Abstract
Colonization of the nasopharynx by a middle ear pathogen is the first step in the development of otitis media in humans. The establishment of an animal model of nasopharyngeal colonization would therefore be of great utility in assessing the potential protective ability of candidate vaccine antigens (especially adhesins) against otitis media. A chinchilla nasopharyngeal colonization model for nontypeable Haemophilus influenzae (NTHI) was developed with antibiotic-resistant strains. This model does not require coinfection with a virus. There was no significant difference in the efficiency of NTHI colonization between adult (1- to 2-year-old) and young (2- to 3-month-old) animals. However, the incidence of middle ear infection following nasopharyngeal colonization was significantly higher in young animals (83 to 89%) than in adult chinchillas (10 to 30%). Chinchillas that had recovered either from a previous middle ear infection caused by NTHI or from an infection by intranasal inoculation with NTHI were completely protected against nasopharyngeal colonization with a homologous strain and were found to be the best positive controls in protection studies. Systemic immunization of chinchillas with inactivated whole-cell preparations significantly protected animals not only against homologous NTHI colonization but also partially against heterologous NTHI infection. In all protected animals, significant serum anti-P6 and anti-HMW antibody responses were observed. The outer membrane P6 and high-molecular-weight (HMW) proteins appear to be promising candidate vaccine antigens to prevent nasopharyngeal colonization and middle ear infection caused by NTHI.
Haemophilus influenzae is an important cause of both local and systemic diseases. More specifically, nontypeable H. influenzae (NTHI) is associated with a number of mucosal diseases, including sinusitis, bronchitis, conjunctivitis, and otitis media, the most common childhood illness requiring a doctor’s visit. Most children have had at least one episode of otitis by their third birthday, and one-third have experienced three or more ear infections (29). It has been well accepted that otitis media due to NTHI begins with the colonization of the nasopharyngeal (NP) epithelium, followed by a contiguous spread through the eustachian tube, leading to infection of the middle ear space (7, 14). A strong relationship has been found between NP colonization with NTHI and the incidence of otitis media in children (13), although not all episodes of otitis media were due to NTHI. The nature of the interactions between NTHI and the host involved in colonization and the subsequent onset of disease are not completely understood; however, host immunity is believed to play an important role in this sequence of events, and antibody directed against surface antigens of H. influenzae are thought to be central to host protection (10, 14, 30).
In recent years, research efforts have focused on the development of an otitis media vaccine. The availability of a relevant animal model would greatly facilitate our understanding of the pathogenesis of otitis media and the search for candidate vaccine antigens. The chinchilla is currently used in the experimental model of otitis media, since it is not susceptible to middle ear infections, which naturally occur in guinea pigs and rabbits, and since its middle ear structure is anatomically similar to that of humans. In the traditional intrabulla inoculation model, freshly grown NTHI is directly introduced into the middle ear space of chinchillas via the epitympanic bulla (1, 8) and inflammation of the tympanic membrane is monitored every 2 to 3 days after challenge. Effusion is sampled by middle ear aspiration via the epitympanic bulla, the sample is cultured on chocolate agar, and bacteria are quantified 24 h later. This model has been a useful tool for investigating the importance of host immunity in the prevention of NTHI-related disease and for screening potential vaccine antigens against the bacteria (2, 6, 11). However, interpretation of the results obtained from this model remains difficult, since the mechanism of ear infection induced by the pathogen is not analogous to that in humans. This model is particularly unsatisfactory when applied to studies of NTHI adhesins, which play a crucial role in the initial step of NP colonization but become less important in the pathogenesis of middle ear infection.
The objective of the present investigation was to establish an NP colonization model with NTHI in chinchillas and to use it as a tool for screening potential otitis media vaccine antigens, in particular adhesins. Our results indicate that chinchillas, when immunized with an inactivated NTHI whole-cell preparation or having recovered from a previous NTHI infection, were well protected against a subsequent challenge with the homologous NTHI strain. These animals were also protected to a certain degree against a heterologous NTHI challenge.
(These data were presented in part at the 97th General Meeting of the American Society for Microbiology, 4 to 8 May 1997, Miami Beach, Fla., [30a].)
MATERIALS AND METHODS
Animals.
Grey chinchillas (Chinchilla laniger), 2 to 3 months old (∼215 g), 4 to 5 months old (∼370 g), or 1 to 2 years old (∼575 g), that were free of middle ear disease (as determined by otoscopy and tympanometry) were purchased from Moulton Chinchilla Ranch (Rochester, Minn.). A total of 32 to 36 chinchillas were used for each study, with an average of 8 to 9 animals per group.
Bacterial strains.
NTHI 12 was kindly provided by S. Barenkamp (St. Louis University, St. Louis, Mo.). NTHI LCDC2 was a gift from the Laboratory Center for Disease Control (Ottawa, Ontario, Canada). No significant genetic or phenotypic differences were found between strains 12 and LCDC2, and both strains expressed high-molecular-weight (HMW) adhesin proteins.
Generation of streptomycin-resistant NTHI strains.
NTHI 12 and LCDC2 were grown as previously described (1). A 700-μl portion of freshly grown culture (absorbance at 578 nm ≈0.5 to 0.7) was streaked on Mueller-Hinton agar plates (Becton-Dickinson) supplemented with hemin (BDH Inc.) and NAD (Sigma), both at 2 μg ml−1, and 10 μg of streptomycin ml−1. The plates were incubated at 37°C for 48 h or until colonies were seen. Bacteria were harvested with saline and streaked again on the above plates containing 25 μg of streptomycin ml−1. The above procedures were repeated with gradually increasing concentrations of streptomycin. The colonies growing in the presence of 100 μg of streptomycin ml−1 were harvested with brain heart infusion medium (Difco Laboratories) supplemented with hemin and NAD and stored at −70°C.
SDS-PAGE and immunoblotting.
Protein samples were subjected to discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) through 12.5% polyacrylamide gels as described by Laemmli (17) and visualized with Rapid Coomassie blue stain (Diversified Biotech). For immunoblot analysis, the proteins were electrophoretically transferred to Immobilon polyvinylidene difluoride (Millipore) membranes and probed with either 1:500 dilutions of chinchilla sera, 1:20 dilutions of chinchilla nasal lavage fluids, or 1:1,000 dilutions of guinea pig anti-P6 antiserum. Recombinant protein G conjugated to alkaline phosphatase (Zymed Laboratories) was used as the secondary reagent to detect immunoglobulin G (IgG) responses. To measure specific IgA responses in chinchilla nasal secretions, the polyvinylidene difluoride membranes were probed with 1:20 dilutions of nasal lavage fluids followed by guinea pig anti-α-chain antiserum and goat anti-guinea pig (Fc-specific) antibody conjugated to either alkaline phosphatase or horseradish peroxidase (HRP; Jackson ImmunoResearch Laboratories). The LumiGlo substrate (Kirkegaard & Perry Laboratories) was used to detect HRP activity.
