Abstract
Alveolar capillary dysplasia (ACD) is a fatal disorder that typically presents in the neonatal period with refractory hypoxemia and pulmonary hypertension. Lung biopsy is traditionally required to establish the diagnosis. We report a 22-mo-old male who presented with anemia, severe pulmonary hypertension, and right heart failure. He had a complicated hospital course resulting in cardiac arrest and requirement for extracorporeal membrane oxygenation. Computed tomography of the chest showed a heterogenous pattern of interlobular septal thickening and pulmonary edema. The etiology of his condition was unknown, lung biopsy was contraindicated because of his medical fragility, and discussions were held to move to palliative care. Rapid whole-genome sequencing (rWGS) was performed. In 2 d it resulted, revealing a novel FOXF1 gene pathogenic variant that led to the presumptive diagnosis of atypical ACD. Cases of atypical ACD have been reported with survival in patients using medical therapy or lung transplantation. Based on the rWGS diagnosis and more favorable potential of atypical ACD, aggressive medical treatment was pursued. The patient was discharged home after 67 d in the hospital; he is currently doing well more than 30 mo after his initial presentation with only one subsequent hospitalization and no requirement for lung transplantation. Our case reveals the potential for use of rWGS in a critically ill child in which the diagnosis is unknown. rWGS and other advanced genetic tests can guide clinical management and expand our understanding of atypical ACD and other conditions.
Keywords: pulmonary hypoplasia, respiratory insufficiency
INTRODUCTION
Alveolar capillary dysplasia (ACD) typically presents in the neonatal period with refractory hypoxemia and pulmonary hypertension and has a near-100% mortality in infancy. It is estimated to affect 1 in 100,000–200,000 live births (Slot et al. 2018). Historically the gold standard for diagnosis has been histologic examination of lung tissue. However, lung biopsy in critically ill patients can result in severe complications or death, and many ACD diagnoses are made on postmortem studies.
Recently, 13 patients with atypical ACD were reported who presented at older ages, with a more heterogenous disease course, and responded to treatment with targeted pulmonary vasodilators, oxygen, or lung transplantation (Ito et al. 2015; Towe et al. 2018; Edwards et al. 2019; Yost et al. 2020). Here, we present a boy with unexplained cardiac and respiratory failure in whom palliative care was considered because of his severe hospital course. Because the cause of his symptoms was unknown, we obtained rapid whole-genome sequencing (rWGS), which led to the diagnosis of atypical ACD, realization of the potential for recovery, and changes in therapy. This case supports the use of rWGS in lieu of lung biopsy for ACD and widens the scope of rWGS diagnostic use in critically ill neonates and children in whom the etiology of disease is not known.
CASE
A 22-mo-old Hispanic boy presented to his pediatrician with several months of a “bounding heart” and diaphoresis, with several days of increased fussiness, generalized fatigue, pallor, shortness of breath, decreased oral intake, and decreased urine output. On exam he was afebrile, with tachycardia, periorbital edema, and pulmonary rales. He was admitted to the hospital for an evaluation of anemia and respiratory distress. Key events, findings, and interventions during his hospital course are summarized in Supplemental Table 1.
His history was significant for being an appropriate-for-gestational-age-size male born at 38 wk gestation via normal spontaneous vaginal delivery. He had respiratory failure at birth requiring intubation and admission to the newborn intensive care unit (NICU) for treatment of presumed sepsis, hypoxic respiratory failure, and hyperbilirubinemia. Following treatment, he was discharged home at 2 wk of life on room air. Subsequent medical history was notable for normal growth, mild speech delay, and a heart murmur noted by his pediatrician. His family history was notable for consanguinity of his paternal grandfather and paternal grandmother (first cousins). There was a history of systemic lupus erythematosus in his maternal grandmother. There was otherwise no family history of congenital heart disease, cardiomyopathy, arrhythmias, sudden cardiac death, or unexplained death. He had a healthy 5-yr-old sister, and his mother had a history of one miscarriage prior to his birth.
