Abstract
We used flight traps baited with unmated female navel orangeworm Amyelois transitella (Walker) (Lepidoptera: Pyralidae) to examine, over two growing seasons, seasonal changes in the abundance of males in fig orchards and the impact of release of 48 mg per ha per day of the pheromone component (Z,Z)-11,13-hexadecadienal from peripherally-located timed-release dispensers on the ability of males to find unmated females within 16-ha treatment plots. Material was placed out and mating disruption was commenced at the beginning of April in the first year, and at the beginning of July the second year. This technique effectively prevented males from finding females in female-baited traps placed throughout the plot. Navel orangeworm abundance was high in figs during the first and third flight, but lower in June and July during the second flight. Since Calimyrna figs are not susceptible to attack by navel orangeworm until mid-to-late July, these findings suggest that materials cost can be reduced by beginning treatment later. Implications for insect pest management in figs and other California crops are discussed.
Keywords: mating disruption; (Z,Z)-11,13-hexadecadienal; pheromone
Introduction
The navel orangeworm Amyelois transitella (Walker) is a pest of California almonds, pistachios, walnuts, and figs (Bentley et al. 2000, 2003; Zalom et al. 2002; Coviello et al. 2003). In California tree nuts, recommendations for management of this pest have focused primarily on cultural practices (Zalom et al. 1984) and, if populations still threaten economic loss, on application of chemical insecticides as soon as the nuts are susceptible and ovipositing females are present (Bentley et al. 2000, 2003; Zalom et al. 2002). Malathion is registered for control of nitidulid beetles in figs and could conceivably reduce navel orangeworm loss (Coviello et al. 2003), but growers avoid using it because of problems with post-harvest intervals and worker re-entry, and have instead used post-harvest fumigation to mitigate insect damage to the fruit (Burks personal observation).
The development of efficient systems of monitoring and mating disruption for navel orangeworm management has lagged behind that of other lepidopteran pests of horticultural crops, due in part to technical limitations. Only the principal component of the female sex pheromone has been identified (Coffelt et al. 1979). This component, (Z,Z)-11,13-hexadecadianal, is not sufficient to efficiently bring males to a point source and is particularly vulnerable to degradation in the field. Monitoring of this pest is therefore dependent on oviposition traps, which are out-competed by the presence of a susceptible host in the orchard (Rice 1976; van Steenwyck and Barnett 1985). An aerosol timed-release system, in which the pheromone is stored in a liquid organic solvent prior to being released at timed intervals, has been one method of avoiding problems with degradation (Shorey and Gerber 1996). While Minks and Cardé (1988) suggested that ideally the optimal blend should be characterized in the laboratory prior to large-scale field trials, they indicated that, in two of the 11 species reviewed, the natural blend was not the best disruptant. There have been subsequent examples in which researchers have concluded that use of more simple blends (Evenden et al. 1999) or a single pheromone component (van Deventer and Blommers 1992; Ryne et al. 2001) was the most pragmatic and cost-effective approach for mating disruption. Previous studies of mating disruption for control of navel orangeworm in almonds have shown that release of (Z,Z)-11,13-hexadecadienal can reduce male capture in female-mated flight traps, mating of unmated females, and reduce crop damage (Curtis et al. 1985; Shorey and Gerber 1996). Studies with this and other lepidopteran pests have suggested that aerosol timed release systems placed around the perimeter of plots to be protected could be equally efficacious in prevention of location of females by males, and could save labor costs compared to such devices placed evenly through the protected plot (Shorey and Gerber 1996; Shorey et al. 1996).
