Abstract
Polyandry in hymenopteran social insects is surprisingly rare, despite its likely colony-level fitness benefits. Ordinarily, a male's fitness will be at odds with that of a colony when the genetic representation within it is diluted by multiple mating by the queen. Consequently, males are expected to be under selection to limit female re-mating, for example via secretions of their accessory glands. I hypothesized that if accessory glands in some way regulate female mating frequency, an evolutionary transition from single to multiple mating would likely be accompanied with a change in the morphology of the accessory glands. The accessory gland morphology was examined in the fungus-gardening ants, which have made a single transition from single to multiple mating. The evolution of polyandry within this clade corresponds to the loss of male accessory glands, lending tentative support to the idea that they may be involved in regulating mating frequency.
| Abbreviation: | |
|---|---|
| MAG | Male accessory gland |
Keywords: fire ants, mating plugs, fatty acids
Introduction
Polyandry, multiple mating by females, occurs frequently in animals. By mating with several males, females gain benefits such as reduced harassment (Wilcox 1984), nuptial gifts (Zeh and Smith 1985), fertilization insurance (Ridley 1983), or genetic variety among offspring (Keller and Reeve 1994). However, it is generally believed that the proportion of eggs fertilized due to polyandry, is not in the in the interests of a male. Indeed, males have evolved numerous tactics preventing females from re-mating, from mate guarding (Birkhead and Parker 1997), to mating plugs (Devine 1977).
Hymenopteran social insects (ants, bees and wasps) are a notable exception to the general prevalence of polyandry, with most females mating just once and using sperm from that copulation for the rest of their lives. To date polyandry has been documented in only about a half-dozen genera of ants, bees and wasps (Boomsma and Sundström 1998, Strassmann 2001). Although their mates do not provide nuptial gifts or parental care, as they die shortly after mating, social insect females are nonetheless expected to benefit from polyandry. In particular, females may either acquire a potentially advantageous mixture of genes for their offspring (Page and Metcalff 1982; Crozier and Page 1985; Sherman et al. 1988; Keller and Reeve 1994), obtain a quantity of sperm necessary for long term production of workers and large colony sizes (Cole 1983) or reduce kin conflict with daughters over sex ratios and male parentage (Starr 1984; Moritz 1985). Indeed, genetic advantages of polyandry have been demonstrated both in naturally polyandrous harvester ants (Cole and Wiernasz 1999) and, by artificial insemination, in singly mating bumblebees (Baer and Schmid-Hempel 1999).
Mating frequency in social insects determines the relatedness of workers and thus the genetic structure of the colony, which affects numerous aspects of behavior. For example, changes in relatedness brought upon by polyandry may be involved in resolving conflicts over male parentage and offspring sex ratios (Ratnieks 1988; Boomsma and Grafen 1990). Accordingly, knowledge of the forces underlying the evolution of multiple mating by queens is fundamental to our understanding of social evolution.
The interests of males have been largely ignored when considering social insect polyandry, probably because of the secondary roles males appear to play in these societies, but see Hölldobler and Bartz (1985) and Boomsma and Sundström (2000), for alternative perspectives. However, secretions of male accessory glands (MAGs), which attenuate re-mating by females, have been documented in numerous non-social insects (Chen 1984). More recently mating plugs were discovered in a bumblebee species (Sauter et al. 2001). These plugs, which consist principally of a mixture of four fatty acids, were found to inhibit re-mating by females (Baer et al. 2001). Male fire ants transfer at least two of the same four fatty acids to females during mating (Mikheyev, 2003) suggesting that re-mating suppression of polyandry may occur in many hymenopteran social insects (Sauter et al. 2001), and may involve the same chemical mechanism as the bumblebees.
The fungus gardening ants have been the focus of several phylogenetic (Schultz and Meier 1995, Wetterer et al. 1998) and paternity studies (Kerr 1961, Moser 1967, Corso and Serzedello 1981, Villesen et al. 1999, Murakami et al. 2000). While the basal species are singly mating, leaf cutters in the genera Atta and Acromyrmex, made a single transition to polyandry (Villesen et al. 2002). Thus, these ants provide a means of examining the possible association between MAGs and the evolution of polyandry. Specifically, if MAG secretions in some way affect female mating frequency, then the evolutionary transition from monandry to polyandry would likely be correlated with morphological changes in the glands themselves.
