this house: Cytosolic sensing of viruses. Curr. Opin. Virol. 2017;22:36–43. doi: 10.1016/j.coviro.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gratton R., Agrelli A., Tricarico P.M., Brandao L., Crovella S. Autophagy in Zika Virus Infection: A Possible Therapeutic Target to Counteract Viral Replication. Int. J. Mol. Sci. 2019;20:1048. doi: 10.3390/ijms20051048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Echavarria-Consuegra L., Smit J.M., Reggiori F. Role of autophagy during the replication and pathogenesis of common mosquito-borne flavi- and alphaviruses. Open Biol. 2019;9:190009. doi: 10.1098/rsob.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lin H., Li H.F., Chen H.H., Lai P.F., Juan S.H., Chen J.J., Cheng C.F. Activating transcription factor 3 protects against pressure-overload heart failure via the autophagy molecule Beclin-1 pathway. Mol. Pharmacol. 2014;85:682–691. doi: 10.1124/mol.113.090092. [DOI] [PubMed] [Google Scholar]
- 159.Carthy C.M., Granville D.J., Watson K.A., Anderson D.R., Wilson J.E., Yang D., Hunt D.W., McManus B.M. Caspase activation and specific cleavage of substrates after coxsackievirus B3-induced cytopathic effect in HeLa cells. J. Virol. 1998;72:7669–7675. doi: 10.1128/JVI.72.9.7669-7675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rothman A.L. Immunity to dengue virus: A tale of original antigenic sin and tropical cytokine storms. Nat. Rev. Immunol. 2011;11:532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- 161.Li J., Yan X., Li B., Huang L., Wang X., He B., Xie H., Wu Q., Chen L. Identification and validation of ferroptosis-related genes in patients infected with dengue virus: Implication in the pathogenesis of DENV. Virus Genes. 2023;59:377–390. doi: 10.1007/s11262-023-01985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tripathy M.K., Abbas W., Herbein G. Epigenetic regulation of HIV-1 transcription. Epigenomics. 2011;3:487–502. doi: 10.2217/epi.11.61. [DOI] [PubMed] [Google Scholar]
- 163.Verdin E., Paras P., Jr., Van Lint C. Chromatin disruption in the promoter of human immunodeficiency virus type 1 during transcriptional activation. EMBO J. 1993;12:3249–3259. doi: 10.1002/j.1460-2075.1993.tb05994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.el Kharroubi A., Martin M.A. cis-acting sequences located downstream of the human immunodeficiency virus type 1 promoter affect its chromatin structure and transcriptional activity. Mol. Cell Biol. 1996;16:2958–2966. doi: 10.1128/MCB.16.6.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rabbi M.F., Saifuddin M., Gu D.S., Kagnoff M.F., Roebuck K.A. U5 region of the human immunodeficiency virus type 1 long terminal repeat contains TRE-like cAMP-responsive elements that bind both AP-1 and CREB/ATF proteins. Virology. 1997;233:235–245. doi: 10.1006/viro.1997.8602. [DOI] [PubMed] [Google Scholar]
- 166.Van Lint C., Amella C.A., Emiliani S., John M., Jie T., Verdin E. Transcription factor binding sites downstream of the human immunodeficiency virus type 1 transcription start site are important for virus infectivity. J. Virol. 1997;71:6113–6127. doi: 10.1128/jvi.71.8.6113-6127.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Henderson A., Bunce M., Siddon N., Reeves R., Tremethick D.J. High-mobility-group protein I can modulate binding of transcription factors to the U5 region of the human immunodeficiency virus type 1 proviral promoter. J. Virol. 2000;74:10523–10534. doi: 10.1128/JVI.74.22.10523-10534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wallace V.C., Blackbeard J., Pheby T., Segerdahl A.R., Davies M., Hasnie F., Hall S., McMahon S.B., Rice A.S. Pharmacological, behavioural and mechanistic analysis of HIV-1 gp120 induced painful neuropathy. Pain. 2007;133:47–63. doi: 10.1016/j.pain.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bekeredjian-Ding I., Stein C., Uebele J. The Innate Immune Response Against Staphylococcus aureus. Curr. Top. Microbiol. Immunol. 2017;409:385–418. doi: 10.1007/82_2015_5004. [DOI] [PubMed] [Google Scholar]