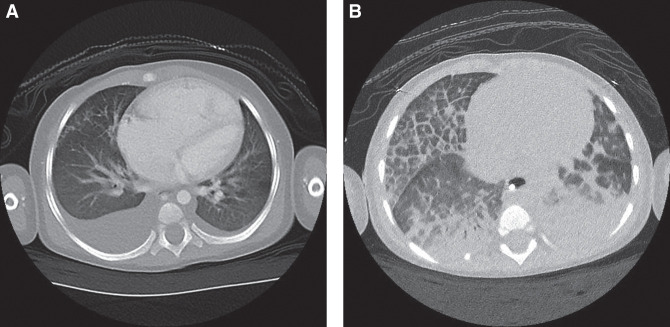
Blood tests showed iron deficiency anemia with a hemoglobin value of 6.5 g/dL. He was treated with supplemental iron and transfusions of red blood cells. He had radiographic findings of cardiomegaly, peribronchial thickening, asymmetric interstitial opacities in the right lung concerning for atelectasis or unilateral pulmonary edema, and a right pleural effusion. Computed tomography (CT) angiogram of the chest showed enlargement of the right atrium, right ventricle, and pulmonary arteries with scattered bilateral ground glass opacities and bilateral small pleural effusions (Fig. 1A). A transthoracic echocardiogram showed right ventricular systolic dysfunction and a tricuspid valve regurgitation gradient consistent with severe pulmonary hypertension. His B-type natriuretic peptide was markedly elevated (8368 pg/mL; normal range <100 pg/mL). He was treated with milrinone 0.5 mcg/kg/min to potentially improve his heart function.
Figure 1.
(A) Computed tomography (CT) scan of the chest prior to initiation of pulmonary vasodilators. (B) CT scan of the chest after initiation of pulmonary vasodilators, revealing extensive heterogenous interlobular septal thickening and pulmonary edema most pronounced in the right middle lung.
On day 3 of hospitalization, cardiac catheterization was performed and is summarized in Supplemental Table 2. He developed bradycardia and pulseless electrical activity with the induction of anesthesia and endotracheal intubation. He required resuscitation with chest compressions, epinephrine, oxygen, and nitric oxide. He had suprasystemic pulmonary arterial pressures on resuscitative medications. His pulmonary arterial pressures markedly decreased after adding intravenous epoprostenol 20 ng/kg/min. He was continued on inhaled nitric oxide and epoprostenol as well as started on sildenafil and ambrisentan. He continued to have high left ventricular filling pressures.
While continuing treatment with pulmonary vasodilators, serial chest radiographs showed progressive right pulmonary edema. A high-resolution CT scan of the chest was performed showing marked septal thickening of the right middle and right lower lung segments (Fig. 1B). This finding raised concern for potential heterogenous pulmonary veno-occlusive disease, pulmonary capillary hemangiomatosis, or ACD. Risks and benefits of performing a lung biopsy were considered. The substantial risks and medical fragility ultimately led to a decision not to perform a biopsy.
The patient then had a second episode of bradycardia and pulseless electrical activity while attempting to wean sedation. He was placed on venous/arterial extracorporeal membrane oxygenation (ECMO), but it was necessary to terminate ECMO after 3 d because of the presence of thrombi in the arterial limb of the ECMO circuit. Balloon atrial septostomy was performed to potentially decompress the right ventricle and maintain systemic perfusion during periods of stress, even though his resting left ventricular filling pressure was greater than his right ventricular filling pressure at rest.