Materials and Methods
Males from seven attine genera (seventeen species) were dissected under magnification, their internal reproductive systems were photographed and the volumes of their male accessory glands (MAGs) and seminal vesicles measured by approximating them as either cylinders or spheroids. In addition, a photograph of the male reproductive system (Hung and Vinson 1975) of the polyandrous (Moser 1967) At. texana was included in the analysis. See Table 1 for a complete list of species.
Table 1.
Species used for morphological and histological study. Numbers in parentheses indicate sample size.
Males of the fire ant Solenopsis invicta, a known singly mated species (Ross and Fletcher 1985) with a well-studied reproductive system (Ball and Vinson 1984), were dissected and included as an outgroup. S. invicta, which belongs in the same subfamily as the attines (Myrmicinae), illustrates the internal reproductive morphology common to most ants studied previously (Beck 1972; Hung and Vinson 1975; Wheeler and Krutzsch 1992), which appears to be the primitive condition in ants.
The seminal vesicles and MAGs of the multiply mating genera Acromyrmex and Atta, and a few singly mates species, were stained and sectioned. All the specimens were stored in 70% ethanol. The MAGs and seminal vesicles were dissected out, fixed for 16–24 h with either Bouins solution or Z-5, then dehydrated with a succession of 95% ethanol (15 min), 100% ethanol (15 min), methyl salicylate (30 min) and two changes of xylene. The tissues were then infiltrated with two changes of Paraplast Plus (Fisher Scientific, www.fishersci.com/) mixed with a few drops of honeybee wax (1 hour each infiltration). Tissues were sectioned at 10 µm, mounted on slides and dried overnight. After being deparaffinized and hydrated with distilled water, the slides were stained with hematoxylin (30 sec) and eosin-Y (15 sec). The estimates of MAG volumes for Acromyrmex and Atta species were made from stained sections mounted on slides. In this manner sperm could be clearly visible in the seminal vesicles, while MAG contents stained a solid red.
Statistical analysis
Male ants are highly specialized for locating and mating with females. A male's gaster contains the bare minimum of life support organs with the rest being occupied by the reproductive structures; MAGs and the seminal vesicles. As there appeared to be a trade-off between allocating space to MAGs versus seminal vesicles, the relative changes in their morphology were expressed as a ratio of MAG volume over seminal vesicle volume.
To eliminate any potential problems caused by phylogenetic non-independence due to unequal genetic distance between species, phylogenetic information derived from (Wetterer et al. 1998) and T. R. Schultz, (pers. comm. 2002) was incorporated to compute a set of phylogenetically independent contrasts (Felsenstein 1985) using the CONTRAST module of PHYLIP (version 3.5).
Results
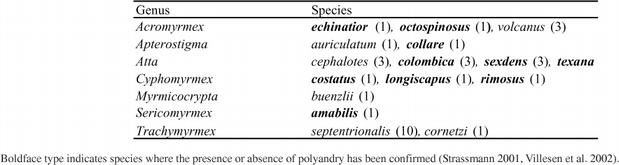
The loss or extreme reduction of MAGs exactly corresponds with the appearance of polyandry in the fungus gardening ants. While the MAGs of the basal attines and fire ants are prominent, with conspicuous lobes protruding laterally off the ejaculatory duct, Atta possess minute accessory glands, relative to the size off their seminal vesicles, and Acromyrmex appear to lack MAGs entirely. Figure 1 shows the presence or absence of large MAGs mapped onto the attine phylogeny (Schultz and Meier 1995, Wetterer et al. 1998), as well as characteristic MAG morphologies. The reproductive system of attine males, excluding the leaf cutter clade, is remarkably similar to that of fire ants and to that of most ants studied to date in that they all possess slender seminal vesicles and prominent lobed MAGs. In the singly mating species, MAGs were about equal in volume to the seminal vesicles. However, a major transition in internal morphology occurs in the leaf cutting genera with the extreme reduction of MAGs in Atta and their complete disappearance in Acromyrmex. Figure 2 illustrates the inverse relationship between the volumes of MAGs relative to seminal vesicle volume and a species' effective paternity, which is a measure of the genetic diversity within a colony due to multiple matings. The effective mating frequency controls for unequal sperm usage by weighing the relative contribution to fertilizations of the different mates of the queen. The phylogenetically independent contrasts of relative MAG volume and effective paternity were negatively correlated (r=−0.77, N=6) for all the species listed in Figure 2, except Ac. echinatior and At. colombica, which lacked phylogenetic distance information and were not included in the analysis.