- 170.De Luca A., Pariano M., Cellini B., Costantini C., Villella V.R., Jose S.S., Palmieri M., Borghi M., Galosi C., Paolicelli G., et al. The IL-17F/IL-17RC Axis Promotes Respiratory Allergy in the Proximal Airways. Cell Rep. 2017;20:1667–1680. doi: 10.1016/j.celrep.2017.07.063. [DOI] [PubMed] [Google Scholar]
- 171.Choi S.M., McAleer J.P., Zheng M., Pociask D.A., Kaplan M.H., Qin S., Reinhart T.A., Kolls J.K. Innate Stat3-mediated induction of the antimicrobial protein Reg3gamma is required for host defense against MRSA pneumonia. J. Exp. Med. 2013;210:551–561. doi: 10.1084/jem.20120260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Robinson K.M., Choi S.M., McHugh K.J., Mandalapu S., Enelow R.I., Kolls J.K., Alcorn J.F. Influenza A exacerbates Staphylococcus aureus pneumonia by attenuating IL-1beta production in mice. J. Immunol. 2013;191:5153–5159. doi: 10.4049/jimmunol.1301237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ritchie N.D., Ritchie R., Bayes H.K., Mitchell T.J., Evans T.J. IL-17 can be protective or deleterious in murine pneumococcal pneumonia. PLoS Pathog. 2018;14:e1007099. doi: 10.1371/journal.ppat.1007099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wonnenberg B., Jungnickel C., Honecker A., Wolf L., Voss M., Bischoff M., Tschernig T., Herr C., Bals R., Beisswenger C. IL-17A attracts inflammatory cells in murine lung infection with P. aeruginosa. Innate Immun. 2016;22:620–625. doi: 10.1177/1753425916668244. [DOI] [PubMed] [Google Scholar]
- 175.Lu Y.J., Gross J., Bogaert D., Finn A., Bagrade L., Zhang Q., Kolls J.K., Srivastava A., Lundgren A., Forte S., et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008;4:e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Park J.M., Ng V.H., Maeda S., Rest R.F., Karin M. Anthrolysin O and other gram-positive cytolysins are toll-like receptor 4 agonists. J. Exp. Med. 2004;200:1647–1655. doi: 10.1084/jem.20041215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Annane D., Bellissant E., Cavaillon J.M. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 178.Sawa T. The molecular mechanism of acute lung injury caused by Pseudomonas aeruginosa: From bacterial pathogenesis to host response. J. Intensive Care. 2014;2:10. doi: 10.1186/2052-0492-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Deshpande R., Zou C. Pseudomonas Aeruginosa Induced Cell Death in Acute Lung Injury and Acute Respiratory Distress Syndrome. Int. J. Mol. Sci. 2020;21:5356. doi: 10.3390/ijms21155356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Peyron P., Vaubourgeix J., Poquet Y., Levillain F., Botanch C., Bardou F., Daffe M., Emile J.F., Marchou B., Cardona P.J., et al. Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog. 2008;4:e1000204. doi: 10.1371/journal.ppat.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Russell D.G., Cardona P.J., Kim M.J., Allain S., Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat. Immunol. 2009;10:943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wu Y.P., Cao C., Wu Y.F., Li M., Lai T.W., Zhu C., Wang Y., Ying S.M., Chen Z.H., Shen H.H., et al. Activating transcription factor 3 represses cigarette smoke-induced IL6 and IL8 expression via suppressing NF-kappaB activation. Toxicol. Lett. 2017;270:17–24. doi: 10.1016/j.toxlet.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 183.Wang J., Yang K., Zhou L., Minhaowu, Wu Y., Zhu M., Lai X., Chen T., Feng L., Li M., et al. MicroRNA-155 promotes autophagy to eliminate intracellular mycobacteria by targeting Rheb. PLoS Pathog. 2013;9:e1003697. doi: 10.1371/journal.ppat.1003697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Feng L., Hu J., Zhang W., Dong Y., Xiong S., Dong C. RELL1 inhibits autophagy pathway and regulates Mycobacterium tuberculosis survival in macrophages. Tuberculosis. 2020;120:101900. doi: 10.1016/j.tube.2020.101900. [DOI] [PubMed] [Google Scholar]