Given that some genetic variants are associated with his pulmonary findings such as pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis (associated with variants in EIF2AK4) and ACD (OMIM #265380, which is associated with variants in FOXF1), rWGS was performed on the patient and his parents. rWGS identified a novel, de novo heterozygous frameshift pathogenic variant in the FOXF1 gene, c.1070_1080del, p.His357ArgfsTer50 (OMIM #601089), 3 d after the test was sent and 2 d after it was received by the testing laboratory (Table 1). Results were confirmed in 2 wk by Sanger sequencing. Based on the available evidence (below), the c.1070_1080del (p.His357ArgfsTer50) variant was classified as Pathogenic (Richards et al. 2015): This frameshift variant is found in the last exon of FOXF1 and it is therefore predicted to escape nonsense-mediated mRNA decay (NMD) (PVS1 criteria); loss-of-function variants downstream from c.1070_1080del (p.His357ArgfsTer50) have been reported as disease-causing in the ClinVar database and Human Gene Mutation Database (HGMD); the c.1070_1080del (p.His357ArgfsTer50) variant is absent from the gnomAD population database and thus is presumed to be rare (PM2 criteria); and analysis of the parental samples was negative for the variant, indicating this variant likely occurred as a de novo event (PS2 criteria) (however, low-level parental mosaicism cannot be excluded).
Table 1.
Variant table
| Gene | Chromosome | HGVS DNA reference | HGVS protein reference | Variant type | Predicted effect | dbSNP/dbVar ID | Genotype | ClinVarID |
|---|---|---|---|---|---|---|---|---|
| FOXF1 | 16:86546618 | c.1070_1080del | p.His357ArgfsTer50 | Deletion, frameshift | Pathogenic | Pending | Heterozygous | SCV003922026 |
The pathogenic FOXF1 variant, along with the heterogenous findings of septal thickening preferentially affecting the right middle and lower lobes, supported a diagnosis of atypical ACD. This led to changing goals of care, from discussion of palliative treatments to aggressive medical treatment and potential referral for lung transplantation.
His subsequent hospital course had continued complications with pneumonia, bilateral pneumothoraces, and respiratory failure, requiring antibiotics, prolonged mechanical ventilation, chest tube placement, and intermittent prone positioning, but his clinical condition gradually improved. Cardiac catheterization repeated on day 52 of the hospitalization showed a moderate-to-large left-to-right atrial level shunt and mildly increased pulmonary vascular resistance. He had a favorable pulmonary vasodilatory response to inhaled nitric oxide and intravenous nicardipine. His pulmonary venous oxygen saturation measurements were only decreased in the right lower pulmonary vein. His left ventricular filling pressure had improved, suggesting that a primary myocardial process was not present.
He was discharged home on hospital day 67. More than 30 mo later he is being treated with supplemental oxygen, sildenafil, bosentan, amlodipine, digoxin, spironolactone, and furosemide. He has been weaned off subcutaneous treprostinil. He had a subsequent hospitalization requiring a prolonged period of assisted ventilation associated with respiratory syncytial virus and rhinovirus infection 12 mo after his initial hospitalization. He also had a subsequent evaluation in the emergency department because of a rhinovirus infection that did not require hospitalization. He has otherwise done well without lung transplantation.
DISCUSSION
To our knowledge, this is the first diagnosis of atypical ACD demonstrated through rWGS. In this case, rWGS allowed the medical team to arrive at a diagnosis of atypical ACD without the need for a dangerous and complex biopsy procedure and avoided transition to palliative care. Atypical ACD usually presents with refractory pulmonary hypertension, and diagnosis is made by histology or genetic testing (Jourdan-Voyen et al. 2020). Management with medical therapy (Ito et al. 2015; Yost et al. 2020) and lung transplantation (Boston et al. 2013; Towe et al. 2018) has led to survival into childhood (Jourdan-Voyen et al. 2020; Yost et al. 2020). Although variants in the FOXF1 gene are usually a harbinger for poor prognosis in typical ACD, our patient's later presentation, favorable response to vasodilators, focal lung involvement, and novel genotype were more consistent with the courses of patients with atypical ACD. Because of the possibility of recovery with medical management, this diagnosis guided discussions away from palliative care options toward aggressive medical management, with a good response to therapy and possible long-term survival. With a good initial response to treatment, we are hopeful that the patient we report will survive long-term with medical therapy, although lung transplantation may be needed if progressive pulmonary parenchymal or vascular disease occurs.