In the current study, we examine the impact of this timed-release system in Calimyrna figs. Calimyrnas, like other commercial fig varieties in California, mature and dry over an extended time and are generally harvested several times at 5–10 day intervals in August and September (Ferguson et al. 1990). Unlike the other varieties, Calimyrna figs have only one significant crop maturing in August and September rather than an additional spring crop that would be harvested as dried fruit around the end of June. The single Calimyrna crop typically does not loose its latex and soften until late July (Smilanick 1979). In this study, we examined the ability of timed release aerosol dispensers placed around 16-ha plots of Calimyrna figs, with treatment beginning in either April or July, to completely prevent capture of males in female-baited flight traps throughout this plot. We also use our trapping data to compare seasonal abundance of navel orangeworm with an established degree-day model.
Materials and Methods
Mating disruption
Mating disruption treatments were applied to two square 16-ha experimental plots of Calimyrna figs at two separate ranches, located in Madera and southern Merced counties east and northeast of Chowchilla, California. Each of these ranches contained over 400 ha of contiguous plantings of figs of various varieties. Comparison plots, located on the same ranch and 0.5 to 2 km from the treatment plots, were also monitored. Both treatment and comparison plots contained Calimyrna figs with drip irrigation. The rows were in a north-south orientation at site A, and an east-west orientation at site B, and were 7.3 m wide at both sites. The trees at site 1 were approximately 20 years old in 2001, averaged 4.5 m in height, and had 4.2 m tree spacing. The trees at the 2001 comparison plot of site 2 were approximately 10 years old in 2001, averaged 3.5 m of height, and had 3 m tree spacings. The 2001 treatment plot at site 2 contained 5-year old trees averaging 2 m in height and planted 2.7 m tree-to-tree. The treatment plots for 2001 were used as untreated control plots in 2002, and vice versa.
Mating disruption treatments were applied using the Suterra Puffer system (Suterra LLC, http://suterra.com), which is substantially similar to the Puffers described by Shorey and Gerber (1996), except that it consists of a pressured aerosol canister inside a programmed cabinet, and an evaporator target was not used. Cabinets containing microprocessors were placed at the recommended density of 5 cabinets per ha (i.e., 80 dispenser units in each treatment plot in both years), suspended at about two-thirds canopy height at approximately 20 m intervals in the alley of the first row or one tree in from the ends of rows (Fig. 1). The cabinets were loaded with aerosol cans containing (Z,Z)-11,13-hexadecadienal of ≥90% purity in either ethanol (in 2001) or a mixture of hexane and acetone (in 2002). The amount of pheromone and solvent used was calculated to be sufficient to provide pheromone and propellant from when mating disruption was started until 1 October. Mating disruption began on 26 March 2001 and 1 July 2002. The cabinets were programmed to propel 0.2 mg of active ingredient every 15 minutes between 6:00 p.m. and 6:00 a.m. Pacific daylight saving time, thus delivering, in principle, 48 mg active ingredient per ha per night. Cabinets were checked on a regular basis through the season, and we estimate a rate of malfunction of approximately 10% in each season.
Figure 1.

Diagramatic illustration of the distribution of 80 pheromone dispensers in the treatment plots and the 16 flight traps within the treatment and comparison plots at each of the two experimental sites.
Flight traps
Male prevalence and the ability of males to locate calling females were monitored using unmated females as a pheromone source. Groups of three females were sealed in a mesh bag that was then suspended from the top of a wing trap (Pherocon IC, Trécé Inc., www.trece.com/stgdprod.html) as described by Curtis and Clark (1984).
Moths for this experiment were obtained as eggs from a laboratory colony originally obtained in 1966 from the University of California, Berkeley, and maintained on a wheat bran diet (Tebbets et al., 1978). Larvae were held at 26 °C 16:8 L:D for 21 days, after which last instar larvae were sorted by sex. Males were identified using the testes, visible as a dark spot through the dorsal cuticle, and discarded. Groups of 100 females were placed in 3.9 liter glass jars with the bottom covered with bran diet to a depth of 2 cm and held at 26 °C 16:8 L:D. Jars were examined on a daily basis, and any moths that had eclosed in the previous scotophase were isolated in transparent plastic vials with screen mesh lids, examined to confirm sex, and held for experiments. Where possible, females were enclosed in mesh bags and placed in the field the first morning after they emerged, and moths were always used within 48 hours of eclosion. When it was necessary to use moths eclosed on two different days, they were grouped so that each bag of three moths contained the same number of 1-day-old and 2-day-old females.