Figure 1.
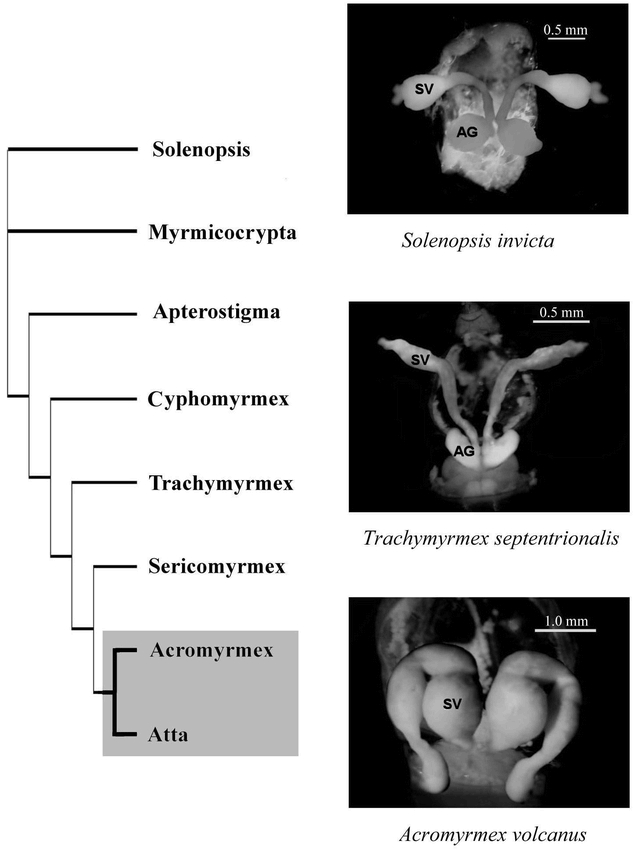
Trait mapping on the attine phylogeny. The transition indicated on the phylogeny corresponds to both the evolution of polyandry, as well as to the reduction of MAGs in the monophyletic leafcutter clade including Acromyrmex and Atta. Representative dissections of males, illustrating the primitive and derived morphologies, are shown alongside the phylogeny. An outgroup, the fire ants (Solenopsis) has been included for an informal comparison. AG = accessory glands, SV = seminal vesicles. Note that Ac. volcanus does not have accessory glands.
Figure 2.
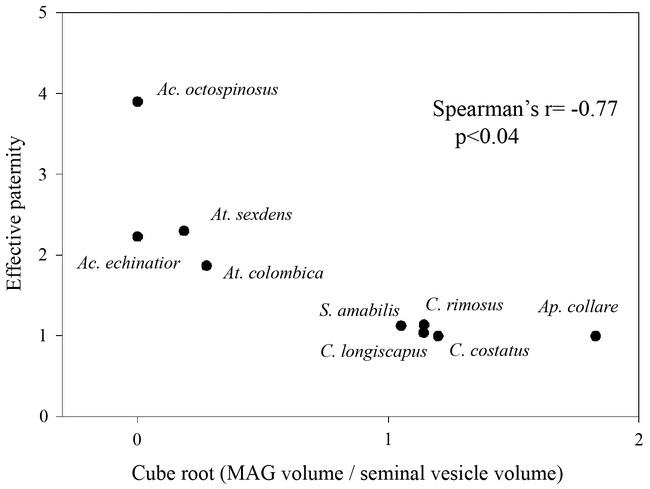
Relationship between relative MAG volume and the number of matings by queens of attine ants (Strassmann 2001, Villesen et al. 2002). The polyandrous species (Acromyrmex and Atta) have much smaller MAGs relative to their seminal vesicles than the monandrous genera (Apterostigma, Cyphomyrmex and Sericomyrmex).
Discussion
The change in MAG volume, which decreases in multiply mating species, suggests that these glands may be involved in regulating multiple mating by females. Ideally, behavioral assays using instrumentally inseminated females should be used to test the function of MAGs, as has been done in bumblebees (Baer et al. 2001). However, to the best of my knowledge, no attine ant has ever been instrumentally inseminated or mated in a laboratory setting. Thus, for now, morphological correlations, somewhat corroborated by evidence for fatty acid transfer from fire ant MAGs (Mikheyev, 2003), provide the best, though inconclusive evidence that MAGs regulate multiple mating in attine ants.