Although variants in FOXF1 have historically indicated poor prognosis in typical ACD, our patient's older age of presentation, favorable response to vasodilators, focal lung involvement, and novel variant were consistent with other patients with atypical ACD.
The past five years have shown an expansion in our understanding of ACD, with typical and atypical forms and different FOXF1 variants exhibiting a spectrum of severity. Novel variants in FOXF1 are being identified in a number of patients with milder presentations of atypical ACD. These patients tend to have more heterogeneous disease and there has been wide phenotypic variation even within familial cases (Towe et al. 2018). With additional cases, it will become possible to delineate disease severity by an underlying genetic etiology and to determine genotype–phenotype correlations. With rWGS and other expanded genetic testing, more cases of ACD may be diagnosed without needing a lung biopsy. This will be particularly helpful in medical decision-making for families and medical teams caring for critically ill neonates and children.
Missense, nonsense, and frameshift variants and upstream 16q24.1 deletions have been identified in both typical and atypical ACD. Several variants identified in patients with atypical ACD are located in the DNA-binding domain, where a majority of previously identified variants have been seen in typical ACD (Sen et al. 2013). At this time full gene deletions and larger copy-number variations (CNVs) encompassing FOXC2 in addition to FOXF1 have not been reported in published atypical ACD cases reviewed here (Supplemental Table 3). With limited atypical cases reported, a clear genotype–phenotype correlation has not been established at this time.
This case expands our understanding of the phenotypic variation of atypical ACD. Additional investigation is needed to determine whether there are individuals with variants in the FOXF1 gene with even milder symptoms, such as those with no pulmonary parenchymal or vascular disease. Identifying these cases would provide additional insights into pathophysiology and scope of ACD disease burden. Our case reveals the importance of rWGS in a critically ill child in which the diagnosis is unknown. rWGS and other advanced genetic tests can guide clinical management and expand our understanding of atypical ACD and other conditions.
METHODS
Consent was obtained for the proband and his parents for rWGS, performed at Rady Children's Institute for Genomic Medicine (RCIGM). Polymerase chain reaction (PCR)-free library preparation was performed, and sequencing was conducted on a NovaSeq6000 system using S1 flow cells. Genome sequences were aligned to human genome assembly GRCh37 (hg19), and the Edico Dragen processor (Illumina) was used for rapid alignment and nucleotide variant calling. Variants were filtered to retain those with allele frequencies of <0.5% in gnomAD. Automated nucleotide and CNV annotation and ranking were performed through Opal Clinical (Fabric Genomics) with variants reviewed and classified according to the American College of Medical Genetics and Genomics/Association of Molecular Pathology (ACMG/AMP) guidelines (Table 1; Richards et al. 2015; Palmquist et al. 2022). The FOXF1 gene was classified as pathogenic and orthogonally confirmed by Sanger sequencing to be heterozygous in the patient and absent from the parental samples.
ADDITIONAL INFORMATION
Data Deposition and Access
The causative variant has been deposited in ClinVar (https://www.ncbi.nih.nlm.gov/clinvar/), under accession number SCV003922026. All data associated with this study are present in the paper or are available under a material transfer agreement or data use agreement, as appropriate, and subject to the limitations of the informed consent documents for the subject.
Ethics Statement
The review and publication of data related to this case were approved by the Institutional Review Board at the University of Utah and the Privacy Board of Intermountain Healthcare with a waiver of consent and authorization.
Acknowledgments
Thank you to our pediatric intensivist Dr. Jill Sweney, pulmonologist Dr. Brian M. McGinley, and geneticist Dr. Nicola Longo, who provided excellent care of this patient. We thank the parents of our patient, who provided consent to include their child in the medical literature.
Author Contributions
D.R.T. and R.W.D. prepared the initial draft of the manuscript. All authors collected data, wrote the paper, and reviewed and provided final approval.
