Abstract
DNA sequence of European corn borer, Ostrinia nubilalis (Hübner) (Lepidoptera: Crambidae), mitochondrial cytochrome c oxidase I (cox1) and II (cox2) genes were characterized and used for population genetic analysis. Twenty-six point mutations were identified from a 2,156 bp DNA sequence alignment. The frequency of polymorphic cox1 DdeI and HaeIII, and cox2 Sau3AI and MspI restriction sites were determined from 1,414 individuals by polymerase chain reaction restriction fragment length polymorphism. Ten haplotypes were observed. A single haplotype was present among 90% of individuals examined, and a HaeIII haplotype was not present in samples from the Atlantic coast. Significant genetic differentiation existed between Atlantic coast and midwestern United States samples, and between sympatric uni- and bivoltine ecotypes. These genetic markers identify regional and ecotype differences in the North American O. nubilalis population.
| Abbreviation: | |
|---|---|
| D | genetic distance |
| PCR-RFLP | Polymerase chain reaction restriction fragment length polymorphism |
Keywords: voltinism variation, geographic variation, Lepidoptera, Crambidae
Introduction
Population structure and mating barriers affect rates of gene flow within a species (Mutebi et al. 2002). The European corn borer, Ostrinia nubilalis (Hübner), is an introduced insect pest of agricultural crops in North America (Mason et al. 1996). O. nubilalis is endemic to Europe and western Asia, and by 1917 it was observed in the eastern United States. It migrated westward, and reached North Dakota by 1950 (Chiang 1972; Showers 1993). The North American O. nubilalis population shows phenotypic variation in pheromone production and perception, and the number of generations per year (voltinism; Showers 1993; Mason et al. 1996).
The pheromone of female O. nubilalis, 11-tetradecenyl acetate, is produced in stereoisomeric forms (E and Z) and in varying blend ratios (Roelofs et al. 1972; Klun et al. 1973). Two ecotypes, the E-strain (New York type) and Z-strain (Iowa type), use 98:2 and 1:99 mixtures of (E)- and (Z)-11-tetradecenyl acetates, respectively (Glover et al. 1987). The Z-pheromone-emitting and responding populations are distributed in the central and eastern United States and southern Canada (Showers et al. 1974), whereas the E-strain inhabits the northeastern United States and Quebec (McLeod et al. 1979). The distribution of pheromone races overlap in the eastern United States and intermating of pheromone races has been documented (Roelofs et al. 1985; Durant et al. 1995).
The uni-, bi-, and multivoltine ecotypes of O. nubilalis differ in response to photoperiod and temperature for diapause induction, resulting in differences in the number of degree-days required for pupation (Showers 1979). Genes that determine voltinism are male sex-linked, and show temperature- and scotophase-dependent dominance (Showers et al. 1972; Showers 1981). Univoltine ecotypes may have a selective advantage in northern climates where short growing seasons favor a single generation, whereas in more temperate southern regions, extended larval dormancy may result in increased mortality (Showers 1993). Genetic and environmental factors determine voltinism phenotypes, resulting in north-south clines that correspond to barrier latitudes that restrict univoltine ecotypes to a northern distribution (Showers 1979; Showers 1993). Movement of bivoltine ecotypes into traditionally univoltine regions, without reciprocal migration, supports the concept of southern barrier latitudes for univoltine migration (Chiang et al. 1965; McEwen et al. 1968). Variation in post-diapause developmental time between voltinism ecotypes (Calvin and Song 1994; Hoard and Weiss 1995) may promote asynchrony in mating period that minimizes genetic exchange (Roelofs et al. 1985). To date, no significant genetic differences have been detected between co-existing (sympatric) voltinism ecotypes.
Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) evaluation of four mitochondrial DNA fragments (Marcon et al. 1999), and variation at six allozyme markers (Bourguet et al. 2000) showed no genetic differences among voltinism ecotypes. PCR-random amplification of polymorphic DNA (RAPD) markers indicated that multivoltine ecotypes (>2 generations per year) were genetically different from both uni- and bivoltine ecotypes (Pornkulwat et al. 1998), but voltinism differences were confounded by geographic variance. Coates and Hellmich (2003) showed regional variation of northern (Minnesota) subpopulations from all other sample sites at two sex-linked microsatellite marker loci. In the current study polymorphism in O. nubilalis cox1 and cox2 genes was used to investigate contributions of voltinism, pheromone race, and geographic location to North American population structure.
Materials and Methods
Sample preparation
Second-flight O. nubilalis adults were obtained from 14 North American locations (Fig. 1) in light or pheromone bait traps. At Lamberton, Minnesota, and Rosemount, Minnesota, season-long monitoring separated peaks of uni- and bivoltine ecotypes. Two pheromone bait traps, one E- and the other Z-, were placed in proximity at Cape Elizabeth, and Oxford Maine. An E-pheromone trap was used at the Newark, Delaware location. The last sample site was the Ithaca, New York colony of Z-pheromone individuals were originally collected May 1996 near Geneva, New York, or E-pheromone individuals were in continuous culture since April 1994, after initial collection from fields in Bouchville, New York. The laboratory colony was maintained at the New York State Agricultural Experiment Station, Geneva, New York. All other samples were collected in light or pheromone traps (see Fig. 1). DNA extraction was performed on individual thoraces as described by Marcon et al. (1999). DNA was suspended in 100 µl of TLE (10 mM Tris, 0.1 mM EDTA, pH 7,5), and DNA concentrations were adjusted to 50 ng/µl with TLE, and stored at −20° C.