Purification of chinchilla IgA from milk and chinchilla IgA heavy chain (α chain).
Chinchilla milk collected within the first 5 days after delivery was purchased from Moulton Chinchilla Ranch. The milk was diluted threefold with phosphate-buffered saline (PBS) and then centrifuged at 20,000 × g for 30 min. The top layer (fat) and bottom layer (mainly casein) were discarded. The middle layer which contained the Igs, was filtered through a 0.45-μm-pore-size filter (Millex-HV; Millipore), and 2 ml of the filtrate was loaded onto a Superdex 200 column (two tandem columns, 1.6 by 60 cm each [Pharmacia]) preequilibrated in PBS. The IgM was recovered in the void volume. The second protein peak fractions, which contained both IgA and IgG, were pooled and then concentrated 10-fold with a Centriprep 30 (Millipore). The concentrated solution was then dialyzed against 0.1 M Tris-HCl (pH 8.0) at 4°C overnight. After dialysis, 5 ml of the solution was incubated with 1 ml of protein G-Sepharose (Pierce) for 30 min at room temperature. IgG bound to the column, whereas IgA was recovered in the flowthrough fraction.
The heavy chain (55 kDa) and the light chain (25 kDa) of IgA were separated by SDS-PAGE (12.5% polyacrylamide) (13). The 55-kDa protein band was recovered from the gel slice by electroelution with a Bio-Rad apparatus for 12 h at room temperature in 50 mM NH4HCO3 (pH 8.2) containing 0.1% SDS.
Purification of chinchilla IgG from serum.
Chinchilla IgG was purified from chinchilla serum as previously described (1).
Generation and purification of guinea pig anti-chinchilla α chain and IgG antibodies.
Guinea pigs (Charles River) were immunized intramuscularly (i.m.) on day 1 with 5 μg of either purified chinchilla IgG or electroeluted α chain emulsified in complete Freund’s adjuvant (CFA) (Difco Laboratories). The animals were given booster immunizations on days 14 and 28 with the same dose of protein emulsified in incomplete Freund’s adjuvant (IFA) (Difco Laboratories). Guinea pig antisera were collected 2 weeks after the last injection. Protein A affinity purification of guinea pig anti-chinchilla IgG and anti-α-chain antibodies was performed by the method of Barenkamp (1).
Whole-cell enzyme-linked immunosorbent assays (ELISAs).
NTHI 12 was grown as previously described (1). Cell pellets were collected by centrifugation, washed with PBS, and resuspended in 50 mM carbonate-bicarbonate buffer (pH 9.6). The optical density of the suspension was adjusted to 0.5 at 490 nm, and 100 μl of a 1:100 dilution of NTHI 12 preparation was used to coat microtiter wells. The plates were air dried at 37°C overnight and then blocked with PBS–0.1% bovine serum albumin at 37°C for 1 h (250 μl per well). After three washes with PBS–0.1% Tween 20, 200 μl of antisera or nasal wash solutions at an appropriate dilution (in PBS–0.1% gelatin) was added to the wells, and the mixture was further incubated at 37°C for 2 h. Affinity-purified guinea pig anti-chinchilla α chain (5 μg ml−1) or anti-IgG antibody (1 μg ml−1) was used as a secondary antibody. Affinity-purified F(ab′)2 fragment of goat anti-guinea pig IgG (heavy plus light-chain) antibodies conjugated to HRP (Jackson ImmunoResearch Laboratories) was used as a reporter. The reaction products were developed with tetramethylbenzidine (TMB)–H2O2 (Aldrich Chemical Co.), and absorbances were measured at 450 nm (with 540 nm as a reference wavelength) in a Flow Multiskan MCC microplate reader (ICN Biomedicals). The reactive titer of an antiserum was defined as the reciprocal of the dilution consistently showing a twofold increase in absorbance over that obtained with the prebleed serum sample.
Protection studies.
Groups of eight or nine animals were immunized three times i.m. with either 2 × 109 CFU of heat-inactivated (56°C for 10 min) NTHI 12 or LCDC2 in alum or alum alone on days 0, 14, and 28. Serum samples and nasal wash samples were taken on day 42 for measurement of anti-NTHI whole-cell antibody titers by an ELISA. The weight of each animal was recorded on days 0 and 42.
On day 44, the animals were lightly anesthetized with xylazine-ketamine HCl by i.m. injection (0.06 mg of xylazine and 0.3 mg of ketamine HCl per kg of body weight). Intranasal inoculations were performed via passive inhalation (50 μl per nares, for a total of 0.1 ml per animal) of freshly cultured streptomycin-resistant NTHI 12 in brain heart infusion medium supplemented with hemin and NAD both at 2 μg ml−1. The dose of challenge bacteria was 108 CFU per animal.
Nasopharyngeal lavages were performed 4 days postinoculation. A sterile open-end Tom Cat catheter (5.5 in. long, French size 3 1/2 [Sherwood Medical]) was connected (through the female luer opening) to a 3CC 23G1 syringe (without the needle) and 1 ml of sterile saline was withdrawn. The open-end smooth beveled tip of the catheter was gently inserted into one nares of an anesthetized chinchilla (xylazine-ketamine HCl, same route and dose as on day 44) which was laid on one side, until a resistance was felt (about 1 cm of the tip can be easily inserted into the nares). The animal was then held with its head slightly down and forward. Secretions were obtained by irrigating the nasopharynx with 1 ml of sterile saline and collecting fluid from the contralateral nares. Normally, about 500 μl of fluid was collected from each animal and 25 μl of sample was plated on a chocolate agar plate in the presence of 50 μl of streptomycin (20 mg ml−1). Otoscopy and tympanometry were performed on anesthetized animals (same route and dose as above) before bacterial inoculation, and the procedures were repeated 4 and 7 days postchallenge. Tympanometry was performed with a MicroTymp with medium tips (no. 23622; Welch Allyn Inc.). For each test, a tympanogram (including static admittance, volume, and middle ear pressure) was recorded. A measured static admittance peak of less than 0.3 Ya-mmho (where Ya is acoustic admittance) and a wide gradient of greater than 150 daPa (1 daPa = 1.04 mm of H2O) was interpreted as middle ear infection (see Fig. 3D for an example). The animals were further assessed by otoscopy for signs of tympanic membrane inflammation, which was recorded as either normal or positive (noticeable inflammation with or without effusion). In some studies, middle ear fluid was collected through the chinchilla epitympanic bulla 4 days after bacterial challenge as described by Barenkamp (1, 2) and immediately mixed with 200 μl of brain heart infusion medium. The aspirates were then plated onto chocolate agar plates in the presence or absence of streptomycin (50 μl of 20-mg-ml−1 streptomycin solution).