Sixteen flight traps were placed in each treatment and comparison plots. They were arranged in 4 × 4 grids such that the each trap was 1.5 m above the ground, ≥100 m from the nearest other traps, and ≥50 m from the edge of the plot (Fig. 1). Each week all traps were examined, mesh bags containing unmated females were replaced, and liners were replaced if they contained moths or were dirty.
We did not perform mating assays in this study. A larger and more recent study of mating disruption for control of navel orangeworm in almonds and pistachios, using the same dosage and formulation as in 2002, showed that mating disruption with this single component reduced mating in both crops and crop damage in almonds (BS Higbee and CS Burks unpublished). In that study, in which abundance ranged from means of 0 to >100 moths per trap per week, flight traps baited with unmated females and mating assays led to the same conclusion concerning sexual communication. The flight trap data, however, were more economical to collect over a large scale and were amenable to more statistically powerful ANOVA techniques, whereas analysis of mating assay data required non-parametric techniques.
Survival, calling, and seasonal abundance
After observing low trap captures in the summer of 2001, we performed a separate experiment to quantify the survival and the ability of unmated females in flight traps to attract males under summer conditions. Two flight traps were hung ca. 1.5 m from the ground and 100 m apart in a 1.6 ha mixed planting of commercial and experimental almond varieties at our Parlier location. Mesh bags containing 3 freshly-eclosed females were placed in these flight traps on 9, 15, 22, and 31 July and 7 August, 2002. Each day the number of navel orangeworm in the trap liner and the number of females remaining alive were counted.
Degree-day calculations and statistical analysis
Degree-day accumulations were calculated with DDU (DNAR 1990), using the double triangle and vertical cut-off options (Sanderson et al. 1989). Climate data for Madera County for 2001 and 2002 (station 145), and for Parlier for 2002 (station 39), were obtained from the California Department of Water Resources web site, http://wwwcimis.water.ca.gov.
All statistical analysis was performed using the SAS System (SAS 1999). For comparison of the effect of mating disruption weekly flight trap observations were transformed as log10 (x+1) (Sokhal and Rohlf 1995) and analyzed separately for 2001 and 2002 as a three-way factorial arrangement on a randomized complete block design using the GLM procedure with the mating disruption treatment, the north-south, and the east-west position of the trap within the grid as factors; the sites as blocks; and weeks as repeated measures. For comparison of the observed distribution of moths between the 4 central and 12 peripheral traps of the 16-trap grid, the FREQ procedure was used to perform Fisher's Exact test for the 138 of the 192 location × treatment × week combinations for the two years which had at least 5 moths in both central and peripheral traps. To examine survival, observations for the 10 week × location combinations were pooled and logistic regression was calculated for female survival using the LOGISTIC procedure. To examine proportional of trap capture as a function of time the MIXED Procedure was used with a logit link via the %GLIMMIX Macro (http://ftp.sas.com/techsup/download/stat/glmm800.html), with location as a random effect and day and week as fixed effects. All figures and tables show means and standard errors of untransformed data.