Figure 1.
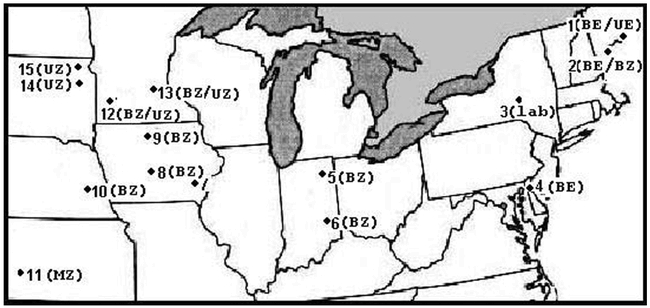
The location of 15 North American Ostrinia nubilalis collection sites. Ecotypes obtained from each collection site are indicated; univoltine Z pheromone race (UZ), bivoltine Z pheromone race (BZ), univoltine E pheromone race (UE), or bivoltine E pheromone race (BE). The New York laboratory colony data (site 3; lab) was omitted from statistical analysis due to haplotype fixation. Collection site ID: 1 Oxford Maine; 2 Cape Elizabeth, Main, 3 Ithaca, New York; 4 Newark, Delaware; 5 Columbia City, Indiana; 6 Franklin County, Indiana; 7 Crawfordsville, Iowa; 8. Hubbard, Iowa; 9 Kanawha, Iowa; 10 Mead, Nebraska; 11 Garden City, Kansas; 12 Lamberton, Minnesota; 13 Rosemount, Minnesota; 14 Brookings, South Dakota; 15 South Shore, South Dakota.
DNA sequencing
Mitochondrial cox1, trnLUUR, and cox2 gene sequences were compared among 14 individual adult corn borers. PCR of individual DNA samples used 50 µl reactions with 2.5 mM MgCl2, 0.8 µM dNTPs, 10.0 pmol of each primer TY-J-1460 and TK-N-3785 (Simons et al. 1994), 400 ng of DNA, and 1.7 U of Taq polymerase (Promega, www.promega.com). A temperature cycle of 94° C for 3 min., followed by 40 cycles of 94° C for 40 sec, 53° C for 50 sec, and 72° C for 2 min was carried out on a PTC-100 thermocycler (MJ Research, http://www.mjr.com/). Purification of PCR reactions used Qiaquick PCR purification columns (Qiagen, www.qiagen.com) according to manufacturer directions, and 200 ng used in dye-terminator cycle sequencing quick start (DTCS-quick) primer extension reactions (Beckman-Coulter, www.beckman-coulter.com). Dye-terminator cycle sequencing (DTCS) reaction products were injected at 110kV for 15 sec onto a CEQ8000 capillary electrophoresis system (Beckman-Coulter, www.beckman-coulter.com) and separated at 110kV for 120min. Sequence reaction data were imported into Vector NTI Suite 7.0 (Informax, www.informax.com), and individual contigs were constructed. Cox1 and cox2 sequences were aligned with homologous regions the O. nubilalis (GenBank AF442957) and O. furnicalis (GenBank AF467260) mitochondrial genomes by using AlignX software (Informax; gap penalty of 5). Amino acid sequence was determined with Sequin 4.00 (www.ncbi.nlm.nih.gov).
PCR-RFLP methods
Three PCR primer pairs, TY-J-1460 (Simons et al. 1994) with OnCox-D-R (5′-TCCA GGATTACCTAATTCAGCTC-3′), OnCox-H-F (5′-CACGAGCTTACTTTACCTCAGCA-3′) with OnCox-H-R (5′-CCAGCTAGCCCTAAGAAATGTTG-3′), and OnCox-SM-F (5′-GGCTA GC TGGTATACCTCGAC-3′) with OnCox-SM-R (5′-GGAGAGGCTCTATTTTGTAGACTA-3′) spanned polymorphic regions. All PCR amplifications used 1.5 mM MgCl2, 0.5 uM dNTPs, 5 pmol of each primer, 0.225 U of Taq DNA polymerase (Promega), and 150 ng of DNA in a 12.5 µl reaction volume. Thermocycler conditions were 94° C for 2 min, followed by 40 cycles of 94° C for 30 sec, 52° C for 30 sec, and 72° C for 20 sec. Single 25 µl digests with DdeI, HaeIII, MspI, or Sau3AI including 5.0 µl of PCR product, 2.5 µl 10X buffer (Promega), 0.1 mg/µl bovine serum albumin, and 0.5 U of enzyme (Promega) were incubated at 37° C for 8 to 14 hr. Reactions were loaded on 1.0 mm by 16 cm 6% polyacrylamide (29:1 acrylamide: bisacrylamide) 0.5X Tris borate EDTA gels, and separated at 140 V for 4 h with a 25-bp step-ladder (Promega) for size comparison. Gels were stained with ethidium bromide, and digital images were taken on a PC-FOTO/Eclipse documentation system (Fotodyne, www.fotodyne.com).