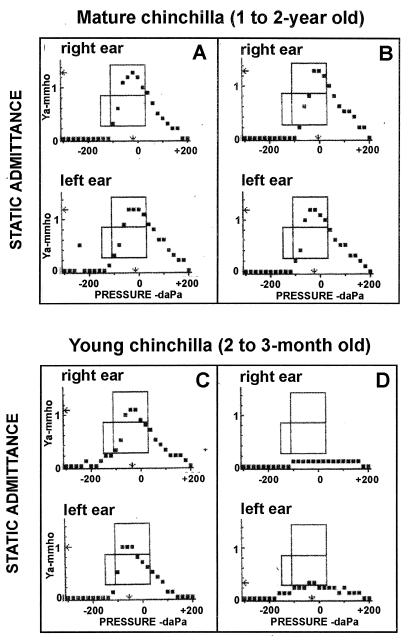
FIG. 3.
Tympanograms of mature and young chinchillas. The animals were challenged intranasally with freshly cultured streptomycin-resistant NTHI 12 as described in Materials and Methods. The development of middle ear infection was monitored by otoscopic examination and tympanometry. Tympanograms were recorded before bacterial challenge (A and C) and again at 4 days postinoculation (B and D).
Convalescent animals.
Animals were inoculated intranasally with freshly grown NTHI 12 as described above. NP colonization or middle ear infection caused by NTHI were monitored. Infected animals usually recovered fully after 2 to 3 weeks as determined by otoscopy and tympanometry (for animals with middle ear infection) or by negative bacterial recovery from nasal lavage fluids (for animals with NP colonization).
Purification of P6 from Hib strain Eagan and generation of guinea pig anti-P6 antisera.
P6 protein was purified from H. influenzae type b (Hib) Eagan as described elsewhere (31), and monospecific anti-P6 antisera were raised in guinea pigs (31).
Statistical analysis.
Comparisons of the protection results between appropriate groups were made by Student’s unpaired t test. Additional analysis was performed by nonparametric tests including the Mann-Whitney and Fisher exact tests. P < 0.05 was considered to be significant.
RESULTS
Development of an NP colonization model in chinchillas with streptomycin-resistant NTHI strains.
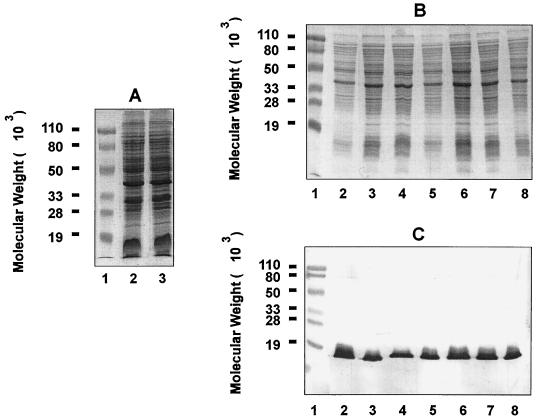
The chinchilla NP is naturally colonized with both gram-positive cocci and gram-negative bacilli (28), which are culturable on chocolate agar plates. To distinguish NTHI used for inoculation from resident bacteria, we used the streptomycin-resistant NTHI strain 12. We found that by mixing 1 mg of streptomycin (50 μl of 20-mg-ml−1 streptomycin solution) with 25 μl of nasal lavage fluids, the growth of contaminating bacteria on chocolate agar plates was completely inhibited whereas that of the antibiotic-resistant NTHI strain 12 was not affected. Therefore, 1 mg of streptomycin was systematically included in the plating of chinchilla nasal lavage fluids throughout the study. The streptomycin-resistant bacteria and the bacteria cultured from NP lavages of infected chinchillas in the presence of antibiotic were confirmed to be NTHI based on similar SDS-PAGE banding patterns with the parent strain 12 (Fig. 1A and B) and the presence of the H. influenzae-specific P6 outer membrane protein on immunoblot analysis (Fig. 1C).
FIG. 1.
SDS-PAGE and immunoblot analysis of bacteria recovered from chinchilla nasal lavage fluids or middle ear effusion. (A) SDS-PAGE was performed on a 12.5% polyacrylamide gel, and proteins were visualized by Rapid Coomassie blue staining. Lanes: 1, prestained molecular weight markers; 2, parent NTHI 12; 3, streptomycin-resistant strain 12 generated through spontaneous mutation. (B and C) Young chinchillas (2 to 3 months old) were challenged intranasally with freshly cultured streptomycin-resistant NTHI 12 as described in Materials and Methods. NP lavage and aspiration of middle ear fluid were performed 4 days after bacterial inoculation. Lanes: 1, prestained molecular weight markers; 2, whole-cell lysate of streptomycin-resistant NTHI 12 inoculum; 3 and 4, whole-cell lysate of bacteria recovered from nasal lavage fluids in the presence of streptomycin; 5 and 6, whole-cell lysate of bacteria recovered from middle ear fluids in the absence of streptomycin; 7 and 8, whole-cell lysate of bacteria recovered from the same middle ear fluids in the presence of streptomycin. Proteins were visualized by Rapid Coomassie blue staining (B), and immunoblotting was performed with guinea pig anti-P6 antiserum (C).
Specificity of anti-chinchilla IgG and IgA reagents.
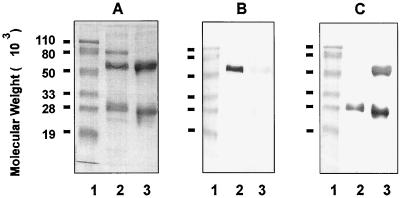
The specificities of anti-chinchilla IgG and anti-chinchilla IgA antisera were tested by immunoblotting. As shown in Fig. 2, guinea pig antisera raised against the electroeluted α chain reacted specifically with the IgA H chain (Fig. 2B, lane 2) but not with the H or L chains of polyclonal IgG molecules (lane 3) on immunoblots. Guinea pig anti-chinchilla IgG antisera recognized both the H and L chains (Fig. 2C, lane 3), as well as the IgA L chain (lane 2), as expected. An 80-kDa protein band was found to be copurified with the IgA molecule based on SDS-PAGE analysis under reducing conditions (Fig. 2A, lane 2). This protein is likely to be the secretory component, a glycoprotein associated with polymeric IgA present in secretion fluids (16, 26).