- 185.Glaser N., Stopper H. Patulin: Mechanism of genotoxicity. Food Chem. Toxicol. 2012;50:1796–1801. doi: 10.1016/j.fct.2012.02.096. [DOI] [PubMed] [Google Scholar]
- 186.Heussner A.H., Dietrich D.R., O’Brien E. In vitro investigation of individual and combined cytotoxic effects of ochratoxin A and other selected mycotoxins on renal cells. Toxicol Vitr. 2006;20:332–341. doi: 10.1016/j.tiv.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 187.Wu T.S., Liao Y.C., Yu F.Y., Chang C.H., Liu B.H. Mechanism of patulin-induced apoptosis in human leukemia cells (HL-60) Toxicol. Lett. 2008;183:105–111. doi: 10.1016/j.toxlet.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 188.Wu Q., Dohnal V., Kuca K., Yuan Z. Trichothecenes: Structure-toxic activity relationships. Curr. Drug Metab. 2013;14:641–660. doi: 10.2174/1389200211314060002. [DOI] [PubMed] [Google Scholar]
- 189.Naik S., Mohammed A. Coexpression network analysis of human candida infection reveals key modules and hub genes responsible for host-pathogen interactions. Front. Genet. 2022;13:917636. doi: 10.3389/fgene.2022.917636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhu G.D., Xie L.M., Su J.W., Cao X.J., Yin X., Li Y.P., Gao Y.M., Guo X.G. Identification of differentially expressed genes and signaling pathways with Candida infection by bioinformatics analysis. Eur. J. Med. Res. 2022;27:43. doi: 10.1186/s40001-022-00651-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Olivier M., Gregory D.J., Forget G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: A signaling point of view. Clin. Microbiol. Rev. 2005;18:293–305. doi: 10.1128/CMR.18.2.293-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pham N.K., Mouriz J., Kima P.E. Leishmania pifanoi amastigotes avoid macrophage production of superoxide by inducing heme degradation. Infect. Immun. 2005;73:8322–8333. doi: 10.1128/IAI.73.12.8322-8333.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liu H., Kuang X., Zhang Y., Ye Y., Li J., Liang L., Xie Z., Weng L., Guo J., Li H., et al. ADORA1 Inhibition Promotes Tumor Immune Evasion by Regulating the ATF3-PD-L1 Axis. Cancer Cell. 2020;37:324–339.e328. doi: 10.1016/j.ccell.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 194.Lu Z., McBrearty N., Chen J., Tomar V.S., Zhang H., De Rosa G., Tan A., Weljie A.M., Beiting D.P., Miao Z., et al. ATF3 and CH25H regulate effector trogocytosis and anti-tumor activities of endogenous and immunotherapeutic cytotoxic T lymphocytes. Cell Metab. 2022;34:1342–1358.e7. doi: 10.1016/j.cmet.2022.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Di Marcantonio D., Martinez E., Kanefsky J.S., Huhn J.M., Gabbasov R., Gupta A., Krais J.J., Peri S., Tan Y., Skorski T., et al. ATF3 coordinates serine and nucleotide metabolism to drive cell cycle progression in acute myeloid leukemia. Mol. Cell. 2021;81:2752–2764.e6. doi: 10.1016/j.molcel.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Huo S., Wang Q., Shi W., Peng L., Jiang Y., Zhu M., Guo J., Peng D., Wang M., Men L., et al. ATF3/SPI1/SLC31A1 Signaling Promotes Cuproptosis Induced by Advanced Glycosylation End Products in Diabetic Myocardial Injury. Int. J. Mol. Sci. 2023;24:1667. doi: 10.3390/ijms24021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ku H.C., Cheng C.F. Master Regulator Activating Transcription Factor 3 (ATF3) in Metabolic Homeostasis and Cancer. Front. Endocrinol. 2020;11:556. doi: 10.3389/fendo.2020.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data were used for the research described in the article.