Data analysis
Pairwise effective number of migrants (Nm) were calculated from linearized FST estimates (Slatkin, 1995) and measured between Atlantic coast and Midwestern samples by using estimated F-statistic; Nm = ((1/FST) − 1) (Hartl and Clark 1997). Genetic distance and analysis of molecular variance (AMOVA) were calculated as described by Weir and Cockerham (1984) and Excoffier et al. (1992) by using Arlequin v1.1 (Schneider et al. 1997). AMOVA tested regional population subdivision (Atlantic coast verses Midwestern samples) and sympatric voltinism ecotype differentiation at Lamberton and Rosemount collection sites. Haplotype relationships were investigated by phylogenetic analysis of PCR-RFLP and DNA sequence alignment data using parsimony that incorporated 1,000 bootstrap resampling steps performed using Seqboot, followed by Mix or DNAPars, and Consense programs in the PHYLIP package (Felsenstein 1989). A haplotype network was constructed from RFLP data using the program TCS version 1.12 (available at http://inbio.byu.edu/Faculty/kac/crandall_lab/programs.htm). Genetic differentiation by geographic distance model was tested by comparison of log10 distance (km) to log10 genetic distance estimates (Slatkin 1993) using NTSYS-pc v. 1.70 (Rohlf 1992), and MXCOMP of NTSYS-pc V. 1.70 to perform Mantel tests with 1000 iterations to test significance. Three levels of comparison were made using European corn borer genetic distance (D) estimates 1) among Atlantic coast and 2) among Midwestern sample sites, and 3) between Atlantic and Midwestern sample sites. ANOVA was performed using Proc Mixed of the SAS statistical software (v. 8).
Results and Discussion
DNA sequence analysis
Mitochondrial cox1, tRNA-LeuUUR, and cox2 genes were sequenced from 14 O. nubilalis moths to survey genetic variation within the species. A 2,156 bp sequence alignment showed 26 point mutations of which 7 resulted in nonsynonymous amino acid changes (Table 1). Individual mutations were rare and resided in only one or two sequences. Cox1 and cox2 gene sequences were highly similar (> 99.5 and > 99.2%, respectively), and no mutations were observed in tRNA-LeuUUR. Screening of 1,414 O. nubilalis moths from 15 collection sites (Fig. 1) revealed low polymorphism at DdeI, HaeIII, MspI, and Sau3AI sites, and a non-digested mitochondrial haplotype among 90% of individuals (1,267 out of 1,414; Table 2). Low O. nubilalis mitochondrial polymorphism agreed with reports by Marcon et al. (1999), and was congruent with allozyme markers (Harrison and Vawter 1977; Cianchi et al. 1980; Glover et al. 1991; Bourguet et al. 2000) and sex-linked microsatellite data (Coates and Hellmich 2003). Low mitochondrial variation may result from a genetic bottleneck at North American O. nubilalis introduction, selection, or mitochondrial DNA fixation by genetic drift. Similar results were observed from moth species, the gypsy moth, Lymantria dispar (Harrison et al. 1983), and tobacco budworm, Heliothis virescens (Roehrdanz et al. 1994), suggesting little intraspecific differentiation within the genera.
Table 1.
Twenty-six variable nucleotide positions observed between 14 North American Ostrinia nubilalis mitochondrial cox1 and cox2 sequences. Random collection of sequences used to survey level and location of polymorphism among samples, such that PCR-RFLP assays could be developed for higher sample throughput. Positions with respect to the O. nubilalis mitochondrial genome sequence (GenBank AF442957), and – indicates sequence identical to the consensus sequence. RFLPs: D = DdeI, H = HaeIII, S = Sau3AI, and M = MspI are indicated. See Table 2 for location detail of collection site ID.
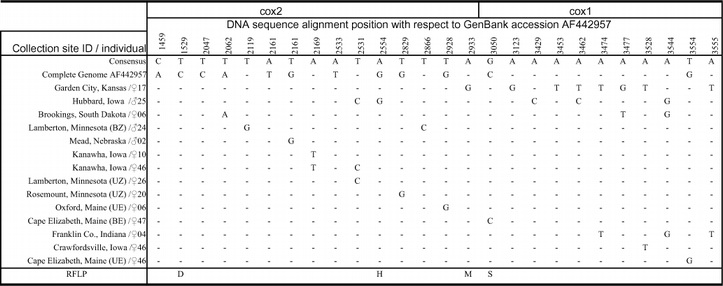
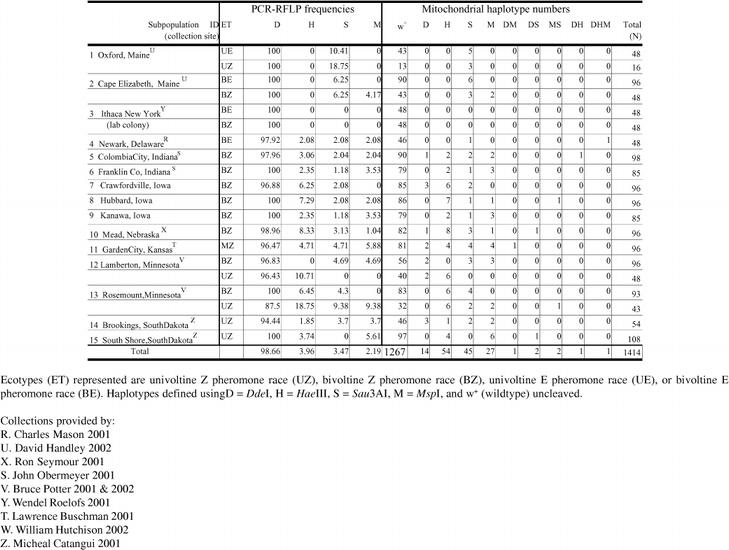
Table 2.