FIG. 2.
SDS-PAGE and immunoblot analysis of anti-chinchilla IgA and IgG antibodies. SDS-PAGE was performed on a 12.5% polyacrylamide gel under reducing conditions (4% 2-mercaptoethanol). Lanes: 1, prestained molecular weight markers; 2, purified chinchilla IgA; 3, purified chinchilla IgG. Proteins were visualized by Rapid Coomassie blue staining (A), and immunoblotting of samples in panel A was performed with guinea pig anti-α chain of chinchilla IgA (B) or guinea pig anti-chinchilla IgG (C).
Effect of the challenge dose on NP colonization and middle ear infection caused by NTHI 12.
To select the optimal dose of bacterial challenge, chinchillas (2 to 3 months old) were inoculated with increasing doses (102, 105, or 108 CFU) of NTHI 12 on day 0. The animals were then monitored for NP colonization on day 4 and for middle ear infection on days 4 and 7. As shown in Table 1, the number of animals with positive NP colonization slightly declined as the challenge dose decreased (e.g., 100% of chinchillas showed positive NP colonization for the highest dose whereas 83% did so for the lowest dose). However, the incidence of subsequent middle ear infection was significantly different between these groups 7 days after NTHI inoculation. In the group of animals challenged with 108 CFU of bacteria, 86% of chinchillas developed middle ear infection. In contrast, in the group of animals which received 105 or 102 CFU, the percentages that developed middle ear infection were 14 and 0%, respectively (Table 1). The number of CFU recovered from nasal lavage fluids also declined significantly when the bacterial challenge dose was reduced from 105 to 102 CFU (Table 1). Thus, a 108 CFU challenge dose was chosen for protection experiments.
TABLE 1.
Effect of challenge dose on NP colonization and middle ear infection caused by NTHI 12 in chinchillasa
| Group | Challenge dose (CFU) | NP colonization
|
Middle ear infection (no. of animals infected/total no. of animals challenged) (%) | |
|---|---|---|---|---|
| No. of animals infected/total no. of animals challenged (%) | Mean CFU/25-μl nasal lavage | |||
| 1 | 108 | 7/7 (100) | 1,000 | 6/7 (86) |
| 2 | 105 | 7/7 (100) | 1,030 | 1/7 (14)c |
| 3 | 102 | 5/6 (83) | 325b | 0/6 (0)c |
Chinchillas (2 to 3 months old) were challenged intranasally with various doses of NTHI 12 as described in Materials and Methods. NP lavages were performed 4 days postinoculation. Middle ear infections were monitored at days 4 and 7 postchallenge by otoscopy and tympanometry.
Student’s unpaired t test. Statistical significance compared to the animals in groups 1 or 2 was found (P < 0.05).
Fisher exact test. Statistical significance compared to animals in group 1 was found (P < 0.05).
Effect of nasal lavage fluid sampling on NP colonization.
We next determined the optimal time for sampling and collecting nasal lavage fluids and investigated whether it was possible to collect sequential nasal lavage fluids from the same animal on different days. To this end, we performed the following studies. Study A included seven groups of six to eight chinchillas each (2 to 3 months old), whereas study B included single group of seven chinchillas (2 to 3 months old). On day 0, all animals in both groups were intranasally inoculated with 108 CFU of NTHI 12. In study A, nasal washes were performed only once on animals from each of the seven groups on day 1, 2, 4, 6, 8, 16, or 23. As shown in Table 2, 100% of the chinchillas in study A remained colonized with NTHI 12 up to day 4. This percentage declined to 63% on day 8, and no bacteria could be detected by day 23. In study B, all the animals from a single group underwent five successive nasal washes on days 1, 2, 4, 6, and 8. The NP of all animals remained colonized with NTHI 12 as late as day 8 (Table 2). These results indicated that repeating nasal washes on the same animal may artificially promote the persistence of NP colonization. Similar results were observed in older chinchillas, i.e., 4 to 5 months old and 1 to 2 years old (data not shown). To give the chinchillas sufficient time to develop potentially protective anti-NTHI responses while ensuring that a sufficient number of control animals remained positive for NP colonization, nasal lavages were performed 4 days after bacterial challenge to optimize the model.
TABLE 2.
Effect of nasal lavage fluid sampling on NP colonization by NTHI 12 in chinchillasa
| Days after bacterial challenge | No. of animals with positive NP colonization/total no. of animals challenged (%) in:
|
|
|---|---|---|
| Study A | Study B | |
| 1 | 7/7 (100) | 7/7 (100) |
| 2 | 7/7 (100) | 7/7 (100) |
| 4 | 7/7 (100) | 7/7 (100) |
| 6 | 5/6 (83) | 7/7 (100) |
| 8 | 5/8 (63) | 7/7 (100) |
| 16 | 1/6 (17) | ND |
| 23 | 0/6 (0) | ND |
Chinchillas (2 to 3 months old) were challenged intranasally with 108 CFU of NTHI 12 on day 0 as described in Materials and Methods. In study A, NP lavage was performed on each group only once. In study B, NP lavage was performed five times on the same group.
ND, not determined.
Effect of chinchilla age on NP colonization and middle ear infection.
We further explored whether the age of animals influenced the efficiency of NTHI colonization and the subsequent induction of middle ear infection. Three age groups of animals (2 to 3 months old, 4 to 5 months old and 1 to 2 years old) were examined. As shown in Table 3, NP colonization (80 to 100%) with NTHI 12 was observed in animals of all age groups up to day 4. The median CFU of bacteria recovered from 25 μl of nasal lavage fluid of infected animals in various groups ranged from 600 to 800 and was not age dependent. In contrast, there was a statistically significant difference in the frequency of middle ear infections between young chinchillas (2 to 3 months old) and older animals (4 to 5 months old and 1 to 2 years old). About 80% of the young chinchillas developed culture-positive otitis following NP colonization, whereas only 10 to 30% of animals in the other two groups developed culture-positive middle ear infection (Table 3). We confirmed that bacteria recovered from infected middle ears via the epitympanic bulla were streptomycin resistant and indeed were NTHI (Fig. 1, lane 7 and 8). Figure 3 displays representative tympanograms of mature (1- to 2-year-old) or young (2- to 3-month-old) chinchillas before (Fig. 3A and C) and after (Fig. 3B and D) intranasal challenge with NTHI. Four days after inoculation, most young animals (∼80%) developed symptoms of middle ear infection as reflected by a significant reduction in the height of the tympanometric peak (Fig. 3D) whereas the majority of adult chinchillas (70 to 90%) had a normal tympanogram (Fig. 3B). In general, the static admittance in a normal young animal (Fig. 3C) was slightly lower than that in a normal adult chinchilla (Fig. 3A), consistent with what is clinically observed in children and adults (18).