Results
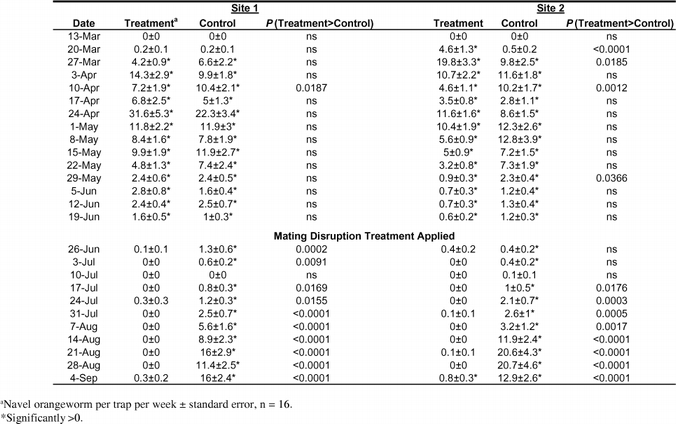
While the mating disruption technique used significantly reduced the number of moths captured in traps at both years and in both sites (Tables 1 and 2), it was more effective in 2002 than in 2001 and in site 1 than in site 2. In 2001 there were significant differences between treatment and control trap means in 21 of the 22 weeks at site 1, but only in 11 of the 22 weeks in site 2. In 2002 there was no significant difference between mean trap counts in the treatment and control plots in most of the 15 weeks prior to the beginning of the mating disruption treatment on 1 July. In the cases in which there were significant differences, the mean count in the control plot (i.e., the treatment plot from the previous year) was greater than that in the treatment plot in three cases and less in two. Following the beginning of pheromone application in the week of 26 June 2002, there was a significant difference between the mean trap capture in the last 9 of the 11 weeks of mating disruption treatment in both sites. In the instances in 2002 when mating disruption was applied and there was no significant difference between captures in treatment and control plots, the number of males captured in the control plots was also not significantly different from zero (i.e., 10 July at both sites and 26 June and 3 July at site 2).
Table 1.
Effect of mating disruption on the number of moths captured in flight traps in 2001.

Table 2.
Effect of mating disruption on the number of moths captured in flight traps in 2002.
No significant correlations between location and trap capture were demonstrated using the ANOVA or parametric techniques such as Pearson's correlation, but Fisher's Exact test demonstrated that, in the absence of mating disruption, moths were usually more likely to be found in the 12 peripheral traps of the 4 × 4 grids than in the 4 central traps. Of the 111 weekly trap counts from plots not under mating disruption (the controls in both years and treatments from 13 March to 19 June of 2002) that met the criteria for calculation of Fisher's Exact Test (i.e., a total of more than 5 moths in both the 4 central and in the 12 peripheral traps), the central traps contained fewer moths than 25% of the total count in 102 cases (54 of which differed significantly) and more moths in 9 cases (1 of which differed significantly) (Fig. 2A). Of the 28 cases of plots under mating disruption in 2001 and 2002 from which the weekly trap counts met the criteria for calculation of Fisher's Exact Test, the central traps contained more than 25% of the moths captured in 14 cases and 25% or less in 14 cases, and none of these differed significantly from 25% (Fig. 2B).
Figure 2.
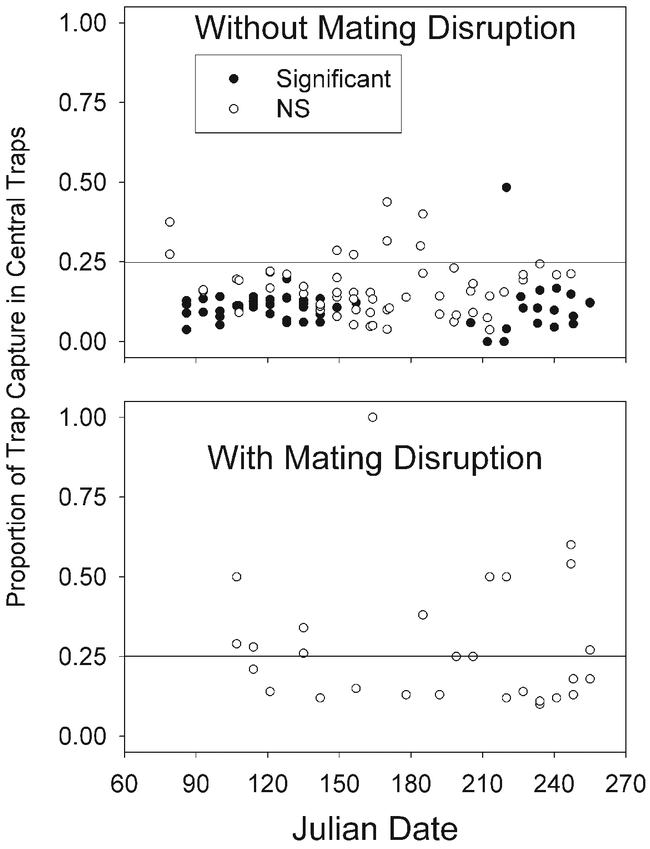
Distribution of total capture between the four central and 16 peripheral traps, without mating disruption (upper), and with mating disruption (lower). The cross bar at 0.25 illustrates the expected distribution if all traps are equally likely to capture moths. The solid circles represent observed that differ significantly from the expected values for a 0.25/0.75 central/peripheral capture ratio, and the unfilled circles represent observations not significantly different from the expected values.