The distribution of Ostrinia nubilalis mitochondrial RFLP frequencies and haplotypes in samples from 15 North American collection sites. Frequency of digestion (PCR-RFLP frequency), and number of O. nubilalis with a given haplotype at each collection site (mitochondrial haplotype number) are provided. Note: Maine (ME) and Minnesota (MN) samples were divided into ecotype (ET) for genetic distance estimation (see Table 4).
Phylogenetic methods and haplotype networks can construct haplotype relationships. Parsimony trees constructed from DNA sequence (Fig. 2) or PCR-RFLP haplotypes (not shown) were inconclusive with respect to haplotype relationships. The parsimony-based phylogenetic tree using DNA sequence data produced bootstrap branch support above a 50% threshold (n = 1,000 bootstrap resample steps) at only 4 of 10 nodes. Nested clade analysis (NCA; Templeton et al. 1992) was similarly unsuitable due to the generation of a circular haplotype-network (data not shown). Inability to resolve haplotype relationships using phylogenetic methods may reside in the absence of sufficient differentiation among O. nubilalis cox1 and cox2 haplotypes (Page and Holmes 1998).
Figure 2.
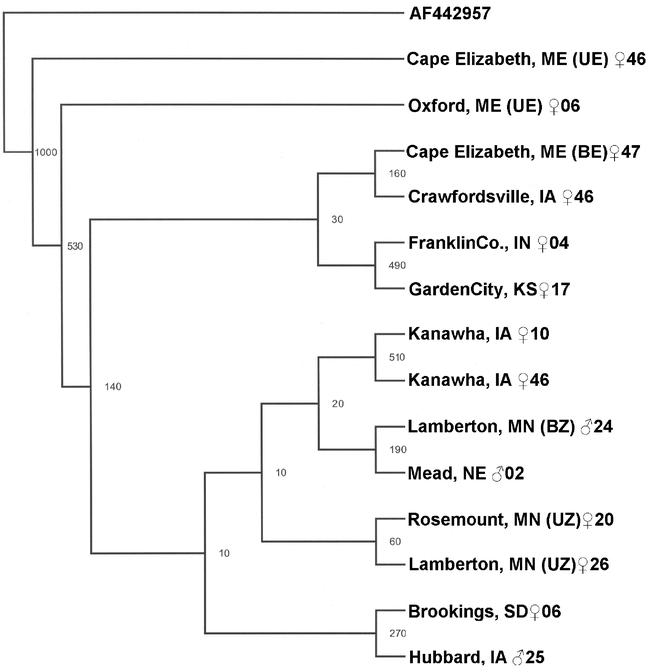
Parsimony tree constructed from a 2,156 bp alignment of 14 mitochondrial cox1, tRNA-LeuUUR, and cox2 DNA sequence (Table 1) randomly selected from the 1,414 Ostrinia nubilalis samples evaluated.
Geographic population differentiation
Polymorphism was absent from Ithaca, New York laboratory colony samples, and these data were omitted resulting in 14 collection sites for analysis. An isolation-by-distance model was rejected as the basis of genetic differentiation between 14 North American O. nubilalis collection sites. Using the program NYSYS-pc v. 1.70 (Rohlf 1992), genetic distance versus geographic distance (km) matrices were plotted, and showed no correlation (R2 = 0.1132, P = 0.9759). Therefore, geographic distance between sample sites may not be correlated with genetic differentiation. Alternatively, the geographic region of the collection site, Midwestern or Atlantic coast, was investigated as the basis of genetic variation. A direct comparison of HaeIII haplotype frequency between Midwestern (5.1%; 54 of 1,055) and Atlantic coast samples (0.0%), indicated presence of a region specific haplotype (private allele; Table 2).
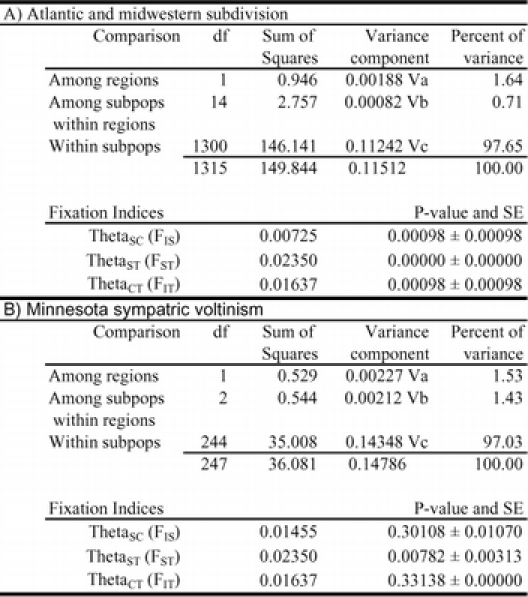
Population differentiation imparted by the absence of HaeIII haplotypes from Atlantic coast samples was investigated using modified fixation indices (θ-statistics; Weir and Cockerham, 1984; Excoffier et al. 1992), analysis of molecular variance (AMOVA; Excoffier et al. 1992), and genetic distance estimates (Nei 1972; Schneider et al. 1997). A comparison of Midwestern and Atlantic coast DdeI, HaeIII, MspI, and Sau3AI RFLP haplotype frequencies generated a modified fixation index, θST, of 0.024 that indicated moderate inbreeding and significantly higher relatedness of haplotypes within each region (P < 0.0001). Low but significant mitochondrial haplotype differentiation between O. nubilalis corroborates prior studies (Harrison and Vawter 1977; Cianchi et al. 1980; Glover et al. 1991; Coates and Hellmich 2003). Analysis of molecular variance (AMOVA) comparing Midwestern and Atlantic coast haplotype frequencies indicated 1.64% of total variation within North America might be due to regional differences (Table 3; Panel A). The presence of a single non-digesting haplotype among 90% of the individuals (w+; Table 2) may contribute to low variation between geographic regions. The absence of the HaeIII haplotype from the Atlantic coast samples suggest that it may be the basis of the low-level geographic (regional) differentiation, and its relative contribution was estimated by excluding it from fixation index estimation and comparison to original values. After removal of HaeIII PCR-RFLP haplotypes, the fixation index (θST) decreased to 0.017 (P < 0.010; data not shown), suggesting that HaeIII haplotypes contributed 29% of the measurable increase in relatedness of haplotypes within each geographic region ((0.024−0.017)/0.024 @ 0.29). These analyses and raw haplotype frequency data suggest the HaeIII haplotype might have regional geographic specificity or reflect North American O. nubilalis population subdivision.