TABLE 3.
Effect of chinchilla age on NP colonization and middle ear infection caused by NTHI 12a
| Group | Age of animals | NP colonization
|
Middle ear infection (no. of animals infected/total no. of animals challenged) (%) | |
|---|---|---|---|---|
| No. of animals infected/total no. of animals challenged (%) | Median CFU/25-μl nasal lavage | |||
| 1 | 2–3 mo | 6/6 (100) | 600–800 | 5/6 (83.3) |
| 2 | 2–3 mo | 8/9 (88.9) | 8/9 (88.9) | |
| 3 | 4–5 mo | 7/8 (87.5) | 600–800 | 1/8 (12.5) |
| 4 | 4–5 mo | 7/8 (87.5) | 2/8 (25.0) | |
| 5 | 4–5 mo | 8/10 (80.0) | 3/10 (30.0) | |
| 6 | 1–2 yr | 8/9 (88.9) | 600–800 | 1/9 (11.1) |
| 7 | 1–2 yr | 6/6 (100) | 2/6 (33.3) | |
Three days before bacterial challenge, the animals were examined for preexisting middle ear infections by otoscopy and tympanometry. They were challenged intranasally with 108 CFU of NTHI 12 as described in Materials and Methods. NP lavage was performed 4 days postinoculation. Middle ear infections were monitored at 4 and 7 days postchallenge by otoscopy and tympanometry.
NP colonization with NTHI in convalescent animals or animals immunized with heat-inactivated NTHI whole-cell preparation.
The protective effect of parenteral immunization with heat-inactivated NTHI 12 or LCDC2 preparations on NP colonization of chinchillas with NTHI 12 was determined. As shown in Table 4, 90% of the control animals which were immunized only with alum had culture-positive nasal lavage fluids. In contrast, 70 to 88% of animals immunized with the inactivated homologous NTHI 12 preparation were culture negative. In animals immunized with a heterologous NTHI strain (LCDC2), 44% were infected. Although this percentage was not statistically significantly different from that obtained for the control group, the bacterial counts in infected animals were significantly lower (63 to 75 versus 600 CFU/25 μl of nasal lavage fluids) (P < 0.05), indicating that immunization with an inactivated NTHI cell lysate induced a certain degree of cross-protection against heterologous NTHI infection. Full protection was observed in convalescent chinchillas, since none of the 15 convalescent animals (from two separate studies) was infected.
TABLE 4.
NP colonization with NTHI 12 in convalescent animals or animals that received i.m. immunizations with inactivated NTHI whole cells
| Group | Animals | No. of infected animals/total no. of animals challenged (%)a | Mean CFU/25-μl nasal lavage | Median CFU/25-μl nasal lavage |
|---|---|---|---|---|
| 1 | Convalescent | 0/9 (0)b | 0c | 0d |
| 2 | 0/6 (0)b | 0c | 0d | |
| 3 | Immunized with strain 12 | 3/10 (30.0)b | 14c | 4d |
| 4 | 1/8 (12.5)b | 10c | 3d | |
| 5 | Immunized with strain LCDC2 | 4/9 (44) | 129c | 63d |
| 6 | 4/9 (44) | 135c | 75d | |
| 7 | Control | 9/10 (90.0) | 550 | 600 |
| 8 | 8/9 (88.9) | 680 | 600 |
Infected animals are defined as having >10 CFU of bacteria recovered from 25 μl of nasal lavage fluid.
Statistical significance compared to control animals (P < 0.05) in the Fisher exact test.
Statistical significance in Student’s unpaired t test.
Statistical significance in the Mann-Whitney rank sum test.
Analysis of antibody responses in chinchilla immune sera and nasal lavage fluids.
Table 5 summarizes the anti-NTHI whole-cell antibody responses in chinchilla immune sera as determined by whole-cell ELISA. All animals, whether convalescent from a previous NTHI 12 infection (group 1) or immunized i.m. with an inactivated NTHI cell lysate preparation (groups 2 and 3), generated significantly higher anti-NTHI 12 antibody responses (IgG and IgA) in serum than did control animals (group 4). However, neither significant IgG nor significant IgA responses were observed in nasal lavage fluids of any animals by whole-cell ELISA (data not shown).
TABLE 5.
Analysis of anti-NTHI 12 whole-cell antibody responses in chinchilla immune sera by whole-cell ELISA
| Group | Animals | Anti-NTHI 12 antibody response in serum
|
|||
|---|---|---|---|---|---|
| Mean titer
|
Median titer
|
||||
| IgG response | IgA response | IgG response | IgA response | ||
| 1 | Convalescent from strain 12 infection | 970a | 13a | 670b | 14b |
| 2 | Immunized with strain 12 whole cells | 15,000a | 90a | 10,390b | 81b |
| 3 | Immunized with strain LCDC2 whole cells | 9,970a | 53a | 6,890b | 44b |
| 4 | Control | 60 | 1.6 | 40 | 1.3 |
Statistical significance compared to control animals (P < 0.05) in Student’s unpaired t test.
Statistical significance compared to control animals (P < 0.05) in the Mann-Whitney rank sum test.
Characterization of antibodies present in chinchilla immune sera.