However, as is the case for all correlative evidence, these observations are consistent with a number of other alternative hypotheses. For instance, MAGs could be the source of nuptial gifts, which are no longer offered by males of Acromyrmex and Atta due to some unknown change in the direction of sexual selection accompanying polyandry. Though Sauter et al. (2001) argued that the bumblebees' mating plug is too small to provide measurable nutritional support and the fire ants secretion also appears miniscule (Mikheyev, 2003), there are no data on size or composition of MAG secretions in the attine ants and this hypothesis cannot be excluded. Likewise, MAG secretions may be used for intersexual signaling that happens differently in singly and multiply mated species. Additionally, the evolution polyandry and subsequent sperm competition may have reduced the adaptive value of large MAGs relative to the need for large seminal vesicles. Doubtless, more hypotheses could be offered.
Assuming that MAG secretions do enforce monandry, we can speculate about forces causing the evolution of polyandry. For instance, polyandry may have evolved when higher attine females, through an unknown mechanism, were able to overcome the male's manipulative strategy. If so, males would not be expected reduce MAG size, but to pursue a coevolutionary arms race, perhaps even leading to MAG hypertrophy. More likely, the evolution of polyandry in the fungus gardeners was driven not solely by female preference for multiple mating but by selection on the males themselves. In theory, polyandry (i.e. increased genetic diversity) amplifies the fitness of a multiply mated colony, relative to a singly mated colony (Page and Metcalff 1982; Sherman et al. 1988). If a queen's multiple mating confers a colony-level fitness boost sufficient to overcompensate her mates for effects of genetic dilution due to polyandry, males will be selected to stop monopolizing females. The beneficial effects of polyandry in harvester ants described by Cole and Wiernasz (1999) appear sufficient to give the necessary fitness amplification, especially considering the high rates of mortality among the more genetically homogeneous colonies. Given the relative rarity of polyandry in the social insects, more needs to be known about the specific benefits of this behavior. Comparative studies may yield clues about why it evolved in only a few lineages.
The inhibition of female re-mating via MAG secretions may be a widespread phenomenon in insects (Chen 1984). The high relatedness within social insect colonies, which has been proposed for the evolution of eusociality through kin selection (Hamilton 1964), presupposes monandry. Thus, MAG-enforced single mating by females in some insect groups may be yet another factor facilitating the evolution of complex kin-based social systems.
Acknowledgments
I thank TR Schultz, JK Wetterer and A Wild for supplying attine males for this study from their collections. I am in debt to UG Mueller and his lab for arranging my accommodations during a collecting trip in Panama and sharing specimens from their collections. I am grateful to WR Tschinkel and his lab for their support throughout the project and assistance with laboratory equipment and supplies. DM Watson performed the sectioning of specimens and the histological stains. I thank R Rogers for allowing me to use the GC/MS at the National High Magnetic Field Laboratory and CA Hughey for making sure the device was up and running when I needed it. Thanks to ES Adams, UG Mueller, D Houle, JN Seal, C Smith, S Soucy and WR Tschinkel for their comments on the manuscript. Funding for this project has been provided by an Explorer's Club grant and a Sigma Xi Grant-in-Aid of Research.