Table 3.
AMOVA and modified F-statistics (Theta (θ)-statistics; Weir and Cockerham, 1984). A) Subdivision of North American Ostrinia nubilalis haplotypes into Atlantic coast (sites 1, 2, and 5) and Midwestern collection sites (sites 5–15, Table 2; the Ithaca, New York laboratory colony haplotype data were omitted). B) Subdivision of sympatric univoltine (UZ) and bivoltine (BZ) ecotypes from Lamberton and Rosemount, Minnesota collection sites (sites 12 and 13; Table 2).
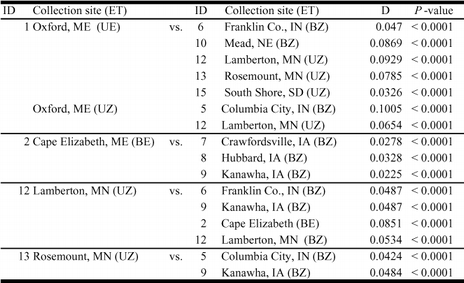
Genetic distance estimates from 14 O. nubilalis collection sites were generated in a pairwise manor (Ithaca, New York laboratory colony haplotypes omitted), and comparisons supported evidence of O. nubilalis geographic differentiation previously inferred from fixation indices and AMOVA (Table 3A). Genetic distances were estimated separately for Lamberton and Rosemount, Minnesota voltinism ecotypes, and Cape Elizabeth and Oxford, Main pheromone races (i.e. treated as separate subpopulations and analyzed as separate entities; Table 2; discussed in next section). Genetic distance estimates suggested differentiation between 16 of 153 comparisons using a Bonferroni corrected significance threshold (P < 0.0003; Table 4). No significant difference in genetic distance was predicted between any two collection sites or ecotypes on the Atlantic coast, suggesting greater genetic similarity within Atlantic coast regions compared to the Midwest. Geographic similarity was further investigated by one-way ANOVA comparing genetic distance (D) between Atlantic coast and Midwestern samples, and resulted in significant differentiation (F = 14.53; df = 2, 150; P < 0.0001).
Table 4.
Significantly different pairwise Nei genetic distance (D) comparisons from an 18 × 18 matrix of 14 Ostrinia nubilalis sample sites (Ithaca, New York laboratory colony data omitted). Empirical P values were determined after 10,000 Markov chain steps (permutations), and significance threshold was set using the Bonferroni adjustment for multiple tests (0.05/153 = 0.0003267). Note: Haplotypes from Lamberton and Rosemount, Minnesota (MN), and Cape Elizabeth and Oxford, Main (ME) collection sites subdivided into ecotype (Table 2). See Table 2 for collection site ID and ecotype (ET) definition.
Exclusion of the HaeIII haplotype from Atlantic coast regions suggest 1) low frequency of HaeIII haplotype migration, or 2) separate and distinct regional O. nubilalis introductions. Assuming Hardy-Weinberg equilibrium, a historic effective female migration rate (Nfm = ((1/FST) − 1); Hartl and Clark 1997) between Atlantic coast and Midwestern regions was estimated at 40 to 41 female migrants per generation (using θST = 0.024; Table 3). Migration estimates are crude due to unrealistic island model assumptions (Hartl and Clark 1997), but suggest a historically moderate level of haplotype exchange. Migration rate estimates may be lower due to inability to differentiate many O. nubilalis mitochondrial haplotypes. The second hypothesis assumes that Atlantic and Midwestern differences result from separate North American O. nubilalis introductions. Records indicate O. nubilalis infestations in eastern North America near Boston (1913) and eastern New York State (1919), and a single Midwestern introduction near Lake Erie (Vinal 1917; Showers 1993). If Boston and New York introductions populated Atlantic coast regions and the single Lake Erie introduction populated the Midwest, present day genetic differentiation might be indicative of European founders. Alternatively, HaeIII haplotype extinction in the Atlantic coast region could have resulted after introduction due to random genetic drift within a small founder population or selection within new habitats. Sampling error in the current study is unlikely to be due to collection sizes at multiple regional locations (Fig. 1; Table 2). Additional sampling is required for follow up confirmatory studies.