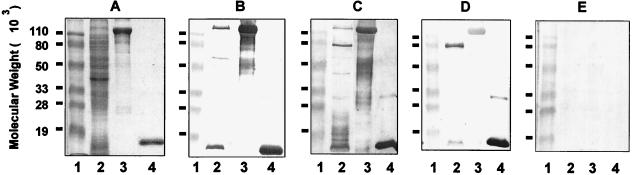
Immunoblotting studies were performed to further determine the specificity of antibodies present in chinchilla immune sera. As shown in Fig. 4, convalescent chinchillas and animals immunized with inactivated NTHI 12 mounted a similar and predominant IgG response against two proteins present in an NTHI whole-cell lysate (Fig. 4B and C, lanes 2). The molecular masses of the two proteins were about 120 and 16 kDa, respectively. The 120-kDa protein(s) was identified by immunoblot analysis as an HMW H. influenzae adhesin, since both chinchilla sera strongly reacted with a mixture of HMW1 and HMW2 proteins purified from NTHI 12 (Fig. 4B and C, lanes 3). The 16-kDa protein was found to be P6, a highly conserved lipoprotein of H. influenzae (Fig. 4B and C, lanes 4). In sera collected from animals immunized with the heterologous NTHI LCDC2 bacteria, the predominant IgG response was shown to be directed against P6 (Fig. 4D, lanes 2 and 4) whereas only a weak but noticeable reactivity against HMW1 and HMW2 proteins of NTHI 12 (Fig. 4D, lanes 2 and 3) was observed, indicating that there are cross-reactive B-cell epitopes among HMW proteins from different NTHI strains. Immune sera collected from animals immunized i.m. with either NTHI 12 or LCDC2 whole cells strongly reacted with a 70-kDa protein present in whole-cell lysate (Fig. 4C and D, lanes 2). This reactivity, however, was not observed with sera from convalescent animals (Fig. 4B, lane 2).
FIG. 4.
SDS-PAGE and immunoblot analysis of Ig responses in chinchilla convalescent-phase and immune sera. SDS-PAGE was performed on a 12.5% polyacrylamide gel. Lanes: 1, prestained molecular weight markers; 2, whole-cell lysate of NTHI 12; 3, HMW proteins (HMW1 + HMW2) purified from NTHI 12; 4, P6 protein purified from Hib Eagan. Proteins were visualized by Rapid Coomassie blue staining (A), and immunoblotting was performed with chinchilla convalescent-phase sera (B) or immune sera collected from animals immunized i.m. with inactivated NTHI 12 whole cells (C) or with NTHI LCDC2 (D) or normal chinchilla sera (E).
DISCUSSION
Chinchilla models have been widely used for investigating pathogenic mechanisms of bacterial otitis media and evaluating candidate vaccine antigens. The model was first described for Streptococcus pneumoniae by Giebink et al. (9) and later adapted for NTHI (8, 15). Two challenge methods have been used in chinchillas for NTHI-induced otitis media, one being a direct bacterial intrabulla challenge (8) and the other involving NP inoculation followed by negative-pressure aspiration of NTHI up into the middle ear through the eustachian tube (12). The chinchilla intrabulla challenge model has been widely used to test vaccine candidates against NTHI in recent years (2, 6, 11). However, since infection with the pathogen is not analogous to that observed in humans, interpretation of the results obtained from this model remains difficult. The NP inoculation method mimics the natural route of infection in humans and has the advantage of allowing colonization factors of NTHI to be exposed. Thus, candidate vaccines neutralizing these colonization factors can be tested for their efficacy in this model. However, the disadvantages of this challenge model are the low infection rates in negative control groups and the additional stress imposed on the animals. Other models have taken advantage of the synergistic effect of viruses (adenovirus or respiratory syncytial virus) on NTHI adherence, colonization, and/or development of middle ear infection (24, 28). These studies have greatly contributed to a better understanding of the pathogenesis of virus-augmented NTHI disease.
An ideal animal protection model for screening vaccine candidates against NTHI-related otitis media should mimic the initial NP colonization step and the subsequent development of middle ear infection. Due to the limitations and problems associated with the current chinchilla intrabulla and intranasal challenge models, we thought that it would be beneficial to develop an NP colonization model involving NTHI only. Such a model has several potential advantages and applications. First, it would be particularly useful in screening NTHI adhesins, which play a crucial role in the attachment of bacteria to the nasopharyngeal epithelium but contribute much less to the development of otitis media. Second, the use of NTHI alone as a pathogen, in contrast to viral coinfection, should allow a simpler interpretation of experimental data. Third, avoiding the practice of negative pressure or eliminating the coinfection step with a virus would significantly reduce the additional stress imposed on chinchillas.
The observation that NTHI strains could colonize the NP epithelium of chinchillas was mentioned in several earlier reports (6, 19, 28). However, the use of this model for testing vaccine antigens was severely limited by the massive contamination of the chinchilla NP with resident bacteria. In the present study, this problem was obviated by using an antibiotic-resistant NTHI strain for bacterial challenge. With this approach, we have developed a quantitative NTHI colonization model in chinchillas and evaluated its potential for screening vaccine candidates which would prevent the attachment of NTHI to NP epithelial cells. The development of a vaccine that inhibits colonization should potentially be efficacious in preventing middle ear infection. In this study, we have (i) examined the effect of animal age on NP colonization and middle ear infection, (ii) optimized the dose of challenge bacteria and the time of nasal lavage fluid sampling, (iii) established a protection model in chinchillas, and (iv) examined the immune responses elicited in protected animals. Although only the studies with strain 12 are presented in this report, similar results were also observed when a second strain, LCDC2, was used as the challenge organism. No significant differences were found between strain 12 and strain LCDC2, either genetically or phenotypically, and both strains expressed HMW adhesin proteins. Whether these HMW proteins play an important role in the colonization of NTHI to chinchilla nasopharyngeal epithelium remains to be studied.
It was interesting that although the age of the animals did not significantly influence the degree of NP colonization with NTHI, it had a marked effect on the subsequent development of middle ear infection. In humans, the eustachian tube in infants and young children is about half as long as that in adults (5). The eustachian tube in adults lies at an angle of 45° in relation to the horizontal plane, whereas in infants this inclination is only 10° (25). In addition, the immunological defense system in infants and young children is not yet fully developed. Thus, these anatomical and immunological factors probably make young children more susceptible to bacterial NP colonization and middle ear infection than adults. One can speculate that the same factors might be responsible for the age-dependent middle ear infection observed in chinchillas after intranasal inoculation. To study only NTHI colonization of the NP epithelium, chinchillas of any age (2 to 3 months old up to 1 to 2 years old) would be suitable in the model. In contrast, for studies requiring both NP colonization and middle ear infection, the use of young animals (2 to 3 months old) is crucial.
Although a dose as low as 105 CFU was sufficient to promote NP colonization, the dose required for induction of middle ear infection was 1,000-fold higher (108 CFU) in 2- to 3-month-old chinchillas. We speculate that the higher dose of challenge bacteria might have created a more severe inflammatory environment (e.g., inflammation of eustachian tube or tympanic membrane), which facilitated the development of middle ear infection.