Unmated females in mesh bags in the flight traps lived at least 4 days under mid-summer field conditions (Fig. 3). The proportion of the eventual trap capture was ∼60% after the first night, 87% after the third night, and 97% after the fifth night. The total number of navel orangeworms caught in the two traps was 41 and 30 (9–14 July 2002), 43 and 34 (15–21 July), 78 and 60 (22–28 July), 67 and 64 (31 July–6 August), and 85 and 83 (7–13 August). The maximum daily and minimum nightly temperatures during these periods were (40.2, 36.7, 35.6, 36.3, 38.4 °C) and (17.8, 16.2, 13.6, 12.3, 12.2 °C), respectively. The average daily temperature range during these periods was 18.8 ± 0.4 °C (n = 34). The mean daily minimum relative humidity (RH) was 27 ± 1% (n = 34), and the mean nightly maximum was 84 ± 1% RH.
Figure 3.

Predicted and observed values for female survival (dashed lines and unfilled circles) and number of males in the traps (sollid lines and circles). The light dashed lines represent 95% confidence intervals for the two logit predictions.
Degree-day calculations were made for 2002 using 24 March as a biofix (Fig. 4). An accumulation of 410 DD was attained on 9 June, and 607 DD was attained on 30 June. Using 30 June as the start of a theoretical second flight for 2002, 410 DD were accumulated on 3 August and 607 DD were accumulated on 23 August. By comparison, mean moths per flight trap in the absence of mating disruption fell from ∼10 to ∼2 through the month of May, from ∼2 to ∼0 in June, and remained low until ∼24 July, after which steady increases in trap capture were observed.
Figure 4.

Mean and standard error of control (1a and 2a) plots for the two locations for 2002, along with values for the treatment plots (1b and 2b) first 15 weeks in which mating disruption was not applied in 2002. Estimated accumulations of 410 and 607 degree-days (°C) starting from 24 March and 30 June are illustrated above.
Discussion
The data presented here for the navel orangeworm provide the most rigorous verification to date of the hypothesis of Shorey and co-workers (Shorey and Gerber 1996, Shorey et al. 1996), that peripherally-arranged aerosol dispensers can very effectively disrupt lepidopteran sexual communication throughout large plots. Previously, timed release of (Z,Z)-11,13-hexadecadienal using peripherally-placed aerosol dispensers was shown to significantly reduce trap capture of navel orangeworm in 16-ha plots in almonds, pistachios, and walnuts (Shorey and Gerber 1996) but, the tests, involving large (16-ha) plots, were conducted for only 1 week, and the time of year was not specified. The present data demonstrate that time of year and seasonal changes in abundance are important in such tests.