Sympatric ecotype differentiation
Coexisting (sympatric) pheromone races were present in the Oxford (site 1) and Cape Elizabeth, Main (site 2) pheromone traps. Comparison of mitochondrial haplotypes between pheromone races showed no significant inbreeding effects within ecotype at Oxford and Cape Elizabeth, Main locations (θST = 0.021; P = 0.081), suggesting gene flow between pheromone ecotypes and corroboration of pheromone hybrid females observed in the field (Roelofs et al. 1985; DuRant et al. 1995). Sympatric voltinism ecotypes also were collected from the same light trap at Lamberton (site 12) and Rosemount (site 13) Minnesota. Comparison of haplotype frequency among sympatric voltinism ecotypes may represent a measure of ecotype variation in the absence of confounding geographic effects. In contrast to pheromone ecotypes, differences were detected when haplotypes from Minnesota collection sites were separated into voltinism ecotypes and analyzed using modified fixation indices (Weir and Cockerham, 1984; Excoffier et al. 1992), and analysis of molecular variance (AMOVA; Excoffier et al. 1992). The level of relatedness was significantly higher for haplotypes of the same voltinism ecotype at the Lamberton and Rosemount, Minnesota collection sites (θST = 0.030; P = 0.008; Table 3). This suggested inbr,eeding within and reduced gene flow between, voltinism ectypes. Furthermore, only 1.53% of total genetic variance between haplotypes was due to voltinism, again suggesting presence of low but significant levels of differentiation.
Sympatric voltinism ecotype differentiation was also detected via a pairwise comparison of genetic distance estimates (Table 4). Specifically, the sympatric uni- and bivoltine ecotypes from Lamberton, Minnesota showed a significantly different pairwise genetic distance (D = 0.0534, P < 0.0001; Table 4), and may represent a measure of ecotype variation without confounding geographic effects. In contrast, no significant genetic distance was observed between uni- and bivoltine haplotype frequencies from Rosemount, Minnesota (D = 0.0198; P = 0.054; data not shown). Genetic differentiation between sympatric voltinism ecotypes at Lamberton, Minnesota suggests a possible mating barrier that may be attributable to mating period asynchrony (Eckenrode et al. 1983; Roelofs et al. 1985). Recent northern bivoltine movement into Minnesota might be responsible for voltinism differences. Univoltine ecotypes migrated to Minnesota in the early 1940s (Chiang 1961) and reached North Dakota in 1950 (Chiang 1972), and a second O. nubilalis migration entered southern Minnesota in 1952 which was suggested to be a northern expansion of bivoltine moths (Chiang et al. 1965; McLeod 1978; Palmer et al. 1985). Bivoltine migration into univoltine areas, and minimal gene flow due to non-overlapping mating periods, may have maintained ecotype differences. Alternatively, genetic drift in smaller northern populations may cause yearly fluctuation in haplotype frequencies that caused the Rosemount, Minnesota samples to be above threshold when sampled. However, the Bonferroni-corrected significance thresholds may be unrealistically high.
Significant differences in genetic distance estimates, θ-statistics, and AMOVA suggest little interspecific gene flow. Genetic diversity observed between sympatric O. nubilalis voltinism types may reflect the effects of reproductive isolation or ecological adaptation (Showers 1979; Showers 1993). Ecotype differentiation not associated with change in plant host range appears rare except in the pea aphid, gallflies, soapberry bug, and brown plant hopper (see Berlocher and Feder 2002 for references). The extent of O. nubilalis ecotype (reproductive) isolation in regions of sympatry remains to be determined, but development of more polymorphic genetic markers and more detailed sampling might help address this question.
Conclusions
Low but significant geographic subdivision of the North American O. nubilalis population may exist (Table 2 and 4). Exclusion of an O. nubilalis HaeIII mitochondrial haplotype from Atlantic coast populations might indicate a genetic bottleneck among European founders, rarity of HaeIII among immigrants from Midwestern region of the United States, or a migrant barrier imparted by the Appalachians. Data also showed genetic differentiation between sympatric O. nubilalis voltinism ecotypes, which may be maintained by asynchrony of mating periods and a reduced level of intermating (McLeod et al. 1979; Eckenrode et al. 1983), or may be anartifact of recent northern expansion of bivoltine ecotypes. Additional genetic markers and more detailed sampling are required to further investigate these observations and currently are under development.
Disclaimer
Mention of a proprietary product does not constitute an endorsement or a recommendation by USDA or Iowa State University for its use.
Acknowledgments
This research was a joint contribution from the USDA Agricultural Research Service and the Iowa Agriculture and Home Economics Experiment Station, Ames, IA (project 3543). This article reports the results of research only. Thank you to all contributors of biological material from collection sites.