We found that performing consecutive nasal washes on the same chinchillas promoted the persistence of NP colonization. There are two possible explanations for this observation. First, since the chinchilla NP is not the natural habitat for NTHI, the inoculated pathogen will eventually die or be cleared from the area. The saline solution used in the nasal washes might have provided extra moisture to the NP that was beneficial to NTHI growth. Second, the first nasal wash might have significantly diluted IgG or IgA responses at the mucosal surface, which in turn could have weakened the first level of immunological defense and allowed the rapid multiplication of bacteria already attached to the NP epithelium. Therefore, to obtain consistent results from this model, the use of a second nasal wash on the same animal is not recommended.
Significant antibody responses against NTHI 12 were detected in the chinchilla immune sera, whether collected from animals that had recovered from previous middle ear infection or NP colonization (convalescent animals) or from animals immunized with heat-inactivated NTHI 12 or LCDC2 whole-cell preparations. Immunoblotting analysis allowed us to further identify specific NTHI proteins which are predominantly recognized by immune sera. In sera collected from convalescent animals or animals immunized with inactivated whole cells of NTHI 12, a predominant IgG response was directed against proteins identified as HMW adhesin and the surface-exposed lipoprotein, P6. However, neither significant IgG responses nor significant IgA responses were detected in the nasal lavage fluids from protected animals. Since the detection methods used in this study have relatively low sensitivity, no further conclusion can be drawn from these results.
The detection of anti-HMW antibodies in chinchilla immune sera was consistent with earlier studies (1). Barenkamp reported that a pool of immune sera prepared by immunizing chinchillas with killed NTHI cells had anti-HMW protein antibodies. Passive immunization of chinchillas with this antiserum pool also protected the animals against middle ear infection induced by challenge with a homologous strain. It was subsequently demonstrated that NTHI surface-exposed HMW proteins were not only the major targets of human serum antibodies but were also related to the filamentous hemagglutinin of Bordetella pertussis (2, 3). Thus, these HMW proteins may be acting as adhesins and playing an important role in the attachment of NTHI to human epithelial cells (27). The role of serum antibodies against HMW adhesins in the prevention or reversal of NTHI attachment to the chinchilla NP requires further study.
The detection of anti-P6 antibodies in chinchilla convalescent-phase sera and immune sera was not surprising. P6 is a highly conserved, surface-exposed peptidoglycan-associated lipoprotein in the outer membrane of all strains of NTHI and Hib (21, 22). The potential of P6 as a candidate vaccine against NTHI and Hib has been extensively studied by several laboratories (6, 11, 23, 31). Anti-P6 antibodies are protective against Hib infection in the infant rat model of bacteremia (31). In a recent study, DeMaria et al. (6) found that active immunization of chinchillas with P6 protein purified from NTHI resulted in the production of NTHI-specific bactericidal antibodies and a reduction in the incidence of NTHI-induced otitis media. However, it did not affect the extent or the duration of NP colonization with NTHI in chinchillas (6). It was unclear whether there was any difference in the quality of anti-P6 antibodies induced in serum by the detergent-extracted P6 protein in the study by DeMaria et al. as opposed to the membrane-associated P6 in our experiment. The contribution of anti-P6 antibodies in convalescent-phase sera or immune sera in protection against NTHI colonization of the chinchilla NP remains to be studied.
NTHI exhibits substantial strain heterogeneity (4, 20). We have shown that active immunization of chinchillas with inactivated NTHI whole cells from two different strains could protect the animals not only against infection caused by a homologous strain but also, to a certain degree, against infection caused by a heterologous strain. By examining the specificity of the IgG response in chinchilla sera, it was found that P6 was the main target antigen for antibodies elicited by both immunizations. Since P6 is highly conserved among H. influenzae strains, anti-P6 antibodies may potentially contribute to the apparent cross-protection observed against heterologous NTHI infection in chinchillas.
In conclusion, we have developed an NP colonization model in chinchillas by using antibiotic-resistant NTHI strains and have demonstrated for the first time that through active parenteral immunization with heat-killed NTHI cells, chinchillas were largely protected against NP colonization with a homologous NTHI strain. In all protected animals, significant anti-P6 and anti-HMW antibody levels in serum were detected. Further evaluation of the antibody responses in the immune sera and/or nasal secretions should provide us with new insights into the understanding of local immune responses following systemic immunization and in the identification of important candidate vaccine antigens.
ACKNOWLEDGMENTS
We thank S. Barenkamp, who kindly provided us with purified HMW proteins. We also thank D. Coleman, M. Haer, D. Persaud, J. Shortreed, A. Truscott, and W. Williams for their excellent technical assistance.
REFERENCES
- 1.Barenkamp S. Protection by serum antibodies in experimental nontypeable Haemophilus influenzae otitis media. Infect Immun. 1986;52:572–578. doi: 10.1128/iai.52.2.572-578.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barenkamp S. Immunization with high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae modifies experimental otitis media in chinchillas. Infect Immun. 1996;64:1246–1251. doi: 10.1128/iai.64.4.1246-1251.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barenkamp S, Leininger E. Cloning, expression, and DNA sequence analysis of genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect Immun. 1992;60:1302–1313. doi: 10.1128/iai.60.4.1302-1313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barenkamp S J, Munson R S, Jr, Granoff D M. Outer membrane protein and biotype analysis of pathogenic nontypeable Haemophilus influenzae. Infect Immun. 1982;36:535–540. doi: 10.1128/iai.36.2.535-540.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bluestone C D. Eustachian tube and nasopharynx. In: Bernstein J M, Ogra P L, editors. Immunology of the ear. New York, N.Y: Raven Press; 1987. pp. 39–61. [Google Scholar]