We attempted to examine spatial distribution of moths within the 16-ha plots because of potential concern about the peripherally-placed dispensers adequately affecting all parts of the plot. While we found statistically greater captures in the peripheral parts of the untreated block but not of the treated block, the more important finding was that there is no evidence that males were consistently able to find females in the center of the protected plots farthest from the peripheral dispensers. The present data show that, under favorable circumstances, we can completely prevent capture of moths in traps throughout a 16-ha treatment plots in spite of captures of 10–20 moths per trap in comparison plots. The greater efficacy in 2002 compared to 2001 may be due to a more compatible solvent and/or less exposure to solar radiation and heat (i.e., in June). It also seems likely that the sparser canopy at site B compared to A, and the greater solar radiation and heat likely experienced by the dispenser units at that site, were factors in the effectiveness of the system. The observations of similar trap counts and no systematic significant differences in trap counts between the control and treatment plots from 13 March to 19 June 2002 (i.e., before pheromone treatments) demonstrates similar abundance in the plots used in this study, and shows that there was no detectable residual effect of pheromone applied the previous year on the ability of navel orangeworm males to locate calling females in the traps.
The data presented here indicate that navel orangeworm abundance is low in figs during the second flight, in June and July. The model used for the degree-day predictions illustrated in Fig. 4 was developed using development data obtained from navel orangeworm on old-crop and fresh almonds (Sanderson et al. 1989), and it must be recognized that navel orangeworm larvae may develop at different rates on old-crop and fresh figs. Regardless of possible differences in development rates between crops, the empirical data in this study, which show low abundance during much of June and July, is important because mating disruption generally works better under conditions of low initial population density (Cardé and Minks 1995). We characterized survival and calling at our location under summer conditions in order to be certain that the low activity that we observed in June and July was not an artifact of the effect of heat stress on survival and calling of the live females in our monitoring traps.
A previous study of the effect of fruit maturity on susceptibility to infestation by nitidulid beetles (Smilanick 1979) showed that Calimyrna figs are not susceptible to infestation by nitidulids prior to softening, loss of latex, and enlargement of the ostiole (stage 2 of Smilanick 1979), which first occurs in late July or early August. In extensive sampling of ripening Calimyrna figs taken from trees at a different location over a 10 week period in 2000, we found no navel orangeworm in 1219 stage 1 or 2 examined fruit, and none prior to 22 August. These findings suggest that delaying the beginning of mating disruption from the beginning of the first flight until shortly before the appearance of susceptible figs (i.e., as we did in 2002) would provide the same or better protection while reducing material costs. This is similar to the practice of Curtis et al. (1985), who started mating disruption around 1 July in order to begin shortly before hull split in Nonpareil almonds. Other data show that the population dynamics for navel orangeworm is similar in figs and almonds (present data cf. Rice 1976; Sanderson et al. 1989).
We assessed crop damage in the current study (Burks and Brandl 2004). This was complicated by the facts that insect damage is generally internal to the fruit and often not apparent from the outside, and that the insect pest has often left the fruit by the time its damage is identified. Sixteen 50-fig samples were taken from figs on the ground at each of the 16 flight trap points for each of 3 harvests in 2001 and 2002. In 2001 the figs were examined by the official market order inspectors who determine only whether a fig is insect-infected. In 2002 the procedure was repeated but the infected figs were turned back to us, and we determined the proportion of figs damaged by navel orangeworm, nitidulids, and other insect pests. While we usually found less total insect damage in treated then the control plots, the differences generally weren't statistically significant and, more importantly, we found that navel orangeworm was responsible for 10–20% of the insect damage at these 2 sites in 2002, and for ∼30% of the insect damage in samples taken from the same locations in 2003. In both cases nitidulid beetles were responsible for the majority of the insect damage. These findings demonstrate the importance improved identification of insect pests responsible for fig damage, and indicate that, if species-specific pest control tactics must be used, those directed against nitidulid beetles should be emphasized over those directed against the navel orangeworm. The findings from this study are nonetheless relevant to almonds, in which the navel orangeworm is a serious pest.