References
- Berlocher SH, Feder JL. Sympatric speciation in phytophagous insects: Moving beyond controversy? Annual Review of Entomology. 2002;47:773–815. doi: 10.1146/annurev.ento.47.091201.145312.0066-4170(2002)047<0773:SSIPIM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bourget D, Bethenod MT, Pasteur N, Viard F. Gene flow in the European corn borer O. nubilalis : implications for the sustainability of transgenic insecticidal maize. Proceeding of the Royal Society of London B. 2000;267:117–122. doi: 10.1098/rspb.2000.0975.0962-8452(2000)267<0117:GFITEC>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin DD, Song PP. Variability in postdiapause development periods of geographically separate Ostrinia nubilalis (Lepidoptera: Pyralidae) populations in Pennsylvania. Environmental Entomology. 1994;23:431–436.0046-225X(1994)023<0431:VIPDPO>2.0.CO;2 [Google Scholar]
- Cianchi R, Maini R, Bullini L. Genetic distance between pheromone strains of the European corn borer Ostrinia nubilalis different contribution of variable substrate regulatory, and nonregulatory enzymes. Heredity. 1980;45:383–388.0018-067X(1980)045<0383:GDBPSO>2.0.CO;2 [Google Scholar]
- Chiang HC. Fringe populations of the European corn borer, Pyrausta nubilalis: Their characteristics and problems. Annals of the Entomological Society of America. 1961;54:378–387.0013-8746(1961)054<0378:FPOTEC>2.0.CO;2 [Google Scholar]
- Chiang HC. Dispersion of the European corn borer (Lepidoptera: Pyralidae) in Minnesota and South Dakota, 1945 to 1970. Environmental Entomology. 1972;1:157–161.0046-225X(1972)001<0157:DOTECB>2.0.CO;2 [Google Scholar]
- Chiang HC, Sisson V, Ewert MA. Northerly movement of corn borer moth, Ostrinia nubilalis in southern Minnesota. Proceeding of the Minnesota Academy of Sciences. 1965;33:17–19. [Google Scholar]
- Coates BS, Hellmich RL. Two sex-chromosome-linked microsatellite loci show geographic variance among North American Ostrinia nubilalis. Journal of Insect Science. 2003;3:29. doi: 10.1093/jis/3.1.29.1536-2442(2003)003<0029:TSMLSG>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant JA, Fescemyer HW, Mason CE, Udayagiri S. Effectiveness of four blends of European corn borer (Lepidoptera: Pyralidae) sex pheromone isomers at three locations in South Carolina. Journal of Agricultural Entomology. 1995;12:241–253.0735-939X(1995)012<0241:EOFBOE>2.0.CO;2 [Google Scholar]
- Eckenrode CJ, Robbins PS, Andaloro JT. Variations in flight patterns of European corn borer (Lepidoptera: Pyralidae) in New York. Environmental Entomology. 1983;12:393–396.0046-225X(1983)012<0393:VIFPOE>2.0.CO;2 [Google Scholar]
- Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics. 1992;131:479–491. doi: 10.1093/genetics/131.2.479.0016-6731(1992)131<0479:AOMVIF>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. PHYLIP-phylogeny inference package (v.3.2) Cladistics. 1989;5:164–166.0748-3007(1989)005<0164:PIPV>2.0.CO;2 [Google Scholar]
- Glover TJ, Tang XH, Roelofs WL. Sex pheromone blend discrimination by male moths from E and Z strains of European corn borer. Journal of Chemical Ecology. 1987;13:143–151. doi: 10.1007/BF01020358.0098-0331(1987)013<0143:SPBDBM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Glover TJ, Knodel JJ, Robbins PS, Eckenrode CJ, Roelofs WL. Gene flow among three races of European corn borers (Lepidoptera: Pyralidae) in New York State. Environmental Entomology. 1991;20:1356–1362.0046-225X(1991)020<1356:GFATRO>2.0.CO;2 [Google Scholar]
- Harrison RG, Vawter AT. Allozyme differentiation between pheromone strains of the European corn borer, Ostrinia nubilalis. Annals of the Entomological Society of America. 1977;70:717–720.0013-8746(1977)070<0717:ADBPSO>2.0.CO;2 [Google Scholar]
- Harrison RG, Wintermeyer SF, Odell TM. Patterns of genetic variation within and among gypsy moth, Lymantria dispar (Lepidoptera: Lymantriidae), populations. Annals of the Entomological Society of America. 1983;76:652–656.0013-8746(1983)076<0652:POGVWA>2.0.CO;2 [Google Scholar]
- Hartl DL, Clark AG. 1997 Principles of Population Genetics. Sinauer, Sunderland, MA. [Google Scholar]
- Hoard MW, Weiss MJ. Influence of postdiapause development on the voltinism of the European corn borer (Lepidoptera: Pyralidae) in North Dakota. Environmental Entomology. 1995;24:564–570.0046-225X(1995)024<0564:IOPDOT>2.0.CO;2 [Google Scholar]
- Hudson M, LeRoux EJ. Biology and population dynamics of the European corn borer (Ostrinia nubilalis) with special reference to sweet corn in Quebec. I. Systematics, morphology, geographical distribution, host range, economic importance. Phytoprotection. 1986;67:39–54.0031-9511(1986)067<0039:BAPDOT>2.0.CO;2 [Google Scholar]
- Klun JA, Chapman OL, Mattes KC, Wojtkowski PW, Beroza M, Sonnet PE. Insect sex pheromones: Minor amount of opposite geometrical isomer critical to attraction. Science. 1973;181:661–663. doi: 10.1126/science.181.4100.661.0193-4511(1973)181<0661:ISPMAO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Marcon PCRG, Taylor DB, Mason CE, Hellmich RL, Siegfried BD. Genetic similarity among pheromone and voltinism races of Ostrinia nubilalis (Hübner) (Lepidoptera: Crambidae) Insect Molecular Biology. 1999;8:213–221. doi: 10.1046/j.1365-2583.1999.820213.x.0962-1075(1999)008<0213:GSAPAV>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mason CE, Rice ME, Calvin DD, Van Duyn JW, Showers WB, Hutchison WD, Witkowski JF, Higgins RA, Onstad DW, and Dively GP. 1996 European corn borer: ecology and management. Bulletin NC-327, Iowa State University, Ames, IA. [Google Scholar]