References
- Baer B, Morgan ED, Schmid-Hempel P. A nonspecific fatty acid within the bumblebee mating plug prevents females from remating. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3926–3928. doi: 10.1073/pnas.061027998.0027-8424(2001)098<3926:ANFAWT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer B, Schmid-Hempel P. Experimental variation in polyandry affects parasite loads and fitness in a bumble-bee. Nature. 1999;397:151–154.0028-0836(1999)397<0151:EVIPAP>2.0.CO;2 [Google Scholar]
- Ball DE, Vinson SB. Anatomy and histology of the male reproductive system of the fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae) International Journal of Insect Morphology and Embryology. 1984;13:283–294.0020-7322(1984)013<0283:AAHOTM>2.0.CO;2 [Google Scholar]
- Beck VH. Vergleichende histologische Untersuchungen an Polyergus rufenscens und Raptiformica sanguinea. Insectes Sociaux. 1972;19:301–342.0020-1812(1972)019<0301:VHUAPR>2.0.CO;2 [Google Scholar]
- Birkhead TR, Parker GA. 1997 Sperm competition and mating systems. In: Krebs JR, Davies NB, editors. Behavioral Ecology: An Evolutionary Approach. 121–145.Oxford: Blackwell Science. [Google Scholar]
- Boomsma JJ, Grafen A. Intraspecific variation in ant sex satios and the Trivers-Hare hypothesis. Evolution. 1990;44:1026–1034. doi: 10.1111/j.1558-5646.1990.tb03823.x.0014-3820(1990)044<1026:IVIASS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Boomsma JJ, Sundström L. Patterns of paternity skew in Formica ants. Behavioral Ecology and Sociobiology. 1998;42:85–92.0340-5443(1998)042<0085:POPSIF>2.0.CO;2 [Google Scholar]
- Boomsma JJ, Sundström L. Reproductive alliances and posthumous fitness enhancement in male ants. Proceedings of the Royal Society of London B Biological Sciences. 2000;257:1439–1444. doi: 10.1098/rspb.2000.1161.0962-8452(2000)257<1439:RAAPFE>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PS. The functional morphology and biochemistry of insect male accessory glands and their secretions. Annual Review of Entomology. 1984;29:233–255.0066-4170(1984)029<0233:TFMABO>2.0.CO;2 [Google Scholar]
- Cole BJ. Multiple mating and the evolution of social behavior in the Hymenoptera. Behavioral Ecology and Sociobiology. 1983;12:191–201.0340-5443(1983)012<0191:MMATEO>2.0.CO;2 [Google Scholar]
- Cole BJ, Wiernasz DC. The selective advantage of low relatedness. Science. 1999;285:891–893. doi: 10.1126/science.285.5429.891.0193-4511(1999)285<0891:TSAOLR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Corso CR, Serzedello A. A study of multiple mating habit in Atta laevigata based on DNA content. Comparative Biochemistry and Physiology. 1981;69:901–902.1095-6433(1981)069<0901:ASOMMH>2.0.CO;2 [Google Scholar]
- Crozier RH, Page RE. On being the right size: male contributions and multiple mating in social Hymenoptera. Behavioral Ecology and Sociobiology. 1985;18:105–115.0340-5443(1985)018<0105:OBTRSM>2.0.CO;2 [Google Scholar]
- Devine MC. Copulatory plugs, restricted mating opportunities and reproductive competition among male garter snakes. Nature. 1977;167:345–346. doi: 10.1038/267345a0.0028-0836(1977)167<0345:CPRMOA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. American Naturalist. 1985;125:1–15. doi: 10.1086/703055.0003-0147(1985)125<0001:PATCM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hamilton WD. The genetical evolution of social beahviour. II. Journal of Theoretical Biology. 1964;7:17–52. doi: 10.1016/0022-5193(64)90039-6.0022-5193(1964)007<0017:TGEOSB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hölldobler B, Bartz SH. 1985 Sociobiology of reproduction in ants. In: Hölldobler B, Lindauer M, editors. Experimental Behavioral Ecology and Sociobiology. 237–257.Sunderland: Sinauer Associates. [Google Scholar]
- Hung ACF, Vinson SB. Notes on the male reproductive system in ants (Hymenoptera: Formicidae) Journal of the New York Entomological Society. 1975;83:192–197.0028-7199(1975)083<0192:NOTMRS>2.0.CO;2 [Google Scholar]
- Keller L, Reeve HK. Genetic-variability, queen number, and polyandry in social Hymenoptera. Evolution. 1994;48:694–704. doi: 10.1111/j.1558-5646.1994.tb01354.x.0014-3820(1994)048<0694:GQNAPI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kerr WE. Acasalamento de rainhas com vários machos em duas espécies da tribu Attini (Hymenoptera: Formicidae) Revista Brasileira de Biologia. 1961;21:45–48.0034-7108(1961)021<0045:ADRCVM>2.0.CO;2 [Google Scholar]