Funding
This work was supported by the Primary Children's Center for Personalized Medicine.
Competing Interest Statement
The authors have declared no competing interest.
Footnotes
[Supplemental material is available for this article.]
REFERENCES
- Boston US, Fehr J, Gazit AZ, Eghtesady P. 2013. Paracorporeal lung assist device: an innovative surgical strategy for bridging to lung transplant in an infant with severe pulmonary hypertension caused by alveolar capillary dysplasia. J Thorac Cardiovasc Surg 146: e42–e43. 10.1016/j.jtcvs.2013.06.014 [DOI] [PubMed] [Google Scholar]
- Edwards JJ, Murali C, Pogoriler J, Frank DB, Handler SS, Deardorff MA, Hopper RK. 2019. Histopathological and genetic features of alveolar capillary dysplasia with atypical late presentation and prolonged survival. J Pediatr 210: 214–219.e2. 10.1016/j.jpeds.2019.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Akimoto T, Cho K, Yamada M, Tanino M, Dobata T, Kitaichi M, Kumaki S, Kinugawa Y. 2015. A late presenter and long-term survivor of alveolar capillary dysplasia with misalignment of the pulmonary veins. Eur J Pediatr 174: 1123–1126. 10.1007/s00431-015-2543-3 [DOI] [PubMed] [Google Scholar]
- Jourdan-Voyen L, Touraine R, Masutti JP, Busa T, Vincent-Delorme C, Dreyfus L, Molin A, Savey B, Mounzer A, Assaf Z, et al. 2020. Phenotypic and genetic spectrum of alveolar capillary dysplasia: a retrospective cohort study. Arch Dis Child Fetal Neonatal Ed 105: F387–F392. 10.1136/archdischild-2019-317121 [DOI] [PubMed] [Google Scholar]
- Palmquist R, Jenkins SM, Bentley D, Miller C, Mao R, Meibos B, Bayrak-Toydemir P, Tvrdik T, Nadauld LD, Bleyl SB, et al. 2022. Evaluating use of changing technologies for rapid next-generation sequencing in pediatrics. Pediatr Res 92: 1364–1369. 10.1038/s41390-022-01965-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. 2015. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17: 405–424. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen P, Yang Y, Navarro C, Silva I, Szafranski P, Kolodziejska KE, Dharmadhikari AV, Mostafa H, Kozakewich H, Kearney D, et al. 2013. Novel FOXF1 mutations in sporadic and familial cases of alveolar capillary dysplasia with misaligned pulmonary veins imply a role for its DNA binding domain. Hum Mutat 34: 801–811. 10.1002/humu.22313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot E, Edel G, Cutz E, van Heijst A, Post M, Schnater M, Wijnen R, Tibboel D, Rottier R, de Klein A. 2018. Alveolar capillary dysplasia with misalignment of the pulmonary veins: clinical, histological, and genetic aspects. Pulm Circ 8: 2045894018795143. 10.1177/2045894018795143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towe CT, White FV, Grady RM, Sweet SC, Eghtesady P, Wegner DJ, Sen P, Szafranski P, Stankiewicz P, Hamvas A, et al. 2018. Infants with atypical presentations of alveolar capillary dysplasia with misalignment of the pulmonary veins who underwent bilateral lung transplantation. J Pediatr 194: 158–164.e1. 10.1016/j.jpeds.2017.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost CE, Putnam AR, Dishop MK, Jorgensen LO, Wirkus PE, Day RW. 2020. A long-term survivor with alveolar capillary dysplasia. JACC Case Rep 2: 1492–1495. 10.1016/j.jaccas.2020.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The causative variant has been deposited in ClinVar (https://www.ncbi.nih.nlm.gov/clinvar/), under accession number SCV003922026. All data associated with this study are present in the paper or are available under a material transfer agreement or data use agreement, as appropriate, and subject to the limitations of the informed consent documents for the subject.