Disclaimer
This is a report of research results only. The use of trade, firm, or corporation names in this report is for the convenience of the reader, and does not constitute or imply endorsement or approval by the United States Department of Agriculture or the Agricultural Research Service of any product or service to the exclusion of others that may be suitable.
Acknowledgments
We thank Kevin Herman and Richard Debenedetto for providing the research sites, Bruce Mackey for statistical advice, Roland Gerber for advice on use of the Suterra Puffer system, Jennifer Estrada, Maria Madrigal, JaiJai Chen and Kai-Mei Loo for technical assistance, and LPS Kuenen, James Campbell, Thomas Phillips, and three anonymous reviewers for useful comments on earlier versions of this manuscript. This work was partially funded by the California Dried Fig Board, and Suterra LLC loaned the dispenser cabinets for the study and donated the material used in 2002.
References
- Bentley WJ, Beede WJ, Daane KM, Michailides TJ, Teviotdale BL, and Westerdahl BB. 2003 UC PIM Pest Management Guidelines: Pistachio. Publication 3471. University of California Agriculture and Natural Resources, Oakland, CA. [Google Scholar]
- Bentley WJ, Coates WW, Hasey J, Hendricks LC, Olson WH, Pickel C, Sibbett GS, Van Steenwyk RK, Teviotdale BL, Gubler WD, Westerdahl BB, Kodira UC, Elmore CL, Elkins RB, Grant JA, Kelley KM, Reil WO, and Sibbett GS. 2000 UC IPM Pest Management Guidelines: Walnut. Publication 3471. University of California Agriculture and Natural Resources, Oakland, CA. [Google Scholar]
- Burks CS, Brandl DG. 2004 Quantitative Assessment of Insect Pest to Figs. Crop Management. [In press]. [Google Scholar]
- Cardé RT, Minks AK. Control of moth pests by mating disruption: successes and constraints. Annual Review of Entomology. 1995;40:559–585.0066-4170(1995)040<0559:COMPBM>2.0.CO;2 [Google Scholar]
- Coffelt JA, Vick KW, Sonnet PE, Doolittle RE. Isolation, identification and synthesis of a female sex pheromone of the navel orangeworm, Amyelois transitella. Journal of Chemical Ecology. 1979;6:955–966.0098-0331(1979)006<0955:IIASOA>2.0.CO;2 [Google Scholar]
- Coviello RL, Bentley WJ, Michailides TJ, Ferguson L, and Westerdahl BB. 2003 UC IPM Pest Management Guidelines: Fig. Publication 3447. University of California Agriculture and Natural Resources, Oakland, CA. [Google Scholar]
- Curtis CE, Clark JD. Pheromone application and monitoring equipment used in field studies of the navel orangeworm (Lepidoptera: Pyralidae) Journal of Economic Entomology. 1984;77:1057–1061.0022-0493(1984)077<1057:PAAMEU>2.0.CO;2 [Google Scholar]
- Curtis CE, Landolt P, Clark JD. Disruption of navel orangeworm (Lepidoptera: Pyralidae) mating in large-scale plots with synthetic pheromone. Journal of Economic Entomology. 1985;78:1425–1430.0022-0493(1985)078<1425:DONOLP>2.0.CO;2 [Google Scholar]
- (DANR) Division of Agriculture and Natural Resources University of California. 1990 DDU Degree-Day Utility. UC IPM Publication 9. University of California Press, Oakland, California. 61. pp. [Google Scholar]
- Evenden ML, Judd GJR, Borden JH. Pheromone-mediated mating disruption of Choristoneura rosaceana: Is the most attractive blend really the most effective? Entomologia Experimentalis et Applicata. 1999;90:37–47.0013-8703(1999)090<0037:PMDOCR>2.0.CO;2 [Google Scholar]