- 6.DeMaria T F, Murwin D M, Leake E R. Immunization with outer membrane protein P6 from nontypeable Haemophilus influenzae induces bactericidal antibody and affords protection in the chinchilla model of otitis media. Infect Immun. 1996;64:5187–5192. doi: 10.1128/iai.64.12.5187-5192.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freijd A, Bygdeman S, Rynnel-Dagoo B. The nasopharyngeal microflora of otitis-prone children, with emphasis on H. influenzae. Acta Otolaryngol. 1984;97:117–126. doi: 10.3109/00016488409130971. [DOI] [PubMed] [Google Scholar]
- 8.Giebink G S. Experimental otitis media due to Haemophilus influenzae in the chinchilla. In: Sell S H, Wright P F, editors. Haemophilus influenzae. New York, N.Y: Elsevier Biomedical Press; 1982. pp. 73–80. [Google Scholar]
- 9.Giebink G S, Payne E E, Mills E L, Juhn S K, Quie P G. Experimental otitis media due to Streptococcus pneumoniae: immunopathogenic response in the chinchilla. J Infect Dis. 1976;134:595–604. doi: 10.1093/infdis/134.6.595. [DOI] [PubMed] [Google Scholar]
- 10.Gnehm H E, Pelton S I, Gulati S, Rice P A. Characterization of antigens from nontypeable Haemophilus influenzae recognized by human bactericidal antibodies: role of Haemophilus outer membrane proteins. J Clin Invest. 1985;75:1645–1658. doi: 10.1172/JCI111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green B A, Metcalf B J, Quinn-Dey T, Kirkley D H, Quataet S A, Deich R A. A recombinant non-fatty acylated form of the Hi-PAL (P6) protein of Haemophilus influenzae elicits biologically active antibody against both nontypeable and type b H. influenzae. Infect Immun. 1990;58:3272–3278. doi: 10.1128/iai.58.10.3272-3278.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green B A, Doyle W J, Cowell J L. Chinchilla model of experimental otitis media for study of nontypeable Haemophilus influenzae vaccine efficacy. Methods Enzymol. 1994;235:59–68. doi: 10.1016/0076-6879(94)35131-7. [DOI] [PubMed] [Google Scholar]
- 13.Harabuchi Y, Faden H, Yamanaka N, Duffy L, Wolf J, Krystofik D, Williamsville T. Nasopharyngeal colonization with nontypeable Haemophilus influenzae and recurrent otitis media. J Infect Dis. 1994;170:862–866. doi: 10.1093/infdis/170.4.862. [DOI] [PubMed] [Google Scholar]
- 14.Jorgensen F, Andersson B, Larsson S. Nasopharyngeal bacterial flora in otitis prone children treated with immunoglobin. Acta Oto-Laryngol. 1992;112:530–538. doi: 10.3109/00016489209137436. [DOI] [PubMed] [Google Scholar]
- 15.Karasic R B, Trumpp C E, Gnehm H E, Rice P A, Pelton S I. Modification of otitis media in chinchillas rechallenged with nontypeable Haemophilus influenzae and serological response to outer membrane antigens. J Infect Dis. 1985;151:273–279. doi: 10.1093/infdis/151.2.273. [DOI] [PubMed] [Google Scholar]
- 16.Kuhn L C, Kraehenbuhl J-P. Interaction of rabbit secretory component with rabbit IgA dimer. J Biol Chem. 1979;254:11066–11071. [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Margolis R H, Heller J. Screening tympanometry: criteria for medical referral. Audiology. 1987;26:197–208. doi: 10.3109/00206098709081549. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto N, Bakaletz L O. Selective adherence of non-typeable Haemophilus influenzae (NTHi) to mucus or epithelial cells in the chinchilla eustachian tube and middle ear. Microb Pathog. 1996;21:343–356. doi: 10.1006/mpat.1996.0067. [DOI] [PubMed] [Google Scholar]
- 20.Murphy T F, Apicella M A. Antigenic heterogeneity of outer membrane proteins of nontypeable Haemophilus influenzae is a basis for a serotyping system. Infect Immun. 1985;50:15–21. doi: 10.1128/iai.50.1.15-21.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy T F, Bartos L C, Campagnare A A, Nelson M B, Apicella M A. Antigenic characterization of the P6 protein of nontypeable Haemophilus influenzae. Infect Immun. 1986;54:774–779. doi: 10.1128/iai.54.3.774-779.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy T F, Nelson M B, Dudas K C, Mylotte J M, Apicella M A. Identification of a specific epitope of Haemophilus influenzae on a 16,600-dalton outer membrane protein. J Infect Dis. 1985;152:1300–1307. doi: 10.1093/infdis/152.6.1300. [DOI] [PubMed] [Google Scholar]
- 23.Murphy T F, Bartos L C, Campagnari A M, Nelson M B, Dudas K C, Apicella M A. Identification of a 16,600 dalton outer membrane protein of nontypeable Haemophilus influenzae as a target for human bactericidal antibody. J Clin Invest. 1986;78:1020–1027. doi: 10.1172/JCI112656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel J, Faden H, Sharma S, Ogra P L. Effect of respiratory syncytial virus on adherence, colonization and immunity of non-typable Haemophilus influenzae: implications for otitis media. Internat J Pediatr Otorhinolaryngol. 1992;23:15–23. doi: 10.1016/0165-5876(92)90075-z. [DOI] [PubMed] [Google Scholar]
- 25.Proctor B. Embryology and anatomy of the eustachian tube. Arch Otolaryngol. 1967;86:503–526. doi: 10.1001/archotol.1967.00760050505008. [DOI] [PubMed] [Google Scholar]
- 26.Schiff J M, Fisher M M, Underdown B J. Secretory component as the mucosal transport receptor: separation of physicochemically analogous human IgA fractions with different receptor-binding capacities. Mol Immunol. 1986;23:45–56. doi: 10.1016/0161-5890(86)90170-7. [DOI] [PubMed] [Google Scholar]
- 27.St. Geme J W., III The HMW1 adhesin of nontypeable Haemophilus influenzae recognizes sialylated glycoprotein receptors on cultured human epithelial cells. Infect Immun. 1994;62:3881–3889. doi: 10.1128/iai.62.9.3881-3889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki K, Bakaletz L O. Synergistic effect of adenovirus type 1 and nontypeable Haemophilus influenzae in a chinchilla model of experimental otitis media. Infect Immun. 1994;62:1710–1718. doi: 10.1128/iai.62.5.1710-1718.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teele D W, Klein J O, Rosner B the Greater Boston Otitis Media Study Group. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective cohort study. J Infect Dis. 1989;160:83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 30.van Alphen L, Eijk P, Kayhty H, van Marle J, Dankert J. Antibodies to Haemophilus influenzae type b polysaccharide affect bacterial adherence and multiplication. Infect Immun. 1996;64:995–1001. doi: 10.1128/iai.64.3.995-1001.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.Yang Y-P, Loosmore S, Klein M. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Nasopharyngeal colonization with nontypeable Haemophilus influenzae in chinchillas, abstr. B-404; p. 98. [Google Scholar]
- 31.Yang Y P, Munson R S, Jr, Grass S, Chong P, Harkness R E, Gisonni L, James O, Kwok Y, Klein M H. Effect of lipid modification on the physicochemical, structural, antigenic and immunoprotective properties of Haemophilus influenzae outer membrane protein P6. Vaccine. 1997;15:976–987. doi: 10.1016/s0264-410x(96)00296-4. [DOI] [PubMed] [Google Scholar]