- McEwen FL, Adams JA, Davis AC, Rinick HB Jr. Corn borer in western New York. N.Y. Food Science Q. 1968;1:15–16. [Google Scholar]
- McLeod DGR. Genetics of diapause induction and termination in the European corn borer, Ostrinia nubilalis (Lepidoptera: Pyralidae), in southwestern Ontario. Canadian Entomologist. 1978;110:1351–1353.0008-347X(1978)110<1351:GODIAT>2.0.CO;2 [Google Scholar]
- McLeod DGR, Ritchot C, Nagei T. Occurrence of a two-generation strain of the European corn borer, Ostrinia nubilalis (Lepidoptera: Pyralidae), in Quebec. Canadian Entomologist. 1979;11:233–236.0008-347X(1979)011<0233:OOATSO>2.0.CO;2 [Google Scholar]
- Mutebi JP, Alexander JB, Lanzaro GC. Genetic differentiation among populations of Lutzomyia longipalpis (Diptera: Psychodidae) in central and South America. Annals of the Entomological Society of America. 2003;95:740–752.0013-8746(2003)095<0740:GDAPOL>2.0.CO;2 [Google Scholar]
- Nei M. The genetic distance between populations. American Naturalist. 1972;106:283–292.0003-0147(1972)106<0283:TGDBP>2.0.CO;2 [Google Scholar]
- Page RDM, Holmes E. 1998 Molecular Evolution: A Phylogenetic Approach. Blackwell, Boston, MA. [Google Scholar]
- Palmer DE, Schenk TC, and Chiang HC. 1985 Dispersal and voltinism adaptation of the European corn borer in North America, 1917–1977. Minnesota Agriculture Experiment Station Bulletin AD-SB-2716. [Google Scholar]
- Pornkulwat S, Skoda SR, Thomas GD, Foster JE. Random amplified polymorphic DNA used to identify genetic variation in ecotypes of the European corn borer (Lepidoptera: Pyralidae) Annals of the Entomological Society of America. 1998;91:719–725.0013-8746(1998)091<0719:RAPDUT>2.0.CO;2 [Google Scholar]
- Roelofs WL, Cardé RT, Bartelt RJ, Tierney PG. Sex attractant trapping of the European corn borer in New York. Environmental Entomology. 1972;1:606–608.0046-225X(1972)001<0606:SATOTE>2.0.CO;2 [Google Scholar]
- Roelofs WL, Du JW, Tang XH, Robbins PS, Eckenrode CJ. Three European corn borer populations in New York based on sex pheromones and voltinism. Journal of Chemical Ecology. 1985;11:829–836. doi: 10.1007/BF01012071.0098-0331(1985)011<0829:TECBPI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Roehrdanz RL, Lopez JD, Loera J, Hendricks DE. Limited mitochondrial DNA polymorphism in North American populations of Heliothis virescens (Leptidoptera; Noctuidae) Annals of the Entomological Society of America. 1994;87:856–864.0013-8746(1994)087<0856:LMDPIN>2.0.CO;2 [Google Scholar]
- Rohlf FJ. 1992 NTSYS-pc numerical taxonomy and multivariate system. Exeter Publishing, Ltd., New York, NY. [Google Scholar]
- Schneider S, Kueffer JM, Roessli D, and Excoffier L. 1997 Arlequin ver.1.1: a software for population genetic data analysis. Genetics and Biometry Laboratory, University of Geneva, Switzerland. [Google Scholar]
- Showers WB, Brindley TA, Reed GL. Survival and diapause characteristics of hybrids of three geographical races of the European corn borer. Annals of the Entomological Society of America. 1972;65:450–457.0013-8746(1972)065<0450:SADCOH>2.0.CO;2 [Google Scholar]
- Showers WB, Reed GL, Oloumi-Sadeghi H. European corn borer: Attraction of males to synthetic lure and to females of different strains. Environmental Entomology. 1974;3:51–58.0046-225X(1974)003<0051:ECBAOM>2.0.CO;2 [Google Scholar]
- Showers WB. 1979 Effects of diapause on the migration of the European corn borer into the doutheastern United States. In: Rabb RL, Kennedy GG editors. Movement of Highly Mobile Insects: Concepts and Methodology in Research pp. 420–430.North Carolina State University Press. [Google Scholar]
- Showers WB. 1981 Geographic variation of the diapause response in the European corn borer. In: Denno RF, Dingle H editors. Insect Life History Patterns, Habitat and Geographic Variation, pp. 97–111.Springer Verlag, New York, NY. [Google Scholar]
- Showers WB. 1993 Diversity and variation of European corn borer populations. In: Kim KC, McPherson BA editors. Evolution of Insect Pests/Patterns of Variation, pp. 287–309.Wiley and Sons Inc., New York, NY. [Google Scholar]
- Simons C, Frati F, Bechenback A, Crespi B, Liu H, Flook P. Evolution, weighting, and phylogentic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Annals of the Entomological Society of America. 1994;87:651–701.0013-8746(1994)087<0651:EWAPUO>2.0.CO;2 [Google Scholar]
- Templeton AR, Crandall KA, Sing CF. A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. III. Cladogram estimation. Genetics. 1992;132:619–633. doi: 10.1093/genetics/132.2.619.0016-6731(1992)132<0619:ACAOPA>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinal SC. The European corn borer, a recently established pest in Massachusetts. Massachusetts Agriculture Experiment Station Bulletin. 1917;178:147–152. [Google Scholar]
- Weir BS, Cockerham CC. Estimating F-statistics for the analysis of population structure. Evolution. 1984;38:1358–1270. doi: 10.1111/j.1558-5646.1984.tb05657.x.0014-3820(1984)038<1358:EFFTAO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]