- Mikheyev AS. Evidence for mating plugs in the fire ant Solenopsis invicta. Insectes Sociaux. 2003;50:401–402.0020-1812(2003)050<0401:EFMPIT>2.0.CO;2 [Google Scholar]
- Moritz RFA. The effects of multiple mating on the worker-queen conflict in Apis mellifera L. Behavioral Ecology and Sociobiology. 1985;16:375–377.0340-5443(1985)016<0375:TEOMMO>2.0.CO;2 [Google Scholar]
- Moser JC. Mating activities of Atta texana (Hymenoptera: Formicidae) Insectes Sociaux. 1967;14:295–312.0020-1812(1967)014<0295:MAOATH>2.0.CO;2 [Google Scholar]
- Murakami T, Higashi S, Windsor D. Mating frequency, colony size, polyethism and sex ratio in fungus-growing ants (Attini) Behavioral Ecology and Sociobiology. 2000;48:276–284.0340-5443(2000)048<0276:MFCSPA>2.0.CO;2 [Google Scholar]
- Page RE, Metcalff RA. Multiple mating, sperm utilization and social evolution. American Naturalist. 1982;119:263–281.0003-0147(1982)119<0263:MMSUAS>2.0.CO;2 [Google Scholar]
- Ratnieks FLW. Reproductive harmony via mutual policing in eusocial Hymenoptera. American Naturalist. 1988;132:217–236.0003-0147(1988)132<0217:RHVMPI>2.0.CO;2 [Google Scholar]
- Ridley M. Mating frequency and fecundity in insects. Biological Reviews. 1983;63:509–549.0006-3231(1983)063<0509:MFAFII>2.0.CO;2 [Google Scholar]
- Ross KG, Fletcher DJC. Comparative study of genetic and social structure in two forms of the fire ant, Solenopsis invicta. Behavioral Ecology and Sociobiology. 1985;17:349–356.0340-5443(1985)017<0349:CSOGAS>2.0.CO;2 [Google Scholar]
- Sauter A, Brown MJF, Baer B, Schmid-Hempel P. Males of social insects can prevent queens from multiple mating. Proceedings of the Royal Society of London B Biological Sciences. 2001;268:1449–1454. doi: 10.1098/rspb.2001.1680.0962-8452(2001)268<1449:MOSICP>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz TR, Meier R. A phylogenetic analysis of the fungus-growing ants (Hymenoptera: Formicidae: Attini) based on morphological characters of the larvae. Systematic Entomology. 1995;20:337–370.0307-6970(1995)020<0337:APAOTF>2.0.CO;2 [Google Scholar]
- Sherman PW, Seeley TD, Reeve HK. Parasites, pathogens, and polyandry in social Hymenoptera. American Naturalist. 1988;131:602–610. doi: 10.1086/286127.0003-0147(1988)131<0602:PPAPIS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Starr CK. 1984 Sperm competition, kinship and sociality in the aculeate Hymenoptera. In: Smith RL, editor. Sperm Competition and the Evolution of Animal Mating Systems. 35–79.Academic Press, Orlando. [Google Scholar]
- Strassmann J. The rarity of multiple mating by females of social Hymenoptera. Insectes Sociaux. 2001;48:1–13.0020-1812(2001)048<0001:TROMMB>2.0.CO;2 [Google Scholar]
- Villesen P, Gertsch PJF, Frydenberg J, Mueller UG, Boomsma JJ. Evolutionary transition from single to multiple mating in fungus-growing ants. Molecular Ecology. 1999;8:1819–1824. doi: 10.1046/j.1365-294x.1999.00767.x.0962-1083(1999)008<1819:ETFSTM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Villesen P, Murakami T, Schultz TR, Boomsma JJ. Identifying the transition between single and multiple mating of queens in fungus-growing ants. Proceedings of the Royal Society of London B Biological Sciences. 2002;269:1541–1548. doi: 10.1098/rspb.2002.2044.0962-8452(2002)269<1541:ITTBSA>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterer JK, Schultz TR, Meier R. Phylogeny of fungus-growing ants (Tribe Attini) based on mtDNA sequence and morphology. Molecular Phylogenetics and Evolution. 1998;9:42–47. doi: 10.1006/mpev.1997.0466.1055-7903(1998)009<0042:POFATA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wheeler DE, Krutzsch PH. Internal Reproductive system in adult males of the genus Camponotus (Hymenoptera: Formicidae: Formicinae) Journal of Morphology. 1992;211:307–317. doi: 10.1002/jmor.1052110308.0362-2525(1992)211<0307:IRSIAM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wilcox RS. Male copulatory guarding enhances female foraging success in a water strider. Behavioral Ecology and Sociobiology. 1984;15:171–174.0340-5443(1984)015<0171:MCGEFF>2.0.CO;2 [Google Scholar]
- Zeh DW, Smith RL. Paternal investment in terrestrial arthropods. American Zoologist. 1985;25:785–805.0003-1569(1985)025<0785:PIITA>2.0.CO;2 [Google Scholar]