- Ferguson L, Michailides TJ, Shorey HH. The California fig industry. Horticultural Reviews. 1990;12:409–490.0163-7851(1990)012<0409:TCFI>2.0.CO;2 [Google Scholar]
- Minks AK, Cardé RT. Disruption of pheromone communication in moths: Is the natural blend really most efficacious? Entomologia Experimentalis et Applicata. 1988;49:25–36.0013-8703(1988)049<0025:DOPCIM>2.0.CO;2 [Google Scholar]
- Rice RE. A comparison of monitoring techniques for the navel orangeworm. Journal of Economic Entomology. 1976;69:25–28.0022-0493(1976)069<0025:ACOMTF>2.0.CO;2 [Google Scholar]
- Ryne C, Svensson GP, Löfstedt C. Mating disruption of Plodia interpunctella in small-scale plots: Effects of pheromone blend, emission rates, and population density. Journal of Chemical Ecology. 2001;27:2109–2124. doi: 10.1023/a:1012251106037.0098-0331(2001)027<2109:MDOPII>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- SAS Institute. 1999 SAS/STAT User's Guide, Version 8. SAS Institute, Inc., Cary, NC. [Google Scholar]
- Sanderson JP, Barnes MM, Seaman WS. Synthesis and validation of a degree-day model for navel orangeworm (Lepidoptera: Pyralidae) development in California almond orchards. Environmental Entomology. 1989;18:612–617.0046-225X(1989)018<0612:SAVOAD>2.0.CO;2 [Google Scholar]
- Shorey HH, Gerber RG. Use of puffers for disruption of sex pheromone communication among navel orangeworm moth (Lepidoptera: Pyralidae) in almonds, pistachios, and walnuts. Environmental Entomology. 1996;25:1154–1157.0046-225X(1996)025<1154:UOPFDO>2.0.CO;2 [Google Scholar]
- Shorey HH, Sisk CB, Gerber RG. Widely separated pheromone release sites for disruption of sex pheromone communication in two species of Lepidoptera. Environmental Entomology. 1996;25:446–451.0046-225X(1996)025<0446:WSPRSF>2.0.CO;2 [Google Scholar]
- Smilanick JM. Colonization of ripening figs by Carpophilus spp. Journal of Economic Entomology. 1979;72:557–559.0022-0493(1979)072<0557:CORFBC>2.0.CO;2 [Google Scholar]
- Sokal RR, Rohlf FJ. 1995 Biometery: The Principles and Practice of Statistics in Biological Research. Freeman and Co., New York. [Google Scholar]
- Tebbets JS, Curtis CE, Fries RD. Mortality of immature stages of the navel orangeworm stored at 3.5 °C. Journal of Economic Entomology. 1978;71:875–876.0022-0493(1978)071<0875:MOISOT>2.0.CO;2 [Google Scholar]
- van Deventer P, Blommers LHM. Mating disruption of several leaf feeding orchard leaf-roller species with a single sex pheromone component. Acta Phytopathologica et Entomologica Hungarica. 1992;27:615–620.0238-1249(1992)027<0615:MDOSLF>2.0.CO;2 [Google Scholar]
- van Steenwyk RK, Barnett WW. Improvements of navel orangeworm (Lepidoptera: Pyralidae) egg traps. Journal of Economic Entomology. 1985;78:282–286.0022-0493(1985)078<0282:IONOLP>2.0.CO;2 [Google Scholar]
- Zalom FG, Barnett WW, Weakley CV. Efficacy of winter sanitation for managing the navel orangeworm, Paramyelois transitella (Walker), in California almond orchards. Protection Ecology. 1984;7:37–41.0378-4339(1984)007<0037:EOWSFM>2.0.CO;2 [Google Scholar]
- Zalom FG, Van Steenwyk RK, Bentley WJ, Coviello RL, Rice RE, Hendricks LC, Pickel C, Freeman MW, Teviotdale BL, Gubler WD, Stapleton JJ, McKenry MV, and Prather TS. 2002 UC IPM Pest Management Guidelines: Almond. Publication 3431. University of California Agriculture and Natural Resources, Oakland, CA. [Google Scholar]


